Abstract
Biological tissues have developed structures that fulfil their various specific requirements. Mineralized tissues, such as tooth and bone, are often of mechanical competence for load bearing. Tooth enamel is the hardest and toughest mineralized tissue. Despite a few millimeters thick and with minimal regenerative capacity, human tooth enamel maintains its functions throughout a lifetime. Bone provides skeletal support and essential metabolism to our body. Degenerative diseases and ageing induce the loss of mechanical integrity of the bone, increasing the susceptibility to fractures. Tooth and bone share certain commonalities in chemical components and material characteristics, both consisting of nanocrystalline apatite and matrix proteins as their basic foundational structural units. Although the mechanical properties of such mineralized hard tissues remain unclear, it is plausible that they have an inherent toughening mechanism. Nanoindentation is able to characterize the mechanical properties of tooth enamel and bone at multiscale levels, and the results suggest that such toughening mechanisms of enamel and bone may be mainly associated with the smallest-scale structure–function relationships. These findings will benefit the development of advanced biomaterials in the field of material science and will further our understanding of degenerative bone disease in the clinical community.
Keywords: Nanoindentation, Mineralized tissue, Bone, Tooth, Mechanical property
1. Introduction
Nature sculpts biological tissues with unique microstructures, which largely determine the mechanical properties of the tissue and play critical roles in governing its functioning and environmental adaptation. The characteristics of mineralized hard tissues such as tooth, bone, and seashell are able to fulfill various multifunctional requirements, often showing compelling advantages over those of similar engineered materials [1], [2], [3], [4]. We can learn useful lessons from such well-designed natural biological materials. Therefore, the structural motifs found in hard tissues have long inspired researchers to develop and conceptualize new multifunctional materials for clinical and/or industrial implements. In other words, material development has entered an era of biomimicry and bioduplication. Comprehending the knowledge of the structure–function relationships of mineralized tissues is the primary step towards furthering the development of biomimetic load-bearing biomaterials.
From a dentistry perspective, new dental materials with similar mechanical properties to those of tooth enamel are required for dental fillings and/or prosthetic crowns. Dental ceramics such as yttrium-stabilized zirconia are now widely used in dental clinics, mainly because they have a favorable white appearance and are relatively strong compared with dental porcelain [5], [6]. The replacement of metallic materials with new dental ceramics may meet with some success, but the considerable mechanical discontinuity at the interfaces between the host tooth and the ceramic remains a clinical challenge, jeopardizing treatment outcomes. Moreover, the toughness and durability of the new ceramics remain inferior to those of traditional metallic dental materials or the host tooth [6], [7], [8].
The intrinsic toughening mechanisms of tooth and bone depend, at least in part, on their complex hierarchical structures. For instance, inherent structural motifs, such as enamel rods surrounded by protein-rich sheath layers [9] and the haversian osteon in cortical bone [10], play important roles in producing interfaces that open up in the presence of potentially dangerous crack, thereby making crack propagation energetically expensive [3], [11]. In other words, the distribution, orientation, periodicity and the wellness of alignment of tissues’ local motifs would affect their material properties[12], resulting the mesoscopic mechanical heterogeneity of biological tissues and contributing to their macroscopic mechanical performance. However, such multi-scale structural complicity and heterogeneity of mineralized tissues compromise the precise mechanical characterization of the underlying toughening mechanism at the material level (nanoscale). In the present study, the terminology ‘material level’ of the hard tissues needs to be re-defined as the local material characteristics determined solely by the smallest structural units consisting of nanocrystalline apatite and matrix proteins. A cutting-edge technology that enables mechanical characterization at the material level would probably address the numerous questions related to the toughening mechanism of mineralized tissues; i.e., there is a paucity of information regarding the abnormal bone fragility associated with the progression of some diseases (and with ageing), which is probably due to the loss of the intrinsic toughening mechanism [2], [13], [14], [15], [16], [17].
In the present study, we investigated the mechanical properties of mineralized hard tissues with respect to their smallest-scale structure–function relationships. Our objective was to illustrate the favorable design developed by nature, and to demonstrate the necessity of improving the design and selection of the microstructures of dental materials. An advanced nanomechanical characterization technique that is applicable to biological materials is also introduced. For the sake of brevity, we limited the present review to an exploration of the mechanical behaviors of enamel and cortical bone (or dentin) among the numerous natural mineralized tissues (e.g., shells, arthropod exoskeletons, antlers, tusks, and the beaks of birds). We have also included a brief discussion of the importance of the hierarchical (multiscale) structures of tooth and bone suggested by conventional universal testing. We hope that those involved in bone research and the development of dental material will adjust to the concept of the structure–function relationship in mineralized hard tissues at the material level.
2. Tooth enamel
Although it is only millimeters thick, enamel is the hardest and stiffest biological tissue, and provides remarkable durability throughout our lives. The enamel is thought to be capable of bearing bite forces up to 1000 N with a minimal contact area (0.4–2.2 mm2) [18], generating a contact stress of 0.45–2.5 GPa [18], [19]. In addition, huge shear stresses are generated during mastication on enamel by the oppose teeth. It remains under studied that, being a nonregenerative tissue, how does the enamel endure such a harsh physical environment throughout the life? Comprehending the knowledge of enamel’s inherent toughening mechanism is important for the development of alternative restorative materials and dental crowns.
