Abstract
The NOD-like receptor pyrin domain 3 (NLRP3) inflammasome is activated during atherogenesis, but how this occurs is unclear. Here, we explored the mechanisms activating and regulating NLRP3 inflammasomes via the acid sphingomyelinase (ASM)-ceramide signaling pathway. As a neointima formation model, partial left carotid ligations were performed on endothelial cell (EC)-specific ASM transgene mice (Smpd1trg/ECcre) and their control littermates (Smpd1trg/WT and WT/WT) fed on the Western diet (WD). We found neointima formation remarkably increased in Smpd1trg/ECcre mice over their control littermates. Next, we observed enhanced colocalization of NLRP3 versus adaptor protein ASC (the adaptor molecule apoptosis-associated speck-like protein containing a CARD) or caspase-1 in the carotid ECs of WD-treated Smpd1trg/ECcre mice but not in their control littermates. In addition, we used membrane raft (MR) marker flotillin-1 and found more aggregation of ASM and ceramide in the intima of Smpd1trg/ECcre mice than their control littermates. Moreover, we demonstrated by in situ dihydroethidium staining, carotid intimal superoxide levels were much higher in WD-treated Smpd1trg/ECcre mice than in their control littermates. Using ECs from Smpd1trg/ECcre and WT/WT mice, we showed ASM overexpression markedly enhanced 7-ketocholesterol (7-Ket)-induced increases in NLRP3 inflammasome formation, accompanied by enhanced caspase-1 activity and elevated interleukin-1β levels. These 7-Ket-induced increases were significantly attenuated by ASM inhibitor amitriptyline. Furthermore, we determined that increased MR clustering with NADPH oxidase subunits to produce superoxide contributes to 7-Ket-induced NLRP3 inflammasome activation via a thioredoxin-interacting protein-mediated controlling mechanism. We conclude that ceramide from ASM plays a critical role in NLRP3 inflammasome activation during hypercholesterolemia via MR redox signaling platforms to produce superoxide, which leads to TXNIP dissociation.
Supplementary keywords: carotid artery, endothelial cells, lysosomal ceramide, redox signaling, amitriptyline, NADPH, atherogenesis, 7-ketocholesterol, IL-1β, caspase-1
Abbreviations: Ami, amitriptyline; ASC, the adaptor molecule apoptosis-associated speck-like protein containing a CARD; ASM, acid sphingomyelinase; CTXB, cholera toxin B; DHE, dihydroethidium; EC, endothelial cell; FLICA, fluorescent labeled inhibitor of caspase; IHC, immunohistochemistry; IL, interleukin; 7-Ket, 7-ketocholesterol; MR, membrane raft; ND, normal diet; NLRP3, NOD-like receptor pyrin domain 3; NOX, NADPH oxidase; PLCA, partial ligated carotid artery; ROS, reactive oxygen species; α-SMA, α-smooth muscle actin; TXNIP, thioredoxin-interacting protein; vWF, von Willebrand factor; WD, Western diet
It has been demonstrated that activation of NOD-like receptor pyrin domain 3 (NLRP3) inflammasome is importantly involved in atherogenesis (1). During NLRP3 inflammasome activation, NLRP3 protein as a sensor protein recognizes endogenous and exogenous danger signals and recruits the accumulation of the adaptor protein ASC (the adaptor molecule apoptosis-associated speck-like protein containing a CARD) and the effector protein procaspase-1 to form a proteolytic complex. In such a complex, procaspase-1 is activated to form active caspase-1 leading to the production of mature interleukin (IL)-1β and IL-18 by cleavage of their precursors (2). Recent studies have indicated that endothelial dysfunction and consequent vascular injury and inflammation are associated with the formation and activation of NLRP3 inflammasome (3, 4). In this regard, endothelial NLRP3 inflammasome was found to be activated upon different proatherogenic stimuli such as cholesterol crystals (5), ATP (6), uric acid (7), hyperhomocysteinemia (8), and damage-associated molecular patterns (9) via different pathways. However, the precise mechanisms by which NLRP3 inflammasomes are activated and regulated remain poorly understood.
In general, NLRP3 inflammasome activation has been proposed to relate to increased ionic potassium flux, enhanced production of reactive oxygen species (ROS), and lysosomal rupture (2, 10). Our recent studies have demonstrated the crucial role of ROS-dependent NLRP3 inflammasomes in the regulation of functions of glomeruli or arterial endothelial cells (ECs) and the development of atherosclerosis during hyperlipidemia (3, 11) and the progression of glomerular sclerosis during hyperhomocysteinemia (12, 13, 14). Many endothelial injurious factors including FasL, TNF-α, oxidized LDL, visfatin, and endostatin were found to induce the formation of membrane raft (MR) clusters, in which NADPH oxidase (NOX) subunits such as gp91 and p47 are aggregated to amplify NOX activity in arterial ECs (15). These membrane MR-NOX clusters or complexes that possess a redox signaling function have been referred to as MR redox signaling platforms (16, 17, 18). This led us to hypothesize that the MR redox signaling platforms may play a critical role in endothelial NLPR3 inflammasomes, which have been tested in the present study.
Acid sphingomyelinase (ASM, a lysosomal hydrolase encoded by the Smpd1 gene) has been shown to importantly participate in the aggregation of NOX subunits in ceramide-enriched MR microdomains promoting the formation of MR redox signaling platform in ECs (15, 19, 20). Upon stimulation, lysosome traffick and fuses to the MR area via a SNARE-centered exocytic machinery (21, 22), where ASM hydrolyzes sphingomyelin into ceramide serving to reorganize and cluster MR-associated signaling molecules (e.g., NOX subunits) in ceramide-enriched MR microdomains (23). Our recent study linked the ASM-MR redox signaling with endothelial NLRP3 inflammasome activation, which was demonstrated by the fact that deficiency of the Smpd1 gene inhibited the activation of endothelial NLRP3 inflammasomes and neointimal lesion formation in a mouse model of Niemann-Pick disease using Smpd1 gene global knockout mice (24). The role of ASM-MR redox signaling in NLRP3 inflammasome activation was confirmed in cultured ECs (24). However, the usage of the global gene knockout mouse model could not elucidate whether the ASM-MR redox signaling is derived from an endothelial source that contributes to endothelial NLRP3 inflammasome activation and neointimal lesion formation in vivo. In this study, we generated EC-specific Smpd1 transgenic mice (Smpd1trg/ECcre) and examined whether the EC-specific overexpression of the Smpd1 gene could enhance the production of ceramide and the formation of MR redox signaling platforms, promote the activation of endothelial NLRP3 inflammasomes, and thereby result in endothelial dysfunction and atherogenesis. Moreover, using the primary cultured ECs isolated from Smpd1trg/ECcre mice, we examined whether ASM-MR redox signaling is coupled with endothelial NLRP3 inflammasome activation via a redox sensor, thioredoxin-interacting protein (TXNIP).
Materials and Methods
Mice
All animal experiments were performed following the National Institutes of Health guidelines for the care and use of laboratory animals. The protocols were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. Eight- to 12-week-old male and female C57BL/6J WT (WT/WT), Smpd1trg/WT mice, and EC-specific Smpd1 transgenic mice were used in the current study. EC-specific Smpd1 transgenic mice were generated by crossbreeding EC-specific Cre transgenic (Tie2 [tunica intima endothelial kinase 2]-Cre) mice with Smpd1trg/WT mice and genotyped in a similar method as we described for other tissue-specific transgenic mice with Smpd1 gene overexpression in podocytes (25) and smooth muscle cells (26). Mice were maintained in a controlled environment of 20°C and 40–50% humidity, with a 12-h light/dark cycle. Mice were separated into six groups randomly and fed with the WD (0.21% cholesterol, D12079; Research Diet) for 60 days.
Partial ligated carotid artery
Partial ligated carotid artery (PLCA) surgery was performed as previously reported by others (27, 28, 29, 30, 31). The surgery was performed after 30 days of WD treatment. Briefly, mice were anesthetized with 2% isoflurane inhalation for 5 min and epilated in the neck and then continued being anesthetized through a nose cone. A ventral midline incision was made in the neck and disinfected with 70% ethanol, and then the muscle layers were separated with curved forceps to expose the left carotid artery after blunt dissection. The external carotid, internal carotid, and occipital artery were ligated with a piece of 6.0 silk suture, whereas the superior thyroid artery was left intact, which provided the sole source for blood circulation. The right carotid artery was not ligated and served as an internal control. After closing the incision and disinfection, the mice were kept on a heating pad until they gained consciousness. After 4 weeks of PLCA, mice were sacrificed, and both their carotid arteries were perfused and isolated for frozen sections and paraffin sections. The slides were used for immunohistochemistry (IHC), dual fluorescence staining, and confocal analysis, respectively.
Morphologic examination and medial thickening analysis
To study the morphological changes, H&E staining of carotid artery sections was used as described previously (32). Briefly, the carotid artery was perfused with cold PBS for 5 min and 4% cold paraformaldehyde for another 5 min. Then the carotid artery was separated and stored into 10% neutral-buffered formalin. After at least 48 h, the tissues were embedded in paraffin and cut into 5 μm serial sections for histopathological evaluation. For H&E staining, the sections were heated for 10 min at 65°C, and deparaffinization was performed twice in 100% xylene for 10 min. The samples were rehydrated with 100, 95, 90, and 75% ethanol to tap water and immersed in hematoxylin and hydrochloride alcohol. Once the nucleus turned blue, the sections were stained with eosin. After that, the sections were rinsed with running tap water and dehydrated with ethanol of 75, 90, 95, and 100%. Finally, dibutyl phthalate polystyrene xylene was used to mount the slides. Intimal-medium thickening of carotid arteries was measured using Image-Pro Plus 6.0 software (Media Cybernetics, Inc, Bethesda, MD).
Immunofluorescence staining
Cells cultured on the sterilized cover slides or frozen carotid artery sections were rinsed three times for 2 min with PBS and fixed in 4% paraformaldehyde in PBS for 15 min. Then the samples were washed three times with PBS for 10 min and permeabilizated with 0.1% Triton X-100 in PBS by washing for 10 min followed three times for 5 min with PBS. After that, the samples were blocked with 3% bovine serum albumin for 1 h. Primary antibody dilution and catalog information are NLRP3 (1:200 dilution, catalog no.: MAB7578; R&D Systems), ASC (1:200 dilution, catalog no.: SAB4501314; Santa Cruz), caspase-1 (1:200 dilution, catalog no.: SC-392736; Santa Cruz), flotillin-1 (1:200 dilution, catalog no.: 610820; BD Biosciences; catalog no.: ab41927; Abcam), gp91 (1:200 dilution, catalog no.: 611415; BD Biosciences), p47 (1:200 dilution, catalog no.: 610355; BD Biosciences), ASM (1:200 dilution, catalog no.: LS-C334919; LSBio), cholera toxin B (CTXB; 1:2000 dilution, catalog no.: C34775; Invitrogen), ZO-1 (1:200 dilution, catalog no.: 40-2200; Invitrogen), ZO-2 (1:200 dilution, catalog no.: 71-1400; Invitrogen), von Willebrand factor (vWF; 1:300 dilution, catalog no.: ab11713; Abcam), ceramide (1:200 dilution, catalog no.: ALX-804-196; Enzo). Primary antibodies were incubated overnight at 4°C followed by incubation with secondary antibodies for 1 h at room temperature in the darkroom. Finally, the nucleus was stained by 4',6-diamidino-2-phenylindole and mounted with nail polish. Pictures were taken by a confocal laser scanning microscope (Fluoview FV1000; Olympus, Tokyo, Japan). The fluorescence intensity was measured and analyzed with ImageJ software (National Institutes of Health, Bethesda, MD). The colocalization of NLRP3 with ASC and caspase-1 was analyzed by the Image-Pro Plus, version 6.0 software. These summarized colocalization efficiency data were expressed as Pearson correlation coefficient as described previously (4, 33).
