Abstract
Expression of the cytokine osteopontin (OPN) is elevated in granulomas caused by Mycobacterium tuberculosis. We tested the hypothesis that OPN contributes to host protection in a mouse model of mycobacterial infection. When infected with Mycobacterium bovis BCG, mice lacking a functional OPN gene had more severe infections characterized by heavier bacterial loads and a delayed clearance of the bacteria. The OPN-null mice had greater granuloma burdens consistent with the elevated bacterial load. The ability of osteopontin to facilitate the clearance of mycobacteria was most pronounced early after infection and appeared to be independent of known mediators of resistance to infection by mycobacteria: antigen-specific T-cell immunity, gamma interferon production, and nitric oxide production. BCG grew more rapidly in macrophages derived from OPN-null mice than in those from wild-type mice, demonstrating that the null phenotype was due to an intrinsic macrophage defect. These results indicate that osteopontin augments the host response against a mycobacterial infection and that it acts independently from other antimycobacterial resistance mechanisms.
Tuberculosis is a pandemic infection that involves much of the world’s population and is caused by Mycobacterium tuberculosis. The World Health Organization has raised concern over the epidemic potential of this organism because of increasing antimicrobial resistance (36). Recently, an isolate of M. tuberculosis with an extraordinary growth rate and with increased rates of transmission was isolated (46). These findings have generated interest in understanding the unique interactions of host cells and mycobacteria: M. tuberculosis can elude host immune responses and persist in a latent state for years. The granuloma is a feature of this host-pathogen interaction. Granulomas are characterized by a mononuclear cell infiltration of macrophages and lymphocytes, by the formation of giant cells and epithelioid cells, and by fibrosis, sometimes with calcification (2, 12). The public health concerns and the unusual pathology of tuberculosis led us to question what is normally involved in the host macrophage response after infection by mycobacteria.
We have undertaken a study of macrophage gene expression changes after infection by mycobacteria. By differential screening of a cDNA library and probes derived from a murine macrophage cell line, one gene isolate, osteopontin (OPN), was identified repeatedly in cells infected by mycobacteria compared with cells infected by Escherichia coli (35). We found that osteopontin gene expression in human pulmonary macrophages increased after infection with virulent M. tuberculosis and that OPN protein expression was widespread in human tuberculosis pathology (35).
OPN is a secreted, phosphorylated, glycoprotein that is associated with several host inflammatory states. The gene encoding OPN, known as spp1, cosegregates with resistance to lethal infection by Orientia (Rickettsia) tsutsugamushi in mice (16, 23). The protein stimulates the migration of macrophages (22, 34, 42) and of smooth muscle cells (29, 51). OPN is found in the inflammatory macrophages of rat myocardium after cryoinjury (33), in arterial atherosclerosis (21, 24), and in several granulomatous conditions (3, 35). OPN has been labeled a cytokine based on its proinflammatory properties (38, 45) and is a likely candidate for mediating or modulating mononuclear cell infiltration and chronic inflammation.
We and others have identified genes whose expression changes in mammalian cells after infection (35, 52). These findings merit further studies to determine the roles of these genes in infection. The work detailed here describes the use of OPN knockout mice (28) to investigate whether OPN influences the host response against infection by mycobacteria. Animals lacking OPN had more severe infections and were delayed in eliminating the mycobacteria after infection. The findings identify a novel role for OPN as an accessory molecule to activate macrophages and to augment the clearance of inflammatory stimuli.
MATERIALS AND METHODS
Animals.
OPN mutant mice (spp1tm1) and wild-type controls 5 to 8 weeks old and matched for age and sex were used for experiments. The generation of the OPN mutant mice has been described elsewhere (28). The experiments described herein were done with wild-type and mutant animals on a (129 × Black Swiss) hybrid background as was used previously (28). Genotyping was confirmed on all breeding pairs with a PCR analysis. All animal procedures were performed according to the guidelines of the Massachusetts Institute of Technology Committee on Animal Care.
Infections.
Mycobacterium bovis bacillus Calmette-Guérin (BCG) (ATCC 35734; Trudeau mycobacterial culture collection no. 1011, “BCG Pasteur”) was grown from a frozen stock for 6 to 7 days (to an optical density at 600 nm of ∼1.4) in Middlebrook 7H9 broth with 0.5% glycerol, 0.05% Tween 80, and ADC enrichment (Difco, Detroit, Mich.). On the day of infection, bacteria were resuspended in phosphate-buffered saline (PBS), and an inoculum of approximately 107 CFU was delivered intraperitoneally. This route of inoculation was selected because of previous studies of OPN and infection (23, 37) and because intraperitoneal infections can successfully generate systemic mycobacterial infections (32). At various times after infection, organs were harvested for analysis. For CFU analysis, liver sections were weighed and disrupted in PBS with an electric homogenizer (Polytron PT 1200B; Kinimatica). Serial dilutions were made in 7H9 medium, and aliquots were spread on 7H10 plates. CFU were enumerated 3 to 4 weeks later.
Immunohistochemistry.
