Key Points
Question
Is the combination of avelumab and talazoparib effective in patients with pathogenic BRCA1/2 or ATM alterations, regardless of tumor type?
Findings
In this phase 2b nonrandomized controlled trial with 200 patients, neither the BRCA1/2 nor ATM cohort met the prespecified target of an objective response rate of 40% across cancer types. Durable clinical activity was observed in patients with BRCA1/2-associated tumor types (eg, ovarian, breast, prostate, and pancreatic cancers) vs those with non–BRCA-associated cancer types; a notable exception were patients with BRCA1/2-altered uterine leiomyosarcoma, who had prolonged responses to treatment.
Meaning
These findings suggest that a pan-cancer, tumor-agnostic approach with this combination is not an optimal clinical strategy for treating patients with BRCA1/2-altered tumors.
This phase 2b nonrandomized controlled trial evaluates whether the combination of avelumab and talazoparib is effective in patients with pathogenic BRCA1/2 or ATM alterations, regardless of tumor type.
Abstract
Importance
Nonclinical studies suggest that the combination of poly(ADP-ribose) polymerase and programmed cell death 1/programmed cell death–ligand 1 inhibitors has enhanced antitumor activity; however, the patient populations that may benefit from this combination have not been identified.
Objective
To evaluate whether the combination of avelumab and talazoparib is effective in patients with pathogenic BRCA1/2 or ATM alterations, regardless of tumor type.
Design, Setting, and Participants
In this pan-cancer tumor-agnostic phase 2b nonrandomized controlled trial, patients with advanced BRCA1/2-altered or ATM-altered solid tumors were enrolled into 2 respective parallel cohorts. The study was conducted from July 2, 2018, to April 12, 2020, at 42 institutions in 9 countries.
Interventions
Patients received 800 mg of avelumab every 2 weeks and 1 mg of talazoparib once daily.
Main Outcomes and Measures
The primary end point was confirmed objective response (OR) per RECIST 1.1 by blinded independent central review.
Results
A total of 200 patients (median [range] age, 59.0 [26.0-89.0] years; 132 [66.0%] women; 15 [7.5%] Asian, 11 [5.5%] African American, and 154 [77.0%] White participants) were enrolled: 159 (79.5%) in the BRCA1/2 cohort and 41 (20.5%) in the ATM cohort. The confirmed OR rate was 26.4% (42 patients, including 9 complete responses [5.7%]) in the BRCA1/2 cohort and 4.9% (2 patients) in the ATM cohort. In the BRCA1/2 cohort, responses were more frequent (OR rate, 30.3%; 95% CI, 22.2%-39.3%, including 8 complete responses [6.7%]) and more durable (median duration of response: 10.9 months [95% CI, 6.2 months to not estimable]) in tumor types associated with increased heritable cancer risk (ie, BRCA1/2-associated cancer types, such as ovarian, breast, prostate, and pancreatic cancers) and in uterine leiomyosarcoma (objective response in 3 of 3 patients and with ongoing responses greater than 24 months) compared with non–BRCA-associated cancer types. Responses in the BRCA1/2 cohort were numerically higher for patients with tumor mutational burden of 10 or more mutations per megabase (mut/Mb) vs less than 10 mut/Mb. The combination was well tolerated, with no new safety signals identified.
Conclusions and Relevance
In this phase 2b nonrandomized controlled trial, neither the BRCA1/2 nor ATM cohort met the prespecified OR rate of 40%. Antitumor activity for the combination of avelumab and talazoparib in patients with BRCA1/2 alterations was observed in some patients with BRCA1/2-associated tumor types and uterine leiomyosarcoma; benefit was minimal in non–BRCA-associated cancer types.
