Abstract
Background:
The eye region of the face is particularly relevant for decoding threat-related signals, such as fear. However, it is unclear if gaze patterns to the eyes can be influenced by fear learning. Previous studies examining gaze patterns in adults find an association between anxiety and eye gaze avoidance, although no studies to date examine how associations between anxiety symptoms and eye-viewing patterns manifest in children. The current study examined the effects of learning and trait anxiety on eye gaze using a face-based fear conditioning task developed for use in children.
Methods:
Participants were 82 youth from a general population sample of twins (aged 9–13 years), exhibiting a range of anxiety symptoms. Participants underwent a fear conditioning paradigm where the conditioned stimuli (CS+) were two neutral faces, one of which was randomly selected to be paired with an aversive scream. Eye tracking, physiological, and subjective data were acquired. Children and parents reported their child’s anxiety using the Screen for Child Anxiety Related Emotional Disorders.
Results:
Conditioning influenced eye gaze patterns in that children looked longer and more frequently to the eye region of the CS+ than CS– face; this effect was present only during fear acquisition, not at baseline or extinction. Furthermore, consistent with past work in adults, anxiety symptoms were associated with eye gaze avoidance. Finally, gaze duration to the eye region mediated the effect of anxious traits on self-reported fear during acquisition.
Conclusions:
Anxiety symptoms in children relate to face-viewing strategies deployed in the context of a fear learning experiment. This relationship may inform attempts to understand the relationship between pediatric anxiety symptoms and learning.
Keywords: Eye gaze, face processing, anxiety, conditioning, psychophysiology
Introduction
This study explores associations between anxiety and eye gaze patterns to faces that have acquired aversive qualities through conditioning. Humans spend considerable time fixating on eyes during social interactions (Grossmann, 2017; Haith, Bergman, & Moore, 1977; Haxby, Hoffman, & Gobbini, 2002; Johnson, Dziurawiec, Ellis, & Morton, 1991), particularly when a face conveys threat (Green, Williams, & Davidson, 2003; Öhman, Flykt, & Lundqvist, 2000). Of note, eye-viewing patterns also relate to symptoms of anxiety disorders (Horley, Williams, Gonsalvez, & Gordon, 2003); anxious individuals are more likely than nonanxious individuals to view eye gaze as a source of threat (Öhman, 1986). Also, consistent with associations between anxiety and avoidance behavior, anxious individuals tend to avoid eye contact (Baker & Edelmann, 2002; Farabee, Ramsey, & Cole, 1993; Weeks, Howell, & Goldin, 2013), especially in response to threatening faces (Horley, Williams, Gonsalvez, & Gordon, 2004; Horley et al., 2003). It is unclear if eye-viewing patterns are influenced by learning and how the relationship between anxiety symptoms and eye-viewing patterns manifests in children. The current study addresses these two questions.
Threatening stimuli like fearful or angry faces receive prioritized visual processing and are detected more rapidly than nonthreatening stimuli (LoBue, Matthews, Harvey, & Stark, 2014; Öhman, Flykt, & Esteves, 2001; Öhman, Lundqvist, & Esteves, 2001). Learning may further amplify this innate tendency. Visual stimuli that have previously been associated with an aversive event (conditioned stimuli, CS+), such as an aversive noise or electrical shock, can capture and modulate spatial attention (Armony & Dolan, 2002) and are more readily detected compared to neutral stimuli (Padmala & Pessoa, 2008). Innate tendencies and those acquired through experience may interact to influence attention for some stimuli (Mineka & Öhman, 2002). Through such forms of conditioning, associations can be formed between aversive unconditioned stimuli (UCS) and conditioned stimuli (CS) to elicit conditioned responses (Michalska et al., 2016) and preferential orienting compared to neutral stimuli. However, no prior study examines whether effects of fear conditioning lead eye movements to preferentially target specific internal features, such as the eye region of a conditioned face. Eyes are salient targets (Lundqvist, Esteves, & Ohman, 1999) to which individuals direct their gaze in an effort to locate the source of a threat (Öhman, Flykt et al., 2000). We investigated whether fear-conditioned neutral faces evoke eye movements to the eye region.
Anxious individuals exhibit biased attention orienting toward threats (i.e. Shechner et al., 2013, 2017). However, paradoxically, direct eye gaze, particularly by faces expressing negative emotions, also can elicit gaze avoidance in highly anxious participants (Heuer, Rinck, & Becker, 2007; Horley et al., 2003, 2004; Lange, Keijsers, Becker, & Rinck, 2008; Roelofs et al., 2009; Schneier, Rodenbaugh, Blanco, Lewin, & Liebowitz, 2011; Van Peer et al., 2007; Van Peer, Spinhoven, van Dijk, & Roelofs, 2009). In eye tracking studies, anxious relative to nonanxious adults show less gaze fixations on the eyes of faces expressing negative emotions (Horley et al., 2003, 2004; Moukheiber et al., 2010). Such gaze avoidance may function acutely to reduce anxiety, but over time avoidance may reduce opportunities to counter a biased view of other people as critical or rejecting or minimalize extinction learning. Moreover, other people may misinterpret such gaze avoidance as a sign of disinterest, diminishing opportunity for positive social interaction.
Findings on eye gaze patterns in anxiety could reflect either the innately threatening qualities of emotional faces or learned properties of faces. To consider how fear learning impacts eye gaze, this study examines associations between anxiety and eye gaze using faces that have acquired aversive properties through conditioning. Understanding whether anxious individuals avoid eye contact in the context of learned associations may clarify factors that lead to maladaptive learning behaviors and impact subsequent learning in anxiety disorders. Most research on eye gaze and anxiety examines adults, finding eye gaze avoidance in anxious patients. The only relevant study in youth focused on temperament as opposed to anxiety symptoms, and the pattern in that study was opposite from the pattern found in adults (Brunet, Heisz, Mondloch, Shore, & Schmidt, 2009). Mapping relations between anxiety and eye gaze in children, particularly in a preclinical population at risk for anxiety, may identify factors that predict conversion to clinical anxiety. Learned responses to threatening stimuli may contribute to this process. Such work is particularly needed in preadolescence, a sensitive period in socioemotional development (Blakemore & Mills, 2014).
To address this need, we examined associations between eye gaze and pediatric anxiety symptoms using a face-based fear conditioning task developed for use with children (Britton et al., 2013; Lau et al., 2008; Lau et al., 2011). To monitor the effectiveness of the conditioning procedure, we assessed skin conductance response (SCR) and self-reported fear, in addition to eye tracking, as measures of fear learning. The study tested two hypotheses. First, irrespective of trait anxiety levels, aversive conditioning was expected to impact eye gaze patterns toward the face. Due to mixed evidence in the adult literature (Green et al., 2003; Horley et al., 2003, 2004), we were agnostic regarding the direction of this effect. Second, based on data linking eye gaze avoidance and anxiety in adults, increasing levels of trait anxiety were expected to predict increasingly strong eye gaze avoidance across preacquisition, acquisition, and extinction. Finally, in an exploratory analysis, we examined the degree to which eye gaze during acquisition mediated the relationship between trait anxiety symptoms and fear learning. Reduced gaze patterns to the eye region during learning may lead the viewer to miss social information and maintain anxious behavior.
