Abstract
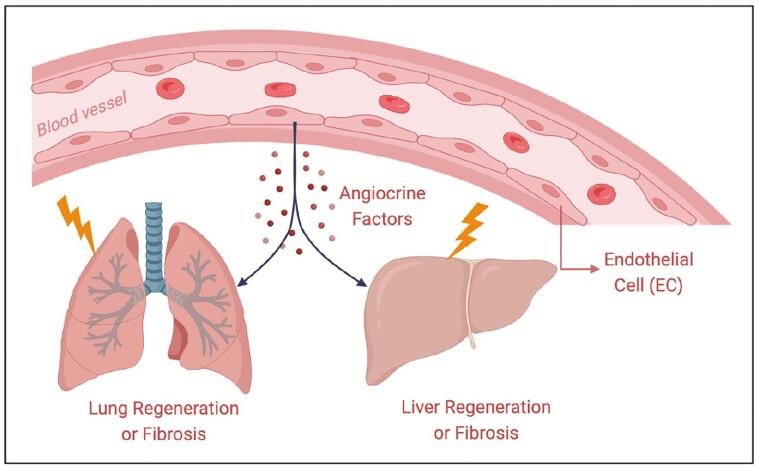
The vasculature occupies a large area of the body, and none of the physiological activities can be carried out without blood vessels. Blood vessels are not just passive conduits and barriers for delivering blood and nutrients. Meanwhile, endothelial cells covering the vascular lumen establish vascular niches by deploying some growth factors, known as angiocrine factors, and actively participate in the regulation of a variety of physiological processes, such as organ regeneration and fibrosis and the occurrence and development of cancer. After organ injury, vascular endothelial cells regulate the repair process by secreting various angiocrine factors, triggering the proliferation and differentiation process of stem cells. Therefore, analyzing the vascular niche and exploring the factors that maintain vascular homeostasis can provide strong theoretical support for clinical treatment targeting blood vessels. Here we mainly discuss the regulatory mechanisms of the vascular niche in organ regeneration and fibrosis.
Keywords: angiocrine, endothelial cells, regeneration, fibrosis, vascular
Graphical Abstract
Graphical Abstract.
Significance Statement.
This review introduces that vascular niche modulates organ regeneration and fibrosis by deploying angiocrine factors. Understanding the mechanisms that organ regeneration and homeostasis are directed by tissue-specific microvascular endothelial cells is important for clinical treatments that potentiate organ repair without scaring.
Introduction
The diffusion distance of oxygen is limited in the organs, and each cell is approximately 100-150 μm away from the nearest capillary. The vascular system runs over the whole body.1 The total length of blood vessels in adults reaches 90,000 km, and the inner side of blood vessels is covered with approximately 10-60 trillion endothelial cells (ECs), which occupy a large area of the body. Endothelial cells are closely arranged to form the lumen of the blood circulation system, which is composed of arteries, veins, and widely distributed capillaries. The surface area of capillaries accounts for more than 95% of the total circulating surface area, except for some special cases (such as cartilage and cornea).2-5 Blood vessels are involved in almost every physiological and pathological activity; therefore, it is very necessary to deeply explore the fields of blood vessels.
Vasculature develops from endothelial precursor cells derived from the mesoderm at the embryonic stage. It is a very complex and interrelated system that can transport oxygen, nutrients, metabolites, and carbon dioxide.6-11 In recent decades, with the development of technology, our knowledge about blood vessels has also been advancing; blood vessels can not only serve as passive conduits but also play an active regulatory role through paracrine signaling, thus guiding organ regeneration and sustaining homeostasis and metabolism.12 The microvascular circulation composed of vascular endothelial cells is responsible for these functions. Paracrine factors derived from endothelial cells are known as angiocrine factors. They play an irreplaceable role in maintaining organ homeostasis, balancing the self-renewal and differentiation of stem cells, and coordinating organ regeneration without fibrosis and tumor growth.3
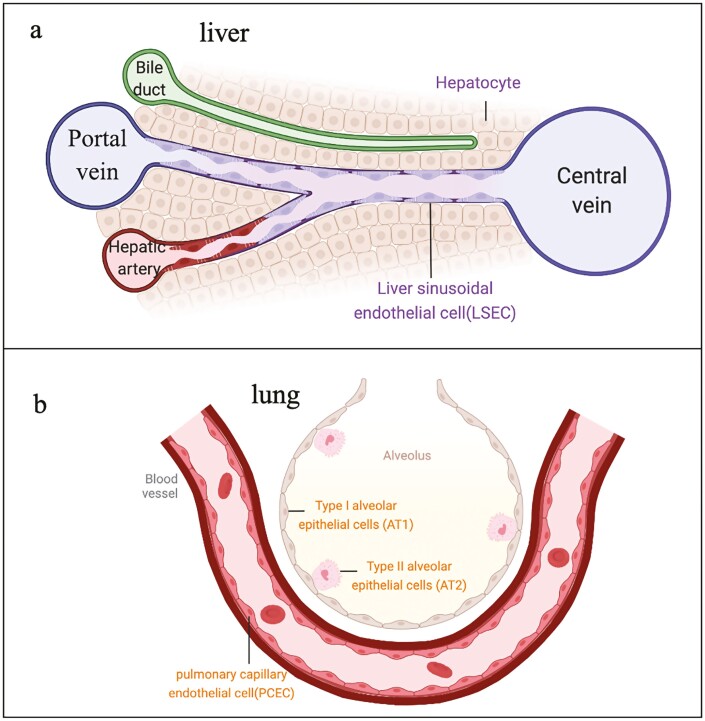
Endothelial cells, a barrier between the circulatory system and tissues, are arranged in a monolayer and attached to the inner side of the capillaries. Endothelial cells allow water- and fat-soluble substances to diffuse into the surrounding interstitial fluid and prevent substances such as circulating cells and plasma proteins from passing through blood vessels.13 The architecture of capillaries is not immutable since they have tissue specificity with different functional environments. For example, liver sinusoidal endothelial cells (LSECs) are specialized ECs with nondiaphragmed fenestrae and without a basement membrane that contributes to material exchange between hepatocytes and the circulatory system(Fig. 1a).14 The ECs in the lungs and skin are closely arranged and have a complete basement membrane, serving as a barrier between internal organs and the external environment(Fig. 1b).1,15,16 In addition, ECs have a special tight intercellular connection, no fenestrae structure, and quite low endocytosis efficiency in the blood-brain barrier system. These structures can protect the brain from harmful substances; however, the effects of targeted therapy drugs on the brain have been greatly hindered because of these structures.17-22
Figure 1.
