Abstract
Myopia is a widespread and complex refractive error in which a person’s ability to see distant objects clearly is impaired. Its prevalence rate is increasing worldwide, and as per WHO, it is projected to increase from 22% in 2000 to 52% by 2050. It is more prevalent in developed, industrial areas and affects individuals of all ages. There are a number of treatments available for the control of myopia, such as glasses, contact lenses, laser surgery, and pharmaceuticals agents. However, these treatments are less beneficial and have significant side effects. A novel molecule, 7-methylxanthine (7-MX), has been found to be a highly beneficial alternate in the treatment of myopia and excessive eye elongation. Many preclinical and clinical studies showed that 7-MX is effective for the treatment of myopia and is presently under phase II of clinical investigation. We have also investigated preclinical toxicity studies such as acute, sub-acute, sub-chronic, and chronic on rats. In these studies, 7-MX was found to be non-toxic as compared to other reported anti-myopic agents. Moreover, as an ideal drug, 7-MX is observed to have no or low toxicity, brain permeability, non-allergic, higher oral administration efficacy, and low treatment costs and thus qualifies for the long-term treatment of myopia. This review article on 7-MX as an alternative to myopia treatment will highlight recent findings from well-designed preclinical and clinical trials and propose a potential future therapy.
Keywords: 7-methylxanthine, myopia, prevalence, treatments
The term “myopia” derives its meaning from the Greek word “muopia,” that is, to close the eyes. It is a more common visual refractive error also known as near-sightedness. Both genetic and environmental factors further make it a complex disease. While numerous studies have shed light on the causes of myopia, the exact cause of myopia remains unknown.[1] This occurs when parallel light rays from an object fall on the eye and pass through the lens and converge in front of the retina in the vitreous body. It means that light rays bend through the eye so that the picture can be transmitted to the brain. As a result, the eye is unable to see distant objects clearly; however, close objects can be seen. In contrast, in a normal eye, the light rays fall on the eye and converge on the retina at fovea centralize, which is the most light-sensitive part of the retina in the eye. The retina is the only part of the central nervous system that can be seen by an ophthalmoscope. On the contrary, when the light rays from the object fall on the eye and pass through the lens and converge behind the retina, it is called hyperopia. Myopia is characterized as a refractive error of more than 0.25 or 0.50 D.[2] When myopia is greater than 6 D, the risk of blindness is increased, which is known as extreme myopia. Most infants are hyperopic at birth, and when they get older, their hyperopia reduces as the eye grows with age, the eyeball elongates, the lens is thin, and the cornea flattens. Normal axial growth of the eye in children is 14–24 mm, but in the case of myopia, the axial length increases from 24 mm to 28 mm at the age of 14. Myopia is first noticed in children after the age of 6 years, accompanied by excessive elongation of the axial length of the eye, and steadily increases until the age of 14, after which it gradually decreases[3] and stabilizes in the early twenties.[2] Myopia is caused by the elongation of the eyeball and reduction in the focal length of the eye lens, resulting in the eyeball taking on a long or “egg” shape.[4] Refractive errors such as retinal detachment, cataract, macular degeneration, and glaucoma are more likely with increased axial duration.[3] Increased eye axial length is connected with changes in the sclera.[5] Changes in the sclera composition occur when the connective tissue synthesis and collagen 1 decrease. The increased occurrence of myopia is due to the weakness of scleral tissues. When compared to non-inflammatory diseases, inflammatory diseases such as juvenile diabetes (7.9%), uveitis (3.7%), and systemic lupus erythematosus (3.5%) are responsible for a higher prevalence of myopia in patients.[6] Myopia also has protective effects against diabetic retinopathy in population and clinical-based studies. In myopic eyes, when a spherical equivalent of <−5 D decreases, the axial length increases in each millimeter and decreases the risk of developing diabetic retinopathy.[7]
There are currently no successful therapies that effectively stop and slow down the development of myopia without causing side effects.[8] Various forms of laser surgery, spectacle lenses, contact lenses, and prescription agents such as atropine and pirenzepine have also been studied recently. However, they have significant side effects and minor benefits. The development of myopia and axial eye growth is also minimized by pharmaceutical agents available in the market, such as atropine (non-selective antagonist) and pirenzepine (selective antagonist, M1).[9,10] However, they have significant side effects and small benefits. Atropine and pirenzepine mechanisms have not been identified but are supposed to be independent of their action on lodging and can depend on receptors of the retina or sclera.[3]
A novel molecule, 7-methylxanthine (7-MX), has been found to be beneficial in the treatment of myopia development and excessive eye elongation. In young rabbits, 7-MX, caffeine, and theobromine metabolite have been shown to increase the thickness, content of collagen-related amino acids as well as the diameter of the collagen fibrils in the posterior sclera.[11,12] In guinea pigs, 7-MX has been shown to minimize myopia form deprivation associated with eye elongation and counteracts the posterior sclera and collagen fibril thinning.[13] This review article focuses on the use of 7-MX as a myopia treatment alternative, highlights recent findings from well-designed preclinical and clinical trials, and proposes a potential future therapy.
Prevalence
Its prevalence rate in the last few years has increased and varied through regions and races in various parts of the world. Peoples in developed countries are more myopic due to strong environmental and competitive educational systems.[14,15] Increased risk factors for myopia have been identified as more time spent on near-sighted work (computer, cell phone, video game), time spent indoors, higher educational standards, and a family history of myopia. The incidence is even higher in urban areas and East-Asian populations[16] such as Taiwan and Singapore, with 25% of Americans and 50% of Europeans affected.[17]
The prevalence in Asian children, especially in the Chinese population, is higher than that in the western population.[1] It is also much more prevalent in urban and developed areas than in non-industrialized and rural areas.[18] The rate of myopia rises in all groups of individuals and increases with age.[4] In 2014, Rajendran et al.[19] has been published, upto 80% adult population suffered from myopia to 0.5D and 41% suffered with 1D myopia in India. In Taiwan, the prevalence rate in 7-, 12-, and 18-year-old children was 5.8%, 21%, and 10.9% (1983), and increased to 36.7%, 61%, and 21%, respectively, in 2000. In a recent population-based study, it was found that more time spent outside, near work, lifestyle, and reading behavior are responsible for the development of myopia in childhood and significantly a faster onset at a younger age.[20] In the United States, the prevalence of myopia was 28.1% for whites and 19.4% for blacks in the Baltimore Eye Survey, 21.9% in African-Americans aged 40–84 years in the Barbados Eye Study, 26.2% in the Beaver Dam Eye Study, and 26.2% in the Los Angeles Latino Eye Study for those aged 40 years and older.[1,21] The high prevalence rate seen in these studies suggests that myopia is on the rise, as are the challenges of permanent blindness conditions such as retinal detachment, macular degeneration, and glaucoma.[17] According to the World Health Organization (2015), the prevalence of myopia in 2000 was projected to be 22%, and by 2050, it is expected to affect 52% of the global population. Table 1 lists several additional studies that indicate the prevalence of myopia.
Table 1.
