Summary
Memory consolidation, the process by which newly encoded and fragile memories become more robust, is thought to be supported by the reactivation of brain regions - including the hippocampus - during post-learning rest. While hippocampal reactivations have been demonstrated in humans in the declarative memory domain, it remains unknown whether such a process takes place after motor learning. Using multivariate analyses of task-related and resting state fMRI data, here we show that patterns of brain activity within both the hippocampus and striatum elicited during motor learning persist into post-learning rest, indicative of the reactivation of learning-related neural activity patterns. Moreover, results indicate that hippocampal reactivation reflects the spatial representation of the learned motor sequence. These results thus provide insights into the functional significance of neural reactivation after motor sequence learning.
Subject areas: Biological sciences, Neuroscience, Cognitive neuroscience
Graphical abstract
Highlights
-
•
Multivoxel brain patterns were examined during motor learning and subsequent rest
-
•
Hippocampal and striatal patterns during learning persist into subsequent rest
-
•
Such persistence is indicative of the reactivation of learning-related neural patterns
-
•
Hippocampal reactivation reflects the spatial representation of motor sequences
Biological sciences; Neuroscience; Cognitive neuroscience
Introduction
Memory consolidation is thought to be supported by the replay of hippocampal activity during subsequent offline episodes, i.e. during post-encoding rest.1,2,3 Hippocampal replay, initially reported in the rodent literature, has recently been translated to human research with the development of multivariate pattern analyses of fMRI data.1 Using these approaches, several studies have shown that hippocampal brain patterns elicited by declarative learning can persist into subsequent rest episodes and that this spontaneous memory reactivation is related to better memory [e.g.,4,5,6.
Despite these recent advances, several critical knowledge gaps remain. First, hippocampal reactivations have predominantly been limited to examinations in the context of declarative memory, which is surprising given that the role of the hippocampus in motor (sequence) memory processes is now well established [see7,8 for reviews]. Specifically, previous research has not only shown that the hippocampus is recruited during both implicit and explicit versions of motor sequence learning (MSL) [e.g.,9,10,11,12,13,14, but also that hippocampal activity and connectivity patterns elicited by task practice are linked to subsequent motor memory consolidation.10,15 In line with this, research using multivariate approaches has shown that task-related hippocampal patterns can discriminate newly learned as compared to consolidated motor sequences.16 While there is recent evidence to suggest that the hippocampus is involved in micro-offline motor memory processes [i.e., fast consolidation occurring during short rest intervals between practice blocks; see17,18], persistence of hippocampal patterns after motor learning has never been reported. Second, whereas previous rodent research has demonstrated that hippocampal replays consolidate previously learned spatial relationships (e.g.,19), the functional significance of hippocampal reactivation in humans is poorly understood.
The current study aims to address these knowledge gaps. Resting state (RS) scans from 55 young healthy participants were acquired immediately before and after participants completed a motor sequence learning (MSL) task inside the MR scanner (Task A; Figure 1A). Participants were then subsequently assigned to one of the two experimental groups and completed an MSL task variant (Task B) that probed the allocentric (i.e., spatial) or egocentric (i.e., motor) representation of the acquired motor sequence that is known to be dependent on hippocampo- or striato-cortical regions, respectively.20 Task B was then followed by a third RS scan. In three regions of interest (ROIs) known to be involved in motor memory consolidation (the hippocampus, striatum, and primary motor cortex (M1)), we examined: a) whether multivoxel patterns of neural activity showed evidence of persistence into post-learning rest; and, b) the functional significance of pattern persistence (i.e., whether pattern persistence reflected specific representations of the acquired motor sequence). Based on the aforementioned literature on the hippocampus as well as previous evidence demonstrating that (i) the hippocampus can lead striatal replay21 and (ii) task-related patterns in the primary motor cortex (M1) can be subsequently reactivated offline,22,23 the following hypotheses were made. We predicted that response patterns elicited by motor task practice in our three ROIs would persist into post-learning rest, reflecting the reactivation of learning-related patterns. Moreover, and in line with our earlier research,20 we postulated that pattern persistence in the hippocampus would reflect the reactivation of the spatial features of the task whereas persistence in the striatum and M1 would reflect the reinstatement of the motoric component of the task.
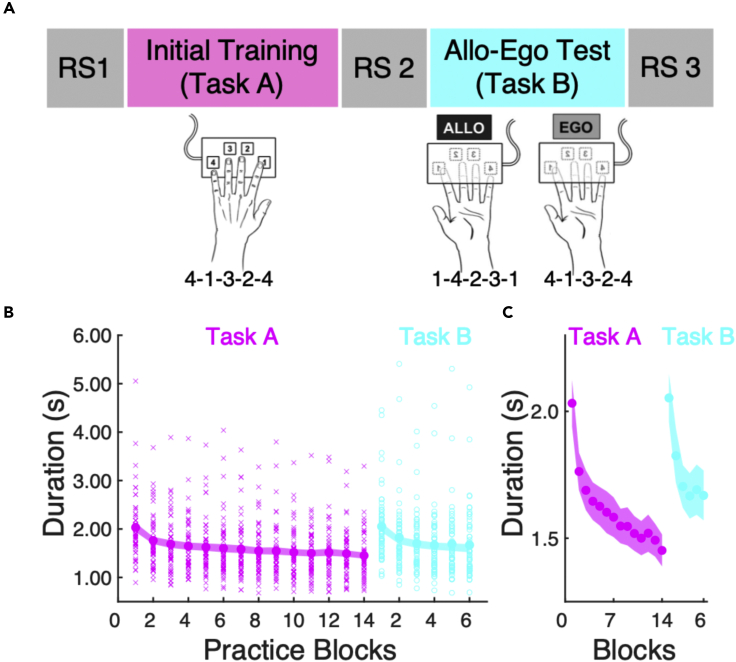
Figure 1.
Experimental design and behavioral results
(A) Resting state (RS) scans were acquired immediately prior to (RS1) and after (RS2) the initial training of a motor sequence learning task (Task A) in 55 young healthy individuals as well as following (RS3) a test session (Task B) assessing allocentric (Allo; n = 26) or egocentric (Ego; n = 29) sequence representations. Both tasks consisted of explicitly known 5-element sequences completed with the left non-dominant hand (1 = index finger; 2 = middle finger, 3 = ring finger, 4 = little finger). The egocentric representation Task B was assessed by having the participants complete the same sequence of finger presses as the initial training (i.e., 4-1-3-2-4); however, the spatial locations of the movement responses were now novel as both the keyboard and hand were inverted. For the allocentric representation, the spatial representation of the sequence was preserved by having the participants complete the mirrored finger sequence (i.e., 1-4-2-3-1) after the inversion of the keyboard and hand. Note that all task and RS runs were completed inside the MRI scanner.