Tissue specificity of blood vessels. a. Vascular structure in liver. Hepatic vascular system is composed of central veins, portal veins, hepatic arteries, and sinusoids. the unique feature of liver sinusoidal endothelial cells (LSECs) is fenestration and lack a typical basement membrane, facilitating macromolecular transport. b. Alveolar-capillary barrier. Type 1 epithelial cells (AT2) cover 95% of the internal surface of each alveolus. They share the basement membrane with pulmonary capillary endothelial cells (PCECs), forming a vast gas exchange surface. Type 2 epithelial cells (AT2) are progenitor cells which have an ability to differentiate to AT1 after injury.
Many studies have demonstrated its tissue-specific regulatory function. Carmen M et al. reported that the deficiency of angiocrine factors derived from mature ECs can destroy the homeostasis of stem cells and impede organ regeneration but has no influence on the transportation of blood.23 The diversity of angiogenesis modes is crucial to metabolic tissues.24,25 In the state of pathological stress or mechanical injury, silent tissue-specific stem cells are regulated by specific angiocrine factors derived from activated vascular ECs and drive the process of regeneration and steady-state environment reconstruction.26,27
It was announced that local production of polyamines in ECs stimulates adipocyte lipolysis and regulates white adipose tissue homeostasis in mice.28Yu et al. demonstrated that angiopoietin-like2 (ANGPTL2) secreted by the vascular niche endothelial cells serves as the seminal paracrine angiocrine factor, guiding hematopoietic stem cell (HSC) homeostasis and regeneration after myelosuppression.29
Mammalian organs have a certain ability to repair and regenerate after acute injury. On the contrary, after chronic injury, this ability will be inhibited and develop into fibrosis (scarring). According to statistics, 45% of the causes of death worldwide are related to fibrosis. Fibrosis is a pathological extension of the normal wound healing process and excessive deposition of connective tissue in response to injury. Over accumulation of extracellular matrix (ECM) destroys the normal physiological structure of organs, leading to organ dysfunction, and eventually leading to high mortality.30 Using single-cell RNA-seq and genetic fate-tracing, naked cuticle homolog 2 (Nkd2) serves as a myofibroblast-specific target in human kidney fibrosis.31 Heart regeneration is a challenge for scientists all over the world. Reversible reprogramming of cardiomyocytes (CMs), short-time expression of c-Myc, induces adult CMs to dedifferentiate, conferring regenerative capacity to adult hearts.32 Meanwhile, Roman et al. described a cell-autonomous repair process of skeletal muscle without stem cells. Mouse muscle injury triggers a signaling cascade that attracts myonuclei to the damaged site via microtubules and dynein.33
In the process of injured organ repairing, regeneration and fibrosis are dynamic balance process, which is regulated by the vascular niche. Next, we focus on the regulation of vascular niche in the repair process of 2 crucial organs, liver and pulmonary.
Regeneration and Fibrosis in the Liver
The liver is the largest internal organ of the human body and undertakes a variety of physiological functions, including regulating blood volume, arranging growth factors, and maintaining a steady state of metabolites. The endothelial cells attached to the medial side are known as hepatic sinusoidal endothelial cells (LSECs) with nondiaphragmed fenestrate and without a basement membrane. This special structure provides a channel for material transportation between blood and hepatic parenchymal cells. Because LSECs have a large number of fenestrae and no basement membrane, the liver microcirculation is the most porous of all endothelial barriers.34-36
Pericytes, as an important part of the vascular microenvironment, are distributed around blood vessels and play a certain role in supporting blood vessels. Pericytes in the liver are known as hepatic stellate cells (HSCs), whose main function is to store vitamin A in a resting state. After being activated, they are highly expressed α SMA, which secretes extracellular matrix (ECM). Once suffering from chronic injury, HSCs is continuously activated, over secreting extracellular matrix such as collagen, destroying the normal physiological structure of the liver, inhibiting the regeneration of damaged organs, and promoting fibrosis37,38
Chronic or overwhelming injury destroys the self-repair ability of the liver, which can be repaired by ectopic transplantation of parenchymal cells.39-42 However, the vascular niche of damaged tissues reduces the survival and proliferation efficiency of implanted cells. Studies have shown that double editing vascular endothelial cells and perivascular fibroblasts of the damaged tissues make the vascular niche better, which can “nourish” the transplanted exogenous parenchymal cells. Ectopic and synergistic inhibition of perivascular NOX4 by hepatocyte growth factor (HGF) derived from LSECs can promote the functional implantation of mouse and human hepatocytes in the injured liver. In the process of liver regeneration, NOX4 in the perivascular of HGF iΔEC/ iΔECmice shows an abnormal upward trend, and eventually liver fibrosis occurs. However, silencing NOX4 in HGF iΔEC/ iΔECmice by a special liver tissue enrichment administration method can reverse liver fibrosis.43 This phenomenon indicates that endothelial HGF can promote liver regeneration without fibrosis by inhibiting the expression of NOX4 in perivascular fibroblasts. This phenomenon also showed the same results in the cholestasis model caused by bile duct ligation in mice, as well as in clinical samples. TGF- β promotes the expression of NOX4 in fibroblasts. The co-culture system of endothelial cells and hepatic stellate cells in vitro also proves that overexpression of HGF in endothelial cells reduces the fibrogenic factor TGF- β, leading to downregulation of abnormal NOX4 expression in hepatic stellate cells.
