Abstract
Objectives:
To develop pediatric-specific models that predict liver stiffness and hepatic steatosis in non-alcoholic fatty liver disease (NAFLD), based on clinical and laboratory data.
Methods:
Children with NAFLD, who had undergone magnetic resonance imaging with proton density fat fraction (MRI-PDFF) for steatosis quantification and/or magnetic resonance elastography (MRE) for liver stiffness assessment were included. We used data from patients imaged between April 2009 to July 2018 to develop a predictive model for fat fraction and stiffness. We validated the performance of the models using data from a second cohort, imaged between 2018 and 2019.
Results:
The first cohort (n = 344) consisted of predominantly non-Hispanic (80%), male (67%) adolescents. MRE data were available for 343 children, while PDFF data were available for 130. In multivariable regression, ethnicity, insulin levels, platelet count, and aspartate aminotransferase independently predicted liver stiffness and these variables were used to develop the predictive model. Similarly, sex, ethnicity, alanine aminotransferase, and triglycerides levels independently predicted liver PDFF and were used in the PDFF model. The AUC of the optimal cutoff for the model that predicted a stiffness of >2.71 kPa was 0.70 and for the model that predicted PDFF >5% was 0.78. The validation group (n=110) had similar characteristics. The correlation coefficient of the model with the measured liver stiffness was 0.30 and with the measured liver PDFF was 0.26.
Conclusions:
Pediatric-specific models perform poorly at predicting exact liver stiffness and steatosis; however, in the absence of magnetic resonance imaging can be used to predict the presence of significant steatosis (>5%) and/or significant stiffness (>2.71). Thus, imaging remains an invaluable adjunct to laboratory investigations in determining disease severity.
Keywords: hepatic steatosis, liver fibrosis, magnetic resonance elastography, non-alcoholic fatty liver disease, predictive modeling, proton density fat fraction
Non-alcoholic fatty liver disease (NAFLD) is a highly prevalent condition affecting more than one-third of the global population, with a growing prevalence in preschool ages children (1,2). NAFLD diagnosis traditionally requires a liver biopsy for confirmation, but magnetic resonance imaging (MRI) has significantly expanded our ability to accurately detect and measure steatosis and advanced fibrosis noninvasively (3–6). Liver biopsies are typically obtained on patients with persistently and significantly elevated liver enzymes, specifically alanine aminotransferase (ALT) (7); however, liver biopsies are invasive and, hence, far from ideal as the sole diagnostic tool of such a prevalent condition (6,8). In addition, clinicians are often hesitant to obtain liver biopsies for the indication of NAFLD (6). Serologic markers, such as ALT, are of limited value, as NAFLD can occur in patients with normal ALT levels (9–11). Similarly, ultrasonography, which is also often used for the identification of NAFLD, currently has suboptimal sensitivity and specificity in determining the presence and quantifying the severity of steatosis and fibrosis (8). In contrast, MRI-proton density fat fraction (PDFF) and MR elastography (MRE) can noninvasively detect and quantify hepatic steatosis and fibrosis.
MRI-PDFF can be achieved with a rapid (<1 minute) scan and does not require the use of intravenous contrast material (12–15). In addition, MRE, which can be performed in the same examination as MRI-PDFF, allows the noninvasive measurement of liver stiffness, which reflects liver fibrosis (3,5). Assessing the latter is useful, as in adults with NAFLD, fibrosis is the sole determinant of long-term liver outcomes (16). An advantage of MRI over histology is that it assesses the entire liver, rather than a microscopic fraction, which is important for a disease like NAFLD, which can be patchy in its distribution; however, considering the lack of widespread availability of MR-based imaging, and the costs associated with MRI, it is of great interest to develop novel, non-invasive approaches to assess the disease severity of children with NAFLD. In adult NAFLD, combinations of routine clinical and laboratory measurements have been used to develop mathematical equations that predict liver disease severity. These equations provide an estimate of either hepatic steatosis or fibrosis with variable accuracy (17–21). Similarly, a pediatric equation has been developed for pediatric fibrosis estimation using liver biopsy data (19). To date, there is no way to non-invasively estimate steatosis severity, or to predict the stiffness of the entire liver without using advanced imaging methodologies.
The objectives of this study were, therefore, to develop and validate pediatric-specific models using routinely used clinical and laboratory data to predict liver stiffness and steatosis as measured by MRI.
PATIENTS AND METHODS
Subjects and Study Design
This was a retrospective study performed at Cincinnati Children’s Hospital Medical Center. Institutional Review Board approval and a waiver of informed consent were obtained before the initiation of data collection. Inclusion criteria were patients 10–19 years of age with presumed or histologically confirmed NAFLD, who had undergone at least one MRI-PDFF and/or MRE examination from April 1, 2009 to July 30, 2018 for the first cohort (model development group), and from August 1, 2018 to August 30, 2019 for the second cohort (validation group). Exclusion criteria were secondary cases of liver steatosis (eg, genetic or medication-induced), evidence of other liver diseases, and history of bariatric surgery.
Clinical records were reviewed for age, sex, race and ethnicity, anthropometrics (weight, height, body mass index (BMI), and waist circumference) within 6 months of the MRI. Diagnosis of type 2 diabetes mellitus (T2DM), prescribed medication list, and blood pressure measurements at the time of the MRI were collected. Laboratory data obtained within 3 months of the MRI (those closest to the MRI, including levels of ALT, aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), alkaline phosphatase (ALP), fasting glucose, fasting insulin, hemoglobin A1C (HbA1c), platelet count, and lipid profile) were also collected. MRI examinations were reviewed to collect information regarding the liver volume (mL), liver PDFF (%), and liver stiffness (kPa).