2.1. Material classification of enamel
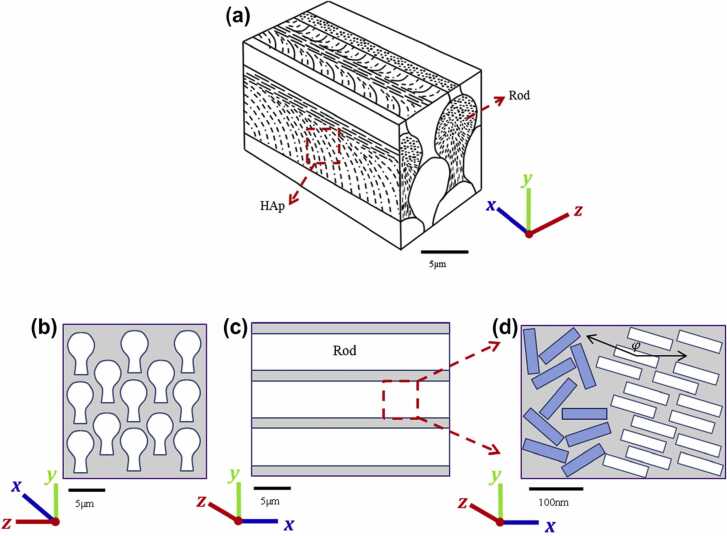
Ameloblasts differentiate from the inner enamel epithelium [20]. Unlike other hard tissues such as bone and dentin, enamel is an ectodermal tissue. The inner enamel epithelium differentiates into secretory ameloblasts immediately after early dentin formation, leading to the formation of enamel [20], [21]. The Tomes’ processes secrete enamel proteins to increase the thickness of the enamel [22]. During this period, biomineralization also occurs within the enamel, albeit incompletely [23]. Ameloblasts then transition to mature ameloblasts. At the material level, immature enamel is degraded by enzymes produced by the ameloblasts themselves, and is replaced by hydroxyapatite crystals, leaving with highly mineralized mature enamel [24]. Under the microscope, enamel appears as a regular columnar structure; each enamel column (enamel rod) is composed of four ameloblasts, and the direction of the rod is determined by the orientation of the Tomes’ process. Enamel rods grow in such a way that they intersect in layers, and are thought to be responsible for macroscopic stiffness. In mature enamel, amelogenin is almost completely degraded and is not detected. However, enamelin and interstitial proteins remain in enamel as degradation products, and function as the glue between crystalline apatite “nanowires” and the interstitium, respectively. Therefore, enamel, which attains a mineralization ratio of nearly 90% by volume [25], is a composite material with a sophisticated hierarchical structure (Fig. 1).
Fig. 1.
Hierarchical structure of tooth enamel [26] (license: Creative Commons CC-BY). (a) A schematic 3D structure of enamel, showing keyhole-like rods aligned in parallel. The rods contain organized and bundled apatite crystals. (b) Rods viewed along their longitudinal direction. (c) Rods viewed along their transverse direction. (d) The structure of partially aligned apatite nanowires viewed from a cross-section parallel to the direction of the rods. The alignment angle φ with respect to the global X-axis is also shown.
2.2. Nanoindentation characterization of tooth enamel at the material level
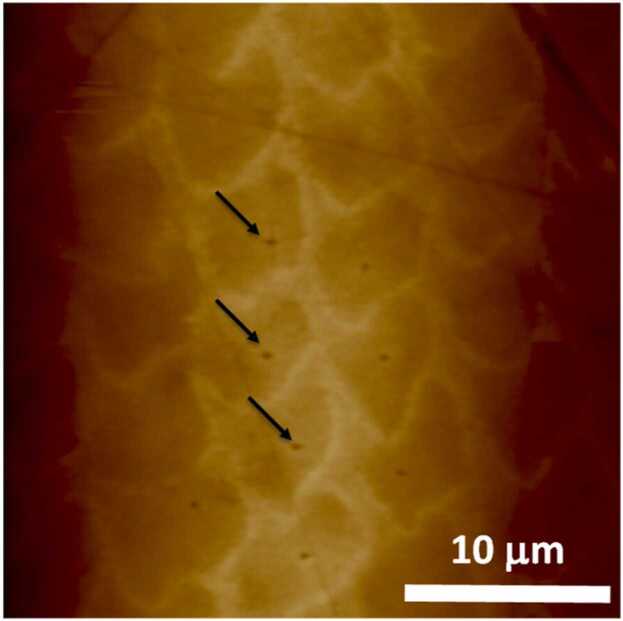
The elastic modulus of enamel, a foundational mechanical property of all materials, has been reported with large discrepancy, mainly due to difference in measurement approach (i.e., the scale of concern and measurement direction). Relatively low elastic moduli are generally reported in the macroscopic range, whereas relatively high moduli (greater than 90 GPa) are generally reported in the microscopic range (micro- to nanoscale) [27]. The basic smallest mesoscopic unit of enamel is the enamel rod, which is approximately 5 µm in diameter. Because values measured using macroscopic mechanical tests vary significantly depending on the inherent mesoscopic biological structures, micro- and nanoscale evaluation methods without influence of mechanical behavior related to the enamel rod’s structure and sheath protein are desirable for evaluating the local mechanical properties of enamel at the material level (Fig. 2).
Fig. 2.
Nanoindentation tests of enamel rods [3] (license number: 5375690944827). Representative atomic force microscopy image of a polished enamel surface. The indentation points are marked with arrows.
The Vickers hardness test is a widely-adopted technique for measuring the hardness of dental materials within a small range. The test comprises bringing a pyramid-shaped diamond indenter into contact with the material surface at a constant load; the hardness is determined from the applied load versus the projected contact area of the indenter. The Vickers hardness test depends on the assumption that the perfect pyramidal shape will predominate the contact area. However, such perfect pyramidal shape at the “tip” part of indenter cannot be guaranteed, hence the above assumption makes it impossible to measure extremely small scale (<micron scale) hardness. That is to say, the Vickers hardness test requires a large scale contact area (>micron scale) so that changes in the indenter shape due to the intrinsic “tip shape” and wear caused by repetitive testing can be ignored. Therefore, such traditional hardness tests cannot be used to assess the mechanical property of enamel at the material (smallest) level without the influence of mesoscopic structures.
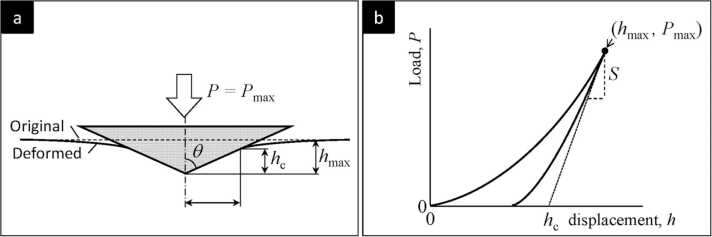
Since the postulate of the Sneddon’s theorem in 1960 s, it is evident that, during elastic deformation, the contact area between the indenter and the material surface is determined by the contact stiffness of the material [28]. In 1992, Oliver and Pharr proposed a nanoindentation method using a triangular pyramidal Berkovich diamond indenter (Fig. 3a), and suggested that the hardness and elastic modulus of a small area of material can be obtained from the measured force–displacement (p-h) curves (Fig. 3b) [29]. During nanoindentation, tests with various loads are performed on a standard calibration material, such as fused quartz (reduced modulus = 69.6 GPa), and the contact area versus the contact depth of the indenter can be determined from the contact stiffness obtained from the onset of the unloading curve (Fig. 3b). This tip area function enables the measurement of an extremely small region of interest on the surface of the tooth enamel. Modern nanoindentation devices are interfaced with an atomic force microscope, enabling the diamond indenter to obtain scanning images of the sample surface and the mechanical properties can be obtained in simultaneously [3], [30].
