Abstract
Purpose:
To compare image characteristics of retinal nerve fiber layer (RNFL) between glaucoma patients and healthy controls using adaptive optics scanning laser ophthalmoscopy (AOSLO).
Methods:
This was a cross-sectional pilot study with two groups: a glaucoma group with patients with moderate or severe glaucoma as per the Hodapp–Parrish–Anderson classification system and a control group with healthy individuals. The optic nerve damage in moderate glaucoma was predominantly located in only one hemisphere; the other hemisphere was un- or minimally affected on optical coherence tomography and automated perimetry and is referred to as early glaucoma. The structure of RNFL bundles and gain (%) in RNFL images with mean pixel values between 15 and 35 were analyzed. Imaging was performed one degree away from the optic disc margin at two and four cardinal clock positions in the glaucoma and control groups, respectively. The field of view was 1.3° at 2.3 m resolution. We studied one eye per participant.
Results:
There were 11 glaucoma patients and 7 healthy controls. Imaging was successful at 88% of the locations in controls and early glaucoma; the reflectivity differed significantly (0.51 and 0.56, respectively, P < 0.001) but not the structure of RNFL bundles (Cohen’s Kappa 0.11) between them. In patients with moderate and severe glaucoma, imaging was successful only at 46% of the locations; RNFL bundles were not discernible, and RNFL reflectivity did not differ from those with early glaucoma (P < 0.11).
Conclusion:
The recorded gain (%) of RNFL images obtained using AOSLO could be an objective indicator of early glaucoma.
Keywords: Adaptive optics scanning laser ophthalmoscopy (AOSLO) in glaucoma, early detection of glaucoma, reflectivity of retinal nerve fiber layer
Glaucoma is a neurodegenerative disease caused by progressive retinal ganglion cell (RGC) loss associated with characteristic structural and functional changes in the optic nerve. Early detection of glaucoma is important to maximize the benefits of treatment and minimize the economic burden of the disease. While there are several methods to assess the structure of the optic nerve, the function of the nerve is largely measured using differential light sensitivity test with standard automated perimetry (SAP). Both structural and functional changes result from the loss of RGCs and their axons. However, these structural and functional measures exhibit wide variability in glaucoma patients.[1] In the early stage of glaucoma, significant loss of RGCs would correspond to relatively small changes in mean deviation (MD) on SAP. The converse is true in severe glaucoma, wherein anatomical measures have a residual nonneural component. One of the reasons for this nonlinear structure–function relationship is the logarithmic scaling of perimetric stimulus for threshold measurement and reporting.[2,3]
The structural assessment of the optic nerve is best done by optical coherence tomography (OCT), as this method is expected to detect thinning of retinal nerve fiber layer (RNFL) and macular ganglion cell complex (GCC) in glaucoma. However, the diagnostic performance of OCT in detecting early glaucoma is not as good as its performance in detecting moderate or severe glaucoma.[4,5,6] Wide anatomic variations of the optic nerve head limit the diagnostic ability of OCT.[7] In addition, several sources of error and the database having a limited number of subjects of any single ethnicity limit the diagnostic performance of OCT in detecting glaucoma.[8]
Reflectivity of RNFL has recently been proposed as a marker for glaucoma.[9,10] Incident light rays are reflected from the cytoskeleton of the ganglion cell axons.[11] A change in reflectivity of incident light rays preceded thinning of RNFL in an experimental animal model.[9] In a longitudinal study, the interaction between reflectance and thickness of RNFL improved prediction of rate of change of MD on SAP.[10] Attempts have been made to quantify the reflectance of RNFL using OCT,[10] multispectral imaging,[12] and second harmonic imaging[13]; however, these have not been successful as OCT does not provide a robust enough quantification system, and the latter two techniques can only be carried out ex-vivo at this point in time.