Liver Stiffness and Fat Fraction Measured by Magnetic Resonance Imaging
Per standard clinical practice (22,23), abdominal MRI examinations had been performed without intravenous contrast material and with an active-passive driver system operated at 60 Hz, utilizing either a two-dimensional gradient recalled echo or two-dimensional spin-echo echo-planar imaging elastography sequence. Four axial slices through the mid liver had been obtained to generate shear wave and elastogram images. Regions of interest had been drawn manually by dedicated Department of Radiology imaging postprocessors for the measurement of liver stiffness (guided by 95% confidence maps), and overall liver stiffness was expressed as the weighted mean of the mean liver stiffness values for each of the four elastograms (22).
Liver PDFF imaging was performed with IDEAL IQ (GE Healthcare; Waukesha, WI) or mDIXON Quant (Philips Healthcare; Best, The Netherlands). The same postprocessors drew ovoid regions of interest for PDFF measurements that included as much liver parenchyma as possible while excluding large vessels. PDFF measurements were performed on four slices through the mid-liver with overall liver PDFF expressed as a mean of these values.
Definitions
To categorize obesity severity, patients were defined as overweight (BMI: 85th to <95th percentile for age and sex based on Centers for Disease Control and Prevention growth charts), obese class I (BMI: 95th percentile to <120% of the 95th percentile), severe obesity class II (BMI: 120% to <140% of the 95th percentile), or severe obesity class III (BMI > 140% of the 95th percentile) (24). Diagnosis of T2DM was defined as HbA1c >6.4%, oral glucose tolerance test with plasma glucose >200 mg/dL at 2 hours or confirmation of T2DM diagnosis by an endocrinologist.
Elevated MRI-PDFF was considered a result >5% (consistent with NAFLD), whereas an elevated liver stiffness was considered a result >2.71 kPa (consistent with increased risk of advanced fibrosis) (5).
Candidate Variables for Non-Alcoholic Fatty Liver Disease Liver Fat and Liver Stiffness Models
The following variables were studied in modeling of liver fat fraction and stiffness:
Clinical variables: age, sex, ethnicity, diagnosis of T2DM, anthropometrics (weight, height, BMI, and their respective z scores) and blood pressure measurements at the time of the MRI
Laboratory variables (within 3 months of the MRI): serum levels of ALT, AST, GGT, ALP, insulin, lipid panel for both models, and platelet count for the liver stiffness model.
Statistical Analyses
Statistical analyses were performed using SAS v. 9.4 (SAS Institute Inc, Cary, NC). Significance was set at a threshold of P value <0.05. Descriptive statistics (medians and interquartile ranges (IQRs) for continuous variables and counts and percentages for categorical variables) were used to present the demographics and clinical characteristics of the cohort.
To build the new pediatric model for liver PDFF and for liver stiffness in our cohort, univariable linear regression and one-way analysis of variance (ANOVA) were used to describe the relationship between variables and liver PDFF and liver stiffness. Multivariable analysis of covariance (ANCOVA) analyses were used to build the prediction models. All variables found to be significantly associated with liver PDFF or liver stiffness in the univariable analyses were included in a multivariable stepwise model selection procedure based on the predicted residual sum of squares. Non-normally distributed data were used after logarithmic (base e) transformation. The correlation between the observed values and predicted models was investigated by Pearson correlation coefficient. We also evaluated the performance of the model using the adjusted coefficient of determination (R2). The receiver operating characteristic (ROC) curve of each final model was used to identify the optimal cut-off point using the Youden index, the low cut-off point that corresponds to 95% specificity and the high cut-off point corresponds to 95% sensitivity for both NAFLD (fat fraction > 5%) (24,25) and advanced fibrosis (liver stiffness > 2.71 kPa) (3,22). Lastly, Pearson correlation coefficient and the adjusted R2 were used to validate the newly developed equations in the validation group.
RESULTS
The first cohort (model development group, n=344) consisted of predominantly non-Hispanic, male adolescents (Table 1). Liver stiffness data (MRE) were available for 343 of the 344 children, while PDFF data were available for 130 of the 344 patients.
TABLE 1.