The above results show that HGF derived from ECs enhances liver regeneration.44 Can endothelial overexpression of HGF promotes the transplantation of exogenous hepatocytes in the liver injury model? This problem can be verified by the pseudovirus-specific transport system. The virus surface has the ability to bind to immunoglobulin.45,46 When the pseudovirus recognizes and couples the endothelial cell surface antigen CD31, the virus successfully infects endothelial cells. The intrasplenic injection can enrich the virus in LSECs to achieve specific overexpression of HGF in LSECs.47After pseudovirus combined with a NOX4 inhibitor (GKT137831) was used to treat mice, it was found that using a dual editing system, overexpression of HGF, as well as inhibition of NOX4 in LSECs, could alleviate liver fibrosis in recipient mice and improve the transplantation efficiency of exogenous hepatocytes by ameliorating the vascular niche.
The liver has a strong regeneration ability. The liver of mice can recover to the original liver weight within 7-10 days after the surgical resection of up to 70% of the liver’s mass, which is called partial hepatectomy (PH). The procedure of liver regeneration can hardly start without the participation of angiocrine. This model can be used to study the regulation of the hepatic vascular niche in the process of liver regeneration. Liver regeneration is a precisely regulated process by angiocrine secreted by ECs to ensure the functional regeneration of the liver. During the inductive phase of liver regeneration (1-4 days after PH), the expression of angiotensin-2 derived from LSECs decreased, thereby inhibiting the expression of TGF-β1. During the angiogenic phase of liver regeneration (4-8 days after PH), angiotensin 2 returns to normal levels and promotes VEGFR2-dependent angiogenesis.3,48
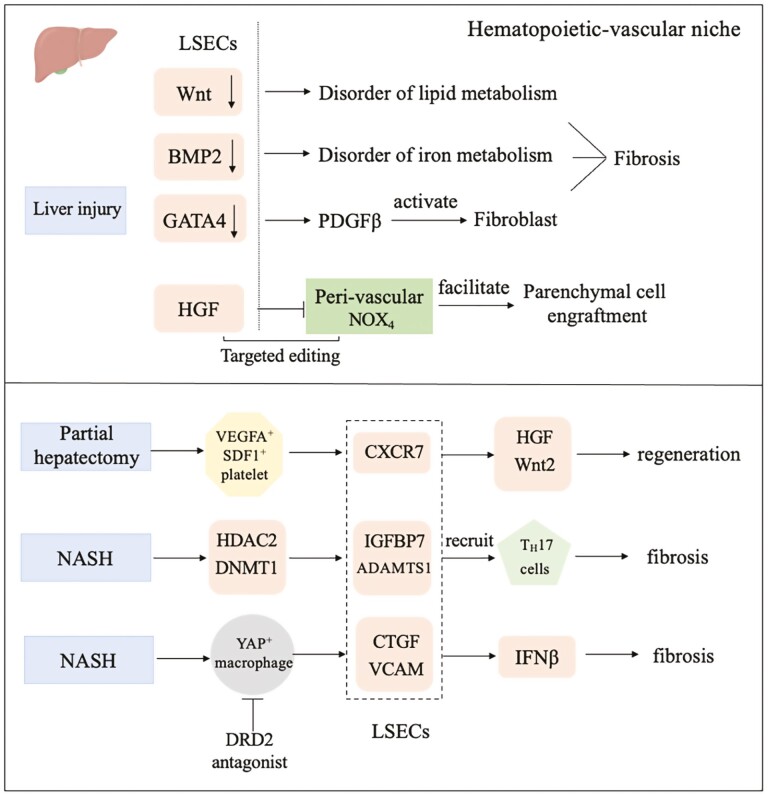
By studying the development of the liver in the embryonic stage, it was found that vessel perfusion in the liver vascular system can activate β1 integrin and VEGFR3 in LSECs. Notably, these 2 angiocrines are strictly required for the proliferation of hepatic parenchymal cells.49 In vitro perfusion of adult mouse liver and mechanical tensile of human LSECs both illustrate that mechanical force alone is sufficient for endothelial cells to secrete β1 integrin and VEGFR3. The deletion of GATA4 in adult mouse LSECs induces a series of liver diseases, such as fibrosis and inhibition of liver regeneration. The specific pathological manifestation was that LSECs, which express activator PDGF β of hepatic stellate, lost fenestrate and basement membrane, and showed capillarization. It was also found that GATA4 in endothelial cells also had a protective effect on liver fibrosis induced by a high-fat diet.50-52 Wnt signaling derived from LSECs plays an important role in regulating metabolic zonation of the liver. The deficiency of angiocrine Wnt signaling in LSECs reduces the liver/body weight ratio and disrupts lipid metabolism homeostasis.53,54 In the process of liver fibrosis, P300, a main regulator of gene transcription in LSECs, interacts with NF- κB and BRD4 to promote the expression of CCL2, which can lead to the recruitment and aggregation of macrophages in the liver, leading to portal hypertension and liver fibrosis.55 The specific deletion of the BMP2 gene in mouse LSECs causes a large amount of iron overload in the liver, a significant increase in serum iron content, and a higher level of iron deposition in the heart or other organs, indicating that the angiocrine signal secreted by the liver vascular not only regulates the physiological process of the liver itself but also has an important impact on the maintenance of systemic homeostasis(Fig.2).56
Figure 2.
Angiocrine signals in the liver modulate regeneration and fibrosis after injury.
