Abstract
S100A9/S100A8 (calprotectin), a member of the S100 protein family, has been shown to play a pivotal role in innate immunity activation. Calprotectin plays a critical role in the pathogenesis of rheumatoid arthritis (RA), as it triggers chemotaxis, phagocyte migration and modulation of neutrophils and macrophages. Higher calprotectin levels have been found in synovial fluid, plasma, and serum from RA patients. Recent studies have demonstrated better correlations between serum or plasma calprotectin and composite inflammatory disease activity indexes than c-reactive protein (CRP) or the erythrocyte sedimentation rate (ESR). Calprotectin serum levels decreased after treatment, independently of the DMARD type or strategy. Calprotectin has shown the strongest correlations with other sensitive techniques to detect inflammation, such as ultrasound. Calprotectin independently predicts radiographic progression. However, its value as a biomarker of treatment response and flare after tapering is unclear. This update reviews the current understanding of calprotectin in RA and discusses possible applications as a biomarker in clinical practice.
Keywords: calprotectin, rheumatoid arthritis, biomarker, acute phase reactants, CRP - C-reactive protein
1 Introduction
Rheumatoid arthritis (RA) is a heterogeneous disease of unknown origin, characterized by chronic polyarthritis that may lead to joint destruction, disability, and increased mortality. Extraarticular manifestations are not uncommon. RA affects 0.5-1% of the adult population, predominantly females. Genetic and environmental factors have been implicated in the susceptibility to RA. Autoimmunity, with the presence of characteristic autoantibodies such as rheumatoid factor or anticitrullinated peptide autoantibodies, are implicated in the pathogenesis of RA, although recent data confirms the role of the innate immune system in this disease (1).
The innate immune system plays a central role in initiating local inflammation, contributing to the pathogenesis of RA by promoting the production of inflammatory cytokines and chemokines. Pattern recognition receptors (PRRs) are a family of receptors of the innate immune system that bind to damage-associated molecular pattern molecules (DAMP) (2). The most important PRRs are Toll-like receptors (TLRs), which allow the activation of monocytes, neutrophils, dendritic cells, natural killer (NK) cells and B cells (3).
S100A9/S100A8 (calprotectin) a member of the S100 protein family has been studied as an important proinflammatory factor of innate immunity as an endogenous DAMP via TLR4 activation. Calprotectin plays a critical role in the development of inflammation loops in RA as a trigger for chemotaxis, phagocyte migration and modulation of various macrophage functions (4–6).
This update reviews the current understanding of calprotectin in RA and discusses possible applications as a biomarker in clinical practice.
2 Calprotectin and the S100 protein family
The first members of the S100 protein family were purified from bovine brain in the early 1980s. The protein complex was denominated “S100” because of its 100% solubility in ammonium sulphate solution (7). The S100 protein family is specifically linked to innate immune functions by their expression in cells of myeloid origin.
The S100 protein family is widely expressed, although they are not ubiquitous, and several have highly restricted distributions. The functions of these proteins vary widely between individual members, functioning as both intracellular and extracellular signaling molecules. S100 protein functions include cytoskeletal function, homeostasis, tumor-suppression, antimicrobial response, chemotactic activity, atherogenesis and protection from oxidative cell damage in brain tissue. The main tissue cell locations and functions are summarized in Table 1 .
Table 1.
S100 protein family. Main tissue cell localization and function of S100 protein family.
| S100 protein | Tissue and subcellular location | Function |
|---|---|---|
| S100A1 | It is expressed in skeletal muscle fibers, cardiomyocytes and some neuronal populations (8) | Accelerates deterioration of cardiac performance and transition to heart failure (9) |
| S100A2 | It is expressed in the lung, kidney, prostate, skin and salivary and mammary glands (10) | Tumor-suppressing function (11) |
| S100A3 | Highly expressed in hair root cells and some astrocytomas (12). | Tumor-suppressing function and epithelial cell differentiation (11) |
| S100A4 | It is overexpressed in breast cancer, gastric cancer and non-small cell lung cancer (NSCLC) (13) | Apoptosis, cell motility, and tumorigenesis (14) |
| S100A5 | It is upregulated in bladder cancers and recurrent grade I meningiomas (15) | Not described |
| S100A6 | It is overexpressed in lung, bile duct, and brain cancer, and non-small cell lung adenocarcinoma (16–19) | Cell proliferation, cytoskeletal dynamics and tumorigenesis (20, 21) |
| S100A7 | It is overexpressed in inflammatory skin diseases. lymphocytes, monocytes and granulocytes (22) | Antimicrobial response, chemotactic activity, prevent generation of amyloidogenic peptides in Alzheimer’s disease. psoriasis, and atopic dermatitis (23–25). |
| S100A8 | It is found in macrophages, dendritic cells, microvascular endothelial cells but not endothelial cells from larger vessels, epithelial cells (e.g., keratinocytes) and fibroblasts (26) | Regulation of inflammation. Effect on leukocyte adhesion and neutrophil adhesion (e.g., asthma) (5). |
| S100A9 | It is found in myeloid cells: (macrophages and neutrophils) some cancer cells (e.g., breast cancer, esophageal squamous cell carcinoma) (27). | Leukocyte migration, adhesion and transmigration from blood vessels, thus it has anti-inflammatory properties. In some cancer cells it promotes growth suppression (5). |
| S100A10 | It is found in cell types throughout the body though it is located predominantly in the lungs and kidneys (28). | It is involved in the trafficking of proteins to the plasma membrane (angiogenesis and endothelial cell function) (28) and can be expressed on the cell surface as a receptor with clinical implications in some malignancies (e.g., tumor migration, hemorrhagic phenotype of promyelocytic leukemia) (29). |
| S100A11 | It is induced/released by chondrocytes (30) | Stimulates cell growth by enhancing the level of epidermal growth factor (EGF) family proteins, promoting hypertrophic chondrocyte differentiation (31). |
| S100A12 | It is constitutively expressed in neutrophils and inducible in macrophages and smooth muscle cells. it is expressed in human aortic aneurysms (32). | Expression in epithelial cells is associated with growth arrest. Potentiation of atherogenesis (33). |
| S100A13 | It is found in multiple cell types, including fibroblasts, osteoblasts and melanoma cells (20) | It may play a pivotal role in angiogenesis (34). |
| S100A14 | It can be found in an esophageal squamous cell carcinoma cells (35). | It may function as a cancer suppressor, playing a dual role in tumor cells (enhancing or decreasing tumor cell invasiveness) (36). |
| S100A15 | It is expressed in keratinocytes in inflamed skin (37). | Antimicrobial activity against E. coli (38). |
| S100A16 | It is expressed in theca cells, urothelial cells, pancreatic endocrine cells, prostatic glandular cells, squamous epithelial cells, basal prostatic cells, and suprabasal keratinocytes. It is upregulated in several tumors (39). | Recently, a role in insulin sensitivity regulation has been described (40). |
| S100B | It is expressed in astrocytes, certain neuronal populations, Schwann cells, melanocytes, chondrocytes, adipocytes, skeletal myofibers and associated satellite cells, certain dendritic cell and lymphocyte populations and other cell types (41). | It induces neurogenesis and reduces delayed neuronal injury and might contribute significantly to neuroinflammation (42). |
| S100P | It can be expressed in urothelial cells, pancreas, syncytiotrophoblasts, gastric mucus-secreting cells (43) | It promotes transendothelial migration of tumor cells and, potentially, metastasis (44). |
| S100Z | The highest levels were found in the spleen and leukocytes, and in some tumor tissues (e.g., prostate) (45). | Tumor growth. Recently, it has been associated with pulmonary systemic sclerosis (45). |
Among the S100 protein family, calprotectin (S100A8/S100A9) is primarily expressed in innate immune cells, particularly in neutrophils, monocytes, and macrophages, which constitute approximately 40% of the cytosolic proteins in these cells. However, under specific stimuli, calprotectin may be expressed in other cell lines, such as osteoclasts and keratinocytes (46–48). The gene encoding calprotectin subunits is located in the gene cluster on human chromosome 1q12-1q21 (49, 50).
Although calprotectin has been recognized for almost 40 years, it has only recently become of interest as a biomarker of disease activity and damage in rheumatology and was previously known as L1 protein (51), cystic fibrosis-associated antigen (CFA) (52), calgranulins A and B (53), S-1OOa and b (54) or myeloid related protein 8 and 14 (MRP 8/14) (48). Calprotectin is a well-established biomarker in other medical areas, such as calprotectin in faeces in inflammatory bowel disease (55–59).
2.1 Calprotectin molecular structure
Calprotectin is a calcium- and zinc-binding heterodimeric molecule of 36.5 kDa, with two heavy and one light chain non-covalently linked (51). It has two subunits, S100A8 and S100A9, which are 8.3 kDa and 13.3 kDa, respectively (60). S100A8 is the active subunit and S100A9 acts as the regulatory subunit, preventing early degradation of S100A8 (61). The EF-hand is composed of two α-helices flanking a central calcium-binding loop, resulting in classical helix-loop-helix domains (62). Calprotectin is mostly found in the form of heterodimers (S100A9-S100A8) and tetramers (S100A9-S100A8)2 in a calcium dependent-manner (63).
Two independent calprotectin activation pathways have been proposed to explain calprotectin release to extracellular compartments. The first, a canonical pathway, includes protein kinase C, which is induced by different inflammatory stimuli (e.g., bacteria). The second, a non-classical secretion avoiding the Golgi-associated pathway, requires the elevation of intracellular calcium levels, induced by contact between phagocytes and pre-activated endothelial cells by tumor necrosis factor (TNF), resulting in active secretion of calprotectin by phagocytes (63). Recently, a new activation mechanism has been proposed, namely activation via chromatin in neutrophil extracellular traps (NETs) (64). In addition, calprotectin may be secreted passively from apoptotic cells (61).