IHC
To perform the IHC staining, the sections in 10% formalin were dehydrated and embedded in paraffin. Immunohistochemical examinations were performed following the manufacturer's protocol for CHEMICON IHC Select HRP/DAB Kit (EMD Millipore, MA). Briefly, after antigen recovery was performed with pH 6.1 citrate buffer, the sections were blocked by using 3% bovine serum albumin for 1 h at room temperature. After that, primary antibodies (IL-1β, 1:200 dilution, catalog no.: P420B; Invitrogen and α-smooth muscle actin [α-SMA], 1:2000 dilution, catalog no.: ab5694, Abcam) were developed overnight at 4°C and subsequently with biotinylated secondary antibodies and a streptavidin peroxidase complex (PK-7800; Vector Laboratories, Burlingame, CA) for 1 h separately. Finally, the sections were counterstained with hematoxylin and dehydrated as well as mounted using dibutyl phthalate polystyrene xylene. The area percentage of the positive staining was examined using Image-Pro Plus 6.0 software.
In situ analysis of caspase-1 activity
The caspase-1 activity in the carotid arterial endothelium was analyzed in situ by labeling the active caspase-1 proteins with Fluorescent Labeled Inhibitor of Caspases (FLICA™) probes (ImmunoChemistry Technologies, LLC, Bloomington, MN) as described previously (34). The FLICA probes are comprised of three moieties including a caspase-1 recognition sequence tyrosine-valine-alanine-aspartic acid (YVAD) that binds to active caspase-1, a fluoromethyl ketone moiety that results in irreversible binding with the enzyme, and a fluorescent tag carboxyfluorescein reporter. After entering the cells, the FLICA reagent carboxyfluorescein-YVAD-fluoromethyl ketone becomes covalently coupled to the active caspase-1, whereas any unbound FLICA reagent diffuses out of the cell and is washed away. The remaining green fluorescent signal is a direct measure of the active caspase-1 enzyme activity in the cell or tissue samples. To detect caspase-1 activity in the carotid arterial endothelium, frozen artery section slides were first fixed in acetone and incubated overnight at 4°C with sheep anti-vWF (1:200 dilution; Abcam, Waltham, MA). These slides were then costained with fluorescence-conjugated anti-sheep secondary antibody and FLICA reagent (1:10 dilution) for 1.5 h at room temperature, washed, mounted, visualized, and analyzed by confocal microscopy as described previously.
Cell culture
Isolation of mouse carotid arterial ECs was performed and characterized as previously described (15). ECs were cultured in DMEM (Gibco), supplemented with 10% FBS (Gibco) and 1% penicillin-streptomycin (Gibco) in humidified 100% air and 5% CO2 mixture at 37°C. ECs were primed with a low dose of lipopolysaccharide (1 ng/ml) for 3 h before any experiments. For the proatherogenic stimulation, cells were treated with 7-ketocholesterol (7-Ket; 15 μg/ml) and then incubated for 21 h. In the case of inhibitors used, the cells were pretreated with amitriptyline (Ami) (20 μM) for 30 min.
Western blot analysis
Western blot analysis was performed as we described previously (35). Briefly, whole-cell lysate protein concentrations were measured and resuspended to 2 μg/μl. Cell lysates were run on an SDS-PAGE gel at a voltage of 100 V for 2 h and transferred into a polyvinylidene difluoride membrane at a voltage of 100 V for 1 h. The membrane was blocked with 5% nonfat milk for 1 h, followed by incubation with the following primary antibodies: cleaved caspase-1 (1:500 dilution; Santa Cruz) overnight at 4°C. Then the secondary antibody was labeled with HRP for 1 h at room temperature, and the membrane was washed three times with Tris-buffered saline with Tween-20. The intensity of the bands was quantified using ImageJ 6.0.
ELISA analysis of IL-1β secretions
The fresh culture medium was collected for IL-1β product measurement with an IL-1β ELISA kit according to the manufacturer's instructions as we described (36). In brief, at least 200 μl of the culture medium was used and incubated for 2 h at room temperature. Then, the samples were incubated with IL-1β conjugate for another 2 h at room temperature. Thorough washes were performed between and after the two incubations. About 100 μl of substrate solution was applied to generate chemiluminescence. Chemiluminescent absorbance was examined using a microplate reader at λ = 450.
ESR analysis of O2•− production
ESR detection of O2•− was performed as previously described (37). Briefly, cellular protein concentrations were measured in a lysis buffer and then prepared for analysis by resuspension in a modified Kreb's-Hepes buffer containing deferoxamine (100 μM) and diethyldithiocarbamate (5 μM). NOX-dependent O2•− production was examined by the addition of 1 mM NADPH as a substrate in 20 μg protein in the presence or the absence of superoxide dismutase (200 U/ml) to produce O2•−. Then, 10 mM 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine, a superoxide-specific spin-trapping compound, was added to trap O2•− before the mixture was loaded into the glass capillaries and immediately measured the O2•− production kinetically for 10 min using a Miniscope MS200 ESR spectrometer (Magnettech, Germany). The ESR settings were as follows: biofield, 3,350; field sweep, 60 G; microwave frequency, 9.78 GHz; microwave power, 20 mW; modulation amplitude, 3G; 4,096 points of resolution; receiver gain, 100; and kinetic time, 10 min. Superoxide dismutase-sensitive components of ESR signals were used to calculate changes in O2•− production or its level, which were shown as the fold changes of control.
In situ O2•− production in mouse carotid arteries
Dihydroethidium (DHE) is a lipophilic cell-permeable dye that is one of the most widely used fluorogenic probes for the detection of intracellular superoxide (38). In brief, the frozen tissue slides were incubated with 10 mM DHE in phosphate-buffered saline at room temperature for 30 min. Then, the slides were washed, fixed, and subjected to confocal microscopic analysis (Fluoview FV1000; Olympus).
Trichrome stain in mouse carotid arteries
Trichrome stain (Abcam; catalog no.: ab150686) was used to detect extracellular matrix and fibrosis following the manufacturer’s instructions. Deparaffinize sections and hydrate in distilled water. Preheat Bouin’s fluid in a water bath to 56–64°C in a fume hood or very well-ventilated area. Place slide in preheated Bouin’s fluid for 60 min followed by a 10-min cooling period. Rinse the slide in tap water until the section is completely clear. Rinse once in distilled water. Mix equal parts of Weigert’s (A) and Weigert’s (B) and stain the slide with working Weigert’s iron hematoxylin for 5 min. Rinse slide in running tap water for 2 min. Apply Biebrich Scarlet/Acid Fuchsin solution to slide for 15 min. Rinse the slide in distilled water. Differentiate in phosphomolybdic/phosphotungstic acid solution for 10–15 min or until the collagen is not red. Without rinsing, apply Aniline Blue solution to the slide for 5–10 min. Rinse the slide in distilled water. Apply acetic acid solution (1%) to slide for 3–5 min. Dehydrate very quickly in two changes of 95% alcohol, followed by two changes of absolute alcohol. Clear in xylene or xylene substitute and mount in synthetic resin. The images are quantified using ImageJ 6.0.
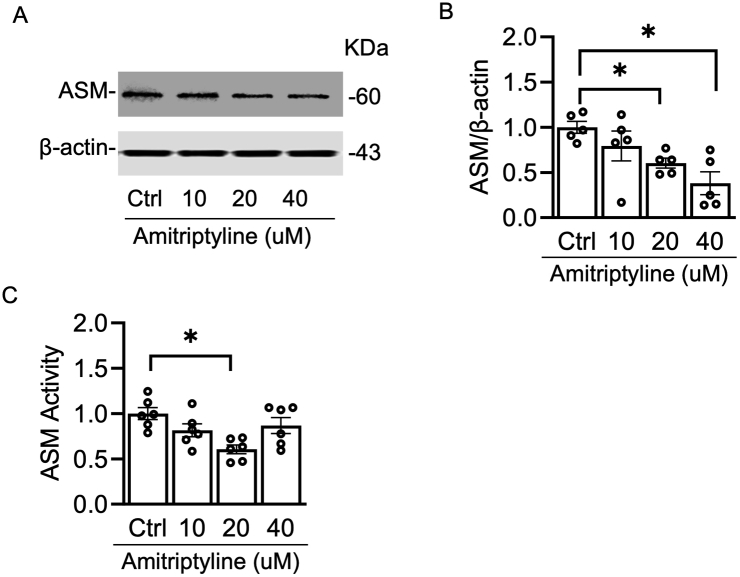
ASM activity assay
ASM activity was measured using the Acid Sphingomyelinase Assay Kit (catalog no.: ab190554; Abcam) following the manufacturer’s instructions. The WT/WT ECs were treated with different concentrations of Ami (Ctrl, 10, 20, 40 μM). Then cell homogenization was used for the activity assay.
Statistics
Data are presented as means ± SEM. Significant differences between and within multiple groups were examined using ANOVA for repeated measures, followed by Duncan's multiple range test. The statistical analysis was performed by Sigmaplot 12.5 software (Systat Software, San Jose, CA). P < 0.05 was considered statistically significant.
Results
EC-specific overexpression of the Smpd1 gene exacerbates atherosclerotic lesions in mouse carotid arteries
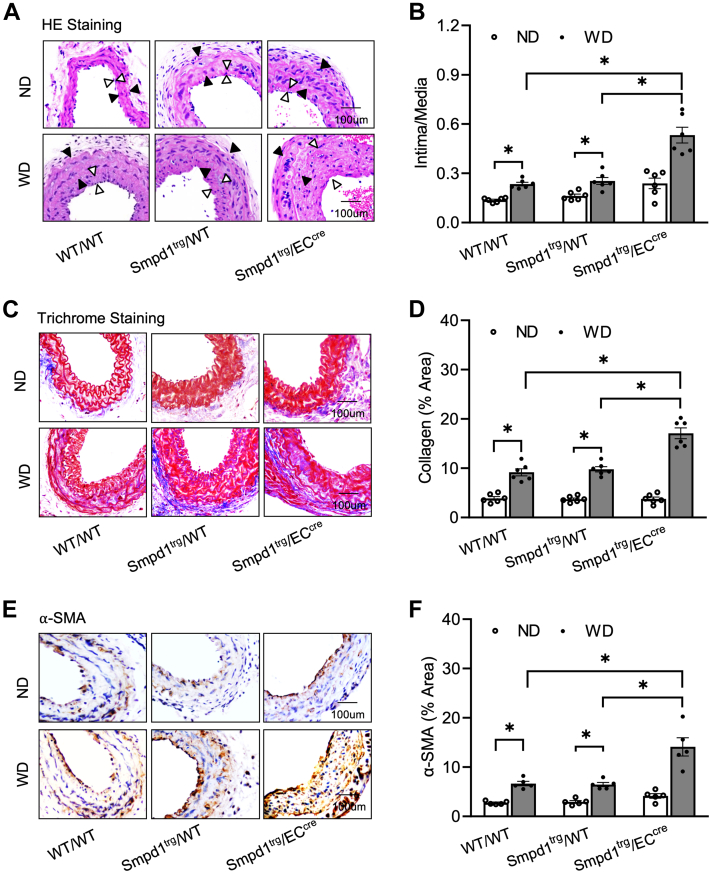
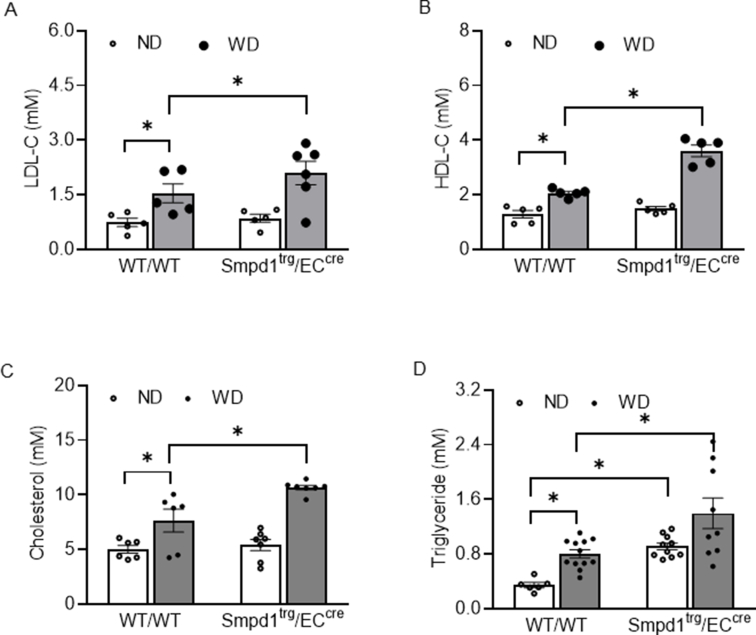
To induce neointima formation in carotid arteries, a PLCA model was surgically prepared in mice fed with a Western diet (WD) for 4 weeks as we described previously (3, 24, 39). Two control mice were used in our studies including WT (WT/WT) mice and floxed Smpd1 transgene (Smpd1trg/WT) mice. The EC-specific Smpd1 transgene (Smpd1trg/ECcre) mice were generated by crossing Smpd1trg/WT mice with EC-specific Tie2-Cre mice as described (40). Tie2 promoter-driven Cre recombinase activity results in overexpression of the Smpd1 gene in ECs. We first confirmed the animal model by measuring the serum lipids (LDL-C, HDL-C, total cholesterol, and triglyceride) and found that Smpd1trg/ECcre mice enhanced the lipid levels induced by the WD (supplemental Fig. S3A–D). As shown in Fig. 1A, the neointima formation was not observed in PLCAs of normal diet (ND)-treated WT/WT mice or Smpd1trg/WT mice. ND-treated Smpd1trg/ECcre mice exhibited mild neointima thickening, but there is no statistically significant increase in the ratio of intima/media (Fig. 1A, B). In contrast, WD treatment significantly increased the neointima formation and the intima/media ratio in PLCAs of WT/WT and Smpd1trg/WT mice, which were more significantly enhanced in Smpd1trg/ECcre mice (Fig. 1A). These data indicate that hypercholesterolemia-induced neointimal lesions were exacerbated when the Smpd1 gene is overexpressed in ECs.