Descriptive statistics of the first cohort (n=344) and univariate analyses with the stiffness outcome
| Variable name | |||
|---|---|---|---|
|
| |||
| Summary stats | Parameter estimates (coefficients) | Relationship with stiffness* | |
| Age (y) | 15 (12,16) | −0.10 | 0.163 |
| Sex: male, n (%) | 232 (67.4) | 0.91 | 0.685 |
| Ethnicity: non-Hispanic, n (%) | 275 (79.9) | 0.92 | 0.028 |
| Type 2 diabetes mellitus, n (%) | 26 (7.6) | 0.03 | 0.013 |
| Medication usage, n (%) | |||
| Insulin | 14 (4.1) | 1.03 | 0.026 |
| Metformin | 87 (25.3) | 0.97 | 0.001 |
| Vitamin E supplementation | 22 (6.4) | −0.11 | 0.315 |
| Statin | 9 (2.6) | −0.04 | 0.675 |
| Physical examination data | |||
| Obesity group, n (%) | 0.005 | ||
| Overweight | 10 (2.9) | 0.12 | |
| Obese Class I | 72 (20.9) | −0.24 | |
| Severe obesity Class II | 93 (27.0) | −0.06 | |
| Severe obesity Class III | 168 (48.8) | 0.93 | |
| BMI (kg/m2) | 36.5 (32.1, 41) | 0.01 | <0.001 |
| BMI z score | 2.48 (2.18, 2.68) | 0.01 | <0.001 |
| Systolic blood pressure (mmHg) | 127 (120, 134.5) | 0.003 | 0.007 |
| Diastolic blood pressure (mmHg) | 69 (63, 76) | 0.004 | 0.035 |
| Laboratory data | |||
| ALT (U/L)† | 73 (52, 105) | 0.003 | <0.001 |
| AST (U/L)† | 35 (26, 53) | 0.08 | <0.001 |
| AST_ALT ratio† | 0.49 (0.42, 0.59) | 0.07 | 0.947 |
| GGT (U/L)† | 39 (25, 60) | −0.003 | <0.001 |
| ALP (U/L)† | 155.5 (103, 255) | 0.000 | 0.273 |
| Platelet (109/L) | 293 (255, 335) | 0.06 | 0.051 |
| Fasting glucose (mg/dL)† | 90.5 (85, 96) | 0.16 | 0.154 |
| Fasting insulin level (mU/L) | 24.7 (15.4, 36.5) | 0.10 | <0.001 |
| HbA1C (%)† | 5.3 (5, 5.5) | 0.04 | 0.067 |
| Triglycerides (mg/dL)† | 140 (101.5, 201.5) | 0.001 | 0.066 |
| LDL (mg/dL) | 87 (68, 110) | −0.001 | 0.088 |
| HDL (mg/dL) | 38 (32, 45) | −0.02 | 0.579 |
| MRI data | |||
| Liver volume (mL) | 2214.5 (1861, 2586.5) | – | |
| Liver stiffness (kPa) | 2.47 (2.12, 2.81) | – | |
Data are presented as medians and interquartile ranges or N (%). ALP = alkaline phosphatase; ALT = alanine aminotransferase; ANOVA = analysis of variance; AST = aspartate aminotransferase; BMI = body mass index; GGT = gamma glutamyl transpeptidase; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein; LDL = low-density lipoprotein; MRI = magnetic resonance imaging.
One-way ANOVA was used for categorical variable and linear regression was used for continuous variables.
Variables were log-transformed for the tests.
Development of Stiffness Prediction Model
The median age of the first cohort was 15 years (IQR 12–16). The majority of patients had severe obesity (n=261; 76%). At the time of imaging, metformin, insulin, statins, and vitamin E were used by 25%, 4%, 3%, and 6% of patients, respectively. T2DM and metabolic syndrome were diagnosed in 26 of 344 (8%) and 98 of 185 (53%) of patients, respectively. The remaining of the clinical and laboratory data are summarized in Table 1.
The median liver volume measured by MRI was 2215 mL (IQRs: 1861–2587). The median stiffness was 2.47 kPa (IQRs: 2.12–2.81). Thirty percent (n=103) of patients had evidence of increased stiffness (>2.71 kPa).
In univariate analyses, log-transformed liver stiffness was associated with multiple clinical and laboratory parameters (Table 1). Stepwise multivariable regression analyses defined ethnicity, serum insulin levels, platelet count, and serum AST levels as independently predicting liver stiffness (details on model selection for Stiffness outcome are included in Table 1, Supplemental Digital Content, http://links.lww.com/MPG/C619). Using this model, liver stiffness could be calculated as follows:
The Pearson correlation coefficient between the measured (by MRI) and calculated (using the new prediction model) liver stiffness was 0.5 (P < 0.0001). The adjusted R2 of the model was 0.19.
The ROC curve for predicting the binary presence or absence of increased liver stiffness (>2.71 kPa) using the calculated value from this model is shown in Figure 1. The cutoff point of a predicted liver stiffness value of 2.445 kPa has a sensitivity of 0.77, specificity of 0.60, and area under the curve (AUC) of 0.70 for predicting the presence of an elevated liver stiffness (>2.71 kPa).
FIGURE 1.
