Abstract
Acne scars are classified into various types based on their appearances, ranging from hypertrophic to atrophic. Abnormal wound healing processes play an important role in the pathogenesis of scars; however, the exact mechanisms involved in various scar appearances have still not been elucidated. In this study, we used immunofluorescence and immunohistochemistry techniques to detect the presence of myofibroblasts, B cells, and mast cells in each type of acne scar persisting longer than 6 months. We found the highest density of myofibroblasts in hypertrophic acne scars, while in the other atrophic scars, we could not identify any myofibroblast-rich areas in our specimens. B-cell infiltration was mild and found in only 23% (4/17) of all acne scar specimens. Interestingly, mast cells were identified in all specimens, ranging from minimal to high density, and a high number of mast cells in acne scars were associated with obesity. In conclusion, myofibroblasts are abundant only in hypertrophic acne scars, and mast cells, but not B cells, might play an important role in the pathogenesis of long-standing acne scars.
Keywords: Acne scars, Fibroblasts, Fibrosis, Inflammation, Mast cells
Introduction
Acne vulgaris is a common chronic inflammatory disease of the pilosebaceous units affecting approximately 80% of adolescents [1, 2]. In up to 95% of these patients, scarring occurs as common sequelae. The chronicity and visibility of scars can negatively affect the patients' quality of life and self-esteem [3, 4].
Based on their morphologies, dermatologists practically classify acne scars into four different types. The three types of atrophic acne scars (AASs) include boxcar scar (BCS), rolling scar (RS), and pitting scar (PS), and the fourth type is hypertrophic acne scar (HAS). Most acne scars result from comedonal and inflammatory acne lesions [5]. After the inflammatory phase subsides, all types of scars go into a “similar” wounding process in the vicinity of the lesions. The exact mechanisms that result in the diversity of scar appearances are not yet fully understood. Several cellular components, namely, myofibroblasts and other inflammatory cells predominantly found in the skin might hold an essential key to these mechanisms.
Myofibroblasts are one of the most important cells in the process of wound healing, especially during the proliferative phase. Alpha smooth muscle actin (SMA), an actin isoform providing a highly contractile force, is the most important component in myofibroblast differentiation [6, 7]. During the wound healing process, alpha SMA is highly upregulated in the myofibroblasts [8], along with extracellular matrix-degrading enzymes, including matrix metalloproteinases (MMPs), and their inhibitors expressed in the dermis. The homeostasis of these components is a critical factor in determining the architecture of wound appearance. It is well-known that the persistence of alpha-SMA-positive myofibroblasts is a prominent feature of scarring and fibrosis in scars, especially surgical scars [9]. However, the role of myofibroblasts, especially in AASs and HASs, is still inconclusive [10, 11].
Besides myofibroblasts, B cells and plasma cells could play significant roles in AAS formation. In a recent study, B cells and plasma cells were seen in AASs of three-week-old acne papules in scar-prone patients [12]. The scientific evidence, particularly from long-standing AASs, is scarce. Our study aimed to investigate correlations between the number and distribution of myofibroblasts, B cells, and plasma cells using immunofluorescence and immunohistochemistry techniques in different types of acne scars, in subjects with long-standing acne scars.
Materials and Methods
Patients and Tissue Preparation
A total of 17 patients who visited the OPD clinic, between January 2019 and June 2019, were included in our study. The inclusion criterion was persistence of acne scars at least 6 months before the enrollment. A dermatologist classified the acne scar types based on the appearance of lesions in accordance with previous studies [5].
Exclusion criteria included patients whose graded acne severity was greater than one according to the Investigator Global Assessment, and those who had generalized hypertrophic scars or keloids, or a history of allergy to anesthetic drugs. Patients with a history of prior surgical procedures or systemic treatments, namely, tetracyclines and isotretinoin for acne and acne scars for 6 months before the study were also excluded.
After investigators collected the demographic data and history of acne scars, a representative scar was selected and biopsied using a 3-mm sterile punch under an aseptic technique. Specimens were fixed in 10% buffered formalin solution for at least 6 h before being embedded in paraffin.