Fig. 1.
Atherosclerotic lesions in the carotid arteries in EC-specific Smpd1 transgenic mice during hypercholesterolemia. A: H&E staining showing the neointima and media on the mouse carotid arterial wall. AOI: the media area (black arrowheads) and the intima area (white arrowheads). B: Quantitative analysis of atherosclerotic lesions in PLCA represented by calculation of the ratio between arterial intima and media area. C: Trichrome staining showing the collagen deposition on the mouse carotid arterial wall. D: Quantification of the blue color of Trichrome staining. E: Representative microscopic images of tissue slide with immunohistochemical staining that shows α-SMA expression on the arterial wall. F: Summarized data showing the density of α-SMA stained with anti-α-SMA antibody. Data are expressed as means ± SEM, n = 5. ∗P < 0.05 is defined as significant. The scale bar represents 100 μm.
To further investigate the histological changes in the carotid arteries of all groups, Masson’s trichrome staining and α-SMA expression on the carotid wall by IHC staining was performed. We also found that WD treatment significantly showed active collagen production (Fig. 1C, D) and increased α-SMA expression (Fig. 1E, F) on the carotid media and intima layer of WT/WT and Smpd1trg/WT mice, which were more significantly enhanced in Smpd1trg/ECcre mice.
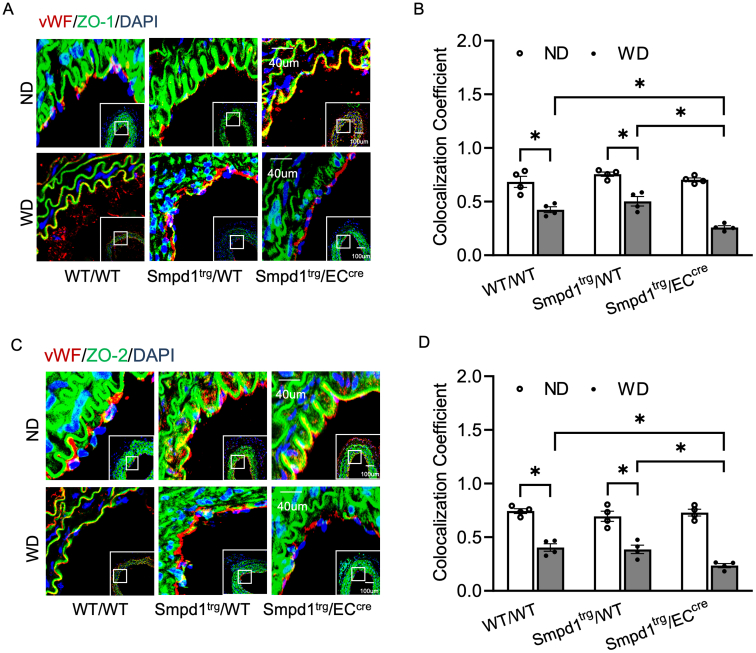
We also studied the effects of ASM overexpression on the endothelial injury. As shown in supplemental Fig. S1A, C, intact endothelium in ND-treated mice expresses similar higher ZO-1 and ZO-2 than in WD-treated mice; however, Smpd1trg/ECcre mice significantly attenuated WD-induced decrease. supplemental Fig. S1B, D showed clear changes.
EC-specific overexpression of the Smpd1 gene enhances endothelial inflammasome activation in the carotid arteries
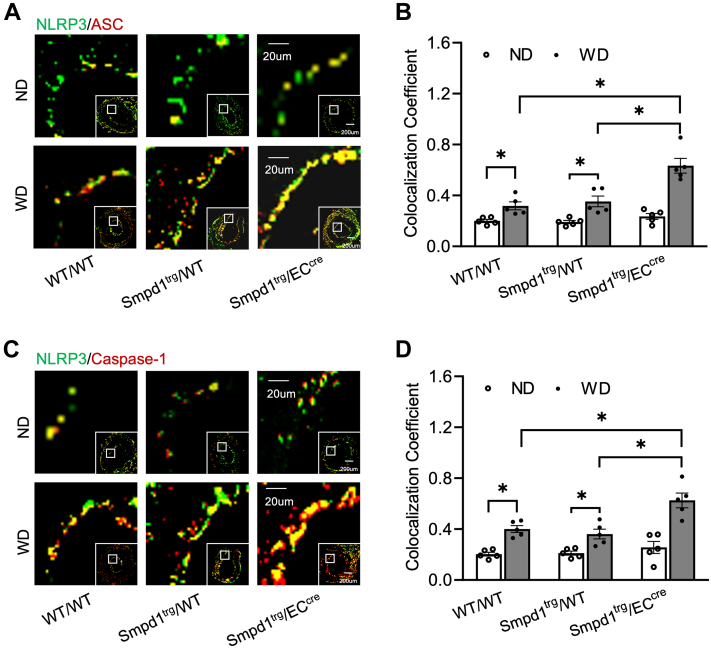
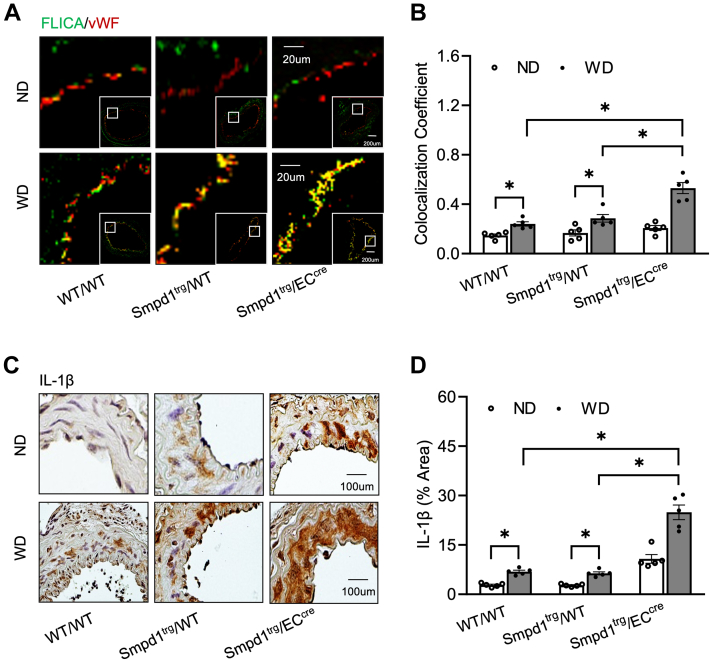
The NLRP3 inflammasome formation in PLCAs was analyzed by examining the colocalization of NLRP3 inflammasome subunits using confocal immunofluorescence microscopy. As shown in Figure 2A, C, in WT or Smpd1trg/WT control mice, WD significantly increased the colocalization of NLRP3 with ASC or caspase-1 compared with ND as shown by yellow spots in the intima of the carotid arteries. Moreover, the WD-induced increase in the colocalization of NLRP3 subunits was enhanced in Smpd1trg/ECcre mice compared with WT/WT or Smpd1trg/WT mice. The quantified colocalization coefficient data are summarized in Figure 2B, D. In addition to their formation, the activation of the NLRP3 inflammasome was analyzed by examining the caspase-1 activity using FLICA probes (Fig. 3A, B) and IL-1β expression using IHC staining (Fig. 3C, D). As shown in Figure 3A, B, there was no significant colocalization of FLICA with endothelial marker vWF in all mouse strains with ND treatment suggesting a basal level of NLRP3 inflammasome activity in these mice. In contrast, WD treatment significantly increased the FLICA/vWF colocalization in WT/WT and Smpd1trg/WT control mice, and such WD-induced colocalization was more significantly increased in Smpd1trg/ECcre mice. Accompanied by enhanced caspase-1 activity by WD, Smpd1trg/ECcre mice showed a more significant increase in the levels of IL-1β expression by WD in the carotid intima region compared with that in WT/WT and Smpd1trg/WT mice (Fig. 3C, D). Together, these data suggest that EC-specific Smpd1 gene overexpression enhances endothelial NLRP3 inflammasome formation and activation in the carotid arteries of mice during hypercholesterolemia.
Fig. 2.
Endothelial NLRP3 inflammasome formation in the carotid arteries of endothelium-specific Smpd1 transgenic mice during hypercholesterolemia. A: Representative fluorescent confocal microscope images displaying the yellow dots or patches showing the colocalization of NLRP3 (green) with ASC (red). B: The summarized data showing the colocalization coefficient of NLRP3 with ASC. C: Representative fluorescent confocal microscope images showing the colocalization of NLRP3 (green) with caspase-1 (red). D: The summarized data show the colocalization coefficient of NLRP3 with caspase-1. Data are expressed as means ± SEM, n = 5. ∗P < 0.05 is defined as significant. The scale bar represents 20 μm or 200 μm.
Fig. 3.
Endothelial NLRP3 inflammasome activation in the carotid arteries of EC-specific Smpd1 transgenic mice during hypercholesterolemia. A: Representative fluorescent confocal microscope images displaying the yellow dots or patches showing the colocalization of FLICA (green) with vWF (red). B: The summarized data show the colocalization coefficient of FLICA with vWF. C: Representative microscopic images of tissue slide with immunohistochemical staining that shows IL-1β accumulation on the arterial wall. D: Summarized data showing the density of IL-1β stained with selective anti-IL-1β antibody. Data are expressed as means ± SEM, n = 5. ∗P < 0.05 is defined as significant. The scale bar represents 20 μm or 200 μm for A and 100 μm for C.