Trial Registration
ClinicalTrials.gov Identifier: NCT03565991
Introduction
Poly(ADP-ribose) polymerase (PARP) inhibitor clinical development has focused on tumor types associated with pathogenic germline BRCA1/2 alterations.1 BRCA1/2 alteration carriers have a heritable risk of breast, ovarian, prostate, and pancreatic tumors (termed BRCA1/2-associated cancer types), and PARP inhibitors are approved for these indications in the relapsed and/or maintenance settings. Germline alterations in other DNA damage repair genes, such as ATM, can also confer heritable cancer risk. Additionally, BRCA1/2 and ATM alterations are present in other solid tumors with limited treatment options.2 For example, homozygous somatic BRCA2 deletions have been identified in uterine sarcomas, with anecdotal response to PARP inhibitors reported.3,4,5,6
Preclinical data suggest that immune checkpoint inhibitors (ICIs) may be effective in BRCA1/2-altered tumors and synergize with PARP inhibitors due to their complementary mechanisms of action.7 Early-phase trials of PARP inhibitors combined with anti–programmed cell death 1/programmed cell death–ligand 1 (anti–PD-1/PD-L1) antibodies showed preliminary antitumor activity and tolerable safety in patients with selected cancers.8,9 We therefore hypothesized that combining PARP inhibition with PD-L1 inhibition could represent a promising therapeutic strategy in BRCA1/2- and ATM-altered solid tumors.
Talazoparib is a potent oral PARP inhibitor that is approved for the treatment of patients with deleterious or suspected deleterious germline BRCA1/2-altered, ERBB2 (formerly HER2)–negative, locally advanced or metastatic breast cancer. Talazoparib has also shown clinical activity in ovarian, pancreatic, and prostate cancers, harboring germline and/or somatic BRCA1/2 alterations.10,11,12,13,14
Avelumab is a human immunoglobin G1 anti–PD-L1 monoclonal antibody approved as monotherapy for metastatic Merkel cell carcinoma and advanced urothelial carcinoma (first-line maintenance or second-line therapy). It has been approved in combination with axitinib for first-line treatment of advanced renal cell carcinoma.15,16,17 In this phase 2b tumor-agnostic trial (JAVELIN BRCA/ATM), we investigated avelumab plus talazoparib in patients with BRCA1/2- or ATM-altered cancers, regardless of tumor histology, to evaluate whether PD-L1 inhibitors enhanced the efficacy observed with PARP inhibitor monotherapy in BRCA1/2-associated tumor types and extended the clinical benefit to other BRCA- or ATM-altered tumors.
Methods
Patients
Eligible patients (aged ≥18 years; ≥20 years in Japan) had histologically diagnosed locally advanced or metastatic solid tumors not amenable to treatment with curative intent, had received at least 1 prior line of standard-of-care treatment for locally advanced or metastatic disease (unless otherwise specified), and had pathogenic or likely pathogenic germline or somatic alterations in BRCA1, BRCA2, or ATM, as determined by local testing in a College of American Pathologists/Clinical Laboratory Improvement Amendments–certified (or comparable locally or regionally certified) laboratory using either germline or tumor DNA. Prior treatment with ICIs and PARP inhibitors was not allowed. Except for patients with metastatic castration-resistant prostate cancer (mCRPC), patients were required to have investigator-assessed measurable disease per RECIST 1.1. Complete eligibility criteria are detailed in the trial protocol, which appears in Supplement 1. This study followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.
The trial was performed in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, the International Conference on Harmonisation Guidelines for Good Clinical Practice, and the Declaration of Helsinki. All patients provided written informed consent before enrollment. The protocol was approved by the institutional review board or independent ethics committee at each participating center.
Study Design and Treatment
JAVELIN BRCA/ATM is an open-label, multicenter, pan-cancer, phase 2b nonrandomized clinical trial investigating avelumab plus talazoparib in patients with BRCA1/2- or ATM-altered solid tumors. The study was conducted at 42 centers in 9 countries. Patients were enrolled into 1 of 2 cohorts based on their qualifying mutation: BRCA1/2 or ATM alterations; patients with concurrent BRCA1/2 and ATM alterations were enrolled in the BRCA1/2 cohort. All patients received avelumab 800 mg as a 1-hour intravenous infusion every 2 weeks and talazoparib 1 mg orally once daily (0.75 mg for patients with moderate kidney impairment). To mitigate infusion-related reactions, patients received premedication with antihistamine and acetaminophen prior to the first 4 avelumab infusions. Treatment was continued until disease progression, unacceptable toxic effects, or patient withdrawal. For toxic effects, avelumab dosing could be delayed; talazoparib dosing could be delayed or reduced. Treatment could continue beyond initial disease progression if the investigator judged that the patient was experiencing clinical benefit from either study drug.