Endothelial cells communicate with blood cells (platelets, myeloid cells/macrophages, and T cells) to orchestrate liver regeneration and fibrosis. Targeted editing of this “hematopoietic-vascular niche” might help develop clinical treatments for hepatic disorders. After a liver injury in a mouse model, activated platelets secrete SDF1 and VEGFA to stimulate CXCR7+ liver endothelial cells (LSECs) and VEGFR+ myeloid cells. Both CXCR7+ LSECs and VEGFR+ myeloid cells promote the expression of HGF and Wnt2, 2 crucial angiocrine factors initiating hepatocyte proliferation.57 In both murine and minipig non-alcoholic steatohepatitis (NASH) models, DRD2 antagonism selectively targets YAP-dependent fibrogenic crosstalk between macrophages and the CTGF+VCAM+ vascular niche, promoting liver regeneration and bypassing fibrosis.58 Meanwhile, multiomics analysis showed that crosstalk between histone deacetylase 2 (HDAC2) and DNA methyltransferase1 (DNMT1) promoted liver ECs maladaptation to promote the production of angiocrine IGFBP7 and ADAMTS1 in extracellular vesicles, recruiting fibrogenic TH17 cells to the liver(Fig. 2).59
Pulmonary Regeneration and Fibrosis
The lung is an organ that exchanges gas with the outside world of mammals. The pulmonary vascular system is composed of arteries, veins, and capillaries. Because of their specific function, alveolar epithelial cells and pulmonary capillary endothelial cells (PCECs) are closely combined in structure for extensive exchange surface exchange to maintain the orderly progression of normal physiological activities of the whole body.60-65
The lung is continuously exposed to the air and directly contacts the external environment, which makes it more vulnerable. After an acute injury, the pulmonary has a certain self-repair ability called functional regeneration. However, most pulmonary outcomes caused by persistent damage are fibrosis, which is commonly referred to as scarring.66-73 Endothelial cells interact with other cells (such as platelets, macrophages, and pericytes) by secreting some angiocrine factors to form the vascular niche, which modulates the fate of lung progenitor cells.63,65,74
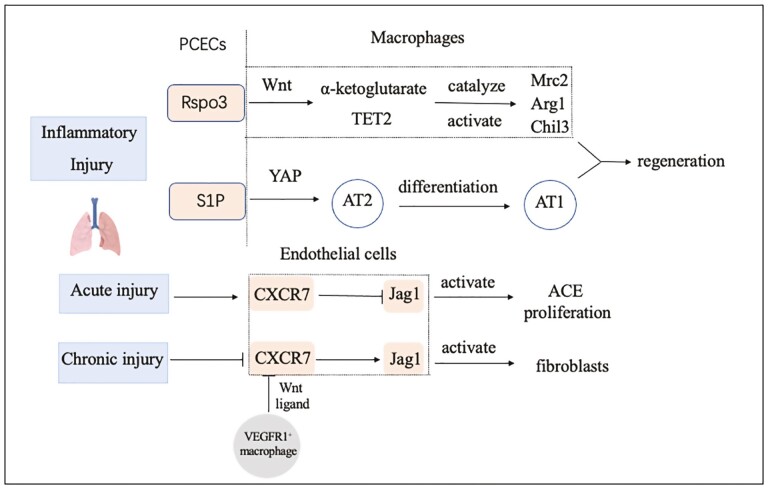
Single or multiple intratracheal injections of bleomycin can induce acute or chronic lung injury models. Repeated lung injury activates pulmonary capillary endothelial cells (PCECs) and perivascular macrophages, which hamper alveolar repair and cause fibrosis. After a single bleomycin injury, the chemokine receptor CXCR7, which is highly expressed on PCECs, protects epithelial cells from bleomycin injury and effectively alleviates fibrosis. In contrast, multiple bleomycin injections inhibit the expression of CXCR7 in PCECs and recruit perivascular VEGFR1+ macrophages. This recruitment promotes the upregulation of the Notch ligand Jagged1 in PCECs and then stimulates perivascular fibroblasts and activates the fibrotic response.75 In response to inflammatory injury, PCECs release the Rspondin3 signaling factor to activate the Wnt/β-Catenin signaling pathway in macrophages and increase mitochondrial respiration through glutaminolysis. α-Ketoglutarate generated in this process acts as a cofactor of the epigenetic regulator TET2 to catalyze the methylation of anti-inflammatory factor DNA in macrophages to activate the transcription of related genes and finally impede the inflammatory response in damaged lung tissue and initiate its repair and regeneration process.76 After bacterial injury of lung tissue caused by Pseudomonas aeruginosa, PCECs release S1P signaling molecules, act on type II alveolar epithelial cells through its receptor S1PR2, trigger the Yap signal pathway, and then promote the differentiation of type II alveolar epithelial cells into type I alveolar epithelial cells that perform gas exchange functions.77
Unilateral pneumonectomy (PNX), which stimulates PCECs to produce paracrine factors and modulate the proliferation of alveolar epithelial progenitor cells, is a canonical model to study lung regeneration. After lung resection, alveolar capillary endothelial cells secrete VEGFR2 and FGFR1 to promote the production of MMP14. Then MMP14 initiates and maintains the formation and development of alveoli by activating the extracellular domain of EGF and the EGF receptor EGFR. The vascular niche plays an important regulatory role in lung regeneration and repair (Fig. 3).78 Meanwhile, deficiency of interaction between pericytes and endothelial cells leads to poorly organized and dysfunctional vascular in non–small cell lung cancer. High expression of hexokinase 2 (HK2)-driven glycolysis in tumor pericytes, which upregulates ROCK2-MLC2 mediated contractility leading to impaired blood vessel supporting function.79
Figure 3.
Angiocrine signals of the pulmonary instruct regeneration and fibrosis after injury. AEC: Alveolar epithelial cells.