Organs were harvested for histologic analysis at the times indicated after infection. Tissues were fixed in buffered formalin and processed by standard histologic techniques. For immunohistochemistry, paraffin-embedded tissue sections were deparaffinized in HistoClear (National Diagnostics, Atlanta, Ga.), rehydrated in graded ethanol washes, and washed in PBS. The F4/80 antibody (Harlan Bioproducts, Indianapolis, Ind.) was used according to the manufacturer’s recommendations. Rabbit antiserum against holo-NOS2 (49) was a gift from Qiao-wen Xie and Carl Nathan and was used as described previously (30), except that a 1:750 dilution was incubated with specimens at room temperature for 2 h. Biotinylated secondary antibodies, avidin-biotin-horseradish peroxidase conjugates, and substrates (Vector Labs, Burlingame, Calif.) were used according to the manufacturer’s directions. Slides were counterstained with hematoxylin (Dako, Carpinteria, Calif.). After the completion of staining, specimens were dehydrated in graded ethanol and ClearRite 3 (Richard-Allan Scientific, Kalamazoo, Mich.) before being mounted with Permount (Fisher, Fair Lawn, N.J.).
Quantitation of granuloma burden.
The percent granuloma burden, the total percentage of liver surface area covered by granulomas, was measured by macrophage immunostaining and a Bioquant image analysis system (Nashville, Tenn.) (26, 27). The Bioquant system is an operator-interactive image analysis device linked to stage x-y encoders, utilizing a Dage MTI CCD72 video camera system with a Leitz Aristoplan microscope. A systematically random sampling scheme was used to select fields for measurement of granuloma burden: a grid was overlaid over the liver section, and from a random start point, every eighth 500-μm by 500-μm field was captured by video under a ×16 objective with the fluorescein cube. The counting chamber consisted of a 500-μm by 500-μm sampling box with extended exclusion lines. For each field, a threshold optical density was obtained, which discriminated staining from background. Manual editing of each field eliminated artifacts, as well as excluding granulomas touching the lower and left borders of the counting chamber, while including granulomas touching the upper and right borders. Granulomas were defined as areas of immunoreactivity greater than 150 μm2, or greater than approximately two to three macrophages. The total number of granulomas, the area of each granuloma, and the sum of the areas of the granulomas divided by the total area sampled (granuloma burden) were determined over four to five liver sections for each animal. Some background counts could be attributed to the confluence of Kupffer cells that registered above the threshold filter. Statistical analysis was by t test comparing homozygous spp1tm1 and wild-type mice. The appropriateness of the sampling scheme was evaluated by calculating the precision of the estimates, expressed as the coefficient of error (CE) (48). The CE was <0.10, suggesting that a minimal amount of variance in the granuloma burden measurement was from the sampling technique and that measured differences reflected true biological variability. All measurements were performed by a single examiner (G.N.) blinded to treatment group.
In vitro cell stimulation.
Spleens were harvested at the times indicated after infection and were crushed through nylon mesh to obtain single-cell suspensions. Cells were washed and resuspended to 4 × 106 cells/ml, and 1 ml was plated per well of a Falcon 24-well plate (Becton Dickinson, Lincoln Park, N.J.) with various antigens or concanavalin A (ConA). The purified protein derivative (PPD) from M. tuberculosis was generously donated by Lederle Laboratories (Pearl River, N.Y.). Supernatants were harvested 72 h later. The gamma interferon (IFN-γ) MiniKit from Endogen (Woburn, Mass.) was used to assay the culture supernatants.
NO measurement.
Peritoneal exudate cells (PEC) were isolated 5 to 7 days after intraperitoneal inoculation of 1 ml of 3% thioglycolate. In some instances, macrophages were purified by adherence and recovered by dispase treatment and scraping (7). The production of nitric oxide (NO) by the PEC was assessed by measuring the accumulation of nitrite in culture supernatants. Griess reagents were used as described previously (25).
In vitro assay of BCG growth.
The measurement of BCG growth in macrophages was performed by using a [3H]uracil assay that has been widely used to measure the growth of mycobacteria (5, 17, 18, 40); uptake closely correlates with CFU (17). Thioglycolate-derived PEC (2 × 105) and BCG (multiplicity of infection, 5 to 10 bacteria per 1 macrophage) were cocultured with RPMI (Life Technologies, Gaithersburg, Md.) with 1% heat-inactivated fetal calf serum in U-bottom plates (ICN Biomedicals, Inc., Aurora, Ohio). After 4 h of coincubation, the wells were washed with warm Hank’s balanced salt solution (Life Technologies) to remove extracellular BCG and nonadherent cells and RPMI medium–1% fetal calf serum was added. At various times after the infection, [3H]uracil was added to the wells, and after 18 h, the contents of the wells were harvested with a cell harvester (Skatron, Inc., Sterling, Md.) by using Triton X-100 for macrophage lysis and trichloroacetic acid.
RESULTS
Reduced clearance of M. bovis BCG in OPN-deficient mice.