Method
Participants
Families were recruited as part of a larger multisite twin study (the Virginia Commonwealth University Juvenile Anxiety Study, VCU-JAS) recruited through the Mid-Atlantic Twin Registry (MATR). Families were tested at one of the two sites, based on proximity. The present study is from a subsample of this larger cohort, reporting data from all subjects studied at the National Institute of Mental Health (NIMH) in Bethesda, Maryland. Participants were enrolled who provided written assent and whose primary caregivers provided written informed consent. Inclusion criteria were being a twin identified through MATR between the ages of 9 and 13. Exclusion criteria were use of medication; suicidal ideation; lifetime history of mania, psychosis, or pervasive developmental disorder; active medical problems; and IQ < 70. Study procedures were approved by the National Institute of Mental Health Institutional Review Board. The paradigm consisted of one visit that included the fear conditioning and extinction phases. During that visit, all participants and their parents completed a structured psychiatric interview and reported on the child’s anxiety symptoms using total scores and subscores from the Screen for Child Anxiety Related Emotional Disorders (SCARED) (Birmaher et al., 1997). Families were compensated for their time.
A total of 125 children between the ages 9 and 13 years participated. Thirteen children (10%) discontinued participation when they became anxious. Children who discontinued did not differ on SCARED total scores from children who completed the procedures (p = .67). One additional child withdrew consent prior to starting procedures, leaving 111 contributing data. From these 111 children, data from 29 were excluded because of technical problems, leaving 82 in the eye tracking analysis (27 complete twin pairs: 11 MZ, 12 DZ, four pairs missing zygosity information). Of these, 78 children had complete SCR data (26 complete twin pairs). Subjects whose data were lost because of technical problems did not differ on SCARED total scores from subjects with no data loss (p = .84). Data from two participants were excluded from SCR analyses because they were outliers (see Data Analysis section for details on how outliers were determined). Therefore, 76 children were included in the final SCR analysis. Twenty-five children had SCARED scores in the clinically significant range, and five children were currently experiencing an anxiety disorder, as confirmed by a clinician (see Table 1 for demographic information and Appendix S1 for diagnosis details).
Table 1.
Demographic characteristics of the children in the analyses
| Measure |
N
|
Age in years (M, SD) | IQ | SCARED Parent | SCARED Child | |
|---|---|---|---|---|---|---|
| Total | Female (%) | |||||
| Eye tracking | 82 | 59.80 | 11.81 (1.31) | 113.30 (9.64) | 14.52 (10.66) | 17.38 (10.83) |
| Skin conductance | 76 | 55.60 | 11.77 (1.32) | 113.61 (9.86) | 14.56 (10.91) | 17.09 (10.55) |
Procedure
Fear conditioning task.
Participants underwent a differential fear conditioning paradigm used previously (Britton et al., 2013; Lau et al., 2011). Fear conditioning consisted of a preacquisition phase and an acquisition phase. In preacquisition, children passively viewed neutral faces of two women, the CS, in the absence of the unconditioned stimulus (UCS). During acquisition (Figure 1), one woman, the CS+, predicted the UCS, a 1 s image of a fearful face coterminating with a loud, aversive 95 dB scream, while the other woman, the CS–, did not. The two faces were counterbalanced for CS+ and CS–. The CS+ was followed by the UCS with an 80% reinforcement schedule. Participants were instructed that they could learn to predict when the UCS would occur but were not informed of the CS/UCS contingency. During fear extinction, the CS+ and CS– were presented repeatedly in the absence of the UCS. Fear extinction occurred immediately after participants filled out subjective fear ratings following acquisition. Throughout all phases, the CS+ and CS– were presented for 7–8 s, followed by an interstimulus interval (ISI) of a blank, gray screen for 8–21 s (mean = 15 s).
Figure 1.

Example heat map and schematic representation of experimental design. During fear acquisition, one female face [conditioned stimulus (CS+)] was paired with a fearful face coterminating with a scream [unconditioned stimulus (UCS)]. The other female face (CS–) was never paired with the UCS. During extinction, the two female faces were presented without the UCS
The task was presented using E-prime version 3.2.1 on a desktop computer and 10.75″ × 13.5″ monitor, approximately 90 cm from the seated child. Eye movements were recorded at 60 Hz using a remote infrared eye tracking system (T60; Tobii Technology, Stockholm, Sweden, www.tobii.com) with an estimated 0.5° of recording error, after the software was calibrated to the participant’s pupils using a 9-point calibration matrix.
Measures
Screen for Child Anxiety Related Emotional Disorders.
We collected parent and child ratings of anxiety symptoms using the SCARED (Birmaher et al., 1997). Items are rated on a 3-point Likert scale ranging from 0 (not true or hardly ever true) to 2 (true or often true). The SCARED produces a total score (41 items; Cronbach’s α = .92) and five symptom dimensions (generalized anxiety, social anxiety, panic, separation anxiety, and school anxiety). The SCARED has been shown to be a reliable and valid measure of child and adolescent anxiety symptomatology (Birmaher et al., 1999; Hale, Crocetti, Raaijmakers, & Meeus, 2011; Monga et al., 2000), with higher scores indicating greater symptom severity and functional impairment. Parent and child versions are identical in content, and prior investigations have identified optimal cutoff scores of 25 (child) and 17 (parent) to best identify clinically significant anxiety (Birmaher et al., 1999). The instrument has good convergent and divergent validity when compared to formal psychiatric diagnoses (Birmaher et al., 1999). We used the combined parent and child total score in our main analyses as we find that the two scores load on the same factor in latent variable approaches, suggesting that they are indicators of the same factor (Kircanski et al., 2017). Furthermore, combining the two scores reduces the number of statistical tests, and we thus commonly use this approach when examining cognitive neuroscience constructs (Guyer et al., 2008; Shechner et al., 2017). We also considered the symptom dimensions of generalized and social anxiety, as they previously have been associated with abnormalities in processing threat faces (Clark, 1999; Mogg & Bradley, 1999). Child and parent total scores were correlated at r = .306, p < .001. The average of the parent and child generalized and social anxiety subscales also were correlated (r = .445, p < .001).
Eye tracking.