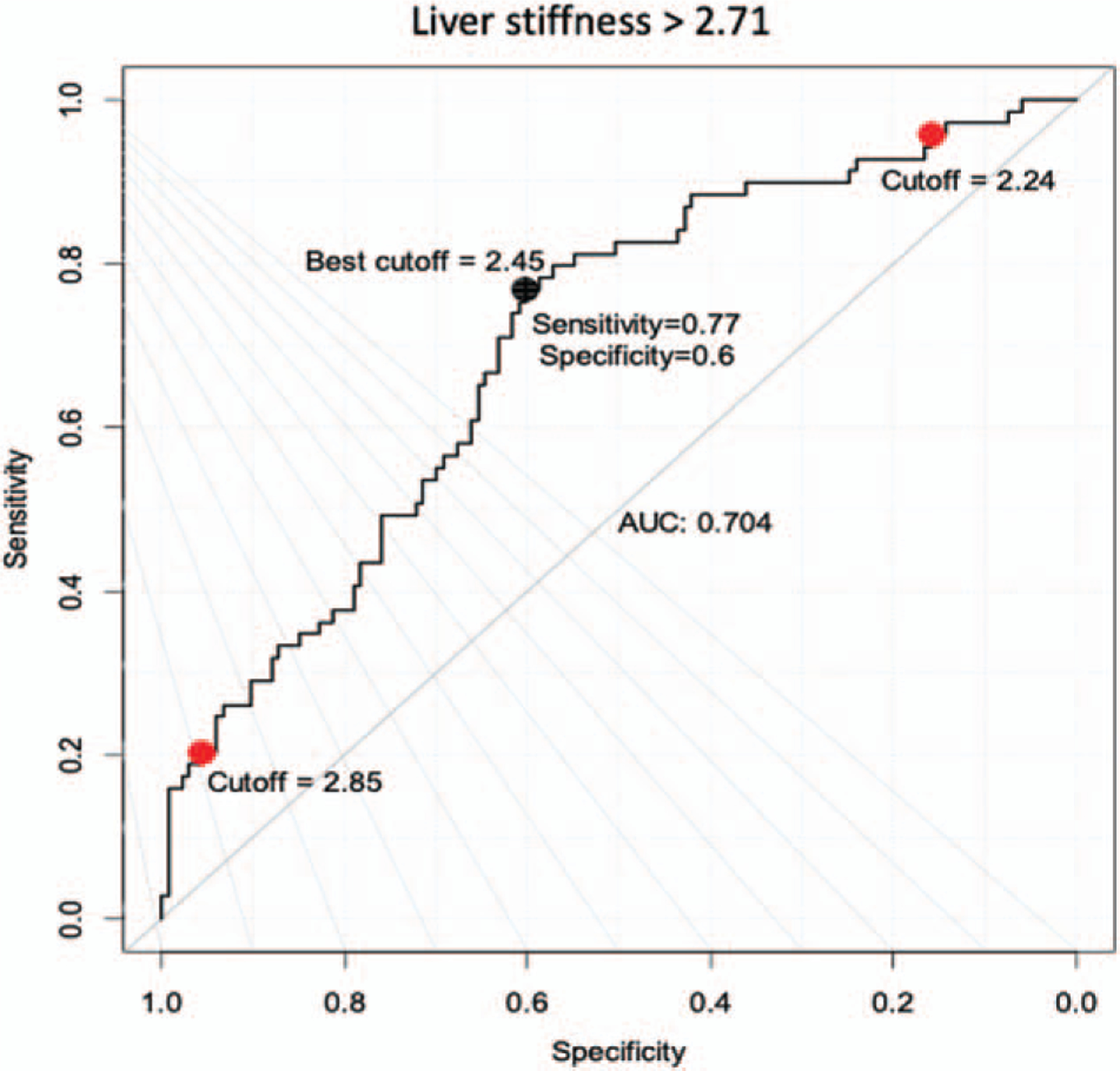
ROC curve showing the performance of the calculated liver stiffness value in predicting the presence of increased liver stiffness (>2.71 kPa). The black dot is the optimal cutoff point (2.445) that maximizes the distance to the diagonal line (Youden’s method). The two red dots are the cutoff points can predict the presence of MRI-measured liver stiffness >2.71 kPa with a minimum sensitivity and specificity of 95%, respectively. MRI = magnetic resonance imaging; ROC = receiver operating characteristic.
Development of the Fat Fraction Model (n = 130)
The median age of this cohort was 15 years (IQR 13–17); 72% of the patients were male and 21% were Hispanic. The majority of patients had severe obesity (n=104; 80%). At the time of imaging, metformin, insulin, statins, and vitamin E were used by 23%, 4%, 3%, and 4% of patients, respectively. T2DM was diagnosed in 12 of 130 (9%) of patients. The remaining of the clinical and laboratory data are summarized in Table 2.
TABLE 2.
MRI-PDFF cohort (n=130)—descriptive statistics and univariate analyses with the liver fat fraction (liver PDFF) outcome
| Variable name | |||
|---|---|---|---|
|
| |||
| Summary stats | Parameter estimates (coefficients) | Relationship with liver PDFF* P value |
|
| Age (y) | 15 (13,17) | −0.21 | 0.564 |
| Sex: male, n (%) | 94 (72.3) | 22.51 | 0.008 |
| Ethnicity: non-Hispanic, n (%) | 103 (79.2) | 19.47 | 0.003 |
| Type 2 diabetes mellitus, n (%) | 12 (9.2) | 28.28 | 0.015 |
| Medication usage | |||
| Insulin | 5 (3.8) | 30.40 | 0.050 |
| Metformin | 30 (23.1) | 24.23 | 0.062 |
| Vitamin E | 5 (3.8) | 22.49 | 0.750 |
| Statin | 4 (3.1) | 15.05 | 0.277 |
| Physical examination data | |||
| Obesity group, n (%) | 0.592 | ||
| Overweight | 4 (3.1) | −3.79 | |
| Obese Class I | 22 (16.9) | −3.17 | |
| Severe obesity Class II | 31 (23.8) | −1.94 | |
| Severe obesity Class III | 73 (56.2) | 22.06 | |
| BMI (kg/m2) | 37.4 (32.6, 41.2) | 0.06 | 0.705 |
| BMI z score | 2.56 (2.26, 2.73) | 2.13 | 0.423 |
| Systolic blood pressure (mmHg) | 126 (118, 134) | 0.01 | 0.897 |
| Diastolic blood pressure (mmHg) | 69 (63, 76) | 0.17 | 0.126 |
| Laboratory data | |||
| ALT (U/L)† | 75 (56, 105) | 7.34 | <0.001 |
| AST (U/L)† | 35 (28, 52) | 5.56 | 0.001 |
| AST_ALT ratio† | 0.48 (0.4, 0.59) | −6.68 | 0.028 |
| GGT (U/L)† | 42.5 (28, 64) | 1.54 | 0.279 |
| ALP (U/L)† | 145 (101, 237) | 1.52 | 0.438 |
| Platelet (109/L) | 294 (268, 334) | 0.04 | 0.054 |
| Fasting glucose (mg/dL)† | 90 (86, 96) | 10.43 | 0.124 |
| Fasting insulin level (mU/L) | 24.1 (15.7, 32.4) | 0.06 | 0.347 |
| HbA1C (%)† | 5.3 (5.1, 5.6) | 24.97 | 0.005 |
| Triglycerides (mg/dL)† | 144 (98, 200) | −0.24 | 0.898 |
| LDL (mg/dL) | 87 (65, 112) | −0.01 | 0.834 |
| HDL (mg/dL) | 38 (33, 45) | −0.10 | 0.371 |
| MRI data | |||
| Liver volume (mL) | 2333 (1943, 2745) | – | |
| Liver PDFF (%) | 21.1 (11, 29.9) | – | |
ALP = alkaline phosphatase; ALT = alanine aminotransferase; ANOVA = analysis of variance; AST = aspartate aminotransferase; BMI = body mass index; GGT = gamma glutamyl transpeptidase; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein; LDL = low-density lipoprotein; MRI = magnetic resonance imaging; PDFF = proton density fat fraction.
