Abstract
The present study was undertaken to test the hypothesis that tumor necrosis factor (TNF) and/or interleukin-1 (IL-1) activity mediates lipopolysaccharide (LPS)-induced bone resorption in vivo. To test this hypothesis, Escherichia coli LPS or Porphyromonas gingivalis LPS was injected into the subcutaneous tissues overlying mouse calvariae. Histological sections, prepared from the center of the lesion, were stained for tartrate-resistant acid phosphatase, and histomorphometric analysis was performed to quantify the osteoclast number and the area of bone resorption. In time course experiments using normal mice, a peak of bone resorption occurred 5 days after endotoxin stimulation. In dose-response experiments, IL-1 receptor type 1 deletion (IL-1R−/−), TNF double-receptor p55/p75 deletion (TNF p55−/−/p75−/−), combined TNF p55 and IL-1 receptor type 1 deletion (TNF p55−/−/IL-1R−/−), and IL-1β-converting enzyme-deficient (ICE−/−) mice and the respective wild-type mice were injected with 500, 100, or 20 μg of P. gingivalis LPS and sacrificed 5 days after LPS injection. At the highest dose (500 μg), significant decreases in osteoclast number occurred in mutant mice compared to wild-type mice: (i) a 64% reduction for the TNF p55−/−/IL-1R−/− mice, (ii) a 57% reduction for the IL-1R−/− mice, (iii) a 41% reduction for the TNF p55−/−/p75−/− mice, and (iv) a 38% reduction for the ICE−/− mice. At the two lower doses, bone resorption was apparent but no significant differences between mutant and wild-type animals were observed. The present data indicate that at higher doses, LPS-induced bone resorption is substantially mediated by IL-1 and TNF receptor signaling. Furthermore, IL-1 receptor signaling appears to be slightly more important than TNF receptor signaling. At lower LPS doses, other pathways leading to osteoclast activity that are independent of TNF and IL-1 are involved.
Lipopolysaccharide (LPS) is a biologically active substance found in the cell walls of gram-negative bacteria. LPS exerts its effects by binding to host cells, but the cellular mechanism by which LPS stimulates cells has not been fully elucidated. The consequences of LPS stimulation can be severe, as injection of purified LPS into animals or endotoxin stimulation during infection with gram-negative bacteria elicits an inflammatory response as well as an immune host response that can ultimately lead to systemic shock (10, 23).
Local reactions to endotoxin are also characterized by inflammatory and immune responses. They include vascular changes associated with recruitment of leukocytes and the subsequent release of proinflammatory mediators, such as prostaglandins, leukotrienes, and cytokines (5, 9). It is believed that prolonged or excessive production of cytokines such as tumor necrosis factor (TNF), interleukin-6 (IL-6), IL-8, and IL-1 represents an important etiologic factor in inflammatory diseases ranging from arthritis to periodontal disease (2, 8, 15, 18).
Periodontitis is an infectious disease that causes the loss of tooth-supporting tissues, including alveolar bone resorption. Porphyromonas gingivalis has been considered to be one of the important pathogenic microorganisms associated with periodontal disease, particularly adult periodontitis (45). The virulence of this pathogen is attributed to many of its cell wall components, especially LPS (45). Endotoxin or LPS has been identified as one of the principal bacterial factors in stimulating bone resorption, but its exact mechanism of action is unknown (7, 16, 17, 24, 42, 45). It has been suggested that LPS can penetrate gingival connective tissue and induce a local inflammatory response that leads to periodontal bone resorption (34, 40). Recent studies indicate that live or heat-killed P. gingivalis stimulates resorption in the calvarial model (46). The fact that live and killed bacteria have similar activities suggests that a cell wall component such as LPS plays an important role. However, it is likely that LPS stimulates osteoclastic bone resorption indirectly, based on findings that LPS does not directly stimulate resorptive activity on isolated osteoclasts in vitro (37).
Although LPS may not directly stimulate osteoclasts, it is possible that LPS could induce osteoclast activity indirectly by first stimulating other cell types, such as osteoblasts. In the periodontium, LPS could induce inflammation and tissue damage through the induction of cytokines such as IL-1, TNF, or IL-6 that may be produced by several cell types, including gingival fibroblasts, fibroblastic cells in the periodontal ligament, or recruited leukocytes (6, 13, 33, 39). The role of IL-1 and TNF in periodontal inflammation and bone loss was recently demonstrated by the reduction of these parameters when TNF and IL-1 activities were antagonized with function-blocking soluble receptors (4, 14).
