Abstract
Introduction
Pancreatic cancer often invades the duodenum; however, it rarely causes duodenal bleeding.
Presentation of case
We describe a case of a 77-year-old Japanese woman admitted to our hospital with hematemesis, who presented with pancreatic head cancer and received radiochemotherapy (radiotherapy + gemcitabine). The following day, she developed hemorrhagic shock, and an emergency endoscopy was performed, which revealed a bleeding ulcerative lesion in the second portion of the duodenum. We chose surgical treatment over other therapies (interventional radiology or endoscopy). Pancreaticoduodenectomy was successfully performed to control hemorrhage and the Child's method was used for reconstruction. The patient's postoperative course was uneventful. After her condition improved, she was treated for residual cancer 2 months after surgical treatment; therefore, complementary radiation with concurrent chemotherapy based on GEM was administrated. However, she died 12 months after the surgery.
Discussion and conclusion
During the treatment of pancreatic cancer, it is necessary to avoid bleeding as much as possible by considering prophylactic treatment, including periodic gastrointestinal scrutiny and resection or embolization, depending on the case.
Keywords: Pancreatic head cancer, Hemorrhagic shock, Invading the duodenum
Highlights
-
•
Duodenal invasion of pancreatic cancer is often experienced, but bleeding is rare.
-
•
We suggests that the number of pancreatic cancer patients who bleed directly from the tumor may increase due to the effects of radiation chemotherapy and tumor necrosis or as more patients receive antithrombotic medications as they age.
-
•
Routine gastrointestinal scrutiny and prophylactic treatment should be considered in some cases to avoid gastrointestinal bleeding during pancreatic cancer treatment.
1. Introduction
Although pancreatic cancer generally invades the duodenum easily, bleeding within the tumor or at the site of tumor invasion is rare due to the poor blood flow in the tumor [1]. In contrast, with the increasing incidence of pancreatic cancer and the advancement and development of multidisciplinary treatment, the oncologic emergencies in pancreatic cancer, perforation, rupture, and hemorrhage are less common, and biliary infections owing to bile duct obstruction and obstructive symptoms due to gastrointestinal invasion are more common. Earlier recognition of emergency signs and appropriate treatment is necessary to achieve the best outcome for patients in an oncologic emergency [2]. However, non-operative cases of locally advanced pancreatic head cancer can be challenging to treat in terms of surgical technique, complications, and prognosis.
In this report, we describe the case of a patient with locally advanced pancreatic cancer invading the duodenum who had hemorrhagic shock during radiochemotherapy and was successfully treated with emergency surgery. This paper has been reported in line with SCARE criteria [3].
2. Presentation of case
A 77-year-old woman presented to our hospital with a complaint of general fatigue. Blood biochemical analysis evidenced liver dysfunction (total bilirubin 1.38 mg/dl, aspartate transaminase 34 IU/l, and alanine transaminase 38 IU/l), and carcinoembryonic antigen (8.14 ng/ml) and carbohydrate antigen 19–9 (55,124.63 U/ml) were increased. However, white blood cell count, C-reactive protein, and biliary enzymes (alkaline phosphatase 277 IU/l and ɤ-glutamyltransferase 47 IU/l) were within the normal ranges. Abdominal computed tomography (CT) demonstrated a mass sized 50 mm in the pancreas head invading to the duodenum and with encasement of celiac artery, superior mesenteric artery. Magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS) showed a 40 mm hypoechoic mass at the head of the pancreas, which was invasive and fine needle biopsies using by EUS identified as an adenocarcinoma. Positron emission tomography CT showed intense abnormal fluorodeoxyglucose uptake (standardized uptake value max 15) in the head of the pancreas, which invaded surrounding vessels. However, there was no evidence of distant metastasis such as liver or lung metastasis or bone metastasis. Due to the result of some imaging, she was deemed surgically unresectable and chemoradiotherapy was initiated.