One-way ANOVA was used for categorical variable and linear regression was used for continuous variables.
Variables were log-transformed for the tests.
The median liver volume was 2333 mL (IQRs: 1943–2745). The median liver PDFF was 21.1% (IQR: 11–29.9). In univariable analyses, liver PDFF was associated with sex, ethnicity, T2DM diagnosis, use of insulin, as well as serum ALT, AST, and HbA1c levels (Table 2). Under stepwise model selection, sex, ethnicity, serum ALT and triglycerides levels, and three interactions were selected for liver PDFF (details on selected model selection for liver fat fraction outcome are included in Table 2, Supplemental Digital Content, http://links.lww.com/MPG/C620). Using this model, the mean liver PDFF could be calculated as follows:
The Pearson correlation coefficient between the calculated and measured liver PDFF was 0.59 (P < 0.0001). The adjusted R2 that measures the model performance was 0.31.
The ROC curve of using predicted liver fat fraction value to predict presence of MRI-measured fat fraction (>5%) is shown in Figure 2. The best cutoff point (18.3%) that maximized the distance to the diagonal line using the Youden index achieves sensitivity of 0.74, specificity of 0.75, and AUC of 0.78.
FIGURE 2.
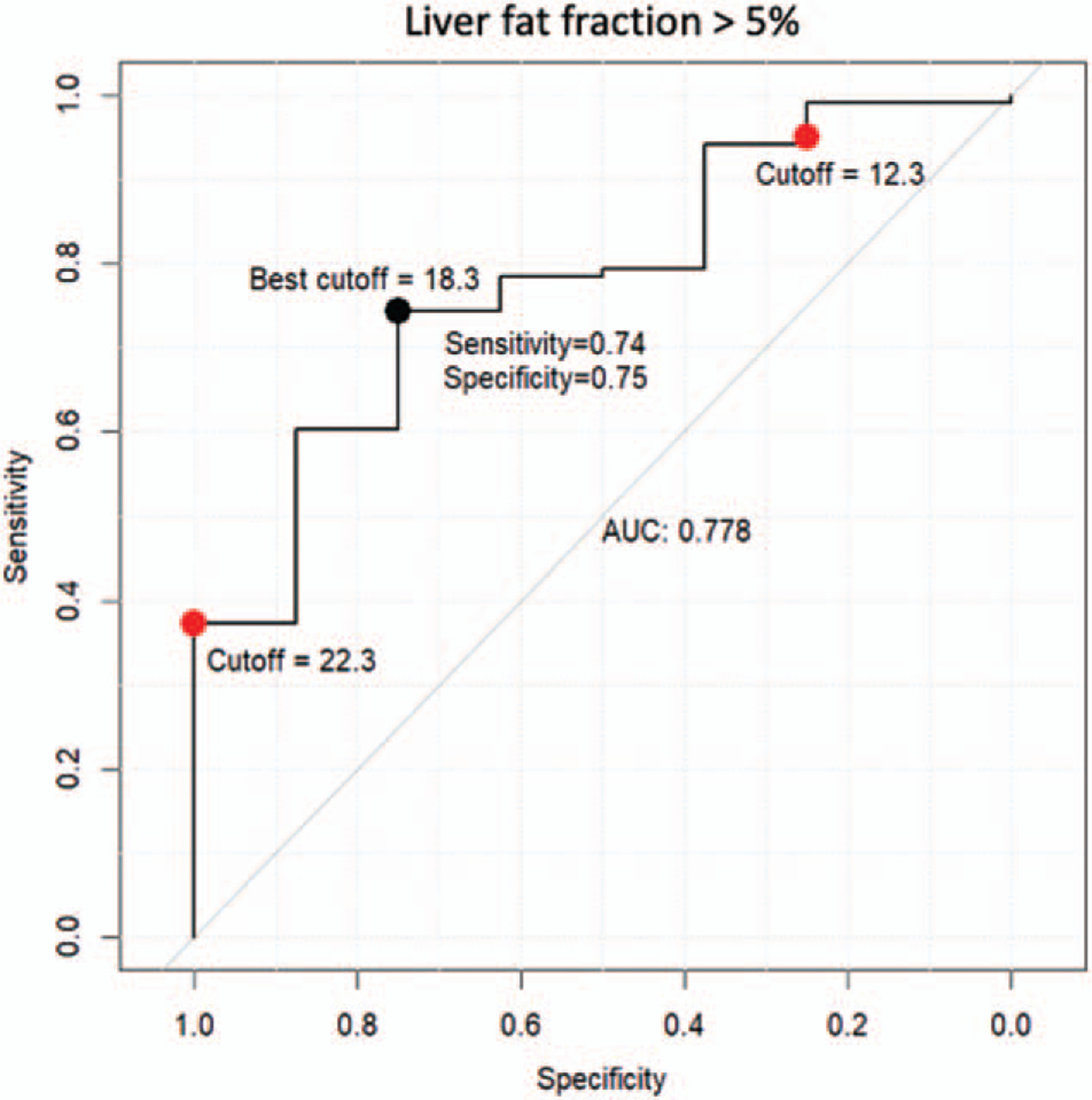
ROC curve showing the performance of the calculated liver PDFF (MRI-measured fat fraction) value in predicting the presence of increased liver PDFF (>5%). The black dot is the best cutoff point (18.3%) that maximize the distance to the diagonal line (Youden’s method). The two red dots are the cutoff points can predict the presence of PDFF with at least 95% of sensitivity and with at least 95% of specificity respectively. MRI=magnetic resonance imaging; PDFF = proton density fat fraction; ROC = receiver operating characteristic.