Immunofluorescence Studies
We performed an immunofluorescence technique described previously [13]. Briefly, we first retrieved the antigens by manually heating 4-μm-thick tissues on a glass slide in sodium citrate buffer (pH 6) in a water bath for 20 min after the deparaffinizing process. The tissues were then incubated in 10% normal goat serum (Abcam, Cambridge, MA, USA) in phosphate-buffered saline for 1 h and 0.3% Triton X-100 (Biosesang, Seongnam, Republic of Korea) for 10 min at room temperature. Myofibroblasts were identified by double-positive staining of a mouse anti-vimentin antibody (Abcam, Cambridge, MA, USA) and a rabbit anti-alpha SMA (Abcam, Cambridge, MA, USA) at 10-μg/mL concentration. Before continuing with the detection of secondary antibodies on the following day, the primary antibodies were incubated at 4°C overnight. On the second day, sections were incubated for 2 h at room temperature with Alexa Flour@ 488-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA, USA) and Alexa Flour@ 555-conjugated goat anti-rabbit IgG (Abcam, Cambridge, MA, USA). After rinsing with phosphate-buffered saline, we used 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA, USA) at a concentration of 300 nM for nuclear counterstaining. Finally, the samples were mounted with Prolong Diamond Antifade Mountant (Invitrogen, Carlsbad, CA, USA) and subsequently examined on a confocal laser scanning microscope (Zeiss LSM 800, White Plains, NY, USA).
Immunohistochemical Assays
After being deparaffinized, the 4-μm-thick sections were processed by the VENTANA-BenchMark-XT computerized automated slide system (Roche, Indianapolis, IN) with the ultraView Universal Red v3 detection. The primary antibodies included anti-human anti-CD20 mouse monoclonal antibody and anti-CD117 (Dako, Santa Clara, CA, USA) and CD117, rabbit monoclonal antibody (Cell Marque, Rocklin, CA, USA). The staining protocols of VENTANA-BenchMark-XT computerized automated system were followed. All specimens were also stained with hematoxylin and eosin (H&E). Dermatologists blinded to the study scored them using a visual scoring scale from 0 to 3 (0, none; 1, minimal, <10% positive staining; 2, moderate, 10–50% positive staining; 3, strong, >50% of positive staining).
Results
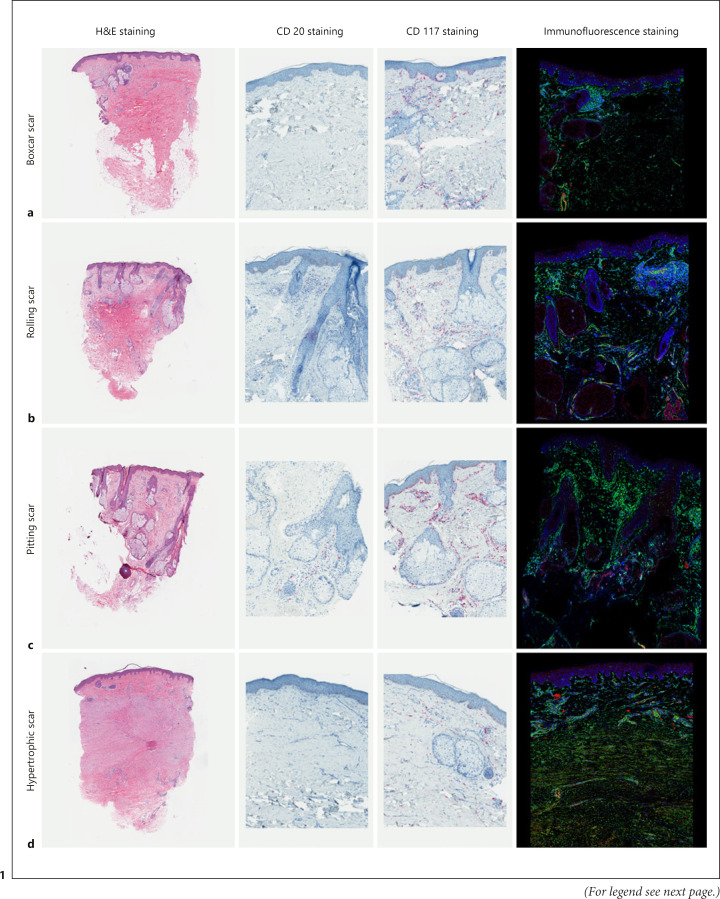
This study included ten women and seven men, aged between 24 and 39 years. From a total of 17 acne scar samples (five BCSs, five RSs, five PSs, and five HASs), the mean duration of the acne scars was 7.3 (standard deviation) [5.9] years. Most of the acne scars were on the cheeks of participants; however, two RSs and all HASs were on the temporal area and chin, respectively. Only two participants (1 PS and 1 HAS) had undergone previous laser treatments for acne scars more than 6 months before the study. Five participants (1 RS, 2 PSs, and 2 HASs) were obese, defined as more than 25 kg/m2 body mass index [14] (Table 1). All the representative specimens of each type of acne scar are displayed in Figure 1.