End Points and Assessments
The primary end point was confirmed objective response (OR; best overall response of complete response or partial response) assessed by blinded independent central review (BICR) per RECIST 1.1; bone disease in patients with mCRPC was assessed per Prostate Cancer Clinical Trials Working Group 3. Secondary end points included time to response (TTR), duration of response (DOR), progression-free survival (PFS) by BICR and investigator assessments, confirmed OR by investigator, overall survival (OS), safety, and biomarker assessments. End point definitions and biomarker methods are provided in eAppendix 1 and eAppendix 2 in Supplement 2.
Statistical Analysis
Data analyses were conducted in SAS version 9.4 (SAS Institute). The planned sample size was 200 patients: 150 and 50 patients in the BRCA1/2 and ATM cohorts, respectively. Assuming a beta distribution (0.5, 0.5) prior, the posterior probability of a true OR rate (ORR) of 40% or greater was 0.80 or greater with 66 responders of 150 patients (ORR, 44%) in the BRCA1/2 cohort and with 23 responders of 50 patients (ORR, 46%) in the ATM cohort. An interim analysis for each cohort was planned after at least 20 patients had been treated and followed up for 24 weeks to allow early termination of enrollment for futility according to prespecified criteria (ie, if the probability of a true ORR ≥40% was ≤0.05, then the cohort would be stopped for futility). The ORR was calculated by cohort, with corresponding exact 2-sided 95% CIs using the Clopper-Pearson method. Median PFS, OS, and DOR were estimated using the Kaplan-Meier method.
Results
Patients and Treatment
Between July 2, 2018, and April 12, 2020, 200 patients were enrolled and treated: 159 patients in the BRCA1/2 cohort and 41 patients in the ATM cohort (Figure 1). Median patient age was 59 (range, 26-89) years; 132 patients (66.0%) were women; there were 15 (7.5%) Asian, 11 (5.5%) African American, and 154 (77.0%) White participants; most patients were heavily pretreated (Table 1). The BRCA1/2 and ATM cohorts included 23 and 16 different tumor types, respectively (Table 1). At data cutoff (October 12, 2020), combination