The location, duration, and frequency of fixations were calculated from areas of interest (AOIs) drawn around the eyes, the remainder of the face, and the rest of the computer screen. Two primary outcome measures were obtained: (a) fixation duration, that is the duration of time spent in each AOI and (b) fixation count, that is the number of fixations made to each AOI. Both measures were calculated separately for each CS type using Tobii eye tracking software version 3.2.1. After aggregating the data for each CS type during each phase of the task, the total fixation duration and total fixation count for each individual was divided by the number of events in the phase of the task to account for different amounts of time in each phase of the paradigm.
Skin conductance.
Skin conductance response to the CS+ and CS– was one measure of fear acquisition and extinction. SCR was recorded from two Ag/AgCl electrodes from the middle and ring finger of the nondominant hand, using a BioPac MP150 system (GSR100C; Biopac Systems, Goleta, CA) together with AcqKnowledge 4.3 (Biopac) software continuously sampled skin conductance data at 1,000 Hz.
Subjective ratings.
Self-reported anxiety was a second measure of fear acquisition and extinction. Following each of the three phases, children completed subjective fear ratings when viewing the CS+ and CS– using a 10-point Likert scale (1 = none, 10 = extreme).
Data analysis
Data on eye-scanning patterns, SCR, and self-reported fear to each face were analyzed as fear measures for each experimental phase. SCR for each CS+ and CS– was determined by the difference between baseline and peak amplitude within 5 s after stimulus onset. Raw values for each trial were normalized to that trial’s average baseline SCR and expressed as a percent change from that baseline value. We used the maximum value within the stimulus period to represent the response magnitude for each trial (Balderston & Helmstetter, 2010). Outliers were determined by computing the global SCR average (across all trials) for each subject. Individual trials that fell above or below 3 SD of the global average were regarded as artifacts and replaced with that participant’s global average. Data from subjects who had >20% outlier trials or missing data were excluded from analyses (Boucsein, 2012).
Reinforcement learning models measure a latent learning variable that is not detected using traditional analyses. Thus, it may be more sensitive to individual variation in fear learning than averaged responses. We fit a reinforcement learning model to the SCRs of each subject on a trial-by-trial basis. The model updates the predicted skin conductance, v, of the CS+ using the response to the UCS, r, in trial t as follows:
Thus, the updated predicted SCR of the CS+ is given by its value during the immediately preceding trial, vi(t – 1) plus a change based on the difference between the actual response to the UCS and the predicted response (r(t) – vi(t – 1)), multiplied by the learning rate parameter, α. At the beginning of each conditioning phase, the value, v, of the CS+ was set to 0. The learning rate parameter α was fit by minimizing the sum of squares between the measured skin conductance and the model’s prediction of the skin conductance.
Self-report measures were submitted to a repeated measures analysis of variance (ANOVA), with phase (preacquisition, acquisition, and extinction) and stimulus (CS+, CS–) as within-subject factors. Significant results were further examined for specific effects using post hoc analysis. For all analyses, statistical significance was set to α = .05.
The main analyses relied on data for eye gaze patterns. To test the first hypothesis that conditioning impacts eye gaze patterns, we conducted two separate omnibus repeated measures ANOVAs for total fixation duration and total fixation count with phase (preacquisition, acquisition, and extinction), stimulus (CS–, CS+), and AOI (eye region, rest of face, and rest of screen) as within-subject factors. Greenhouse–Geisser-corrected p-values are reported when the sphericity assumption was violated, while follow-up comparisons were examined with post hoc paired t-tests. We only examined effects that emerged in the full model, with all data and variables included. However, for the sake of completeness, we also provide Bonferroni-adjusted values for comparison where relevant. Analyses were conducted using IBM SPSS Statistics Version 21.
To test the second hypothesis that increasing levels of trait anxiety predict increasingly strong eye gaze avoidance, we tested whether dimensional measures of anxiety (SCARED total composite scores) influenced the two eye tracking measures (fixation duration and fixation count). We conducted a univariate analysis of covariance (ANCOVA), with SCARED scores as the continuous predictor and phase (preacquisition, acquisition, and extinction), AOI (eyes, rest of face, and rest of screen), and stimulus type (CS–, CS+) as within-subject variables. We used Pearson’s correlations to assess associations between the severity of anxiety symptoms (SCARED total composite scores) and the two eye tracking measures. Statistical significance was determined using a two-tailed a priori alpha of .05. Because prior research implicates both dependent measures in psychopathology, tests of hypotheses for these two measures were considered independent.
Because this was a twin sample, we conducted additional analyses to account for nonindependence due to the familial nature of the data. Mixed models used family unit as the random effect and specified the compound symmetric covariance structure to evaluate task effects on eye tracking. We also adjusted for child sex in the mixed models and calculated partial correlations after covarying child sex.
Finally, in an exploratory analysis, we examined whether eye gaze during acquisition mediated the relationship between anxious traits and self-reported fear toward the conditioned stimuli, using the PROCESS tool for SPSS. This regression-based approach estimates the indirect effect of an independent variable (IV) on a dependent variable (DV) via a mediator. The indirect effect is calculated as the product of the effect of the IV on the mediator and the effect of the mediator on the DV controlling for the IV. To determine the significance of the indirect effect, PROCESS uses bootstrapped confidence intervals (CI; significant when not overlapping zero; Hayes, 2013). This approach to mediation analysis is preferable to the traditional Sobel test because the bootstrapping procedure does not assume a parametric sampling distribution of the indirect effect; indeed, this distribution is typically skewed (Preacher & Hayes, 2008; Zhao, Lynch, & Chen, 2010). Additionally, the bootstrapping mediation analysis increases power to detect indirect effects without increasing the Type 1 error rate (Preacher & Hayes, 2008).
Results
Fear conditioning
SCR results.
We compared the SCRs to the CS+ and the CS– during the conditioning phase. There was a main effect of stimulus type, such that the SCR responses were stronger to the CS+ than to the CS–, F(1, 75) = 22.26, p < .001. However, this was qualified by a stimulus × trial interaction, such that there was a stronger SCR response to the CS+, relative to the CS– in the early acquisition phase trials (stim × trial, F(9, 675) = 4.74, p < .001). Thus, the SCR response demonstrated rapid conditioning, which habituated relatively quickly.
We further characterized the SCRs by fitting a reinforcement learning model. This model characterizes the extent to which the SCR to the CS, trial-by-trial, depends on the previous UCSs. We found a significant learning rate (M = 0.27, SEM = 0.03, t (75) = 8.9, p < .001) (Figure 2). There was no correlation between the learning rate and the SCARED total score for individual subjects (r = −.03, p = .78).
Figure 2.