Myopia prevalence in various countries
| Study ID | Study design (Country) | No. of Participants | Age (Year) | Prevalence Rate | Degree of Myopia |
|---|---|---|---|---|---|
| Zhang et al.[22] | Comparative study in (Xiamen, China, & Singapore) | 132, 104, 146 | 6-7 | 3.9%, 9.1%, 12.3% | −0.5 D |
| Wong et al.[23] | Study on adult Chinese (Singapore) | 1232 | 40-79 | 38.7% | <−0.5D |
| Saw et al.[24] | Concurrent cohort study (Singapore) | 153 | 6-12 | −0.59 D | |
| Mavracanas et al.[25] | Study on school children (Greece) | 1738 | 15-18 | 36.8% | −4.0 D |
| Pokharel et al.[26] | RESC (Nepal) | 4977 | 5-15 | 1.2% | −0.5 D |
| Maul et al.[27] | RESC (China) | 5293 | 5-15 | 6.8% | ≤−0.5 D |
| Zhao et al.[28] | RESC (China) | 5882 | 5-15 | 21.6% | ≤−0.5 D |
| Hung et al.[29] | Taiwan school children (Taiwan) | 10889 | 7, 12, 15, 16-18 | 20%, 61%, 81%, 84% | ≤−0.25 D |
| Wu et al.[30] | Singapore military conscripts (Singapore) | 15095 | 16-25 | 79.3% | ≤−0.5 D |
| Saw et al.[31] | Cross sectional study Singapore chinese children (Singapore) | 1453 | 7, 8, 9 | 29%, 34.7%, 53.1% | ≤−0.5 D |
| Saw et al.[32] | Singapore, Xiamen, and China School children | 957 | 7-9 | 36.7% (Singapore), 18.5% (China) | ≤−0.5 D |
| Edward et al.[33] | Study on children (Hong kong) | 138 | 7-10.5 | No significant difference | ≤−0.5 D |
| Dandona et al.[34] | RESC (India) | 4074 | 5-15 | 4.1% | ≤−0.5 D |
| Murthy et al.[35] | RESC (India) | 6447 | 5-15 | 7.4% | ≤−0.5 D |
| Vannas et al.[36] | Army Conscripts (Finland) | 3524 | 17-30 | 22.2% | - |
| Naidoo et al.[37] | RESC (South Africa) | 4890 | 5-15 | 4% | ≤ -0.5 D |
| Kleinstein et al.[38] | Community population-based study (USA) | 2523 | 5-17 | 9.2% | -0.75 D |
| Villarreal et al.[39] | Study on school children (Monterrey Mexico) | 1035 | 12-13 | 37% | ≥ −0.5 D |
| Zadnik et al.[40] | Study on school children (USA) | 2583 | 6–14 | 11.6%, 10.1% | −0.50 D, −0.75 D |
| He et al.[41] | RESC (south China) | 4364 | 5–15 | 38.1% | ≤−0.5 D |
| Woo et al.[42] | Study on medical students (Singapore) | 157 | 19–23 | 89.8% | ≤−0.5 D |
| Raju et al.[43] | Population-based study (India) | 4800 | >39 | 26.99% | ≤−0.5 D |
| Fan et al.[14] | Study on school children (Hong kong) | 7560 | <7, 7, 8, 9, 10, ≥11 | 17%, 28.9%, 37.5%, 43.1%, 48.2%, 53.1% | ≤−0.5 D |
| Goh et al.[44] | RESC (Malayasia) | 5528 | 5-15 | 20.7% | ≤−0.5 D |
| Dayan et al.[45] | Population based surveys from 1990 to 2002 (Israel) | 919,929 | 16-22 | 28.3% | −0.50 to−3.0 D: mild, −3.25 to−6.0 D: moderate, >−6.0 D: high |
| Ojaimi et al.[46] | Population-based study on School (Australia) | 1765 | 5.5-8.4 | 1.43% | ≤−0.5 D |
| Khader et al.[47] | Study on school children (Jordan) | 1777 | 12-17 | 17.6% | −0.5 D |
| Ip et al.[48] | Population-based study on children (Australia) | 2353 | 11.1-14.4 | 11.9% | ≤−0.5 D |
| Saw et al.[49] | Population-based, cross-sectional study in the right eye (Singapore) | 2974 | 40-80 | 30.7% | ≤−0.5 D |
| Jobke et al.[50] | Refractive errors study (Germany) | 516 | 2–6, 7-11, 12-17, 18-35 | 0%, 5.5%, 21%, 41.3% | ≤−0.5 D |
| Krishnaiah et al.[51] | A population-based cross-sectional epidemiologic study (India) | 10293 | ≥40 | 34.6% | ≤−0.5 D |
| Pan et al.[52] | Cross-sectional study in Singapore Indians (Singapore) | 3400 | >40 | 28.0% | ≤−0.5 D |
| Guo et al.[53] | National health interview survey (Taiwan) | 20609 | ≥12 | 46.7% | ≤−0.5 D |
| Rajendran et al.[19] | Study on school children (India) | 68 | 10-12 | 51.47% | ≤−0.5 D |
| Hansen et al.[55] | Cross-sectional clinic-based study, medina (Saudi Arabia) | 1215 | 3-14 | 3.5% | ≤−0.5 D |
| Alemam et al.[54] | Prospective, population-based, observational study (Denmark) | 1443 | 16-17 | 25% | ≤−0.5 D |
Types of Myopia
Myopia has been labeled in a variety of ways. Different researchers have proposed classification schemes for myopia. Myopia has been classified in different ways. According to the clinical entity, myopia is classified into five groups by Gross and Eskridge (1987): basic (simple) myopia, nocturnal myopia, pseudo-myopia, pathological myopia, and induced myopia. Myopia was defined by Grosvenor and Gross (1999) based on the age of onset (i.e., congenital, youth onset, early adult-onset, late adult-onset) and degree (i.e., mild, medium, or high) given in Table 2. Every form of myopia has a different diagnosis and treatment plan.
Table 2.
Classification of myopia based on different entities
| Classification | Cause |
|---|---|
| Based on clinical entity | |
| Basic (simple) myopia | -- (<6 D) hinge on axial length, optical power of the cornea, and crystalline lens of the eye[56,57] |
| Nocturnal myopia | -- Low illumination of light and darkness are responsible[58] |
| Pseudo-myopia | -- Due to ciliary muscle spasm or unrelaxed accommodation system |
| Pathological myopia | -- Associated with degenerative changes at the posterior pole and retinal periphery |
| Induced myopia | -- Occurs by some external pharmaceutical agents or by disease conditions. It is reversible.[57] |
| Based on age | |
| Congenital myopia | --At birth to whole life |
| Youth onset myopia | --From 5 to 20 years |
| Early adult-onset myopia | --From 20 to 40 years |
| Late adult-onset myopia | --After 40 years |
| Based on degree | |
| Low myopia | <−3 D |
| Medium myopia | −3 to <−6 D |
| High myopia | >−6 D |
Classification of myopia according to the clinical entity
Simple myopia
Simple myopia depends on the crystalline lens, the optical power of the cornea, and the axial length.[56] Simple myopia appears in childhood and becomes severe.[59,60] The rate of progression of childhood myopia is 0–1.0 D per year. Its rate of progression ceases or becomes slow in the middle of teenage years.[61]
Nocturnal myopia
It is also called night myopia. It occurs in darkness or under low illumination conditions.[58] Over a number of days, the accommodative dark focus tends to be relatively constant. Night myopia is characterized by blurred distant vision in dim light. Patients may complain about trouble seeing road signs at night.[62,63]
Pseudo-myopia
Pseudo-myopia is due to ciliary muscle spasm or unrelaxed accommodation system. Pseudo-myopia generally occurs in younger patients due to excessive close work.[64] A distant blur is transient, especially greater after near work, which may indicate pseudo-myopia.
Degenerative myopia
It is linked to degenerative changes in the retinal periphery and posterior pole. Swelling of the eye in degenerative myopia can damage the optic nerve. The retina is stretched away from the optic nerve temporarily. Blur distant vision occurs because of the significant degree of myopia.[57]
Induced myopia
Induced myopia refers to myopia that occurs by some external pharmaceutical agents or diseases. It depends on the initiating conditions or agents. This type of myopia is referred to as reversible and temporary myopia. Patients also report blurred distant vision.[57] There are some pharmaceutical agents that induce myopia mentioned in Table 3.
Table 3.
List of myopia-inducing pharmaceutical agents
| Class | Pharmaceutical agents |
|---|---|
| Cholinergic agonists | Acetylcholine, Neostigmine, Physostigmine, Pilocarpine |
| Antibiotics | Tetracycline, Sulfonamides, Isoniazid |
| Antihypertensives | Adrenergic drugs, Thiazide diuretics |
| Hormonal agents | Corticosteroids, Oral contraceptives |
| CNS agents | Opium, Morphine |
Classification of myopia according to age
Pathological, school-age, and adult-onset myopia are the three broad categories of myopia [Tables 2 and 3].
Pathological myopia
Pathological myopia results from atypical and severe elongation of the axial length of the eye, and it usually occurs before the age of six.[65]
School age myopia
Between the ages of 6 and 18 years, school-age myopia occurs, which usually stabilizes by the late teens or early twenties,[66] higher intelligence, and increased reading time.[67,68] Urban and industrial areas have a higher prevalence of school-age myopia.[69]
Adult-onset myopia
This myopia may develop between the ages of 20 and 40 years (early adult-onset) or after the age of 40 years (late adult-onset). It is linked to concentrating issues and jobs that need a lot of close vision, including computer work.[70]
Risk Factors of Myopia
Near-sightedness is caused by a combination of environmental and hereditary factors.[71] When compared to non-myopic children, children who have myopic parents are more likely to develop myopia.[18] Twin studies show that monozygotic twins are significantly more concordant in myopia as well as three ocular components (axial length, corneal radius of curvature, and lens power) than dizygotic twins.[72] In genetic studies, several different types of myopia have been identified for a variety of myopia severities.[73] The environmental factors responsible for the development of myopia include indoor activities such as more time spent near the TV, mobile phone usage, playing games, and reading books from less than 30-cm distance. Multiple reading styles and long periods of reading in low illumination are also factors in the progression of myopia.[19] A study also shows that outdoor activities protect against the development and progression of myopia in children and university students.[74] Stature, parental smoking, and intelligence quotient are other predictable environmental risk factors that may also affect myopia.[67]
Current Available Treatment for Controlling Myopia
Myopia can currently be treated with glasses, contact lenses, laser surgery, and pharmaceutical agents.[75] According to the findings of these studies, the vast majority of myopia treatments provide only minor treatment benefits that last only for a short time and have significant side effects. While these treatments correct myopia, they do not slow the accompanying eye growth or physiological changes caused by excessive axial elongation. The WHO has set a goal of eliminating preventable blindness caused by refractive error, including myopia, by the year 2020.[76]
Eye glasses and contact lenses
The most straightforward treatment option preferred by the majority of near-sighted patients is dependent on the degree of myopia. Either the patient requires a full-time need of glasses, contact lenses, or when clear distant vision, such as typing, reading from computer, driving, watching a movie, or looking at a chalkboard. Myopia can be corrected with spectacles or contact lenses. However, they are powerless in preventing the eye from lengthening.