EC-specific overexpression of Smpd1 gene amplifies ceramide-MR-mediated redox signaling in the carotid artery walls
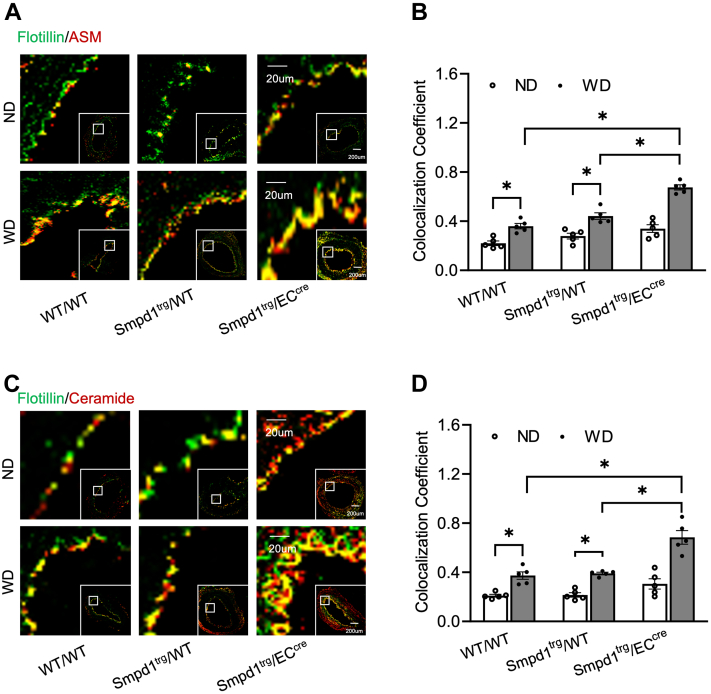
Earlier reports from our laboratory have demonstrated that ASM plays an essential role in forming ceramide-enriched MR clusters in ECs, in which NOX subunits are aggregated and activated leading to redox signaling and consequent endothelial NLRP3 inflammasome activation and arterial neointima formation during hypercholesterolemia (18, 24). Here, we observed that WD treatment increased the colocalization of ASM or ceramide within flotillin (MR marker) in the intima region of PLCAs of WT/WT or Smpd1trg/WT mice compared with ND-treated controls, whereas such increases were further enhanced in Smpd1trg/ECcre mice (Fig. 4A, C). These changes were quantified as a colocalization coefficient as summarized in Figure 4B, D. It should be noticed that under basal conditions (ND groups), Smpd1 gene overexpression only caused a statistically insignificant increase in either the ASM protein expression or ceramide in MR clusters in the carotid intima of Smpd1trg/ECcre mice compared with WT/WT or Smpd1trg/WT mice (Fig. 4A–D). Thus, our data suggest that EC-specific overexpression of the Smpd1 gene sensitizes the hypercholesterolemia-induced formation of ceramide-enriched MR clusters in the carotid arterial intima.
Fig. 4.
Accumulation of ASM and ceramide in endothelial membrane rafts on the carotid artery wall of EC-specific Smpd1 transgenic mice during hypercholesterolemia. A: Representative fluorescent confocal microscopic images showing the colocalization of MR marker, flotillin (green) with ASM (red). B: Summarized data showing the colocalization coefficient of flotillin with ASM. C: Representative fluorescent confocal microscopic images showing the colocalization of MR marker, flotillin (green) with ceramide (red). D: Summarized data showing the colocalization coefficient of flotillin with ceramide. Data are expressed as means ± SEM, n = 5. ∗P < 0.05 as defined is significant. The scale bar represents 20 μm or 200 μm.
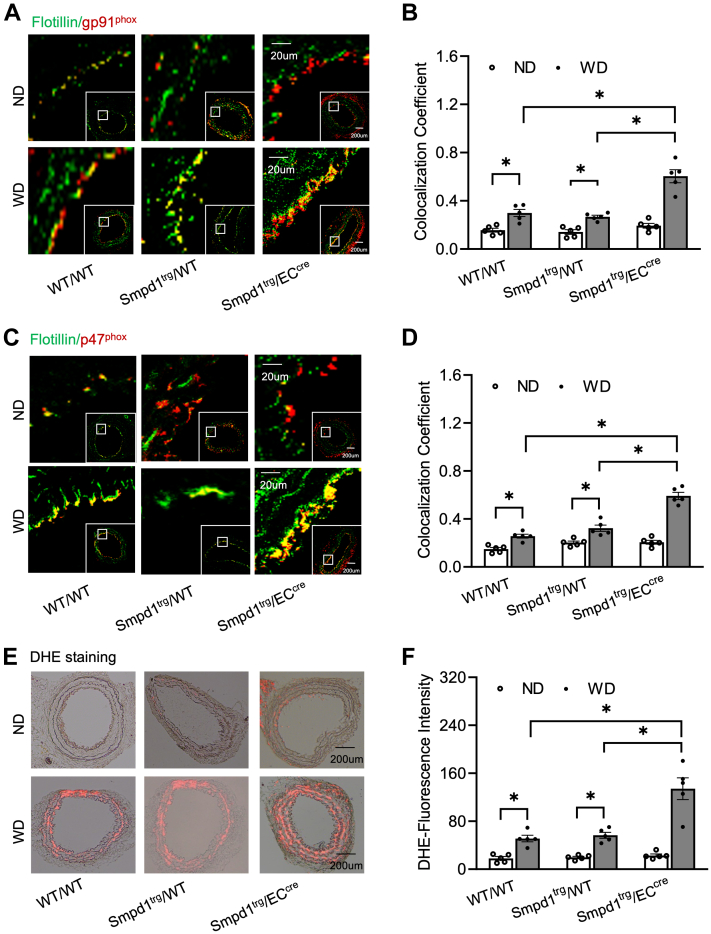
Consistent with the increased formation of ceramide-enriched MR clusters by WD in Smpd1trg/ECcre mice, we also observed that WD induced more significant increases in the aggregation of NOX subunits gp91 or p47 in ceramide-enriched MR clusters in these mice (Fig. 5A–D). Furthermore, O2•− production in situ was detected by DHE staining on the carotid arterial wall. As shown in Figure 5E, F, WD caused a more significant increase in DHE-O2•− signal in Smpd1trg/ECcre mice compared with that in WT/WT or Smpd1trg/WT mice. Together, these data suggest that hypercholesteremia-induced MR-redox signaling is amplified in the carotid arterial wall of mice when the Smpd1 gene is overexpressed in ECs.
Fig. 5.
NOX subunit aggregation and superoxide production in the carotid artery wall during hypercholesterolemia. A: Representative fluorescent confocal microscopic images showing the colocalization of MR marker, flotillin (green) with NOX subunit, gp91phox (red). B: Summarized data showing the colocalization coefficient of flotillin with gp91phox. C: Representative fluorescent confocal microscopic images showing the colocalization of flotillin (green) with NOX subunit, gp47phox (red). D: Summarized data showing the colocalization coefficient of flotillin with gp47phox. E: Representative merged images (DHE red fluorescence merged with transmission light) showing the O2•− production in situ in mouse carotid arteries. F: Summarized data showing the DHE fluorescence intensity. Data are expressed as means ± SEM, n = 5. ∗P < 0.05 is defined as significant. The scale bar represents 20 μm or 200 μm for A and C and 200 μm for E.
Smpd1 gene overexpression enhances NLRP3 inflammasome formation and activation in cultured ECs
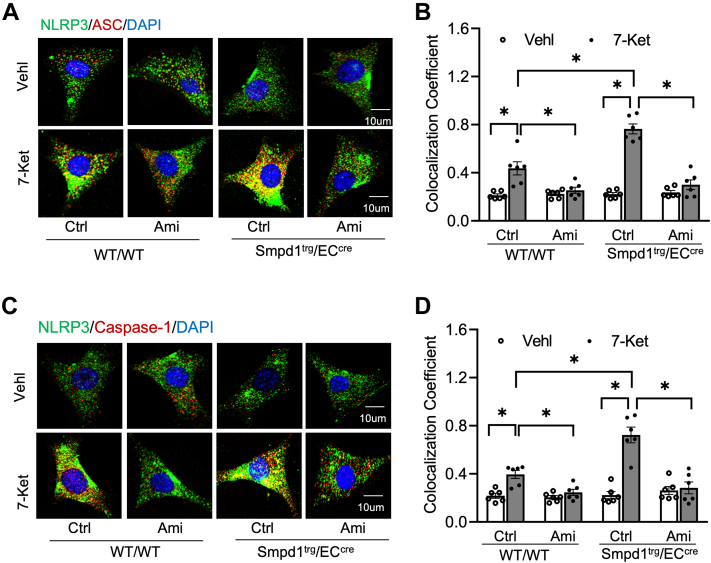
Next, we aimed to confirm the contribution of Smpd1 gene overexpression to NLRP3 inflammasome activation and formation in primary cultured ECs from carotid arteries of WT/WT and Smpd1trg/ECcre mice as previously described (41, 42). First, we confirmed that Ami, an ASM inhibitor, dose-dependently decreased ASM protein by Western blot and ASM activity detected by ELISA kit (supplemental Fig. S2A–C). Next, it was found that 7-Ket-induced colocalization of NLRP3 with ASC or caspase-1 was more significantly increased in Smpd1trg/ECcre ECs compared with that in WT/WT ECs, whereas pretreatment of both ECs with Ami, an ASM inhibitor, abolished such 7-Ket-induced increases (Fig. 6A–D).
Fig. 6.
Effects of ASM blockade on NLRP3 inflammasome formation in the primary cultures of ECs from EC-specific Smpd1 transgenic mice. Primary cultured carotid arterial ECs were treated with 7-Ket (15 μg/ml) for 24 h followed by treatment of ASM inhibitor Ami (20 μmol/l). A: Representative fluorescent confocal microscope images displaying the yellow dots or patches showing the colocalization of NLRP3 (green) with ASC (red). B: The summarized data show the colocalization coefficient of NLRP3 with ASC. C: Representative fluorescent confocal microscope images showing the colocalization of NLRP3 (green) with caspase-1 (red). D: The summarized data show the colocalization coefficient of NLRP3 with caspase-1. Data are expressed as means ± SEM, n = 6. ∗P < 0.05 is defined as significant. The scale bar represents 10 μm.
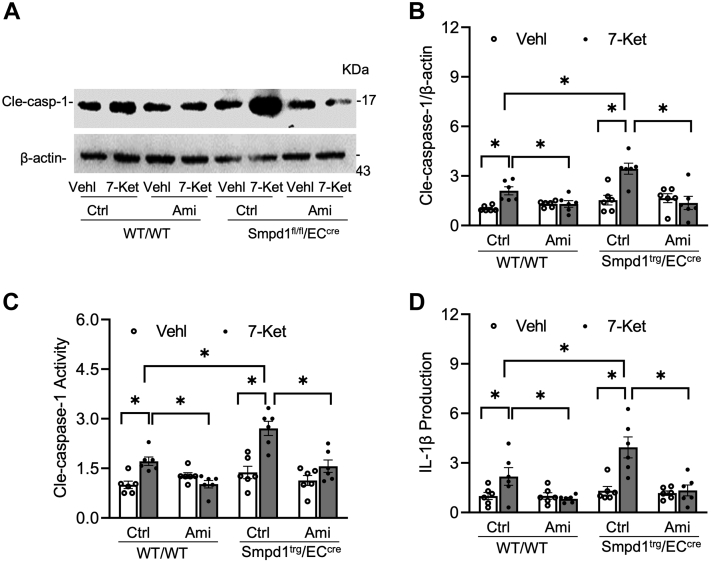
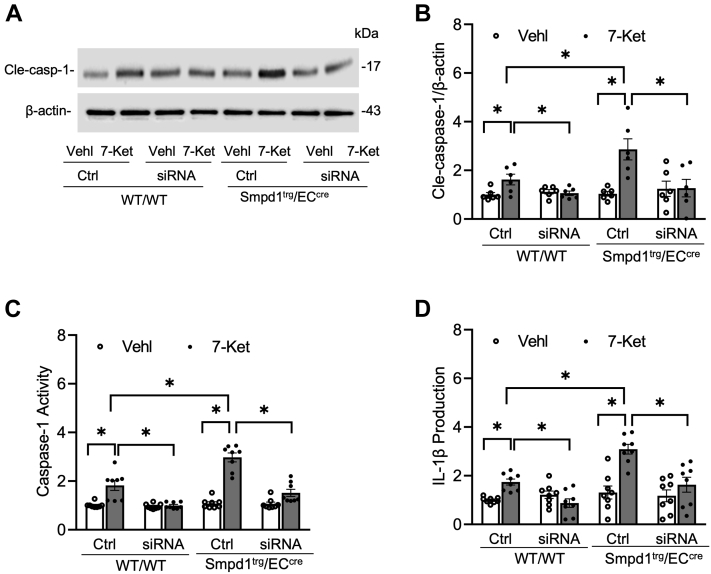
The NLRP3 inflammasome activation in cultured ECs was further assessed by Western blot analysis of the caspase-1 cleavage, caspase-1 activity assay, and ELISA analysis of IL-1β production. As shown in Figure 7A, B, 7-Ket remarkably increased cleaved caspase-1 expression in WT/WT ECs, which was further enhanced in Smpd1trg/ECcre ECs. Such enhancement in caspase-1 cleavage in Smpd1trg/ECcre ECs was correlated with increased caspase-1 activity (Fig. 7C) and IL-1β production (Fig. 7D). Moreover, 7-Ket-induced increases in caspase-1 cleavage, caspase-1 activity, and IL-1β production were prevented by Ami in both WT/WT and Smpd1trg/ECcre ECs (Fig. 7A–D). It should be noticed that under basal conditions (vehicle), Smpd1trg/ECcre ECs did not have significantly higher levels of NLRP3 inflammasome formation (Fig. 6A–D) and activation (Fig. 7A–D) compared with WT/WT ECs. Therefore, Smpd1 gene overexpression alone seems to be insufficient to trigger NLRP3 inflammasome formation and activation. However, our data indicate that Smpd1 gene overexpression in ECs could enhance 7-Ket-induced NLRP3 inflammasome formation and activation in vitro, which is large because of increased ASM activity.