Trial-by-trial skin conductance response (SCR) to CS+ and CS– during four preacquisition and 10 acquisition trials. Learning α: M = 0.27, SEM = 0.03, t(75) = 8.9, p < .001
Self-report ratings
We examined self-reported fear to the CS+ and the CS– at each phase. A repeated measures ANOVA yielded a significant main effect of phase, F(2, 158) = 9.89, p < .001, , a main effect of stimulus type, F(1, 79) = 46.18, p < .001, , and a phase-by-stimulus type interaction, F(2, 158) = 23.10, p < .001, . Follow-up contrasts revealed no difference between CS+ (M = 2.48 ± 2.22) and CS– (M = 2.54 ± 2.03) during preacquisition, t(81) = −.28, p = .781, d = .03, whereas ratings were greater to the CS+ relative to the CS– during both acquisition, (CS+: M = 4.37 ± 2.62; CS– : M = 2.51 ± 1.80), t(81) = 7.27, p < .001, d = .80 and extinction, (CS+: M = 3.54 ± 2.49; CS– : M = 2.14 ± 1.75), t(79) = 6.37, p < .001, d = .71. Of note, unlike SCR, self-reported fear persisted into the extinction period. Moreover, at preacquisition, an association between SCARED total scores and self-reported fear to the CS+ (r = .29, p = .01) and the CS– (r = .36, p = .001) was observed, indicating that the higher children’s anxiety, the more fear they reported to both types of stimuli prior to conditioning. No correlation between SCARED total scores and self-reported fear to the CS+ and CS– was found at either acquisition or extinction (all ps > .45).
Fixation duration and fixation count
The first study hypothesis concerned the expectation that eye gaze patterns to faces change as a result of conditioning. This hypothesis was tested with a repeated measures ANOVA on fixation duration with a Greenhouse–Geisser correction, with phase (preaquisition, acquisition, and extinction) × stimulus (CS+, CS–) × AOI (eyes, face, other). As shown in Table 2, three findings unrelated to the main hypothesis emerged. First, a main effect of phase emerged. Children spent overall more time fixating on the display during the preacquisition phase than the acquisition and extinction phases, indicating habituation. Second, a main effect of AOI emerged, with eyes being overall more salient than noneye regions. Third, the phase-by-AOI interaction was significant. Follow-up contrasts collapsed across CS types revealed participants to spend more time viewing the eye region at preacquisition than acquisition, t(81) = 4.46, p < .001 and extinction, t(81) = 3.82, p < .001, also indicating habituation.
Table 2.
ANOVA results for phase-by-stimulus-by-region for the two eye tracking measures
| Effect | Looking duration |
Looking count |
||||
|---|---|---|---|---|---|---|
| df | F | Sig | df | F | Sig | |
| Effects of conditioning | ||||||
| Phase | 2, 162 | 13.63 ** | p < .001 | 2, 162 | 15.13 ** | p < .001 |
| Stimulus | 1, 81 | 0.37 | p = .547 | 1, 81 | 0.45 | p = .503 |
| Region | 2, 162 | 42.31 ** | p < .001 | 2, 162 | 34.73 ** | p < .001 |
| Phase × Stimulus | 2, 162 | 0.67 | p = .480 | 2, 162 | 1.08 | p = .334 |
| Phase × Region | 4, 324 | 5.37 ** | p < .001 | 4, 324 | 3.72 * | p = .014 |
| Stimulus × Region | 2, 162 | 0.95 | p = .390 | 2, 162 | 2.05 | p = .132 |
| Phase × Stimulus × Region | 4, 324 | 5.89 ** | p < .001 | 4, 324 | 2.79 * | p = .037 |
| Effects of Anxiety (SCARED Total: Parent and Child Composite) | ||||||
| Anxiety | 1, 80 | 3.09 | p = .083 | 1, 80 | 1.88 | p = .174 |
| Phase × Anxiety | 2, 160 | 1.89 | p = .155 | 2, 160 | 0.72 | p = .472 |
| Stimulus × Anxiety | 1, 80 | 2.37 | p = .128 | 1, 80 | 0.45 | p = .505 |
| Region × Anxiety | 2, 160 | 5.32 * | p = .010 | 2, 160 | 4.66 * | p = .014 |
| Phase × Stimulus × Anxiety | 2, 160 | 0.82 | p = .418 | 2, 160 | .480 | p = .585 |
| Phase × Region × Anxiety | 4, 320 | 2.00 | p = .095 | 4, 320 | 2.60 | p = .057 |
| Stimulus × Region × Anxiety | 2, 160 | 0.18 | p = .821 | 2, 160 | 1.46 | p = .236 |
| Phase × Stimulus × Region × Anxiety | 4, 320 | 0.41 | p = .753 | 4, 320 | .62 | p = .615 |
| Effects of Anxiety (SCARED Social: Parent and Child Composite) | ||||||
| Anxiety | 1, 80 | 5.72 * | p = .042 | 1, 80 | 4.40 * | p = .039 |
| Phase × Anxiety | 2, 160 | 2.11 | p = .132 | 2, 160 | 1.89 | p = .160 |
| Stimulus × Anxiety | 1, 80 | 1.94 | p = .168 | 1, 80 | 1.82 | p = .181 |
| Region × Anxiety | 2, 160 | 3.38 * | p = .049 | 2, 160 | 4.09 * | p = .024 |
| Phase × Stimulus × Anxiety | 2, 160 | 1.11 | p = .321 | 2, 160 | 1.08 | p = .332 |
| Phase × Region × Anxiety | 4, 320 | 0.65 | p = .548 | 4, 320 | 1.05 | p = .369 |
| Stimulus × Region × Anxiety | 2, 160 | 0.15 | p = .844 | 2, 160 | 0.61 | p = .544 |
| Phase × Stimulus × Region × Anxiety | 4, 320 | 0.52 | p = .673 | 4, 320 | 0.84 | p = .483 |
| Effects of Anxiety (SCARED GAD: Parent and Child Composite) | ||||||
| Anxiety | 1, 80 | 0.78 | p = .381 | 1, 80 | 0.08 | p = .775 |
| Phase × Anxiety | 2, 160 | 1.77 | p = .173 | 2, 160 | 0.25 | p = .749 |
| Stimulus × Anxiety | 1, 80 | 1.12 | p = .293 | 1, 80 | 0.01 | p = .934 |
| Region × Anxiety | 2, 160 | 2.61 | p = .090 | 2, 160 | 1.94 | p = .153 |
| Phase × Stimulus × Anxiety | 2, 160 | 0.95 | p = .370 | 2, 160 | 0.30 | p = .698 |
| Phase × Region × Anxiety | 4, 320 | 4.33 * | p = .010 | 4, 320 | 3.07 * | p = .031 |
| Stimulus × Region × Anxiety | 2, 160 | 0.64 | p = .529 | 2, 160 | 0.90 | p = .409 |
| Phase × Stimulus × Region × Anxiety | 4, 320 | 0.94 | p = .424 | 4, 320 | 0.406 | p = .765 |
d.f = degrees of freedom. Significant effects are indicated in bold.
P < .05;
P < .001.