Fig. 3.
a) The typical schema of performing nanoindentation with a Berkovich diamond indenter. Generally, the test is performed by applying the controlled load () and then, recording the material’s deformation by sensing the contact depth () while the force ramping and unloading. b) A typical nanoindentation force-displacement curve from the test of an elastic-plastic material [31] (license: 5375740175063). The hardness and the elastic modulus of the material can be obtained by analysing the contact stiffness (S) of unloading segment. The plasticity is indicated as the displacement is not returning to zero.
2.3. Contact mechanics of tooth enamel
Earlier nanoindentation measurements revealed that tooth enamel has a size-dependent property [32]. This means that the indenter becomes increasingly sensitive to soft interstitial proteins with the increase in the contact area of the enamel surface (Fig. 1 and Fig. 2). Enamel is not a homogeneous inorganic material like ceramic. Rather, it is a biocomposite that is highly complexed with residual enamel proteins and apatite nanocrystals in the tissue.
From the classical perspective, the fracture resistance of enamel depends on its anisotropic hierarchical structure. In fact, in one study, crack extension in a sample of enamel tested for fracture was significantly longer in the vertical (enamel rod) direction than in the lateral direction [27]. Accordingly, fracture toughness varied significantly depending on the direction of measurement [33]. However, enamel is reportedly being three times tougher than hydroxyapatite crystals of equivalent density, suggesting that the biological production of hydroxyapatite crystals—complexed with enamel proteins during the embryonic stage—is responsible for the excellent material properties of tooth enamel [34].
The enamel toughening mechanism may also be explained by the time-dependency (viscoelasticity) of the organic component, such as remnant enamel protein. The stress intensity of the microcracks in enamel may be relieved by the delayed response of the organic component, preventing further crack extension and substantial fracture. Similar to typical polymers, during nanoindentation, enamel exhibits large creep and stress relaxation with different strain rate under load and displacement, respectively [35]. In general, the peptide chains of proteins are folded into each other by hydrogen bonds between amino acids, and enamel proteins are thought to be similar. Enamel proteins deform against indentation loading strain, and the folded proteins unfold in a time-dependent manner, requiring extra energy expenditure. Hydrogen bonds are restored upon unloading, allowing recovery of the local geometrical profile over time. This time-dependent property is not only a barrier to crack propagation, but also plays an important role in maintaining enamel morphology through significant dimensional recovery.
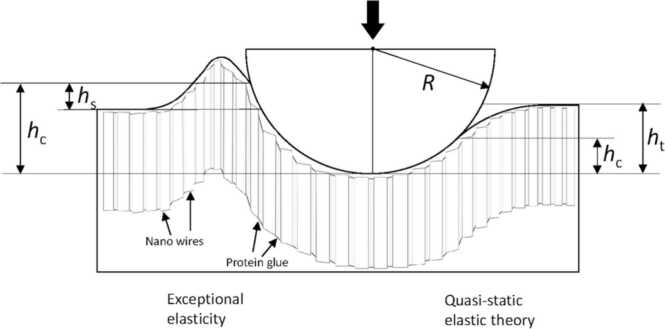
Viscoelastic creep and recovery are thought to be partly responsible for enamel toughness, but this delayed response of enamel cannot be fully responsible for the high strain rates that often occur during occlusal contacts. Recent nanoindentation stress–strain tests using a spherical indenter have revealed that the enamel complex comprising the smallest apatite nanowire and protein glue is responsible for the exceptional contact elasticity of enamel via temporary pile-up against high strain rate indentation (Fig. 4). As a consequence, the complexes produce an extension of the contact area so that enamel can resist the initiation of microcracks by virtue of its enhanced apparent resilience [3]. This smallest level mechanism may reinforce the enamel toughening mechanism aside from delayed viscoelastic behavior, as mentioned above.
Fig. 4.
Schematic illustration of the exceptional elastic response (left) and the estimated theoretical elastic deformation (right) of enamel induced by a spherical indenter. The figure shows the indenter tip radius (R), the total penetration depth (ht), the contact depth (hc), and the (temporary) pile-up depth (hs). The h values are much smaller than R in reality. An extension of the contact area prevents frictional sliding between nanocrystals or detachment of the protein glue, and there is therefore an enhanced elastic limit (left). Enhanced contact stiffness resulting from an increase in the true contact area (left) as a function of the estimated contact area calculated from quasi-static elastic theory (right) results in an apparent high elastic modulus [3] (license: 5375750655737).
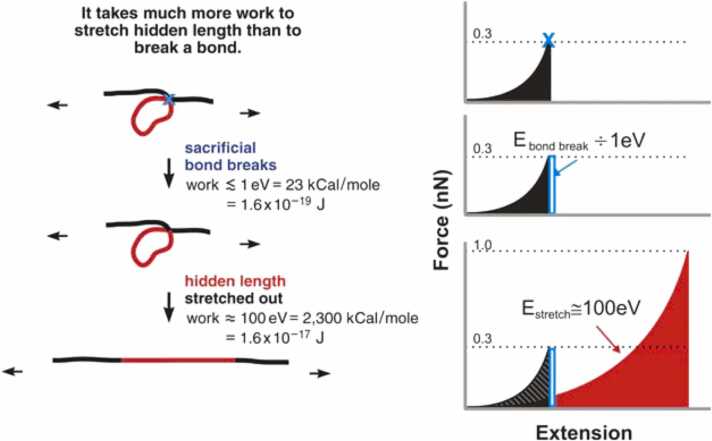
Non-collagenous proteins, such as enamel proteins, fold intricately between crystals or within the interstitium to form a series of nano-level humped nodules (sacrificial bonds) [36]. In response to tensile stress, sacrificial bonds may unfold sequentially, preventing rapid crack extension [27], [37]. The existence of this mechanism in bone tissue is widely recognized, and has attracted much attention in the elucidation of the fracture toughness of mineralized tissues, including enamel (Fig. 5).