After four courses of therapy (gemcitabine [GEM] 900 mg × 4 times + radiation 60 Gy), she was admitted for hematemesis. Gastrointestinal endoscopy (GIS) was performed, it recognized a small amount of black clots in the stomach and the deep ulceration at the second portion of the duodenum, but exposed blood vessels and active hemorrhage were not seen. On the next day of the hospitalization, she suddenly developed massive hematemesis and progressed to a state of shock: her consciousness level was somnolent, blood pressure was 60 mmHg on palpation, heart rate was 125 /min, respiratory rate was 28/min, body temperature was 35.7 °C in the deep part of the body, and anemia was observed on the conjunctiva of the eyelids. The abdomen was flat with mild tenderness in the pericardial area, but no muscular defense. Blood biochemistry analysis showed marked anemia (RBC 193 × 104/μl, H b 6.5 g/dl, H t 19.8 %), biliary enzymes (ALP 168 IU/l, ɤ GTP 65I U/l) were elevated, but coagulation system was still intact. Tumor markers were better than before chemotherapy (CEA 4.0 ng/ml, CA19–9 U/ml). In the emergency room, two IV routes were immediately secured in both upper extremities, a urinary balloon and a 16 Fr gastric tube were placed, and the patient was started on high volume IV fluids, blood transfusion (6 U of fresh concentrated red blood cells and 8 U of fresh frozen plasma), and whole body warming. The blood pressure rose, and emergency GIS was per formed. A large amount of blood clots were found in the stomach, and blood clots and a pancreatic cancer-infiltrated ulcer bed were found in the posterior duodenal bulb, but it was difficult to identify the obvious exposed vessels. The diagnosis of upper gastrointestinal bleeding due to duodenal invasion of pancreatic cancer was made, but endoscopic hemostasis was not performed. Emergent abdominal CT demonstrated a mass sized 40 mm in the pancreas head invading to the duodenum and with encasement of celiac artery, superior mesenteric artery, common hepatic artery, portal vein, and some lymph node metastases; however, could not confirm the extravasation, aneurysm, and other abnormal lesions of the duodenum (Fig. 1A, B). Moreover, an emergency GIS was performed; however, we could not recognize the bleeding point of the ulcerative lesion in the second portion of the duodenum (Fig. 1C). As we could not confirm the arterial bleeding from surrounding pancreatic arches; therefore, we judged to prefer an operation to intervention therapy (IVR), endoscopic and other therapies for her condition.
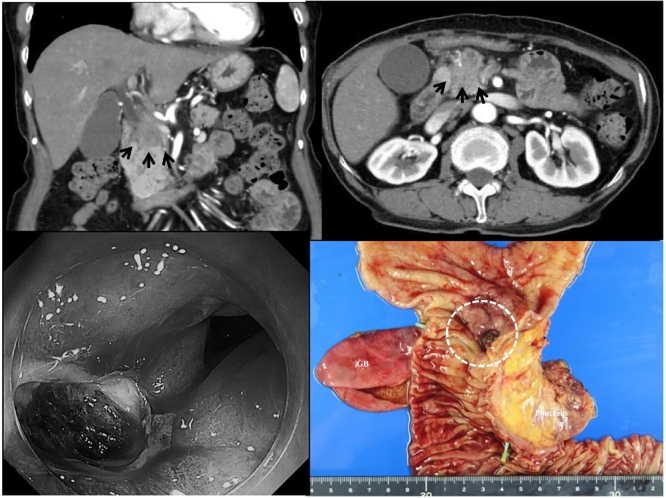
Fig. 1.
Emergency abdominal computed tomography (CT) shows a mass of size 40 mm in the pancreas head invading to the duodenum and with encasement of the celiac artery superior mesenteric artery, common hepatic artery, portal vein, and some lymph node metastasis; however, the extravasation aneurysm, and other abnormal lesions of the duodenum cannot be confirmed (A, B, black arrows). The emergency gastrointestinal endoscopy (GIS) shows some coagulations and huge ulceration at the second portions of the duodenum; however, the bleeding point of the ulcerative lesion cannot be identified (C). The macroscopic view of the resected specimen shows ulcerations with the tumor of the duodenum (D).