Adaptive optics scanning laser ophthalmoscopy (AOSLO) could fill this gap in imaging of the retina.[14] This technique combines adaptive optics to remove optical aberrations from images obtained from scanning laser ophthalmoscopy. This has enabled the imaging of living retinal cells in reflective mode.[15] Alterations in the reflectance of photoreceptors detected using AOSLO have been used as biomarkers for inherited retinal diseases.[16]
In this study, we use AOSLO to detect alterations in the reflectance of the RNFL between glaucoma patients with varying degrees of severity and healthy controls. Our AOSLO system set up has been published elsewhere.[17] As far as we are aware, this is the first report on using measures of reflectance of the RNFL using AOSLO as an indicator for glaucoma.
Methods
Study design
This was a cross-sectional pilot study. Written informed consent was obtained from all participants. The study followed the tenets of the Declaration of Helsinki. Ethics committee approval was obtained from the institutional review board.
Study center
The study was carried out at a large, tertiary care, referral eye institute.
Study population
Consecutive patients visiting glaucoma clinic over 4 months and fulfilling the inclusion criteria (described below) were enrolled in the glaucoma group. Healthy controls were selected from general clinic. Only one eye per participant was studied.
Study procedure and definitions
The participants underwent comprehensive ophthalmic evaluation which included an account of their medical history, refraction, slit-lamp examination, intraocular pressure measurement with Goldmann applanation tonometry, dark room gonioscopy with 4-mirror gonioprism, and dilated fundus evaluation. In addition, the glaucoma patients underwent OCT (Carl Zeiss Meditec AG, Jena, Germany) and Humphrey visual field (HVF) testing.
Glaucoma was diagnosed based on characteristic structural changes in the optic nerve head with corresponding visual field loss. The severity of glaucoma was defined using the Hodapp–Parrish–Anderson (H-P-A) criteria.[18] A visual field defect on HVF 24-2 program is labeled “moderate” when: (a) the MD is between -6 and -12 dB, (b) <50% points are depressed below the 5% level and <20 points are depressed below the 1% level on the pattern deviation plot, (c) no point in the central 5 degrees has a sensitivity of 0 dB, and (d) only one hemifield has a point with sensitivity <15 dB within 5 degrees of fixation. Similarly, by the H-P-A criteria, a defect is labeled “severe” when: (a) the MD is worse than -12 dB, (b) >50% points are depressed below the 5% level and >20 points are depressed below the 1% level on the pattern deviation plot, (c) at least 1 point in the central 5 degrees has a sensitivity of 0 dB, and (d) both hemifields have points with sensitivity <15 dB within 5 degrees of fixation.[18]
The glaucoma group included patients with established glaucoma in moderate or severe stage. Patients with moderate glaucoma had asymmetric optic nerve damage across the horizontal meridian; the less affected hemisphere had normal or borderline RNFL thinning as assessed by OCT and unaffected or minimally affected function (better than the definition of “moderate” glaucoma as per the H-P-A criteria) on HVF. This hemisphere was considered to represent, and will henceforth be called early glaucoma. The patients in the control group had no ocular abnormalities. Additional inclusion criteria for all the participants were best-corrected visual acuity ≥20/25, spherical equivalent ≤3 Diopters, and intraocular pressure ≤22 mm Hg with or without antiglaucoma medication(s).
The participants were scheduled for AOSLO imaging on a specific day. The imaging was performed using a 790 nm wavelength laser source (Superlum, Ireland) with a light power of 180 mW and fixed system settings. We established the relationship between the gain of the detector [Photon Multiplier Tube (PMT), H7422-50 Hamamatsu, Japan] and the mean pixel intensity of the image through system calibration using a model eye.
The locations for imaging were 1 degree away from the margin of optic disc at 3, 6, 9, and 12 o’clock hours. For the control group, the imaging was done at all four locations. Imaging was done at 6 and 12 o’clock, i.e. the affected hemisphere and the un- or minimally affected hemisphere (referred to as early glaucoma) in patients with moderate glaucoma and both the affected hemispheres in patients with severe glaucoma. The patients with severe glaucoma were imaged at 3 and 9 o’clock, if no RNFL structure was identified at 6 and 12 o’clock. The field of view was 1.3° at 2.3 m resolution.