Fig. 5.
Schematic drawing of the basic principle of the sacrificial bond–hidden length mechanism [37] (license: 5374611137473). Before a sacrificial bond is broken, only the black length of the molecule contributes to entropic spring, and therefore to the force with which the molecule resists stretching. The red length of the molecule is hidden from the applied force by the sacrificial bond. When the bond breaking force is reached, only a small amount of energy is needed to break the sacrificial bond. Subsequently, the whole length (black plus red) contributes to the entropy of the molecule. In total, the increase in the energy required to break the molecule can be determined from the shaded area under the first peak.
2.4. Enamel-inspired nanocomposite
As explained at the beginning of this article, tooth enamel comprises varying orientations of inorganic apatite prisms (rods) and nanowires with a soft protein matrix (Fig. 1). Such structural motifs have been used to develop advanced engineering nanocomposites [38]. Yeom et al. reported a method for the replication of columnar enamel by the sequential growth of a zinc oxide nanowire and the deposition of a polymeric matrix [38]. The resulting nanocomposite had mechanical properties (such as hardness) similar to those of tooth enamel, despite the lower inorganic content of the nanocomposite compared with that of enamel. However, tooth enamel has been ‘shaped' with favorable properties over a long period of evolution and adaptation, and its toughening mechanism has not been fully elucidated. The hidden mechanism behind the exceptional durability of tooth enamel must be elucidated before enamel-inspired materials can be further developed.
3. Bone
Bone forms the skeleton supporting human body and provides a life-support system for active mineral metabolism. Degenerative diseases such as osteoporosis are becoming more prevalent as the population ages. Bone must have a macroscopic morphology and a volume fraction that are commensurate with biomechanical loading. The prevalence of pathological bone fractures associated with aging is often thought to be due to a decrease in volume fraction and bone mineral density. Recent studies suggest that healthy bone may possess nano-level mechanisms that resist substantial fracture [36], [39]. Therefore, various pathological fractures could be caused not only by bone loss but also by degradation at the nano (material) level. The nanomechanical characterization of bone may provide an important insight into the prevention and treatment of these degenerative diseases.
3.1. The morphological and mechanical characteristics of bone
Bones are classified into long, short, flat, and irregular on the basis of their overall morphology. The bone comprises four tissues: bony tissue, the periosteum, bone marrow, and articular cartilage. The cortical bone has distinct macroscopic features from trabecular bone. Cortical bone is composed of numerous laminae with abundant blood vessels and nerves. In cortical bone, vessels and nerves run through the central canal of the concentric laminae (the Haversian canal), and the penetrating canal runs through the laminae (the Volkmann’s canal). This vascular system maintains cellular activity and mineral dynamics between the bone marrow and the cortical bone.
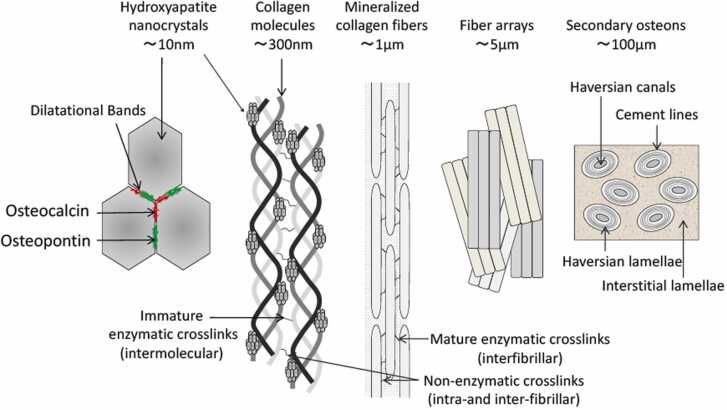
With regard to histological characteristics, the cortical bone lamina comprises bone units (osteons) of approximately 10 µm centered on a Haversian canal, and collagen fibers oriented with nanoscale hydroxyapatite crystals at the molecular level (Fig. 6). The collagen fibers in bone are composed of type I collagen. Some lysine or hydroxylysine residues in the collagen molecule are converted to allysine or hydroxylysine with aldehyde bonds by lysyl oxidase; this is followed by an aldol condensation between allysine and other lysines [40]. Crosslinks are formed between collagen molecules via aldol condensations between allysines on adjacent chains and Schiff base formation with other lysines. Type I collagen is the dominant bone matrix protein, and accounts for 90% of the total amount of collagen; it coexists with remnant non-collagenous proteins. Collagen crosslinking in the bone matrix is an important determinant of the mechanical properties of bone as a biocomposite [40]. Osteocalcin is the most abundant non-collagenous protein in bone, and its expression level has been used as a marker of osteoblast differentiation [41], [42]. It has a strong affinity for calcium and binds to hydroxyapatite crystals; it is linked to collagen fibers via osteopontin [2], [39]. Osteopontin has also attracted attention owing to its role in sensing mechanical stimuli [43]. The minimal bone structure formed by hydroxyapatite crystals and these non-collagenous proteins functions as a system of dilatational bands [2], [39] to distribute strain among the collagen fibers in response to dynamic stress (Fig. 6). Such non-collagenous protein complexes also act as sacrificial bonds (see Section 2.3) [10] in response to further strain [36].
Fig. 6.
Schematic illustrations of bone hierarchical structures [2], [39] (license: 5375790444716).
Such complicated hierarchical microstructure of bone (Fig. 6) makes it a heterogeneous and anisotropic material [10], [44], resulting the elastic modulus of bone vary in a wide-range (5–20 GPa) [10]. In other words, the mechanical properties of bone exhibit differently when measuring at different scale and location and from different direction. To be more specific, the local alignment of the collagen and osteons in the bone makes the mechanical responses different in the directions along and transverse to the alignment. In addition, the macroscopic measurement captures the combined responses and interactions of the different components and phases within the bone, often resulting in a relevantly lower elastic modulus, whereas the elastic modulus would be higher when measuring the cortical bone matrix at microscale. Therefore, at the material level, the superior mechanical properties of bone depend on its micro- and nanoscale dynamic mechanical response to strain, and dynamic mechanical testing in the nanoscale local region may elucidate the mechanism.