Table 1.
Clinico-demographic data and pathological grading of special staining in each patient
| Type of scars | Demographic data |
H&E staining |
Special staining |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| age, years | sex | BMI, kg/m2 | waist circumferences, cm | sites of biopsy | duration of scar, years | duration after the last laser treatment, years | lymphocytic infiltration | fibrosis | myofibroblast | CD20 | CD117 | |
| BCS | 38 | Female | 23.88 | 80 | Right cheek | 2 | −† | 1+ | 1+ | None | None | 1+ |
| 27 | Female | 23.23 | 75 | Right cheek | 3 | − | 1+ | 1+ | None | None | 1+ | |
| 26 | Female | 24.56 | 74 | Right cheek | 2 | − | 1+ | 2+ | None | None | 1+ | |
| 31 | Female | 21.87 | 70 | Right cheek | 10 | − | 1+ | 3+ | None | None | 1+ | |
| 26 | Female | 20.20 | 74 | Right temporal | 3 | − | 1+ | 2+ | None | None | 1+ | |
|
| ||||||||||||
| RS | 27 | Male | 22.15 | 77.5 | Right temporal | 4 | − | 1+ | 1+ | None | None | 1+ |
| 28 | Male | 23.31 | 78 | Left temporal | 4 | − | 1+ | 2+ | None | 1+ | 1+ | |
| 26 | Female | 24.97 | 77 | Left cheek | 4 | − | 2+ | 1+ | 1+ at periglandular | None | 2+ | |
| 34 | Male | 26.78 | 94 | Right cheek | 17 | − | 1+ | 1+ | None | 1+ | 3+ | |
| 24 | Male | 22.23 | 79 | Left cheek | 4 | − | 2+ | 1+ | 1+ at periglandular | None | 2+ | |
|
| ||||||||||||
| PS | 28 | Male | 2■.76 | 77 | Right cheek | 5 | − | 1+ | 1+ | None | None | 2+ |
| 32 | Male | 23.38 | 78 | Right cheek | 17 | − | 1+ | 1+ | None | 1+ | 3+ | |
| 29 | Female | 28.72 | 88 | Right cheek | 5 | − | 2+ | 1+ | None | 1+ | 2+ | |
| 27 | Male | 24.44 | 88 | Right cheek | 4 | − | 1+ | 1+ | None | None | 1+ | |
| 39 | Female | 30.11 | 92 | Right cheek | 21 | − | 1+ | 2+ | None | None | 2+ | |
|
| ||||||||||||
| HAS | 33 | Female | 25.71 | 85 | Right chin | 10 | − | 1+ | 3+ | 2+ at interstitial | None | 1+ |
| 31‡ | Female | 28.26 | 92 | Right chin | 10 | 0.5 | 1+ | 1+ | None | None | 1+ | |
H&E, hematoxylin and eosin.
Not done.
Turned out to be sebaceous gland hyperplasia.
Fig. 1.
Histopathological differences in the examined acne scar specimens. a Boxcar: increased in fibrosis. b Rolling: minimal infiltration of CD117. c Pitting: rich in sebaceous glands. d Hypertrophic: abundant myofibroblasts (yellow cells in immunofluorescence staining). Magnification, ×10 in H&E staining. ×200 in CD20, CD117, and immunofluorescence staining.