Fig. 7.
Effects of ASM blockade on NLRP3 inflammasome activation in the primary cultures of ECs from EC-specific Smpd1 transgenic mice. A: Representative Western blot gel documents showing the expression of cleaved caspase-1 in the primary cultures of carotid ECs from endothelium-specific Smpd1 transgenic mice. B: Summarized data showing the expression of cleaved caspase-1 in the primary cultures of carotid ECs from endothelium-specific Smpd1 transgenic mice. C: Summary data showing caspase-1 activity in the primary cultures of carotid ECs from endothelium-specific Smpd1 transgenic mice. D: Summarized data showing IL-1β production in the primary cultures of carotid ECs from endothelium-specific Smpd1 transgenic mice. Data are expressed as means ± SEM, n = 5. ∗P < 0.05 is defined as significant.
Smpd1 gene overexpression enhances the formation and activation of MR-redox signaling platforms in cultured ECs
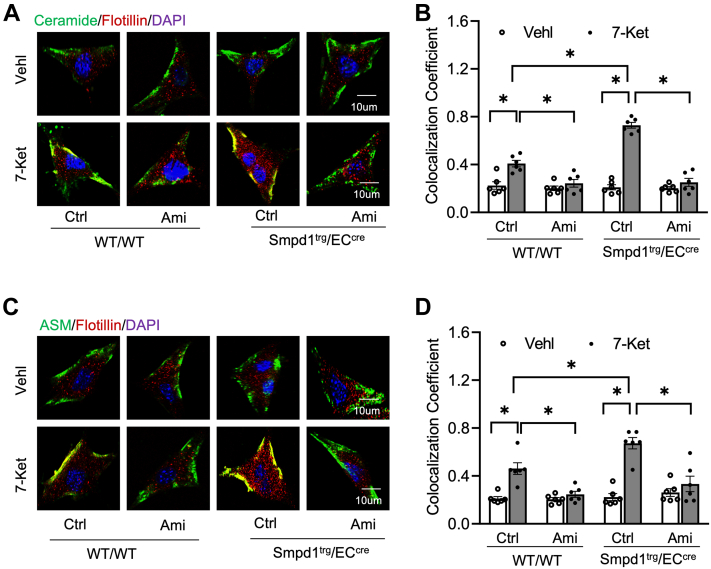
As shown in Figure 8A–D, untreated Smpd1trg/ECcre ECs had a similar level of colocalization of ceramide or ASM with flotillin compared with that of WT/WT ECs, suggesting that Smpd1 gene overexpression does not trigger ceramide-enriched MR clustering under basal condition (vehicle groups). Interestingly, 7-Ket stimulated the colocalization of ceramide or ASM expression with flotillin in WT/WT ECs. Such 7-Ket-induced effects were enhanced in Smpd1trg/ECcre ECs, which were significantly inhibited by treating the ECs with ASM inhibitor Ami. Therefore, our data indicate that Smpd1 gene overexpression could enhance 7-Ket-induced ceramide-enriched MR clustering in ECs.
Fig. 8.
Formation of ASM-ceramide MR signaling platforms in the primary cultures of ECs from EC-specific Smpd1 transgenic mice. A: Representative fluorescent confocal microscopic images showing the colocalization of MR marker, flotillin (red) with ASM (green). B: Summarized data showing the colocalization coefficient of flotillin with ASM. C: Representative fluorescent confocal microscopic images showing the colocalization of MR marker, flotillin (red) with ceramide (green). D: Summarized data showing the colocalization coefficient of flotillin with ceramide. Data are expressed as means ± SEM, n = 5. ∗P < 0.05 is defined as significant. The scale bar represents 10 μm.
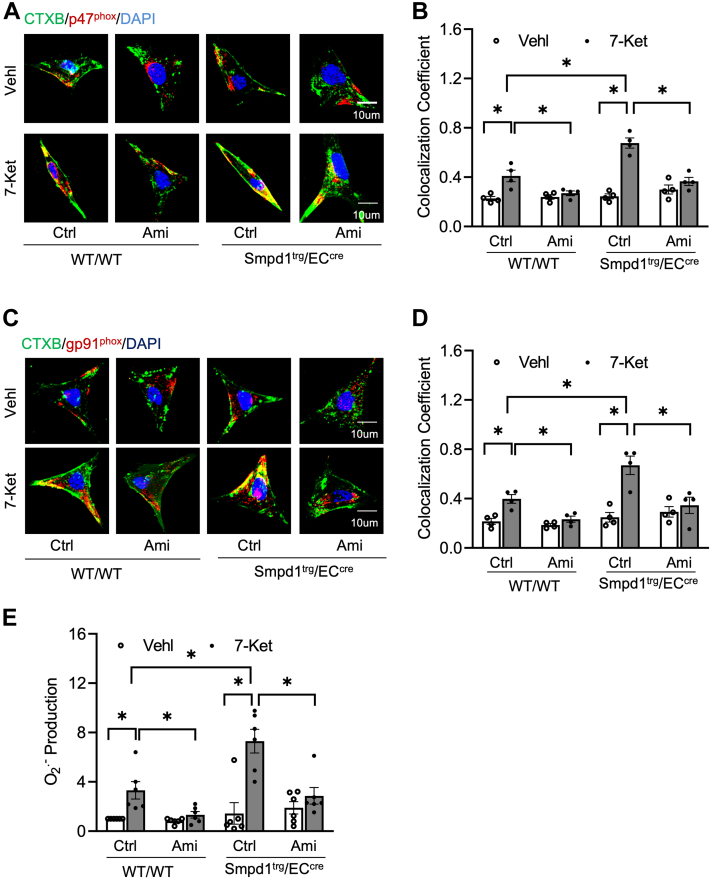
We then examined the effects of Smpd1 gene overexpression on the formation of MR-redox signaling platforms in ECs. MR was labeled with antibodies against CTXB (an MR marker). It was found that the expression of NOX subunits gp91 or gp47 in CTXB-labeled MR clusters was similar between WT/WT ECs and Smpd1trg/ECcre ECs under ND conditions (control groups). 7-Ket increased the levels of colocalization of gp91phox or gp47phox with CTXB in WT/WT ECs, which were enhanced in Smpd1trg/ECcre ECs and inhibited by Ami. Using ESR spectrometry, we measured the O2•− production in cultured ECs. As shown in Figure 9E, 7-Ket increased O2•− production in WT/WT ECs, an effect enhanced in Smpd1trg/ECcre ECs. Treating both types of ECs with Ami significantly attenuated 7-Ket-induced O2•− production. Together, these data suggest that Smpd1 gene overexpression enhances the 7-Ket-induced formation of MR-redox signaling platforms in ECs.
Fig. 9.
Aggregation and activation of NOXs in MR signaling platforms in the primary cultures of ECs from EC-specific Smpd1 transgenic mice. A: Representative fluorescent confocal microscopic images showing the colocalization of MR marker, CTXB (green) with NOX subunit, gp47phox (red). B: Summarized data showing the colocalization coefficient of CTXB with gp47phox. C: Representative fluorescent confocal microscopic images showing the colocalization of LR marker, CTXB (green) with NOX subunit, gp91phox (red). D: Summarized data showing the colocalization coefficient of CTXB with gp91phox. E: NOX-mediated O2•− production as measured by ESR spectrometry. Data are expressed as means ± SEM, n = 5. ∗P < 0.05 is defined as significant. The scale bar represents 10 μm.
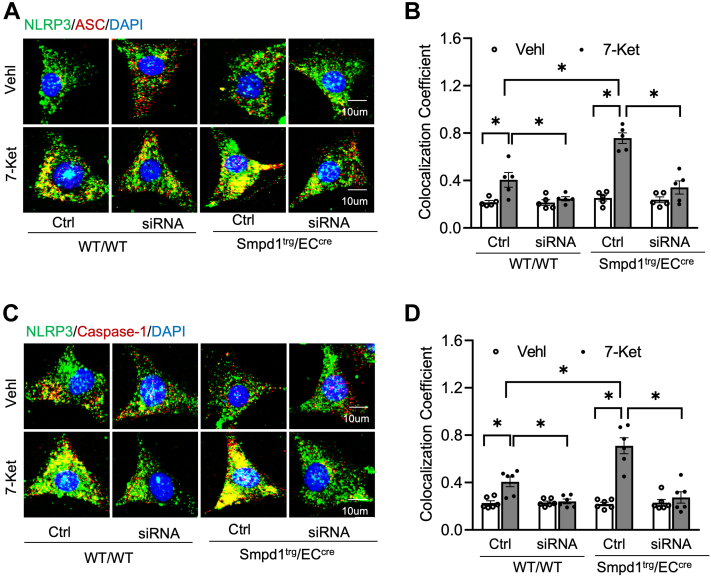
Txnip gene silencing abrogates 7-ket-increased NLRP3 inflammasome formation and activation in ECs with Smpd1 gene overexpression
Previous studies demonstrated that TXNIP binds NLRP3 protein and triggers NLRP3 inflammasome formation and activation (43). Here, we examined the role of TXNIP in 7-Ket-induced NLRP3 inflammasome formation and activation in Smpd1trg/ECcre ECs. As shown in Figure 10A–D, Txnip gene silencing by Txnip siRNA transfection markedly attenuated 7-Ket-induced increases in the colocalization of NLRP3 with ASC or caspase-1 in both WT/WT ECs and Smpd1trg/ECcre ECs. 7-Ket-induced increases in caspase-1 cleavage, caspase-1 activity, and IL-1β production were significantly abolished by Txnip gene silencing in WT/WT ECs or Smpd1trg/ECcre ECs (Fig. 11A–D). Together, these results demonstrate that targeting the Txnip gene abrogated 7-Ket-induced NLRP3 inflammasome activation and formation in ECs with Smpd1 gene overexpression.
Fig. 10.
TXNIP gene silencing abrogated NLRP3 inflammasome formation in the primary cultures of ECs from EC-specific Smpd1 transgenic mice. A: Representative fluorescent confocal microscope images displaying the yellow dots or patches showing the colocalization of NLRP3 (green) with ASC (red). B: The summarized data show the colocalization coefficient of NLRP3 with ASC. C: Representative fluorescent confocal microscope images showing the colocalization of NLRP3 (green) with caspase-1 (red). D: The summarized data show the colocalization coefficient of NLRP3 with caspase-1. Data are expressed as means ± SEM, n = 6. ∗P < 0.05 is defined as significant. The scale bar represents 10 μm.
Fig. 11.