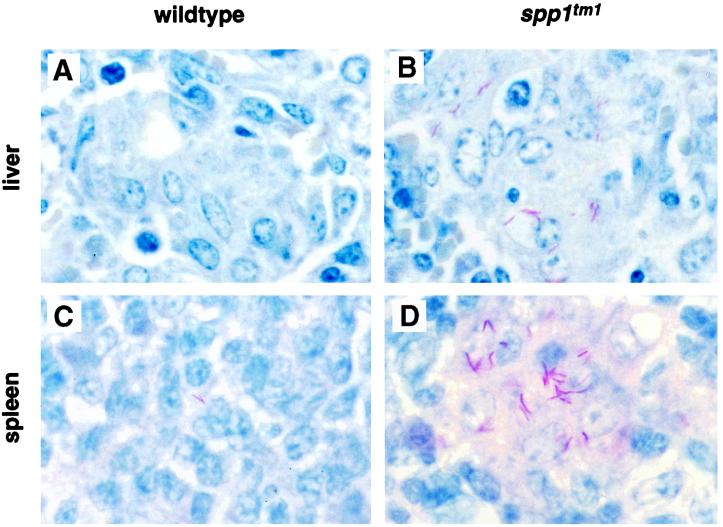
Wild-type and OPN-null mice were infected with BCG, and the bacterial load in liver sections was assessed over 12 weeks by using standard tissue staining for acid-fast bacilli. Four independent experiments were performed, and representative results are shown in Fig. 1. When sections of liver and spleen obtained 4 weeks after infection from wild-type mice were stained for acid-fast organisms, few bacilli were detected (Fig. 1A and C). In contrast, the mycobacteria were easily identified in the livers and spleens of OPN-null animals (Fig. 1B and D). Similar results were seen in lung sections (data not shown).
FIG. 1.
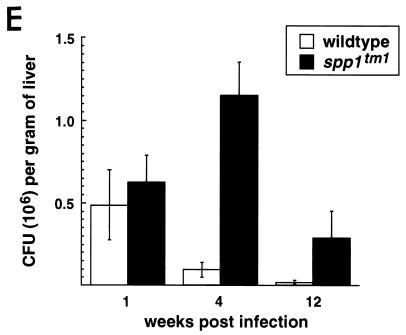
Mycobacterial organism burden in tissues of OPN-null mice after intraperitoneal infection with BCG. (A to D) Ziehl-Nielson stains of liver (A and B) and spleen (C and D) from wild-type (A and C) and spp1tm1 (B and D) animals. The mycobacteria stain red. Similar findings were observed in 15 animals per genotype from four different experiments. Original magnification, ×500. (E) CFU determination from liver samples of animals infected intraperitoneally at time 0 weeks. Symbols represent means ± standard errors for three to four animals per group. A 10- to 40-fold difference in CFU between wild-type and spp1tm1 animals at 4 weeks was observed in three different experiments. P < 0.05 at 4 weeks postinfection. A similar 10-fold difference in CFU was also observed in these animals’ spleens at the 12-week time point.
The burden of mycobacteria in infected tissue was measured by serial dilutions of homogenized liver samples from the wild-type and OPN-null mice (Fig. 1E). Similar numbers of bacilli were found in wild-type and spp1tm1 mice immediately after infection. However, there was a significant difference in the mycobacterial burden 4 weeks after infection. In three independent experiments, OPN-null mice had 10- to 40-fold more bacteria per gram of liver tissue than wild-type mice. The spp1tm1 animals were, however, capable of reducing the mycobacterial burden by 12 weeks. These results show that the OPN-null mice have impaired clearance of BCG early after infection compared to that in wild-type mice.
Increase in granuloma number and size in OPN mutants.
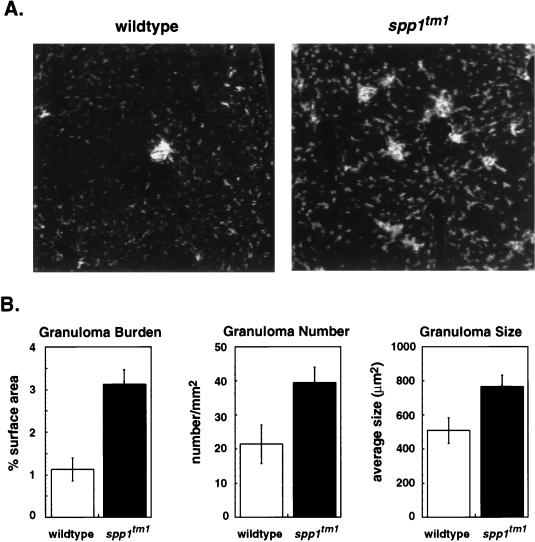
Several biological activities have been attributed to OPN that suggested it would be involved in granuloma formation: OPN is known to activate macrophage migration in vitro and in vivo (22, 34, 42), and it can bind calcium (6). However, histologic analyses of tissues after infection showed that granulomas were present in OPN-null animals; immunohistochemical analyses showed that these granulomas were comprised of both macrophages and T cells similar to those of the wild type (data not shown). The macrophage staining did indicate a difference in the total amount of hepatic tissue involved with granulomas. This difference was quantitated by measuring the granuloma burden. Liver sections stained for macrophages with the F4/80 monoclonal antibody and the Vector red alkaline phosphate substrate were subjected to a stereologic evaluation to measure the granuloma burden. The fluorescent emissions from the Vector red substrate clearly demonstrated that the OPN-null mice had more macrophages within the liver and overall a greater burden of granulomas (Fig. 2A). The granuloma burdens of liver specimens from 20 animals were systematically measured as described in Materials and Methods. The wild-type animals had an estimated 1.1% of the liver tissue area involved with granulomas (Fig. 2B). In contrast, the OPN-null mice had 3.1% of the liver tissue area involved with granulomas. Statistically significant differences were also observed when the sections were analyzed for the number of granulomas and the average granuloma size (Fig. 2B). The inability of OPN-null mice to clear BCG was reflected in more extensive granulomatous inflammation.
FIG. 2.