IL-1 and TNF are cytokines that have considerable overlap in their biological effects, including leukocyte activation, prostaglandin formation, cytokine gene expression, endothelial cell activation, and bone resorption (6, 9). Numerous in vitro studies have shown that IL-1 causes dramatic increases in osteoclastic bone resorption (8, 27, 32, 38). TNF has also been shown to have potent osteolytic properties, although it does not appear to be as potent as IL-1 (19, 27, 29, 41). Simultaneous addition of IL-1 and TNF to multinucleated cell cultures suggests a synergistic effect between IL-1 and TNF in osteoclast formation and activation (27). Two forms of IL-1 exist as precursors (pro-IL-1). Pro-IL-1α is fully active as a precursor and remains intracellular. In contrast, pro-IL-1β is not fully active after synthesis and acquires its activity after secretion by cleavage with a specific intracellular protease, IL-1β-converting enzyme (ICE) (6). ICE-deficient animals displayed impaired production of IL-1β upon stimulation with LPS. Homozygous mutants were highly resistant to endotoxic shock (22). Cells that respond to IL-1 and TNF have specific high-affinity cell surface receptors. There are at least two forms of IL-1 receptors: type 1 and type 2. The type 1 receptor is capable of mediating a biological signal, while the type 2 receptor is thought to function as a decoy receptor (36). Two types of high-affinity receptors, p55 and p75, have been identified for TNF molecules. Most but not all TNF activity has been shown to be mediated by the TNF p55 receptor (3, 26, 30, 31). Recent evidence obtained with mice lacking p75 suggests that p75 may act to suppress TNF-mediated inflammatory responses (3, 31).
Virtually complete inhibition of cytokine activity can be obtained when cytokine or cytokine receptors are genetically deleted from experimental animals. Targeted deletions of IL-1 and TNF receptors have been invaluable tools in dissecting out the roles of these cytokines in disease processes, particularly in the inflammatory response to LPS. It has been reported that the response to endotoxin is diminished in ICE-deficient mice (which lack soluble IL-1β) (22) and in mice lacking TNF receptors (3). In addition, a reduction in LPS-induced osteoclastogenesis in transgenic mice lacking type 1 TNF receptors has recently been reported (1). Together, these data support the predominant role of IL-1 and TNF as proinflammatory mediators in LPS-induced toxicity. However, the relative contribution of IL-1 or TNF to LPS-induced bone resorption has not been addressed. The objective of the present in vivo study was to compare and contrast the osteoclast responses to local injection of multiple doses of LPS in mice lacking IL-1 type 1 receptor or TNF p55/p75 receptor signaling. The results indicate that at higher doses, LPS-induced osteoclastogenesis and bone resorption is substantially mediated by IL-1 and TNF receptor signaling, while at lower doses, it is not.
MATERIALS AND METHODS
LPS.
Purified Escherichia coli serotype O55:B5 LPS was purchased from Sigma Chemical Co. P. gingivalis LPS was extracted from P. gingivalis A7436 by using a modification of the procedure described by Westphal and Jann (44). Briefly, P. gingivalis was plated on Laked blood agar plates (Carr Scarborough Microbiologicals, Augusta, Ga.) for 5 to 7 days in an anaerobic chamber at 37°C. The bacteria were then transferred into Schaedler broth suspension and grown for 5 to 6 days. After incubation, the bacterial solution was centrifuged at 4, 650 × g at 4°C for 30 min, washed twice with 0.9% saline solution, and centrifuged again under the same conditions. Each washed bacterial pellet was resuspended in 5 ml of distilled water. Five milliliters of phenol (90%) was slowly added to each tube while vortexing, and the tubes were then placed in a 68°C water bath for 15 min, with vortexing every 2 to 3 min. The emulsion was chilled on ice, and the phases were separated by centrifugation at 4,650 × g for 30 min at 4°C. The upper aqueous layer containing LPS was removed and chilled on ice. An equal volume of water (68°C) was added to the phenol phase for two additional extractions. The aqueous phase of the extraction was pooled and dialyzed in distilled water for 72 h. The phenol-water LPS extract was lyophilized and further purified on a CsCl (Boehringer Mannheim, Indianapolis, Ind.) isopycnic density gradient.
The lyophilized phenol-water-extracted P. gingivalis LPS was resuspended and dissolved in distilled water (at 1 mg/ml) by sonication, layered over CsCl (2.8 g per 4.8-ml sample), and subjected to centrifugation (80,000 × g at 4°C) for 60 to 72 h. Fractions with densities of 1.42 to 1.52 g/cm3 containing peak endotoxin activity were collected. These fractions were pooled and dialyzed for 72 h in distilled water with two changes of water per day. The purified material was identified as LPS by using a Limulus amoebocyte lysate assay (Biowhittaker, Walkersville, Md.). The number of endotoxin units was found to be 5.05 × 104/mg (the potency of endotoxin is expressed as endotoxin units with respect to EC-6, which is the currently accepted U.S. reference standard endotoxin). The highly purified LPS was also tested with a micro bicinchoninic acid protein assay (Pierce, Rockford, Ill.), and the protein content was less than 1%.
Injection of LPS.
In the initial time course experiments, 20 wild-type mice (C57BL/6 X129) received 500 μg of E. coli LPS subperiosteally and were sacrificed at days 2, 5, 9, and 14. E. coli LPS was used initially because of its commercial availability and for comparison with other studies using E. coli LPS (2, 24). In the dose dependency experiment, 81 male mice, between 8 and 12 weeks old, were given local calvarial injections of P. gingivalis LPS (strain A7436) and then sacrificed after 5 days. In addition, five mice were injected with 100 μl of 0.9% saline solution and served as negative controls.