Validation of the New Equations in Validation Group with Pediatric Non-Alcoholic Fatty Liver Disease
The validation group (n=110) also consisted of predominantly non-Hispanic, male adolescents (details on the validation group are included in Table 3).
TABLE 3.
Comparison between the equation group and the validation group of subjects with pediatric NAFLD
| Variables | Equation group (n=344) | Validation group (n=110) | P value |
|---|---|---|---|
| Age | 15 (12, 16) | 14 (12, 17) | 0.867 |
| Sex, male; n (%) | 232 (67%) | 80 (73%) | 0.298 |
| Ethnicity; n (%) | 0.057 | ||
| White, Hispanic | 69 (20%) | 32 (29%) | |
| Non-Hispanic | 275 (80%) | 78 (71%) (80%) | |
| Type 2 diabetes, n (%) | 26 (7.6%) | 6 (5.5%) | 0.453 |
| Medications | |||
| Insulin, n (%) | 14 (4%) | 1 (1%) | 0.106 |
| Metformin, n (%) | 87 (25%) | 25 (23%) | 0.587 |
| Vitamin E, n (%) | 22 (6%) | 8 (7%) | 0.747 |
| Statin, n (%) | 9 (3%) | 2 (2%) | 0.636 |
| BMI (kg/m2) | 36 (32, 41) | 35 (32, 40) | 0.100 |
| z score | 2.5 (2.2, 2.7) | 2.4 (2.2, 2.7) | 0.463 |
Data are presented as medians (ranges) or n (%). BMI = body mass index; NAFLD = non-alcoholic fatty liver disease.
The Pearson correlation coefficient between the measured (by MRI) and calculated (using the new prediction model) liver stiffness was 0.30 (P=0.005). The adjusted R2 of the model was 0.052. The Pearson correlation coefficient between the calculated and measured liver PDFF was 0.26 (P=0.008). The adjusted R2 that measures the model performance was 0.081.
DISCUSSION
In this study, we used all available clinical and laboratory information from a large pediatric cohort of patients with NAFLD who had undergone MRI-PDFF and MRE to develop novel mathematical models that estimate the liver stiffness and liver PDFF for patients with presumed NAFLD. Liver stiffness and liver PDFF values predicted based on these newly developed stiffness and fat fraction models had fair correlation with the observed MRI measurements. While the performance of these new models in predicting elevated liver stiffness (>2.71 kPa) and liver PDFF (>5%) were acceptable (with ROC 0.70 and 0.78, respectively), the predicted liver fat fraction and stiffness estimates of the validation group correlated poorly with the measured values.
Liver stiffness measured by MRE is a surrogate marker for fibrosis (26). The presence and severity of fibrosis are important predictors of the long-term risk for cirrhosis, hepatocellular carcinoma, and liver-related mortality (16,26). A variety of equations have been developed to predict fibrosis in adults, and fewer in children; however, these have not been found to be accurate. Jackson et al recently tested the accuracy of the following fibrosis scoring systems: AST to ALT ratio, AST to Platelet Ratio Index (APRI) (16), Pediatric NAFLD Fibrosis Index (18), Pediatric NAFLD Fibrosis Score (19), and Fibrosis-4 (20) in a cohort of 146 children with NAFLD and found them to perform poorly (APRI was the best performing score with an AUC of only 0.67) (27). Similarly, in our study, a novel model that was developed based on a combination of clinical and laboratory markers to predict liver stiffness also performed poorly. This suggests that liver stiffness measured by imaging is complementary to the clinical information and laboratory investigations obtained and should remain part of the work up of patients with NAFLD, when available; however, in the absence of available MRE, our novel stiffness model could be used to predict the presence of increased stiffness (>2.71 kPa) and contribute to clinical decision-making, such as when to proceed with a liver biopsy.