Vascular Niche in Aging Individuals
It is estimated that the global population aged 65 and over will increase from 524 million in 2010 to 1.5 billion in 2050, accounting for 16% of the total population.80-83 Elderly individuals are prone to chronic diseases. Aging can lead to the decline of many functions of the human body and cause a variety of diseases. At least part of this is caused by fibrosis, which is caused by the excessive deposition of extracellular matrix (ECM) components. In this process, the functional parenchymal organs are replaced by fibrotic tissues, and organ function is seriously weakened. In the process of organ fibrosis caused by aging, the circulating microenvironment composed of vascular endothelial cells and immune cells plays a regulatory role.84,85
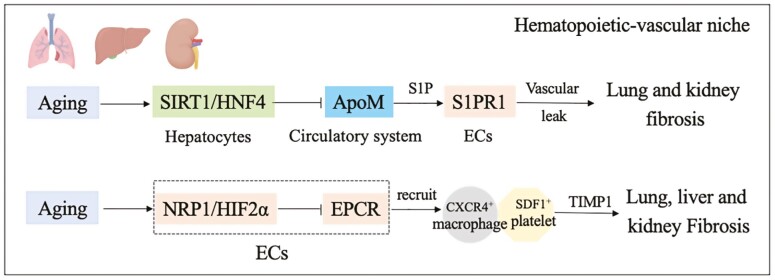
Stress on aged organs often causes vascular endothelial cell reprogramming, leading to fibrosis at the expense of regeneration. Aging induces NRP1/HIF2α to suppress the expression of anti-thrombotic and anti-inflammatory EPCR, causing the formation of profibrotic platelet-macrophage rosettes. Under this condition, platelets activated by IL1α chemokine synergize with angiocrine factors derived from ECs to recruit fibrogenic TIMP1hi macrophages. In mouse models, genetic targeting of endothelial Neuropilin-1-HIF2α, platelet interleukin-1α, or macrophage TIMP1 normalized the profibrotic hematopoietic-vascular niche and restored the regenerative capacity of old organs.86
ApoM derived from the liver shows transcriptional suppression in aging mice. This inhibition in the liver is transmitted to other organs through the circulatory system to play a further regulatory role, which can be transmitted to the lung and kidney, resulting in downregulation of the S1PR1-S1P pathway of ECs. This signal leads to a reduction in resistance to vascular leakage, which eventually causes organ fibrosis. The reason for hepatic ApoM transcriptional suppression is downregulation of the Sirt1-HNF4 pathway (Fig. 4).87
Figure 4.
Angiocrine signals orchestrate regeneration and fibrosis of aging organs.
Age-associated vascular changes and their relation to organ aging and pathology are fundamental for tissue fibrosis and regeneration. It is published that vascular attrition characterized by pericyte to fibroblast differentiation precedes the appearance of cellular hallmarks of aging, such as senescence. Age-associated organ-specific molecular changes in the endothelium drive vascular loss and dictate pericyte to fibroblast differentiation, and drive organ fibrosis.88
Technology
The study of vascular regeneration and homeostasis is inseparable from vascular imaging technology. With the application of fluorescent reporter mice and the development of optical microscopy and electron microscopy, the trend in angiography is toward in vivo and high resolution. The advantage is that researchers can observe a series of dynamic changes in blood vessels. However, the depth and resolution of imaging still need to be further improved89,90 By improving the fluorescent dye, excitation wavelength, and other methods, it is now possible to achieve an imaging depth of 200μm.91 The commonly used vascular imaging techniques include immunofluorescence staining. Zhang et al. announced that angiocrine factors produced by distinct blood vessels are responsible for the specific spatial organization of hematopoiesis using in suit mapping technology.92Vascular endothelial markers have tissue specificity, but generally express CD31, VE-Cad, CD34, and VEGFR, and are negative for CD45. The advantage of a vascular cast is that sample can be kept for a long time, moreover, the blood vessels of whole organs can be seen.
Tissue-specific endothelial cells modulate organ development and regeneration, but human ECs cultured ex vivo lose this ability.1,93 The development of organoids has great therapeutic potential in solving the problem of limited donors for human organ transplantation. The cultivation of organoids in vitro is inseparable from the functional construction of the vascular system.94-97 Embryonic-restricted ETS variant transcription factor 2 (ETV2) in mature human endothelial cells cultured in serum-free 3-dimensional matrix composed of laminin, annexin, and type IV collagen. The reprogrammed endothelial cells form perfusion and plastic vascular plexuses in vitro and interact with 3-dimensional cocultured organoids. This technology can not only replace the biomimetic chemical materials used as vascular cavities in organoid culture but also actively regulate organoids in vitro through the secretion of vascular factors.98,99
Conclusion and Future Perspectives
The vascular niche formed by endothelial cells can actively regulate organ regeneration and fibrosis, which makes a qualitative breakthrough in the traditional understanding of passive vascular transport. With the proposal of this concept, the research on the vascular niche is becoming increasing in depth. The existing evidence shows that the blood vessels of different organs have heterogeneity, and even the vasculature of the same organ has the heterogeneity of arteries, veins, capillaries, and lymphatic capillaries. Further study of this heterogeneity is useful to improve the accuracy of disease treatment. Fortunately, with the continuous innovation of biotechnology, especially now combined with single-cell sequencing, this problem can be further solved, but the problem of sequencing depth and subsequent joint analysis still needs to be improved by researchers in future studies.
It is indispensable for researchers to further clarify the regulatory mechanism of angiocrine factors. Cellular mechanisms, such as the communication between macrophages, platelets, and vascular endothelial cells, and molecular mechanisms, such as the epigenetic modification or protein post-translational modification, need further study. These studies enable us to intervene with upstream and downstream targets at the same time, which is helpful to improve the targeting of drugs or therapeutic means.
The vasculature participates in various physical activities of each cell. Abnormalities in the vascular niche constitute a potential pathogenic cause of various diseases. Therefore, an in-depth analysis of the vascular niche will be an effective way to solve some human health problems.