The patient was successfully treated by emergency cephalic pancreaticoduodenectomy for hemorrhage. The laparotomy was opened through an upper midline abdominal incision, with a small amount of serous ascites, no hepatic metastasis, peritoneal dissemination, or Schnitzler metastasis. The pancreatic head was considered edematous and continued to bleed, so a distal gastrectomy was performed prior to gastrointestinal resection. The jejunum was then dissected about 20 cm anal side from the Treitz ligament. Kocher's manoeuvre was performed, and the transverse colon was taken down to confirm the SMV (superior mesenteric vein). To separate the pancreas from the cancerous area, common hepatic artery (CHA) was confirmed at the superior margin of the pancreas, tunneling was performed on the left side of the portal vein (PV), and the pancreas was removed with an electrocautery scalpel. The pancreatic head and hepatoduodenal mesentery were blurred due to vascular and tissue fusion caused by concomitant pancreatitis, cholangitis, and radiation chemotherapy, and the PV and CHA-proper hepatic artery (PHA) - gastroduodenal artery (GDA) were difficult to detach due to tumor invasion. The bile duct was dissected caudal to the right hepatic artery (RHA), the jejunum was pulled out on the right side after dissection, PV-SMV continuity was confirmed, and pancreaticoduodenectomy was completed with dissection with some tumor remaining. Reconstruction was performed using by Child's variant method, with the pancreatic duct tube being led out from the jejunal transection with an internal/external fistula, and the pancreatic-jejunal anastomosis was a full-layer suture with the pancreatic parenchyma and the jejunal wall in close contact. The bile duct jejunum was sutured in one layer with retrograde-transhepatic bile drainage (RTBD), and the gastric-jejunal anastomosis was sutured with Albert-Lemmbert anastomosis, and the operation was closed by placing intraperitoneal drains on both sides (operating time: 4 h 55 min, blood loss: 400 ml). Resected the specimen confirmed the diagnosis of a tumor of the head of the pancreas (Fig. 1D) with infiltrating duodenum. Pathologically, its tumor consisted of a moderately differentiated tubular adenocarcinoma (Fig. 2A, B, C and D), classified as pT3N0M0 (stage IIIB) (Invasive ductal carcinoma, post CRT; Tubular adenocarcinoma, associated with perforating duodenal ulcer, TS2, 35 × 30 mm, pT3 [pCH1, pDU0, pS0, pRP0, pPV0, pA0, pPL0, pOO0], sci, INFc, ly0, v0, ne0, pN0 [0/17], mpd0, pPCM0, pBCM1, pDPM1, pN0, pR1, therapeutic grade 2).
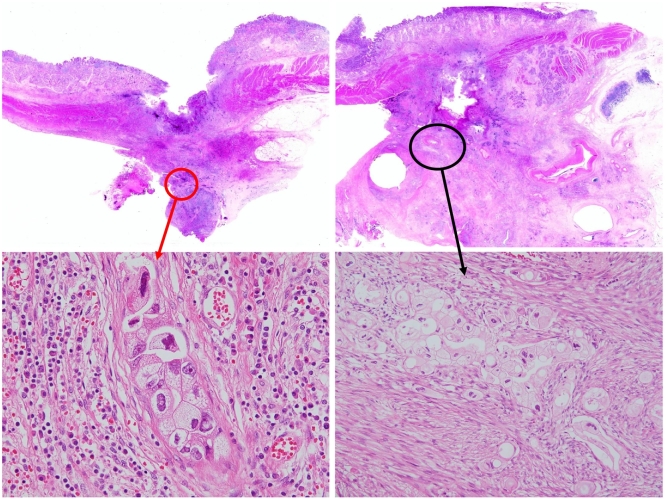
Fig. 2.
Pathologically, the specimens demonstrate deep ulceration through the whole layer in the duodenum with many coagulations. Traditional feature findings invasive ductal carcinoma with tubular pattern surrounding massive fibrosis in pancreas head area (A and C: HE ×40), and the tumors invades the bottom of the ulcer in the duodenum (B and D: HE ×40).
The patient's postoperative course was uneventful, and she was discharged on postoperative day 25. After her condition improved, she was treated for residual cancer 2 months after surgical treatment; therefore, complementary radiation with concurrent chemotherapy based on GEM was administrated. However, she died 12 months after the surgery.
3. Discussion
With the increase in numbers of patients with cancer, including pancreatic cancer, the frequency of oncologic emergencies is increasing [1]. Most surgical problems associated with oncologic emergencies are obstruction, infection, hemorrhage, perforation, or rupture. To achieve the best possible outcomes for patients with such conditions, early recognition of the signs of an emergency, and appropriate treatment, are necessary [2]. With pancreatic cancer, the main challenges are general obstructive symptoms, that is, obstructive jaundice and gastrointestinal obstruction, and associated biliary infections and bacterial translocation, and hemorrhage [1], perforation, and rupture [4] are relatively uncommon.