Assessment of granuloma burden after infection with BCG. (A) Fluorescent images of liver sections from mice of each genotype captured in the BioQuant system. A low-power-view of granuloma burden in OPN-null and wild-type mice viewed by fluorescent microscopy under a fluorescein cube is shown. The original images were captured with a ×16 objective lens. (B) Comparison of granuloma burden, granuloma number, and average granuloma size of wild-type versus spp1tm1 animals. Measurements were performed with 10 OPN-null and 10 wild-type animals from four separate experiments. OPN-null animals had more extensive hepatic inflammation by all measures. Values are ± standard error. P < 0.05 for each comparison. Livers from uninfected control animals of each genotype showed granuloma burdens of <0.05%.
Macrophage recruitment in OPN-null mice.
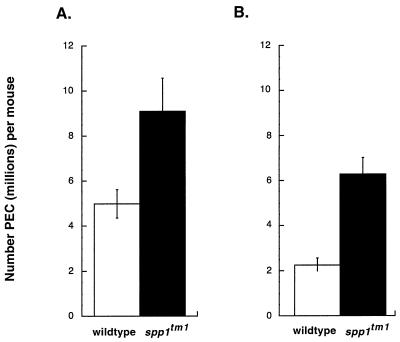
OPN induces inflammatory infiltrates when administered in vivo (42), and it is expressed rapidly after intraperitoneal administration of inflammatory stimuli (37). Because we used an intraperitoneal route of infection, it was possible that the elevated bacterial counts in spp1tm1 mice were a result of an early defect in inflammatory cell accumulation after inoculation. To test this possibility, we quantitated the acute inflammatory response after infection. Seventy-two hours is sufficient time to allow peak OPN gene expression after instillation of a peritoneal irritant (37). At this time after BCG inoculation, OPN-null mice unexpectedly had more cellular exudates than wild-type mice (Fig. 3A). To assess the specificity of this response, intraperitoneal injection of thioglycolate was used to elicit inflammatory cells. In repeated experiments, the PEC from spp1tm1 mice outnumbered those from wild-type mice (Fig. 3B). Cytospin and fluorescence-activated cell sorter analyses of the inflammatory exudates demonstrated there was an abundance of macrophages in PEC from both genotypes (data not shown). These results indicate that spp1tm1 mice did not have a defect in macrophage recruitment; in fact, these animals had a exaggerated inflammatory responses to peritoneal irritants.
FIG. 3.
Exaggerated inflammatory response of spp1tm1 mice compared to wild-type animals after exposure to thioglycolate or BCG. (A) A total of 107 BCG organisms were inoculated intraperitoneally, and PEC were harvested 3 days later. Viable cells were counted on a hemocytometer by trypan blue exclusion. Results are means ± standard errors of 10 animals per group from two separate experiments. P < 0.05 for the significance of differences. (B) PEC accumulation 3 days after intraperitoneal injection of thioglycolate. Results are means ± standard errors for five animals per group. P < 0.05 for the significance of differences. Significant differences in cell counts were observed in five other experiments in which PEC pooled from OPN-null mice outnumbered those from wild-type mice by 1.6- to 6-fold 5 to 7 days after administration of thioglycolate.
IFN-γ production and NO metabolism are normal in OPN-null mice.
The production of IFN-γ (9, 19) and reactive nitrogen intermediates (RNI) (4, 5, 13, 30) is crucial to the successful eradication of M. tuberculosis infections. It was possible that the OPN-null mice were deficient in producing one or both of these important factors after infection by BCG.
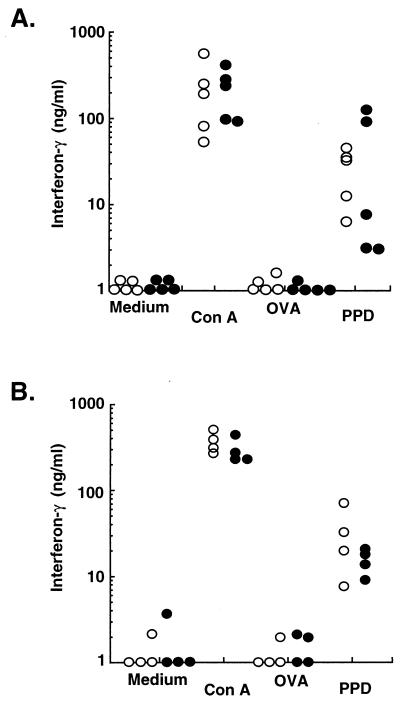
We analyzed the induction of antigen-specific immunity in spp1tm1 versus that in wild-type animals. Splenocytes from animals infected 4 weeks earlier were stimulated in vitro with ConA, an irrelevant antigen (ovalbumin [OVA]), the relevant mycobacterial antigen (purified protein derivative [PPD]), or culture medium only. The results in Fig. 4A demonstrate that splenocytes from OPN-null animals were capable of producing IFN-γ specifically after stimulation with the relevant antigen, PPD. The level of IFN-γ produced by splenocytes from null animals was comparable to that produced by splenocytes from wild-type animals; this occurred when the difference between the CFU was the greatest. There was no difference in the kinetics of induction of T-cell immunity, because splenocytes of both genotypes produced similar levels of IFN-γ 1 week after infection (Fig. 4B). In addition, splenocytes from wild-type and OPN-null mice did not differ in their production of interleukin-4 (IL-4) or IL-10 after stimulation (data not shown). Therefore, the OPN mutant mice mounted appropriate T-cell responses after infection. In addition, there was not a defect in antigen presentation.
FIG. 4.