Boxcar Scars
The tissues biopsied from BCSs showed minimal infiltration with mononuclear cells, mostly lymphocytes. Although these specimens showed the highest intensity of fibrosis among all other types of acne scars, neither double-positive cells nor CD20-positive cells were identified. Minimal CD117-positive cells were found in all specimens.
Rolling Scars
Sections revealed mild lymphocytic infiltration and fibrosis in almost all specimens except in two, one of which had moderate lymphocytic infiltration and one with moderate fibrosis. The double-positive cells were predominantly found in the periglandular areas in two specimens, and the CD20-positive cells were mildly positive in two samples. Interestingly, one tissue from an obese patient showed marked infiltration of CD117-positive cells.
Pitting Scars
Of the five specimens, only one was moderately infiltrated with lymphocytes and was moderately fibrotic, while the others were mildly infiltrated. No double-positive cells were found in the immunofluorescence study. The CD20-positive cells were minimal and were found only in two specimens; CD117-positive cell infiltration varied from mild to strong in this type of scar. Moderate infiltration of CD117-positive cells was identified in specimens from all obese patients with PSs. This type of scar was also found to be most abundant in the area with sebaceous glands compared to other types of scars.
Hypertrophic Acne Scars
Of the two specimens in this type of acne scar, one (ID17) was an extra-large sebaceous gland hyperplasia, while the other specimen showed marked fibrosis and abundant myofibroblasts. No CD20-positive cells were identified, and the sample was only mildly infiltrated by CD117-positive cells.
Discussion/Conclusion
Myofibroblasts, one of several subtypes of fibroblasts, are involved in the fibrotic and scarring processes in many tissues, including acne scars. In our study, by detecting double-positive (vimentin and alpha-SMA staining) cells using the immunofluorescence technique, we were able to demonstrate myofibroblasts in HASs and RSs. However, only the HASs showed abundant double-positive myofibroblasts. Previous studies showed that in hypertrophic scars, the number of myofibroblasts are increased in, both, volume and density as they failed to undergo apoptosis days after the complete closure of the wound [15, 16]. We believe that HASs might share the same pathogenesis as that of hypertrophic scars. We also noted double-positive myofibroblasts in two RSs. This observation further supports a hypothesis of the pathomechanism of RSs in which fibrosis draws the skin surface downward, leading to the “rolling appearance” [17].
The difference in myofibroblast numbers in our samples might be associated with the location of acne scars. In this study, the biopsied lesions were taken from various areas of the faces, as shown in Table 1. We observed that the locations of HASs and RSs were on the sites with higher tensile strength compared to other parts of the face. The result reflects the complexity of the acne scar formation related to the nature of facial skin from different locations [1, 18].
Several inflammatory cells in the skin play a dominant role in acne scar formation. Recently, B cells were persistently identified in the 3-week-old scars in acne scar-prone subjects [12]. In wound healing studies, mature B cells, both in acute or chronic scars, are associated with an increased myofibroblast proliferation [19] and regulated collagen synthesis in fibroblasts [20]. Our results, however, were inconsistent with previous studies. We were not able to demonstrate the significance of CD20-positive cells in the acne scars. We speculate that this inconsistency resulted from the longer scar duration since the mean duration of acne scars in our study was approximately 7 years. The result indicated that B cells might not be involved in long-standing acne scars.
Mast cells play a pivotal role in the wound healing process. They can regulate the inflammatory response and collagen remodeling in wounds by producing several cytokines and growth factors, modulating myofibroblast functions and cause pruritus, which is a common feature of scars, especially hypertrophic scars [21, 22]. In the literature, mast cells are active in the early inflammatory phase; however, our results showed the presence of CD117-positive cells in all specimens. Moreover, we found five specimens with moderate and two with strong staining in RSs and PSs but mild staining in HASs. Further studies should be focused on the role of mast cells in acne scars, focusing on HASs, since mast cell-targeted therapies could be a promising alternative treatment.