Although perforation and rupture owing to increased intratumor pressure are unlikely to occur in hypovascular pancreatic cancer, some tumors, such as anaplastic pancreatic ductal carcinoma, are hypervascular tumors, and occasional cases of spontaneous rupture due to rapid growth have been reported [4].
With regard to hemorrhage, duodenal invasion is relatively frequent with pancreatic cancer, yet rarely leads to upper gastrointestinal hemorrhage. With cases of upper gastrointestinal hemorrhage, the frequency is estimated to be 0.35 %–2.6 % [2], [5]. In general, the conditions under which malignant tumors lead to hemorrhage are “invasive growth” of cancer, and “plentiful supply of blood vessels to the tumor.” However, there are two mechanisms by which pancreatic cancer may cause gastrointestinal hemorrhage. Firstly, there is a hemorrhage in the pancreatic duct. There have been occasional reports of rupture of peripancreatic aneurysms associated with obstructive pancreatitis (hemosuccus pancreaticus [6]), hemorrhage from cystic pancreatic tumors, direct invasion of the pancreatic duct by anaplastic pancreatic cancer [7], and metastatic pancreatic tumors [8], [9]. Secondly, there is direct hemorrhage from pancreatic cancer, and there have been reports of resulting from the direct invasion at the duodenum from pancreatic cancer, the development of ectopic pancreatic cancer in the duodenum [10], hemorrhage due to necrosis at the tumor site in preoperative chemotherapy [11], [12]. Pancreatic cancer is rich in fibrous stromal components; however, poor in solid components. Therefore, it grows invasively; however, is poorly supplied with blood vessels, and intraductal hemorrhage is considered to be rare. There have been only a few reports of hemorrhage from infiltrating blood vessels or due to a vascular arcade being trapped in the head region of the cancerous pancreas [13], [14]. However, in general, ordinary type pancreatic cancer is considered to have a large fibrous stromal component but a small substantial component, and it rarely causes intraductal hemorrhage because it grows invasively but lacks tumor blood vessels.
The number of reported treatment of cases of hemorrhagic duodenal invasive pancreatic cancer is 23, including the present case, in 18 reports [1], [6], [7], [8], [13], [14], [15], [16], [17], [18], [19], [20] (Table 1). The mean age was 66 (27–83) years, the respective numbers of men and women were 13 and 10, and no significant differences in age or sex were found. These cases included 12 cases of hemorrhagic shock (52.2 %). The sites of the hemorrhage were the first section of the duodenum (pylorus to the bulb) in six cases (26.1 %), the second section of the duodenum (descending leg) in 10 cases (43.5 %), the third section of the duodenum (horizontal leg) in five cases (21.7 %), and not stated in two cases. Hemorrhage from the distal duodenum (second and third sections) was observed frequently.
Table 1.
Reports of pancreatic cancer invading the duodenum with bleeding.