OPN-null animals are capable of developing antigen-specific immunity comparable to wild-type animals. Splenocytes were stimulated in vitro 4 weeks after infection (A) or 1 week after infection (B). IFN-γ secretion was measured in supernatants harvested after 72 h of incubation with culture medium only, ConA (5 μg/ml), OVA (10 μg/ml), or PPD (10 μg/ml). Each symbol represents an individual animal: solid symbols are spp1tm1 animals, and open symbols are wild-type animals. Those symbols on the x axis represent cytokine levels below the smallest y axis value or that were undetectable in the enzyme-linked immunosorbent assay.
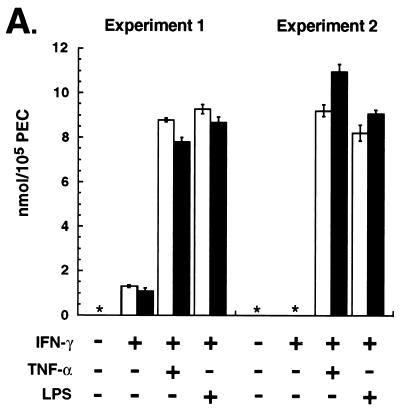
We next tested macrophages for RNI production by measuring the surrogate marker for NO, NO2− (14). Figure 5A shows the production of RNI by PEC from wild-type and spp1tm1 mice treated with inflammatory stimuli. Cells from both strains of mice produced comparable RNI after stimulation by IFN-γ plus lipopolysaccharide (LPS) or plus tumor necrosis factor alpha (TNF-α).
FIG. 5.
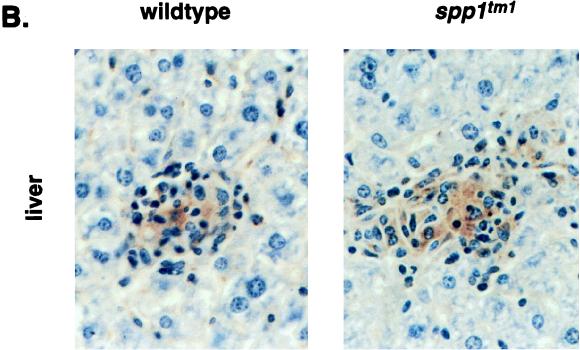
Macrophages from OPN-null and wild-type animals produce similar amounts of NO in vitro and NOS2 in vivo. (A) Nitrite production from PEC stimulated in vitro. Thioglycolate-elicited PEC were stimulated with IFN-γ (100 ng/ml) for 6 (experiment 1) or 16 (experiment 2) h followed by addition of TNF-α (10 ng/ml [experiment 1] or 100 ng/ml [experiment 2]) or LPS (5 μg/ml) for 48 h. Values are means ± standard errors of quadruplicate cultures; open bars and solid bars are PEC from wild-type and spp1tm1 animals, respectively. ∗, none detected. There was no detectable nitrite produced by cells stimulated with either TNF-α or LPS alone. (B) Immunohistochemical stain for NOS2 4 weeks after infection with BCG. Representative animals are shown for each genotype. Similar results were observed with 14 animals in three separate experiments. The resident macrophages of control, uninfected animals did not stain for NOS2. Original magnification, ×200.
Because the requirements of NO production in vivo may differ from the conditions used in the in vitro culture system, the expression of NOS2 was assessed in vivo. Immunohistochemical staining of liver sections revealed widespread NOS2 protein in the granulomas of both wild-type and OPN mutant animals 4 weeks after infection (Fig. 5B). Macrophages infiltrating the livers of both wild-type and mutant animals 1 week after infection also stained faintly for NOS2 (data not shown). As was observed with the T-cell activity (Fig. 4A), the NOS2 was present in tissues at the time of the greatest difference in CFU between the genotypes.
Macrophages from OPN-null mice are defective in killing BCG.
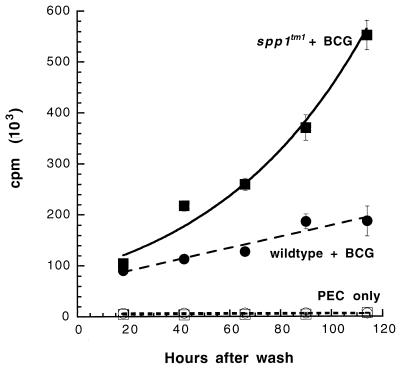
The presence of specific T-cell immunity (Fig. 4) and the apparently normal induction of NOS2 (Fig. 5) were consistent with the fact that OPN-null animals eventually reduce the numbers of BCG (Fig. 1E). It was possible, however, that there was a difference in the ability of the macrophages from spp1tm1 mice to limit the growth of the BCG in the absence of antigen-specific immunity. We tested the antimycobacterial activity of macrophages from naïve wild-type and null mice by infecting them with BCG in vitro. As shown in Fig. 6, [3H]uracil incorporation by BCG, which correlates directly with CFU (17), increased exponentially in macrophages derived from OPN-null mice. In contrast, the incorporation of [3H]uracil by BCG cultured with wild-type macrophages was linear throughout the experiment (Fig. 6). Thus, the OPN-null mice have an intrinsic defect in their macrophages that renders them more susceptible to BCG growth.
FIG. 6.