Laser surgery
Surgical options are available, but they are more expensive and may pose a greater risk than wearing contact lenses. To remove small amounts of tissue from the cornea, these procedures use laser technology or manual incisions. Laser surgery is typically performed as an outpatient procedure, which does not necessitate an overnight stay in the hospital. The three most common types of laser surgeries are listed below.
Photo-refractive keratectomy (PRK)
In this procedure, a small portion of the surface of the cornea is removed with the help of the laser to change the form of the cornea.
Laser epithelial keratomileusis (LASEK)
The treatment is identical to PRK, except that alcohol is used to loosen the cornea’s surface until it is lifted out of the way. It is used to alter the form of the cornea in the same way as PRK is used.
Laser in situ keratectomy (LASIK)
The most common laser refractive surgery is LASIK. The treatment is similar to LASEK, except that it includes a small corneal flap. A surgical knife is used to cut the corneal flap, which is then folded back into place and kept in place by natural suction rather than being removed.
Pharmaceutical agents used for the treatment of myopia
Myopia development and axial eye growth can be minimized with the use of topical muscarine antagonists viz. non-selective antagonist (atropine) and M1-selective antagonist (pirenzepine).[10,31] However, they have significant side effects and small benefits. The mode of action of atropine and pirenzepine is assumed to be dependent on the retina or sclera receptors.[77] Reduced pupillary accommodation and dilation are an issue with atropine and, to a lesser extent, with pirenzepine.
Atropine
Atropine occurs naturally in plants and is used topically either as an eye drop or ointment in the treatment of the eye. It is a non-selective muscarinic acetylcholine receptor antagonist. These muscarinic receptors M1–M5 are present in the ciliary body and pupillary spinture. Atropine competitively blocks these muscarinic acetylcholine receptors and inhibits acetylcholine from attaching to these receptors. Doctors use atropine for pupil dilation to examine the eye. Atropine has been shown in a number of studies to slow the development of myopia and axial elongation. Several preclinical studies on guinea pigs, rats, mice, monkeys, chicken, tree shrews, Syrian hamsters, and clinical studies on humans have shown the inhibitory effect of atropine on myopia.[6] In recent years, several clinical studies and trials demonstrating the effectiveness of atropine in reducing myopia progression have been reported.[78]
Dyer et al.[79] compared the atropine community to a group of children wearing only glasses for a span of 2–8 years in their study, “The Role of Cycloplegics in Progressive Myopia.” They reported that 1% atropine was successful in slowing the development of myopia and there can be a permanent reduction in the degree of myopia.
Yen et al.[80] compared the effect of cycloplegics atropine (1%) and cyclopentolate (1%), on myopia with the control group. A total of 96 people were included in the report. Atropine and cyclopentolate are both involved in slowing the development of myopia, according to the findings. Though the effectiveness of atropine was reported to be better than that of cyclopentolate, all the patients on atropine treatment had photophobia as a side effect.
Chou et al.[81] looked at the use of 0.5% atropine once per night for treating myopia in 20 Taiwanese children aged 7–14 years who had high degrees of myopia (>6.0 D). They discovered that atropine at a lower concentration of 0.5% had a constrictive effect in regulating high degrees of myopia (>6.0 D) and was effective in controlling its progression.
Shih et al.[82] compared the effects of different doses of topical atropine on regulating myopia in myopic children (0.5%, 0.25%, and 0.1%). Myopic children (n = 186) between the ages of 6 and 13 years were chosen for this research and were treated for 2 years. They found that the mean myopia development was −0.04 D/year in the 0.5% atropine group (n = 41), −0.45 D/year in the 0.25% atropine group (n = 47), −0.47 D/year in the 0.1% atropine group (n = 49), and −1.06 D/year in the control group (n = 49). When compared to the control group, all atropine groups showed less mean progression of myopia. Atropine (0.5%) was found to be more effective than other concentrations, indicating that atropine has a dose-dependent effect.
Shih et al.,[83] in a randomized 18-month clinical research on a total of 227 school children aged 6–13 years, studied myopia progression reduction by using atropine and/or multifocal lenses. They discovered that combining 0.5% atropine with multifocal lenses slows the development of myopia as compared to using multifocal lenses alone or a control group.
Chua et al.[17] examined the effectiveness and safety of topical atropine (1%) in Asian children in their study, “Atropine for the treatment of childhood myopia.” A total of 400 children aged 6–12 years with refractive error 1–6 D participated in the research, with 346 completing the two-year study. When comparing the atropine-treated group to the placebo group, the mean development of myopia was reduced by 77%. In Asian children, a higher tolerance and effectiveness of topical atropine in slowing low and moderate myopia development and ocular axial elongation was observed. Atropine was well tolerated in this study with no significant side effects reported. Chua et al. in 2012 studied the safety and efficacy of lower atropine doses of 0.5%, 0.1%, and 0.01%. Children (n = 400, aged 6–12 years) with myopia were administered 0.5%, 0.1%, and 0.01% doses of atropine for 2 years. They discovered that 0.01% atropine has fewer side effects than other higher doses used and that it is statistically different from 0.5% atropine in terms of efficacy in controlling the progression of myopia.
Lee et al.[84] compared and estimated the effectiveness of 0.05% atropine-treated group with the untreated control group. A total of 57 school children between the age of 6 and 12 years were chosen and divided into two groups. They found statistically decreased mean myopia progression in the 0.05% atropine-treated group (0.28 ± 0.26 D/year; n = 21) compared to the control group (0.75 ± 0.35 D/year). They concluded that 0.05% atropine reduces the mean myopia progression in school children. Group A (n = 21) received 0.05% atropine eye drops, while group B (n = 36) received no treatment. They found that the 0.05% atropine-treated group had a statistically lower mean myopia progression rate (−0.28 ± 0.26 D/year; n = 21) than the control group (−0.75 ± 0.35 D/year). They concluded that 0.05% atropine slows the development of myopia in school-aged children.
Fan et al.[78] studied the effectiveness and protection of 1% atropine ointment in children with moderate to extreme myopia compared to a control group in terms of retarding myopic progression and axial length. In this study, a total of 23 children aged 5–10 years were chosen for each group (1% atropine and control group). After a year, they found that 1% atropine is safe and successful in reducing mild to extreme myopia development and axial duration in infants. The 1% atropine group (+0.06 D/year) had a lower mean progression rate of myopia than the control group (−1.19 D/year) after 1 year. In the atropine group (0.09 mm), axial length growth was also significantly slower than in the control group (0.70 mm).
Liang et al.[85] compared the atropine group alone to the combined treatment of atropine and auricular acupoint stimulation. In this study, school children were randomly assigned to one of three groups. Group 0.25A (n = 22) received 0.25% atropine alone, group 0.5A (n = 23) received 0.5% atropine alone, and group (0.25A + E) received 0.25% atropine with auricular acupoint stimulation. They discovered that in the atropine group 0.25A, the mean myopia progression was 0.38 ± 0.32 D/year, in the 0.5A atropine group, it was 0.15 ± 0.15 D/year, and in the 0.25A + E group, it was 0.21 ± 0.23 D/year. The 0.25A + E group was more effective than the 0.25A group and nearly as effective as the 0.5A group, indicating that stimulating auricular acupoints increases the effect of 0.25% atropine and is nearly as effective as 0.5% atropine.
Fang et al.[86] found the efficacy of 0.025% atropine solution on pre-myopic children and compared it to the control group in their retrospective cohort study. A total of 50 children were chosen, with 24 (avg. age: 7.6 years) receiving 0.025% atropine treatment and 26 (avg. age: 8.2 years) receiving no treatment. The mean spherical refraction myopia in the 0.025% atropine group was −0.14 ± 0.24 D/year, which was significantly lower than that in the control group (-0.58 ± 0.34 D/year) (P = 0.0001). They discovered that using 0.025% atropine eye drops on a daily basis for 1 year can prevent myopia onset and change in pre-myopic children.