2.2 Calprotectin functions
2.2.1 Intracellular functions
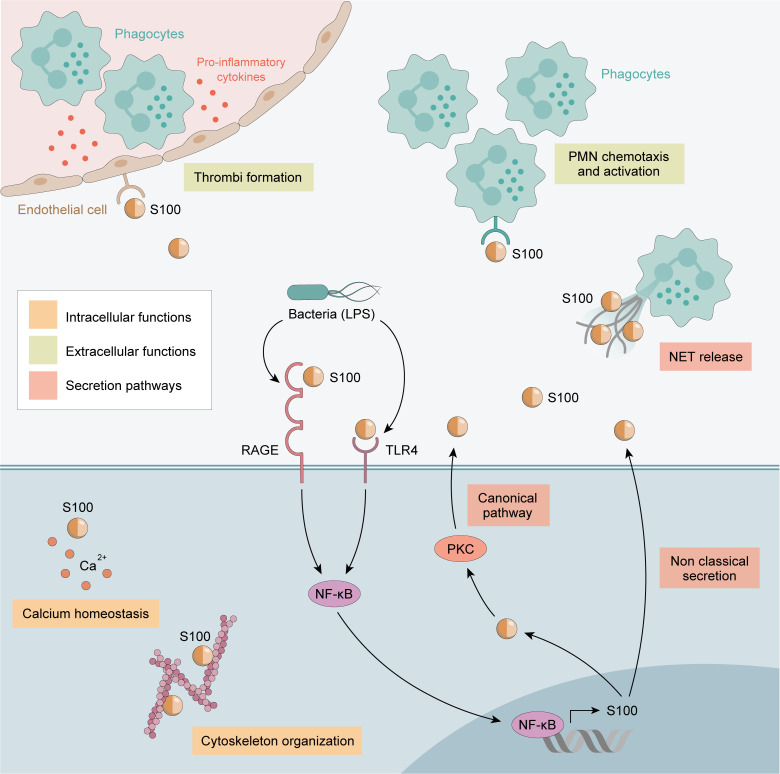
Calprotectin complexes are known to interact with cytoskeletal components such as actin filaments, keratin, vimentin, and microtubules in a calcium-dependent manner. High calcium concentrations induce a rearrangement of calprotectin into tetramers, allowing translocation to the cell membrane and tubulin polymerization (61). This process is regulated by the phosphorylation of the threonine at position 113 in S100A9 by p38 MAPK ( Figure 1 ).
Figure 1.
Calprotectin Functions. Intracellular functions are shown in orange and extracellular functions in green. Calprotectin Intracellular functions includes cytoskeleton cell migration and calcium homeostasis. Extracellular functions involve endothelial cells activation, promoting the adhesion of phagocytes to the vascular endothelium and thrombi formation. Also increase chemotaxis and the activation of PMN. Finally, calprotectin exerts a strong antimicrobial action against a variety of bacterial and fungal pathogens.
2.2.2 Extracellular functions
Extracellular calprotectin complexes interact with endothelial cells by binding to heparan sulfate and, specifically, carboxylated glycans, and up-regulate integrin receptors on leukocytes, resulting in the activation of endothelial cells. Activated endothelial cells express a pro-inflammatory cytokine profile (e.g., IL-1, IL-8 MCP-1), and thus calprotectin plays a central role in promoting the adhesion of phagocytes to the vascular endothelium and thrombi formation (65, 66). Calprotectin generates a positive feedback loop, increasing chemotaxis and the activation of PMN, which are the main source of calprotectin: therefore, calprotectin has an autocrine and paracrine function (67). Notably, calprotectin interaction with non-activated endothelium inhibits its secretion, meaning calprotectin is only released at sites of inflammation by activated phagocytes ( Figure 1 ).
Calprotectin exerts a strong antimicrobial action against a variety of bacterial and fungal pathogens. Calprotectin recognizes proteins related to bacteria, such as lipopolysaccharides (LPS), up-regulating the production of pro-inflammatory profile cytokines, such as TNF-α, IL-1β, and IL-12 locally (68, 69). It is recognized as an endogenous DAMP and binds to the TLR4 receptor and RAGE, amplifying the innate immune response and inducing PMN recruitment to inflamed tissues (3). The antibacterial activity of calprotectin results from the sequestering of transition metals by chelation of nutrient Mn2+ and Zn2+ (70). Engulfment of bacteria by macrophages leads to decreased Zn2+ uptake and increased Zn2+ efflux from the cytoplasm and the efflux of Mn2+ and Fe2+ from the phagosome by NRAMP family transporters (71). Chelation is mediated through two high-affinity binding sites, both of which can bind Zn2+ with nanomolar affinity, while only one binds Mn2+ with this affinity (71).
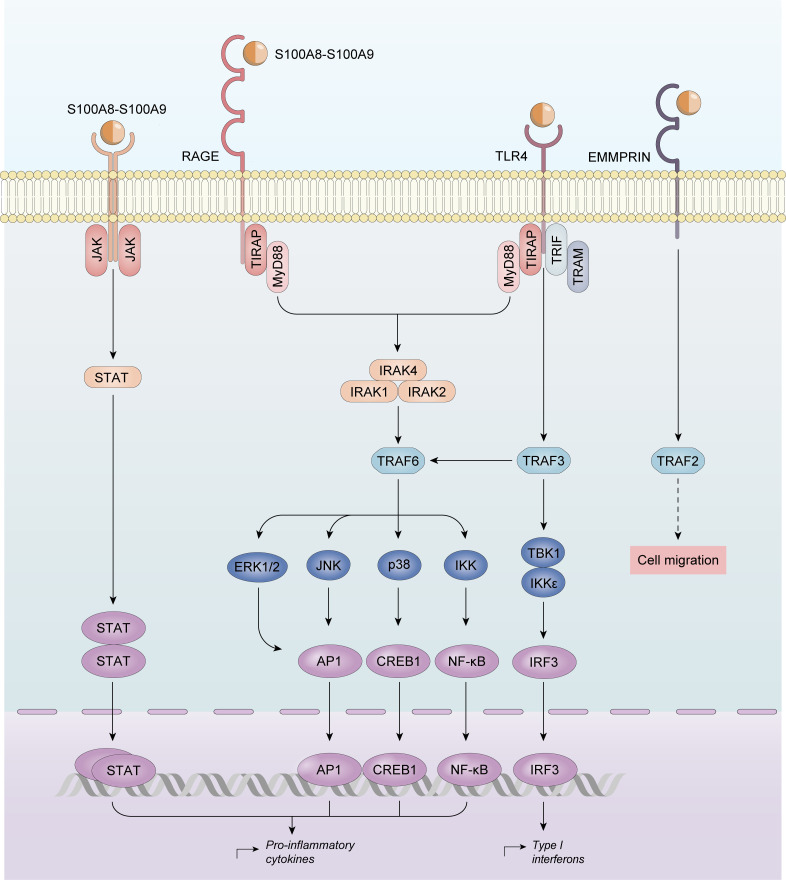
Calprotectin also activates the MyD88-dependent and TIR domain-containing adaptor protein inducing IFNβ (TRIF; also known as TICAM1)-dependent signaling pathways downstream of TLR4, resulting in NF-κB-mediated and interferon regulatory factor (IRF)-mediated gene transcription. EMMPRIN, a transmembrane glycoprotein of the immunoglobulin superfamily is also able to bind S100A9. However, its biological function has not yet been described (72).
2.3 Calprotectin in health and disease
Studies have shown that calprotectin levels are minimal in serum and stool samples from healthy population compared with patients with inflammatory conditions (73–76). High calprotectin serum levels were also observed in patients with infectious disease and sepsis (77). Recently, the accuracy of calprotectin as a biomarker of bacterial respiratory disease was found to be even higher than procalcitonin (78).
Increased calprotectin expression has been found in patients with rheumatic diseases (See Table 2 ). Higher calprotectin levels have been found in plasma, serum and faecal samples from patients with RA (100), spondyloarthropathies (SpA) (101), inflammatory bowel disease (IBD) (102) and type 2 diabetes (T2D) (103). In IBD, faecal calprotectin has been shown to be a more sensitive indicator of inflammatory activity and is being used for both the diagnosis and follow-up in routine clinical practice (104). Calprotectin levels are not affected by age or gender (105).
Table 2.
Calprotectin in Rheumatic Diseases. This table summarizes the main findings of calprotectin levels and rheumatic diseases other than RA.
| Disease | Calprotectin source | Main finding |
|---|---|---|
| Reactive arthritis |
|
• CLP correlated with CRP and disease activity (79). • CLP was the first biomarker to return to baseline levels after disease improvement (79). |
| Ankylosing spondylitis | Serum | • CLP correlated with PGA, pain VAS, BASDAI, BASFI, and ASDAS (80, 81). • CLP is an independent marker for radiographic progression (82). |
| Psoriatic arthritis | Serum | • CLP levels are significantly higher in active PsA patients than in healthy controls (83). • Higher CLP levels were found in the polyarticular group than in patients with mono/oligoarticular disease (84). • CLP levels are sensitive to change, and its levels decrease after TNFi and IL17i treatment (83). |
| Systemic juvenile idiopathic arthritis | Serum | • CLP levels are sensitive to change (85). • CLP more accurately predicts disease relapse (86). |
| Adult onset Still disease | Serum | • CLP levels are significantly higher than RA, SLE patients or controls (87, 88). |
| Gout | Serum, synovial biopsy and tophi | • CLP levels were elevated in the synovium, tophi, and serum of patients with gout (89). • CLP levels correlated with disease activity (89). |
| Systemic lupus erythematosus | Urine | • CLP levels in SLE-LN patients (90). • CLP levels correlated with disease activity (91). |
| Primary Sjogren syndrome | Serum, saliva and salivary gland biopsy. | • Increased levels in pSS patients (92, 93). |
| Systemic sclerosis | Serum, broncho-alveolar lavage fluid, skin biopsy | • Increased levels in SSc patients (94, 95). • Increased levels in diffuse cutaneous SSc patients (96). |
| Behcet’s disease | Serum | • CLP levels are significantly higher than healthy controls (97). • CLP correlated with CRP and disease activity (97) |
| Idiopathic inflammatory myopathies | Serum and muscle biopsy | • Increased levels in IIM patients (98). • CLP promotes myoblast activation (99). |
CLP, calprotectin; PGA, patient global assessment; pain VAS, pain visual analogue scale; BASDAI, Bath AS disease activity index; BASFI, the Bath AS functional index; ASDAS, Ankylosing Spondylitis Disease Activity Score; TNFi, TNFn inhibitors; IL17i; interleukin 17 inhibitor; SLE-LN, SLE-lupus nephritis.