treatment was ongoing for 27 patients (16.4%) in the BRCA1/2 cohort and 1 patient (2.4%) in the ATM cohort (eTable 1 in Supplement 2). Median duration of treatment with avelumab and talazoparib was 5.3 (range, 0.5-26.7) months for both treatments in the BRCA1/2 cohort and 3.7 and 3.8 months, respectively, (range for both, 0.5-18.6 months) in the ATM cohort (eTable 1 in Supplement 2). The median duration of follow-up for OS in the BRCA1/2 and ATM cohorts was 13.5 (95% CI, 12-15.2) months and 16.7 (95% CI, 16-19.9) months, respectively.
Figure 1. Study Flow Diagram.
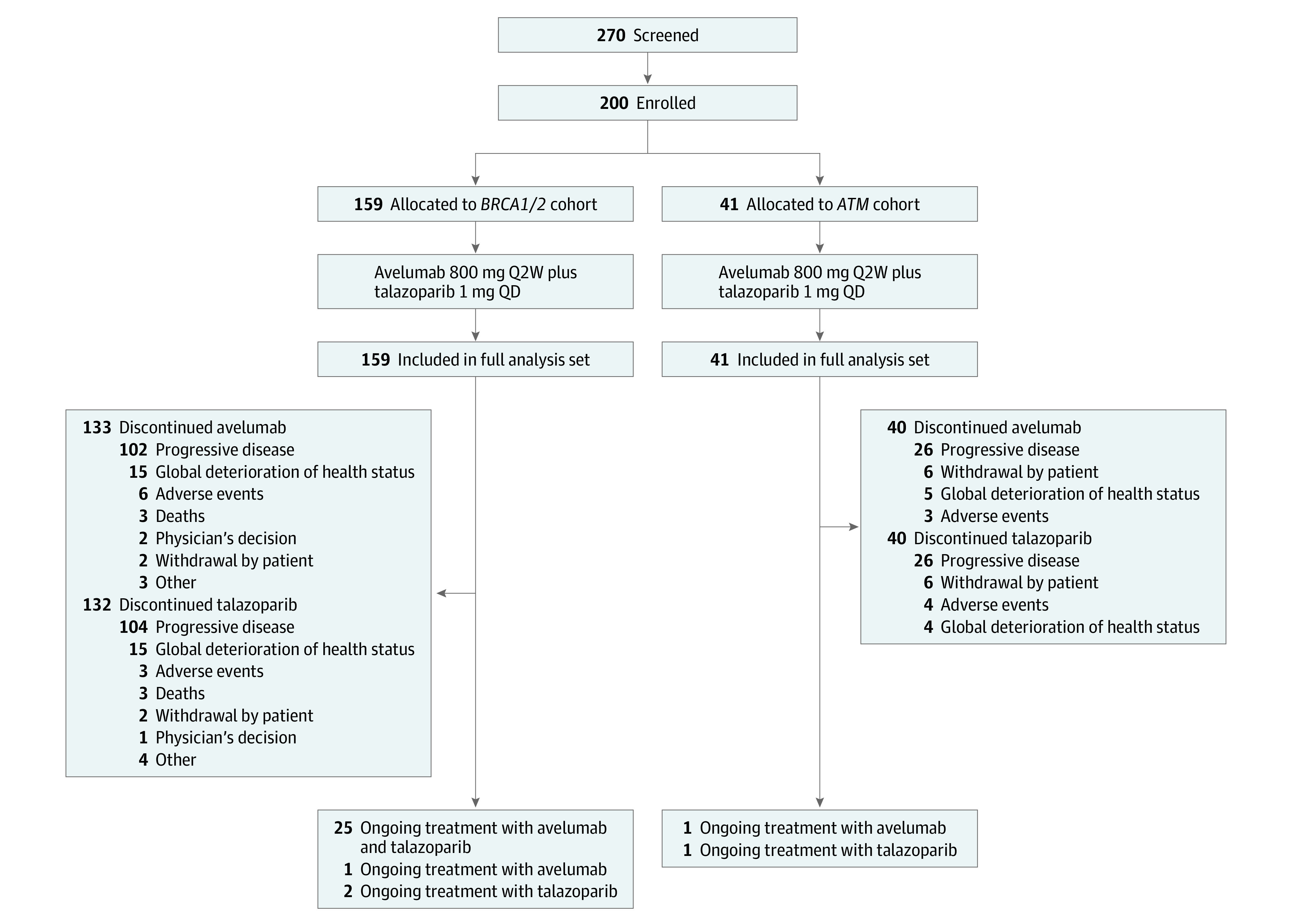
Q2W indicates every 2 weeks; QD, once daily.
Table 1. Patient Demographic and Baseline Characteristics.
| Characteristic | No. (%) | ||
|---|---|---|---|
| BRCA1/2 cohort (n = 159) | ATM cohort (n = 41) | All patients (N = 200) | |
| Age, median (range), y | 57.0 (26.0-84.0) | 61.0 (32.0-89.0) | 59.0 (26.0-89.0) |
| Sex | |||
| Female | 108 (67.9) | 24 (58.5) | 132 (66.0) |
| Male | 51 (32.1) | 17 (41.5) | 68 (34.0) |
| Race | |||
| African American | 8 (5.0) | 3 (7.3) | 11 (5.5) |
| American Indian or Alaska Native | 1 (0.6) | 0 | 1 (0.5) |
| Asian | 15 (9.4) | 0 | 15 (7.5) |
| White | 117 (73.6) | 37 (90.2) | 154 (77.0) |
| Not reported | 18 (11.3) | 1 (2.4) | 19 (9.5) |
| Pooled geographic region | |||
| North America | 89 (56.0) | 37 (90.2) | 126 (63.0) |
| Europe | 61 (38.4) | 4 (9.8) | 65 (32.5) |
| Asia | 9 (5.7) | 0 | 9 (4.5) |
| ECOG PS | |||
| 0 | 76 (47.8) | 13 (31.7) | 89 (44.5) |
| 1 | 81 (50.9) | 28 (68.3) | 109 (54.5) |
| 2a | 2 (1.3) | 0 | 2 (1.0) |
| Primary tumor subgroup | |||
| Breast cancer | |||
| Any | 51 (32.0) | 6 (14.6) | 57 (28.5) |
| HR+/ERBB2− | 26 (16.4) | 6 (14.6) | 32 (16.0) |
| Triple negative | 25 (15.7) | 0 | 25 (12.5) |
| Ovarian cancer | 26 (16.4) | 3 (7.3) | 29 (14.5) |
| mCRPC | |||
| Any | 26 (16.4) | 5 (12.2) | 31 (15.5) |
| Measurable disease (investigator)b | 18 (69.2) | 3 (60.0) | 21 (67.7) |
| Pancreatic cancer | 16 (10.1) | 5 (12.2) | 21 (10.5) |
| Colorectal cancer | 8 (5.0) | 9 (22.0) | 17 (8.5) |
| Cholangiocarcinoma | 8 (5.0) | 1 (2.4) | 9 (4.5) |
| Endometrial cancer | 2 (1.3) | 3 (7.3) | 5 (2.5) |