Analysis of RNFL structure
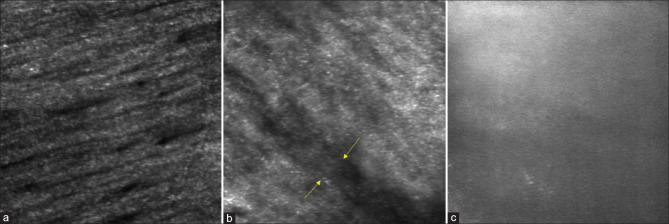
We captured videos by focusing the AOSLO system on the RNFL using the standard technique.[19] Images were obtained by processing these videos. At the outset, we prepared a set of test images for training. The set consisted of five AOSLO images each, focused on the RNFL of healthy control eyes and those with different severities of glaucoma. Two fellowship-trained and experienced glaucoma specialists were trained on the areas of missing, indistinguishable, or absent RNFL bundles due to glaucoma [Fig. 1]. None of the images used for training were used in the assessment.
Figure 1.
Images of the retinal nerve fiber layer (RNFL) obtained with AOSLO (adaptive optics scanning laser ophthalmoscopy). (a) Healthy RNFL; (b) RNFL in moderate glaucoma; note areas of missing (marked by arrows) and indistinguishable RNFL bundles; (c) RNFL in severe glaucoma; note complete loss of RNFL bundles
The glaucoma specialists qualitatively assessed the structures of RNFL bundles from AOSLO images to distinguish between controls and early, moderate, or severe glaucoma on the basis of the appearance of the RNFL.
Analysis of RNFL reflectivity
We measured the reflected light from the RNFL on AOSLO. The gain could be adjusted between 0 and 1 (PMT control voltage) and the pixel value could vary between 0 and 255 on the intensity scale.[20] The gain (%) was recorded after achieving a mean pixel value between 15 and 35. At each location, a stream of 230 images was captured in 10 s and the mean pixel value was obtained.
The gain adjustment depended on the reflectivity of the object. A highly reflective object reflects a large number of photons and requires a small gain to achieve the desired mean pixel intensity. On the other hand, an object with low reflectance requires a higher gain to achieve the desired mean pixel intensity. While the structures in both high and low reflective images may appear similar, further investigation into the gain that was required to capture a picture of desired pixel intensity can give us the measure of reflectivity.
Statistical analysis
The data is presented as median along with Q1 (first quartile) and Q3 (third quartile). Cohen’s Kappa was calculated to assess interobserver agreement in differentiating between controls and patients with early glaucoma. Intergroup comparison of gain value was done by a nonparametric test. Statistical analyses were performed using statistical software Stata 12.1 (StataCorp, College Station, TX).
Results
We included 18 participants in this study with 11 glaucoma patients (seven with moderate glaucoma and four with severe glaucoma) and 7 healthy controls.
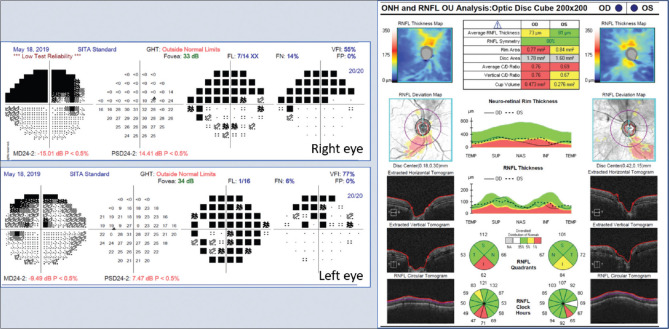
Glaucoma group: The median age of the patients included in this group (of eight men and three women) was 56 (Q1 = 41, Q3 = 62) years. Eight patients had primary open-angle glaucoma, while three had primary angle closure disease. The median IOP on the day of examination prior to AOSLO imaging was 15 mm Hg (Q1 = 13, Q3 = 18) on two (Q1 = 1, Q3 = 2) topical anti-glaucoma medications. The MD on the 24-2 program of HVF in patients with moderate and severe glaucoma was -9.43 (Q1= -6.94, Q3= -10.81) and -25.44 (Q1= -19.79, Q3= -28.8) dB, respectively. The thickness of the RNFL corresponding to the un- or minimally affected segment of the optic nerve in patients with moderate glaucoma was 59 (Q1 = 49, Q3 = 103) mm; these segments were classified as “within normal limits” or “borderline thinning” by the OCT machine classifier. The corresponding hemifield on the 24-2 program of HVF was classified as “better than moderate stage glaucoma” as per the H-P-A criteria [Fig. 2]. In two patients with severe glaucoma, the RNFL images could be obtained only at the nasal clock hour with AOSLO.