Recently, a new disorder, characterized by recurrent infections, hepatosplenomegaly, anemia, cutaneous vasculitis, and evidence of systemic inflammation has been described. These patients have shown hyperzincemia with hypercalprotectinaemia (106).
3 Calprotectin in rheumatoid arthritis
There is abundant evidence that the innate immune system is persistently activated in RA, as predominately macrophages are found in rheumatoid synovium (107).
In RA, calprotectin induces nitric oxide synthase (iNOS) by nuclear factor κB (NF-κB) activation (108). Calprotectin allows the phosphorylation of multiple protein kinase-mediated signal transduction pathways, including c-Jun-N-amino-terminal kinase (JNK), extracellular-regulated kinase 1/2 (ERK1/2), and Janus kinase/signal transducers and activators of transcription (JAK/STAT) (109). However, calprotectin activation converges in multiple pathways whose activation enhances the production of proinflammatory cytokines, namely tumor necrosis factor-α (TNF-α), IL-6, IL-8, IL-12/23, and IL-18 (110), which are known to be physiopathologically- and clinically-relevant in RA ( Figure 2 ).
Figure 2.
Calprotectin activation in Rheumatoid Arthritis. Calprotectin activates nuclear factor κB (NF-κB), and protein kinase-mediated signal transduction pathways, including c-Jun-N-amino-terminal kinase (JNK), extracellular-regulated kinase 1/2 (ERK1/2), and Janus kinase/signal transducers and activators of transcription (JAK/STAT) upregulating proinflammatory cytokines production.
Calprotectin expression is noted predominantly in the macrophages of the synovial lining layer in tissues adjacent to the cartilage-pannus junction (CPJ), suggesting altered activation and differentiation of lining layer macrophages at the CPJ, which is the site of maximum cartilage destruction in RA (111). Calprotectin is synthesized in fibroblast-like synoviocytes (FLSs), which are crucial players in the pathogenesis of synovitis in RA. IL-22 enhanced FLS proliferation and up-regulated MMP1 and S100A8/A9 production (112). Interestingly, when stimulated FLSs were treated with a JAK 2 and JAK3 inhibitor, there was a significant decrease in IL-22-induced S100A8/A9 production.
Calprotectin is released from activated leukocytes leading to increased concentrations in RA plasma and serum (113, 114). a study focused on determining the association of calprotectin in EDTA-plasma or in serum with disease activity, found stronger plasma associations with all measures of disease activity (115). However, ELISA commercial kits are designed to determine both, and most evidence available is drive by from serum determinations.
3.1 Calprotectin as a biomarker of rheumatoid arthritis
Higher calprotectin levels have been found in synovial fluid (SF), plasma and serum from RA patients (113, 114). A recent study demonstrated that the most enhanced proteins in RA SF were the S100A8, S100A9 and S00A12 proteins, using proteomic fingerprints of RA patients’ serum (116). A German study found that calprotectin and other S100 proteins were the most up-regulated proteins in SF in RA patients. Their expression was about 10-fold higher than that observed in the SF of osteoarthritis patients (OA). Not only was calprotectin the best RA biomarker identified in this study, but higher calprotectin levels were found in the SF of RA compared with SpA patients: therefore, calprotectin may differentiate RA from other inflammatory arthritis (117).
Interestingly, calprotectin levels correlated with rheumatoid factor. Furthermore, higher calprotectin plasma levels were found in seropositive than in seronegative patients. The correlation with ACPA titres remains unclear (118–126).
Calprotectin also significantly contributes to comorbid conditions in RA patients, such as cardiovascular disease. High calprotectin levels have been associated with precocious atheroma formation and the accelerated development of atherosclerosis (127).
In summary, serum calprotectin levels may provide information on macrophage activation, supporting their potential role as a biomarker of disease activity, radiographic progression and therapeutic response.
3.2 Calprotectin as a marker of disease activity
In RA, changes in the amount of synovial sublining macrophages correlate with disease activity (128): these macrophages are the major source of calprotectin, and thus their levels may provide reliable information on their activation 74). Recent studies have demonstrated better correlations between calprotectin and DAS28, swollen joint count (SJC), CDAI, SDAI and physical global VAS than C-reactive protein (CRP) or the erythrocyte sedimentation rate (ESR) (121–123, 129). This was especially pronounced in patients receiving IL-6 inhibitors, with a pronounced decrease in CRP serum levels independently of disease activity (129). These correlations were independent of the disease stage. Studies in both recent-onset patients (105, 130) and those with established disease (123) have demonstrated a significant correlation between calprotectin and SJC, DAS28, SDAI and CDAI (73, 75, 76, 131, 132). In a recent metanalysis of 16 studies, the relationship between calprotectin and disease activity was confirmed (74).
In clinical remission, CRP and ESR may be normal, although there may be residual inflammatory activity, especially in patients receiving biological therapy. Calprotectin levels, but not CRP or ESR levels, were significantly lower in patients with no swollen joints than in those with ≥ 1 swollen joint, supporting the hypothesis that calprotectin levels reflect local ongoing inflammation rather than a systemic inflammatory response (118, 120, 126). Studies by our research group have demonstrated that calprotectin more accurately stratifies disease activity in RA patients in remission or low disease activity receiving TNF inhibitors (133) or the biologic agent, tocilizumab (129). This effect might lead overestimates of the response rate when disease activity indices including ESR or CRP are used (134). In these patients, calprotectin, but not CRP or ESR, can distinguish between patients without swelling from those with ≥1 swollen joint. Calprotectin serum levels, but not CRP, are independent of trough serum tocilizumab levels (129). Recently, these results have been replicated in an independent large cohort (135).
RA patients with normal CRP pose a therapeutic challenge in daily clinical practice. Therefore, calprotectin may have a potential role in assessing disease activity in patients with remission or low disease activity, identifying patients with subclinical synovitis more accurately.
3.2.1 Calprotectin serum levels are sensitive to change
Calprotectin serum levels decreased after treatment, independently of the DMARD type or strategy (105). A significant reduction in serum calprotectin levels was observed after three months of csDMARD treatment (105, 123, 136). The same occurs during biological therapy. Infliximab significantly decreases serum calprotectin levels in RA patients, as confirmed by immunohistochemical staining for S100A8 on serial synovium sections, which showed a progressive decrease in the number of infiltrating S100 A9-positive macrophages. Similar results were observed in RA patients receiving adalimumab and etanercept (105, 137–139).
3.2.2 Calprotectin and ultrasound synovitis
Musculoskeletal ultrasound (US) is a non-invasive diagnostic technique widely used in rheumatology to assess joint inflammation with greater sensitivity (140). A pilot study explored the associations between calprotectin and comprehensive US examination in 20 RA patients starting treatment with adalimumab and found a significant association with B-mode and the power Doppler score, and there was a correlation between calprotectin and the number of swollen joints (141) and the results were recently replicated (132, 142). In a one-year prospective cohort study of patients with established RA patients, calprotectin showed the overall strongest correlations with US scores and SJC, even after adjustment for several variables (143). We found that RA and PsA patients in remission or low disease activity with a US power Doppler signal had significantly-higher calprotectin levels than those without, and calprotectin correlated better with US power Doppler, synovial hypertrophy and US global scores than ESR or CRP (144). Taken together, serum calprotectin and power Doppler are both identifying local active synovitis in patients with inflammatory arthritis, even those with low levels of disease activity.
3.2.3 Calprotectin and radiographic progression.
Murine arthritis models have shown that overexpression of IL-17 and TNFα strongly enhanced up-regulation of calprotectin, resulting in bone erosion. In contrast, calprotectin deficiency in mice protected against the IL-17/TNFα effect in cartilage (145).
In RA, calprotectin antigens were located in the synovial cartilage, suggesting a pivotal role in cartilage destruction and subchondral bone erosions, a typical hallmark in active RA patients (111). In this regard, a study of 145 RA patients showed that baseline calprotectin levels were independently associated with the van der Heijde modified Sharp score (SvH) and the Rheumatoid Arthritis Articular Damage Score (RAAD score), even when adjusted for CRP, ESR, rheumatoid factor, DAS28, sex and age (123). The prospective follow up of this cohort found that calprotectin was an independent predictor of radiographic joint damage after 10 years. Patients with normal baseline calprotectin levels had less joint damage; again, calprotectin was independently associated with progression in the SvH and RAAD scores (125). Similarly, a longitudinal study with a median of 8-years of follow-up, demonstrated that calprotectin predicts erosive disease and joint space narrowing (73). An exploratory analysis has correlated calprotectin significantly with joint bone marrow edema on MRI in RA patients in clinical remission (146). Recently, the same results have been replicated in a large early-RA cohort from the ARTIC trial, where high levels of calprotectin were associated with radiographic progression in multivariate models (130).
3.3 Calprotectin and treatment response
Prediction of the individual response to treatment has become a major clinical challenge in RA. Recent studies and post-hoc clinical trials have provided evidence of calprotectin’s accuracy in predicting the response to csDMARD and bDMARD therapy in RA. In patients receiving csDMARDs, the results are unclear, and decreases in serum calprotectin levels, rather than CRP, were associated with improvements in the SJC over time (105). Patients who achieved remission had a significant reduction in serum calprotectin levels. Likewise, a study shown that baseline serum calprotectin levels decreased rapidly in responders after csDMARD treatment but remained stable in non-responders (122). In early RA, a post-hoc analysis of the prospective ESPOIR cohort found that calprotectin was not an independent predictive factor of the response to MTX. Although the study excluded a large proportion of cohort participants, its results suggested a potential interest in calprotectin as a part of a multivariable score for personalized medicine in these patients (147). Similar results have been seen in RA patients receiving biological therapy: a prospective cohort study evaluated 170 RA patients receiving biological therapy (adalimumab, infliximab, and rituximab). Calprotectin levels were measured at 0, 4 and 16 weeks after biologic drug initiation. As previously described, responders had higher baseline calprotectin levels than non-responders. Higher baseline calprotectin levels increased the odds of being classified as a responder by up to 55-fold, and levels decreased after treatment. In contrast, non-responders had stable calprotectin levels during the study (148). The authors developed a treatment algorithm based on a prediction score using calprotectin and concluded that it may have potential in personalized treatment in RA (149).