Transgenic mice with targeted deletions of IL-1 receptor type 1 (IL-1R−/−), TNF double receptors p55 and p75 (TNF p55−/−/p75−/−), and both TNF p55 and IL-1 receptor type 1 (TNF p55−/−/IL-1R−/−) (generously provided by Immunex Corp.) (12, 26) and wild-type C57BL/6 X129 hybrid mice (purchased from Jackson Laboratory, Bar Harbor, Maine) with the same genetic background were used in this study. In selected experiments, ICE-deficient (ICE−/−) mice (generously provided by BASF Corp.) (22) and genetically matched wild-type (164BBC) mice were studied. All procedures involving animals were approved by an institutional animal care and use committee at Boston University School of Medicine.
All animals were anesthetized intramuscularly with a ketamine-xylazine solution (a combination of 1 ml of ketamine [Ketaset, Fort Dodge, Iowa], 1 ml of xylazine [Rompum, Columbus, Ohio], and 6 ml of sterile phosphate-buffered saline [Gibco BRL, Grand Island, N.Y.]). Approximately 5 μl of anesthetic per g of body weight was administered. The heads of the anesthetized mice were shaved to receive subperiosteal injections of LPS. The injections were administered with a 30.5-gauge needle at a point on the midline of the skull located between the ears and eyes. Wild-type, TNF p55−/−/p75−/−, IL-1R−/−, and TNF p55−/−/IL-1R−/− mice were divided into three groups. Each group received a different dose of LPS (500, 100, or 20 μg). The ICE−/− mice and the respective wild-type mice were divided into two groups; one group received 500 μg of LPS, and the other received 100 μg.
Tissue preparation.
After the injection, the mice were sacrificed in a CO2 chamber at 5 days unless stated otherwise. The entire calvarial bone was dissected and fixed in 4% paraformaldehyde for 4 h at 4°C. The specimens were then washed for 15 min each with 5, 10, and 15% glycerol–phosphate-buffered saline solutions. The calvarial bones were decalcified with 15% glycerol in 15% EDTA solution (pH 7.1) for 21 days at 4°C. Following decalcification, the anterior half of the frontal bone and most of the occipital bone were trimmed off, and the specimens were stored in 30% sucrose overnight and then transferred to −80°C prechilled 2-methylbutane.
Histochemical staining.
The parietal bones were sectioned in half through the center of the bone lesion. The two halves were embedded side by side with HISTO PREP compound. Transverse 5-μm serial sections were made by cryostat sectioning. Fifty slides were obtained for each specimen, and every 10th slide was kept for staining. The sections were stained for tartrate-resistant acid phosphatase (TRAP). The TRAP solution was prepared as follows: 9.6 mg of naphthol AS-BI phosphate substrate (Sigma) was dissolved in 0.6 ml of N,N- dimethylformamide (Sigma) with 60 ml of 0.2 M sodium acetate buffer (pH 5.0) (Sigma), which contained 84 mg of fast red-violet LB diazonium salt (Sigma), 58.2 mg of tartaric acid (Sigma), and 240 μl of 10% MgCl2. The mixture was filtered through a 0.22-μm-pore-size filter. Slides were incubated for 5 min in the staining solution at 37°C in the dark. The slides were then washed with water for 30 min, followed by counterstaining with hematoxylin for 5 to 6 min.
Bone histomorphometry.
For each animal, four slides, each containing two tissue sections with the largest number of bone marrow cells (eight specimens total), were analyzed. For each tissue section, the microscopic fields with the most resorption were studied. The osteoclast index, which represents the number of osteoclasts per millimeter of trabecular bone surface, was measured. The percentage of bone surface covered by osteoclasts was also measured. This was calculated as the sum of the lengths of the osteoclasts containing lacunae (active eroded area) divided by the total trabecular bone perimeter. Individual osteoclast activity was calculated as the ratio between osteoclastic resorption area (micrometers) and osteoclast number (43). These histomorphometric parameters adhere to the recommended American Society of Bone and Mineral Research nomenclature (25).
Statistics.
The statistical significance of the data was determined by Fisher’s one-way analysis of variance and Student’s t test. Significance was determined at the P < 0.01 level.
RESULTS
Since previous data (3, 35) demonstrated that E. coli LPS and P. gingivalis LPS exhibit similar local inflammatory activities, we used E. coli LPS for the time course study and P. gingivalis LPS for the dose-response study.
Time course study.