Lin et al.[87] compared the low-dose atropine 0.125% with the orthokeratology lens group and analyzed the effect of atropine and orthokeratology lens on reduction in the mean myopia progression and elongation of axial length. The study was conducted on 210 subjects: 105 patients treated with low-dose atropine 0.125% and 105 treated with orthokeratology lens for 3 years. Over 3 years, the increase in myopia in the atropine 0.125% group was 0.31 ± 0.19, 0.35 ± 0.25, and 0.32 ± 0.23, and 0.29 ± 0.31, 0.27 ± 0.24, and 0.28 ± 0.31 in the orthokeratology lens group. In 3 years, the axial length of the atropine group was 0.38 ± 0.09, 0.37 ± 0.12, and 0.36 ± 0.08, while the axial length of the orthokeratology lens group was 0.28 ± 0.08, 0.30 ± 0.09, and 0.27 ± 0.10. They found that orthokeratology lenses are more effective than atropine at reducing mean myopia progression and axial length elongation over a three-year period.
Clark & Clark[88] compared the 0.01% atropine eye drops with the control to slow childhood myopic progression in Asian populations over a wide range of myopia. Children (n = 60) aged 6–15 years were enrolled for this study. As compared to the control group (−0.6 ± 0.4 D/year), the atropine-treated group had substantially (P = 0.001) lower mean development of myopia (−0.1 ± 0.6 D/year). They discovered that atropine 0.01% significantly decreases the rate of mean progression of myopia over a year with few side effects.
Lee et al.[89] studied and compared the effects of different concentrations of atropine (0.125% and 0.25%) on intraocular pressure measurements and myopia progression in school children aged 6–12 years for 1 year in Taiwan. Lower concentrations of atropine were shown to be effective in the treatment of myopia.
Yam et al.[90] compared the efficacy of 0.05%, 0.025%, and 0.01% atropine in a double-masked study for 2 years. In this study, 383 randomly selected children aged 4–12 years with −1.0-D myopia were administered different doses of atropine for 2 years. They discovered that 0.05% atropine has double efficacy as compared to 0.01% atropine and reduces the progression of myopia.
Pirenzepine
Pirenzepine is a selective M1 muscarinic antagonist. This has been shown to slow the development of myopia in children. While the precise location of pirenzepine’s action is unknown, numerous studies have shown that it acts in the sclera. Stone et al.[91] demonstrated that pirenzepine is effective in reducing myopia progression in chicks with form-deprivation myopia. As compared to atropine, pirenzepine is less effective at the dilation of the pupils. Other studies have shown that pirenzepine can minimize type-deprivation myopia and axial length elongation in animals.[10,92,93,94]
Leech et al.[94] determined the effect of different doses of pirenzepine administered by injection through intravitreal and subconjunctivally. The intravitreal injection had six groups (n = 7 in each group), which were injected with different doses of pirenzepine such as 3.5, 20, 100, 200, 250, and 500 mg. Subconjunctivally injected had six groups (n = 6 in each group); the doses administered were 3.5 mg, 500 mg, 750 mg, 1 mg, 5 mg, and 7.5 mg, while the control group received 0.9% saline only. They demonstrated the daily intravitreal injection was more effective in preventing form-deprivation myopia. The dose of 500-mg pirenzepine administered through intravitreal injection showed prevention in deprived induced myopia (+0.9 D vs. −13.7 D) and axial length elongation (−0.14 mm vs. +0.32 mm). Pirenzepine was more effective through intravitreal injection as compared to the subconjunctivally injected, and pirenzepine may be effective on the muscarinic receptors present on the retina and choroid.
Cottriall and McBrien[92] reported that M1 muscarinic antagonist pirenzepine reduces the experimentally induced monocularly deprived myopia in tree shrew as compared to sham-injected and saline-injected groups.
Siatkowski et al.[95] conducted a retrospective study to find out the efficacy of pirenzepine in school-aged myopic children aged 8–12 years with a spherical equivalent of −0.75 to −4.00 D. The patients were split into two groups, administered 2% pirenzepine gel (n = 117) and a placebo control (n = 57) in a 2:1 ratio for 1 year. After 12 months, the mean myopia progression was 0.26 D in the pirenzepine-treated group and 0.53 D in the placebo-controlled group (P = 0.001). They showed that pirenzepine is effective and safe for the treatment of myopia, with a 50% reduction in mean myopia progression after one year.
Tan et al.[77] conducted a 1-year double-masked study on myopia school children (n = 353, aged 6–12 years) to assess the safety and efficacy of M1 muscarinic antagonist pirenzepine. The mean increase myopia progression in 2% pirenzepine gel was 0.47 D, significantly less as compared to that for placebo/gel (0.70 D) and placebo/placebo (0.84 D). They demonstrated that 2% pirenzepine gel effectively reduces the mean progression of myopia and is safe over 1 year.
In another randomized, double-masked, placebo-controlled study by Siatkowski et al.,[10] parallel safety and efficacy of 2% pirenzepine ophthalmic gel in myopic children were estimated for 2 years. In the second year, they found that the pirenzepine group had a 0.58-D reduction in mean progression myopia compared to the control group, which had a 0.99-D reduction (P = 0.008). They demonstrated that 2% pirenzepine is effective and well-tolerated in school-aged myopic children over the 2-year treatment period.
A recently found ideal molecule for myopia treatment, 7-MX has emerged as a novel molecule of prime interest. In preclinical and clinical studies, 7-MX has been reported to inhibit the progression of myopia and axial length of the eye without any significant side effects.
7-MX
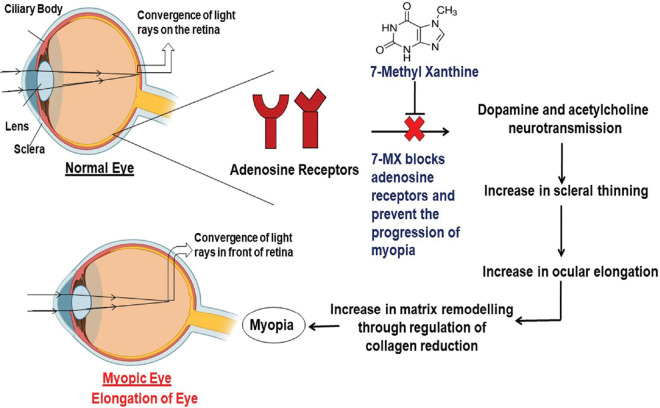
7-MX is a novel molecule that has been shown in phase II clinical trials to be effective in the treatment of myopia. 7-MX is an anti-myopic agent that is a non-selective adenosine antagonist.[12] 7-MX is a metabolite of various methylxanthines (theobromine, theophylline, and caffeine). It is found naturally in cocoa fruit. It is a purine base component that is commonly found in human tissues and fluids. It belongs to the class of methylxanthines that are consumed daily in diet such as tea, coffee, chocolate, and beverages. In the last few years, a number of experiments have been conducted to study the pharmacological actions of various methylxanthines in various human disorders such as respiratory, cardiac, nervous, renal, and male fertility and obesity.[96] However, 7-MX is the first molecule from the xanthines family to be used for the effective treatment of myopia development and axial length of the eye [Fig. 1].
Figure 1.
Flowchart shows the 7-MX preventing excessive eye elongation
7-MX is a methylxanthine that has a similar structure to theobromine (3,7, dimethyl xanthine) and caffeine (1,3,7 trimethyl xanthine). Theobromine, the main ingredient of chocolate, is more closely related to 7-MX. Both plants and humans naturally metabolize theobromine to 7-MX. 7-MX has been shown to be successful in treating myopia development and excessive eye elongation in both preclinical and clinical trials.[96,98] Myopia is a progressive condition that can begin as early as the age of six and progress until the age of 14 or 16 (childhood myopia), necessitating long-term care. As a result, an ideal drug should have no or low toxicity, brain permeability, non-allergic properties, oral administration efficacy, and low treatment costs. 7-MX satisfies nearly all of the ideal properties of the drug for the chronic treatment of myopia because it occurs naturally in the body as a metabolite of theobromine and caffeine.