Figure 2.
Investigation reports of a typical patient with moderate glaucoma included in the study. This patient’s left eye was studied. Note that the glaucoma predominantly affected the inferior hemisphere in this eye
Control group: Of the seven control participants (six men and one woman), four were age-decade matched and three were in their 20s. The median age of this group was 34 (Q1 = 21, Q3 = 50) years.
RNFL structure
Fig. 3 shows AOSLO images focused on the RNFL at two locations each in the controls and patients with early glaucoma. One glaucoma specialist (RK) could correctly identify three out of five randomly picked images from the control group and five out of seven images from the patients with early glaucoma. The interobserver agreement between the two glaucoma specialists in differentiating between RNFL images of controls from the patients with early glaucoma was poor (Cohen’s Kappa Statistic = 0.11). The evaluators easily identified AOSLO images of RNFL damage in later stages of glaucoma.
Figure 3.
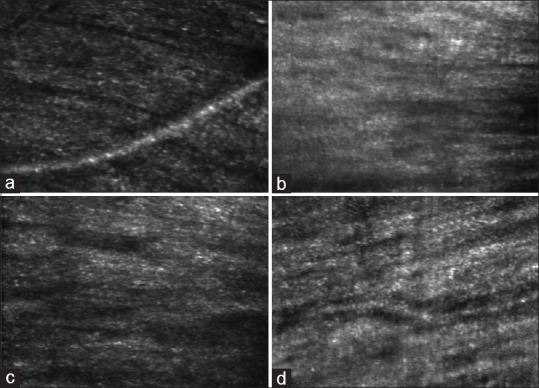
Images of retinal nerve fiber layer (RNFL) obtained with AOSLO (adaptive optics scanning laser ophthalmoscopy). Images (a and b) belong to the control group and (c and d) belong to patients with early glaucoma. Note that there are no clear differences in the structures of the RNFL between controls and patients with early glaucoma
RNFL reflectivity
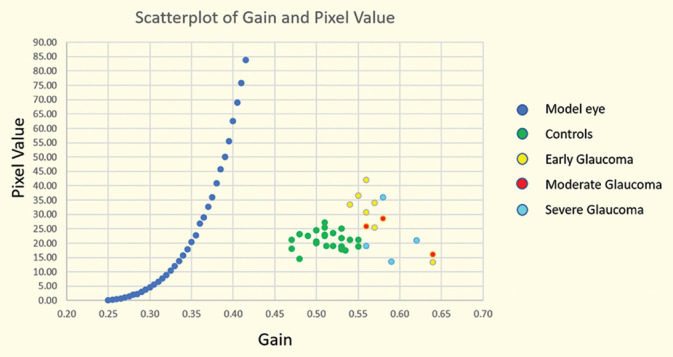
At the outset, we imaged a model eye to establish the intrinsic relationship between the detector’s gain (%) and pixel value [blue dots in Fig. 4]. The slope of the curve is dependent on the detector and the amplifier, but not on the object being imaged. If the same system is used with analogous settings to image multiple objects with varying reflectivity, the curve will shift on either side but its slope will remain constant.
Figure 4.