Interestingly, 75% of obese participants in the study showed moderate to strong CD117-positive cell infiltration. As seen in previous studies, an increased mast cell proliferation in the skin was observed in patients with metabolic syndrome [23, 24]. We speculate that the existence of mast cells after the inflammatory process could be for a longer period, especially in obese patients compared to the nonobese population. It is also possible that the higher expression of mast cells in the acne scars might be correlated with body weight. Weight reduction might be beneficial for the management of acne scars.
Another proposed pathomechanism of AASs is the loss of pilosebaceous units [25, 26]. The disappearance of sebaceous glands, detected by the downregulation of lipid metabolism in lesions from acne-prone patients, was hypothesized [12]. In contrast, our study found relatively normal or increased number of sebaceous glands in all AASs; the largest number was particularly seen in PSs. We instead believe that the process of skin atrophy resulting in AASs is related to a permanent tissue loss at the scar location. As the pilosebaceous units have a natural potential regrowth capacity, their presence in long-lasting scars can be expected.
The limitations of our study include the study design from which causal relationships between the findings could not be demonstrated. An extra-large sebaceous gland hypertrophy can imitate the appearance of HASs, as was seen in one of the studied lesions clinically classified as HAS. The aid of other investigating tools, such as dermoscopy, could help to distinguish between these lesions before deciding on an appropriate treatment. Since we did not perform collagen and elastic fiber stains in our study, we could not confirm the abnormal changes in the fibers resulting in acne scars. The cross-sectional nature of this study and the fact that one scar was taken from each individual also precludes any conclusion regarding the sites and temporal associations.
In conclusion, we demonstrated that HASs had an abundance of myofibroblasts and might share similar pathological pathways with other hypertrophic scars. We did not identify any associations between myofibroblasts or B cells and AASs. The presence of mast cells could be related to AASs, and they might persist longer particularly in AASs in obese patients. For better strategies to prevent and treat acne scars, prospective longitudinal studies focusing on the pathological process in different types of scars are necessary in the future.
Statement of Ethics
The study was conducted following the Declaration of Helsinki and was approved by the Institutional Review Board of Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB No. 618/61). Written informed consent was obtained from participants to participate in the study.
Conflict of Interest Statement
The authors declare no conflict of interest.
Funding Sources
The research was funded by Ratchadaphisek Somphot Endowment Fund, Grant No. RA. 62/092, Graduate School, Chulalongkorn University.
Author Contributions
Data collection and formal analysis: B.C. Validation and visualization, conclusion, and manuscript writing: B.C. and C.K. Review and editing: C.K. and P.A.
Data Availability Statement
The data collected for this study will not be available to the public. Investigators interested in the deidentified participant data are encouraged to contact author at after publication of this article for data sharing and collaboration.
Acknowledgments
We are grateful for Dr. Trailak Pisitkul, Mr. Chatikorn Boonkrai, and Mr. Wittavit Aksornkit (Center of Excellence in Systems Biology, Chulalongkorn University) for the technical assistance.
Funding Statement
The research was funded by Ratchadaphisek Somphot Endowment Fund, Grant No. RA. 62/092, Graduate School, Chulalongkorn University.