Acute and sub-acute toxicity studies [Table 4] were conducted to evaluate the maximum tolerated dose (MTD) and LD50, which is necessary for the toxicity evaluation in rodents compared to the other clinically used xanthines, that is, caffeine and theobromine. In the acute toxicity study, 7-methylxanthine was administered in a single dose. This study was done on two different species, namely Wistar mice and rats, as per the OECD guideline no. 423. In this acute toxicity study, both rodent species did not show any form of morbidity or mortality upon 7-MX administration, while 66.6% (mice) and 33.3% (rat) mortality was observed on treatment with caffeine and theobromine groups, respectively. There were no significant changes in body weight and feed intake after administration of 7-MX, which were similar to those treated with caffeine and theobromine. In the sub-acute study, 7-MX was orally administered daily for a period of 28 days at the dose of 250, 500, and 1000 mg/kg. Each group comprised 10 animals, with 5 males and 5 females in each. Body weight was monitored weekly, and feed intake was monitored daily. The blood biochemistry and hematology were done on the 0th day and 28th day. There were no changes in body weight and percentage body weight during the experimental period. A histopathological study done on the 28th day did not reveal any pathological changes in any animals sacrificed, and animals did not show any toxicity sign and mortality at any administered doses of 7-MX.[96]
Table 4.
Preclinical toxicity studies of 7-methylxanthine conducted on animals
In another study, sub-chronic (90-day repeated dose toxicity) and chronic toxicity (180-day repeated dose toxicity) evaluation, 7-MX was found to be non-toxic as compared to caffeine and theobromine as per the OECD guidelines 408 and 452. The data obtained from these toxicity studies showed that 7-MX is non-toxic in nature and can be used clinically for the chronic treatment of myopia. As per regularity guidelines for new molecules, toxicity sub-chronic and chronic toxicity study is mandatory for a drug developed for long-term administration. 7-MX is developed for long-term treatment of myopia starting from the age group of 6–8 years and up to 16–18 years.[97]
In Table 5, several preclinical and clinical studies illustrate that 7-MX halts myopia progression and eye elongation. It also demonstrates that 7-MX is safe and has no side effects. 7-MX has emerged as a promising new molecule. 7-MX is a purine component of urinary calculi, which is a methyl derivative of xanthine. It is a methylxanthine metabolic byproduct (caffeine, theophylline, and theobromine). Caffeine metabolism is primarily catalyzed by CYP1A2 and xanthine oxidase, which results in the formation of 14 different metabolites, including 7-MX.[99]
Table 5.
Summary of preclinical and clinical studies in which 7-MX showed effective results in the treatment of myopia
| Study ID | Study name/design | Animal used | Inference |
|---|---|---|---|
| Trier et al.[11] | Preclinical | 30 pigmented rabbits (age: 8 weeks) | 7-MX was found to be effective. |
| Trier et al.[3] | Clinical study (36-month pilot study) | 68 myopic children (mean age: 11.3 years) | 7-MX was found to be effective. |
| Cui et al.[13] | Preclinical | 20 guinea pigs (3 weeks old) | 7-MX was found to be effective |
| Nie et al.[12] | Preclinical | 16 pigmented rabbits (age: 10 days) | 7-MX was found to be effective. |
| Hung et al.[100] | Preclinical | 16 rhesus monkeys (age: 2-3 weeks) | 7-MX was found to be effective. |
In the preclinical study, the effects on the ultrastructure and biochemical makeup of rabbit sclera were examined for the first time. The collagen concentration and collagen fibril diameter in the posterior sclera increase.[11] In mammals, experimental myopia involves a decrease in proteoglycan and collagen scleral levels with reversals during regeneration. A rear sclera dilution and an increase in the number of small collagen fibrils are found in mammalian models of high myopia. Thus, 7-MX was used to determine its effect on the posterior sclera in form-deprivation myopia for pigmented rabbits in another study. The previous results were confirmed and myopia formation in pigmented rabbits was prevented.[12] 7-MX has also been found to reduce myopia (by around 50%) and eye elongation by counteracting the thinning of the fibril sclera and collagen in the back sclera in a model of deprivation of the shape.[13]
In addition, the 7-MX clinical studies showed promising results in the treatment of childhood myopia. In a primary study report, 68 children were hired and received either 7-MX or placebo for 12 months, followed by all 7-MX participants for the next 12 months. Lengths at −6, 0, 12, 24, and 36 months were measured. In children receiving 7-MX during 24 months, axial growth reduced compared with children who received only for 12 months. The growth of myopia and axial eye growth was delayed in the 7-MX therapy, while it continued with treatment stoppage. There was, therefore, a recommendation that 7-MX treatment should be continued until the age of 18–20 as myopia generally stops around this age.[3] 7-MX has been in clinical use only in Denmark since 2006 under the rule of Magisterial Pharmacy. According to this rule, only two local pharmacies have permission for dispensing 7-MX to children with myopia by the Danish Medical Agency. Therefore, the preclinical and clinical toxicity evaluation is unavoidably crucial for the registration of 7-MX as a new drug entity as the majority of applications of the NDA require toxicity data, which are compulsory to register and use any drug-related molecule.
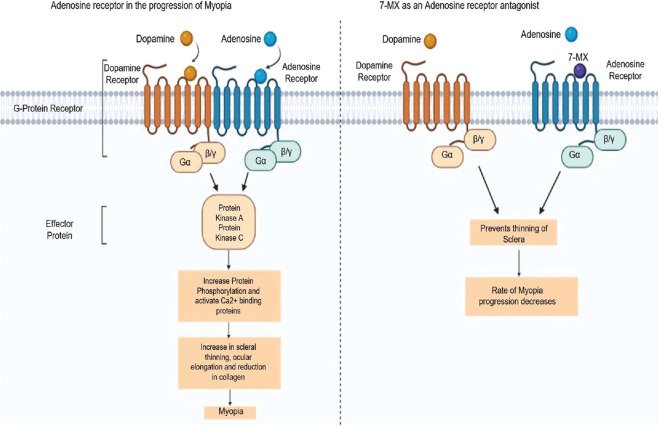
Methylxanthines have been shown to modulate GABA receptors, inhibit phosphodiesterases, and mobilize intracellular calcium by acting as an antagonist for adenosine receptors.[101] 7-MX is a non-selective adenosine receptors antagonist that acts as a competitive inhibitor for adenosine receptors due to its structural similarity with other methylxanthines (caffeine, theophylline, and theobromine). In humans, there are four subtypes of adenosine receptors: A1, A2A, A2B, and A3. These adenosine receptors have been found in the sclera, retinal pigment epithelium, choroid, and retina from guinea pig, rat, and human eyes. The sclera is the main part of the eye that maintains the visual apparatus and biochemical properties and may lead to excessive elongation of the eye. Collagen is responsible for the strength and rigidity of scleral tissues. Collagen accounts for up to 80% dry weight of the sclera.[11] The axial length of the eye is connected to the remodeling in the sclera, reduction of scleral tissues, and increased collagen degradation, resulting in the altered composition of the sclera. Smith et al.[102] showed that the photoreceptors and retinal pigment epithelial of the retina play a role in the modulation of eye growth by sending signals to scleral tissue remodeling. Adenosine receptors are directly involved in eye growth.[3]
Adenosine receptors also play a role in the activity of various retinal neurotransmitters in form-deprivation myopia. Acetylcholine and dopamine orchestrate downstream events by acting on adenosine receptors, which can be responsible for myopia. 7-MX competitively blocks the muscarinic acetylcholine or dopamine adenosine receptors. These receptors are directly involved in modulating the various retinal neurotransmitters observed in form-deprivation myopia or drug-induced experimental myopia, which interferes with neurotransmission as demonstrated in Fig. 2. 7-MX inhibits the progression of myopia and axial length induced by form-deprivation myopia in guinea pigs and pigmented rabbits and increases the collagen fibril diameter and concentration in the posterior sclera.[11,12,13]
Figure 2.
Flowchart showing the mechanism of 7-MX in preventing the progression of myopia
Conclusion
As we know, the treatment of myopia is long. To cure myopia, the treatment should be effective and non-toxic. As per the present review and data compiled from currently available studies, treatments have small benefits with some adverse effects. Only 7-MX, which is a new molecule, has been found to be effective and non-toxic in both preclinical and clinical studies. An ideal drug should have no or minimal toxicity. 7-MX achieves almost all the required properties of a drug for the long-term treatment of myopia. 7-MX may thus provide another pharmaceutical treatment option for myopia. In support of this new pharmaceutical molecule, many published preclinical and clinical studies showed that 7-MX is effective in myopia progression and eye elongation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors are grateful to the Department of Science & Technology, New Delhi for financial assistance to the Department of Pharmaceutical Sciences, under the DST-FIST scheme (sanction no. SR/FST/LSI-657). The authors are also grateful to the University Grant Commission (UGC), New Delhi for providing grants in aid to Guru Nanak Dev University, Amritsar under component 4.0 of RUSA 2.0 scheme to establish the Center for Basic and Translational Research in Health Sciences (CBTRHS). The authors are also thankful to V.B. Medicare Pvt. Ltd., Hosur, Bangalore for providing 7-MX.