Scatterplot of detector’s gain (%) and mean pixel value
The gain (%) in the control group images was 0.51 (Q1 = 0.50, Q3 = 0.53). Similarly, the gain in images of the patients with early glaucoma was 0.56 (Q1 = 0.55, Q3 = 0.57) and that of all locations in the glaucoma group was 0.57 (Q1 = 0.56, Q3 = 0.59) (see Fig. 4).
The values of gain at a comparable range of mean pixel values significantly differed between the control group and patients with early glaucoma (P < 0.001, Mann–Whitney U-test). Similarly, the values of gain differed significantly between the control and glaucoma groups (P < 0.001, Mann–Whitney U-test). However, the gain did not differ significantly between the patients with early and later stages of glaucoma (P < 0.11, Mann–Whitney U-test).
The imaging was successfully carried out at 24 out of the possible 28 locations in the control group and at all possible 7 locations affected with early glaucoma. However, images within the desired range of pixel values could be obtained only at three out of the seven locations affected with moderate glaucoma and at four out of the eight locations affected with severe glaucoma.
Discussion
In this study, we have described the use of AOSLO for visualizing RNFL bundles and assessing the reflectivity of the layer for diagnosis of glaucoma; currently, neither of these procedures are possible in-vivo using OCT or any other method.
Significant physiological variation in the structure of the optic disc and peripapillary region is one of the major challenges in the structural diagnosis of early stage of glaucoma.[21] The dependence of automated perimetry on the subject’s performance and potential underestimation of early changes limits the utility of current functional tests in the diagnosis of early glaucoma.[1,2,3] Therefore, taking advantage of topographic differences in the glaucomatous damage to the optic nerve, we included patients with moderate stage of the disease but having asymmetric damage to the optic nerve head. An experimental model of chronic high-pressure glaucoma in Rhesus monkeys has challenged the concept that a mostly diffuse type of optic neuropathy occurs in high-pressure glaucoma by demonstrating a mixture of localized and diffuse pattern of optic nerve damage.[22] The RNFL thickness of the unaffected quadrant on visual fields in eyes with high-pressure glaucoma has been shown to be significantly reduced than the RNFL thickness of the corresponding quadrant of the healthy eyes by scanning laser polarimetry,[23,24] and by both OCT and scanning laser polarimetry.[25] Analogous thinning in the GCC in such cases has also been demonstrated using spectral domain OCT.[26] Thus, our AOSLO imaging of the conventionally un- or minimally affected regions of the optic nerve head in patients with moderate stage glaucoma likely represent an early or even predisease state.
Measuring the thickness of the RNFL using OCT is a well-established method for assessing the structural damage caused by glaucoma. The RNFL also exhibits optical properties attributable to its cytoskeleton components;[11] distortion of the axonal cytoskeleton in a whole-mounted retina in a rat model has been reported as an early sign of glaucomatous damage.[27] Recent studies have attempted to study RNFL reflectivity using OCT.[9,10] However, commercial OCT has several limitations in measuring RNFL reflectivity, which are: a) the direction of RNFL bundles could be a major source of variability in measuring RNFL reflectivity on OCT[12] and b) OCT measures reflectance of RNFL with respect to another layer of the retina. Therefore, while OCT is suitable for monitoring longitudinal changes in RNFL reflectance, it has limited applicability for interindividual comparisons.[10] In addition, parameters like gain, polarization, and focus are automatically adjusted in commercial OCT setups for best visual image reconstruction; this makes the reliability of OCT-based RNFL reflectance measurements questionable. On the other hand, AOSLO provides absolute measurement of RNFL reflectivity due to its scanning principle, high lateral resolution, and manual gain control.[14] In our study, the control group exhibited higher RNFL reflectivity and images with pixel values between 15–35 were obtained at a lower gain. In contrast, higher gain was needed to obtain images within the desired range of pixel values in the glaucoma group. Thus, reflectivity of RNFL, represented by system gain, may be a novel indicator of glaucomatous optic neuropathy. Moreover, this measure is objective and may possibly show a linear relationship with severity of glaucoma.