(B) Participants exhibited significant reductions in the duration to complete a correct sequence (in s) across practice blocks of Task A (magenta), reflective of learning the sequence of movements. Similar results were obtained for Task B (cyan) that followed RS2. These performance improvements were revealed by significant effects of block in one-way repeated measures ANOVAs (14 blocks for Task A and 6 blocks for Task B). As performance speed did not differ between allocentric and egocentric representations of the motor sequence task (see main text for statistical details), the plots above collapsed across the two experimental groups. Large circles represent means, shaded regions represent +/− SEM, small x’s and o’s denote individual data for training and test runs, respectively. (C) Zoomed-in version of behavioral data depicted in panel B to better represent reductions in movement speed as a function of practice. Circles represent means, shaded regions represent +/− SEM Data underlying panels B and C are included in the file Data S1, Figure 1 Data.
Results
Behavior
Participants performed an explicitly known five-element finger sequence with their non-dominant (left) hand while positioned supine in the fMRI scanner and brain images were recorded (Figure 1A). During the initial training session (Task A), the time to perform a correct sequence (in seconds) decreased across blocks of practice (F(13,689) = 36.8, Greenhouse-Geisser corrected p value < 0.001, partial η2 = 0.404; Figures 1B and 1C). Following the post-training RS scan, participants were divided into two groups and completed 6 additional blocks of a task variant (Task B) that probed either the allocentric (i.e., spatial) or egocentric (i.e., motor) representation of the acquired motor sequence (see20 and STAR Methods later in discussion). Similar to Task A, the time to complete a sequence for Task B significantly decreased as a function of practice blocks (F(5,270) = 40.4, corrected p value < 0.001, partial η2 = 0.428). While the brain responses subtending these different task representations are known to be distinct,20 our analyses of the current sample were consistent with previous research20,24 demonstrating no differences in movement speed between the two task variants (group main effect: F(1,53) = 2.56, corrected p value = 0.12, partial η2 = 0.046; group × block interaction: F(5,265) = 0.32, corrected p value = 0.86, partial η2 = 0.006). These results collectively demonstrate the expected finding that participants exhibited evidence of motor sequence learning in both tasks, as reflected by improvements in movement speed.
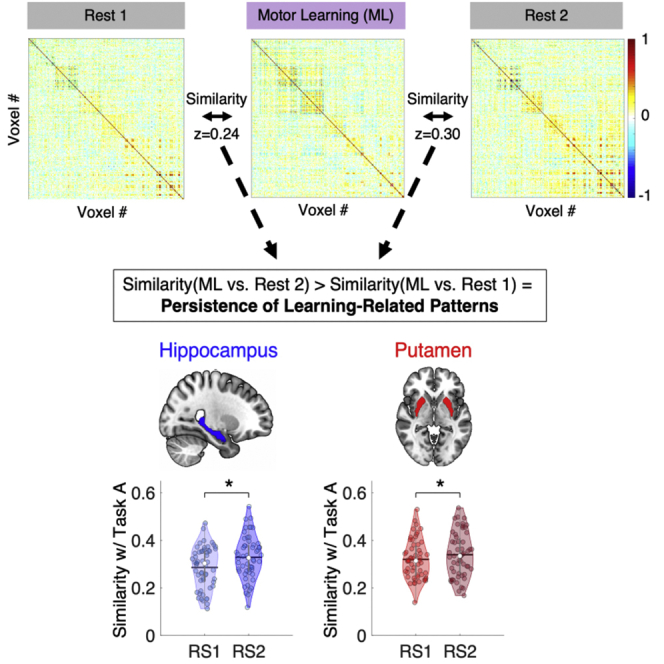
Persistence of task patterns following task A
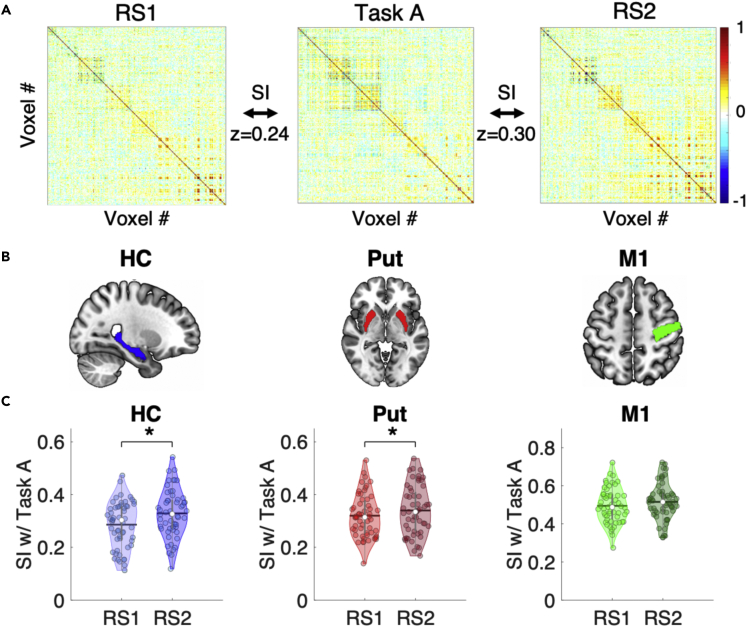
We first examined whether brain patterns during Task A (i.e., initial sequence training) within our three ROIs (Figure 2B) showed evidence of persistence into post-learning rest. To do so, we computed for each ROI and for each fMRI run of interest (i.e., RS1, Task A, and RS2) a multivoxel correlation structure (MVCS; see Figure 2A) reflecting the multivoxel activity pattern of the ROI.4 Next, for each ROI, we measured the similarity between rest- and task-related multivoxel patterns, defined as the r-to-z transformed correlation between the two MVCS matrices. We then tested whether RS1/Task A and Task A/RS2 similarity indices differed, with greater Task A/RS2 similarity indicative of the persistence of the task-related pattern into subsequent rest (i.e., similarity with RS1 serves as a within-subject control).4 Results showed that the multivoxel response patterns within the hippocampus (HC) and putamen (Put) during Task A were significantly more similar to activity patterns in the post-training RS2 scan as compared to the pre-training RS1 scan (HC: (t(54) = 2.86, p(unc) = 0.006, p(FDR) = 0.018, Cohen’s d = 0.39; Put: t(54) = 2.45, p(unc) = 0.018, p(FDR) = 0.027, Cohen’s d = 0.33; Figure 2C). In contrast, the similarity of M1 activity patterns between Task A and the RS scans did not differ between pre- and post-training RS (t(54) = 1.37, p(unc) = 0.18; Cohen’s d = 0.18; Figure 2C). These results indicate that task-related patterns within the hippocampus and striatum—but not in M1—significantly persisted into rest after initial motor sequence training.
Figure 2.
Pattern persistence following initial learning
(A) MVCS matrices are depicted for an exemplar ROI and participant. Each matrix shows the correlation between each of the n voxels of the ROI with all the other voxels of the ROI during the pre-training resting state (RS1), Task A (i.e., motor sequence learning (MSL) training) and post-training resting state (RS2) runs. The similarity index (SI) between two matrices (RS1 vs. Task A and Task A vs. RS2) is defined as the r-to-z transformed correlation between each pair of MVCS matrices. RS1/Task A and Task A/RS2 SIs were compared to assess whether task-related patterns persisted significantly into post-learning rest (results presented in panel C).