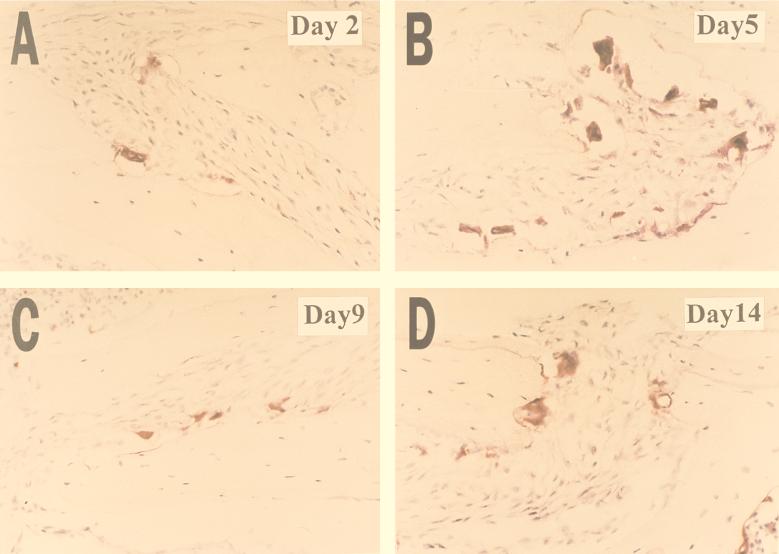
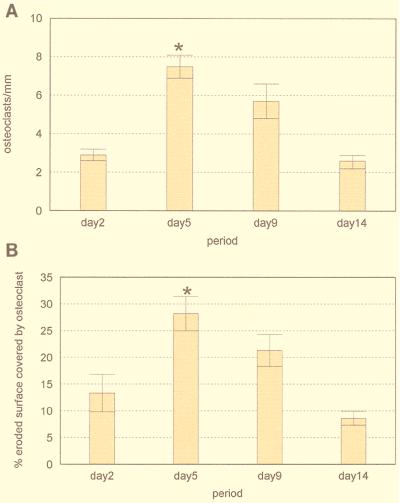
The number of osteoclasts per millimeter of trabecular bone surface and the percentage of bone surface covered by osteoclasts were assessed to quantify bone resorptive activity. A time course experiment was conducted to establish the occurrence of peak active bone resorption after a single injection of E. coli LPS (500 μg). Mice were sacrificed on days 2, 5, 9, and 14. On day 2 there were already signs of osteoclast activation, as indicated by the presence of multinucleated TRAP-positive cells and Howship’s lacunae (Fig. 1A). On day 5, there was a notable increase in the number of osteoclasts, with clear erosion of bone surfaces (Fig. 1B). A decrease in the number of osteoclasts was observed on day 9 (Fig. 1C). By day 14 smooth bone surfaces with very few osteoclasts were present, indicating that osteolytic activity had ceased and bone formation had occurred (Fig. 1D). Quantitative analysis indicated that the number of osteoclasts per millimeter of bone (osteoclast index) and the percentage of bone surface covered by osteoclasts were higher following injection of LPS for all time points compared to controls injected with saline alone (Fig. 2). There was a 2.5-fold increase in the osteoclast index from day 2 to 5 (P < 0.01) (Fig. 2A). By day 14 this had decreased so that there was no difference between the values on day 14 and day 2 (P > 0.01). Similarly, there was a 2.2-fold increase in the percentage of bone surface covered by osteoclasts from day 2 to 5 and a decrease thereafter (Fig. 2B). On the basis of these results, subsequent experiments focused on the 5-day time point.
FIG. 1.
Light micrographs of mouse calveriae injected with 500 μg of LPS. Histological sections of the calvarial bone were stained for TRAP activity. Red-staining cells are osteoclasts in Howship’s lacunae. Magnification, ×200.
FIG. 2.
Time course study of bone resorption induced by a local injection of 500 μg of E. coli LPS (n = 5). Bars represent means and standard errors. Mice were sacrificed after 2, 5, 9, and 14 days, and their calvarial bones were processed for histomorphometry. (A) Osteoclast index; (B) osteoclast-covered surface. The highest peak of bone resorption (P < 0.01) occurred on day 5. ∗, statistical significance at P ≤ 0.01.
Dose-response study.
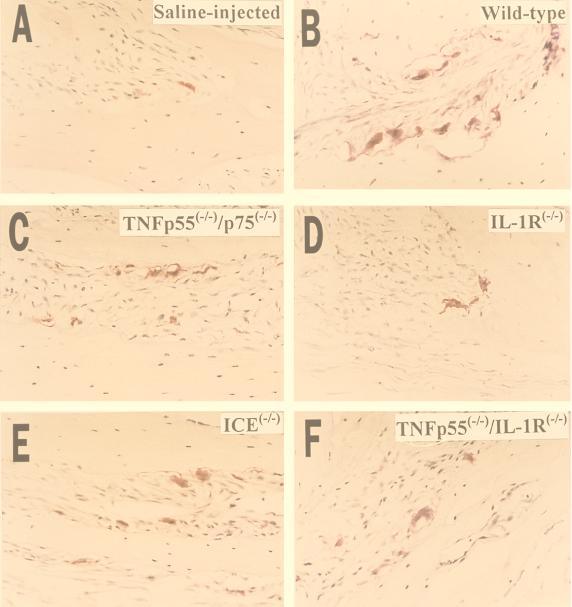
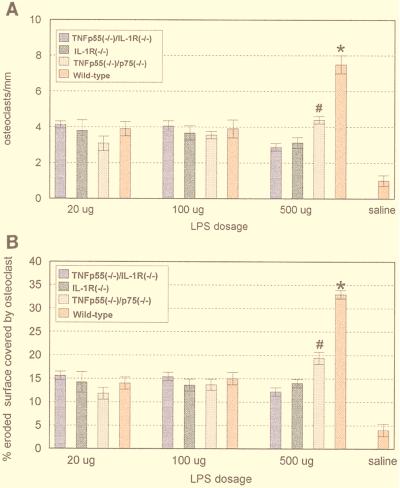
Wild-type mice and mice with targeted mutations of IL-1 receptors, TNF receptors, or ICE were injected with either 500, 100, or 20 μg of LPS purified from P. gingivalis and then sacrificed 5 days later. As shown in Fig. 3, the injection of 500 μg of LPS resulted in fewer osteoclasts in resorption lacunae for all of the mutant mice compared to matched control (wild-type) mice, indicating that at least some of the osteoclast activity was IL-1 or TNF dependent. These observations were further substantiated by a computer-assisted quantitative histomorphometric analysis. As shown in Fig. 4, wild-type mice injected with 500 μg of P. gingivalis LPS exhibited significant increases in the osteoclast index (7.5-fold) and the percentage of bone surface covered by osteoclasts (8-fold) compared to wild-type mice injected with saline alone. In all of the mutant mice the increase was less pronounced, ranging from three- to fourfold for the osteoclast index and from three- to fivefold for the percentage of bone surface covered by osteoclasts. When vehicle alone was injected, wild-type and mutant mice both had similar low values for osteoclast index and percentage of bone surface covered with osteoclasts (data not shown).