| Gallbladder cancer | 3 (1.9) | 1 (2.4) | 4 (2.0) |
| Uterine leiomyosarcoma | 3 (1.9) | 0 | 3 (1.5) |
| Other | 16 (10.1)c | 8 (19.5)d | 24 (12.0) |
| TNM stage | |||
| Stage III | 3 (1.9) | 0 | 3 (1.5) |
| Stage IV | 156 (98.1) | 41 (100) | 197 (98.5) |
| BRCA statuse | |||
| Positive | 159 (100) | 0 | 159 (79.5) |
| Negative | 0 | 24 (58.5) | 24 (12.0) |
| Unknown | 0 | 17 (41.5) | 17 (8.5) |
| BRCA1 statuse | |||
| Positive | 72 (45.3) | 0 | 72 (36.0) |
| Negative | 58 (36.5) | 24 (58.5) | 82 (41.0) |
| Unknown | 29 (18.2) | 17 (43.9) | 46 (23.0) |
| BRCA2 statuse | |||
| Positive | 88 (55.3) | 0 | 88 (44.0) |
| Negative | 47 (29.6) | 23 (56.1) | 70 (35.0) |
| Unknown | 24 (15.1) | 18 (43.9) | 42 (21.0) |
| ATM statuse | |||
| Positive | 4 (2.5) | 41 (100) | 45 (22.5) |
| Negative | 46 (28.9) | 0 | 46 (23.0) |
| Unknown | 109 (68.6) | 0 | 109 (54.5) |
| Prior lines of therapy | |||
| 0 | 2 (1.3) | 1 (2.4) | 3 (1.5) |
| 1 | 31 (19.5) | 8 (19.5) | 39 (19.5) |
| 2 | 50 (31.4) | 8 (19.5) | 58 (29.0) |
| ≥3 | 76 (47.8) | 24 (58.5) | 100 (50.0) |
| Prior lines of therapy in the advanced setting | |||
| 0 | 26 (16.4) | 5 (12.2) | 31 (15.5) |
| 1 | 41 (25.8) | 8 (19.5) | 49 (24.5) |
| 2 | 48 (30.2) | 11 (26.8) | 59 (29.5) |
| ≥3 | 44 (27.7) | 17 (41.5) | 61 (30.5) |
| Breast cancer | |||
| ≥1 Prior cytotoxic therapy in the advanced setting | 30 (58.8) | 5 (83.3) | 35 (61.4) |
| ≥1 Prior platinum regimen | 13 (25.5) | 0 | 13 (22.8) |
| Ovarian cancer | |||
| ≥1 Prior platinum-containing regimen | 26 (100) | 3 (100) | 29 (100) |
| ≥2 Prior platinum regimens | 18 (69.2) | 2 (66.7) | 20 (69.0) |
| ≥3 Prior platinum regimens | 7 (26.9) | 0 | 7 (24.1) |
| Platinum sensitive | 1 (3.8) | 1 (33.3) | 2 (6.9) |
| Platinum resistant | 25 (96.2) | 2 (66.7) | 27 (93.1) |
| mCRPC | |||
| ≥1 Prior taxane-containing regimen | 18 (69.2) | 2 (40.0) | 20 (64.5) |
| Measurable disease at baseline by BICR | 126 (79) | 30 (73) | 156 (78) |
Abbreviations: +, positive, −, negative; BICR, blinded independent central review; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hormone receptor; mCRPC, metastatic castration-resistant prostate cancer.