References
- 1.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32:3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 2.Saw SM, Katz J, Schein OD, Chew SJ, Chan TK. Epidemiology of myopia. Epidemiol Rev. 1996;18:175–87. doi: 10.1093/oxfordjournals.epirev.a017924. [DOI] [PubMed] [Google Scholar]
- 3.Trier K, Ribel-Madsen SM, Cui D, Christensen SB. Systemic 7-methylxanthine in retarding axial eye growth and myopia progression:A 36-month pilot study. J Ocul Biol Dis Infor. 2008;1:85–93. doi: 10.1007/s12177-008-9013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosaka A. Population studies--myopia experience in Japan. Acta Ophthalmol. 1988;185:37–40. doi: 10.1111/j.1755-3768.1988.tb02659.x. [DOI] [PubMed] [Google Scholar]
- 5.Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest Ophthalmol Vis Sci. 1997;38:1726–39. [PubMed] [Google Scholar]
- 6.Lin HJ, Wei CC, Chang CY, Chen TH, Hsu YA, Hsieh YC, et al. Role of chronic inflammation in myopia progression:Clinical evidence and experimental validation. EBioMedicine. 2016;10:269–81. doi: 10.1016/j.ebiom.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Tang L, Gao L, Yang Y, Cao D, Li Y. Myopia and diabetic retinopathy:A systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;111:1–9. doi: 10.1016/j.diabres.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Polkinghorne PJ, Craig JP. Northern New Zealand rhegmatogenous retinal detachment study:Epidemiology and risk factors. Clin Exp Ophthalmol. 2004;32:159–63. doi: 10.1111/j.1442-9071.2004.00003.x. [DOI] [PubMed] [Google Scholar]
- 9.Saw SM, Gazzard G, Eong KA, Tan DT. Myopia:Attempts to arrest progression. Br J Ophthalmol. 2002;86:1306–11. doi: 10.1136/bjo.86.11.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siatkowski RM, Cotter SA, Crockett RS, Miller JM, Novack GD, Zadnik K. Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J AAPOS. 2008;12:332–9. doi: 10.1016/j.jaapos.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Trier K, Olsen EB, Kobayashi T, Ribel-Madsen SM. Biochemical and ultrastructural changes in rabbit sclera after treatment with 7-methylxanthine, theobromine, acetazolamide, orl-ornithine. Br J Ophthalmol. 1999;83:1370–5. doi: 10.1136/bjo.83.12.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie HH, Huo LJ, Yang X, Gao ZY, Zeng JW, Trier K, et al. Effects of 7-methylxanthine on form-deprivation myopia in pigmented rabbits. Int J Ophthalmol. 2012;5:133–7. doi: 10.3980/j.issn.2222-3959.2012.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui D, Trier K, Zeng J, Wu K, Yu M, Hu J, et al. Effects of 7-methylxanthine on the sclera in form deprivation myopia in guinea pigs. Acta ophthalmol. 2011;89:328–34. doi: 10.1111/j.1755-3768.2009.01688.x. [DOI] [PubMed] [Google Scholar]
- 14.Fan DS, Lam DS, Lam RF, Lau JT, Chong KS, Cheung EY, et al. Prevalence, incidence, and progression of myopia of school children in Hong Kong. Invest Ophthalmol Vis Sci. 2004;45:1071–5. doi: 10.1167/iovs.03-1151. [DOI] [PubMed] [Google Scholar]
- 15.Lin LL, Shih YT, Hsiao CT, Chen CT. Prevalence of myopia in Taiwanese schoolchildren:1983 to 2000. Ann Acad Med Singapore. 2004;33:27–33. [PubMed] [Google Scholar]
- 16.Chung K, Mohidin N, O'Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res. 2002;42:2555–9. doi: 10.1016/s0042-6989(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 17.Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113:2285–91. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 18.Saw SM, Goh PP, Cheng A, Shankar A, Tan DT, Ellwein LB. Ethnicity-specific prevalences of refractive errors vary in Asian children in neighbouring Malaysia and Singapore. Br J Ophthalmol. 2006;90:1230–5. doi: 10.1136/bjo.2006.093450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajendran K, Haneef M, Chandrabhanu K, Muhammed M, Pillai RT. A prevalence study on myopia among school going children in a rural area of South India. Indian J Clin Prac. 2014;25:374–80. [Google Scholar]
- 20.Hsu CC, Huang N, Lin PY, Tsai DC, Tsai CY, Woung LC, et al. Prevalence and risk factors for myopia in second-grade primary school children in Taipei:A population-based study. J Chin Med Assoc. 2016;79:625–32. doi: 10.1016/j.jcma.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Klein BE, Klein R, Moss SE. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1994;35:4344–7. [PubMed] [Google Scholar]
- 22.Zhang MZ, Saw SM, Hong RZ, Fu ZF, Yang H, Shui YB, et al. Refractive errors in Singapore and Xiamen, China—A comparative study in school children aged 6 to 7 years. Optom Vis Sci. 2000;77:302–8. doi: 10.1097/00006324-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Wong TY, Foster PJ, Hee J, Ng TP, Tielsch JM, Chew SJ, et al. Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest Ophthalmol Vis Sci. 2000;41:2486–94. [PubMed] [Google Scholar]
- 24.Saw SM, Nieto FJ, Katz J, Schein OD, Levy B, Chew SJ. Factors related to the progression of myopia in Singaporean children. Optom Vis Sci. 2000;77:549–54. doi: 10.1097/00006324-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Mavracanas TA, Mandalos A, Peios D, Golias V, Megalou K, Gregoriadou A, et al. Prevalence of myopia in a sample of Greek students. Acta Ophthalmol Scand. 2000;78:656–9. doi: 10.1034/j.1600-0420.2000.078006656.x. [DOI] [PubMed] [Google Scholar]
- 26.Pokharel GP, Negrel AD, Munoz SR, Ellwein LB. Refractive error study in children:Results from Mechi Zone, Nepal. Am J Ophthalmol. 2000;129:436–44. doi: 10.1016/s0002-9394(99)00453-5. [DOI] [PubMed] [Google Scholar]
- 27.Maul E, Barroso S, Munoz SR, Sperduto RD, Ellwein LB. Refractive error study in children:Results from La Florida, Chile. Am J Ophthalmol. 2000;129:445–54. doi: 10.1016/s0002-9394(99)00454-7. [DOI] [PubMed] [Google Scholar]
- 28.Zhao J, Pan X, Sui R, Munoz SR, Sperduto RD, Ellwein LB. Refractive error study in children:Results from Shunyi District, China. Am J Ophthalmol. 2000;129:427–35. doi: 10.1016/s0002-9394(99)00452-3. [DOI] [PubMed] [Google Scholar]
- 29.Hung T. Epidemiologic study of the prevalence and severity of myopia among schoolchildren in Taiwan in 2000. J Formos Med Assoc. 2001;100:684–91. [PubMed] [Google Scholar]
- 30.Wu HM, Seet B, Yap EP, Saw SM, Lim TH, Chia KS. Does education explain ethnic differences in myopia prevalence?A population-based study of young adult males in Singapore. Optom Vis Sci. 2001;78:234–9. doi: 10.1097/00006324-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Saw SM, Carkeet A, Chia KS, Stone RA, Tan DT. Component dependent risk factors for ocular parameters in Singapore Chinese children. Ophthalmology. 2002;109:2065–71. doi: 10.1016/s0161-6420(02)01220-4. [DOI] [PubMed] [Google Scholar]
- 32.Saw SM, Zhang MZ, Hong RZ, Fu ZF, Pang MH, Tan DT. Near-work activity, night-lights, and myopia in the Singapore-China study. Arch Ophthalmol. 2002;120:620–7. doi: 10.1001/archopht.120.5.620. [DOI] [PubMed] [Google Scholar]
- 33.Edwards MH, Li RW, Lam CS, Lew JK, Yu BS. The Hong Kong progressive lens myopia control study:Study design and main findings. Invest Ophthalmol Vis Sci. 2002;43:2852–8. [PubMed] [Google Scholar]
- 34.Dandona R, Dandona L, Srinivas M, Sahare P, Narsaiah S, Munoz SR, et al. Refractive error in children in a rural population in India. Invest Ophthalmol Vis Sci. 2002;43:615–22. [PubMed] [Google Scholar]
- 35.Murthy GV, Gupta SK, Ellwein LB, Munoz SR, Pokharel GP, Sanga L, et al. Refractive error in children in an urban population in New Delhi. Invest Ophthalmol Vis Sci. 2002;43:623–31. [PubMed] [Google Scholar]