Macrophages from OPN-null mice are more permissive to the viability and growth of BCG in vitro. Infections were performed as described in Materials and Methods. The data are mean counts per minute ± standard errors of quadruplicate cultures; error bars that are not visible are smaller than the symbol for that data point. Time 0 represents the time that the wells were washed after the 4-h infection, the time of the first tritium pulse. The BCG cultured with spp1tm1 PEC grew exponentially (solid squares) (R2 = 0.98). The BCG cultured with wild-type PEC grew linearly (solid circles) (R2 = 0.92). The 95% confidence limits for the best fit lines did not overlap beyond 60 h. No significant [3H]uracil incorporation was observed with wild-type PEC (open circles) or spp1tm1 PEC (open squares) in the absence of BCG. Similar results were seen in two independent experiments.
DISCUSSION
Resistance to tuberculosis in humans involves a complex network of activation signals and effector responses. The more primitive innate immune system relies on pattern recognition of a microorganism to initiate containment of a pathogen (15). Subsequently, initiation of the adaptive immune response enhances the eradication of M. tuberculosis via effector mechanisms such as macrophage activation (10) or direct cytotoxicity (44). The macrophage is a key participant in the innate response and in the effector phase of the adaptive response. Thus, understanding the interaction between macrophages and mycobacteria should help to identify novel host factors that influence resistance to this disease. Our previous analysis of changes in gene expression in macrophages after BCG infection identified one protein, OPN, whose expression was closely linked to tuberculosis infection. Our current studies with an OPN-null mouse demonstrate that OPN enhances host defenses against a mycobacterial infection.
Mice lacking a functional OPN gene suffered from more severe infections by M. bovis BCG than wild-type controls. The OPN-null animals had delayed eradication of live bacilli and exaggerated peritoneal inflammation. The spp1tm1 mice also had a greater hepatic granuloma burden, consistent with persistence of the bacilli and delayed resolution of the inflammation. The defect of the OPN-null mice is intrinsic to the macrophage and the cells’ inability to control the growth of the mycobacteria.
Although it has been labeled a chemoattractant cytokine, OPN’s role in the immune and inflammatory system has been the subject of much speculation. Intradermal injection of the protein can cause inflammation (22, 34, 42). Previous studies have shown that animals treated with anti-OPN serum have a macrophage migration defect (22, 50). In contrast, we found that OPN-null mice had more macrophages accumulate in response to thioglycolate or to an acute or a chronic mycobacterial infection. Similarly, there was no apparent difference in macrophage accumulation between wild-type and spp1tm1 animals after skin incision (28). While these findings do not exclude OPN as a chemoattractant molecule, it appears some stimuli, such as BCG, elicit signals that compensate for the absence of OPN and promote macrophage accumulation.
Rollo and colleagues have demonstrated an inhibitory activity of OPN on NO production and cytotoxicity by macrophages in vitro (39). The production of nitrite after stimulation with LPS and IFN-γ was delayed, a result of failed NOS2 mRNA induction (39). Our studies of the OPN-null mice have not shown a gross abnormality of NO metabolism. While OPN may inhibit NO production under certain conditions, our data indicate that, overall, OPN enhances the eradication of mycobacteria in vivo.
The data presented here, together with those of other studies (41), implicate OPN and its receptor, CD44, as cofactors in granulomatous inflammation. CD44 is believed to be one of several receptors for OPN (47). Mice deficient in CD44 have an exaggerated granulomatous response to intravenous Corynebacterium parvum (41). Likewise, the spp1tm1 mice had an increased granuloma burden after BCG infection. Thus, the phenotypes of the two mutant mice are similar: a 1.5- to 2-fold increase in granuloma size and number was observed in both null animals after a challenge with an agent that induces granuloma formation. A physiologic interaction of OPN with CD44 in granulomatous inflammation would explain the comparable phenotypes observed in both null mice.
The current data support a model in which the OPN-null mice have a defect in an important effector response of macrophages during a mycobacterial infection. OPN expression by macrophages is increased soon after a mycobacterial infection (35). We have now demonstrated that an OPN-null mutation creates an early defect in the eradication of mycobacteria in vivo and a defect in controlling BCG growth in vitro. These results are consistent with findings of reduced debridement of tissue wounds in the OPN-null mouse, probably due to poor macrophage function (28). Together with the observations with the CD44-null mouse described above, these data suggest that OPN enhances macrophage activity to degrade material taken up after phagocytosis, which would eradicate mycobacteria more efficiently.
Multiple factors, such as the production of inflammatory cytokines (10, 11, 19, 20) and the production of NO (4, 30), are involved in host resistance to tuberculosis. Our present results indicate that OPN has a costimulatory role in macrophage activation to enhance the killing of mycobacteria by macrophages. A similar model of accessory molecule stimulation of monocytes has been suggested previously (31). Activation of human monocytes by IFN-γ is enhanced when the cells are cultured on fibronectin (31). In addition, interactions between several cell types and the extracellular matrix can significantly increase the expression of chemokines (43). OPN, like fibronectin, contains an arginine-glycine-aspartic acid (RGD) domain and may act as a soluble protein (42) or may be cross-linked to extracellular matrix proteins (1).
The goal of our initial investigation was to identify macrophage genes whose expression was altered after infection by mycobacteria. The current work confirms the biological relevance of the findings of the genetic screen by identifying an active role of OPN in the clearance of mycobacteria after infection. Further delineation of OPN’s effects on macrophages may lead to new strategies to treat infections, similar to using IFN-γ in patients with multidrug-resistant tuberculosis (8).