Acknowledgments
Due to the limitations of space, the authors apologized for not citing all important studies in this review.
Contributor Information
Yutian Chen, The Department of Endovascular Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China.
Bi-Sen Ding, State Key Laboratory of Biotherapy, West China Second University Hospital, Sichuan University, Chengdu, People’s Republic of China.
Funding
This work was supported by the China Postdoctoral Science Foundation (Grant N0. 2022TQ0299).
Conflict of Interest
The authors declared no competing interests.
Author Contributions
Y.C. and B.-S. D.: wrote the manuscript.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Augustin HG, Koh GY.. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science. 2017;357(6353):eaal2379. [DOI] [PubMed] [Google Scholar]
- 2. Ferland-McCollough D, Slater S, Richard J, Reni C, Mangialardi G.. Pericytes, an overlooked player in vascular pathobiology. Pharmacol Ther. 2017;171:30-42. 10.1016/j.pharmthera.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rafii S, Butler JM, Ding B-S.. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529(7586):316-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quail DF, Joyce JA.. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Apte RS, Chen DS, Ferrara N.. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Potente M, Gerhardt H, Carmeliet P.. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873-887. 10.1016/j.cell.2011.08.039 [DOI] [PubMed] [Google Scholar]
- 7. You L-R, Lin F-J, Lee CT, et al. Suppression of notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435(7038):98-104. 10.1038/nature03511 [DOI] [PubMed] [Google Scholar]
- 8. Wang ZZ, Au P, Chen T, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25(3):317-318. [DOI] [PubMed] [Google Scholar]
- 9. Patsch C, Challet-Meylan L, Thoma EC, et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol. 2015;17(8):994-1003. 10.1038/ncb3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sriram G, Tan JY, Islam I, Rufaihah AJ, Cao T.. Efficient differentiation of human embryonic stem cells to arterial and venous endothelial cells under feeder- and serum-free conditions. Stem Cell Res Ther. 2015;6:261. 10.1186/s13287-015-0260-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Escobedo N, Oliver G.. Lymphangiogenesis: origin, specification, and cell fate determination. Annu Rev Cell Dev Biol. 2016;32:677-691. 10.1146/annurev-cellbio-111315-124944 [DOI] [PubMed] [Google Scholar]
- 12. Eelen G, de Zeeuw P, Treps L, et al. Endothelial cell metabolism. Physiol Rev. 2018;98(1):3-58. 10.1152/physrev.00001.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF.. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11(2):109-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poisson J, Lemoinne S, Boulanger C, et al. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol. 2017;66(1):212-227. 10.1016/j.jhep.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 15. Aird WC. Phenotypic heterogeneity of the endothelium: I. structure, function, and mechanisms. Circ Res. 2007;100(2):158-173. [DOI] [PubMed] [Google Scholar]
- 16. Aird WC. Phenotypic heterogeneity of the endothelium: II. representative vascular beds. Circ Res. 2007;100(2):174-190. 10.1161/01.RES.0000255690.03436.ae [DOI] [PubMed] [Google Scholar]
- 17. Obermeier B, Daneman R, Ransohoff RM.. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ben-Zvi A, Lacoste B, Kur E, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509(7501):507-511. 10.1038/nature13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen LN, Ma D, Shui G, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509(7501):503-506. [DOI] [PubMed] [Google Scholar]
- 20. Zhou Y, Wang Y, Tischfield M, et al. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest. 2014;124(9):3825-3846. 10.1172/JCI76431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Y, Nathans J.. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev Cell. 2014;31(2):248-256. 10.1016/j.devcel.2014.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV.. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163(5):1064-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Warren CM, Iruela-Arispe ML.. Signaling circuitry in vascular morphogenesis. Curr Opin Hematol. 2010;17(3):213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferguson JE, Kelley RW, Patterson C.. Mechanisms of endothelial differentiation in embryonic vasculogenesis. Arterioscler Thromb Vasc Biol. 2005;25(11):2246-2254. [DOI] [PubMed] [Google Scholar]
- 25. Patel-Hett S, D’Amore PA.. Signal transduction in vasculogenesis and developmental angiogenesis. Int J Dev Biol. 2011;55(4-5):353-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Butler JM, Kobayashi H, Rafii S.. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10(2):138-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murakami M, Nguyen LT, Zhuang ZW, et al. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118(10):3355-3366. 10.1172/JCI35298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Monelli E, Villacampa P, Zabala-Letona A, et al. Angiocrine polyamine production regulates adiposity. Nat Metab. 2022;4(3):327-343. 10.1038/s42255-022-00544-6 [DOI] [PubMed] [Google Scholar]
- 29. Yu Z, Yang W, He X, et al. Endothelial cell-derived angiopoietin-like protein 2 supports hematopoietic stem cell activities in bone marrow niches. Blood. 2022;139(10):1529-1540. 10.1182/blood.2021011644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao X, Kwan JYY, Yip K, Liu PP, Liu FF.. Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. 2020;19(1):57-75. 10.1038/s41573-019-0040-5 [DOI] [PubMed] [Google Scholar]
- 31. Kuppe C, Ibrahim MM, Kranz J, et al. Decoding myofibroblast origins in human kidney fibrosis. Nature. 2021;589(7841):281-286. 10.1038/s41586-020-2941-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Y, Lüttmann FF, Schoger E, et al. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science. 2021;373(6562):1537-1540. 10.1126/science.abg5159 [DOI] [PubMed] [Google Scholar]
- 33. Roman W, Pinheiro H, Pimentel MR, et al. Muscle repair after physiological damage relies on nuclear migration for cellular reconstruction. Science. 2021;374(6565):355-359. 10.1126/science.abe5620 [DOI] [PubMed] [Google Scholar]