At the cellular level, alterations in the cytoskeleton are the main cause of altered RNFL reflectance.[11] Glaucoma damages axonal ultrastructure.[28] In an experimental animal model, a change in RNFL reflectivity preceded thinning of the RNFL.[9] In our study, poor agreement between the glaucoma specialists indicates the difficulty in separating those with early glaucoma from healthy controls on the basis of structural changes in RNFL. Varied patterns of abnormality in RNFL in patients with glaucoma have been reported. An abnormal region of RNFL without any bundle was uncommon than bundles of lower contrast or clear bundles sandwiched between regions without bundles.[29] Therefore, one may expect poor agreement or less certainty in detecting “early” structural change in RNFL on AOSLO. Nevertheless, we found a clear distinction between healthy controls and those with early glaucoma on the basis of RNFL reflectivity.
Our study does have a few limitations. The desired mean pixel values of 15–35 could not be achieved in some eyes. The RNFL bundles in patients with glaucoma were seen to be missing, indistinguishable, or lost on AOSLO imaging. If the bundles were indistinguishable, the pixel value was increased to confirm the alterations in their structure. On the other hand, in patients with severe glaucoma, the RNFL bundles were lost, and the pixel value could not be increased even to the level of 15, despite maximizing the gain. The signal on AOSLO in late stages of glaucoma may only represent reflectance from the underlying retinal layers. This observation is analogous to the floor effect in OCT in advanced glaucoma.[30] Thus, the inability to obtain identifiable images in patients with late stages of glaucoma could have resulted in the overlap of RNFL reflectivity between early and other stages of glaucoma. All inclusive, our study has less sample size. Four out of seven controls were age decade matched. The remaining three controls were in 20s, while the lowest age decade was 30s (n = 2) in the study group. At present, AOSLO is an emerging technology, has less tolerance to media opacity, and demands more time as well as cooperation from the patient. We attribute small sample size and intergroup age difference to the current practical limitations of the technology.
In clinical practice, early glaucoma is an area of diagnostic dilemma. We believe we are the first to report usage of AOSLO in measuring reflected light from the RNFL and using this as an indicator to detect glaucoma. This objective indicator is more sensitive to early stage of glaucoma, in contrast to the current gold standard of differential light sensitivity measured on a logarithmic scale. Our choice of site in AOSLO imaging may also aid in early diagnosis of glaucoma as the nerve fiber bundles near the optic nerve head have been shown to be more vulnerable to damage caused by elevated IOP, as exhibited by a rat model[9,31] and by measurements obtained through OCT in humans.[32] Thus, our study on AOSLO possibly identifies a novel, unique, and objective indicator for early diagnosis of glaucoma. These observations need to be validated in a longitudinal study with a larger sample.
Conclusion
Measurement of light reflected from RNFL on AOSLO could be an objective indicator of early stage of glaucoma.
Financial support and sponsorship
Hyderabad Eye Research Foundation, L. V. Prasad Eye Institute, Hyderabad, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We thank Siddharth Dikshit, Glaucoma faculty at VST Glaucoma Center, Dr. Kallam Anji Reddy campus, L V Prasad Eye Institute, Hyderabad, India, for analyzing the images used in the study. We acknowledge the support of David Williams and the Advance Retinal Imaging Alliance (ARIA), Center for Visual Science, Flaum Eye Institute, University of Rochester in building the AOSLO system.