Consistent with the study hypothesis, a significant three-way interaction emerged for phase-by-stimulus-by-AOI, F(4, 324) = 5.89, p < .001, partial η2 = .07, power = .959. Prior eye tracking studies from our laboratory justify expectation of moderate-to-large effects (Shechner et al., 2013, 2017). Examining each phase separately yielded a significant stimulus-by-AOI interaction only during acquisition, F(2, 162) = 12.20, p < .001, partial η2 = .13, but not during preacquisition (all ps > .10) or extinction (all ps > .39). Follow-up contrasts revealed that at acquisition, children spent longer fixating on the eye region of a CS+ face (M = 1.55 ± 1.41) than a CS– face (M = 1.29 ± 1.17), t(81) = −3.57, p < .001, indicating an effect of fear conditioning on eye gaze duration (see Figure 3).
Figure 3.
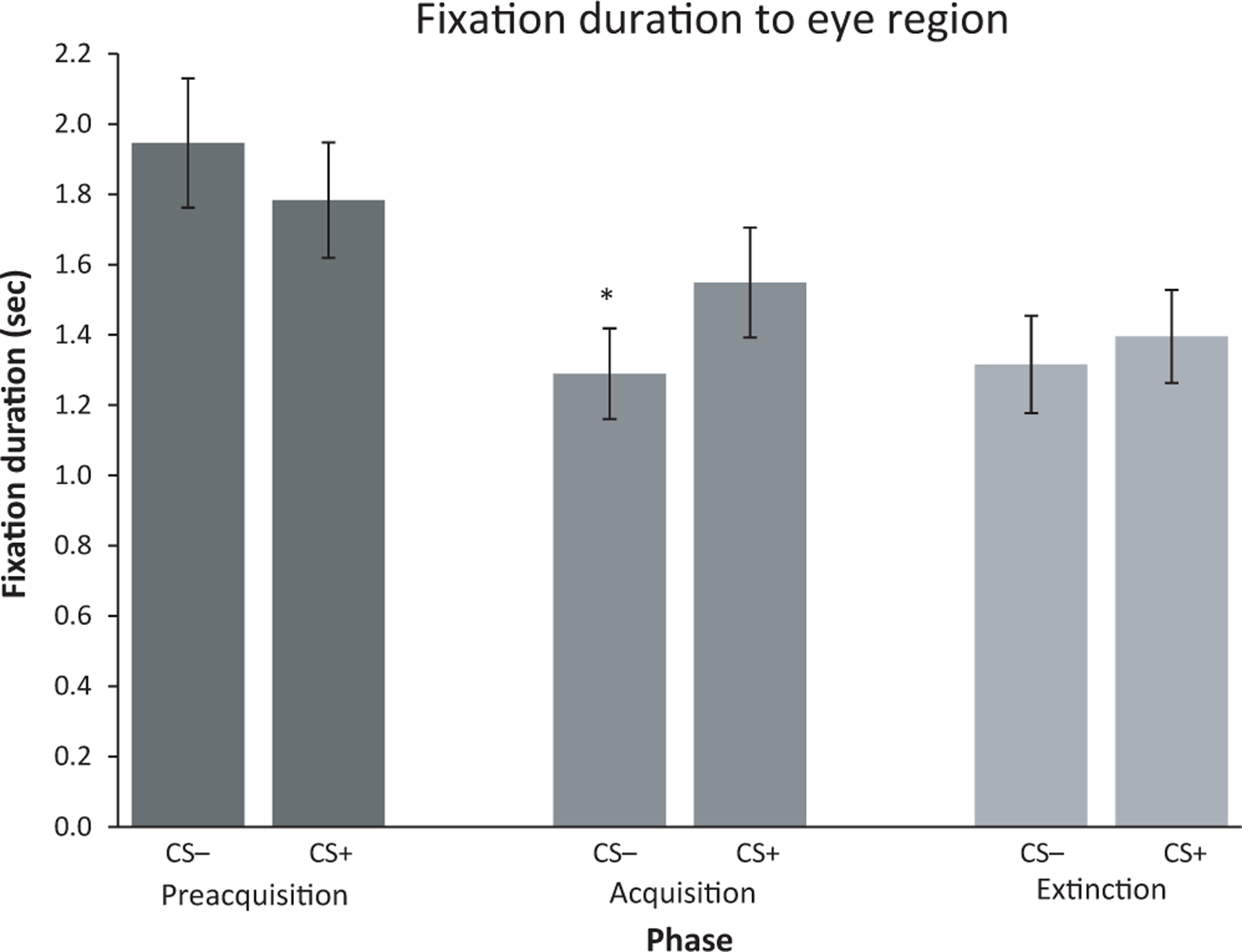
Fixation duration to the eye region for the CS+ and CS– across preacquisition, acquisition, and extinction phases. *p < .001
The same analysis was conducted for fixation count (see Table 2). As with fixation duration, both the main effect of phase and of AOI were significant, with children looking to the eyes more frequently overall and decreasing in their overall looking frequency over time. The two-way phase × AOI interaction was also significant. Follow-up contrasts revealed participants to look more frequently to the eye region of both CS-type faces during preacquisition than acquisition, t(81) = 4.68, p < .001 and extinction, t(81) = 3.96, p < .001. As with the fixation duration data, the phase-by-stimulus-by-AOI was significant, F(4, 324) = 2.79, p = .037, partial η2 = .03, power = .762, although only the fixation duration effect was significant using a corrected α for multiple comparisons (.05/2 = .025). Examining each phase separately yielded a significant stimulus-by-AOI interaction during acquisition, F(2, 162) = 4.39, p < .014, partial η2 = .05. Follow-up contrasts revealed that children looked more frequently to the eye region of a CS+ face than a CS– face, suggesting an effect of fear conditioning on fixation count.
Influence of anxiety symptoms on fixation duration and count
The second study hypothesis concerned the expectation that increasing levels of anxiety symptoms predict increasingly strong eye gaze avoidance. This hypothesis was tested with a repeated measures ANOVA on fixation duration with SCARED total composite scores as the continuous predictor and phase (preacquisition, acquisition, and extinction), AOI (eyes, rest of face, rest of screen) and stimulus type (CS–, CS+) as within-subject variables. The main effect of anxiety was not significant. Consistent with study predictions, a significant anxiety-by-AOI interaction emerged, F(2,160) = 5.32, p = .010, power = .766. Follow-up analyses revealed that across the entire task, there was a significant inverse relationship between fixation duration to the eyes and SCARED total composite scores (r = −.25, p = .022). Thus, higher children’s anxiety scores predicted less time fixating on the eye region. The three- and four-way interactions were not significant (See Table 2).