(B) Depiction of the hippocampus (HC), putamen (Put) and primary motor cortex (M1) ROIs overlaid on the MNI 152 template as part of the software MRIcroGL available at https://www.mccauslandcenter.sc.edu/mricrogl.
(C) Violin plots25 depicting the Similarity Indices (SI; Fisher Z-transformed correlation coefficients) between RS1 and Task A as well as between Task A and RS2 for the HC, Put, and M1. For the HC and Put, the pattern of neural responses observed during Task A persisted into the subsequent rest, reflective of the reactivation of learning-related neural activity. For all violin plots, individual data points are shown as small colored circles jittered on the horizontal axis within the respective plot to increase visualization (N = 55); white circles and horizontal black lines depict group medians and means, respectively. ∗ indicates p < 0.05 for paired t-test after FDR correction for multiple comparisons. Data underlying the figure are included in the file Data S1, Figure 2 Data.
Functional significance of pattern persistence following task A
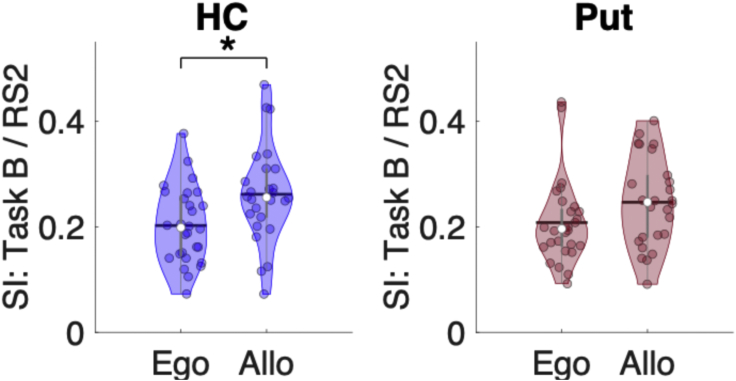
Task B was designed to probe the different task representations (i.e., allocentric and egocentric) that are known to develop during initial motor sequence learning (i.e., during Task A practice, see20,26). Here, we assessed whether post-learning RS2 patterns reflected allocentric and/or egocentric representations of the task. To do so, we tested whether RS2/Task B similarity indices differed between allocentric and egocentric task conditions in the two ROIs showing pattern persistence (i.e., the hippocampus and putamen). Results for the hippocampal ROI showed that the similarity between RS2 and Task B was significantly greater for the allocentric as compared to the egocentric representation (t(52) = 2.67, p(unc) = 0.010, p(FDR) = 0.020, Cohen’s d = 0.73; Figure 3). This suggests that the hippocampal multivoxel patterns reactivated during post-training RS2 reflect the allocentric representation of the sequence task. For the putamen, the difference between the allocentric and egocentric groups did not statistically differ (t(52) = 1.74, p(unc) = 0.09, Cohen’s d = 0.47), suggesting that the persistence of learning-related activity in the putamen does not differentially reflect allocentric or egocentric representations of the motor sequence.
Figure 3.
Functional significance of pattern persistence following Task A
Violin plots25 depicting the Similarity Indices (SI) between MVCS matrices from post-training RS (i.e., RS2) and Task B for the hippocampus (HC; blue) and putamen (Put; red) separately for the egocentric (Ego) and allocentric (Allo) groups. For the HC, SI was greater for the allocentric than the egocentric group, suggesting pattern persistence during RS2 (following initial learning) reflects the reactivation of the allocentric representation of the motor sequence. There were no group differences for the Put. For all violin plots, individual data points are shown as small colored circles jittered on the horizontal axis within the respective plot to increase visualization (N = 28 and 26 in Ego and Allo groups, respectively; one subject in Ego was excluded due to missing data); white circles and horizontal black lines depict group medians and means, respectively. ∗ indicates p < 0.05 for unpaired t-test after FDR correction for multiple comparisons. Data underlying the figure are included in the file Data S1, Figure 3 Data.
Persistence of task patterns following task B
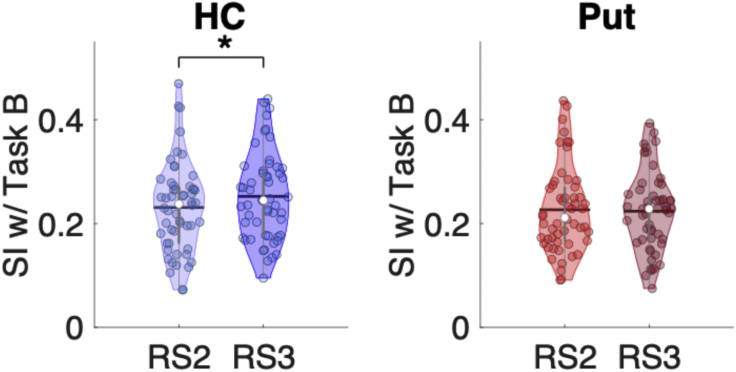
Our next analyses examined whether multivoxel patterns elicited by Task B showed evidence of persistence into the subsequent rest interval. To do so, we tested whether RS2/Task B and Task B/RS3 similarity indices differed, with greater Task B/RS3 similarity indicative of persistence of the task-related pattern into subsequent rest. Results demonstrated that hippocampal multivoxel patterns elicited by Task B (irrespective of allocentric/egocentric representations) indeed persisted into the RS3 epoch. Specifically, the Task B pattern was more similar to RS3 than RS2 (t(53) = 2.33, p(unc) = 0.024, p(FDR) = 0.048, Cohen’s d = 0.32; Figure 4). For the putamen, the multivoxel pattern during Task B did not persist into the subsequent rest interval (t(53) = 0.47, p(unc) = 0.64, Cohen’s d = 0.06).
Figure 4.
Persistence of task patterns following Task B
Violin plots25 depicting the Similarity Indices (SI) between MVCS matrices from Task B and those during RS2 and RS3 for the hippocampus (HC; blue) and putamen (Put; red). For the HC, the pattern of neural responses during Task B was more similar to RS3 than RS2, indicative of persistence. The patterns of neural responses in the Put during Task B were equally similar to the patterns observed during the preceding rest (RS2) and the subsequent rest (RS3) for both allocentric and egocentric conditions. For all violin plots, individual data points are shown as small colored circles jittered on the horizontal axis within the respective plot to increase visualization (N = 54; one subject was excluded due to missing data); white circles and horizontal black lines depict group medians and means, respectively. ∗ indicates p < 0.05 for the paired t-test after FDR correction for multiple comparisons. Data underlying the figure are included in the file Data S1, Figure 4 Data.