Interestingly, of all the clinical and biochemical markers that were significant univariable predictors of liver stiffness, only ethnicity, platelet count, serum AST, and insulin levels were independent predictors of liver stiffness in multivariable analysis. This is not surprising and not different than the variables that have been included in the aforementioned fibrosis scoring systems (17,20,27). Large cohort studies of adults with NAFLD have shown that patients of Hispanic ethnicity have lower fibrosis scores overall and are less likely to have advanced fibrosis than non-Hispanics (28,29). Similarly, in pediatric studies, fasting serum insulin levels and the presence of T2DM have been associated with the severity of liver fibrosis in NAFLD (30,31).
Similar to the model for predicting stiffness, the novel steatosis model developed in this study performed only weakly in terms of predicting the severity of steatosis. While steatosis is not the most important determinant of outcome in patients with NAFLD (16), being able to quantify the degree of steatosis provides an estimate metabolic dysregulation. Furthermore, being able to determine which patients with obesity have a liver fat fraction >5% versus <5%, and as such predicting the presence of NAFLD, can be crucial, as it can eliminate unnecessary testing, such as costly imaging or interventional studies. Currently, steatosis severity can only be assessed using MRI- or computed tomography-based imaging or histology (3–6,32). In our analysis, the variables that independently predicted steatosis and that were used in our model were sex, ethnicity, and serum triglyceride levels. Ethnic differences in steatosis severity have been shown previously, as Hispanic Caucasians have more severe steatosis compared to non-Hispanic Caucasians (28). This may in part be due to the increased prevalence of the patatin-like phospholipase domain containing 3 (PNPLA3) polymorphism (rs738409) in Hispanics, which in turn is associated with steatosis severity (33). In addition, individuals heterozygous for the PNPLA3 polymorphism have higher hepatic triglyceride levels, compared to individuals with the wild type. Higher serum triglyceride levels in the context of NAFLD often also suggest insulin resistance, which is a known driver of hepatic steatosis (34). The differences in steatosis severity among sexes remains unclear; however, population-based studies suggest a higher prevalence of severe liver disease in men during reproductive age (35). This remains to be elucidated further.
Considering the link between pediatric obesity and NAFLD, the significant prevalence of pediatric NAFLD and its rising incidence (1,36), it is important to pursue non-invasive approaches to predict its presence and severity. Specialized MRI with ability to obtain PDFF and elastography measurements is not widely available. Vibration controlled transient elastography (Fibroscan) is becoming more widely available and is superior to MRI in terms of practicality, as it can be used at point-of-care (37); however, it’s use is currently limited by the paucity of pediatric data to allow accurate interpretation of results. The use of adult cutoffs to interpret transient elastography data is problematic considering the differences between adult and pediatric NAFLD histology (38). Therefore, options such as predictive equations are needed clinically.
While our study provides further insight into associations between clinical variables and liver stiffness and PDFF, it has limitations. The limitations of this study include its retrospective nature and the small sample size of the cohort with available liver PDFF measurements. Because of the retrospective design, not all clinical variables of interest that may have been predictive of liver PDFF or stiffness were available. In addition, the stiffness and liver fat fraction models developed in this study remain to be further validated; however, this study included a sample size of patients with MRE and MRI-PDFF studies that was larger than the sample size of most studies that used histology to generate models to predict fibrosis severity (39).
In conclusion, we studied a large cohort of pediatric patients with NAFLD with available MRE and PDFF measurements and developed and validated novel mathematical models to predict stiffness and liver PDFF using only clinical and laboratory parameters. Similar to previously developed models that aimed at predicting the presence of histologically determined steatosis and fibrosis, our models had only weak performance in predicting MRI-based stiffness and PDFF. The models however had acceptable performance when it came to predicting the presence of elevated stiffness (>2.71 kPa) and fat fraction (>5%) and could be used in the absence of advanced imaging methodology. Regardless, our results underscore that imaging remains an important adjunct to the armamentarium of clinicians caring for children with NAFLD.
Supplementary Material
Appendix 1_NAFLD liver stiffness equation.xlsx, http://links.lww.com/MPG/C621.
Appendix 2_NAFLD hepatic fat fraction equation.xlsx, http://links.lww.com/MPG/C622.
What Is Known
Non-alcoholic fatty liver disease (NAFLD) still requires a liver biopsy for confirmation, but magnetic resonance imaging (MRI) techniques have significantly expanded our ability to accurately detect and measure steatosis and advanced fibrosis noninvasively.
Pediatric-specific models that can accurately predict the disease severity of patients with NAFLD in the absence of MRI have not been defined.
What Is New
Pediatric-specific models were developed using clinical and laboratory data to predict MRI-based measurements of fat fraction and stiffness.
These pediatric-specific models had acceptable performance at predicting elevated liver stiffness (>2.71 kPa) and the presence of fatty liver disease (>5%).