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI37869 (to R.A.Y.) and AI01305 (to G.J.N.). B.L.M.H., in whose laboratory the spp1-null mice were generated by L.L., is an Investigator of The Howard Hughes Medical Institute.
We thank Qiao-wen Xie and Carl F. Nathan (Cornell University Medical College, Department of Medicine) for providing the anti-holo-NOS2 reagent. The PPD was kindly provided by Patricia Van Zandt at Lederle Laboratories. We thank Michael C. Irizarry and Bradley T. Hyman (Massachusetts General Hospital, Department of Neurology) for assistance with the immunohistochemistry and stereologic procedures. We thank Sven Holder (Massachusetts General Hospital) and Margo Goetschkes (Boston VA Medical Center) for assistance with histology.
REFERENCES
- 1.Beninati S, Senger D R, Cordella-Miele E, Mukherjee A B, Chackalaparampil I, Shanmugam V, Singh K, Mukherjee B B. Osteopontin: its transglutaminase-catalyzed posttranslational modifications and cross-linking to fibronectin. J Biochem. 1994;115:675–682. doi: 10.1093/oxfordjournals.jbchem.a124395. [DOI] [PubMed] [Google Scholar]
- 2.Boros D. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- 3.Carlson I, Tognazzi K, Manseau E J, Dvorak H F, Brown L F. Osteopontin is strongly expressed by histiocytes in granulomas of diverse etiology. Lab Investig. 1997;77:103–108. [PubMed] [Google Scholar]
- 4.Chan J, Tanaka K, Carroll D, Flynn J, Bloom B R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Bal B S, Gorski J P. Calcium and collagen binding properties of osteopontin, bone sialoprotein, and bone acidic glycoprotein-75 from bone. J Biol Chem. 1992;267:24871–24878. [PubMed] [Google Scholar]
- 7.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. 2nd ed. Vol. 2. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 8.Condos R, Rom W N, Schluger N W. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet. 1997;349:1513–1515. doi: 10.1016/S0140-6736(96)12273-X. [DOI] [PubMed] [Google Scholar]
- 9.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper A M, Flynn J L. The protective immune response to Mycobacterium tuberculosis. Curr Opin Immunol. 1995;7:512–516. doi: 10.1016/0952-7915(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 11.Cooper A M, Magram J, Ferrante J, Orme I M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dannenberg A M, Jr, Rook G A W. Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses—dual mechanisms that control bacillary multiplication. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: ASM Press; 1994. pp. 459–484. [Google Scholar]
- 13.Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991;49:380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- 14.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 15.Fearon D T, Locksley R M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 16.Fet V, Dickinson M E, Hogan B L. Localization of the mouse gene for secreted phosphoprotein 1 (Spp-1) (2ar, osteopontin, bone sialoprotein 1, 44-kDa bone phosphoprotein, tumor-secreted phosphoprotein) to chromosome 5, closely linked to Ric (Rickettsia resistance) Genomics. 1989;5:375–377. doi: 10.1016/0888-7543(89)90074-8. [DOI] [PubMed] [Google Scholar]
- 17.Flesch I, Kaufmann S H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987;138:4408–4413. [PubMed] [Google Scholar]
- 18.Flesch I E, Hess J H, Oswald I P, Kaufmann S H. Growth inhibition of Mycobacterium bovis by IFN-gamma stimulated macrophages: regulation by endogenous tumor necrosis factor-alpha and by IL-10. Int Immunol. 1994;6:693–700. doi: 10.1093/intimm/6.5.693. [DOI] [PubMed] [Google Scholar]
- 19.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 21.Giachelli C M, Bae N, Almeida M, Denhardt D T, Alpers C E, Schwartz S M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Investig. 1993;92:1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giachelli C M, Lombardi D, Johnson R J, Murry C E, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–358. [PMC free article] [PubMed] [Google Scholar]
- 23.Groves M G, Rosenstreich D L, Taylor B A, Osterman J V. Host defenses in experimental scrub typhus: mapping the gene that controls natural resistance in mice. J Immunol. 1980;125:1395–1399. [PubMed] [Google Scholar]
- 24.Hirota S, Imakita M, Kohri K, Ito A, Morii E, Adachi S, Kim H M, Kitamura Y, Yutani C, Nomura S. Expression of osteopontin messenger RNA by macrophages in atherosclerotic plaques. A possible association with calcification. Am J Pathol. 1993;143:1003–1008. [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang S M, Lopez C A, Heck D E, Gardner C R, Laskin D L, Laskin J D, Denhardt D T. Osteopontin inhibits induction of nitric oxide synthase gene expression by inflammatory mediators in mouse kidney epithelial cells. J Biol Chem. 1994;269:711–715. [PubMed] [Google Scholar]