Functional significance of pattern persistence following task B
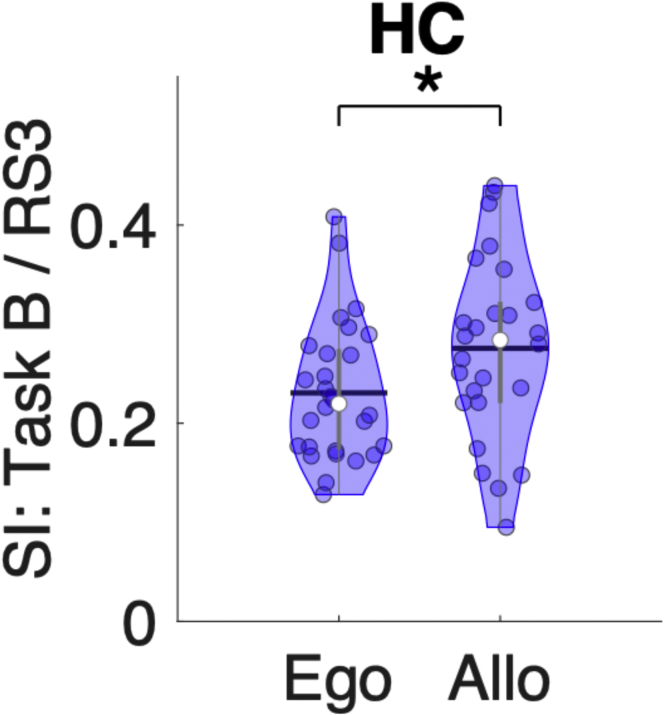
Analogous to the above, we assessed whether the hippocampal persistence following Task B reflected the allocentric or egocentric representation of the task. We, therefore, tested whether Task B/RS3 similarity differed between representations. Results showed that the similarity between RS3 and Task B was significantly greater for the allocentric as compared to the egocentric representation (t(52) = 2.04, p(unc) = 0.047, Cohen’s d = 0.56; Figure 5). This result indicates that the hippocampal multivoxel patterns reactivated during post-test RS3 reflect the allocentric representation of the sequence task. [Note that the putamen did not exhibit pattern persistence following Task B (i.e., see Figure 4) and thus was excluded from the analyses assessing functional significance.]
Figure 5.
Functional significance of pattern persistence following Task B
Violin plots25 depicting the Similarity Indices (SI) between MVCS matrices from the post-test RS (i.e., RS3) and those during Task B for the hippocampus (HC) separately for the egocentric (Ego) and allocentric (Allo) groups. SI was greater for the allocentric than the egocentric group, suggesting pattern persistence during RS3 (following Task B) reflects the allocentric representation of the motor sequence. For all violin plots, individual data points are shown as small colored circles jittered on the horizontal axis within the respective plot to increase visualization (N = 54; one subject was excluded due to missing data); white circles and horizontal black lines depict group medians and means, respectively. ∗ indicates p < 0.05 for independent t-test. Data underlying the figure are included in the file Data S1, Figure 5 Data.
Exploring brain-behavior associations
Based on the results presented above, one could hypothesize that the hippocampal pattern persistence following initial learning would be advantageous for subsequent performance on the allocentric version of the task. To explore this possibility, we correlated a Task A persistence score for the hippocampal ROI with a normalized measure of Task B performance (i.e., normalized to end-of-training Task A performance to account for individual differences in movement speed). Results indicated that there was no relationship between HC persistence and performance on the allocentric task variant (r = −0.11; p = 0.60).
Discussion
In this study, we used multivariate analyses of fMRI data acquired during a motor task and resting state to investigate whether brain patterns elicited by MSL persist into immediate post-learning waking rest. Results indicated that task-related patterns within the hippocampus and the striatum - but not in M1 - were reinstated during subsequent rest and thus provide evidence for the spontaneous reactivation of learning-related activity in awake rest epochs following motor sequence learning. Moreover, pattern persistence in the hippocampus specifically reflected the allocentric (spatial) representation of the acquired sequence, providing insights into the functional significance of hippocampal reactivation following motor learning.
Persistence of task-related hippocampal patterns into post-learning rest has been consistently observed in humans in the declarative memory domain.1,4,5,6,27,28,29,30 It is argued that such pattern persistence reflects “reactivation” or “replay” of patterns that were previously expressed during learning.1 The current study reports task-related hippocampal pattern persistence into post-learning rest in the motor memory domain. These findings extend previous neuroimaging investigations highlighting the crucial role of the hippocampus in motor learning [e.g.,9,10]. Moreover, our findings are in line with recent examinations assessing hippocampal activity on the micro-offline timescale (i.e., during rest blocks interleaved with practice). Jacobacci et al.17 demonstrated that hippocampal activations during these micro-offline rest epochs - as revealed by univariate analyses - tended to be linked to the amplitude of micro-offline gains in performance as well as learning-related changes in hippocampal structure. Additionally, Buch and colleagues18 conducted multivariate pattern analyses of magnetoencephalography (MEG) data and their results demonstrated hippocampal replay during micro-offline epochs. On the macro-offline timescale (i.e., between sessions of motor task practice), results from Buch et al.18 indicated that the replay patterns in the post-task RS epoch were comparable to those observed pre-task and thus evidence of replay or pattern persistence into post-learning rest was lacking.18 The reasons for these discrepant findings between this earlier research and the current results are not immediately clear, but it is worth noting that the two studies employed different imaging modalities (MEG vs. fMRI) with inherently different spatial and temporal resolutions and distinct analytic approaches.
Earlier research from our own group in which a motor sequence task was manipulated to assess different task representations demonstrated that the hippocampus supports the allocentric (i.e., spatial) representation of acquired sequences.26 In line with this finding, results from the current study suggest that the spatial representation of an acquired motor sequence is reactivated in the hippocampus during the early consolidation window following learning. These results are in line with a plethora of rodent studies suggesting that hippocampal replays consolidate previously learned spatial relationships (see19 or a review). The current data thus provide novel insights into the functional significance of hippocampal reactivation following motor sequence learning in humans. That is, the offline reactivation of learning-related hippocampal activity reflects the spatial representation of the motor sequence acquired during online task practice. It is worth noting, however, that the reactivation of the spatial task representation did not lead to subsequent performance enhancements on the allocentric motor task variant. Accordingly, the implications of this hippocampal reactivation for motor performance have yet to be elucidated.
The recurrence of task patterns into subsequent sleep and wakeful rest has been previously reported in rodent research in the ventral striatum [after reward-based learning;31] and putamen [after the exploration of novel objects;32]. While there is evidence from previous human research that striatal multivariate patterns are modulated during motor learning,16,33,34 the current research reports the persistence of motor task-specific striatal patterns into post-learning rest in humans. Interestingly, such reactivation or reverberation of striatal task patterns has been observed in rodents in conjunction with hippocampal replay.21,32 Even though there is no causal evidence that the hippocampus drives replay in the striatum, it has previously been argued that the hippocampus plays a network synchronization role by orchestrating memory trace consolidation in concert with other task-relevant areas such as the striatum.35 Although our results cannot directly speak to the hippocampus as an orchestrator of such inter-regional communications, our data do demonstrate that both the hippocampus and striatum are reactivated during wakefulness following initial learning and thus are consistent with the aforementioned rodent work.21,32
Against our expectations that pattern persistence in the striatum would reflect egocentric (motoric) representations of the motor task,20 the current results indicated that persistence of learning-related activity in the putamen did not differ between allocentric or egocentric task conditions. The functional significance of putamen replay after motor learning, therefore, remains unclear. Based on earlier observations that the striatal system is involved in learning predictive stimulus-response associations (e.g., finger-key mapping,36), one might argue that pattern persistence in the striatum might reflect such task-specific associations during RS2 which, by definition, is not shared with tasks requiring different stimulus-response associations (i.e., task B). This interpretation remains speculative and warrants further investigation. Interestingly, and in contrast to the hippocampus, our data did not provide evidence of pattern persistence following Task B (i.e., allocentric/egocentric test) in the striatum. Specifically, multivoxel activity patterns during Task B were equally similar to the preceding and subsequent RS scans (i.e., RS2 and RS3). It is possible that this null result can be explained by the shorter amount of sequence practice in Task B (i.e., 6 blocks as compared to 14 blocks of Task A). Specifically, it could be argued that this practice duration was not sufficient to elicit the significant reinstatement of task-specific patterns of striatal activity in subsequent rest intervals. The current experimental design unfortunately does not allow us to test this potential explanation.