FIG. 3.
Light micrographs of calveriae of wild-type (A and B) and mutant (C to F) mice injected with 500 μg of LPS (B to F) or saline (A). Histological sections of the calvarial bone were stained for TRAP activity. Red-staining cells are multinucleated cells in Howship’s lacunae. Results similar to those in panel B were obtained for the wild-type controls corresponding to ICE−/− mice (data not shown). Magnification, ×200.
FIG. 4.
Dose-response study. Three doses (20, 100, and 500 μg) of P. gingivalis LPS were applied to the mouse calveriae. Bars represent means and standard errors. Fisher’s analysis of variance (P < 0.01) was performed on histomorphometric data for the calvariae of wild-type, TNF p55−/−/p75−/−, IL-1R−/−, and TNF p55−/−/IL-1R−/− mice (n = 6 in each group) injected with either P. gingivalis LPS or saline. (A) Osteoclast index. *, significant difference for the wild-type mice injected with 500 μg compared with the mutant mice (P ≤ 0.01); #, significant difference for the TNF p55−/−/p75−/− mice compared with the IL-1R−/− and TNF p55−/−/IL-1R−/− mice (P ≤ 0.01). (B) Osteoclast-covered surface. Symbols are as in panel A. A slight increase was seen in the TNF p55−/−/IL-1R−/− mice injected with 100 and 20 μg of LPS compared with the mice injected with 500 μg of LPS. For the osteoclast activity (micrometers per osteoclast), a statistically significant difference was observed only between the mice injected with 500 μg of LPS and the low-dose groups (data not shown).
When mutant and wild-type mice were injected with 500 μg of P. gingivalis LPS and analyzed, the results indicated that the deletion of TNF receptors (p55 and p75) causes a 41% inhibition of both the osteoclast index and the percentage of bone surface covered by osteoclasts compared to wild-type counterparts. In contrast, almost 60% inhibition was observed in IL-1R−/− or TNF p55−/−/IL-1R−/− mice, suggesting that IL-1 activity is relatively more potent than TNF activity in stimulating local induction of osteoclastogenesis (P ≤ 0.05). We also compared ICE−/− mice with their wild-type counterparts (Table 1). An inhibition of approximately 40% in osteoclast index and osteoclast-covered surface was observed in ICE−/− mice compared to wild-type animals.
TABLE 1.
Indices of bone resorption and osteoclast activity in mice injected with P. gingivalis LPS
| Mouse type | Amt (μg) of LPS injected | Osteoclast indexa (mean ± SE) | % Osteoclast-covered surfacea (mean ± SE) |
|---|---|---|---|
| ICE−/− | 500 | 4.7 ± 0.5 | 20 ± 2.2 |
| ICE wild type | 500 | 7.2 ± 0.6b | 32 ± 1.3b |
| ICE−/− | 100 | 2.75 ± 0.3 | 10 ± 0.9 |
| ICE wild type | 100 | 3.47 ± 0.7 | 13 ± 1.3 |
See Materials and Methods.
Significant difference (P ≤ 0.01) compared with mutant mice as calculated by Student’s t test.
When either 100 or 20 μg of P. gingivalis LPS was injected, a 3.5-fold increase in osteoclast index and bone surface covered by osteoclasts was measured for all experimental and wild-type mice compared with those injected with vehicle alone (Fig. 4). No significant differences were observed between mutants and between mutants and their wild-type counterparts for 20 and 100 μg of LPS (Fig. 4). This pattern was consistent for both the osteoclast index and the percentage of bone surface in contact with osteoclasts.