Calprotectin levels at baseline were associated with biological treatment survival (150). A prospective cohort study did not find that baseline calprotectin levels were predictive of the response after 6 months of treatment, although a significant decrease in serum levels was observed in responders (122). A recent study demonstrated a significant decrease in calprotectin in the first month of biological therapy, which was predictive of the EULAR response at 3,6 and 12 months (143).
A recent systematic review summarized the results from 17 studies including 1065 patients and found that calprotectin levels decreased after treatment, although there was a wide range of levels and marked interstudy and intrastudy variability. Baseline calprotectin levels were a significant and independent predictor of erosive progression and therapeutic responses, particularly in patients receiving biological treatment (151).
Taken together, this data supports the idea that calprotectin could potentially help to monitor disease activity and predict the response to bDMARDs in RA, although there is no data available on patients receiving DMARDs. Randomized trials are needed to define the role of calprotectin as a predictor of treatment response, but there is a potential role for calprotectin in the follow-up of RA patients.
3.4 Calprotectin and disease relapse
A clinically relevant unmet need is stratification of the risk of relapse in RA patients in remission or low disease activity. Data demonstrate that calprotectin levels are increased during relapse (152). A prospective one-year follow up cohort study found that calprotectin levels strongly and independently predicted disease relapse in RA with low levels of disease activity during TNFi treatment. In contrast, a prospective cohort study analyzing the role of calprotectin in predicting flares in RA with low disease activity by DAS28 (DAS28<3.2) found that only HAQ-DI remained a significant independent predictor of flares in the multivariate analyses. At the time of the flare, DAS28 and its components significantly correlated with calprotectin, but the correlation was low, suggesting a non-inflammatory component in most events (153).
Another situation is the risk of flare in RA patients before tsDMARD tapering. The capacity of calprotectin in predicting flares in patients undergoing tapering of biologics has been assessed. Calprotectin levels were determined in serum samples from participants in two prospective studies (DRESS and BIO-TOP). Although calprotectin has some predictive value for the clinical response after starting anti-TNF treatment, it has no added value for other clinical factors (154). In contrast, analyses from two tapering studies (IMPROVED study and the RETRO study) showed that calprotectin levels in remission on DMARDs are higher in patients who will flare upon DMARD tapering/cessation (155).
The definitive role of calprotectin as a predictor of disease relapse remains unclear, as there are no specific randomized clinical trials to assess its potential use.
3.5 Calprotectin inhibition as therapeutic approach in RA
Based on the fundamental role of calprotectin in the modulation of acute and chronic inflammation, its inhibition could be a novel target in the treatment of RA. An experimental study investigated the effects of calprotectin inhibition in RA using neutralizing monoclonal antibodies in a mouse collagen-induced arthritis (CIA) model, Murine S100A9 monoclonal antibody and anti-TNFα treatment were compared. Mice treated with anti-S100A9 showed markedly decreased arthritis severity scores compared to the isotype control group. Overall, anti-S100A9 treatment led to an approximately 50% reduction in disease activity, and preserved bone/collagen integrity. No significant differences in disease activity were observed between anti-S100A9 and anti-TNFα-treated animals, suggesting calprotectin might be a novel therapeutic target in RA (156, 157).
4 Discussion and conclusions
There is still an unmet need for robust biomarkers to objectively monitor inflammatory activity and response to therapy in RA and other immune mediated diseases. Based on the pivotal role of calprotectin in the pathophysiology of acute and chronic inflammation, calprotectin blood levels could be a potential biomarker of disease activity in inflammatory arthritis. In RA, there is growing evidence to support the idea that calprotectin more accurately stratifies disease activity than CRP and ESR. Furthermore, recent data has shown that calprotectin serum levels are a potential tool for monitoring disease activity and the therapeutic response in patients receiving biological therapy. However, larger studies and assay standardization are needed in RA patients to ascertain the role of serum calprotectin as a useful biomarker for monitoring disease activity or response to therapy in clinical practice, as occurs with fecal calprotectin in inflammatory bowel disease. Future applications may include potential therapeutic targets, prediction of the response to treatment, or dose-titration of biologics in a personalized medicine approach.
In conclusion, calprotectin plays a pivotal role in innate immune system activation, increasing chemotaxis and the activation of PMN, promoting the production of inflammatory cytokines and chemokines, and contributing to RA pathogenesis. There is growing evidence to support its higher accuracy in stratifying disease activity than CRP and ESR. Calprotectin has shown the strongest correlations with other sensitive techniques to detect inflammation, such as ultrasound. However, its value as biomarker of treatment response and flare after tapering still need larger, standardized studies.
Author contributions
JI-M: investigation, resources, writing (original draft preparation, review and editing). BF-S: investigation, resources, writing (original draft preparation, review and editing). RS: investigation, resources, writing (original draft preparation, review and editing), supervision, and project administration. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors acknowledge David Buss for technical advice, and Antonio García, PhD for Scientific Illustration.
Conflict of interest
JI-M has received honoraria from AbbVie employee.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun (2020) 110:102400. doi: 10.1016/j.jaut.2019.102400 [DOI] [PubMed] [Google Scholar]
- 2. Janeway CA. Approaching the asymptote? evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol (1989) 54 Pt 1:1–13. doi: 10.1101/SQB.1989.054.01.003 [DOI] [PubMed] [Google Scholar]
- 3. Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol (2004) 5(10):987–95. doi: 10.1038/ni1112 [DOI] [PubMed] [Google Scholar]
- 4. Ryckman C, McColl SR, Vandal K, de Médicis R, Lussier A, Poubelle PE, et al. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheumatol (2003) 48(8):2310–20. doi: 10.1002/art.11079 [DOI] [PubMed] [Google Scholar]
- 5. Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol (2003) 170(6):3233–42. doi: 10.4049/jimmunol.170.6.3233 [DOI] [PubMed] [Google Scholar]
- 6. Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the beta 2 integrin mac-1 on neutrophils. J Immunol (1998) 160(3):1427–35. [PubMed] [Google Scholar]
- 7. Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun (1965) 19(6):739–44. doi: 10.1016/0006-291X(65)90320-7 [DOI] [PubMed] [Google Scholar]
- 8. Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol (2001) 33(7):637–68. doi: 10.1016/S1357-2725(01)00046-2 [DOI] [PubMed] [Google Scholar]
- 9. Rohde D, Ritterhoff J, Voelkers M, Katus HA, Parker TG, Most P. S100A1: a multifaceted therapeutic target in cardiovascular disease. J Cardiovasc Transl Res (2010) 3(5):525–37. doi: 10.1007/s12265-010-9211-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ilg EC, Sciiafer BW. Expression pattern of sloo calcium-binding proteins in human tumors. (1996) 68:325–32. doi: [DOI] [PubMed] [Google Scholar]
- 11. van Dieck J, Teufel DP, Jaulent AM, Fernandez-Fernandez MR, Rutherford TJ, Wyslouch-Cieszynska A, et al. Posttranslational modifications affect the interaction of S100 proteins with tumor suppressor p53. J Mol Biol (2009) 394(5):922–30. doi: 10.1016/j.jmb.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 12. Kizawa K, Takahara H, Troxler H, Kleinert P, Mochida U, Heizmann CW. Specific citrullination causes assembly of a globular S100A3 homotetramer: a putative Ca2+ modulator matures human hair cuticle. J Biol Chem (2008) 283(8):5004–13. doi: 10.1074/jbc.M709357200 [DOI] [PubMed] [Google Scholar]
- 13. Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer (2015) 15(2):96–109. doi: 10.1038/nrc3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang DT, Chu WH, Sun HM, Ba HX, Li CY. Expression and functional analysis of tumor-related factor S100A4 in antler stem cells. J Histochem Cytochem (2017) 65(10):579–91. doi: 10.1369/0022155417727263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hancq S, Salmon I, Brotchi J, de Witte O, Gabius HJ, Heizmann CW, et al. S100A5: a marker of recurrence in WHO grade I meningiomas. Neuropathol Appl Neurobiol (2004) 30(2):178–87. doi: 10.1046/j.0305-1846.2003.00525.x [DOI] [PubMed] [Google Scholar]