| No. | Author | Year | Age | Sex | Shock | Site | Treatment | Curability | Prognosis | Periods |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lee | 1994 | 83 | F | NL | 3rd | Operation/UM | NL | Death | NL |
| 2 | 58 | F | NL | 3rd | NL | Non-surgical | NL | NL | ||
| 3 | 72 | F | NL | 2nd | NL | Non-surgical | NL | NL | ||
| 4 | 80 | F | NL | 3rd | Operation/UM | None | Death | NL | ||
| 5 | 67 | M | NL | 2nd | Operation/UM | None | Death | NL | ||
| 6 | Horiguchi | 1994 | 63 | M | Yes | 2nd | TAE | None | Death | 21 days |
| 7 | 59 | M | Yes | 1st | EH → Operation/UM | NL | NL | NL | ||
| 8 | Sugiyama | 1996 | 78 | F | NL | 2nd | EH → Operation/UM | NL | NL | NL |
| 9 | Suzawa | 1996 | 71 | M | Yes | 1st | EH | Non-surgical | Death | NL |
| 10 | Kawano | 2004 | 67 | M | NL | NL | TAE → PD | Yes | Death | 3 months |
| 11 | Lin | 2005 | 77 | M | Yes | 1st | EH | Non-surgical | Death | 10 months |
| 12 | Sakamoto | 2005 | 73 | F | NL | NL | EH → PD | NL | Death | 3 weeks |
| 13 | Tomita | 2006 | 67 | M | Yes | 1st | PD | Yes | Alive | 2 years |
| 14 | Kaneko | 2006 | 75 | M | No | 2nd | EH → TAE → PPPD | Yes | Death | 24 days |
| 15 | Shimada | 2007 | 27 | F | Yes | 1st | PD* | Yes | Alive | 26 months |
| 16 | Hirai | 2008 | 62 | M | Yes | 3rd | TAE → PD | Yes | Death | 113 days |
| 17 | Sunose | 2011 | 65 | M | Yes | 2nd | EH → PD | Yes | NL | NL |
| 18 | Kamei | 2012 | 55 | M | Yes | 3rd | PD | Yes | Death | 8 months |
| 19 | Okumura | 2013 | 66 | M | No | 2nd | EH → PD | Yes | Death | 9 months |
| 20 | Yamada | 2013 | 71 | M | Yes | 1st | EH → IABO | Non-surgical | Death | 9 months |
| 21 | Takada | 2014 | 43 | F | No | 2nd | EH | Non-surgical | Death | 7.5 months |
| 22 | Kagiya | 2017 | 75 | F | Yes | 2nd | EH → PD | Yes | Death | 128 days |
| 23 | Present case | 2022 | 77 | F | Yes | 2nd | PD | None | Death | 368 days |
NL: not listed U/K: unknown operation method.
TAE: transcatheter arterial embolization, PD: pancreaticoduodenectomy, PD*: PD with HA reconstruction.
PPPD: pylorus preserving pancreaticoduodenectomy, IABO: intra-aortic balloon occlusion, EH: endoscopic hemostasis.
Endoscopic hemostasis is the first-line treatment for hemorrhage associated with pancreatic cancer. However, it is difficult to identify the hemorrhage source, and hemostasis is often unsuccessful. In particular, hemorrhage from the distal duodenum is often difficult to stop endoscopically, and in the present case, the hemorrhage was from the second section of the duodenum. Since it is highly challenging to identify the responsible vessel under unstable hemodynamic conditions, pancreaticoduodenectomy as emergency hemostasis is likely to result in serious postoperative complications, and super-selective embolization under angiography is therefore recommended as an emergency hemostasis technique [14]. In patients in a state of shock, in particular, initial treatment includes arterial embolization and aortic occlusion balloon catheterization [15]. If the patient's general condition stabilizes after temporary hemostasis, endoscopic hemostasis, or resection of the primary lesion to remove the hemorrhage source is performed as permanent hemostasis. In contrast, early pancreaticoduodenectomy was performed without IVR in resectable patients who were not in a state of shock [13], [17], [18] (Table 2). Z'graggen et al. [21] reported in their study of non-traumatic, emergency pancreaticoduodenectomy that duodenal hemorrhage due to pancreatic cancer invasion was from multiple collateral blood vessels in the region surrounding the pancreatic head, making hemostasis by IVR challenging; therefore, if hemorrhage occurs repeatedly, emergency pancreaticoduodenectomy must be performed before coagulation abnormalities develop. In contrast, it has been reported that emergency pancreaticoduodenectomy is highly invasive, and should therefore be performed in specialist hospitals where expert surgeons are available, and only when there is no other treatment alternative [22]. In our case, the hemorrhage was a direct bleeding from a tumor that was not curable. There was no ectopic pancreas, and the cause of the hemorrhage was thought to be poorly differentiated adenocarcinoma causing rapid tumor necrosis, tumor necrosis caused by the effect of radiation chemotherapy, and the patient was taking antithrombotic medication. Initially, IVR was considered, but intermittent bleeding from the portal or peripancreatic arteries due to radiation chemotherapy was expected to make it difficult to identify the source of bleeding. Therefore, we opted for surgical treatment and obtained a good outcome.
Table 2.