References
- 1.Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45((1)):109–117. doi: 10.1067/mjd.2001.113451. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrocini G, Annunziata MC, D'Arco V, De Vita V, Lodi G, Mauriello MC, et al. Acne scars: pathogenesis, classification and treatment. Dermatol Res Pract. 2010;2010:893080. doi: 10.1155/2010/893080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuah SY, Goh CL. The impact of post-acne scars on the quality of life among young adults in Singapore. J Cutan Aesthet Surg. 2015;8((3)):153–158. doi: 10.4103/0974-2077.167272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hazarika N, Rajaprabha RK. Assessment of life quality index among patients with acne vulgaris in a suburban population. Indian J Dermatol. 2016;61((2)):163–168. doi: 10.4103/0019-5154.177758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman GJ. Postacne scarring: a review of its pathophysiology and treatment. Dermatol Surg. 2000;26((9)):857–871. doi: 10.1046/j.1524-4725.2000.99232.x. [DOI] [PubMed] [Google Scholar]
- 6.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172((2)):259–268. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63((1)):21–29. [PubMed] [Google Scholar]
- 8.Shi HX, Lin C, Lin BB, Wang ZG, Zhang HY, Wu FZ, et al. The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PLoS One. 2013;8((4)):e59966. doi: 10.1371/journal.pone.0059966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36((6)):1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145((1)):114–125. [PMC free article] [PubMed] [Google Scholar]
- 11.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3((5)):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 12.Carlavan I, Bertino B, Rivier M, Martel P, Bourdes V, Motte M, et al. Atrophic scar formation in patients with acne involves long-acting immune responses with plasma cells and alteration of sebaceous glands. Br J Dermatol. 2018;179((4)):906–917. doi: 10.1111/bjd.16680. [DOI] [PubMed] [Google Scholar]
- 13.Khositseth S, Charngkaew K, Boonkrai C, Somparn P, Uawithya P, Chomanee N, et al. Hypercalcemia induces targeted autophagic degradation of aquaporin-2 at the onset of nephrogenic diabetes insipidus. Kidney Int. 2017;91((5)):1070–1087. doi: 10.1016/j.kint.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Pan WH, Yeh WT. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: an extension of Asian-Pacific recommendations. Asia Pac J Clin Nutr. 2008;17((3)):370–374. [PubMed] [Google Scholar]
- 15.van der Veer WM, Bloemen MC, Ulrich MM, Molema G, van Zuijlen PP, Middelkoop E, et al. Potential cellular and molecular causes of hypertrophic scar formation. Burns. 2009;35((1)):15–29. doi: 10.1016/j.burns.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21((12)):3250–3261. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Z, Ding J, Shankowsky HA, Tredget EE. The molecular mechanism of hypertrophic scar. J Cell Commun Signal. 2013;7((4)):239–252. doi: 10.1007/s12079-013-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuji T, Sawabe M. Elastic fibers in scar tissue: scanning and transmission electron microscopic studies. J Cutan Pathol. 1987;14((2)):106–113. doi: 10.1111/j.1600-0560.1987.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 19.Sîrbulescu RF, Boehm CK, Soon E, Wilks MQ, Ilieş I, Yuan H, et al. Mature B cells accelerate wound healing after acute and chronic diabetic skin lesions. Wound Repair Regen. 2017;25((5)):774–791. doi: 10.1111/wrr.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francois A, Chatelus E, Wachsmann D, Sibilia J, Bahram S, Alsaleh G, et al. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res Ther. 2013;15((5)):R168. doi: 10.1186/ar4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruber BL. Mast cells in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2003;5((2)):147–153. doi: 10.1007/s11926-003-0043-3. [DOI] [PubMed] [Google Scholar]
- 22.Wulff BC, Wilgus TA. Mast cell activity in the healing wound: more than meets the eye? Exp Dermatol. 2013;22((8)):507–510. doi: 10.1111/exd.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurung P, Moussa K, Adams-Huet B, Devaraj S, Jialal I. Increased mast cell abundance in adipose tissue of metabolic syndrome: relevance to the proinflammatory state and increased adipose tissue fibrosis. Am J Physiol Endocrinol Metab. 2019;316((3)):E504–9. doi: 10.1152/ajpendo.00462.2018. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Shi GP. Mast cells and metabolic syndrome. Biochim Biophys Acta. 2012;1822((1)):14–20. doi: 10.1016/j.bbadis.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee WJ, Jung HJ, Lim HJ, Jang YH, Lee SJ, Kim DW. Serial sections of atrophic acne scars help in the interpretation of microscopic findings and the selection of good therapeutic modalities. J Eur Acad Dermatol Venereol. 2013;27((5)):643–646. doi: 10.1111/j.1468-3083.2011.04330.x. [DOI] [PubMed] [Google Scholar]
- 26.Moon J, Yoon JY, Yang JH, Kwon HH, Min S, Suh DH. Atrophic acne scar: a process from altered metabolism of elastic fibres and collagen fibres based on transforming growth factor-β1 signalling. Br J Dermatol. 2019;181((6)):1226–1237. doi: 10.1111/bjd.17851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data collected for this study will not be available to the public. Investigators interested in the deidentified participant data are encouraged to contact author at after publication of this article for data sharing and collaboration.