A similar pattern was observed for fixation count. No main effect of anxiety emerged. As with the fixation duration data, the anxiety × AOI interaction was significant for fixation count, F(2, 160) = 4.66, p = .01. Follow-up analyses revealed an inverse association between fixation count to the eye region and SCARED total composite scores (r = −.24, p = .03), indicating that the higher children’s anxiety scores, the lower the looking frequency to the eye region across all phases. Three- and four-way interactions were not significant (see Table 2). Results for the subscale analyses are presented in Table 2 and Appendix S1.
Mediation analyses
To investigate mediation, we used the simple mediation model in PROCESS (PROCESS Model #4; Hayes, 2013), as described in Preacher and Hayes (2008), with SCARED total score as the independent variable, gaze duration to the eye region of the face as the mediator, and self-reported fear to the CS+ at acquisition as the dependent variable. Mediation analysis revealed that gaze duration to the eye region of the face mediated the effect of anxious traits (SCARED total composite) on self-reported fear during conditioning (95% CI: −0.0433, −0.0035) (see Figure 4). While SCARED scores were not significantly associated with self-reported fear to the CS+ during fear conditioning, recent work indicates that a statistically significant total effect is not required for the testing of potential indirect effects (Zhao et al., 2010). Eye gaze at acquisition did not influence fear ratings at the end of extinction.
Figure 4.

Mediation model and path coefficients illustrating the relationship between trait anxiety (SCARED) and self-reported fear to the conditioned stimuli
Additional analyses
All eye tracking task measures remained significant after accounting for nonindependence in our data due to including twins from the same family, suggesting that results were not impacted by familiality. Results were also not influenced by sex of the child.
Discussion
This study had two objectives. The first was to examine the effects of conditioning on eye gaze patterns evoked by faces in 9- to 13-year-old children. The second was to examine the association between levels of anxiety symptoms and such eye gaze patterns across three experimental conditioning phases: baseline, acquisition, and extinction. Three main findings emerged. First, across children with varying levels of anxiety symptoms, conditioning influenced eye gaze patterns. Specifically, children looked longer and more frequently to the eye region of a CS+ than CS– face; this effect was present only during fear acquisition. Second, when collapsed across conditioning phases, high levels of anxiety symptoms were associated with eye gaze avoidance. Third, the relationship between anxiety symptoms and task-evoked fear was mediated by eye contact during conditioning.
Rapid detection of threat may be evolutionarily advantageous. This could afford organisms an enhanced opportunity to avoid rapidly detected, as compared to more slowly detected, threats. Such an advantage is thought to explain the capacity of threats to capture attention, as reflected in eye gaze patterns (Öhman, Hamm, & Hugdahl, 2000). Fearful faces represent one such class of threat stimulus, which automatically modulates spatial attention (Whalen et al., 2001). The eyes are a highly salient facial feature (Smith, Cottrell, Gosselin, & Schyns, 2005). When threat is imminent, humans orient gaze to the eye region of a face, possibly to detect the threat source (Tipples, 2006). Previous work on eye gaze patterns relies mostly on stimuli with intrinsic threat value, such as faces expressing anger or fear (see Vuilleumier, 2002, for a review). However, this approach cannot evaluate directly the effects of learning.
Aversive conditioning generates defensive responding to a newly acquired threat CS+. Our data suggest that conditioning enhances gaze duration to the eyes, leading the eyes of neutral faces to capture attention. Specifically, during acquisition, gaze duration and frequency to the eyes of the CS+ were greater than to the eyes of the CS–. Prior studies in adults show fearful faces to evoke reflexive orienting toward the eyes (Tipples, 2006). Our results complement these findings by suggesting that facial cues acquiring aversive properties through learning similarly influence gaze patterns. Thus, both innately aversive stimuli and stimuli acquiring aversive properties through experience may be prioritized by the attention system. In our study, assignment of one face as the CS+ varied randomly across subjects, leading any CS+/CS– differences to only reflect learning from experience.
Unlike these overall effects across the entire sample, other aspects of our findings link individual differences in anxiety to gaze avoidance. Specifically, high levels of anxiety symptoms were accompanied by an attenuation of the orienting response to the eye region. This attenuation occurred throughout the task, including at baseline, and therefore manifested even before any opportunities for children to learn in the task. These distinct findings for the entire sample, as opposed to for subjects with high anxiety levels, may inform thinking about anxiety disorders. Eye gaze avoidance could place individuals with anxiety at a disadvantage when trying to decode threat-related facial information.
An avoidant response may hinder effective processing and act to maintain anxiety; responses that may be relevant for treatment targets (Turk, Lerner, Heimberg, & Rapee, 2001). However, more research is needed before effects on treatment can be considered, given inconsistencies in the few available studies on eye gaze avoidance in anxious children. For example, in a free-viewing paradigm, Shechner et al. (2013) found no signs of avoidance in eye movement patterns. However, the study did not examine gaze fixation toward the internal features of faces and did not involve conditioning, differences that could explain the divergent findings. Moreover, while prior research consistently finds threats to influence attention orienting, this research also notes such effects to be complex and shaped by the level of the threat (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007). In general, as threat level increases, initial orienting is replaced with avoidance, which under extreme circumstances can revert again to orienting (Bar-Haim et al., 2007). In this context, our results could reflect nonlinear dynamics between threat intensity and the nature of the orienting response either toward or away from the eyes. One possible interpretation of our findings is that in individuals at risk for anxiety disorders, those dynamics may be less flexible. An initial avoidance for low-level threats may generate a pattern in which learning is compromised when conditions change. This may impair the development of appropriate and adaptive responses within a dynamic context. However, further research in this area is needed.
Our finding relating eye gaze avoidance to high levels of anxiety symptoms is consistent with findings among anxious adults (Horley et al., 2003, 2004; Moukheiber et al., 2010; although see Wieser, Pauli, Alpers, & Mühlberger, 2009). Interestingly, although gaze avoidance is thought to maintain anxiety (Rapee & Heimberg, 1997), ours is the first study to examine face scanning patterns in children exhibiting a range of anxiety symptoms. Given the cross-sectional findings, prospective research might consider if eye gaze patterns predict the course of anxiety. Longitudinal research finds that only a subset of anxious children become anxious adults (Pine, Cohen, Gurley, Brook, & Ma, 1998). The subset manifesting eye gaze avoidance or other correlates of adult anxiety disorders could face the highest risk for persistence.
Of note, our findings contrast with those from the most relevant prior study (Brunet et al., 2009). That prior study examined associations between temper-amental shyness and eye movement patterns during a face identification task. Unlike in our study, shy compared to non-shy children spent longer times viewing the eyes than other regions of neutral faces. Several methodological differences between the two studies may explain the differing results: Our study (a) assessed anxiety symptoms rather than temper-amental shyness and might thus be measuring a different construct (Prior, Smart, Sanson, & Oberklaid, 2000); (b) presented stimuli for longer durations than in the prior study; (c) examined different scan patterns than in the prior study; and (d) did not require children to make face identification judgments. Because anxious children manifest exaggerated responses to their own errors (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006; Meyer, Hajcak, Torpey-Newman, Kujawa, & Klein, 2015), requiring children to identify faces could influence eye gaze patterns. Thus, during briefer exposures to neutral faces, shy children may attend to the eye region, whereas at longer durations and in a threat-relevant context, anxious children may avoid the eye region. Future research might test these hypotheses.