The results observed on M1 patterns stand in contrast with previous animal22 and human23 research showing the replay of task-related M1 activity patterns during subsequent sleep. It could be argued that M1 reactivations preferentially occur during post-learning sleep as compared to wakefulness. However, this remains hypothetical, as earlier studies suggest that M1 does not play a prominent role in sleep-dependent motor memory consolidation [see8 for a review]. Our results are in line with recent evidence showing that, in contrast to premotor areas, activity patterns in M1 do not show motor sequence representations but rather exhibit patterns related to the execution of individual finger movements.37,38 It is thus tempting to speculate that M1 patterns do not code for the motor sequence memory trace per se, which might explain the lack of M1 pattern reactivation during post-learning rest reported in the current study.
In conclusion, our results show that patterns of brain activity within the hippocampus and striatum—but not M1—observed during motor learning persisted into post-learning rest. Importantly, our data suggest that the hippocampus specifically replays the spatial representation of the learned motor sequence. Our results thus provide insights into the functional significance of brain reactivation after motor sequence learning in early consolidation windows consisting of wakefulness. Moreover, our findings add to the growing body of literature demonstrating that the hippocampus plays a vital role in learning and memory processes in the motor memory domain.
Limitations of the study
In contrast to previous research in the declarative domain,4 the current results cannot speak to whether the magnitude of the pattern persistence is related to longer-term memory consolidation or retention processes. It would thus be beneficial for future research to examine whether the reinstatement of task-relevant patterns following motor sequence learning is linked to long-term advantages at the behavioral level. Moreover, although the present data demonstrate pattern persistence during wake intervals following motor learning, it is unclear whether similar reactivation processes occur during post-learning sleep epochs. Given the known role of post-learning sleep in motor memory consolidation processes7 as well as the original place cell work in animal models,39,40 one could expect to observe similar reactivation processes during sleep. It would be interesting for future research to examine this possibility directly. Last, and as with all neuroimaging research, head motion can impact the reported results. One could argue that the deleterious influence of head motion artifacts is magnified in studies that employ a multivoxel analytical approach and assess neural activity across relatively long imaging sessions. Although we adopted multiple approaches to minimize the impact of head movement (e.g., excluding participants with a maximum linear movement within a run that exceeded 3mm, inputting head motion parameters as nuisance regressors, and a scrubbing procedure that discarded high-motion volumes), we cannot completely eliminate the possibility that head motion exerted an influence on the MVCS results.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Data published in previous paper. | Albouy et al.20 | N/A |
| Software and algorithms | ||
| MATLAB 2020B | https://www.mathworks.com/products/matlab.html | RRID:SCR_001622 |
| Cogent2000 | http://www.vislab.ucl.ac.uk/cogent_2000.php | RRID:SCR_015672 |
| FSL | http://www.fmrib.ox.ac.uk/fsl/ | RRID:SCR_002823 |
| SPM12 | http://www.fil.ion.ucl.ac.uk/spm/software/spm12/ | RRID:SCR_007037 |
| MRICroGL | https://www.mccauslandcenter.sc.edu/mricrogl | N/A |
| GitHub | https://github.com/ | RRID:SCR_002630 |
| Code (via Zenodo) | https://doi.org/10.5281/zenodo.7245717 | RRID:SCR_004129 |
Resource availability
Lead contact
-
•
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Genevieve Albouy (genevieve.albouy@health.utah.edu).
Materials availability
-
•
This study did not generate new materials.
Experimental model and subject details
Sixty-two right-handed,41 healthy adults (18-30 years of age) were recruited by local advertisements to participate in this study, which was approved by the Research ethics board of the “Regroupement en Neuroimagerie du Québec (RNQ), Centre de recherche de l’Institut universitaire de gériatrie de Montréal, Université de Montréal”. Participants did not suffer from any known psychological, psychiatric (including anxiety42 and depression43) or neurological disorders, and reported normal sleep during the month preceding the experiment, as assessed with the Pittsburgh Sleep Quality Index.44 None of the subjects were taking medications at the time of testing. Moreover, none received formal training on a musical instrument or as a typist. Of the 62 participants recruited for the study, seven were discarded from all analyses. Three participants were excluded for excessive head motion during functional brain imaging. Specifically, 2 participants had maximum linear translations that exceeded 3mm (∼1 voxel) and an additional participant had more than 50% of the volumes removed as part of the scrubbing procedure (see details below). One participant was excluded because missing data from RS 1 and 2 scans, precluding the assessment of persistence following initial learning. Three additional participants were excluded as they presented outlier similarity indices assessing Task A pattern persistence as part of the multivoxel imaging analyses described below (i.e., > 3 standard deviations away from the group average). Consequently, a total of 55 subjects were included (mean age ± SD: 23.8 ± 3.4 years, 36 females).
Method details
Experimental design
All participants were asked to follow a 4-day constant sleep schedule (according to their own rhythm ± 1 h) before the experiment. Compliance to the schedule was assessed using both sleep diaries and wrist actigraphy measures (Actiwatch AW2, Bio-Lynx scientific equipment Inc., Montréal, Canada). On the experimental day, participants were trained to perform a MSL task (see below) in an MRI scanner at approximately 11:30 am. Resting state (RS) functional scans were acquired immediately prior (RS1) and after (RS2) initial MSL training (referred to as Task A) as well as following (RS3) a task variant assessing allocentric or egocentric motor sequence representations (referred to as Task B; see below for additional details). Note that these 5 functional imaging runs were the first runs of a larger experimental protocol completed by the same participants that examined the effects of sleep on offline motor memory consolidation processes (see20 for full protocol).
Motor sequence learning paradigm
The sequential finger tapping task used in this research was coded in Cogent2000 (http://www.vislab.ucl.ac.uk/cogent.php) and implemented in MATLAB (MathWorks Inc., Sherbom, MA). Participants were instructed to tap a five-element finger sequence on an MR-compatible keyboard with their non-dominant (left) hand as fast and as accurately as possible. The sequence to perform was explicitly provided to the participants prior to training. Blocks of task practice were interleaved with 15-second rest intervals, indicated by green and red fixation crosses, respectively, on the center of the screen. Each practice block consisted of 60 key presses, ideally corresponding to 12 correct sequences. This procedure effectively controlled the number of movements executed in each block. Yet, the duration of the practice blocks progressively decreased with learning, as subjects became faster at executing the 60 key presses. During the rest blocks, participants were instructed to keep their fingers still and look at the red cross. The number and timing of each specific key press within each practice block was recorded and subsequently used for the quantification and statistical analyses described below.