3.2. Quasi-static nanomechanical characterization of bone and its theoretical limitation
Nanoindentation (see Section 2.2) has been widely used for the micro- and nanoscale mechanical characterization of bone tissue [45], [46], [47]. However, the measurement approach for nanoindentation proposed by Oliver and Pharr [29] is for elastic–plastic (time-independent) materials, such as metals and ceramics, and viscoelastic (time-dependent) biological tissues often exceed the theoretical limitation. In particular, bone and dentin contain greater amounts of proteins that behave in a time-dependent manner than tooth enamel does.
Regarding the nanoindentation force–displacement curve, the onset of the unloading slope indicates the contact stiffness and the projected contact area at maximum load, and the hardness and elastic modulus are measurable. For elastic–plastic (time-independent) materials, there is no time lag between the maximum loading point and the onset of unloading, so the contact stiffness and indenter contact area are correlative (Fig. 3).
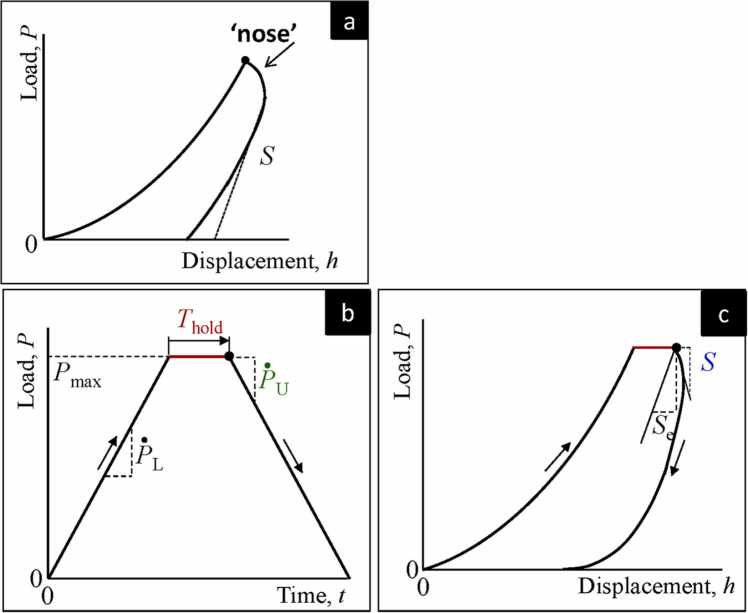
In the case of viscoelastic materials such as bone, the onset of the unloading slope does not respond to indenter withdrawal (unloading) immediately, causing a delay in the strain response (a hysteresis loop). Owing to the hysteresis loop, the unloading portion of the p–h curve may be distorted and may appear more convex than for elastic–plastic materials (Fig. 7a.). The effect of the hysteresis loop can be reduced by applying a holding time of constant stress (or constant displacement) at maximum load (Fig. 7b) to bring the theoretical strain rate close to zero. However, even with the application of a holding period, the deformation may continue to grow upon unloading before the gradually diminishing of the delayed strain response (Fig. 7c) [48]. Nonetheless, the effect of delayed response on the unloading portion may be neglected if the penetration depth grows by less than 1% per minute [49].
Fig. 7.
Typical nanoindentation strategy for viscoelastic materials. a). An example of load-displacement curve for viscoelastic material if the holding time is not applied, showing notable hysteresis loop. The viscosity results a notable ‘nose’ in the curve, indicating the large delay in strain responses. The apparent contact stiffness (S) is negative in this case. b) The loading function for viscoelastic materials. ṖL is the loading rate and Ṗu is the unloading rate at the onset of unloading slope. The applied load is held for a period () when reaches maximum (). c) A correction for elastic modulus determination may be made by replacing S with the effective contact stiffness Se [50]. The viscoelastic hysteresis loop can be reduced with the application of a holding time [31] (license: 5375740175063).
Feng and Ngan proposed that the creep rate is a function of the displacement rate [50]. Their theorem may be useful for nanoindentation tests of viscoelastic materials such as bone, because it allows correction of the contact stiffness based on the relationship between the creep rate and the unloading strain rate. However, for materials with high strain rate sensitivity, the unloading curve may also be strongly affected by the unloading strain rate itself. Bone probably falls into this category and cannot be fully corrected by the Feng–Ngan theorem.
3.3. Potential correlation between bone abnormality and viscoelasticity
There are many challenges in measuring the nanoindentation of viscoelastic materials such as bone, yet the viscoelasticity of bone is almost associated with the mechanical behavior of the matrix proteins. Additionally, many degenerative lesions in bone cause matrix protein abnormalities [51], [52]. By quantifying changes in viscoelasticity, it may be possible to discuss bone abnormalities at the material level.
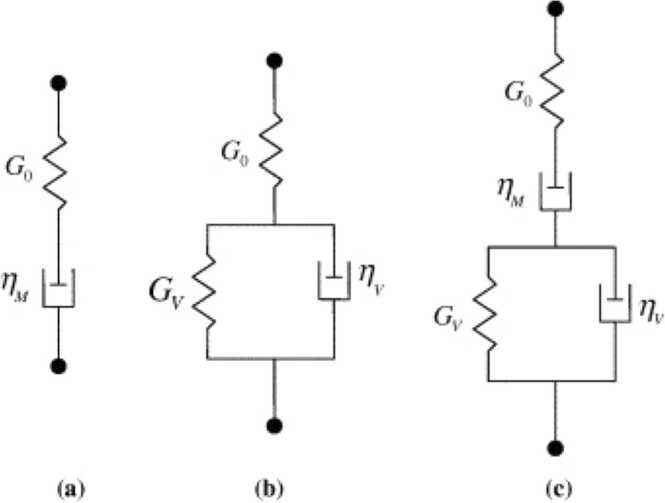
The time-dependent viscoelasticity of matrix proteins can be represented by rheological (spring-and-dashpot) models (Fig. 8). These parameters in the modes can be obtained by fitting the time course of indenter penetration using a suitable mathematical creep function [48]. The simplest parameter for a correlation between matrix degradation and time-dependent viscoelasticity is creep and its recovery. A recent study reported that radiation diminishes the creep deformation recovery of human tooth dentin [4]. The main cause of this behavior is an increase in non-enzymatic crosslinking and the concomitant accumulation of advanced glycation end-products. Similar molecular changes in bone have often been observed during ageing processes and/or abnormal bone metabolism in type II diabetes [4].