Limitations
Several study limitations should be noted. First, technical issues led to data loss for some participants, which may have compromised our ability to detect higher order interactions. Nevertheless, we acquired data from over 75 conditioning sessions in children with varying levels of anxiety symptoms. Second, this study focused on anxiety as a continuous variable, so replication in children with anxiety diagnoses is an important next step. Third, while our sample included twins, the sample size was not sufficiently large to examine the effects of heritability on eye gaze and physiology. Lastly, two additional limitations relate to data acquisition procedures. We did not obtain trial-by-trial measures of self-reported fear. Unlike SCR, self-report was collected only after participants completed each phase, which can differ from trial-by-trial reports (Lipp, Oughton, & LeLievre, 2003). Moreover, we averaged condition-specific eye gaze data patterns over each condition. A trial-by-trial analysis could elucidate more fine-grained aspects of learning. Future studies might consider alternative procedures to extend our findings.
In summary, we obtained three main findings: (a) conditioned faces potentiated fixation to the eyes during acquisition; (b) high levels of anxiety symptoms were associated with eye gaze avoidance across all task phases; and (c) eye gaze duration mediated the association between anxiety symptom levels and levels of reported fear during acquisition. Together, these findings suggest that different face-viewing strategies lead to different responses to facial cues in children.
Supplementary Material
Key points.
Previous studies examining gaze patterns in adults find an association between anxiety and eye gaze avoidance.
The current study examined associations between eye gaze and pediatric anxiety symptoms using a face-based fear conditioning task developed for use in children.
Anxiety symptoms in children relate to face-viewing strategies deployed in the context of a fear learning experiment.
Understanding whether anxious children avoid eye contact in the context of learned associations may help counteract maladaptive learning behaviors and impact subsequent learning.
Acknowledgements
This research was in part supported by the Intramural Research Program of the National Institute of Mental Health (NIMH), Project number ZIAMH00278 National Institutes of Health (NIH) and by R01MH098055 (JMH) and R01MH101815 (RRN). The authors are grateful to Julia Feldman for her help with data entry and to Lance Rappaport and David Pagliaccio for helpful comments. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflicts declared.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Diagnosis details.
References
- Armony JL, & Dolan RJ (2002). Modulation of spatial attention by fear-conditioned stimuli: An event-related fMRI study. Neuropsychologia, 40, 817–826. [DOI] [PubMed] [Google Scholar]
- Baker SR, & Edelmann RJ (2002). Is social phobia related to lack of social skills? Duration of skill-related behaviours and ratings of behavioural adequacy. British Journal of Clinical Psychology, 41, 243–257. [DOI] [PubMed] [Google Scholar]
- Balderston NL, & Helmstetter FJ (2010). Conditioning with masked stimuli affects the timecourse of skin conductance responses. Behavioral Neuroscience, 124, 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, & van IJzendoorn MH (2007). Threat-related attentional bias in anxious and non-anxious individuals: A meta-analytic study. Psychological Bulletin, 133, 1–24. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, & Baugher M (1999). Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): A replication study. Journal of the American Academy of Child and Adolescent Psychiatry, 38, 1230–1236. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, & Neer SM (1997). The screen for child anxiety related emotional disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 545–553. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, & Mills KL (2014). Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology, 65, 187–207. [DOI] [PubMed] [Google Scholar]
- Boucsein W (2012). Electrodermal activity, (2nd ed.). New York: Springer. [Google Scholar]
- Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G, … & Pine DS (2013). Response to learned threat: An FMRI study in adolescent and adult anxiety. American Journal of Psychiatry, 170, 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet PM, Heisz JJ, Mondloch CJ, Shore DI, & Schmidt LA (2009). Shyness and face scanning in children. Journal of Anxiety Disorders, 23, 909–914. [DOI] [PubMed] [Google Scholar]
- Clark DM (1999). Anxiety disorders: Why they persist and how to treat them. Behaviour Research and Therapy, 37, S5–S27. [DOI] [PubMed] [Google Scholar]
- Farabee DJ, Ramsey SL, & Cole SG (1993). Social anxiety and speaker gaze in a persuasive atmosphere. Journal of Research in Personality, 27, 365–376. [Google Scholar]
- Green MJ, Williams LM, & Davidson D (2003). In the face of danger: Specific viewing strategies for facial expressions of threat? Cognition and Emotion, 17, 779–786. [Google Scholar]
- Grossmann T (2017). The eyes as windows into other minds: An integrative perspective. Perspectives on Psychological Science, 12, 107–121. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, … & Nelson EE (2008). Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry, 65, 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith MM, Bergman T, & Moore MJ (1977). Eye contact and face scanning in early infancy. Science, 198, 853–855. [DOI] [PubMed] [Google Scholar]
- Hale WW, Crocetti E, Raaijmakers QA, & Meeus WH (2011). A meta-analysis of the cross-cultural psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED). Journal of Child Psychology and Psychiatry, 52, 80–90. [DOI] [PubMed] [Google Scholar]
- Haxby J, Hoffman EA, & Gobbini MI (2002). Human neural systems for face recognition and social communication. Biological Psychiatry, 51, 59–67. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach New York: Guilford Press. [Google Scholar]
- Heuer K, Rinck M, & Becker ES (2007). Avoidance of emotional facial expressions in social anxiety: The Approach-Avoidance Task. Behaviour Research and Therapy, 45, 2990–3001. [DOI] [PubMed] [Google Scholar]
- Horley K, Williams LM, Gonsalvez C, & Gordon E (2003). Social phobics do not see eye to eye: A visual scanpath study of emotional expression processing. Journal of Anxiety Disorders, 17, 33–44. [DOI] [PubMed] [Google Scholar]
- Horley K, Williams LM, Gonsalvez C, & Gordon E (2004). Face to face: Visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry Research, 127, 43–53. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, & Morton J (1991). Newborn’s preferential tracking of face-like stimuli and its subsequent decline. Cognition, 40, 1–19. [DOI] [PubMed] [Google Scholar]
- Kircanski K, Zhang S, Stringaris A, Wiggins JL, Towbin KE, Pine DS, … & Brotman MA (2017). Empirically derived patterns of psychiatric symptoms in youth: A latent profile analysis. Journal of Affective Disorders, 216, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, & Ryan ND (2006). Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry, 47, 1073–1082. [DOI] [PubMed] [Google Scholar]