For Task A, all participants performed 14 blocks of the sequence 4-1-3-2-4, where 1 through 4 represent the index through little fingers. For Task B (6 blocks), the keyboard and the subject’s hand were turned upside down and participants were assigned to one of two experimental conditions that differed based on whether the allocentric (n=26) or egocentric (n=29) representation of the motor sequence was probed (see20). The egocentric representation was assessed by having the participants complete the same sequence of finger presses (i.e., 4-1-3-2-4); however, with the keyboard/hand turned upside down, the spatial locations of the movement responses were now novel. Conversely, for the allocentric representation, the spatial representation of the sequence was preserved by having the participants complete the mirrored finger sequence (i.e., 1-4-2-3-1).
fMRI data acquisition and processing
Acquisition
Functional MRI-series were acquired using a 3.0 T TIM TRIO scanner system (Siemens, Erlangen, Germany), equipped with a 32-channel head coil. Multislice T2∗-weighted fMRI images were obtained, during both resting state and task practice, with a gradient echo-planar sequence using axial slice orientation (TR = 2650 ms, TE = 30 ms, FA = 90°, 43 transverse slices, 3 mm slice thickness, 10% inter-slice gap, FoV = 220 × 220 mm2, matrix size = 64 × 64 × 43, voxel size = 3.4 × 3.4 × 3 mm3). Slices covering the whole brain were acquired along the z-axis in an ascending direction. During each resting state scan (6 min 40 s), participants were instructed to keep their eyes open and fixate on a white cross in the middle of a black screen. They were also asked to remain still and “not to think about anything in particular”. A structural T1-weighted 3D MP-RAGE sequence (TR = 2300 ms, TE = 2.98 ms, TI = 900 ms, FA = 9°, 176 slices, FoV = 256 × 256 mm2, matrix size = 256 × 256 × 176, voxel size = 1 × 1 × 1 mm3) was also acquired in all subjects. Head movements were minimized using cushions.
ROI definition
Three ROIs were considered in the analyses and consisted of the bilateral hippocampus, bilateral putamen and right M1 (i.e., contralateral to the hand practicing the task). Note that we elected to use a bilateral – as opposed to unilateral – ROI for the putamen based on previous research demonstrating that offline memory consolidation processes are not limited to the contralateral striatum.16,45,46,47,48 The hippocampus and putamen ROIs were created in the native space of each individual using the FMRIB's Integrated Registration Segmentation Toolkit (FSL FIRST; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST). The bilateral hippocampal ROI included an average of 309 voxels (SD = 33; range of 258 – 385) and the bilateral striatal ROI included a mean of 309 voxels (SD = 22; range of 274 – 362). The right M1 ROI was defined by masking the activation map from the group-level main effect of practice map (task > rest) derived from univariate analyses in20 (threshold p <0.05 whole brain FWE corrected) with the anatomically-defined right M1 from the Human Motor Area Template (HMAT).49 As this functional map and anatomical template were provided in MNI space, the M1 ROI was mapped back to native space using the individual’s inverse deformation field output from segmentation of the anatomical image. The right M1 ROI included an average of 254 voxels (SD = 23; range of 204 – 302).
Pre-processing
The fMRI data were preprocessed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/; Wellcome Centre for Human Neuroimaging, London, UK). The reoriented structural image was segmented into gray matter (GM), white matter (WM), cerebrospinal fluid (CSF), bone, soft tissue, and background. Task-based and RS functional volumes of each participant were first slice-time corrected (reference: middle slice). Images were then realigned to the first image of each run and, in a second step, realigned to the mean functional image computed across all the individuals’ fMRI runs using rigid body transformations. The mean functional image was co-registered to the high-resolution T1-weighted anatomical image using a rigid body transformation optimized to maximize the normalized mutual information between the two images. The resulting co-registration parameters were then applied to the realigned functional images. To optimize voxel pattern analyses, functional and anatomical data remained in subject-specific (i.e., native) space, and no spatial smoothing was applied to functional images.4
As part of the custom analysis pipeline written in MATLAB, additional preprocessing of the time series was completed. Specifically, whole-brain signal was detrended and high-pass filtered (cutoff=1/128). The realignment parameters were used to compute framewise displacement (FD). If the FD of any volume n exceeded 0.5mm, volume n and n+1 were discarded from analyses. The percentage of volumes excluded from the RS1, Task B, RS2, Task B and RS3 runs were 0.70 (SD=1.58), 5.90 (SD=8.06), 1.63 (SD=3.22), 7.68 (SD=9.54) and 1.33 (SD = 2.60), respectively. At the ROI level, only voxels with >10% GM probability were included in the analyses. Regression analyses were performed on the fMRI time-series of the remaining voxels in each ROI to remove additional sources of noise. These nuisance regressors included the first three principal components of the signal extracted from the WM and CSF masks created during segmentation of the anatomical image, the 6-dimensional head motion realignment parameters, as well as the realignment parameters squared, their derivatives, and the squared of the derivatives (i.e., a total of 30 nuisance regressors). Last, the number of volumes included in each specific analysis was matched across relevant runs within each individual. To do so, the run with the smallest number of volumes was identified, and for the other subset runs, the corresponding number of volumes centered around the middle volume of that run were selected. For the analysis assessing the persistence following Task A (results presented in Figure 2), the average number of volumes per run included in the analyses was 146 (SD = 6; range 117-150). The remaining analyses included Task B, which consisted of only 6 blocks of motor task practice and thus fewer number of volumes were obtained. For these analyses, the average number of volumes per run included was 94 (SD = 2; range 55-147).
Quantification and statistical analyses
Quantification and statistical analyses were conducted in MATLAB. Cohen’s d, partial η2 and Pearson’s correlation coefficients are reported as indicators of effect size. Results were considered significant if p < 0.05. For MVCS analyses, a false-discovery rate (FDR) correction for testing multiple ROIs50 was applied within each family of hypothesis tests. For behavioral analyses, Greenhouse-Geisser corrections were applied if the sphericity assumption was violated. Statistical details can be found in the results section and figure captions.
Behavior
Performance on the motor sequence learning task was assessed with a measure of movement speed (time to perform a correct sequence in s). As accuracy (i.e., number of correct sequences per block) in this specific experiment was quite high (> 11 correct sequences per block on average; maximum = 12) and stable across blocks of practice (see20), it was not considered further. To ensure participants learned the novel motor sequence, the behavioral measure of movement speed was assessed with separate one-way repeated-measures ANOVAs, with block as the within-subject factor for both Task A (14 blocks) and Task B (6 blocks). As Task B consisted of a motor task variant that assessed either the allocentric or egocentric sequence representation, block (6) x representation (2) ANOVAs were conducted to ensure no performance differences between the two groups (as indicated in the initial analyses reported in20).