TXNIP gene silencing abrogated NLRP3 inflammasome activation in the primary cultures of ECs from EC-specific Smpd1 transgenic mice. A: Representative Western blot gel documents showing the expression of cleaved caspase-1 in the primary cultures of carotid ECs from endothelium-specific Smpd1 transgenic mice. B: Summarized data showing the expression of cleaved caspase-1 in the primary cultures of carotid ECs from EC-specific Smpd1 transgenic mice. C: Summary data showing caspase-1 activity in the primary cultures of carotid ECs from EC-specific Smpd1 transgenic mice. D: Summarized data showing IL-1β production in the primary cultures of carotid ECs from endothelium-specific Smpd1 transgenic mice. Data are expressed as means ± SEM, n = 5. ∗P < 0.05 is defined as significant.
Discussion
The present study revealed the critical contribution of endothelial ASM-ceramide MR redox signaling pathway to hypercholesterolemia-induced NLRP3 inflammasome activation and neointimal hyperplasia. Our results demonstrated that EC-specific overexpression of the Smpd1 gene enhanced hypercholesterolemia-induced neointimal formation, which was accompanied by augmented ASM-ceramide-MR redox signaling and NLRP3 inflammasome formation and activation in the arterial endothelium. Moreover, we demonstrated that TXNIP is a critical mediator that links ASM-ceramide-MR redox signaling to NLRP3 inflammasome formation and activation in ECs. By using Smpd1trg/ECcre mice and the primary cultures of their ECs, our findings provide the first direct evidence that ASM-ceramide-MR redox signaling and downstream redox sensor TXNIP are involved in the formation and activation of NLRP3 inflammasome activation and subsequent neointimal hyperplasia during hypercholesterolemia.
Accumulating evidence has revealed that ASM and ceramide are crucially involved in cardiovascular physiology and pathophysiology (44, 45). During atherosclerosis, lysosome ASM-dependent formation of ceramide-enriched lipid macrodomains in ECs contributes to FasL-induced impairment of the vasodilator response (46, 47) and muscarinic-1 receptor-mediated coronary artery constriction (48), which are both major aggravating factors leading to subsequent atherosclerosis. Recent studies have demonstrated that ASM-derived ceramide mediates NLRP3 inflammasome activation in various tissues including the heart and kidney (49, 50, 51), which suggests NLRP3 inflammasome as one of the effector pathways downstream of the ASM-ceramide pathway. Our recent study demonstrated that ASM deficiency inhibited the endothelial NLRP3 inflammasomes and neointimal lesions (24), which supports the view that ASM-ceramide signaling is involved in endothelial NLRP3 inflammasome activation. However, it remains unknown whether the ASM-ceramide signaling in ECs is directly involved in endothelial NLRP3 inflammasome activation and neointimal lesion formation and how ASM regulates NLRP3 inflammasome activation in response to high cholesterol stimulation in vitro and in hypercholesteremia in vivo. In the present study, we generated EC-specific Smpd1 transgenic mice (Smpd1trg/ECcre), in which the Smpd1 gene was specifically overexpressed in ECs. Their littermate controls (WT/WT or Smpd1trg/WT) were used for the comparison of the studies. Our data first confirmed that Smpd1 gene overexpression in ECs significantly enhanced the WD-induced neointima formation in the carotid artery wall (Fig. 1). Then, confocal microscopic analysis revealed that EC-specific Smpd1 gene overexpression markedly enhanced WD-induced increases in NLRP3 inflammasome formation and activation as characterized by increased colocalization of NLRP3 inflammasome components (Figs. 2 and 3). Consistently, we observed that in Smpd1trg/ECcre mice, the enhanced WD-induced NLRP3 inflammasome activation and neointimal formation were accompanied by higher levels of ASM protein expression and ceramides in the endothelium (Fig. 4). Similarly, using isolated ECs from Smpd1trg/ECcre mice and WT controls, we confirmed that Smpd1 gene overexpression enhanced NLRP3 inflammasome activation (Figs. 6 and 7) and ASM-ceramide signaling (Fig. 8) in cultured ECs stimulated by 7-Ket. Taken together, these results suggest that endothelial ASM-ceramide signaling promotes endothelial NLRP3 inflammasome activation during hypercholesterolemic stimulations, which contributes to the development of neointima formation or atherogenic pathology. To our knowledge, this current study provides the first evidence that endothelium-specific overexpression of ASM exaggerates endothelial NLRP3 inflammasome formation and activation upon pathological stimuli such as hypercholesterolemia.
It is known that ROS generation is one of the first identified triggers of NLRP3 inflammasome activation (52, 53, 54). ROS may be derived from endoplasmic reticulum stress, damaged mitochondria, and NOX, which is an intermediate factor involved in multiple signaling pathways and can trigger the activation of NLRP3 inflammasome (55). Our previous studies have for the first time defined a mechanism mediating NOX activation in response to many different stimuli, termed MR redox signalosomes. These MR signalosomes use MRs as a platform to transduce and amplify the redox signaling and are centered on the enzymatic NOX subunit clustering and activating to produce O2•− (56). NOX-derived ROS can act downstream to transduce transmembrane or intracellular signaling, leading to the redox regulation of cell and organ function (16). We reported that different stimuli such as homocysteine, visfatin, or ATP act on the cell membrane to stimulate ASM to produce and form ceramide-enriched MR platforms and thereby increase NOX-dependent O2•− production in different types of cells, such as podocytes, ECs, and hepatic stellate cells (12, 17, 57, 58, 59, 60, 61, 62). So far, it remains poorly understood whether ASM-ceramide-MR redox signaling platforms are critical in the activation of NLRP3 inflammasomes and atherosclerotic lesions. Using ASM global knockout mice, we recently demonstrated that ASM/ceramide-associated MR clustering with NOX subunits contributes to hypercholesteremia-induced endothelial NLPR3 inflammasome activation and formation (24). In the present study, we further used Smpd1trg/ECcre mice to study the specific role of ASM in the formation and activation of NLRP3 inflammasome and explore related molecular mechanisms. It was found that Smpd1 gene overexpression enhanced WD-induced formation of ceramide-enriched MR redox signaling as shown by increased formation of flotillin-1-positive MR clusters aggregated with ASM/ceramide (Fig. 4) or NOX subunits gp91 and p47 (Fig. 5) and the consequent production of O2•− levels as measured by in situ DHE staining. DHE is well known as a fluorescent probe for superoxide and hydrogen peroxide and is commonly used for the detection of ROS generation in tissues. Our data demonstrated that WD increased arterial fluorescence in the arterial wall including in ECs and SMCs of the media layer, which was further increased when the Smpd1 gene is overexpressed. It is not surprising to observe DHE-ROS fluorescence in the media layer as hydrogen peroxide is cell permeable and could be released into the medial layer. The ROS production in the media layer could also be originated from redox pathways in SMCs that are activated following endothelial activation or injury. Nonetheless, our data indicate that EC-specific Smpd1 gene overexpression enhanced the oxidative stress in the arterial wall. In addition, we confirmed that the Smpd1 gene overexpression enhanced 7-Ket-induced activation of ASM-MR redox signaling in cultured ECs, which were abolished by the ASM inhibitor (Figs. 8 and 9). Together, these results from the ASM overexpression studies provide direct evidence that hypercholesterolemia or cholesterol stimulation instigates the ASM-ceramide pathway to induce MR redox signaling platform formation in ECs and thereby trigger endothelial NLRP3 inflammasomes.
To further study how ASM-mediated activation of MR redox signaling platforms induces NLRP3 inflammasome activation, we determine the role of a redox sensor TXNIP in the process of NLRP3 inflammasome activation. TXNIP is the endogenous inhibitor and regulator of thioredoxin, a major cellular antioxidant, and antiapoptotic system (63). It has been demonstrated that NLRP3 inflammasome activators induce the dissociation of TXNIP from thioredoxin in a ROS-sensitive manner, which allows thioredoxin to bind NLRP3, thereby activating this inflammasome in different cells, such as macrophages (64), ECs (65, 66, 67, 68), and podocytes (69, 70). However, the role of TXNIP in ASM-ceramide-mediated NLRP3 inflammasome activation remains largely unexplored. In the present study, we examined the effects of Txnip gene silencing on the NLRP3 inflammasomes in cultured ECs isolated from WT and Smpd1trg/ECcre mice. Our results demonstrated that Txnip gene silencing abrogated 7-Ket-induced NLRP3 inflammasome formation and activation in ECs from both WT and Smpd1trg/ECcre (Figs. 10 and 11). These data demonstrate that TXNIP is a critical downstream mediator of ASM-ceramide-MR redox signaling that triggers endothelial NLRP3 inflammasome formation and activation. Consistent with our finding, Jiang et al. (71) recently reported that lipopolysaccharide/ATP-induced increase of ASM activity and accumulation of ceramide is attributed to TXNIP/NLRP3 inflammasome activation in J774A.1 cell and THP-1 macrophages. Koka et al. (24) have reported that TXNIP inhibitor verapamil attenuated 7-Ket or cholesterol crystal-induced increases in caspase-1 activity in ECs. In addition, metformin and many other compounds were used to lower aortic TXNIP levels in vivo or endothelial levels in vitro to block NLRP3 inflammasome activation and protect from endothelial dysfunction and cardiovascular risk factors (72, 73, 74, 75, 76). Nrf2 and AMPK are also believed to control the regulation of the NLRP3 inflammasome by the TRX-TXNIP complex (77, 78, 79). Concerning the mechanism responsible for the action of TXNIP, there is evidence that it activates the TLR4-NFκB-NLRP3 inflammasome signaling pathway with increased MyD88, NLPR3 inflammasome, and ASC expression, as well as the increased phosphorylation of IκBα and p65, thus promoting downstream NF-κB activation (80). The overexpression of TXNIP also led to an increased expression of inflammation genes via chromatin modifications and by promoting nuclear translocation of NF-κB (81). These previous studies have indicated that TXNIP may perform its functions through multiple binding partners and thereby directly or indirectly regulate NLRP3 inflammasome formation and activation. The precise mechanism by which TXNIP links ASM-ceramide-MR redox signaling and its downstream effector, NLRP3 inflammasome, deserves future investigation.
Finally, the present study had several limitations, which should be noted. First, our lipid panel analysis demonstrated that Smpd1trg/ECcre mice had increased lipid levels induced by the WD. The mechanism by which serum lipid is increased by endothelial overexpression of ASM remains an intriguing question. Recent evidence identifies that liver sinusoidal EC dysfunction is the main characteristic or early event in the pathogenesis of the nonalcohol fatty liver disease, which contributes to impaired hepatic lipid uptake and metabolism (82). Thus, endothelial ASM overexpression in the liver may cause liver sinusoidal EC dysfunction and hepatic dysregulation of lipid metabolism leading to higher serum lipids, which indirectly contribute to the development of neointimal injury. Second, the EC-specific Smpd1 transgene (Smpd1trg/ECcre) mice were generated by crossing Smpd1trg/WT mice with EC-specific Tie2-Cre mice as described (40). Tie2 is a receptor tyrosine kinase that binds angiopoietin-1 and angiopoietin-2. Tie2 is not only expressed in ECs (83) but also found in hematopoietic cells in fetal liver and adult bone marrow and several differentiated hematopoietic cells (84, 85). The formation and activation of NLRP3 inflammasome in hematopoietic cells are implicated in cardiovascular diseases (86, 87). In this regard, some of the effects may be mediated by ASM overexpression in the hematopoietic cells on cardiovascular diseases.
In summary, the present study demonstrated that EC-specific Smpd1 gene overexpression enhanced the production of ceramide and the formation of MR redox signaling platforms, promoted the activation of endothelial NLRP3 inflammasomes, and thereby resulted in endothelial dysfunction and atherogenesis. These findings provide the first evidence that endothelial ASM-ceramide-MR redox signaling is directly linked with endothelial NLRP3 inflammasomes and neointimal hyperplasia. Moreover, we identified that ASM-ceramide MR redox signaling is coupled with endothelial NLRP3 inflammasome activation via a redox sensor, TXNIP. Our findings may offer novel therapeutic insights into targeting ASM-ceramide-MR redox signaling or TXNIP to suppress the activation of endothelial NLRP3 inflammasomes, thereby preventing and treating vasculopathy associated with hypercholesterolemia.