Fig. 8.
Linear viscoelastic deviator creep models, where G is the elastic spring and η is the viscous dashpot: (a) the 3-parameter Maxwell model; (b) the 4-parameter Kelvin–Voigt model; (c) the 5-parameter combined Kelvin–Voigt–Maxwell model [53] (license: 5375800418098).
3.4. Determination of time-dependent (strain rate) behavior of bone by dynamic nanoindentation tests
Quasi-static nanoindentation tests can be used to evaluate material properties via force–displacement curves, based on the assumption that the effect of the strain experienced by the material surface during loading is dominant at the onset of the unloading slope. The major weakness of the quasi-static nanoindentation theory is that the loading strain and measurement never coincide on the time axis. For viscoelastic materials such as bone, the onset of the unloading strain tends to be delayed (a time-dependent property) and affected by the unloading strain rate. Hence, the unloading curve does not necessarily reflect the previous loading (strain) history.
The dynamic nanoindentation test allows continuous measurement of the indentation stress–strain relationship via sinusoidal oscillations. The viscoelastic properties can be extracted from the strain phase lag, and the elastic modulus (storage modulus) can be determined [54], [55]. The dynamic mechanical response of bone at various strain rates (frequencies) can be discussed at the material level. We reported that, in common with tooth enamel, healthy dense cortical bone exhibits an increase in local (nanoscale) stiffness at high strain rates [2], [3], [55]. Thus, nanoindentation can capture the aforementioned dilatational bands (see Section 3.1), in which the minimal bone structure, composed of hydroxyapatite crystals and non-collagenous proteins, expands in response to high strain rates. Upon unloading, such nanostructures may contribute to morphological recovery and the accompanying large stress relaxation due to protein refolding, resulting in the superior durability and fracture characteristics of healthy bone and tooth enamel. The diminished expression of osteopontin, a non-collagenous protein, has been reported in bone tissue from aging mouse models [56]. As mentioned above (see Section 3.1), the dilatational band comprises nanocrystalline apatite and minor non-collagenous bone proteins such as osteopontin and osteocalcin (Fig. 6). The bone fragility associated with degenerative diseases may be partly dependent on the diminution of the smallest natural protection system, namely the dilatational bands.
3.5. bio-informed bone scaffold
Bone regeneration has been extensively progressed in the past decades and it is recognized scaffold design is one of the critical techniques in regenerative medicine[57]. A scaffold with bio-informed structures and mechanical properties would help avoid the eliciting foreign body response after the surgery and increases the success rate of the tissue regeneration [57], [58]. For example, the hydroxyapatite scaffolds with bio-informed porosity can enhance the bone ingrowth [59]. It is found that the appropriate scaffold composition, such as hydroxyapatite, should resemble the natural composition of bone, and the optimized scaffold porosity and structure can provide a more natural mechanical environment for cellular infiltration and interactions with the external loadings, mimicking their retention of the native bone ECM structures [60], [61]. While the composition of the bone has been extensively studied, the mechanical characterizations of the bone are yet to be comprehended at multiscale level for its sophisticated time-dependent, heterogeneous, anisotropic and viscoelastic nature. Extending the knowledge of multiscale mechanical properties of the bone is urged to bring the current bone regenerative field to the next level.
4. Concluding remarks
The nanoindentation method is an effective analytical tool for elucidating the mechanical properties of enamel. Further research results are expected for the development of advanced restorative and prosthetic materials that mimic the enamel structure–function relationship at the material level. An understanding of the mechanical properties of bone tissue at the material level is important for bone research and clinical communities. Intact bone has excellent fracture resistance that depends on the substructures in nanoscale, which may be compromised in degenerative bone diseases. Nanoindentation is a useful additional tool for distinguishing bone disease, but the limitations of measurement theory must be considered.
Role of the funding source
This work was supported by JSPS KAKENHI Grant Number 19K10188.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Dr. Motohiro Suganuma for his support and technical advice.
Footnotes
Scientific field of dental science: material science
References
- 1.Bar-On B., Wagner H. Structural motifs and elastic properties of hierarchical biological tissues - a review. J Struct Biol. 2013;183:149–164. doi: 10.1016/j.jsb.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Maruyama N., Shibata Y., Mochizuki A., Yamada A., Maki K., Inoue T., et al. Bone micro-fragility caused by the mimetic aging processes in α-klotho deficient mice: In situ nanoindentation assessment of dilatational bands. Biomaterials. 2015;47:62–71. doi: 10.1016/j.biomaterials.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Shimomura N., Tanaka R., Shibata Y., Zhang Z., Li Q., Zhou J., et al. Exceptional contact elasticity of human enamel in nanoindentation test. Dent Mater. 2019;35:87–97. doi: 10.1016/j.dental.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Tobe T., Shibata Y., Mochizuki A., Shimomura N., Zhou J., Wurihan, et al. Nanomechanical characterization of time-dependent deformation/recovery on human dentin caused by radiation-induced glycation. J Mech Behav Biomed Mater. 2019;90:248–255. doi: 10.1016/j.jmbbm.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Güngör M.B., Aydin C., Yilmaz H., Gül E.B. An overview of zirconia dental implants: Basic properties and clinical application of three cases. J Oral Implant. 2014;40:485–494. doi: 10.1563/AAID-JOI-D-12-00109. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki T., Nakamura T., Matsumura H., Ban S., Kobayashi T. Current status of zirconia restoration. J Prosthodont Res. 2013;57:236–261. doi: 10.1016/j.jpor.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Guazzato M., Albakry M., Ringer S.P., Swain M.V. Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part II. Zirconia-based dental ceramics. Dent Mater. 2004;20:449–456. doi: 10.1016/j.dental.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Luthardt R.G., Holzhüter M., Sandkuhl O., Herold V., Schnapp J.D., Kuhlisch E., et al. Reliability and properties of ground Y-TZP-zirconia ceramics. J Dent Res. 2002;81:487–491. doi: 10.1177/154405910208100711. [DOI] [PubMed] [Google Scholar]