Multi-voxel correlation structure (MVCS) analyses
Multi-voxel correlation structure (MVCS) matrices were computed for each ROI and each run of interest with similar procedures as in previous research.4,6 Specifically, Pearson’s correlations were computed between each of n BOLD-fMRI voxel time courses, yielding an n by n MVCS matrix per ROI, run and individual. Pearson’s correlation coefficients were Fisher z-transformed to ensure normality. Each MVCS matrix is thought to reflect the response pattern of the ROI in the specific run.4
To assess the influence of motor learning on multivoxel patterns within our ROIs, similarity indices (SI) that reflect the similarity of the multivoxel patterns between two specific fMRI runs were computed as the r-to-z transformed correlation between the two MVCS matrices of interest.4,27 Four statistical analyses were then conducted to test specific research questions of interest. First, to assess persistence of task-related patterns following Task A (i.e., initial motor sequence learning), SIs were computed between (a) RS1 and Task A and (b) Task A and RS2. The magnitude of the similarity indices between Task A and RS2 reflects the degree of persistence of the learning-related patterns into the subsequent rest interval. To assess whether this persistence is significant, a comparison was made to the SI between the Task A and RS1 (i.e., pre-training) as a within-subject control. Specifically, paired t-tests were computed using MATLAB to compare SIs between RS1/Task A and Task A/RS2. Our second analysis assessed whether the observed pattern persistence revealed in the first analysis reflected the allocentric or egocentric representation of the motor sequence assessed by Task B. Specifically, independent t-tests were conducted to assess group differences (allocentric vs. egocentric) in the similarity between the multivoxel patterns observed during RS2 and Task B. Third, we examined whether multivoxel patterns during Task B showed evidence of persistence into the subsequent rest interval (i.e., RS3). This was achieved by conducting paired t-tests between Task B similarity with RS2 vs. RS3 across task variants. Last, and mirroring our second analysis described above, we assessed whether persistence following Task B (i.e., in RS3) reflected the allocentric or egocentric representation of the motor sequence. Accordingly, independent t-tests were conducted to assess group differences (allocentric vs. egocentric) in the similarity between the multivoxel patterns observed during RS3 and Task B.
Brain-behavior relationships
The link between the persistence of task-related patterns following initial learning (Task A) and subsequent motor performance (i.e., on Task B) was explored via correlation analyses. Specifically, we computed a measure of Task A pattern persistence by dividing the SI between Task A and RS2 by the SI between Task A and RS1. To relate to a measure of performance for Task B, averaged sequence duration for the 6 blocks of Task B was divided by the mean sequence duration from the last 6 blocks of Task A. Essentially, this computation normalized Task B performance by end-of-training performance on Task A to account for individual differences in movement speed. Pearson’s correlation coefficients were then computed to assess brain/behavior relationships.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research (CIHR; MOP 97830) as well as the Ministry of Economic Development, Innovation, and Exportation of Quebec (MDEIE; PSR-SIIRI-704) to JD. MAG received salary support from the Research Foundation Flanders (FWO; project G099516N and predoctoral fellowship 1141320N).
Author contributions
GA and JD conceived and designed the experiment. GA conducted the experiments. GA, BRK, MAG, and DM contributed to analytic tools. BRK analyzed the data. BRK and GA wrote the initial draft of the article. All authors contributed to the subsequent revisions.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse and equitable conduct of research.
Published: December 22, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105498.
Supplemental information
Data and code availability
-
•
The approval granted by the local ethics committee does not permit the sharing of individual raw data. All source data underlying the figures shown in the main text are available in the included file Data S1.
-
•
Original code used for data analyses can be found on GitHub (https://github.com/BradleyRossKing/MVCS.git) and is publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Tambini A., Davachi L. Awake reactivation of prior experiences consolidates memories and biases cognition. Trends Cogn. Sci. 2019;23:876–890. doi: 10.1016/j.tics.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neill J., Pleydell-Bouverie B., Dupret D., Csicsvari J. Play it again: reactivation of waking experience and memory. Trends Neurosci. 2010;33:220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Carr M.F., Jadhav S.P., Frank L.M. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat. Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tambini A., Davachi L. Persistence of hippocampal multivoxel patterns into postencoding rest is related to memory. Proc. Natl. Acad. Sci. USA. 2013;110:19591–19596. doi: 10.1073/pnas.1308499110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruber M.J., Ritchey M., Wang S.F., Doss M.K., Ranganath C. Post-learning hippocampal dynamics promote preferential retention of rewarding events. Neuron. 2016;89:1110–1120. doi: 10.1016/j.neuron.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermans E.J., Kanen J.W., Tambini A., Fernández G., Davachi L., Phelps E.A. Persistence of Amygdala–hippocampal connectivity and multi-voxel correlation structures during awake rest after fear learning predicts long-term expression of fear. Cereb. Cortex. 2017;27:3028–3041. doi: 10.1093/cercor/bhw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albouy G., King B.R., Maquet P., Doyon J. Hippocampus and striatum: dynamics and interaction during acquisition and sleep-related motor sequence memory consolidation. Hippocampus. 2013;23:985–1004. doi: 10.1002/hipo.22183. [DOI] [PubMed] [Google Scholar]
- 8.King B.R., Hoedlmoser K., Hirschauer F., Dolfen N., Albouy G. Sleeping on the motor engram: the multifaceted nature of sleep-related motor memory consolidation. Neurosci. Biobehav. Rev. 2017;80:1–22. doi: 10.1016/j.neubiorev.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Schendan H.E., Searl M.M., Melrose R.J., Stern C.E. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 10.Albouy G., Sterpenich V., Balteau E., Vandewalle G., Desseilles M., Dang-Vu T., Darsaud A., Ruby P., Luppi P.H., Degueldre C., et al. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron. 2008;58:261–272. doi: 10.1016/j.neuron.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Cousins J.N., El-Deredy W., Parkes L.M., Hennies N., Lewis P.A. Cued reactivation of motor learning during sleep leads to overnight changes in functional brain activity and connectivity. PLoS Biol. 2016;14:e1002451. doi: 10.1371/journal.pbio.1002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernández-Seara M.A., Aznárez-Sanado M., Mengual E., Loayza F.R., Pastor M.A. Continuous performance of a novel motor sequence leads to highly correlated striatal and hippocampal perfusion increases. Neuroimage. 2009;47:1797–1808. doi: 10.1016/j.neuroimage.2009.05.061. [DOI] [PubMed] [Google Scholar]
- 13.Gheysen F., Van Opstal F., Roggeman C., Van Waelvelde H., Fias W. Hippocampal contribution to early and later stages of implicit motor sequence learning. Exp. Brain Res. 2010;202:795–807. doi: 10.1007/s00221-010-2186-6. [DOI] [PubMed] [Google Scholar]