DISCUSSION
The results of the present study showed that mice lacking functional TNF and IL-1 receptors exhibited less osteoclast activity when challenged with a high dose of LPS than did wild-type mice. It has previously been reported that TNF signaling through the p55 TNF receptor contributes to LPS-stimulated bone resorption (1). We show that the effect of LPS on osteoclastogenesis is substantially mediated by TNF and IL-1 receptor signaling in response to local injection of relatively high doses of LPS. However, we also found that IL-1 receptor type 1 or TNF p55/p75 receptor signaling does not appear to be involved in the osteoclast response to moderate to low doses of LPS. In addition to significant differences between mice with targeted deletions and their wild-type counterparts, there were also significant differences between the mutant mice. For example, IL-1R−/− mice had significantly less LPS-mediated osteoclastic bone resorption than the TNF p55−/−/p75−/− group. Furthermore, TNF p55−/−/IL-1R−/− mice did not exhibit significantly greater LPS-stimulated osteoclast activity than the IL-1R−/− mice. Based on these findings, it appears that both TNF and IL-1 activities are important for high-dose-LPS-mediated bone resorption; however, IL-1 appears to be relatively more important.
IL-1 exists in two forms, the IL-1α molecule and IL-1β molecule, which have similar activities. Recent reports indicate that IL-1β is involved in some pathological conditions associated with increased bone loss (20, 21) and has significant effects on enhancing granulocyte-macrophage colony-stimulating factor production in bone marrow cell cultures (18). ICE is an intracellular protease which cleaves pro-IL-1β into its mature and active form and as such is responsible for the regulation of IL-1β production by cells. To investigate the relative contribution of processed, active IL-1β, osteoclast activity in ICE−/− mice and wild-type controls was assessed. These mutant mice experienced a 38% decrease in bone resorption, demonstrating that IL-1β is important in mediating LPS-stimulated osteoclastogenesis. However, the finding that IL-1R−/− mice had an even greater decrease in osteoclastogenesis suggests that both IL-1α and IL-1β may be important in mediating LPS-induced osteoclast activity.
As previously stated, our results demonstrated a 60% decrease in bone resorption and a 62% reduction in osteoclast number when both TNF p55 and IL-1 type I receptors were absent. However, active bone resorption was still significantly higher in mutant mice receiving LPS than in normal mice receiving only saline solution injections. Thus, additional LPS-induced osteoclastogenic mechanisms which are independent from IL-1 and TNF signaling are likely to exist. This concept is further supported by the fact that IL-1 and TNF activity appeared to play a role in LPS-mediated osteoclastogenesis only at a high dose of LPS (500 μg), while no statistical differences between mutant and wild-type animals were observed at lower LPS doses (20 or 100 μg). Girasole and colleagues reported that osteoclasts formed in the presence of IL-11, a cytokine produced mainly by marrow stromal cells and osteoclasts, were capable of bone resorption and were unaffected by inhibitors of IL-1 and TNF (11). Thus, there may be a threshold for the concentration of LPS needed to induce sufficient levels of IL-1 and TNF to stimulate osteoclastogenesis. At LPS concentrations below this threshold, the induction of other cytokines such as IL-11 may be largely responsible for LPS-induced osteoclast formation and activation.
The results presented here demonstrated that at high concentrations of LPS, osteoclastic bone resorption is caused primarily by enhanced osteoclastogenesis mediated through TNF and IL-1 receptor signaling. At low concentrations, other pathways independent of TNF and IL-1 activity may be involved. These pathways call for further investigation.
ACKNOWLEDGMENTS
We are indebted to J. Peschon at Immunex Corp. for the generous gift of the transgenic IL-1R−/−, TNF p55−/−/p75−/−, and TNF p55−/−/IL-1R−/− mice and to BASF Corp. for the ICE−/− mutant mice.
This study was supported by National Institutes of Health grants DE10709 (to S. Amar) and DE11254 (to D. T. Graves).
REFERENCES
- 1.Abu-Amer Y, Ross F P, Edwards J, Teitelbanm S L. LPS-stimulated osteoclastogenesis is mediated by TNF via its p55 receptor. J Clin Investig. 1997;100:1557–1565. doi: 10.1172/JCI119679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal S, Piesco N P, Johns L P, Riccelli A E. Differential expression of IL-1 beta, TNF- alpha, IL-6 and IL-8 in human monocytes in response to lipopolysaccharides from different microbes. J Dent Res. 1995;74:1057–1065. doi: 10.1177/00220345950740040501. [DOI] [PubMed] [Google Scholar]
- 3.Amar S, Van Dyke T E, Eugster H P, Schultze N, Koebel P, Bluethmann H. Tumor necrosis factor (TNF)-induced cutaneous necrosis is mediated by TNF receptor 1. J Inflamm. 1996;47:180–189. [PubMed] [Google Scholar]