- 9.Habelitz S., Marshall S.J., Marshall G.W., Balooch M. Mechanical properties of human dental enamel on the nanometre scale. Arch Oral Biol. 2001;46:173–183. doi: 10.1016/s0003-9969(00)00089-3. [DOI] [PubMed] [Google Scholar]
- 10.Rho J.Y., Kuhn-Spearing L., Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20:92–102. doi: 10.1016/s1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 11.Bechtle S., Ang S.F., Schneider G.A. On the mechanical properties of hierarchically structured biological materials. Biomaterials. 2010;31:6378–6385. doi: 10.1016/j.biomaterials.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 12.Auger J.D., Frings N., Wu Y., Marty A.G., Morgan E.F. Trabecular Architecture and Mechanical Heterogeneity Effects on Vertebral Body Strength. Curr Osteoporos Rep. 2020;18:716–726. doi: 10.1007/s11914-020-00640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui J., Shibata Y., Zhu T., Zhou J., Zhang J. Osteocytes in bone aging: advances, challenges, and future perspectives. Ageing Res Rev. 2022:77. doi: 10.1016/j.arr.2022.101608. [DOI] [PubMed] [Google Scholar]
- 14.Ganeko K., Masaki C., Shibata Y., Mukaibo T., Kondo Y., Nakamoto T., et al. Bone aging by advanced glycation end products: A multiscale mechanical analysis. J Dent Res. 2015;94:1684–1690. doi: 10.1177/0022034515602214. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto D., Masaki C., Shibata Y., Watanabe C., Nodai T., Munemasa T., et al. Microstructural and mechanical recovery of bone in ovariectomized rats: the effects of menaquinone-7. J Mech Behav Biomed Mater. 2021:120. doi: 10.1016/j.jmbbm.2021.104571. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura M., Masaki C., Shibata Y., Kondo Y., Mukaibo T., Miyazaki T., et al. Pentosidine correlates with nanomechanical properties of human jaw bone. J Mech Behav Biomed Mater. 2019;98:20–25. doi: 10.1016/j.jmbbm.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez R., Yoshimura K., Shibata Y., Miyamoto Y., Tanaka R., Uyama R., et al. Nanoindentation time-dependent deformation/recovery suggestive of methylglyoxal induced glycation in calcified nodules. Nanomedicine. 2017;13:2545–2553. doi: 10.1016/j.nano.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Waltimo A., Könönen M. Maximal bite force and its association with signs and symptoms of craniomandibular disorders in Young finnish non-patients. Acta Odontol Scand. 1995;53:254–258. doi: 10.3109/00016359509005982. [DOI] [PubMed] [Google Scholar]
- 19.Hayasaki H., Okamoto A., Iwase Y., Yamasaki Y., Nakata M. Occlusal contact area of mandibular teeth during lateral excursion. Int J Prosthodont. 2004;17:72–76. [PubMed] [Google Scholar]
- 20.Warshawsky H. The fine structure of secretory ameloblasts in rat incisors. Anat Rec. 1968;161:211–229. doi: 10.1002/ar.1091610207. [DOI] [PubMed] [Google Scholar]
- 21.Fincham A.G., Simmer J.P. Amelogenin proteins of developing dental enamel. CIBA Found Symp. 1997:118–134. doi: 10.1002/9780470515303.ch9. [DOI] [PubMed] [Google Scholar]
- 22.Warshawsky H. A freeze‐fracture study of the topographic relationship between inner enamel‐secretory ameloblasts in the rat incisor. Am J Anat. 1978;152:153–207. doi: 10.1002/aja.1001520203. [DOI] [PubMed] [Google Scholar]
- 23.Slavkin H.C. Molecular determinants of tooth development: a review. Crit Rev Oral Biol Med. 1990;1:1–16. doi: 10.1177/10454411900010010201. [DOI] [PubMed] [Google Scholar]
- 24.Smith C.E. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- 25.Inage T., Teranishi Y., Toda Y., Iwase T., Moro I., Shimokawa H. Immunocytochemical demonstration of amelogenins and enamelins secreted by ameloblasts during the secretory and maturation stages. Arch Histol Cytol. 1989;52:213–229. doi: 10.1679/aohc.52.213. [DOI] [PubMed] [Google Scholar]
- 26.Sui T., Sandholzer M.A., Baimpas N., Dolbnya I.P., Landini G., Korsunsky A.M. Hierarchical modelling of elastic behaviour of human enamel based on synchrotron diffraction characterisation. J Struct Biol. 2013;184:136–146. doi: 10.1016/j.jsb.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 27.He L.H., Swain M.V. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J Mech Behav Biomed Mater. 2008;1:18–29. doi: 10.1016/j.jmbbm.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Sneddon I.N. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int J Eng Sci. 1965;3:47–57. [Google Scholar]
- 29.Oliver W.C., Pharr G.M. Improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–1580. [Google Scholar]
- 30.Okada M., Hara E.S., Yabe A., Okada K., Shibata Y., Torii Y., et al. Titanium as an Instant Adhesive for Biological Soft Tissue. Adv Mater Interfaces. 2020:7. [Google Scholar]
- 31.Shibata Y., Tanimoto Y., Maruyama N., Nagakura M. A review of improved fixation methods for dental implants. Part II: Biomechanical integrity at bone-implant interface. J Prosthodont Res. 2015;59:84–95. doi: 10.1016/j.jpor.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 32.He L.H., Fujisawa N., Swain M.V. Elastic modulus and stress-strain response of human enamel by nano-indentation. Biomaterials. 2006;27:4388–4398. doi: 10.1016/j.biomaterials.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 33.Xu H.H.K., Smith D.T., Jahanmir S., Romberg E., Kelly J.R., Thompson V.P., et al. Indentation damage and mechanical properties of human enamel and dentin. J Dent Res. 1998;77:472–480. doi: 10.1177/00220345980770030601. [DOI] [PubMed] [Google Scholar]