- 14.Tomassini V., Jbabdi S., Kincses Z.T., Bosnell R., Douaud G., Pozzilli C., Matthews P.M., Johansen-Berg H. Structural and functional bases for individual differences in motor learning. Hum. Brain Mapp. 2011;32:494–508. doi: 10.1002/hbm.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albouy G., Sterpenich V., Vandewalle G., Darsaud A., Gais S., Rauchs G., Desseilles M., Boly M., Dang-Vu T., Balteau E., et al. Interaction between hippocampal and striatal systems predicts subsequent consolidation of motor sequence memory. PLoS One. 2013;8:e59490. doi: 10.1371/journal.pone.0059490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinsard B., Boutin A., Gabitov E., Lungu O., Benali H., Doyon J. Consolidation alters motor sequence- specific distributed representations. Elife. 2019;8:e39324. doi: 10.7554/eLife.39324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobacci F., Armony J.L., Yeffal A., Lerner G., Amaro E., Jovicich J., Doyon J., Della-Maggiore V. Rapid hippocampal plasticity supports motor sequence learning. Proc. Natl. Acad. Sci. USA. 2020;117:23898–23903. doi: 10.1073/pnas.2009576117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buch E.R., Claudino L., Quentin R., Bönstrup M., Cohen L.G. Consolidation of human skill linked to waking hippocampo-neocortical replay. Cell Rep. 2021;35:109193. doi: 10.1016/j.celrep.2021.109193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeiffer B.E. The content of hippocampal “replay. Hippocampus. 2020;30:6–18. doi: 10.1002/HIPO.22824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albouy G., Fogel S., King B.R., Laventure S., Benali H., Karni A., Carrier J., Robertson E.M., Doyon J. Maintaining vs. enhancing motor sequence memories: respective roles of striatal and hippocampal systems. Neuroimage. 2015;108:423–434. doi: 10.1016/j.neuroimage.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 21.Lansink C.S., Goltstein P.M., Lankelma J.V., McNaughton B.L., Pennartz C.M.A. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 2009;7:e1000173. doi: 10.1371/journal.pbio.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramanathan D.S., Gulati T., Ganguly K. Sleep-dependent reactivation of ensembles in motor cortex promotes skill consolidation. PLoS Biol. 2015;13:e1002263. doi: 10.1371/journal.pbio.1002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eichenlaub J.B., Jarosiewicz B., Saab J., Franco B., Kelemen J., Halgren E., Hochberg L.R., Cash S.S. Replay of learned neural firing sequences during rest in human motor cortex. Cell Rep. 2020;31:107581. doi: 10.1016/j.celrep.2020.107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King B.R., Fogel S.M., Albouy G., Doyon J. Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Front. Hum. Neurosci. 2013;7:142. doi: 10.3389/fnhum.2013.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bechtold B. Violin Plots for Matlab. Github Proj. 2016. https://github.com/bastibe/ViolinplotMatlab
- 26.Albouy G., Fogel S., Pottiez H., Nguyen V.A., Ray L., Lungu O., Carrier J., Robertson E., Doyon J. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PLoS One. 2013;8:e52805. doi: 10.1371/journal.pone.0052805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tambini A., Rimmele U., Phelps E.A., Davachi L. Emotional brain states carry over and enhance future memory formation. Nat. Neurosci. 2017;20:271–278. doi: 10.1038/nn.4468. [DOI] [PubMed] [Google Scholar]
- 28.Schapiro A.C., McDevitt E.A., Rogers T.T., Mednick S.C., Norman K.A. Human hippocampal replay during rest prioritizes weakly learned information and predicts memory performance. Nat. Commun. 2018;9:3920. doi: 10.1038/s41467-018-06213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tambini A., D'Esposito M. Causal contribution of awake post-encoding processes to episodic memory consolidation. Curr. Biol. 2020;30:3533–3543.e7. doi: 10.1016/j.cub.2020.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuck N.W., Niv Y. Sequential replay of nonspatial task states in the human hippocampus. Science. 2019;364:eaaw5181. doi: 10.1126/science.aaw5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lansink C.S., Goltstein P.M., Lankelma J.V., Joosten R.N.J.M.A., McNaughton B.L., Pennartz C.M.A. Preferential reactivation of motivationally relevant information in the ventral striatum. J. Neurosci. 2008;28:6372–6382. doi: 10.1523/JNEUROSCI.1054-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribeiro S., Gervasoni D., Soares E.S., Zhou Y., Lin S.C., Pantoja J., Lavine M., Nicolelis M.A.L. Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. PLoS Biol. 2004;2:e24. doi: 10.1371/journal.pbio.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen K.W., Madsen K.H., Siebner H.R. Discrete finger sequences are widely represented in human striatum. Sci. Rep. 2020;10:13189. doi: 10.1038/s41598-020-69923-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berlot E., Popp N.J., Diedrichsen J. A critical re-evaluation of fmri signatures of motor sequence learning. Elife. 2020;9:e55241. doi: 10.7554/eLife.55241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rusu S.I., Pennartz C.M.A. Learning, memory and consolidation mechanisms for behavioral control in hierarchically organized cortico-basal ganglia systems. Hippocampus. 2020;30:73–98. doi: 10.1002/hipo.23167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penhune V.B., Steele C.J. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav. Brain Res. 2012;226:579–591. doi: 10.1016/j.bbr.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 37.Yokoi A., Arbuckle S.A., Diedrichsen J. The role of human primary motor cortex in the production of skilled finger sequences. J. Neurosci. 2018;38:1430–1442. doi: 10.1523/JNEUROSCI.2798-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoi A., Diedrichsen J. Neural organization of hierarchical motor sequence representations in the human neocortex. Neuron. 2019;103:1178–1190.e7. doi: 10.1016/J.NEURON.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Pavlides C., Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J. Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson M.A., McNaughton B.L. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 41.Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 42.Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 43.Beck A.T., Ward C., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 44.Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 45.Debas K., Carrier J., Orban P., Barakat M., Lungu O., Vandewalle G., Hadj Tahar A., Bellec P., Karni A., Ungerleider L.G., et al. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc. Natl. Acad. Sci. USA. 2010;107:17839–17844. doi: 10.1073/pnas.1013176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fogel S., Albouy G., King B.R., Lungu O., Vien C., Bore A., Pinsard B., Benali H., Carrier J., Doyon J. Reactivation or transformation? Motor memory consolidation associated with cerebral activation time-locked to sleep spindles. PLoS One. 2017;12:e0174755. doi: 10.1371/JOURNAL.PONE.0174755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albouy G., King B.R., Schmidt C., Desseilles M., Dang-Vu T.T., Balteau E., Phillips C., Degueldre C., Orban P., Benali H., et al. Cerebral activity associated with transient sleep-facilitated reduction in motor memory vulnerability to interference. Sci. Rep. 2016;6:34948. doi: 10.1038/srep34948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King B.R., Saucier P., Albouy G., Fogel S.M., Rumpf J.J., Klann J., Buccino G., Binkofski F., Classen J., Karni A., Doyon J. Cerebral activation during initial motor learning forecasts subsequent sleep-facilitated memory consolidation in older adults. Cereb. Cortex. 2017;27:1588–1601. doi: 10.1093/cercor/bhv347. [DOI] [PubMed] [Google Scholar]
- 49.Mayka M.A., Corcos D.M., Leurgans S.E., Vaillancourt D.E. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31:1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The approval granted by the local ethics committee does not permit the sharing of individual raw data. All source data underlying the figures shown in the main text are available in the included file Data S1.
-
•
Original code used for data analyses can be found on GitHub (https://github.com/BradleyRossKing/MVCS.git) and is publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.