Abstract
This report shows a case of corneal transplant rejection after vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), short after receiving the BNT162b2 vaccine, in a patient who had undergone keratoplasty more than 20 years ago, with no previous episodes of rejection and no other factor that could lead to the findings on his examinations. After treatment with high doses of topic, oral, and sub-conjunctival corticoids, the patient had a favorable therapeutic response. The signs of corneal transplant rejection must be oriented to the patients and the causing factors actively searched by ophthalmologists so that treatment is rapidly initiated and sequels are avoided. This report raises the question if these events are correlated and whether the patient should receive the second dose of the vaccine against SARS-CoV-2 or not.
Keywords: BNT162b2 vaccine, coronavirus, keratoplasty, penetrating, vaccine
Corneal transplantation is the most frequently performed type of transplant worldwide. It restores visual functions when impairment caused by corneal damage is deemed too severe to provide acceptable quality of life.[1]
The coronavirus disease 2019 (COVID-19) pandemic brought a scenario of systemic and ocular reactions not yet fully elucidated and often with serious consequences. With the introduction of mass vaccination, there was a decrease in cases of COVID; however, adverse reactions to the vaccines began to appear, including ophthalmological changes.
We describe one case of a penetrating allograft rejection following COVID-19 immunization and suggest the possibility of a causal association.
Case Report
A 40-year-old male patient with hypertension using losartan and amlodipine underwent penetrating corneal transplantation in the right eye (OD) in 1998 and in the left eye (OS) in 2014 after complications resulting from keratoconus. There were no previous episodes of corneal graft rejection in both eyes and no vascularization of the graft recipient bed. He was not using any medications prior to immunization. He received the first dose of the Pfizer vaccine against COVID-19 on 02/06/2021. The protocol was previously approved by ethic committee in Brazil under the number 281/2021.
On 12/06/2021, he presented with hyperemia and low visual acuity in the OD mainly in the morning, which improved throughout the day, with progressive worsening. There were no symptoms on the OS. The patient sought ophthalmologic assessment and received the diagnosis of acute transplant rejection. Treatment for corneal allograft was initiated with oral prednisone 20 mg, hourly drops of topic prednisolone acetate 1%, and sub-conjunctival injection of dexamethasone. Prophylactic corticosteroids (prednisolone acetate 6/6 hours) were innitiated after the rejection in the fellow eye.
Examination showed a best-corrected visual acuity of 20/100 in the OD and 20/25 in the OS.
On biomicroscopic examination, findings in the OD included hyperemic conjunctiva, a cornea with epithelial and sub-epithelial bullae, a Khodadoust line, stromal edema and keratic precipitates, a trophic iris, a photo-reactive pupil, and an anterior chamber formed and wide [Figs. 1 and 2]. It was difficult to assess anterior chamber reaction. There were no loose sutures, neovascularization, iris synechiae, or any other stimulus for rejection. OS had no changes. There were no abnormalities in the intra-ocular pressure or in fundoscopy in both eyes.
Figure 1.
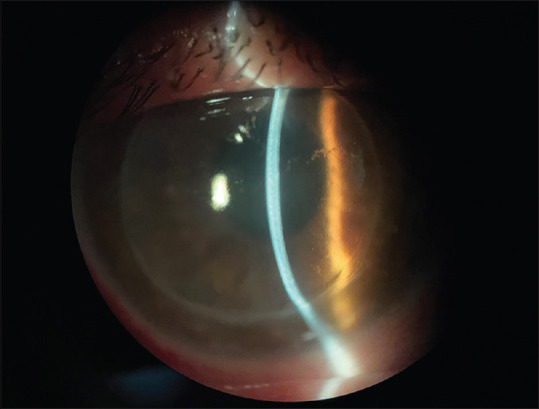
Slit lamp photograph demonstrating ciliary injection and na edematous corneal graft with a Khodadoust line, Descemet folds, and KPs
Figure 2.
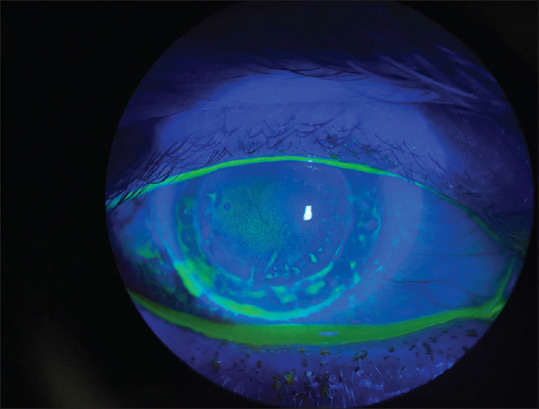
In the illumination with cobalt light, we noticed corneal stromal edema. Areas suggestive of keratitis were not noted
After treatment, the patient made a good recovery and the transplant rejection satisfactorily resolved. Four months after the coronavirus vaccination, the patient is symptom-free, the visual acuity is restored, and the corneal transplant remains clear and with no signs of active rejection.