- 16. Chen D, Luo L, Liang C. Aberrant S100A16 expression might be an independent prognostic indicator of unfavorable survival in non-small cell lung adenocarcinoma. PloS One (2018) 13(5):e0197402. doi: 10.1371/journal.pone.0197402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Filipek A, Leśniak W. S100A6 and its brain ligands in neurodegenerative disorders. Int J Mol Sci (2020) 21(11):3979. doi: 10.3390/ijms21113979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong XH, Dai D, Yang ZD, Yu XO, Li H, Kang H. S100 calcium binding protein A6 and associated long noncoding ribonucleic acids as biomarkers in the diagnosis and staging of primary biliary cholangitis. World J Gastroenterol (2021) 27(17):1973–92. doi: 10.3748/wjg.v27.i17.1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang T, Han S, Du G. S100A6 represses calu-6 lung cancer cells growth via inhibiting cell proliferation, migration, invasion and enhancing apoptosis. Cell Biochem Funct (2021) 39(6):771–9. doi: 10.1002/cbf.3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donato R R, Cannon B, Sorci G, Riuzzi F, Hsu K, Weber D J, et al. Functions of S100 proteins. Curr Mol Med (2013) 13(1):24. doi: 10.2174/156652413804486214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leśniak W, Słomnicki ŁP, Filipek A. S100A6 - new facts and features. Biochem Biophys Res Commun (2009) 390(4):1087–92. doi: 10.1016/j.bbrc.2009.10.150 [DOI] [PubMed] [Google Scholar]
- 22. Gläser R, Meyer-Hoffert U, Harder J, Cordes J, Wittersheim M, Kobliakova J, et al. The antimicrobial protein psorias in (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. J Invest Dermatol (2009) 129(3):641–9. doi: 10.1038/jid.2008.268 [DOI] [PubMed] [Google Scholar]
- 23. Qin W, Ho L, Wang J, Peskind E, Pasinetti GM. S100A7, a novel alzheimer’s disease biomarker with non-amyloidogenic alpha-secretase activity acts via selective promotion of ADAM-10. PloS One (2009) 4(1):e4183. doi: 10.1371/journal.pone.0004183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng Y, Niyonsaba F, Ushio H, Ikeda S, Nagaoka I, Okumura K, et al. Microbicidal protein psoriasin is a multifunctional modulator of neutrophil activation. Immunology (2008) 124(3):357–67. doi: 10.1111/j.1365-2567.2007.02782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolf R, Howard OMZ, Dong HF, Voscopoulos C, Boeshans K, Winston J, et al. Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15. J Immunol (2008) 181(2):1499–506. doi: 10.4049/jimmunol.181.2.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsu K, Champaiboon C, Guenther B, Sorenson B, Khammanivong A, Ross K, et al. Anti-infective protective properties of s100 calgranulins. Antiinflamm Antiallergy Agents Med Chem (2009) 8(4):290–305. doi: 10.2174/187152309789838975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li C, Zhang F, Lin M, Liu J. Induction of S100A9 gene expression by cytokine oncostatin m in breast cancer cells through the STAT3 signaling cascade. Breast Cancer Res (2004) 87(2):123–34. doi: 10.1023/B:BREA.0000041594.36418.f6 [DOI] [PubMed] [Google Scholar]
- 28. Rescher U, Gerke V. S100A10/p11: family, friends and functions. Pflugers Arch (2008) 455(4):575–82. doi: 10.1007/s00424-007-0313-4 [DOI] [PubMed] [Google Scholar]
- 29. O’Connell PA, Madureira PA, Berman JN, Liwski RS, Waisman DM. Regulation of S100A10 by the PML-RAR-α oncoprotein. Blood (2011) 117(15):4095–105. doi: 10.1182/blood-2010-07-298851 [DOI] [PubMed] [Google Scholar]
- 30. Cecil DL, Johnson K, Rediske J, Lotz M, Schmidt AM, Terkeltaub R. Inflammation-induced chondrocyte hypertrophy is driven by receptor for advanced glycation end products. J Immunol (2005) 175(12):8296–302. doi: 10.4049/jimmunol.175.12.8296 [DOI] [PubMed] [Google Scholar]
- 31. Sakaguchi M, Sonegawa H, Murata H, Kitazoe M, Futami JI, Kataoka K, et al. S100A11, an dual mediator for growth regulation of human keratinocytes. Mol Biol Cell (2008) 19(1):78–85. doi: 10.1091/mbc.e07-07-0682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goyette J, Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids (2011) 41(4):821–42. doi: 10.1007/s00726-010-0528-0 [DOI] [PubMed] [Google Scholar]
- 33. Bowman MAH, Gawdzik J, Bukhari U, Husain AN, Toth PT, Kim G, et al. S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein e-null mice by activating an osteogenic gene regulatory program. Arterioscler Thromb Vasc Biol (2011) 31(2):337–44. doi: 10.1161/ATVBAHA.110.217745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayrabedyan S, Kyurkchiev S, Kehayov I. FGF-1 and S100A13 possibly contribute to angiogenesis in endometriosis. J Reprod Immunol (2005) 67(1–2):87–101. doi: 10.1016/j.jri.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 35. Jin Q, Chen H, Luo A, Ding F, Liu Z. S100A14 stimulates cell proliferation and induces cell apoptosis at different concentrations via receptor for advanced glycation end products (RAGE). PloS One (2011) 6(4):e19375. doi: 10.1371/journal.pone.0019375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen H, Yuan Y, Zhang C, Luo A, Ding F, Ma J, et al. Involvement of S100A14 protein in cell invasion by affecting expression and function of matrix metalloproteinase (MMP)-2 via p53-dependent transcriptional regulation. J Biol Chem (2012) 287(21):17109–19. doi: 10.1074/jbc.M111.326975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wolf R, Lewerenz V, Büchau AS, Walz M, Ruzicka T. Human S100A15 splice variants are differentially expressed in inflammatory skin diseases and regulated through Th1 cytokines and calcium. Exp Dermatol (2007) 16(8):685–91. doi: 10.1111/j.1600-0625.2007.00587.x [DOI] [PubMed] [Google Scholar]
- 38. Büchau AS, Hassan M, Kukova G, Lewerenz V, Kellermann S, Würthner JU, et al. S100A15, an antimicrobial protein of the skin: regulation by e. coli through toll-like receptor 4. J Invest Dermatol (2007) 127(11):2596–604. doi: 10.1038/sj.jid.5700946 [DOI] [PubMed] [Google Scholar]
- 39. Sturchler E, Cox JA, Durussel I, Weibel M, Heizmann CW. S100A16, a novel calcium-binding protein of the EF-hand superfamily. J Biol Chem (2006) 281(50):38905–17. doi: 10.1074/jbc.M605798200 [DOI] [PubMed] [Google Scholar]
- 40. Liu Y, Zhang R, Xin J, Sun Y, Li J, Wei D, et al. Identification of S100A16 as a novel adipogenesis promoting factor in 3T3-L1 cells. Endocrinology (2011) 152(3):903–11. doi: 10.1210/en.2010-1059 [DOI] [PubMed] [Google Scholar]
- 41. Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, et al. S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta (2009) 1793(6):1008–22. doi: 10.1016/j.bbamcr.2008.11.009 [DOI] [PubMed] [Google Scholar]
- 42. Kleindienst A, Bullock MR. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J Neurotrauma (2006) 23(8):1185–200. doi: 10.1089/neu.2006.23.1185 [DOI] [PubMed] [Google Scholar]
- 43. Austermann J, Nazmi AR, Müller-Tidow C, Gerke V. Characterization of the Ca2+ -regulated ezrin-S100P interaction and its role in tumor cell migration. J Biol Chem (2008) 283(43):29331–40. doi: 10.1074/jbc.M806145200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Du M, Wang G, Ismail TM, Gross S, Fernig DG, Barraclough R, et al. S100P dissociates myosin IIA filaments and focal adhesion sites to reduce cell adhesion and enhance cell migration. J Biol Chem (2012) 287(19):15330–44. doi: 10.1074/jbc.M112.349787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gribenko AV, Hopper JE, Makhatadze GI. Molecular characterization and tissue distribution of a novel member of the S100 family of EF-hand proteins. Biochemistry (2001) 40(51):15538–48. doi: 10.1021/bi0114731 [DOI] [PubMed] [Google Scholar]
- 46. Grevers LC, de Vries TJ, Vogl T, Abdollahi-Roodsaz S, Sloetjes AW, Leenen PJM, et al. S100A8 enhances osteoclastic bone resorption in vitro through activation of toll-like receptor 4: implications for bone destruction in murine antigen-induced arthritis. Arthritis Rheumatol (2011) 63(5):1365–75. doi: 10.1002/art.30290 [DOI] [PubMed] [Google Scholar]
- 47. Iotzova-Weiss G, Dziunycz PJ, Freiberger SN, Läuchli S, Hafner J, Vogl T, et al. S100A8/A9 stimulates keratinocyte proliferation in the development of squamous cell carcinoma of the skin via the receptor for advanced glycation-end products. PloS One (2015) 10(3):e0120971. doi: 10.1371/journal.pone.0120971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hessian PA, Edgeworth J, Hogg N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol (1993) 53(2):197–204. doi: 10.1002/jlb.53.2.197 [DOI] [PubMed] [Google Scholar]
- 49. Dorin JR, Emslie E, van Heyningen V. Related calcium-binding proteins map to the same subregion of chromosome 1q and to an extended region of synteny on mouse chromosome 3. Genomics (1990) 8(3):420–6. doi: 10.1016/0888-7543(90)90027-R [DOI] [PubMed] [Google Scholar]
- 50. Lagasse E, Clerc RG. Cloning and expression of two human genes encoding calcium-binding proteins that are regulated during myeloid differentiation. Mol Cell Biol (1988) 8(6):2402–10. doi: 10.1128/mcb.8.6.2402-2410.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fagerhol MK, Dale I AT. Release and quantitation of a leukocyte derived protein (L1). Scand J Haematol (1980) 24:393–8. doi: 10.1111/j.1600-0609.1980.tb02754.x [DOI] [Google Scholar]
- 52. Wilson GB, Jahn TL FJR. Demonstration of serum protein differences in cystic fibrosis by isoelectric focusing in thin-layer polyacrylamide gels. Clin Chim Acta (1973) 49(1):79–91. doi: 10.1016/0009-8981(73)90346-X [DOI] [PubMed] [Google Scholar]
- 53. Wilkinson MM, Busuttil A, Hayward C, Brock DJ, Dorin JR, Van Heyningen V. Expression pattern of two related cystic fibrosis-associated calcium-binding proteins in normal and abnormal tissues. J Cell Sci (1988) 91(Pt 2):221–30. doi: 10.1242/jcs.91.2.221 [DOI] [PubMed] [Google Scholar]
- 54. Dorin JR, Novak M, Hill RE, Brock DJ, Secher DS, van Heyningen V. A clue to the basic defect in cystic fibrosis from cloning the CF antigen gene. Nature (1987) 326(6113):614–7. doi: 10.1038/326614a0 [DOI] [PubMed] [Google Scholar]