- 34. Hilmer SN, Cogger VC, Fraser R, McLean AJ, Sullivan D, Le Couteur DG.. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005;42(6):1349-1354. [DOI] [PubMed] [Google Scholar]
- 35. Cogger VC, Hilmer SN, Sullivan D, Muller M, Fraser R, Le Couteur DG.. Hyperlipidemia and surfactants: the liver sieve is a link. Atherosclerosis. 2006;189(2):273-281. [DOI] [PubMed] [Google Scholar]
- 36. Zapotoczny B, Szafranska K, Kus E, et al. Tracking fenestrae dynamics in live murine liver sinusoidal endothelial cells. Hepatology. 2019;69(2):876-888. 10.1002/hep.30232 [DOI] [PubMed] [Google Scholar]
- 37. Fondevila MF, Fernandez U, Heras V, et al. Inhibition of carnitine palmitoyltransferase 1A in hepatic stellate cells protects against fibrosis. J Hepatol. 2022;77(1):15-28. [DOI] [PubMed] [Google Scholar]
- 38. Xi Y, Li Y, Xu P, et al. The anti-fibrotic drug pirfenidone inhibits liver fibrosis by targeting the small oxidoreductase glutaredoxin-1. Sci Adv. 2021;7(36):eabg9241. 10.1126/sciadv.abg9241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eming SA, Martin P, Tomic-Canic M.. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6-265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wynn TA, Ramalingam TR.. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thannickal VJ, Zhou Y, Gaggar A, Duncan SR.. Fibrosis: ultimate and proximate causes. J Clin Invest. 2014;124(11):4673-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hogan BLM, Barkauskas CE, Chapman HA, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15(2):123-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mirza MK, Sun Y, Zhao YD, et al. FoxM1 regulates re-annealing of endothelial adherens junctions through transcriptional control of beta-catenin expression. J Exp Med. 2010;207(8):1675-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hecker L, Logsdon NJ, Kurundkar D, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6(231):231ra47-231ra47. 10.1126/scitranslmed.3008182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou Y, Peng H, Sun H, et al. Chitinase 3-like 1 suppresses injury and promotes fibroproliferative responses in Mammalian lung fibrosis. Sci Transl Med. 2014;6(240):240ra76-240ra76. 10.1126/scitranslmed.3007096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moon J-S, Nakahira K, Chung K-P, et al. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat Med. 2016;22(9):1002-1012. 10.1038/nm.4153 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47. Cao Z, Ye T, Sun Y, et al. Targeting the vascular and perivascular niches as a regenerative therapy for lung and liver fibrosis. Sci Transl Med. 2017;9(405):eaai8710. 10.1126/scitranslmed.aai8710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lefere S, Van de Velde F, Hoorens A, et al. Angiopoietin-2 promotes pathological angiogenesis and is a therapeutic target in murine nonalcoholic fatty liver disease. Hepatology. 2019;69(3):1087-1104. 10.1002/hep.30294 [DOI] [PubMed] [Google Scholar]
- 49. Lorenz L, Axnick J, Buschmann T, et al. Mechanosensing by β1 integrin induces angiocrine signals for liver growth and survival. Nature. 2018;562(7725):128-132. 10.1038/s41586-018-0522-3 [DOI] [PubMed] [Google Scholar]
- 50. Winkler M, Staniczek T, Kürschner SW, et al. Endothelial GATA4 controls liver fibrosis and regeneration by preventing a pathogenic switch in angiocrine signaling. J Hepatol. 2021;74(2):380-393. 10.1016/j.jhep.2020.08.033 [DOI] [PubMed] [Google Scholar]
- 51. Zaret KS, Grompe M.. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322(5907):1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Si-Tayeb K, Lemaigre FP, Duncan SA.. Organogenesis and development of the liver. Dev Cell. 2010;18(2):175-189. [DOI] [PubMed] [Google Scholar]
- 53. Leibing T, Géraud C, Augustin I, et al. Angiocrine Wnt signaling controls liver growth and metabolic maturation in mice. Hepatology. 2018;68(2):707-722. 10.1002/hep.29613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walter TJ, Cast AE, Huppert KA, Huppert SS.. Epithelial VEGF signaling is required in the mouse liver for proper sinusoid endothelial cell identity and hepatocyte zonation in vivo. Am J Physiol Gastrointest Liver Physiol. 2014;306(10):G849-G862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gao J, Wei B, Liu M, et al. Endothelial p300 promotes portal hypertension and hepatic fibrosis through C-C motif chemokine ligand 2-mediated angiocrine signaling. Hepatology. 2021;73(6):2468-2483. 10.1002/hep.31617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Koch P-S, Olsavszky V, Ulbrich F, et al. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood. 2017;129(4): 415-419. 10.1182/blood-2016-07-729822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shido K, Chavez D, Cao Z, et al. Platelets prime hematopoietic and vascular niche to drive angiocrine-mediated liver regeneration. Signal Transduct. Target. Ther. 2017;2:16044. 10.1038/sigtrans.2016.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qing J, Ren Y, Zhang Y, et al. Dopamine receptor D2 antagonism normalizes profibrotic macrophage-endothelial crosstalk in non-alcoholic steatohepatitis. J Hepatol. 2022;76(2):394-406. 10.1016/j.jhep.2021.09.032 [DOI] [PubMed] [Google Scholar]
- 59. Zhang H, Ma Y, Cheng X, et al. Targeting epigenetically maladapted vascular niche alleviates liver fibrosis in nonalcoholic steatohepatitis. Sci Transl Med. 2021;13(614):eabd1206. 10.1126/scitranslmed.abd1206 [DOI] [PubMed] [Google Scholar]
- 60. Beers MF, Morrisey EE.. The three R’s of lung health and disease: repair, remodeling, and regeneration. J Clin Invest. 2011;121(6):2065-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Matthay MA, Howard JP.. Progress in modelling acute lung injury in a pre-clinical mouse model. Eur Respir J. 2012;39(5):1062-1063. [DOI] [PubMed] [Google Scholar]