- 4.Assuma R, Oates T, Cochran D, Amar S, Graves D T. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;16:403–409. [PubMed] [Google Scholar]
- 5.Aznar C, Fitting C, Cavaillon J M. LPS-induced production of cytokines by bone marrow-derived macrophages: destruction between intracellular IL-1 production and IL-1 release. Cytokine. 1990;2:259–265. doi: 10.1016/1043-4666(90)90026-p. [DOI] [PubMed] [Google Scholar]
- 6.Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res. 1993;28:500–510. doi: 10.1111/j.1600-0765.1993.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 7.Bom-van Noorloos A A, van der Meer J W, van de Gevel J S, Schepens E, van Steenbergen T J, Burger E H. Bacteroides gingivalis stimulates bone resorption via interleukin-1 production by mononuclear cells. The relative role for B. gingivalis endotoxin. J Clin Periodontol. 1990;17:409–413. doi: 10.1111/j.1600-051x.1990.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 8.Boyce B F, Aufdemorte T B, Garrett I R, Yates A J, Mundy G R. Effects of interleukin-1 on bone turnover in normal mice. Endocrinology. 1989;125:1142–1150. doi: 10.1210/endo-125-3-1142. [DOI] [PubMed] [Google Scholar]
- 9.Dinarrello C A, Cannon J G, Mier J W, Bernheim H A, LoPreste G, Lynn D L, Love R N, Webb A C, Auron P E, Reuben R C, et al. Multiple biological activities of human recombinant IL-1. J Clin Investig. 1986;77:1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galanos C, Freudenberg M A. Mechanism of endotoxin shock and endotoxin hypersensitivity. Immunobiology. 1993;187:346–356. doi: 10.1016/S0171-2985(11)80349-9. [DOI] [PubMed] [Google Scholar]
- 11.Girasole G, Passeri G, Jilka R L, Manolagas S C. Interleukin-11: a new cytokine critical for osteoclast development. J Clin Investig. 1994;93:1516–1524. doi: 10.1172/JCI117130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaccum M B, Stocking K L, Charrier K, Smith J L, Willis C R, Maliszewski C, Livingston D J, Peschon J J, Morrissey P J. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunology. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 13.Gowen M, Meikle M C. Stimulation of bone resorption in vitro by a non-prostanoid factor released by human monocytes in culture. Biochim Biophys Acta. 1983;762:471–474. doi: 10.1016/0167-4889(83)90014-9. [DOI] [PubMed] [Google Scholar]
- 14.Graves D T, Delima A, Assuma R, Amar S, Oates T, Cochran D. IL-1 and TNF antagonists inhibit the progression of inflammatory cell infiltration toward alveolar bone in experimental periodontitis. J Periodontol. 1998;69:1419–1424. doi: 10.1902/jop.1998.69.12.1419. [DOI] [PubMed] [Google Scholar]
- 15.Hanazawa S, Amano S, Nakada K, Ohmori Y, Miyoshi T, Hirose K, Kitano S. Biological characterization of interleukin-1-like cytokine produced by cultured bone cells from newborn mouse calvaria. Calcified Tissue Int. 1987;41:31–37. doi: 10.1007/BF02555128. [DOI] [PubMed] [Google Scholar]
- 16.Hausmann E, Nair B C, Dziak R. Bacterial components which result in bone loss. In: Genco R J, Mergenhagen S E, editors. Host-parasite interactions in periodontal diseases. Washington, D.C: American Society for Microbiology; 1982. pp. 151–159. [Google Scholar]
- 17.Hausmann E, Weinfeld N. Human dental plaque: stimulation of bone resorption in tissue culture. Arch Oral Biol. 1973;18:1509–1515. doi: 10.1016/0003-9969(73)90126-x. [DOI] [PubMed] [Google Scholar]
- 18.Ishimi Y, Miyaura C, Jin C H, Akatsu T, Abe E, Nakamura Y, Yamaguchi A, Yoshiki S, Matsuda T, Hirano T, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- 19.Johnson R A, Boyce B F, Mundy G R, Roodman G D. Tumors producing human TNF induce hypercalcemia and osteoclastic bone resorption in nude mice. Endocrinology. 1989;124:1424–1427. doi: 10.1210/endo-124-3-1424. [DOI] [PubMed] [Google Scholar]
- 20.Kimble R B, Matayoshi A B, Vannice J L, Kung V T, Williams C, Pacifici R. Simultaneous block of IL-1 and TNF is required to completely prevent bone loss in the early postovariectomy period. Endocrinology. 1995;136:3054–3061. doi: 10.1210/endo.136.7.7789332. [DOI] [PubMed] [Google Scholar]
- 21.Koide M, Suda S, Saitoh S, Ofuji Y, Suzuki T, Yoshie H, Takai M, Ono Y, Taniguchi Y, Hara K. In vivo administration of IL-1 beta accelerates silk ligature-induced alveolar bone resorption in rats. J Oral Pathol Med. 1995;24:420–434. doi: 10.1111/j.1600-0714.1995.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskin M, Rodman L, Salfeld J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 23.Morrison D C, Ryan J L. Endotoxins and disease mechanisms. Ann Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 24.Orcel P, Feuga M. Local bone injection of LPS and M-CSF resorption by different pathways in vivo in rats. Am J Physiol. 1993;264:E391–E397. doi: 10.1152/ajpendo.1993.264.3.E391. [DOI] [PubMed] [Google Scholar]