- 55. Waugh N, Cummins E, Royle P, Kandala NB, Shyangdan D, Arasaradnam R, et al. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess (2013) 17(55):xv-xix, 1–211. doi: 10.3310/hta17550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang Z, Clark N, Park KT. Effectiveness and cost-effectiveness of measuring fecal calprotectin in diagnosis of inflammatory bowel disease in adults and children. Clin Gastroenterol Hepatol (2014) 12(2):253–262.e2. doi: 10.1016/j.cgh.2013.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. D’Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflammation Bowel Dis (2012) 18(12):2218–24. doi: 10.1002/ibd.22917 [DOI] [PubMed] [Google Scholar]
- 58. Khaki-Khatibi F, Qujeq D, Kashifard M, Moein S, Maniati M, Vaghari-Tabari M. Calprotectin in inflammatory bowel disease. Clinica Chimica Acta (2020) 510:556–65. doi: 10.1016/j.cca.2020.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Menees SB, Powell C, Kurlander J, Goel A, Chey WD. A meta-analysis of the utility of c-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol (2015) 110(3):444–54. doi: 10.1038/ajg.2015.6 [DOI] [PubMed] [Google Scholar]
- 60. Odink K, Cerletti N, Brüggen J, Clerc RG, Tarcsay L, Zwadlo G, et al. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature (1987) 330(6143):80–2. doi: 10.1038/330080a0 [DOI] [PubMed] [Google Scholar]
- 61. Vogl T, Ludwig S, Goebeler M, Strey A, Thorey IS, Reichelt R, et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood (2004) 104(13):4260–8. doi: 10.1182/blood-2004-02-0446 [DOI] [PubMed] [Google Scholar]
- 62. Heizmann CW, Fritz G, Schäfer BW. S100 proteins: structure, functions and pathology. Front Biosci (2002) 7:d1356–68. doi: 10.2741/A846 [DOI] [PubMed] [Google Scholar]
- 63. Roth J, Burwinkel F, van den Bos C, Goebeler M, Vollmer E, Sorg C. MRP8 and MRP14, s-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood (1993) 82(6):1875–83. doi: 10.1182/blood.V82.6.1875.1875 [DOI] [PubMed] [Google Scholar]
- 64. McCormick A, Heesemann L, Wagener J, Marcos V, Hartl D, Loeffler J, et al. NETs formed by human neutrophils inhibit growth of the pathogenic mold aspergillus fumigatus. Microbes Infect (2010) 12(12–13):928–36. doi: 10.1016/j.micinf.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 65. Viemann D, Strey A, Janning A, Jurk K, Klimmek K, Vogl T, et al. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood (2005) 105(7):2955–62. doi: 10.1182/blood-2004-07-2520 [DOI] [PubMed] [Google Scholar]
- 66. Srikrishna G, Panneerselvam K, Westphal V, Abraham V, Varki A, Freeze HH. Two proteins modulating transendothelial migration of leukocytes recognize novel carboxylated glycans on endothelial cells. J Immunol (2001) 166(7):4678–88. doi: 10.4049/jimmunol.166.7.4678 [DOI] [PubMed] [Google Scholar]
- 67. Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MAD, et al. Mrp8 and Mrp14 are endogenous activators of toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med (2007) 13(9):1042–9. doi: 10.1038/nm1638 [DOI] [PubMed] [Google Scholar]
- 68. Goebeler M, Roth J, Van Den Bos C, Ader G, Sorg C. Increase of calcium levels in epithelial cells induces translocation of calcium-binding proteins migration inhibitory factor-related protein 8 (MRP8) and MRP14 to keratin intermediate filaments. Biochem J (1995) 309(Pt 2):419–24. doi: 10.1042/bj3090419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roth J, Vogl T, Sorg C, Sunderkötter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol (2003) 24(4):155–8. doi: 10.1016/S1471-4906(03)00062-0 [DOI] [PubMed] [Google Scholar]
- 70. Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science (2008) 319(5865):962–5. doi: 10.1126/science.1152449 [DOI] [PubMed] [Google Scholar]
- 71. Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, et al. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of staphylococcus aureus. Cell Host Microbe (2011) 10(2):158–64. doi: 10.1016/j.chom.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Austermann J, Spiekermann C, Roth J. S100 proteins in rheumatic diseases. Nat Rev Rheumatol (2018) 14(9):528–41. doi: 10.1038/s41584-018-0058-9 [DOI] [PubMed] [Google Scholar]
- 73. Bach M, Moon J, Moore R, Pan T, Nelson JL, Lood C. A neutrophil activation biomarker panel in prognosis and monitoring of patients with rheumatoid arthritis. Arthritis Rheumatol (2020) 72(1):47–56. doi: 10.1002/art.41062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bae SC, Lee YH. Calprotectin levels in rheumatoid arthritis and their correlation with disease activity: a meta-analysis. Postgrad Med (2017) 129(5):531–7. doi: 10.1080/00325481.2017.1319729 [DOI] [PubMed] [Google Scholar]
- 75. Wang Y, Liang Y. Clinical significance of serum calprotectin level for the disease activity in active rheumatoid arthritis with normal c-reactive protein. Int J Clin Exp Pathol (2019) 12(3):1009–14. [PMC free article] [PubMed] [Google Scholar]
- 76. Van Hoovels L, Vander Cruyssen B, Bogaert L, Van Den Bremt S, Bossuyt X. Pre-analytical and analytical confounders of serum calprotectin as a biomarker in rheumatoid arthritis. Clin Chem Lab Med (2019) 58(1):40–9. doi: 10.1515/cclm-2019-0508 [DOI] [PubMed] [Google Scholar]
- 77. Wollmer M, Wändell P, Rosenqvist M, Larsson A, Melander O, Wessman T, et al. Plasma calprotectin in the emergency department: a potential clinical biomarker for patients with infectious diseases. Scand J Clin Lab Invest (2021) 81(7):593–7. doi: 10.1080/00365513.2021.1980223 [DOI] [PubMed] [Google Scholar]
- 78. Havelka A, Sejersen K, Venge P, Pauksens K, Larsson A. Calprotectin, a new biomarker for diagnosis of acute respiratory infections. Sci Rep (2020) 10(1):1–7. doi: 10.1038/s41598-020-61094-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hammer HB, Kvien TK, Glennås A, Melby K. A longitudinal study of calprotectin as an inflammatory marker in patients with reactive arthritis. Clin Exp Rheumatol (1995) 13(1):59–64. [PubMed] [Google Scholar]
- 80. Hu H, Du F, Zhang S, Zhang W. Serum calprotectin correlates with risk and disease severity of ankylosing spondylitis and its change during first month might predict favorable response to treatment. Mod Rheumatol (2019) 29(5):836–42. doi: 10.1080/14397595.2018.1519103 [DOI] [PubMed] [Google Scholar]
- 81. Gupta L, Bhattacharya S, Agarwal V, Aggarwal A. Elevated levels of serum MRP8/14 in ankylosing spondylitis: associated with peripheral arthritis and active disease. Clin Rheumatol (2016) 35(12):3075–9. doi: 10.1007/s10067-016-3448-x [DOI] [PubMed] [Google Scholar]
- 82. Turina MC, Yeremenko N, Paramarta JE, De Rycke L, Baeten D. Calprotectin (S100A8/9) as serum biomarker for clinical response in proof-of-concept trials in axial and peripheral spondyloarthritis. Arthritis Res Ther (2014) 16(4):413. doi: 10.1186/s13075-014-0413-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sokolova MV, Simon D, Nas K, Zaiss MM, Luo Y, Zhao Y, et al. A set of serum markers detecting systemic inflammation in psoriatic skin, entheseal, and joint disease in the absence of c-reactive protein and its link to clinical disease manifestations. Arthritis Res Ther (2020) 22(1):26. doi: 10.1186/s13075-020-2111-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hansson C, Eriksson C, Alenius GM. S-calprotectin (S100A8/S100A9): A potential marker of inflammation in patients with psoriatic arthritis. (2014) 2014:696415. doi: 10.1155/2014/696415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Holzinger D, Frosch M, Kastrup A, Prince FHM, Otten MH, Van Suijlekom-Smit LWA, et al. The toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann Rheum Dis (2012) 71(6):974–80. doi: 10.1136/annrheumdis-2011-200598 [DOI] [PubMed] [Google Scholar]
- 86. Frosch M, Vogl T, Seeliger S, Wulffraat N, Kuis W, Viemann D, et al. Expression of myeloid-related proteins 8 and 14 in systemic-onset juvenile rheumatoid arthritis. Arthritis Rheumatol (2003) 48(9):2622–6. doi: 10.1002/art.11177 [DOI] [PubMed] [Google Scholar]
- 87. Mitrovic S, Fautrel B. New markers for adult-onset still’s disease. Joint Bone Spine (2018) 85(3):285–93. doi: 10.1016/j.jbspin.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 88. Jung JY, Suh CH, Kim HA. The role of damage-associated molecular pattern for pathogenesis and biomarkers in adult-onset still’s disease. Expert Rev Mol Diagn (2019) 19(6):459–68. doi: 10.1080/14737159.2019.1615449 [DOI] [PubMed] [Google Scholar]
- 89. Holzinger D, Nippe N, Vogl T, Marketon K, Mysore V, Weinhage T, et al. Myeloid-related proteins 8 and 14 contribute to monosodium urate monohydrate crystal-induced inflammation in gout. Arthritis Rheumatol (2014) 66(5):1327–39. doi: 10.1002/art.38369 [DOI] [PubMed] [Google Scholar]
- 90. Turnier JL, Fall N, Thornton S, Witte D, Bennett MR, Appenzeller S, et al. Urine S100 proteins as potential biomarkers of lupus nephritis activity. Arthritis Res Ther (2017) 19(1):242. doi: 10.1186/s13075-017-1444-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Haga HJ, Brun JG, Berntzen HB, Cervera R, Khamashta M, Hughes GRV. Calprotectin in patients with systemic lupus erythematosus: relation to clinical and laboratory parameters of disease activity. Lupus (1993) 2(1):47–50. doi: 10.1177/096120339300200108 [DOI] [PubMed] [Google Scholar]
- 92. Cuida M, Brun JG, Johannessen AC, Jonsson R. Immunohistochemical characterization of the cellular infiltrates in sjögren’s syndrome, rheumatoid arthritis and osteoarthritis with special reference to calprotectin-producing cells. APMIS (1996) 104(12):881–90. doi: 10.1111/j.1699-0463.1996.tb04953.x [DOI] [PubMed] [Google Scholar]