- 36.Vannas AE, Ying GS, Stone RA, Maguire MG, Jormanainen V, Tervo T. Myopia and natural lighting extremes:Risk factors in Finnish army conscripts. Acta Ophthalmol Scand. 2003;81:588–95. doi: 10.1046/j.1395-3907.2003.0151.x. [DOI] [PubMed] [Google Scholar]
- 37.Naidoo KS, Raghunandan A, Mashige KP, Govender P, Holden BA, Pokharel GP, et al. Refractive error and visual impairment in African children in South Africa. Invest Ophthalmol Vis Sci. 2003;44:3764–70. doi: 10.1167/iovs.03-0283. [DOI] [PubMed] [Google Scholar]
- 38.Kleinstein RN, Jones LA, Hullett S, Kwon S, Lee RJ, Friedman NE, et al. Refractive error and ethnicity in children. Arch Ophthalmol. 2003;121:1141–7. doi: 10.1001/archopht.121.8.1141. [DOI] [PubMed] [Google Scholar]
- 39.Villarreal GM, Ohlsson J, Cavazos H, Abrahamsson M, Mohamed JH. Prevalence of myopia among 12-to 13-year-old schoolchildren in northern Mexico. Optom Vis Sci. 2003;80:369–73. doi: 10.1097/00006324-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Zadnik K, Manny RE, Yu JA, Mitchell GL, Cotter SA, Quiralte JC, et al. Ocular component data in schoolchildren as a function of age and gender. Optom Vis Sci. 2003;80:226–36. doi: 10.1097/00006324-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 41.He M, Zeng J, Liu Y, Xu J, Pokharel GP, Ellwein LB. Refractive error and visual impairment in urban children in southern China. Invest Ophthalmol Vis Sci. 2004;45:793–9. doi: 10.1167/iovs.03-1051. [DOI] [PubMed] [Google Scholar]
- 42.Woo WW, Lim KA, Yang H, Lim XY, Liew F, Lee YS, et al. Refractive errors in medical students in Singapore. Singapore Med J. 2004;45:470–4. [PubMed] [Google Scholar]
- 43.Raju P, Ramesh SV, Arvind H, George R, Baskaran M, Paul PG, et al. Prevalence of refractive errors in a rural South Indian population. Invest Ophthalmol Vis Sci. 2004;45:4268–72. doi: 10.1167/iovs.04-0221. [DOI] [PubMed] [Google Scholar]
- 44.Goh PP, Abqariyah Y, Pokharel GP, Ellwein LB. Refractive error and visual impairment in school-age children in Gombak District, Malaysia. Ophthalmology. 2005;112:678–85. doi: 10.1016/j.ophtha.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 45.Dayan YB, Levin A, Morad Y, Grotto I, Ben-David R, Goldberg A, et al. The changing prevalence of myopia in young adults:A 13-year series of population-based prevalence surveys. Invest Ophthalmol Vis Sci. 2005;46:2760–5. doi: 10.1167/iovs.04-0260. [DOI] [PubMed] [Google Scholar]
- 46.Ojaimi E, Rose KA, Morgan IG, Smith W, Martin FJ, Kifley A, et al. Distribution of ocular biometric parameters and refraction in a population-based study of Australian children. Invest Ophthalmol Vis Sci. 2005;46:2748–54. doi: 10.1167/iovs.04-1324. [DOI] [PubMed] [Google Scholar]
- 47.Khader YS, Batayha WQ, Abdul Aziz SM, Al Shiekh Khalil MI. Prevalence and risk indicators of myopia among schoolchildren in Amman, Jordan. East Mediterr Health J. 2006;12:434–9. [PubMed] [Google Scholar]
- 48.Ip JM, Huynh SC, Robaei D, Kifley A, Rose KA, Morgan IG, et al. Ethnic differences in refraction and ocular biometry in a population-based sample of 11–15-year-old Australian children. Eye. 2008;22:649–56. doi: 10.1038/sj.eye.6702701. [DOI] [PubMed] [Google Scholar]
- 49.Saw SM, Chan YH, Wong WL, Shankar A, Sandar M, Aung T, et al. Prevalence and risk factors for refractive errors in the Singapore Malay Eye Survey. Ophthalmology. 2008;115:1713–9. doi: 10.1016/j.ophtha.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Jobke S, Kasten E, Vorwerk C. The prevalence rates of refractive errors among children, adolescents, and adults in Germany. Clin Ophthalmol. 2008;2:601–7. doi: 10.2147/opth.s2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krishnaiah S, Srinivas M, Khanna RC, Rao GN. Prevalence and risk factors for refractive errors in the South Indian adult population:The Andhra Pradesh Eye disease study. Clin Ophthalmol. 2009;3:17–27. [PMC free article] [PubMed] [Google Scholar]
- 52.Pan CW, Wong TY, Lavanya R, Wu RY, Zheng YF, Lin XY, et al. Prevalence and risk factors for refractive errors in Indians:The Singapore Indian Eye Study (SINDI) Invest Ophthalmol Vis Sci. 2011;52:3166–73. doi: 10.1167/iovs.10-6210. [DOI] [PubMed] [Google Scholar]
- 53.Guo YH, Lin HY, Lin LL, Cheng CY. Self-reported myopia in Taiwan:2005 Taiwan National health interview survey. Eye. 2012;26:684–9. doi: 10.1038/eye.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohammed Alemam A, Aldebasi MH, Rehmatullah A, Alsaidi R, Tashkandi I. Prevalence of myopia among children attending pediatrics ophthalmology clinic at Ohud Hospital, Medina, Saudi Arabia. J Ophthalmol. 2018;2018:3708409. doi: 10.1155/2018/3708409. doi:10.1155/2018/3708409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hansen MH, Laigaard PP, Olsen EM, Skovgaard AM, Larsen M, Kessel L, et al. Low physical activity and higher use of screen devices are associated with myopia at the age of 16-17 years in the CCC2000 eye study. Acta Ophthalmol. 2020;98:315–21. doi: 10.1111/aos.14242. [DOI] [PubMed] [Google Scholar]
- 56.Stenstrom S, Woolf D. Investigation of the variation and the correlation of the optical elements of human eyes. Am J Optom Arch Am Acad Optom. 1948;58:1–71. doi: 10.1097/00006324-194809000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Ahmad I, Qureshi T, Jan R, Ahmad R, Pandit AK. Myopia:Perspectives and challenges. JK Pract. 2007;14:65–70. [Google Scholar]
- 58.Epstein D. Accommodation as the primary cause of low-luminanace myopia:Experimental Evidence. Acta Ophthalmol. 1983;61:424–30. doi: 10.1111/j.1755-3768.1983.tb01441.x. [DOI] [PubMed] [Google Scholar]
- 59.Hofstetter HW. Some interrelationships of age, refraction, and rate of refractive change. Optom Vis Sci. 1954;31:161–9. doi: 10.1097/00006324-195404000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Hirsch MJ. A longitudinal study of refractive state of children during the first six years of school—a preliminary report of the Ojai study. Optom Vis Sci. 1961;38:564–71. doi: 10.1097/00006324-196110000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Goss DA, Cox VD. Trends in the change of clinical refractive error in myopes. J Am Optom Assoc. 1985;56:608–13. [PubMed] [Google Scholar]
- 62.Charman WN. Night myopia and driving. Ophthalmic Physiol Opt. 1996;16:474–85. [PubMed] [Google Scholar]
- 63.Miller RJ. Temporal stability of the dark focus of accommodation. Am J Optom Physiol Optic. 1978;55:447–50. doi: 10.1097/00006324-197807000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Locke L. Induced refractive and visual changes. Diagnosis and Managment in Vision Care. Boston: Butterworths; 1987. pp. 313–67. [Google Scholar]
- 65.Curtin BJ. Physiologic vs pathologic myopia:Genetics vs environment. Ophthalmology. 1979;86:681–91. doi: 10.1016/s0161-6420(79)35466-5. [DOI] [PubMed] [Google Scholar]
- 66.Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005;24:1–38. doi: 10.1016/j.preteyeres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 67.Saw SM, Tan SB, Fung D, Chia KS, Koh D, Tan DT, et al. IQ and the association with myopia in children. Invest Ophthalmol Vis Sci. 2004;45:2943–8. doi: 10.1167/iovs.03-1296. [DOI] [PubMed] [Google Scholar]
- 68.Wu PC, Tsai CL, Hu CH, Yang YH. Effects of outdoor activities on myopia among rural school children in Taiwan. Ophthalmic Epidemiol. 2010;17:338–42. doi: 10.3109/09286586.2010.508347. [DOI] [PubMed] [Google Scholar]
- 69.Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–85. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 70.Simensen B, Thorud LO. Adult-onset myopia and occupation. Acta Ophthalmol. 1994;72:469–71. doi: 10.1111/j.1755-3768.1994.tb02799.x. [DOI] [PubMed] [Google Scholar]