Data Availability
The data that support the findings of this study are available from the corresponding author on realistic request.
Supplemental Data
This article contains supplemental data.
Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank Mrs. Xiaoyuan Wu for preparing the wax slides and frozen slides for this study. This study was supported by the grants HL057244, HL075316, HL150007, HL122937, and DK120491 from the National Institutes of Health.
Author Contributions
Y. Z. and P.-L. L. conceptualization; X. Y., O. M. B., and Y. Z. formal analysis; X. Y., O. M. B., and Y. Z. investigation; X. Y. writing–original draft; O. M. B., Y. Z., and X. L. writing–review & editing.
Funding and Additional Information
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplemental Data
Figure S1.
Figure S2.
Figure S3.
References
- 1.Duewell P., Kono H., Rayner K.J., Sirois C.M., Vladimer G., Bauernfeind F.G., et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y., Wang H., Kouadir M., Song H., Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia M., Boini K.M., Abais J.M., Xu M., Zhang Y., Li P.L. Endothelial NLRP3 inflammasome activation and enhanced neointima formation in mice by adipokine visfatin. Am. J. Pathol. 2014;184:1617–1628. doi: 10.1016/j.ajpath.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan X., Bhat O.M., Samidurai A., Das A., Zhang Y., Li P.L. Reversal of endothelial extracellular vesicle-induced smooth muscle phenotype transition by hypercholesterolemia stimulation: role of NLRP3 inflammasome activation. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.597423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang M., Lv H., Liu Q., Zhang L., Zhang R., Huang X., et al. Colchicine alleviates cholesterol crystal-induced endothelial cell pyroptosis through activating AMPK/SIRT1 pathway. Oxid. Med. Cell Longev. 2020;2020 doi: 10.1155/2020/9173530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., Yang X., He Y., Wang W., Zhang J., Zhang W., et al. Negative regulation of NLRP3 inflammasome by SIRT1 in vascular endothelial cells. Immunobiology. 2017;222:552–561. doi: 10.1016/j.imbio.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Yin W., Zhou Q.L., OuYang S.X., Chen Y., Gong Y.T., Liang Y.M. Uric acid regulates NLRP3/IL-1beta signaling pathway and further induces vascular endothelial cells injury in early CKD through ROS activation and K(+) efflux. BMC Nephrol. 2019;20:319. doi: 10.1186/s12882-019-1506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y., Li X., Pitzer A.L., Chen Y., Wang L., Li P.L. Coronary endothelial dysfunction induced by nucleotide oligomerization domain-like receptor protein with pyrin domain containing 3 inflammasome activation during hypercholesterolemia: beyond inflammation. Antioxid. Redox Signal. 2015;22:1084–1096. doi: 10.1089/ars.2014.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdei J., Toth A., Balogh E., Nyakundi B.B., Banyai E., Ryffel B., et al. Induction of NLRP3 inflammasome activation by heme in human endothelial cells. Oxid. Med. Cell Longev. 2018;2018 doi: 10.1155/2018/4310816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao N., Li C.C., Di B., Xu L.L. Recent advances in the NEK7-licensed NLRP3 inflammasome activation: mechanisms, role in diseases and related inhibitors. J. Autoimmun. 2020;113 doi: 10.1016/j.jaut.2020.102515. [DOI] [PubMed] [Google Scholar]
- 11.Li X., Zhang Y., Xia M., Gulbins E., Boini K.M., Li P.L. Activation of Nlrp3 inflammasomes enhances macrophage lipid-deposition and migration: implication of a novel role of inflammasome in atherogenesis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abais J.M., Zhang C., Xia M., Liu Q., Gehr T.W., Boini K.M., et al. NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid. Redox Signal. 2013;18:1537–1548. doi: 10.1089/ars.2012.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boini K.M., Xia M., Abais J.M., Li G., Pitzer A.L., Gehr T.W., et al. Activation of inflammasomes in podocyte injury of mice on the high fat diet: effects of ASC gene deletion and silencing. Biochim. Biophys. Acta. 2014;1843:836–845. doi: 10.1016/j.bbamcr.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang D., Li G., Zhang Q., Bhat O.M., Zou Y., Ritter J.K., et al. Contribution of podocyte inflammatory exosome release to glomerular inflammation and sclerosis during hyperhomocysteinemia. Biochim. Biophys. Acta Mol. Basis Dis. 2021;1867 doi: 10.1016/j.bbadis.2021.166146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang A.Y., Yi F., Zhang G., Gulbins E., Li P.L. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;47:74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62. [DOI] [PubMed] [Google Scholar]
- 16.Li P.L., Zhang Y., Yi F. Lipid raft redox signaling platforms in endothelial dysfunction. Antioxid. Redox Signal. 2007;9:1457–1470. doi: 10.1089/ars.2007.1667. [DOI] [PubMed] [Google Scholar]
- 17.Boini K.M., Zhang C., Xia M., Han W.Q., Brimson C., Poklis J.L., et al. Visfatin-induced lipid raft redox signaling platforms and dysfunction in glomerular endothelial cells. Biochim. Biophys. Acta. 2010;1801:1294–1304. doi: 10.1016/j.bbalip.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Y.M., Li X., Xiong J., Abais J.M., Xia M., Boini K.M., et al. Attenuation by statins of membrane raft-redox signaling in coronary arterial endothelium. J. Pharmacol. Exp. Ther. 2013;345:170–179. doi: 10.1124/jpet.112.201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang A.Y., Teggatz E.G., Zou A.P., Campbell W.B., Li P.L. Endostatin uncouples NO and Ca2+ response to bradykinin through enhanced O2∗- production in the intact coronary endothelium. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H686–H694. doi: 10.1152/ajpheart.00174.2004. [DOI] [PubMed] [Google Scholar]
- 20.Bao J.X., Xia M., Poklis J.L., Han W.Q., Brimson C., Li P.L. Triggering role of acid sphingomyelinase in endothelial lysosome-membrane fusion and dysfunction in coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H992–H1002. doi: 10.1152/ajpheart.00958.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han W.Q., Xia M., Xu M., Boini K.M., Ritter J.K., Li N.J., et al. Lysosome fusion to the cell membrane is mediated by the dysferlin C2A domain in coronary arterial endothelial cells. J. Cell Sci. 2012;125:1225–1234. doi: 10.1242/jcs.094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han W.Q., Xia M., Zhang C., Zhang F., Xu M., Li N.J., et al. SNARE-mediated rapid lysosome fusion in membrane raft clustering and dysfunction of bovine coronary arterial endothelium. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H2028–H2037. doi: 10.1152/ajpheart.00581.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrews N.W. Solving the secretory acid sphingomyelinase puzzle: insights from lysosome-mediated parasite invasion and plasma membrane repair. Cell Microbiol. 2019;21 doi: 10.1111/cmi.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koka S., Xia M., Chen Y., Bhat O.M., Yuan X., Boini K.M., et al. Endothelial NLRP3 inflammasome activation and arterial neointima formation associated with acid sphingomyelinase during hypercholesterolemia. Redox Biol. 2017;13:336–344. doi: 10.1016/j.redox.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang D.D., Li G.B., Camus S., Li N.J., Ritter J.K., Li P.L. Contribution of NLRP3 inflammasome activation to glomerular injury during hyperhomocysteinemia with and without enhanced exosome secretion. FASEB J. 2020;34 doi: 10.1096/fasebj.2020.34.s1.06730. [DOI] [Google Scholar]
- 26.Bhat O., Yuan X.X., Cain C., Salloum F.N., Li P.L. Medial calcification in the arterial wall of smooth muscle cell-specific Smpd1 transgenic mice: a ceramide-mediated vasculopathy. J. Cell Mol. Med. 2020;24:539–553. doi: 10.1111/jcmm.14761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korshunov V.A., Berk B.C. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler. Thromb. Vasc. Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 28.Abraham N.G., Sodhi K., Silvis A.M., Vanella L., Favero G., Rezzani R., et al. CYP2J2 targeting to endothelial cells attenuates adiposity and vascular dysfunction in mice fed a high-fat diet by reprogramming adipocyte phenotype. Hypertension. 2014;64:1352–1361. doi: 10.1161/HYPERTENSIONAHA.114.03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin I.J., Shon S.M., Schellingerhout D., Park J.Y., Kim J.Y., Lee S.K., et al. Characterization of partial ligation-induced carotid atherosclerosis model using dual-modality molecular imaging in ApoE knock-out mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nam D., Ni C.W., Rezvan A., Suo J., Budzyn K., Llanos A., et al. A model of disturbed flow-induced atherosclerosis in mouse carotid artery by partial ligation and a simple method of RNA isolation from carotid endothelium. J. Vis. Exp. 2010;22:1861. doi: 10.3791/1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee G., Shim J.H., Kang H., Nam K.M., Song H., Park J.T. Monodisperse Pt and PtRu/C(60) hybrid nanoparticles for fuel cell anode catalysts. Chem. Commun. (Camb) 2009;7:5036–5038. doi: 10.1039/b911068b. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y., Li X., Boini K.M., Pitzer A.L., Gulbins E., Zhang Y., et al. Endothelial Nlrp3 inflammasome activation associated with lysosomal destabilization during coronary arteritis. Biochim. Biophys. Acta. 2015;1853:396–408. doi: 10.1016/j.bbamcr.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan X., Bhat O.M., Lohner H., Zhang Y., Li P.L. Downregulation of lysosomal acid ceramidase mediates HMGB1-induced migration and proliferation of mouse coronary arterial myocytes. Front. Cell Dev. Biol. 2020;8:111. doi: 10.3389/fcell.2020.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M., Li Y., Guo Y., Xu J. Arginine regulates NLRP3 inflammasome activation through SIRT1 in vascular endothelial cells. Inflammation. 2021;44:1370–1380. doi: 10.1007/s10753-021-01422-1. [DOI] [PubMed] [Google Scholar]
- 35.Dong H., Huang H., Yun X., Kim D.S., Yue Y., Wu H., et al. Bilirubin increases insulin sensitivity in leptin-receptor deficient and diet-induced obese mice through suppression of ER stress and chronic inflammation. Endocrinology. 2014;155:818–828. doi: 10.1210/en.2013-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan X., Bhat O.M., Lohner H., Zhang Y., Li P.L. Endothelial acid ceramidase in exosome-mediated release of NLRP3 inflammasome products during hyperglycemia: evidence from endothelium-specific deletion of Asah1 gene. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864 doi: 10.1016/j.bbalip.2019.158532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li G., Huang D., Li N., Ritter J.K., Li P.L. Regulation of TRPML1 channel activity and inflammatory exosome release by endogenously produced reactive oxygen species in mouse podocytes. Redox Biol. 2021;43 doi: 10.1016/j.redox.2021.102013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dikalov S.I., Harrison D.G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal. 2014;20:372–382. doi: 10.1089/ars.2012.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan X., Wang L., Bhat O.M., Lohner H., Li P.L. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: antioxidant action of butyrate. Redox Biol. 2018;16:21–31. doi: 10.1016/j.redox.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payne S., De Val S., Neal A. Endothelial-specific cre mouse models: is your cre CREdibile? Arterioscl. Throm. Vas. 2018;38:2550–2561. doi: 10.1161/ATVBAHA.118.309669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi M., Inoue K., Warabi E., Minami T., Kodama T. A simple method of isolating mouse aortic endothelial cells. J. Atheroscler. Thromb. 2005;12:138–142. doi: 10.5551/jat.12.138. [DOI] [PubMed] [Google Scholar]