Emergency pancreaticoduodenectomy for pancreatic carcinoma with bleeding.
| 1 | No. of reports (including our case) | 23 |
| 2 | Frequency of bleeding | |
| Upper gastrointestinal tract | 0.35–1.9 % | |
| Pancreas head cancer | 0.25–3.0 % | |
| 3 | Age | 66.6 year-old (27–83) |
| 4 | Sex (M/F) | 13:10 |
| 5 | Invading location (1st : 2nd : 3rd : unknown) | 6 : 10 : 5 : 2 |
| 6 | Shock (presence : absence : unknown) | 12 : 03 : 08 |
| 7 | Treatment | |
| Operation | 16 (PD/PPPD11, Unknown 5) | |
| TAE | 1 | |
| Endoscopy | 4 (with IABO 1) | |
| Unknown | 2 | |
| 8 | Prognosis | |
| Alive | 3 | |
| Death | 15 | |
| Unknown | 5 | |
| Mean survival time | 8.5 months | |
| 21 days ∼24 months |
The prognosis of pancreatic cancer with hemorrhage is poor. In our own case, the patient died of the primary disease 12 months after surgery. Among the reported cases, the survival period was relatively long, which was thought to be due to the success of early postoperative chemotherapy for poorly differentiated adenocarcinoma with strong proliferative potential that led to tumor necrosis. With the development of multidisciplinary treatment, the number of cases that can be resected due to the effect of tumor necrosis will increase. On the other hand, it was suggested that the number of cases in which direct bleeding from the tumor due to the effect of radiation chemotherapy or tumor necrosis may increase, as well as cases on antithrombotic medication. Therefore, it is necessary to keep gastrointestinal bleeding in mind even in patients with pancreatic cancer, and prophylactic treatment to avoid gastrointestinal bleeding should be considered in some cases.
4. Conclusions
Hemorrhagic shock arising from pancreatic head cancer with duodenal invasion is rare as pancreatic cancer is a hypovascular tumor. Although duodenal invasion of pancreatic cancer is often experienced, bleeding is rare, but the number of pancreatic cancer patients who bleed directly from the tumor may be increasing due to the anti-tumor effect of radiation chemotherapy or the increasing number of patients receiving anti-thrombotic drugs as they age. In order to predict these cases or to avoid gastrointestinal bleeding, periodic gastrointestinal scrutiny and prophylactic treatment, including resection or embolization, should be considered in some cases to avoid bleeding as much as possible.
List of abbreviations
- GEM
gemcitabine
- CT
computed tomography
- MRI
magnetic resonance imaging
- ERCP
endoscopic retrograde cholangiopancreatography
- CBD
common bile duct
- EUS
endoscopic ultrasound
- PD
pancreaticoduodenectomy
- CBD
common bile duct
- IVR
intervention therapy
Ethical approval
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Y. Wada, S. Taniwaki and H. Yoshimoto performed the operations. Y Wada and K. Hayashi managed the postoperative intensive care. All authors conceived the study and participated in its design and coordination. S. Taniwaki supervised the study and drafted the manuscript. Y Morimitsu contributed to the pathological diagnosis. All authors read and approved the final manuscript.
Guarantor
Yoshito Wada, MD, PhD.
Registration of research studies
This study is not ‘First in Man’ study.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Declaration of competing interest
The authors have no conflict of interest.
Acknowledgments
Acknowledgement
We would like to thank Editage (www.editage.jp) for English language editing.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Contributor Information
Yoshito Wada, Email: yo-wada@kyoaikai.com.
Satoshi Taniwaki, Email: taniwaki@kyoaikai.com.