- 93. Nordal HH, Brun JG, Halse AK, Madland TM, Fagerhol MK, Jonsson R. Calprotectin (S100A8/A9), S100A12, and EDTA-resistant S100A12 complexes (ERAC) in primary sjögren’s syndrome. Scand J Rheumatol (2014) 43(1):76–8. doi: 10.3109/03009742.2013.848930 [DOI] [PubMed] [Google Scholar]
- 94. Xu X, Wu WY, Tu WZ, Chu HY, Zhu XX, Liang MR, et al. Increased expression of S100A8 and S100A9 in patients with diffuse cutaneous systemic sclerosis. a correlation with organ involvement and immunological abnormalities. Clin Rheumatol (2013) 32(10):1501–10. doi: 10.1007/s10067-013-2305-4 [DOI] [PubMed] [Google Scholar]
- 95. Hesselstrand R, Wildt M, Bozovic G, Andersson-Sjöland A, Andréasson K, Scheja A, et al. Biomarkers from bronchoalveolar lavage fluid in systemic sclerosis patients with interstitial lung disease relate to severity of lung fibrosis. Respir Med (2013) 107(7):1079–86. doi: 10.1016/j.rmed.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 96. Van Bon L, Cossu M, Loof A, Gohar F, Wittkowski H, Vonk M, et al. Proteomic analysis of plasma identifies the toll-like receptor agonists S100A8/A9 as a novel possible marker for systemic sclerosis phenotype. Ann Rheum Dis (2014) 73(8):1585–9. doi: 10.1136/annrheumdis-2013-205013 [DOI] [PubMed] [Google Scholar]
- 97. Torgutalp M, Dincer ABK, Yayla EM, Yurteri EU, Okatan EI, Guloksuz GEA, et al. THU0610 serum calprotectin levels in bechet’s disease: relationships between disease activity and clinical parameters. Ann Rheum Dis (2018) 77:504. [Google Scholar]
- 98. Nistala K, Varsani H, Wittkowski H, Vogl T, Krol P, Shah V, et al. Myeloid related protein induces muscle derived inflammatory mediators in juvenile dermatomyositis. Arthritis Res Ther (2013) 15(5):R131. doi: 10.1186/ar4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Seeliger S, Vogl T, Engels IH, Schröder JM, Sorg C, Sunderkötter C, et al. Expression of calcium-binding proteins MRP8 and MRP14 in inflammatory muscle diseases. Am J Pathol (2003) 163(3):947–56. doi: 10.1016/S0002-9440(10)63454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nys G, Cobraiville G, Servais AC, Malaise MG, de Seny D, Fillet M. Targeted proteomics reveals serum amyloid a variants and alarmins S100A8-S100A9 as key plasma biomarkers of rheumatoid arthritis. Talanta (2019) 204:507–17. doi: 10.1016/j.talanta.2019.06.044 [DOI] [PubMed] [Google Scholar]
- 101. Oktayoglu P, Bozkurt M, Mete N, Caglayan M, Em S, Nas K. Elevated serum levels of calprotectin (myeloid-related protein 8/14) in patients with ankylosing spondylitis and its association with disease activity and quality of life. J Investig Med (2014) 62(6):880–4. doi: 10.1097/JIM.0000000000000095 [DOI] [PubMed] [Google Scholar]
- 102. Meuwis MA, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Piver E, et al. Serum calprotectin as a biomarker for crohn’s disease. J Crohns Colitis (2013) 7(12):e678–83. doi: 10.1016/j.crohns.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 103. Pedersen L, Nybo M, Poulsen MK, Henriksen JE, Dahl J, Rasmussen LM, et al. Plasma calprotectin and its association with cardiovascular disease manifestations, obesity and the metabolic syndrome in type 2 diabetes mellitus patients. BMC Cardiovasc Disord (2014) 14(1):196. doi: 10.1186/1471-2261-14-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bressler B, Panaccione R, Fedorak RN, Seidman EG. Clinicians’ guide to the use of fecal calprotectin to identify and monitor disease activity in inflammatory bowel disease. Can J Gastroenterol Hepatol (2015) 29(7):369–72. doi: 10.1155/2015/852723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Andrés Cerezo L, Mann H, Pecha O, Pleštilová L, Pavelka K, Vencovský J, et al. Decreases in serum levels of S100A8/9 (calprotectin) correlate with improvements in total swollen joint count in patients with recent-onset rheumatoid arthritis. Arthritis Res Ther (2011) 13(4):R122. doi: 10.1186/ar3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Resende LO, Jorge MFS, Schmitt JV. Extensive pyoderma gangrenosum-like lesions revealing a case of hyperzincemia and hypercalprotectinemia: when to suspect it? Bras Dermatol (2019) 94(6):713. doi: 10.1016/j.abd.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Youssef PP, Smeets TJ, Bresnihan B, Cunnane G, Fitzgerald O, Breedveld F, et al. Microscopic measurement of cellular infiltration in the rheumatoid arthritis synovial membrane: a comparison of semiquantitative and quantitative analysis. Br J Rheumatol (1998) 37(9):1003–7. doi: 10.1093/rheumatology/37.9.1003 [DOI] [PubMed] [Google Scholar]
- 108. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature (2003) 423(6937):356–61. doi: 10.1038/nature01661 [DOI] [PubMed] [Google Scholar]
- 109. Malemud CJ. Myeloid-related protein activity in rheumatoid arthritis. Int J Inflam (2011) 2011:580295. doi: 10.4061/2011/580295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Malemud C, Pearlman E. Targeting JAK/STAT signaling pathway in inflammatory diseases. Curr Signal Transduct Ther (2009) 4(3):201–21. doi: 10.2174/157436209789057467 [DOI] [Google Scholar]
- 111. Youssef P, Roth J, Frosch M, Costello P, Fitzgerald O, Sorg C, et al. Expression of myeloid related proteins (MRP) 8 and 14 and the MRP8/14 heterodimer in rheumatoid arthritis synovial membrane. J Rheumatol (1999) 26(12):2523–8. [PubMed] [Google Scholar]
- 112. Carrión M, Juarranz Y, Martínez C, González-Álvaro I, Pablos JL, Gutiérrez-Cañas I, et al. IL-22/IL-22R1 axis and S100A8/A9 alarmins in human osteoarthritic and rheumatoid arthritis synovial fibroblasts. Rheumatol (Oxford) (2013) 52(12):2177–86. doi: 10.1093/rheumatology/ket315 [DOI] [PubMed] [Google Scholar]
- 113. Berntzen HB, Ölmez Ü, Fagerhol MK, Munthe E. The leukocyte protein L1 in plasma and synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Scand J Rheumatol (1991) 20(2):74–82. doi: 10.3109/03009749109165280 [DOI] [PubMed] [Google Scholar]
- 114. Uchida T, Fukawa A, Uchida M, Fujita K, Saito K. Application of a novel protein biochip technology for detection and identification of rheumatoid arthritis biomarkers in synovial fluid. J Proteome Res (2002) 1(6):495–9. doi: 10.1021/pr025531w [DOI] [PubMed] [Google Scholar]
- 115. Nordal HH, Fagerhol MK, Halse AK, Hammer HB. Calprotectin (S100A8/A9) should preferably be measured in EDTA-plasma; results from a longitudinal study of patients with rheumatoid arthritis. (2018) 78(1–2):102–8. doi: 10.1080/00365513.2017.1419371 [DOI] [PubMed] [Google Scholar]
- 116. Baillet A. Protéines S100A8, S100A9 et S100A12: marqueurs inflammatoires ou acteurs physiopathologiques de la polyarthrite rhumatoïde. Rev Medecine Interne (2010) 31(6):458–61. doi: 10.1016/j.revmed.2009.10.435 [DOI] [PubMed] [Google Scholar]
- 117. Baillet A, Trocmé C, Berthier S, Arlotto M, Grange L, Chenau J, et al. Synovial fluid proteomic fingerprint: S100A8, S100A9 and S100A12 proteins discriminate rheumatoid arthritis from other inflammatory joint diseases. Rheumatol (Oxford) (2010) 49(4):671–82. doi: 10.1093/rheumatology/kep452 [DOI] [PubMed] [Google Scholar]
- 118. Brun JG, Haga HJ, Bøe E, Kallay I, Lekven C, Berntzen HB, et al. Calprotectin in patients with rheumatoid arthritis: relation to clinical and laboratory variables of disease activity. J Rheumatol (1992) 19(6):859–62. [PubMed] [Google Scholar]
- 119. Brun JG, Jonsson R, Haga HJ. Measurement of plasma calprotectin as an indicator of arthritis and disease activity in patients with inflammatory rheumatic diseases. J Rheumatol (1994) 21(4):733–8. [PubMed] [Google Scholar]
- 120. Berntzen HB, Munthe E, Fagerhol MK. A longitudinal study of the leukocyte protein L1 as an indicator of disease activity in patients with rheumatoid arthritis. J Rheumatol (1989) 16(11):1416–20. [PubMed] [Google Scholar]
- 121. De Seny D, Fillet M, Ribbens C, Marée R, Meuwis MA, Lutteri L, et al. Monomeric calgranulins measured by SELDI-TOF mass spectrometry and calprotectin measured by ELISA as biomarkers in arthritis. Clin Chem (2008) 54(6):1066–75. doi: 10.1373/clinchem.2007.099549 [DOI] [PubMed] [Google Scholar]
- 122. García-Arias M, Pascual-Salcedo D, Ramiro S, Ueberschlag ME, Jermann TM, Cara C, et al. Calprotectin in rheumatoid arthritis: association with disease activity in a cross-sectional and a longitudinal cohort. Mol Diagn Ther (2013) 17(1):49–56. doi: 10.1007/s40291-013-0016-9 [DOI] [PubMed] [Google Scholar]
- 123. Hammer HB, Ødeg̊ard S, Fagerhol MK, Landewé R, van der Heijde D, Uhlig T, et al. Calprotectin (a major leucocyte protein) is strongly and independently correlated with joint inflammation and damage in rheumatoid arthritis. Ann Rheum Dis (2007) 66(8):1093–7. doi: 10.1136/ard.2006.064741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen YS, Yan W, Geczy CL, Brown MA, Thomas R. Serum levels of soluble receptor for advanced glycation end products and of S100 proteins are associated with inflammatory, autoantibody, and classical risk markers of joint and vascular damage in rheumatoid arthritis. Arthritis Res Ther (2009) 11(2):R39. doi: 10.1186/ar2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Berner Hammer H, Ødegård S, Syversen SW, Landewé R, van der Heijde D, Uhlig T, et al. Calprotectin (a major S100 leucocyte protein) predicts 10-year radiographic progression in patients with rheumatoid arthritis. Ann Rheum Dis (2010) 69(1):150–4. doi: 10.1136/ard.2008.103739 [DOI] [PubMed] [Google Scholar]