References
- 1.Malik R, Swanson WH, Garway-Heath DF. 'Structure-function relationship'in glaucoma:Past thinking and current concepts. Clin Exp Ophthalmol. 2012;40:369–80. doi: 10.1111/j.1442-9071.2012.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci. 2001;43:2213–20. [PubMed] [Google Scholar]
- 3.Racette L, Medeiros FA, Bowd C, Zangwill LM, Weinreb RN, Sample PA. The impact of the perimetric measurement scale, sample composition, and statistical method on the structure-function relationship in glaucoma. J Glaucoma. 2007;16:676–84. doi: 10.1097/IJG.0b013e31804d23c2. [DOI] [PubMed] [Google Scholar]
- 4.Medeiros FA, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Influence of disease severity and optic disc size on the diagnostic performance of imaging instruments in glaucoma. Invest Ophthalmol Vis Sci. 2006;47:1008–15. doi: 10.1167/iovs.05-1133. [DOI] [PubMed] [Google Scholar]
- 5.Leite MT, Zangwill LM, Weinreb RN, Rao HL, Alencar LM, Sample PA, et al. Effect of disease severity on the performance of Cirrus spectral-domain OCT for glaucoma diagnosis. Invest Ophthalmol Vis Sci. 2010;51:4104–9. doi: 10.1167/iovs.09-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao HL, Leite MT, Weinreb RN, Zangwill LM, Alencar LM, Sample PA, et al. Effect of disease severity and optic disc size on diagnostic accuracy of RTVue spectral domain optical coherence tomograph in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:1290–6. doi: 10.1167/iovs.10-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao HL, Kumbar T, Addepalli UK, Bharti N, Senthil S, Choudhari NS, et al. Effect of spectrum bias on the diagnostic accuracy of spectral-domain optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2012;53:1058–65. doi: 10.1167/iovs.11-8463. [DOI] [PubMed] [Google Scholar]
- 8.Aref AA, Cheema A, Moore DB. Spectral domain optical coherence tomography in glaucoma [American Academy of Ophthalmology, EyeWiki website. Apr, 2020. [Last accessed on 2022 Jun 06]. https: //eyewiki.aao.org/Spectral_Domain_Optical_Coherence_Tomography_in_Glaucoma .
- 9.Huang XR, Zhou Y, Kong W, Knighton RW. Reflectance decreases before thickness changes in the retinal nerve fiber layer in glaucomatous retinas. Invest Ophthalmol Vis Sci. 2011;52:6737–42. doi: 10.1167/iovs.11-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardiner SK, Demirel S, Reynaud J, Fortune B. Changes in retinal nerve fiber layer reflectance intensity as a predictor of functional progression in glaucoma. Invest Ophthalmol Vis Sci. 2016;57:1221–7. doi: 10.1167/iovs.15-18788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang XR, Knighton RW. Microtubules contribute to the birefringence of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2005;46:4588–93. doi: 10.1167/iovs.05-0532. [DOI] [PubMed] [Google Scholar]
- 12.Huang XR, Knighton RW, Feuer WJ, Qiao J. Retinal nerve fiber layer reflectometry must consider directional reflectance. Biomed Opt Express. 2015;7:22–33. doi: 10.1364/BOE.7.000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharoukhov D, Bucinca-Cupallari F, Lim H. Microtubule imaging reveals cytoskeletal deficit predisposing the retinal ganglion cell axons to atrophy in DBA/2J. Invest Ophthalmol Vis Sci. 2018;59:5292–300. doi: 10.1167/iovs.18-24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns SA, Elsner AE, Sapoznik KA, Warner RL, Gast TJ. Adaptive optics imaging of the human retina. Prog Retin Eye Res. 2019;68:1–30. doi: 10.1016/j.preteyeres.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pallikaris A, Williams DR, Hofer H. The reflectance of single cones in the living human eye. Invest Ophthalmol Vis Sci. 2003;44:4580–92. doi: 10.1167/iovs.03-0094. [DOI] [PubMed] [Google Scholar]