- 71.Saw SM, Shankar A, Tan SB, Taylor H, Tan DT, Stone RA, et al. A cohort study of incident myopia in Singaporean children. Invest Ophthalmol Vis Sci. 2006;47:1839–44. doi: 10.1167/iovs.05-1081. [DOI] [PubMed] [Google Scholar]
- 72.Dirani M, Chamberlain M, Shekar SN, Islam AF, Garoufalis P, Chen CY, et al. Heritability of refractive error and ocular biometrics:The Genes in Myopia (GEM) twin study. Invest Ophthalmol Vis Sci. 2006;47:4756–61. doi: 10.1167/iovs.06-0270. [DOI] [PubMed] [Google Scholar]
- 73.Klein AP, Duggal P, Lee KE, Klein R, Bailey-Wilson JE, Klein BE. Confirmation of linkage to ocular refraction on chromosome 22q and identification of a novel linkage region on 1q. Arch Ophthalmol. 2007;125:80–5. doi: 10.1001/archopht.125.1.80. [DOI] [PubMed] [Google Scholar]
- 74.Dirani M, Tong L, Gazzard G, Zhang X, Chia A, Young TL, et al. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009;93:997–1000. doi: 10.1136/bjo.2008.150979. [DOI] [PubMed] [Google Scholar]
- 75.Gwiazda J. Treatment options for myopia. Optom Vis Sci. 2009;86:624–8. doi: 10.1097/OPX.0b013e3181a6a225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dandona R, Dandona L. Refractive error blindness. Bull World Health Organ. 2001;79:237–43. [PMC free article] [PubMed] [Google Scholar]
- 77.Tan DT, Lam DS, Chua WH, Shu-Ping DF, Crockett RS Asian Pirenzepine Study Group. One-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology. 2005;112:84–91. doi: 10.1016/j.ophtha.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 78.Fan DS, Lam DS, Chan CK, Fan AH, Cheung EY, Rao SK. Topical atropine in retarding myopic progression and axial length growth in children with moderate to severe myopia:A pilot study. Jpn J Ophthalmol. 2007;51:27–33. doi: 10.1007/s10384-006-0380-7. [DOI] [PubMed] [Google Scholar]
- 79.Dyer JA. Role of cycloplegics in progressive myopia. Ophthalmology. 1979;86:692–4. doi: 10.1016/s0161-6420(79)35459-8. [DOI] [PubMed] [Google Scholar]
- 80.Yen MY, Liu JH, Kao SC, Shiao CH. Comparison of the effect of atropine and cyclopentolate on myopia. Ann Ophthalmol. 1989;21:180–2. [PubMed] [Google Scholar]
- 81.Chou AC, Shih YF, Ho TC, Lin LL. The effectiveness of 0.5% atropine in controlling high myopia in children. J Ocul Pharmacol Ther. 1997;13:61–7. doi: 10.1089/jop.1997.13.61. [DOI] [PubMed] [Google Scholar]
- 82.Shih YF, Chen CH, Chou AC, Ho TC, Lin LL, Hung PT. Effects of different concentrations of atropine on controlling myopia in myopic children. J Ocul Pharmacol Ther. 1999;15:85–90. doi: 10.1089/jop.1999.15.85. [DOI] [PubMed] [Google Scholar]
- 83.Shih YF, Hsiao CK, Chen CJ, Chang CW, Hung PT, Lin LL. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmol Scand. 2001;79:233–6. doi: 10.1034/j.1600-0420.2001.790304.x. [DOI] [PubMed] [Google Scholar]
- 84.Lee JJ, Fang PC, Yang IH, Chen CH, Lin PW, Lin SA, et al. Prevention of myopia progression with 0.05% atropine solution. J Ocul Pharmacol Ther. 2006;22:41–6. doi: 10.1089/jop.2006.22.41. [DOI] [PubMed] [Google Scholar]
- 85.Liang CK, Ho TY, Li TC, Hsu WM, Li TM, Lee YC, et al. A combined therapy using stimulating auricular acupoints enhances lower-level atropine eyedrops when used for myopia control in school-aged children evaluated by a pilot randomized controlled clinical trial. Complement Ther Med. 2008;16:305–10. doi: 10.1016/j.ctim.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 86.Fang PC, Chung MY, Yu HJ, Wu PC. Prevention of myopia onset with 0.025% atropine in premyopic children. J Ocul Pharmacol Ther. 2010;26:341–5. doi: 10.1089/jop.2009.0135. [DOI] [PubMed] [Google Scholar]
- 87.Lin HJ, Wan L, Tsai FJ, Tsai YY, Chen LA, Tsai AL, et al. Overnight orthokeratology is comparable with atropine in controlling myopia. BMC Ophthalmol. 2014;14:1–8. doi: 10.1186/1471-2415-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clark TY, Clark RA. Atropine 0.01% eyedrops significantly reduce the progression of childhood myopia. JOcul Pharmacol Ther. 2015;31:541–5. doi: 10.1089/jop.2015.0043. [DOI] [PubMed] [Google Scholar]
- 89.Lee CY, Sun CC, Lin YF, Lin KK. Effects of topical atropine on intraocular pressure and myopia progression:A prospective comparative study. BMC Ophthalmol. 2016;16:1–7. doi: 10.1186/s12886-016-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yam JC, Jiang Y, Tang SM, Law AK, Chan JJ, Wong E, et al. Low-concentration atropine for myopia progression (LAMP) study:A randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126:113–24. doi: 10.1016/j.ophtha.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 91.Stone RA, Lin T, Laties AM. Muscarinic antagonist effects on experimental chick myopia. Exp. Eye Res. 1991;52:755–8. doi: 10.1016/0014-4835(91)90027-c. [DOI] [PubMed] [Google Scholar]
- 92.Cottriall CL, McBrien NA. The M1 muscarinic antagonist pirenzepine reduces myopia and eye enlargement in the tree shrew. Invest Ophthalmol Vis Sci. 1996;37:1368–79. [PubMed] [Google Scholar]
- 93.Tigges M, Iuvone PM, Fernandes A, Sugrue MF, Mallorga PJ, Laties AM, et al. Effects of muscarinic cholinergic receptor antagonists on postnatal eye growth of rhesus monkeys. Optom Vis Sci. 1999;76:397–407. doi: 10.1097/00006324-199906000-00020. [DOI] [PubMed] [Google Scholar]
- 94.Leech EM, Cottriall CL, McBrien NA. Pirenzepine prevents form deprivation myopia in a dose dependent manner. OphthalmicPhysiol Opt. 1995;15:351–6. [PubMed] [Google Scholar]
- 95.Siatkowski RM, Cotter S, Miller JM, Scher CA, Crockett RS, Novack GD, et al. Safety and efficacy of 2% pirenzepine ophthalmic gel in children withmyopia:A 1-year, multicenter, double-masked, placebo-controlled parallel study. Arch Ophthalmol. 2004;122:1667–74. doi: 10.1001/archopht.122.11.1667. [DOI] [PubMed] [Google Scholar]
- 96.Singh H, Sahajpal NS, Singh H, Vanita V, Roy P, Paul S, et al. Pre-clinical and cellular toxicity evaluation of 7-methylxanthine:An investigational drug for the treatment of myopia. Drug Chem Toxicol. 2019;12:1–0. doi: 10.1080/01480545.2019.1635615. [DOI] [PubMed] [Google Scholar]
- 97.Singh H, Singh H, Sahajpal NS, Paul S, Kaur I, Jain SK. Sub-chronic and chronic toxicity evaluation of 7-methylxanthine:A new molecule for the treatment of myopia. Drug Chem Toxicol. 2020;16:1–2. doi: 10.1080/01480545.2020.1833904. [DOI] [PubMed] [Google Scholar]
- 98.Trier K, Munk Ribel-Madsen S, Cui D, Brøgger Christensen S. Systemic 7-methylxanthine in retarding axial eye growth and myopia progression:a 36-month pilot study. J Ocul Biol Dis Infor. 2008;1:85–93. doi: 10.1007/s12177-008-9013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Safranow K, Machoy Z. Simultaneous determination of 16 purine derivatives in urinary calculi by gradient reversed-phase high-performance liquid chromatography with UV detection. J Chromatogr B. 2005;819:229–35. doi: 10.1016/j.jchromb.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 100.Hung LF, Arumugam B, She Z, Ostrin L, Smith EL., III Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Exp Eye Res. 2018;176:147–60. doi: 10.1016/j.exer.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oñatibia-Astibia A, Martínez-Pinilla E, Franco R. The potential of methylxanthine-based therapies in pediatric respiratory tract diseases. Respir Med. 2016;112:1–9. doi: 10.1016/j.rmed.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 102.Smith EL, Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–72. doi: 10.1167/iovs.05-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]