- 26.Hyman B T, Marzloff K, Arriagada P V. The lack of accumulation of senile plaques or amyloid burden in Alzheimer’s disease suggests a dynamic balance between amyloid deposition and resolution. J Neuropathol Exp Neurol. 1993;52:594–600. doi: 10.1097/00005072-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Irizarry M C, Soriano F, McNamara M, Page K J, Schenk D, Games D, Hyman B T. Aβ deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J Neurosci. 1997;17:7053–7059. doi: 10.1523/JNEUROSCI.17-18-07053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liaw L, Birk D E, Ballas C B, Whitsitt J S, Davidson J M, Hogan B L M. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Investig. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liaw L, Skinner M P, Raines E W, Ross R, Cheresh D A, Schwartz S M, Giachelli C M. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J Clin Investig. 1995;95:713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacMicking J D, North R J, La Course R, Mudgett J S, Shah S K, Nathan C F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy J B, Vachhani B V, Wahl S M, Finbloom D S, Feldman G M. Human monocyte binding to fibronectin enhances IFN-gamma-induced early signaling events. J Immunol. 1997;159:2424–2430. [PubMed] [Google Scholar]
- 32.Murray P J, Wang L, Onufryk C, Tepper R I, Young R A. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol. 1997;158:315–321. [PubMed] [Google Scholar]
- 33.Murry C E, Giachelli C M, Schwartz S M, Vracko R. Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol. 1994;145:1450–1462. [PMC free article] [PubMed] [Google Scholar]
- 34.Nasu K, Ishida T, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Expression of wild-type and mutated rabbit osteopontin in Escherichia coli, and their effects on adhesion and migration of P388D1 cells. Biochem J. 1995;307:257–265. doi: 10.1042/bj3070257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nau G J, Guilfoile P, Chupp G L, Berman J S, Kim S J, Kornfeld H, Young R A. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc Natl Acad Sci USA. 1997;94:6414–6419. doi: 10.1073/pnas.94.12.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pablos-Mendez A, Laszlo A, Bustreo F, Binkin N, Cohn D L, Lambregts-van Weezenbeek C S B, Kim S J, Chaulet P, Nunn P, Raviglione M C. Anti-tuberculosis drug resistance in the world. WHO/TB/97.229. Geneva, Switzerland: World Health Organization; 1997. [DOI] [PubMed] [Google Scholar]
- 37.Patarca R, Freeman G J, Singh R P, Wei F Y, Durfee T, Blattner F, Regnier D C, Kozak C A, Mock B A, Morse H D, et al. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J Exp Med. 1989;170:145–161. doi: 10.1084/jem.170.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patarca R, Saavedra R A, Cantor H. Molecular and cellular basis of genetic resistance to bacterial infection: the role of the early T-lymphocyte activation-1/osteopontin gene. Crit Rev Immunol. 1993;13:225–246. [PubMed] [Google Scholar]
- 39.Rollo E E, Laskin D L, Denhardt D T. Osteopontin inhibits nitric oxide production and cytotoxicity by activated RAW264.7 macrophages. J Leukoc Biol. 1996;60:397–404. doi: 10.1002/jlb.60.3.397. [DOI] [PubMed] [Google Scholar]
- 40.Rook G A, Steele J, Fraher L, Barker S, Karmali R, O’Riordan J, Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–163. [PMC free article] [PubMed] [Google Scholar]
- 41.Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, Wakeham A, Shahinian A, Catzavelos C, Rak J, Furlonger C, Zakarian A, Simard J J, Ohashi P S, Paige C J, Gutierrez-Ramos J C, Mak T W. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–2233. [PubMed] [Google Scholar]
- 42.Singh R P, Patarca R, Schwartz J, Singh P, Cantor H. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J Exp Med. 1990;171:1931–1942. doi: 10.1084/jem.171.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith R E, Hogaboam C M, Strieter R M, Lukacs N W, Kunkel S L. Cell-to-cell and cell-to-matrix interactions mediate chemokine expression: an important component of the inflammatory lesion. J Leukoc Biol. 1997;62:612–619. doi: 10.1002/jlb.62.5.612. [DOI] [PubMed] [Google Scholar]
- 44.Stenger S, Modlin R L. Cytotoxic T cell responses to intracellular pathogens. Curr Opin Immunol. 1998;10:471–477. doi: 10.1016/s0952-7915(98)80123-4. [DOI] [PubMed] [Google Scholar]
- 45.Uede T, Katagiri Y, Iizuka J, Murakami M. Osteopontin, a coordinator of host defense system: a cytokine or an extracellular adhesive protein? Microbiol Immunol. 1997;41:641–648. doi: 10.1111/j.1348-0421.1997.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 46.Valway S E, Sanchez M P C, Shinnick T F, Orme I, Agerton T, Hoy D, Jones J S, Westmoreland H, Onorato I M. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N Engl J Med. 1998;338:633–639. doi: 10.1056/NEJM199803053381001. [DOI] [PubMed] [Google Scholar]
- 47.Weber G F, Ashkar S, Glimcher M J, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 48.West M J. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 49.Xie Q W, Cho H J, Calaycay J, Mumford R A, Swiderek K M, Lee T D, Ding A, Troso T, Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 50.Yu X Q, Nikolic-Paterson D J, Mu W, Giachelli C M, Atkins R C, Johnson R J, Lan H Y. A functional role for osteopontin in experimental crescentic glomerulonephritis in the rat. Proc Assoc Am Physicians. 1998;110:50–64. [PubMed] [Google Scholar]
- 51.Yue T L, McKenna P J, Ohlstein E H, Farach-Carson M C, Butler W T, Johanson K, McDevitt P, Feuerstein G Z, Stadel J M. Osteopontin-stimulated vascular smooth muscle cell migration is mediated by beta 3 integrin. Exp Cell Res. 1994;214:459–464. doi: 10.1006/excr.1994.1282. [DOI] [PubMed] [Google Scholar]
- 52.Zhu H, Cong J P, Mamtora G, Gingeras T, Shenk T. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:14470–14475. doi: 10.1073/pnas.95.24.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]