- 16.Litts KM, Cooper RF, Duncan JL, Carroll J. Photoreceptor-based biomarkers in AOSLO retinal imaging. Invest Ophthalmol Vis Sci. 2017;58:BIO255–67. doi: 10.1167/iovs.17-21868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dave VP, Kumar S, Mulani Y, Richhariya A, Pappuru RR, Das T. Foveal cone count reduction in resolved endophthalmitis:An adaptive optics scanning laser ophthalmoscopy (AO-SLO)-based prospective pilot study. Br J Ophthalmol. 2021;105:1520–4. doi: 10.1136/bjophthalmol-2020-317309. [DOI] [PubMed] [Google Scholar]
- 18.Hodapp E, Parrish R, II, Anderson D. Clinical Decisions in Glaucoma. 1st ed. St. Louis: Mosby-Year Book; 1993. [Google Scholar]
- 19.Scoles D, Gray DC, Hunter JJ, Wolfe R, Gee BP, Geng Y, et al. In-vivo imaging of retinal nerve fiber layer vasculature:Imaging histology comparison. BMC Ophthalmol. 2009;9:9. doi: 10.1186/1471-2415-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Roorda A. Photon signal detection and evaluation in the adaptive optics scanning laser ophthalmoscope. J Opt Soc Am A Opt Image Sci Vis. 2007;24:1276–83. doi: 10.1364/josaa.24.001276. [DOI] [PubMed] [Google Scholar]
- 21.Garway-Heath DF. Early diagnosis in glaucoma. Prog Brain Res. 2008;173:47–57. doi: 10.1016/S0079-6123(08)01105-9. [DOI] [PubMed] [Google Scholar]
- 22.Jonas JB, Hayreh SS. Localised retinal nerve fibre layer defects in chronic experimental high pressure glaucoma in rhesus monkeys. Br J Ophthalmol. 1999;83:1291–5. doi: 10.1136/bjo.83.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kook MS, Sung K, Kim S, Park R, Kang W. Study of retinal nerve fibre layer thickness in eyes with high tension glaucoma and hemifield defect. Br J Ophthalmol. 2001;85:1167–70. doi: 10.1136/bjo.85.10.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto C, Shirato S, Haneda M, Yamashiro H, Saito M. Study of retinal nerve fiber layer thickness within normal hemivisual field in primary open-angle glaucoma and normal-tension glaucoma. Jpn J Ophthalmol. 2003;47:22–7. doi: 10.1016/s0021-5155(02)00663-9. [DOI] [PubMed] [Google Scholar]
- 25.Bagga H, Greenfield DS. Quantitative assessment of structural damage in eyes with localized visual field abnormalities. Am J Ophthalmol. 2004;137:797–805. doi: 10.1016/j.ajo.2003.11.060. [DOI] [PubMed] [Google Scholar]
- 26.Saito H, Iwase A, Araie M. Comparison of retinal ganglion cell-related layer asymmetry between early glaucoma eyes with superior and inferior hemiretina damage. Br J Ophthalmol. 2020;104:655–9. doi: 10.1136/bjophthalmol-2019-314563. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Kong W, Zhou Y, Gregori G. Distortion of axonal cytoskeleton:An early sign of glaucomatous damage. Invest Ophthalmol Vis Sci. 2011;52:2879–88. doi: 10.1167/iovs.10-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006;7:66. doi: 10.1186/1471-2202-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen MF, Chui TY, Alhadeff P, Rosen RB, Ritch R, Dubra A, et al. Adaptive optics imaging of healthy and abnormal regions of retinal nerve fiber bundles of patients with glaucoma. Invest Ophthalmol Vis Sci. 2015;56:674–81. doi: 10.1167/iovs.14-15936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sihota R, Sony P, Gupta V, Dada T, Singh R. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci. 2006;47:2006–10. doi: 10.1167/iovs.05-1102. [DOI] [PubMed] [Google Scholar]
- 31.Yu S, Tanabe T, Yoshimura N. A rat model of glaucoma induced by episcleral vein ligation. Exp Eye Res. 2006;83:758–70. doi: 10.1016/j.exer.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Leung CK, Choi N, Weinreb RN, Liu S, Ye C, Liu L, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography:Pattern of RNFL defects in glaucoma. Ophthalmology. 2010;117:2337–44. doi: 10.1016/j.ophtha.2010.04.002. [DOI] [PubMed] [Google Scholar]