Discussion
Our patient had transplantation in OD for more than 20 years, with no previous episode of graft rejection; only 10 days after receiving the BNT162b2 vaccine, he presented himself with all the symptoms described previously in this article.
Corneal allografts are the most common and the most successful form of solid organ transplantation performed worldwide.[2] With the systematic vaccination efforts adopted by countries worldwide, a very large number of patients with corneal transplants have had, or are set to have, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines.[3] Vaccination-associated corneal graft rejection is a rare event and not well understood. Only a few cases of corneal graft rejection after vaccination have been described after influenza, hepatitis B, tetanus toxoid, and yellow fever immunizations.[4]
The BNT162b2 messenger RNA vaccine introduces genetic material into cells, which encodes a membrane-anchored SARS-CoV-2 full-length spike, stabilized in the prefusion conformation, which in turn generates a systemic antibody response.[4,5] This vaccine has demonstrated high levels of neutralizing antibody titers and antigen-specific CD8+ and TH1-type CD4+ T-cell responses.[4]
Vaccination also incites immune response that can induce Class II MHC complex antigens in all layers of the grafted cornea and could trigger allograft rejection like the influenza vaccine. The recent rampant vaccination against COVID-19 has triggered a debate about a similar such effect on endothelial or full thickness keratoplasty.[6]
For our patient, it is of particular importance the local immune tolerance that has been maintained for 20 years after surgery. Moreover, considering the good state of health of the patient, we can exclude other possible etiological factors responsible for the rejection. Also, the 10-day lag between vaccine administration and corneal graft rejection that we observed falls within the window of increased risk described in the specific guidelines (Causality Assessment of an Adverse Event Following Immunization—AEFI).[7] There were no other “qualifying factors” (i.e., previous similar episodes with or without vaccination or exposure to drugs/allergens/over-the-counter products—OTCs) that may be responsible for the rejection. In fact, after surgery for keratoconus, the patient had no other pathologies in progress (no drugs/no OTCs). Moreover, the patient had no previous reactions to vaccines nor exposure to toxic substances.[7]
This case has some similarities with the other cases discussed in the current literature, such as the time required for the appearance of symptoms after immunization with the BNT162b2 vaccine, the similarity between the signs and symptoms manifested by the patients, the occurrence of rejection to the corneal transplant in healthy patients who have never had previous episodes, and the absence of other triggering factors.
This condition must be recognized for patients and ophthalmologists to react promptly if rejection symptoms develop after vaccination. A recent vaccination history should be questioned when reviewing patients with signs of transplant rejection and any temporal association reported to the relevant local agencies.[3]
Conclusion
Case reports are important to record current cases of illnesses, with poorly defined etiology. Currently, more and more people are getting vaccinated because of the COVID-19 pandemic, and this shows the importance of studying the possible effects in patients with corneal transplants.
Our case highlights the possibility of immune corneal graft rejection after COVID-19 vaccination which corneal surgeons should be aware of, so they can identify and treat as soon as possible and thus keep the transplant viable and avoid a new surgical approach or irreversible loss of endothelial cells. It also raises the dilemma regarding the timing of the second dose of vaccination in patients presenting with rejection.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to dedicate this work to all the members of the ophthalmology sector at Complexo Hospitalar Padre Bento de Guarulhos, who work hard to provide quality care to the population.
References
- 1.Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 2.Niederkorn JY, Larkin DF. Immune privilege of corneal allografts. Ocul Immunol Inflamm. 2010;18:162–71. doi: 10.3109/09273948.2010.486100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phylactou M, Li JO, Larkin DF. Characteristics of endothelial corneal transplant rejection following immunisation with SARS-CoV-2 messenger RNA vaccine. Br J Ophthalmol. 2021;105:893–6. doi: 10.1136/bjophthalmol-2021-319338. [DOI] [PubMed] [Google Scholar]
- 4.Wasser LM, Roditi E, Zadok D, Berkowitz L, Weill Y. Keratoplasty rejection after the BNT162b2 messenger RNA vaccine. Córnea. 2021;40:1070–2. doi: 10.1097/ICO.0000000000002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh EE, Frenck RW, Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–50. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravichandran S, Natarajan R. Corneal graft rejection after COVID-19 vaccination. Indian J Ophthalmol. 2021;69:1953–4. doi: 10.4103/ijo.IJO_1028_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nioi M, d'Aloja E, Fossarello M, Napoli PE. Dual corneal-graft rejection after mRNA vaccine (BNT162b2) for covid-19 during the first six months of follow-up:Case report, state of the art and ethical concerns. Vaccines (Basel) 2021;9:1274. doi: 10.3390/vaccines9111274. [DOI] [PMC free article] [PubMed] [Google Scholar]