- 62. Rackley CR, Stripp BR.. Building and maintaining the epithelium of the lung. J Clin Invest. 2012;122(8):2724-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barkauskas CE, Cronce MJ, Rackley CR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123(7):3025-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pardo-Saganta A, Tata PR, Law BM, et al. Parent stem cells can serve as niches for their daughter cells. Nature. 2015;523(7562):597-601. 10.1038/nature14553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Desai TJ, Brownfield DG, Krasnow MA.. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507(7491):190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kotton DN, Morrisey EE.. Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nat Med. 2014;20(8):822-832. 10.1038/nm.3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vaughan AE, Brumwell AN, Xi Y, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517(7536):621-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Petrache I, Natarajan V, Zhen L, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11(5):491-498. 10.1038/nm1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952-961. [DOI] [PubMed] [Google Scholar]
- 70. Farkas L, Farkas D, Ask K, et al. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest. 2009;119(5):1298-1311. 10.1172/JCI36136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lazarus A, Del-Moral PM, Ilovich O, et al. A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Development. 2011;138(11):2359-2368. 10.1242/dev.060723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hergenreider E, Heydt S, Tréguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14(3):249-256. 10.1038/ncb2441 [DOI] [PubMed] [Google Scholar]
- 73. Lee J-H, Bhang DH, Beede A, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156(3):440-455. 10.1016/j.cell.2013.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Evans MJ, Cabral LJ, Stephens RJ, Freeman G.. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22(1):142-150. 10.1016/0014-4800(75)90059-3 [DOI] [PubMed] [Google Scholar]
- 75. Cao Z, Lis R, Ginsberg M, et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med. 2016;22(2):154-162. 10.1038/nm.4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou B, Magana L, Hong Z, et al. The angiocrine Rspondin3 instructs interstitial macrophage transition via metabolic-epigenetic reprogramming and resolves inflammatory injury. Nat Immunol. 2020;21(11):1430-1443. 10.1038/s41590-020-0764-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen Q, Rehman J, Chan M, et al. Angiocrine sphingosine-1-phosphate activation of S1PR2-YAP signaling axis in alveolar type II cells is essential for lung repair. Cell Rep. 2020;31(13):107828. 10.1016/j.celrep.2020.107828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ding B-S, Nolan DJ, Guo P, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147(3):539-553. 10.1016/j.cell.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Meng Y-M, Jiang X, Zhao X, et al. Hexokinase 2-driven glycolysis in pericytes activates their contractility leading to tumor blood vessel abnormalities. Nat Commun. 2021;12(1):6011. 10.1038/s41467-021-26259-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science (80-). 2014;344(6184):630-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kusumbe AP, Ramasamy SK, Itkin T, et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 2016;532(7599):380-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Martinod K, Witsch T, Erpenbeck L, et al. Peptidylarginine deiminase 4 promotes age-related organ fibrosis. J Exp Med. 2017;214(2):439-458. 10.1084/jem.20160530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stahl EC, Haschak MJ, Popovic B, Brown BN.. Macrophages in the aging liver and age-related liver disease. Front Immunol. 2018;9:2795. 10.3389/fimmu.2018.02795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wilhelm K, Happel K, Eelen G, et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529(7585):216-220. 10.1038/nature16498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sinha M, Jang YC, Oh J, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science (80-). 2014;344(6184):649-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen Y, Pu Q, Ma Y, et al. Aging reprograms the hematopoietic-vascular niche to impede regeneration and promote fibrosis. Cell Metab. 2021;33(2):395-410.e4. 10.1016/j.cmet.2020.11.019 [DOI] [PubMed] [Google Scholar]
- 87. Ding B-S, Yang D, Swendeman SL, et al. Aging suppresses sphingosine-1-phosphate chaperone apom in circulation resulting in maladaptive organ repair. Dev Cell. 2020;53(6):677-690.e4. 10.1016/j.devcel.2020.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen J, Sivan U, Tan SL, et al. High-resolution 3D imaging uncovers organ-specific vascular control of tissue aging. Sci Adv. 2021;7(6):eabd7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yang W, Yuste R.. In vivo imaging of neural activity. Nat Methods. 2017;14(4):349-359. 10.1038/nmeth.4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mansour AA, Gonçalves JT, Bloyd CW, et al. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018;36(5):432-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wu T, Liao J, Yu J, et al. In vivo label-free two-photon excitation autofluorescence microscopy of microvasculature using a 520 nm femtosecond fiber laser. Opt Lett. 2020;45(10):2704-2707. 10.1364/OL.394242 [DOI] [PubMed] [Google Scholar]
- 92. Zhang J, Wu Q, Johnson CB, et al. In situ mapping identifies distinct vascular niches for myelopoiesis. Nature. 2021;590(7846):457-462. 10.1038/s41586-021-03201-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Carmeliet P, Jain RK.. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298-307. 10.1038/nature10144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nolan DJ, Ginsberg M, Israely E, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26(2):204-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bhatia SN, Ingber DE.. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32(8):760-772. [DOI] [PubMed] [Google Scholar]
- 96. Pellegata AF, Tedeschi AM, De Coppi P.. Whole organ tissue vascularization: engineering the tree to develop the fruits. Front Bioeng Biotechnol. 2018;6:56. 10.3389/fbioe.2018.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ronaldson-Bouchard K, Vunjak-Novakovic G.. Organs-on-a-chip: a fast track for engineered human tissues in drug development. Cell Stem Cell. 2018;22(3):310-324. 10.1016/j.stem.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Giobbe GG, Crowley C, Luni C, et al.. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat Commun. 2019;10(1):5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Palikuqi B, Nguyen DT, Li G, et al. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature. 2020;585(7825):426-432. 10.1038/s41586-020-2712-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.