- 25.Parfitt A M, Drezner M K, Glorieux F H, Kanis J A, Malluche H, Meunier P J, Ott S M, Recker R R. Bone histomorphometry: standardization of nomenclature, symbols and units. J Bone Min Res. 1987;6:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 26.Peschon J J, Torrance D S, Stocking K L, Glaccum M B, Otten C, Willis C R, Charrier K, Morrissey P J, Ware C B, Mohler K M. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 27.Pfeilschifter J, Chenu C, Bird A, Mundy G R, Roodman G D. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclast-like cells in vitro. J Bone Min Res. 1989;4:113–118. doi: 10.1002/jbmr.5650040116. [DOI] [PubMed] [Google Scholar]
- 28.Pinckard J K, Kathleen C F, Sheehan C D, Arthur C D, Schreiber R D. Constitutive shedding of both p55 and p75 murine TNF receptors in vivo. J Immunol. 1997;158:3869–3873. [PubMed] [Google Scholar]
- 29.Roodman G D. Advances in bone biology: the osteoclast. Endocrine Rev. 1996;17:308–332. doi: 10.1210/edrv-17-4-308. [DOI] [PubMed] [Google Scholar]
- 30.Rothe J, Lesslauer W, Lotscher H, Lang Y, Kobel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz K, Bluethmann H. Mice lacking the tumor necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 31.Ruby J, Bluethmann H, Peschon J J. Antiviral activity of tumor necrosis factor (TNF) is mediated via p55 and p75 TNF receptors. J Exp Med. 1997;186:1591–1596. doi: 10.1084/jem.186.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabatini M, Boyce B, Aufdemorte T, Bonewald L, Mundy G R. Infusions of recombinant human IL-1 α and β cause hypercalcemia in normal mice. Proc Natl Acad Sci USA. 1988;85:5235–5239. doi: 10.1073/pnas.85.14.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saglie F R, Simon K, Merrill J, Koeffler H P. LPS from Actinobacillus actinomycetemcomitan stimulates macrophages to produce IL-1 and TNF mRNA and protein. Oral Microbiol Immunol. 1990;5:256–262. doi: 10.1111/j.1399-302x.1990.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz J, Stinson F L, Parker R B. The passage of tritiated bacterial endotoxin across intact gingival crevicular epithelium. J Periodontol. 1972;43:270–276. doi: 10.1902/jop.1972.43.5.270. [DOI] [PubMed] [Google Scholar]
- 35.Shapira L, Takashiba S, Amar S, Van Dyke T E. Porphyromonas gingivalis lipopolysaccharide stimulation of human monocytes: dependence on serum and CD14 receptor. Oral Microbiol Immunol. 1994;9:112–117. doi: 10.1111/j.1399-302x.1994.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 36.Sims J E, Gayle M A, Slack J L, Alderson M R, Bird T A, Giri J G, Colotta F, Re F, Manotvani A, Shanebeck K, Grabstein K H, Dower S K. IL-1 signaling occurs exclusively via the type I receptor. Proc Natl Acad Sci USA. 1993;90:6155–6159. doi: 10.1073/pnas.90.13.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sismey-Durrant H J, Hopps R M. The effect of lipopolysaccharide from the oral bacterium Bacteroides gingivalis on osteoclastic resorption of sperm-whale dentine slices in vitro. Arch Oral Biol. 1987;32:911–913. doi: 10.1016/0003-9969(87)90106-3. [DOI] [PubMed] [Google Scholar]
- 38.Suda T, Takahashi N, Martin T J. Modulation of osteoclast differentiation. Endocrine Rev. 1992;13:66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- 39.Takada H, Mihara J, Morisaki I, Hamada S. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect Immun. 1991;59:295–301. doi: 10.1128/iai.59.1.295-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takashi T, Miyanchi M, Ogawa I, Ito H, Kobayashi J, Nikai H. Reactive change in proliferative activity of junctional epithelium after topical application of LPS. J Periodontol. 1997;68:531–535. doi: 10.1902/jop.1997.68.6.531. [DOI] [PubMed] [Google Scholar]
- 41.Thomson S M, Mundy G R, Chambers T J. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987;138:775–779. [PubMed] [Google Scholar]
- 42.Umezu A, Kaneko N, Toyama Y, Watanabe Y. Appearance of osteoclasts by injections of LPS in rat periodontal tissue. J Periodontal Res. 1989;24:378–383. doi: 10.1111/j.1600-0765.1989.tb00886.x. [DOI] [PubMed] [Google Scholar]
- 43.Uy H L, Dallas M, Calland J W, Boyce B F, Mundy G R, Roodman G D. Use of an in vivo model to determine the effects of interleukin-1 on cells at different stages in the osteoclast lineage. J Bone Min Res. 1995;10:295–301. doi: 10.1002/jbmr.5650100217. [DOI] [PubMed] [Google Scholar]
- 44.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. In: Whistler R L, editor. Methods in carbohydrate chemistry. 5th ed. New York, N.Y: Academic Press Inc.; 1965. pp. 157–162. [Google Scholar]
- 45.Williams R C. Periodontal disease. N Engl J Med. 1990;322:373–382. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- 46.Zubery Y, Dunstan C R, Story B M, Kesavalu L, Ebersole J L, Holt S C, Boyce B F. Bone resorption caused by three periodontal pathogens in vivo in mice is mediated in part by prostaglandin. Infect Immun. 1999;66:4158–4162. doi: 10.1128/iai.66.9.4158-4162.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]