- 126. Berntzen HB, Munthe E, Fagerhol MK. The major leukocyte protein L1 as an indicator of inflammatory joint disease. Scand J Rheumatol Suppl (1988) 76:251–6. doi: 10.3109/03009748809102976 [DOI] [PubMed] [Google Scholar]
- 127. Bisoendial RJ, Stroes ESG, Tak PP. Critical determinants of cardiovascular risk in rheumatoid arthritis. Curr Pharm Des (2011) 17(1):21–6. doi: 10.2174/138161211795049741 [DOI] [PubMed] [Google Scholar]
- 128. Haringman JJ, Gerlag DM, Zwinderman AH, Smeets TJM, Kraan MC, Baeten D, et al. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis (2005) 64(6):834–8. doi: 10.1136/ard.2004.029751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Inciarte-Mundo J, Ruiz-Esquide V, Hernández MV, Cañete JD, Cabrera-Villalba SR, Ramirez J, et al. Calprotectin more accurately discriminates the disease status of rheumatoid arthritis patients receiving tocilizumab than acute phase reactants. Rheumatol (United Kingdom) (2015) 54(12):2239–43. doi: 10.1093/rheumatology/kev251 [DOI] [PubMed] [Google Scholar]
- 130. Jonsson MK, Sundlisæter NP, Nordal HH, Hammer HB, Aga AB, Olsen IC, et al. Calprotectin as a marker of inflammation in patients with early rheumatoid arthritis. Ann Rheum Dis (2017) 76(12):2031–7. doi: 10.1136/annrheumdis-2017-211695 [DOI] [PubMed] [Google Scholar]
- 131. Hurnakova J, Hulejova H, Zavada J, Hanova P, Komarc M, Mann H, et al. Relationship between serum calprotectin (S100A8/9) and clinical, laboratory and ultrasound parameters of disease activity in rheumatoid arthritis: A large cohort study. PloS One (2017) 12(8):e0183420. doi: 10.1371/journal.pone.0183420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mansour HE, Abdullrhman MA, Mobasher SA, El Mallah R, Abaza N, Hamed F, et al. Serum calprotectin in rheumatoid arthritis: A promising diagnostic marker, how far is it related to activity and sonographic findings? J Med Ultrasound (2017) 25(1):40–6. doi: 10.1016/j.jmu.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Inciarte-Mundo J, Hernández MV, Ruiz-Esquide V, Cabrera-Villalba SR, Ramirez J, Cuervo A, et al. Serum calprotectin more accurately discriminates the inflammatory disease activity of rheumatoid arthritis patients receiving TNF inhibitors than acute phase reactants. Arthritis Care Res (Hoboken) (2015) (Hoboken: ) 68(7):899–906. doi: 10.1002/acr.22795 [DOI] [PubMed] [Google Scholar]
- 134. Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: The role of acute-phase reactants. Arthritis Rheumatol (2011) 63(1):43–52. doi: 10.1002/art.27740 [DOI] [PubMed] [Google Scholar]
- 135. Jarlborg M, Courvoisier DS, Lamacchia C, Martinez Prat L, Mahler M, Bentow C, et al. Serum calprotectin: a promising biomarker in rheumatoid arthritis and axial spondyloarthritis. Arthritis Res Ther (2020) 22(1):18671. doi: 10.1038/s41598-021-98255-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nielsen UB, Bruhn LV, Ellingsen T, Stengaard-Pedersen K, Hornung N. Calprotectin in patients with chronic rheumatoid arthritis correlates with disease activity and responsiveness to methotrexate. Scand J Clin Lab Invest (2018) 78(1–2):62–7. doi: 10.1080/00365513.2017.1413591 [DOI] [PubMed] [Google Scholar]
- 137. De Rycke L, Baeten D, Foell D, Kruithof E, Veys EM, Roth J, et al. Differential expression and response to anti-TNF? treatment of infiltrating versus resident tissue macrophage subsets in autoimmune arthritis. J Pathol (2005) 206(1):17–27. doi: 10.1002/path.1758 [DOI] [PubMed] [Google Scholar]
- 138. Kopec-Medrek M, Kucharz EJ. Fibulin-3 and other cartilage metabolism biomarkers in relationship to calprotectin (MRP8/14) and disease activity in rheumatoid arthritis patients treated with anti-TNF therapy. Adv Clin Exp Med (2018) 27(3):383–9. doi: 10.17219/acem/68362 [DOI] [PubMed] [Google Scholar]
- 139. Yunchun L, Yue W, Jun FZ, Qizhu S, Liumei D. Clinical significance of myeloid-related protein 8/14 as a predictor for biological treatment and disease activity in rheumatoid arthritis. Ann Clin Lab Sci (2018) 48(1):63–8. [PubMed] [Google Scholar]
- 140. Joshua F, Edmonds J, Lassere M. Power Doppler ultrasound in musculoskeletal disease: a systematic review. Semin Arthritis Rheumatol (2006) 36(2):99–108. doi: 10.1016/j.semarthrit.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 141. Hammer HB, Fagerhol MK, Wien TN, Kvien TK. The soluble biomarker calprotectin (a S100 protein) is associated to ultrasonographic synovitis scores and is sensitive to change in patients with rheumatoid arthritis treated with adalimumab. Arthritis Res Ther (2011) 13(5):R178. doi: 10.1186/ar3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hurnakova J, Zavada J, Hanova P, Hulejova H, Klein M, Mann H, et al. Serum calprotectin (S100A8/9): an independent predictor of ultrasound synovitis in patients with rheumatoid arthritis. Arthritis Res Ther (2015) 17:252. doi: 10.1186/s13075-015-0764-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nordal HH, Brokstad KA, Solheim M, Halse AK, Kvien TK, Hammer HB. Calprotectin (S100A8/A9) has the strongest association with ultrasound-detected synovitis and predicts response to biologic treatment: results from a longitudinal study of patients with established rheumatoid arthritis. Arthritis Res Ther (2017) 19(1):3. doi: 10.1186/s13075-016-1201-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Inciarte-Mundo J, Ramirez J, Hernández MV, Ruiz-Esquide V, Cuervo A, Cabrera-Villalba SR, et al. Calprotectin and TNF trough serum levels identify power Doppler ultrasound synovitis in rheumatoid arthritis and psoriatic arthritis patients in remission or with low disease activity. (2016) 18(1):160. doi: 10.1186/s13075-016-1032-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Koenders MI, Marijnissen RJ, Devesa I, Lubberts E, Joosten LAB, Roth J, et al. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: Rationale for combination treatment during arthritis. Arthritis Rheumatol (2011) 63(8):2329–39. doi: 10.1002/art.30418 [DOI] [PubMed] [Google Scholar]
- 146. Ramírez J, Narváez JA, Ruiz-Esquide V, Hernández-Gañán J, Cuervo A, Inciarte-Mundo J, et al. Clinical and sonographic biomarkers of structural damage progression in RA patients in clinical remission: A prospective study with 12 months follow-up. Semin Arthritis Rheumatol (2017) 47(3):303–9. doi: 10.1016/j.semarthrit.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 147. Bernardy C, Lejeune S, Courtier A, Wendling D, Berenbaum F, Nguyen MVC, et al. Calprotectin alone is not sufficient to predict response to methotrexate in early ACR/EULAR 2010 rheumatoid arthritis: Analysis of the ESPOIR cohort. Joint Bone Spine (2020) 87(1):99–100. doi: 10.1016/j.jbspin.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 148. Choi IY, Gerlag DM, Herenius MJ, Thurlings RM, Wijbrandts CA, Foell D, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis (2015) 74(3):499–505. doi: 10.1136/annrheumdis-2013-203923 [DOI] [PubMed] [Google Scholar]
- 149. Nair SC, Welsing PMJ, Choi IYK, Roth J, Holzinger D, Bijlsma JWJ, et al. A personalized approach to biological therapy using prediction of clinical response based on MRP8/14 serum complex levels in rheumatoid arthritis patients. PloS One (2016) 11(3):e0152362. doi: 10.1371/journal.pone.0152362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Martínez-Feito A, Plasencia-Rodríguez C, Navarro-Compán V, Jochems A, Hernández-Breijo B, Peiteado D, et al. Low serum calprotectin levels correlate with the presence of biological drugs after the first year of treatment in patients with rheumatoid arthritis. Scand J Clin Lab Invest (2019) 79(7):538–40. doi: 10.1080/00365513.2019.1669813 [DOI] [PubMed] [Google Scholar]
- 151. Abildtrup M, Kingsley GH, Scott DL. Calprotectin as a biomarker for rheumatoid arthritis: a systematic review. J Rheumatol (2015) 42(5):760–70. doi: 10.3899/jrheum.140628 [DOI] [PubMed] [Google Scholar]
- 152. Aghdashi MA, Seyedmardani S, Ghasemi S, Khodamoradi Z. Evaluation of serum calprotectin level and disease activity in patients with rheumatoid arthritis. Curr Rheumatol Rev (2019) 15(4):316–20. doi: 10.2174/1573397115666190122113221 [DOI] [PubMed] [Google Scholar]
- 153. Bechman K, Tweehuysen L, Garrood T, Scott DL, Cope AP, Galloway JB, et al. Flares in rheumatoid arthritis patients with low disease activity: Predictability and association with worse clinical outcomes. J Rheumatol (2018) 45(11):1515–21. doi: 10.3899/jrheum.171375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tweehuysen L, Den Broeder N, Van Herwaarden N, Joosten LAB, Van Lent PL, Vogl T, et al. Predictive value of serum calprotectin (S100A8/A9) for clinical response after starting or tapering anti-TNF treatment in patients with rheumatoid arthritis. RMD Open (2018) 4(1):e000654. doi: 10.1136/rmdopen-2018-000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. De Moel EC, Rech J, Mahler M, Roth J, Vogl T, Schouffoer A, et al. Circulating calprotectin (S100A8/A9) is higher in rheumatoid arthritis patients that relapse within 12 months of tapering anti-rheumatic drugs. Arthritis Res Ther (2019) 21(1):268. doi: 10.1186/s13075-019-2064-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Cesaro A, Anceriz N, Plante A, Pagé N, Tardif MR, Tessier PA. An inflammation loop orchestrated by S100A9 and calprotectin is critical for development of arthritis. PloS One (2012) 7(9):e45478. doi: 10.1371/journal.pone.0045478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Austermann J, Zenker S, Roth J. S100-alarmins: potential therapeutic targets for arthritis. Expert Opin Ther Targets (2017) 21(7):739–51. doi: 10.1080/14728222.2017.1330411 [DOI] [PubMed] [Google Scholar]