- 34.White S.N., Luo W., Paine M.L., Fong H., Sarikaya M., Snead M.L. Biological organization of hydroxyapatite crystallites into a fibrous continuum toughens and controls anisotropy in human enamel. J Dent Res. 2001;80:321–326. doi: 10.1177/00220345010800010501. [DOI] [PubMed] [Google Scholar]
- 35.He L.H., Swain M.V. Influence of environment on the mechanical behaviour of mature human enamel. Biomaterials. 2007;28:4512–4520. doi: 10.1016/j.biomaterials.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Fantner G.E., Hassenkam T., Kindt J.H., Weaver J.C., Birkedal H., Pechenik L., et al. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat Mater. 2005;4:612–616. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- 37.Fantner G.E., Oroudjev E., Schitter G., Golde L.S., Thurner P., Finch M.M., et al. Sacrificial bonds and hidden length: Unraveling molecular mesostructures in tough materials. Biophys J. 2006;90:1411–1418. doi: 10.1529/biophysj.105.069344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeom B., Sain T., Lacevic N., Bukharina D., Cha S.H., Waas A.M., et al. Abiotic tooth enamel. Nature. 2017;543:95–98. doi: 10.1038/nature21410. [DOI] [PubMed] [Google Scholar]
- 39.Poundarik A.A., Diab T., Sroga G.E., Ural A., Boskey A.L., Gundberg C.M., et al. Dilatational band formation in bone. Proc Natl Acad Sci USA. 2012;109:19178–19183. doi: 10.1073/pnas.1201513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata Y., Suzuki D., Wurihan, Yamada A., Maruyama N., Fujisawa N., et al. Lysyl oxidase like-2 reinforces unsatisfactory ossification induced by bone morphogenetic protein-2: relating nanomechanical properties and molecular changes. Nanomedicine. 2013;9:1036–1047. doi: 10.1016/j.nano.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Iwai-Yoshida M., Shibata Y., Wurihan, Suzuki D., Fujisawa N., Tanimoto Y., et al. Antioxidant and osteogenic properties of anodically oxidized titanium. J Mech Behav Biomed Mater. 2012;13:230–236. doi: 10.1016/j.jmbbm.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Shibata Y., Suzuki D., Omori S., Tanaka R., Murakami A., Kataoka Y., et al. The characteristics of in vitro biological activity of titanium surfaces anodically oxidized in chloride solutions. Biomaterials. 2010;31:8546–8555. doi: 10.1016/j.biomaterials.2010.07.098. [DOI] [PubMed] [Google Scholar]
- 43.Ishijima M., Rittling S.R., Yamashita T., Tsuji K., Kurosawa H., Nifuji A., et al. Enhancement of osteoclastic bone resorption and suppression of osteoblastic bone formation in response to reduced mechanical stress do not occur in the absence of osteopontin. J Exp Med. 2001;193:399–404. doi: 10.1084/jem.193.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katsamenis O.L., Chong H.M.H., Andriotis O.G., Thurner P.J. Load-bearing in cortical bone microstructure: Selective stiffening and heterogeneous strain distribution at the lamellar level. J Mech Behav Biomed Mater. 2013;17:152–165. doi: 10.1016/j.jmbbm.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Lewis G., Nyman J.S. The use of nanoindentation for characterizing the properties of mineralized hard tissues: State-of-the art review. J Biomed Mater Res Part B Appl Biomater. 2008;87:286–301. doi: 10.1002/jbm.b.31092. [DOI] [PubMed] [Google Scholar]
- 46.Roschger P., Paschalis E.P., Fratzl P., Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456–466. doi: 10.1016/j.bone.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 47.Wu D., Isaksson P., Ferguson S.J., Persson C. Young's modulus of trabecular bone at the tissue level: a review. Acta Biomater. 2018;78:1–12. doi: 10.1016/j.actbio.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Menčík J., He L.H., Němeček J. Characterization of viscoelastic-plastic properties of solid polymers by instrumented indentation. Polym Test. 2011;30:101–109. [Google Scholar]
- 49.Chudoba T., Richter F. Investigation of creep behaviour under load during indentation experiments and its influence on hardness and modulus results. Surf Coat Technol. 2001;148:191–198. [Google Scholar]
- 50.Feng G., Ngan A.H.W. Effects of creep and thermal drift on modulus measurement using depth-sensing indentation. J Mater Res. 2002;17:660–668. [Google Scholar]
- 51.Saito M., Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 52.Viguet-Carrin S., Garnero P., Delmas P.D. The role of collagen in bone strength. Osteoporos Int. 2006;17:319–336. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]
- 53.Vandamme M., Ulm F.J. Viscoelastic solutions for conical indentation. Int J Solids Struct. 2006;43:3142–3165. [Google Scholar]
- 54.Li X., Bhushan B. A review of nanoindentation continuous stiffness measurement technique and its applications. Mater Charact. 2002;48:11–36. [Google Scholar]
- 55.Maruyama N., Shibata Y., Wurihan A., Swain M.V., Kataoka Y., Takiguchi Y., et al. Strain-rate stiffening of cortical bone: Observations and implications from nanoindentation experiments. Nanoscale. 2014;6:14863–14871. doi: 10.1039/c4nr03180f. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki M., Hasegawa T., Yamada T., Hongo H., de Freitas P.H.L., Suzuki R., et al. Altered distribution of bone matrix proteins and defective bone mineralization in klotho-deficient mice. Bone. 2013;57:206–219. doi: 10.1016/j.bone.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Kanwar S., Vijayavenkataraman S. Design of 3D printed scaffolds for bone tissue engineering: A review. Bioprinting. 2021:24. [Google Scholar]
- 58.Egan P., Ferguson S.J., Shea K. Design and 3D printing of hierarchical tissue engineering scaffolds based on mechanics and biology perspectives. Proceedings of the ASME Design Engineering Technical Conference2016.
- 59.Eggli P.S., Muller W., Schenk R.K. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. A comparative histomorphometric and histologic study of bone ingrowth and implant substitution. Clin Orthop Relat Res. 1988:127–138. [PubMed] [Google Scholar]
- 60.Cheng A., Schwartz Z., Kahn A., Li X., Shao Z., Sun M., et al. Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Eng Part B: Rev. 2019;25:14–29. doi: 10.1089/ten.teb.2018.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y., Luo D., Wang T. Hierarchical structures of bone and bioinspired bone tissue engineering. Small (Weinh der Bergstr, Ger) 2016;12:4611–4632. doi: 10.1002/smll.201600626. [DOI] [PubMed] [Google Scholar]