References
- 1.Lee P., Sutherland D., Feller E.R. Massive gastrointestinal bleeding as the initial manifestation of pancreatic carcinoma. Int. J. Pancreat. 1994;15:223–227. doi: 10.1007/BF02924198. [DOI] [PubMed] [Google Scholar]
- 2.Johnson P.G. Oxford University Press; 23 May 2002.. RAJ spence OBE: oncologic emergencies. [Google Scholar]
- 3.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline:updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura Y., Iwai K., Kawasaki R., et al. A case of spontaneously ruptured anaplastic carcinoma (giant cell type) of the pancreas with intraabdominal hemorrhage. Jpn J Gastroenterol Surg. 2007;40:456–461. [Google Scholar]
- 5.Sharon P., Stalnikovics R., Rachemilewitz D. Endoscopic diagnosis of duodenal neoplasm causing upper gastrointestinal bleeding. J. Clin. Gastroenterol. 1982;4:35–38. doi: 10.1097/00004836-198202000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Igari K., Watanabe Y., Aihara A., et al. Two cases of hemosuccus pancreaticus. Jpn. J. Gastroenterol. Surg. 2010:431246–431251. [Google Scholar]
- 7.Mizukami Y., Arisato S., Satou K., et al. A case of anaplastic carcinoma of the pancreas, disclosed a hemosuccus pancreaticus. J. Jpn. Panc. Soc. 1997;94:706–711. [PubMed] [Google Scholar]
- 8.Aiko S., Tokura Y., Yamafuji K., et al. A resected case of pancreatic metastasis from renal cell carcinoma presenting with acute duodenal bleeding. J. Jpn. Surg. Assoc. 1993;54:2666–2672. [Google Scholar]
- 9.Meyer A., Behrend M. Is pancreatic resection justified for metastasis of papillary thyroid cancer? Anticancer Res. 2006;26:2269–2273. [PubMed] [Google Scholar]
- 10.Kaneko K., Koyama S., Nomura T., et al. A case of adenocarcinoma arising in heterotopic pancreas with gastrointestinal bleeding. Jpn. J. Gastroenterol. Surg. 2006;39:583–588. [Google Scholar]
- 11.Okumura N., Fujii T., Ishikawa T., et al. A resected case of pancreatic head cancer induced duodenal penetration and bleeding during neoadjuvant chemoradiation. Jpn J Gastroenterol Surg. 2013;46:282–288. [Google Scholar]
- 12.Takada R., Ioka T., Sueyoshi H., et al. Duodenal hemorrhage from pancreatic cancer therapy infiltration controlled through combination therapy with gemcitabine and S-1. Case Rep Gastroenterol. 2014;8:221–226. doi: 10.1159/000364819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunose Y., Hirai K., Yoshinari D., et al. A case of pancreatic cancer invading the duodenum with an initial manifestation of hypovolemic shock from duodenal bleeding: J of abdominal emergency medicine. 2011;31:119–122. [Google Scholar]
- 14.Hirai R., Negoro Y., Hyodo T., et al. Hematemesis as the initial manifestation of pancreatic cancer directly invading the duodenum: a case report. J Jpn Panc Soc. 2008;23:172–179. [Google Scholar]
- 15.Yamada M., Katou K., Kashima R., et al. A case of endoscopic hemostasis with intra-aortic balloon occlusion for severe bleeding from duodenal invasion pancreatic carcinoma. Gastroenterol. Endosc. 2013;55:459–466. [Google Scholar]
- 16.Lin Y.H., Chen C.Y., Chen C.P., et al. Hematoemesis as the initial complication of pancreatic adenocarcinoma directly invading the duodenum: a case report. J. Gastroenterol. 2005;11:767–769. doi: 10.3748/wjg.v11.i5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakamoto N., Onozato Y., Iizuka H., et al. Study of 11 cases which were performed endoscopic hemostasis for bleeding from the second portion of the duodenum. Prog Dig Endosc. 2005;66:26–30. [Google Scholar]
- 18.Tomita H., Osada S., Matsuo M., et al. Pancreatic cancer presenting with hematemesis from directly invading the duodenum: report of an usual manifestation and review. Am. Surg. 2006;72:363–366. [PubMed] [Google Scholar]
- 19.Kamei K., Yasuda T., Nakata Y., et al. Gastrointestinal bleeding as an initial manifestation of pancreatic adenocarcinoma successfully treated by emergency pancreaticoduodenectomy: case report. Acta Med Kinki Univ. 2012;37:95–97. [Google Scholar]
- 20.Kagiya T., Ishido K., Kudo D., et al. A case of pancreatic head cancer with hemorrhagic shock. J Abdominal Emergency Medicine. 2017;37:651–656. [Google Scholar]
- 21.Z’graggen K., Strobel O., Schmied B.M., et al. Emergency pancreaticoduodenectomy in non-trauma patients. Pancreas. 2002;24:258–263. doi: 10.1097/00006676-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Lissidini G., Prete F.P., Piccinni G., et al. Emergency pancreaticoduodenectomy: when is it needed? A dual non-trauma centre experience and literature review. Int. J. Surg. 2015;21:S83–S88. doi: 10.1016/j.ijsu.2015.04.096. [DOI] [PubMed] [Google Scholar]