- Lange WG, Keijsers G, Becker ES, & Rinck M (2008). Social anxiety and evaluation of social crowds: Explicit and implicit measures. Behaviour Research and Therapy, 46, 932–943. [DOI] [PubMed] [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, … & Pine D (2011). Distinct neural signatures of threat learning in adolescents and adults. Proceedings of the National Academy of Sciences of the United States of America, 108, 4500–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, … & Pine DS (2008). Fear conditioning in adolescents with anxiety disorders: Results from a novel experimental paradigm. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp OV, Oughton N, & LeLievre J (2003). Evaluative learning in human Pavlovian conditioning: Extinct, but still there? Learning and Motivation, 34, 219–239. [Google Scholar]
- LoBue V, Matthews K, Harvey T, & Stark SL (2014). What accounts for the rapid detection of threat? Evidence for an advantage in perceptual and behavioral responding from eye movements. Emotion, 14, 816. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Esteves F, & Ohman A (1999). The face of wrath: Critical features for conveying facial threat. Cognition and Emotion, 13, 691–711. [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey-Newman DC, Kujawa A, & Klein DN (2015). Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. Journal of Abnormal Psychology, 124, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska KJ, Shechner T, Hong M, Britton JC, Leibenluft E, Pine DS, & Fox NA (2016). A developmental analysis of threat/safety learning and extinction recall during middle childhood. Journal of Experimental Child Psychology, 146, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, & Öhman A (2002). Phobias and preparedness: The selective, automatic, and encapsulated nature of fear. Biological Psychiatry, 52, 927–937. [DOI] [PubMed] [Google Scholar]
- Mogg K, & Bradley BP (1999). Orienting of attention to threatening facial expressions presented under conditions of restricted awareness. Cognition & Emotion, 13, 713–740. [Google Scholar]
- Monga S, Birmaher B, Chiappetta L, Brent D, Kaufman J, Bridge J, & Cully M (2000). Screen for child anxiety-related emotional disorders (SCARED): Convergent and divergent validity. Depression and Anxiety, 12, 85–91. [DOI] [PubMed] [Google Scholar]
- Moukheiber A, Rautureau G, Perez-Diaz F, Soussignan R, Dubal S, Jouvent R, & Pelissolo A (2010). Gaze avoidance in social phobia: Objective measure and correlates. Behaviour Research and Therapy, 48, 147–151. [DOI] [PubMed] [Google Scholar]
- Öhman A (1986). Face the beast and fear the animal: Animal and social fears as prototypes for evolutionary analyses of emotion. Psychophysiology, 23, 123–145. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, & Esteves F (2001). Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General, 130, 466. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, & Lundqvist D (2000). Unconscious emotion: Evolutionary perspectives, psychophysiological data and neuropsychological mechanisms. Cognitive Neuro-science of Emotion, 296–327.
- Öhman A, Hamm A, & Hugdahl K (2000). Cognition and the autonomic nervous system: Orienting, anticipation, and conditioning. In Cacioppo JT, Tassinary LG & Berntson G (Eds.), Handbook of psychophysiology (2nd edn, pp. 533–575). New York: Cambridge University Press. [Google Scholar]
- Öhman A, Lundqvist D, & Esteves F (2001). The face in the crowd revisited: A threat advantage with schematic stimuli. Journal of Personality and Social Psychology, 80, 381. [DOI] [PubMed] [Google Scholar]
- Padmala S, & Pessoa L (2008). Affective learning enhances visual detection and responses in primary visual cortex. Journal of Neuroscience, 28, 6202–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, & Ma Y (1998). The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry, 55, 56–64. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–891. [DOI] [PubMed] [Google Scholar]
- Prior M, Smart D, Sanson A, & Oberklaid F (2000). Does shy-inhibited temperament in childhood lead to anxiety problems in adolescence? Journal of the American Academy of Child & Adolescent Psychiatry, 39, 461–468. [DOI] [PubMed] [Google Scholar]
- Rapee RM, & Heimberg RG (1997). A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy, 35, 741–756. [DOI] [PubMed] [Google Scholar]
- Roelofs K, van Peer J, Berretty E, de Jong P, Spinhoven P, & Elzinga BM (2009). Hypothalamus–pituitary–adrenal axis hyperresponsiveness is associated with increased social avoidance behavior in social phobia. Biological Psychiatry, 65, 336–343. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Rodenbaugh TL, Blanco C, Lewin H, & Liebowitz MR (2011). Fear and avoidance of eye contact in social anxiety disorder. Comprehensive Psychiatry, 52, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Jarcho JM, Britton JC, Leibenluft E, Pine D, & Nelson EE (2013). Attention bias of anxious youth during extended exposure of emotional face pairs: An eye-tracking study. Depression and Anxiety, 30, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Jarcho JM, Wong S, Leibenluft E, Pine DS, & Nelson EE (2017). Threats, rewards, and attention deployment in anxious youth and adults: An eye tracking study. Biological Psychology, 122, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Cottrell GW, Gosselin F, & Schyns PG (2005). Transmitting and decoding facial expressions. Psychological Science, 16, 184–189. [DOI] [PubMed] [Google Scholar]
- Tipples J (2006). Fear and fearfulness potentiate automatic orienting to eye gaze. Cognition and Emotion, 20, 309–320. [Google Scholar]
- Turk CL, Lerner J, Heimberg RG, & Rapee RM (2001). An integrated cognitive-behavioral model of social anxiety. In Hofmann SG & DiBartolo PM (Eds.), From social anxiety to social phobia: Multiple perspectives (pp. 281–303). Needham Heights, MA: Allyn & Bacon. [Google Scholar]
- Van Peer JM, Roelofs K, Rotteveel M, van Dijk JG, Spinhoven P, & Ridderinkhof KR (2007). The effects of cortisol administration on approach–avoidance behavior: An event-related potential study. Biological Psychology, 76, 135–146. [DOI] [PubMed] [Google Scholar]
- Van Peer JM, Spinhoven P, van Dijk JG, & Roelofs K (2009). Cortisol-induced enhancement of emotional face processing in social phobia depends on symptom severity and motivational context. Biological Psychology, 81, 123–130. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P (2002). Facial expression and selective attention. Current Opinion in Psychiatry, 15, 291–300. [Google Scholar]
- Weeks JW, Howell AN, & Goldin PR (2013). Gaze avoidance in social anxiety disorder. Depression and Anxiety, 30, 749–756. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, & Rauch SL (2001). A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion, 1, 70. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, Alpers GW, & Mühlberger A (2009). Is eye to eye contact really threatening and avoided in social anxiety? – An eye-tracking and psychophysiology study. Journal of Anxiety Disorders, 93–103. [DOI] [PubMed]
- Zhao X, Lynch JG, & Chen Q (2010). Reconsidering Baron and Kenny: Myths and truths about mediation analysis. Journal of Consumer Research, 37, 197–206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