- 42.Li X., Han W.Q., Boini K.M., Xia M., Zhang Y., Li P.L. TRAIL death receptor 4 signaling via lysosome fusion and membrane raft clustering in coronary arterial endothelial cells: evidence from ASM knockout mice. J. Mol. Med. (Berl) 2013;91:25–36. doi: 10.1007/s00109-012-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y., Lian K., Zhang L., Wang R., Yi F., Gao C., et al. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res. Cardiol. 2014;109:415. doi: 10.1007/s00395-014-0415-z. [DOI] [PubMed] [Google Scholar]
- 44.Linardic C.M., Hannun Y.A. Identification of a distinct pool of sphingomyelin involved in the sphingomyelin cycle. J. Biol. Chem. 1994;269:23530–23537. [PubMed] [Google Scholar]
- 45.Andrieu N., Salvayre R., Levade T. Comparative study of the metabolic pools of sphingomyelin and phosphatidylcholine sensitive to tumor necrosis factor. Eur. J. Biochem. 1996;236:738–745. doi: 10.1111/j.1432-1033.1996.00738.x. [DOI] [PubMed] [Google Scholar]
- 46.Jin S., Yi F., Zhang F., Poklis J.L., Li P.L. Lysosomal targeting and trafficking of acid sphingomyelinase to lipid raft platforms in coronary endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:2056–2062. doi: 10.1161/ATVBAHA.108.172478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang A.Y., Yi F., Jin S., Xia M., Chen Q.Z., Gulbins E., et al. Acid sphingomyelinase and its redox amplification in formation of lipid raft redox signaling platforms in endothelial cells. Antioxid. Redox Signal. 2007;9:817–828. doi: 10.1089/ars.2007.1509. [DOI] [PubMed] [Google Scholar]
- 48.Jia S.J., Jin S., Zhang F., Yi F., Dewey W.L., Li P.L. Formation and function of ceramide-enriched membrane platforms with CD38 during M1-receptor stimulation in bovine coronary arterial myocytes. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1743–H1752. doi: 10.1152/ajpheart.00617.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bianco F., Perrotta C., Novellino L., Francolini M., Riganti L., Menna E., et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28:1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cuzzocrea S., Deigner H.P., Genovese T., Mazzon E., Esposito E., Crisafulli C., et al. Inhibition of ceramide biosynthesis ameliorates pathological consequences of spinal cord injury. Shock. 2009;31:634–644. doi: 10.1097/SHK.0b013e3181891396. [DOI] [PubMed] [Google Scholar]
- 51.Grassme H., Carpinteiro A., Edwards M.J., Gulbins E., Becker K.A. Regulation of the inflammasome by ceramide in cystic fibrosis lungs. Cell Physiol. Biochem. 2014;34:45–55. doi: 10.1159/000362983. [DOI] [PubMed] [Google Scholar]
- 52.Zhou R.B., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 53.Sorbara M.T., Girardin S.E. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011;21:558–560. doi: 10.1038/cr.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding W., Guo H.L., Xu C.Y., Wang B., Zhang M.M., Ding F. Mitochondrial reactive oxygen species-mediated NLRP3 inflammasome activation contributes to aldosterone-induced renal tubular cells injury. Oncotarget. 2016;7:17479–17491. doi: 10.18632/oncotarget.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai B.C., Yang Y.Y., Wang Q., Li M., Tian C., Liu Y., et al. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11:776. doi: 10.1038/s41419-020-02985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin S., Zhou F., Katirai F., Li P.L. Lipid raft redox signaling: molecular mechanisms in health and disease. Antioxid. Redox Sign. 2011;15:1043–1083. doi: 10.1089/ars.2010.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin S., Yi F., Li P.L. Contribution of lysosomal vesicles to the formation of lipid raft redox signaling platforms in endothelial cells. Antioxid. Redox Signal. 2007;9:1417–1426. doi: 10.1089/ars.2007.1660. [DOI] [PubMed] [Google Scholar]
- 58.Li X., Gulbins E., Zhang Y. Role of kinase suppressor of ras-1 in lipopolysaccharide-induced acute lung injury. Cell Physiol. Biochem. 2012;30:905–914. doi: 10.1159/000341468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia M., Zhang C., Boini K.M., Thacker A.M., Li P.L. Membrane raft-lysosome redox signalling platforms in coronary endothelial dysfunction induced by adipokine visfatin. Cardiovasc. Res. 2011;89:401–409. doi: 10.1093/cvr/cvq286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yi F., Jin S., Zhang F., Xia M., Bao J.X., Hu J., et al. Formation of lipid raft redox signalling platforms in glomerular endothelial cells: an early event of homocysteine-induced glomerular injury. J. Cell Mol. Med. 2009;13:3303–3314. doi: 10.1111/j.1582-4934.2009.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang C., Boini K.M., Xia M., Abais J.M., Li X., Liu Q., et al. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension. 2012;60:154–162. doi: 10.1161/HYPERTENSIONAHA.111.189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y., He X., Yuan X., Hong J., Bhat O., Li G., et al. NLRP3 Inflammasome Formation and Activation in Nonalcoholic Steatohepatitis: therapeutic target for antimetabolic syndrome remedy FTZ. Oxid. Med. Cell Longev. 2018;2018 doi: 10.1155/2018/2901871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lane T., Flam B., Lockey R., Kolliputi N. TXNIP shuttling: missing link between oxidative stress and inflannmasome activation. Front. Physiol. 2013;4:50. doi: 10.3389/fphys.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou R.B., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:U136–U151. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 65.Xiang M., Shi X.L., Li Y.H., Xu J., Yin L.H., Xiao G.Z., et al. Hemorrhagic Shock Activation of NLRP3 Inflammasome in Lung Endothelial Cells. J. Immunol. 2011;187:4809–4817. doi: 10.4049/jimmunol.1102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yin Y.L., Zhou Z.H., Liu W.W., Chang Q., Sun G.Q., Dai Y.L. Vascular endothelial cells senescence is associated with NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation via reactive oxygen species (ROS)/thioredoxin-interacting protein (TXNIP) pathway. Int. J. Biochem. Cell B. 2017;84:22–34. doi: 10.1016/j.biocel.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Luo X.J., Hu Y.M., He S., Ye Q.R., Lv Z.B., Liu J.X., et al. Dulaglutide inhibits high glucose-induced endothelial dysfunction and NLRP3 inflammasome activation. Arch. Biochem. Biophys. 2019;671:203–209. doi: 10.1016/j.abb.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 68.Li X.X., Ling S.K., Hu M.Y., Ma Y., Li Y., Huang P.L. Protective effects of acarbose against vascular endothelial dysfunction through inhibiting Nox4/NLRP3 inflammasome pathway in diabetic rats. Free Radic. Bio. Med. 2019;145:175–186. doi: 10.1016/j.freeradbiomed.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 69.Gao P., Meng X.F., Su H., He F.F., Chen S., Tang H., et al. Thioredoxin-interacting protein mediates NALP3 inflammasome activation in podocytes during diabetic nephropathy. Biochim. Biophys. Acta. 2014;1843:2448–2460. doi: 10.1016/j.bbamcr.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Abais J.M., Xia M., Li G.B., Chen Y., Conley S.M., Gehr T.W.B., et al. Nod-like Receptor Protein 3 (NLRP3) inflammasome activation and podocyte injury via Thioredoxin-Interacting Protein (TXNIP) during Hyperhomocysteinemia. J. Biol. Chem. 2014;289:27159–27168. doi: 10.1074/jbc.M114.567537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang J.J., Shi Y.N., Cao J.Y., Lu Y.J., Sun G.Y., Yang J. Role of ASM/Cer/TXNIP signaling module in the NLRP3 inflammasome activation. Lipids Health Dis. 2021;20:19. doi: 10.1186/s12944-021-01446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tian Y., Su Y., Ye Q.L., Chen L., Yuan F., Wang Z.Y. Silencing of TXNIP alleviated oxidative stress injury by regulating MAPK-Nrf2 axis in ischemic stroke. Neurochem. Res. 2020;45:428–436. doi: 10.1007/s11064-019-02933-y. [DOI] [PubMed] [Google Scholar]
- 73.Li J., Wang P.P., Chen Z., Yu S.P., Xu H.W. Fenofibrate ameliorates oxidative stress-induced retinal microvascular dysfunction in diabetic rats. Curr. Eye Res. 2018;43:1395–1403. doi: 10.1080/02713683.2018.1501072. [DOI] [PubMed] [Google Scholar]
- 74.Zhou X., Wu Y.J., Ye L.F., Wang Y.T., Zhang K.M., Wang L.J., et al. Aspirin alleviates endothelial gap junction dysfunction through inhibition of NLRP3 inflammasome activation in LPS-induced vascular injury. Acta Pharmaceutica Sin. B. 2019;9:711–723. doi: 10.1016/j.apsb.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang G., Dian F.Q., Li W.X., Wang Y.Q., Zeng C., Hu J., et al. Metformin inhibited Nod-like receptor protein 3 inflammasomes activation and suppressed diabetes-accelerated atherosclerosis in apoE(-)(/-) mice. Biomed. Pharmacother. 2019;119:109410. doi: 10.1016/j.biopha.2019.109410. [DOI] [PubMed] [Google Scholar]
- 76.Mai W.J., Xu Y.Z., Xu J.H., Zhao D., Ye L.Y., Yu G.X., et al. Berberine inhibits nod-like receptor family pyrin domain containing 3 inflammasome activation and pyroptosis in nonalcoholic steatohepatitis via the ROS/TXNIP Axis. Front. Pharmacol. 2020;11:185. doi: 10.3389/fphar.2020.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hou Y.H., Wang Y.T., He Q., Li L.Y., Xie H., Zhao Y., et al. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav. Brain Res. 2018;336:32–39. doi: 10.1016/j.bbr.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y.D., Gao Z., Gao X.H., Yuan Z.G., Ma T., Li G.Y., et al. Tilianin Protects Diabetic Retina through the Modulation of Nrf2/TXNIP/NLRP3 Inflammasome Pathways. J. Environ. Pathol. Tox. 2020;39:89–99. doi: 10.1615/JEnvironPatholToxicolOncol.2020032544. [DOI] [PubMed] [Google Scholar]
- 79.Dinesh P., Rasool M. Berberine, an isoquinoline alkaloid suppresses TXNIP mediated NLRP3 inflammasome activation in MSU crystal stimulated RAW 264.7 macrophages through the upregulation of Nrf2 transcription factor and alleviates MSU crystal induced inflammation in rats. Int. Immunopharmacol. 2017;44:26–37. doi: 10.1016/j.intimp.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 80.Dai Y.X., Wang S., Chang S.F., Ren D.Y., Shali S., Li C.G., et al. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-kappa B/NLRP3 inflammasome signaling pathway. J. Mol. Cell Cardiol. 2020;142:65–79. doi: 10.1016/j.yjmcc.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 81.Perrone L., Devi T.S., Hosoya K.I., Terasaki T., Singh L.P. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J. Cell Physiol. 2009;221:262–272. doi: 10.1002/jcp.21852. [DOI] [PubMed] [Google Scholar]
- 82.Wang X.K., Peng Z.G. Targeting liver sinusoidal endothelial cells: an attractive therapeutic strategy to control inflammation in nonalcoholic fatty liver disease. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.655557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kisanuki Y.Y., Hammer R.E., Miyazaki J., Williams S.C., Richardson J.A., Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 84.Puri M.C., Bernstein A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12753–12758. doi: 10.1073/pnas.2133552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takakura N., Huang X.L., Naruse T., Hamaguchi I., Dumont D.J., Yancopoulos G.D., et al. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 1998;9:677–686. doi: 10.1016/s1074-7613(00)80665-2. [DOI] [PubMed] [Google Scholar]
- 86.Adamiak M., Abdel-Latif A., Bujko K., Thapa A., Anusz K., Tracz M., et al. Nlrp3 inflammasome signaling regulates the homing and engraftment of Hematopoietic Stem Cells (HSPCs) by enhancing incorporation of CXCR4 receptor into membrane lipid rafts. Stem Cell Rev. Rep. 2020;16:954–967. doi: 10.1007/s12015-020-10005-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Louwe M.C., Olsen M.B., Kaasboll O.J., Yang K., Fosshaug L.E., Alfsnes K., et al. Absence of nlrp3 inflammasome in hematopoietic cells reduces adverse remodeling after experimental myocardial infarction. JACC Basic Transl. Sci. 2020;5:1210–1224. doi: 10.1016/j.jacbts.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on realistic request.