The models performed poorly at predicting the exact liver stiffness and fat fraction.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
REFERENCES
- 1.Anderson EL, Howe LD, Jones HE, et al. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One 2015;10:. e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhaliwal J, Chavhan GB, Lurz E, et al. Hepatic steatosis is highly prevalent across the paediatric age spectrum, including in pre-school age children. Aliment Pharmacol Ther 2018;48:556–63. [DOI] [PubMed] [Google Scholar]
- 3.Xanthakos SA, Trout AT, Dillman JR. Magnetic resonance elastography assessment of fibrosis in children with NAFLD: promising but not perfect. Hepatology 2017;66:1373–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dillman JR, Trout AT, Costello EN, et al. Quantitative liver MRI-biopsy correlation in pediatric and young adult patients with nonalcoholic fatty liver disease: can one be used to predict the other? AJR Am J Roentgenol 2018;210:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xanthakos SA, Podberesky DJ, Serai SD, et al. Use of magnetic resonance elastography to assess hepatic fibrosis in children with chronic liver disease. J Pediatr 2014;164:186–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mouzaki M, Ling SC, Schreiber RA, et al. Management of pediatric nonalcoholic fatty liver disease by academic hepatologists in Canada: a nationwide survey. J Pediatr Gastroenterol Nutr 2017;65:380–3. [DOI] [PubMed] [Google Scholar]
- 7.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017;377:2063–72. [DOI] [PubMed] [Google Scholar]
- 8.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr 2017;64:319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–95. [DOI] [PubMed] [Google Scholar]
- 10.Molleston JP, Schwimmer JB, Yates KP, et al. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J Pediatr 2014;164:707–13 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orkin S, Yodoshi T, Sun Q, et al. Can baseline characteristics be used to predict liver disease outcomes in pediatric nonalcoholic fatty liver disease? Obesity (Silver Spring) 2020;29:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease—MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 2012;36:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Middleton MS, Heba ER, Hooker CA, et al. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology 2017;153:753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Younossi ZM, Loomba R, Anstee QM, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology 2018;68:349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Middleton MS, Van Natta ML, Heba ER, et al. Diagnostic accuracy of magnetic resonance imaging hepatic proton density fat fraction in pediatric nonalcoholic fatty liver disease. Hepatology 2018;67: 858–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389.e10–97.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cárdenas E, et al. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol 2008;7:350–7. [PubMed] [Google Scholar]
- 18.Nobili V, Alisi A, Vania A, et al. The pediatric NAFLD fibrosis index: a predictor of liver fibrosis in children with non-alcoholic fatty liver disease. BMC Med 2009;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alkhouri N, Mansoor S, Giammaria P, et al. The development of the pediatric NAFLD fibrosis score (PNFS) to predict the presence of advanced fibrosis in children with nonalcoholic fatty liver disease. PLoS One 2014;9:e104558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–6. [DOI] [PubMed] [Google Scholar]
- 21.Kotronen A, Peltonen M, Hakkarainen A, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009;137:865–72. [DOI] [PubMed] [Google Scholar]
- 22.Mouzaki M, Trout AT, Arce-Clachar AC, et al. Assessment of non-alcoholic fatty liver disease progression in children using magnetic resonance imaging. J Pediatr 2018;201:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yodoshi T, Orkin S, Arce-Clachar AC, et al. Alternative etiologies of liver disease in children with suspected NAFLD. Pediatrics 2021;147: e009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krebs NF, Himes JH, Jacobson D, et al. Assessment of child and adolescent overweight and obesity. Pediatrics 2007;120(Suppl 4): S193–228. [DOI] [PubMed] [Google Scholar]
- 25.Koot BG, van der Baan-Slootweg OH, Bohte AE, et al. Accuracy of prediction scores and novel biomarkers for predicting nonalcoholic fatty liver disease in obese children. Obesity (Silver Spring) 2013;21: 583–90. [DOI] [PubMed] [Google Scholar]
- 26.Schwimmer JB, Behling C, Angeles JE, et al. Magnetic resonance elastography measured shear stiffness as a biomarker of fibrosis in pediatric nonalcoholic fatty liver disease. Hepatology 2017;66:1474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson JA, Konomi JV, Mendoza MV, et al. Performance of fibrosis prediction scores in paediatric non-alcoholic fatty liver disease. J Paediatr Child Health 2018;54:172–6. [DOI] [PubMed] [Google Scholar]
- 28.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010;52:913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bambha K, Belt P, Abraham M, et al. Ethnicity and nonalcoholic fatty liver disease. Hepatology 2012;55:769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patton HM, Yates K, Unalp-Arida A, et al. Association between metabolic syndrome and liver histology among children with nonalcoholic Fatty liver disease. Am J Gastroenterol 2010;105:2093–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xanthakos SA, Jenkins TM, Kleiner DE, et al. High prevalence of nonalcoholic fatty liver disease in adolescents undergoing bariatric surgery. Gastroenterology 2015;149:623.e8–34.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Awai HI, Newton KP, Sirlin CB, et al. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin Gastroenterol Hepatol 2014;12:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballestri S, Nascimbeni F, Baldelli E, et al. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther 2017;34:1291–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahota AK, Shapiro WL, Newton KP, et al. Incidence of nonalcoholic fatty liver disease in children: 2009–2018. Pediatrics 2020;146:e20200771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassinotto C, Boursier J, de Ledinghen V, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 2016; 63:1817–27. [DOI] [PubMed] [Google Scholar]
- 38.Tokuhara D, Cho Y, Shintaku H. Transient elastography-based liver stiffness age-dependently increases in children. PLoS One 2016;11: e0166683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joshi M, Dillman JR, Singh K, et al. Quantitative MRI of fatty liver disease in a large pediatric cohort: correlation between liver fat fraction, stiffness, volume, and patient-specific factors. Abdom Radiol (NY) 2018;43:1168–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1_NAFLD liver stiffness equation.xlsx, http://links.lww.com/MPG/C621.
Appendix 2_NAFLD hepatic fat fraction equation.xlsx, http://links.lww.com/MPG/C622.