Patients had an ECOG PS of 1 at screening and 2 at the time of enrollment.
The percentage was calculated using the number of participants with mCRPC as the denominator.
Other tumor types include esophageal cancer (n = 2), gastric cancer (n = 1), glial tumor (n = 2), malignant tumor, site unspecified (n = 1), non–small cell lung cancer (n = 2), urothelial cancer (n = 1), gastrointestinal stromal tumor (n = 1), leiomyosarcoma (n = 1), metastatic uveal melanoma (n = 1), sarcoma of the uterus (n = 1), testicular cancer (n = 1), uterine carcinoma (n = 1), and uvula squamous carcinoma (n = 1).
Other tumor types include esophageal cancer (n = 1), gastric cancer (n = 1), malignant tumor, site unspecified (n = 1), urothelial cancer (n = 1), glomangiosarcoma of the lung (n = 1), high-grade neuroendocrine tumor (n = 1), papillary thyroid cancer (n = 1), and small bowel adenocarcinoma (n = 1).
Local laboratory testing. Unknown includes uninformative or missing results.
Efficacy in the BRCA1/2 Cohort
In the BRCA1/2 cohort, 42 of 159 patients had a confirmed OR by BICR (ORR, 26.4%; 95% CI, 19.7%-34.0%) (Table 2); ORR by investigator was 33.3% (95% CI, 26.1%-41.2%) (eTable 2 in Supplement 2). In patients with measurable disease at baseline, the ORR by BICR was 32.5% (95% CI, 24.5%-41.5%), and many patients had some degree of tumor shrinkage (eFigure 4 in Supplement 2). In patients with BRCA1/2-dependent tumors, for BRCA1/2 alterations under loss of heterozygosity (LOH), responses occurred in 14 of 47 patients (29.8%; 95% CI, 17.3%-44.9%) compared with 2 of 9 patients (22.2%; 95% CI, 2.8%-60.0%) with heterozygous BRCA alterations. In patients with BRCA1/2-dependent tumors, the response rate was 36.4% (95% CI, 22.4%-52.2%; 16 of 44 patients) for high genomic LOH (gLOH) tumors vs 31.6% (95% CI, 12.6%-56.6%; 6 of 19 patients) for low gLOH tumors. In patients with tumor mutational burden (TMB) of 10 or greater mutations per megabase (mut/Mb) vs less than 10 mut/Mb, responses occurred in 5 of 8 patients (ORR, 62.5%; 95% CI, 24.5%-91.5%) vs 22 of 92 (ORR, 23.9%; 95% CI, 15.6%-33.9%) (eTable 4 in the Supplement). According to BICR, median TTR was 1.8 (range, 1.5-3.6) months and median DOR was 10.9 months (95% CI, 6.2 months to not estimable) (Figure 2). Median PFS was 3.7 (95% CI, 3.1-5.3) months (eFigure 1 in Supplement 2). At data cutoff, responses were ongoing in 17 of 42 patients (40.5%) with a confirmed OR. Efficacy in tumor types with 5 or more patients is given in Table 2 and eTable 3 in Supplement 2.
Table 2. Best Overall Response and Confirmed Objective Response by BICR in BRCA-Dependent Tumors and in the BRCA1/2 Cohort in Tumor Types With at Least 5 Patients.
| Best overall response by BICR | Patients in BRCA1/2 cohort, No. (%) | ATM cohort, FAS (n = 41) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FAS (n = 159) | BRCA categorization | BRCA1/2 by tumor types with ≥5 patients | |||||||||||
| Dependent tumor (n = 122)a | Not dependent tumor (n = 37) | Breast (n = 51)b | HR+ and ERBB2− breast (n = 26) | TNBC (n = 25) | mCRPC (n = 26)c | Ovarian (n = 26) | Pancreatic (n = 16) | Colorectal (n = 8) | Cholangio-carcinoma or gallbladder (n = 10) | Other tumors (n = 22)d | |||
| CR | 9 (5.7) | 9 (7.4) | 0 | 3 (5.9) | 2 (7.7) | 1 (4.0) | 4 (15.4) | 1 (3.8) | 0 | 0 | 0 | 1 (4.5) | 0 |
| PR | 33 (20.8) | 30 (24.6) | 3 (8.1) | 21 (41.2) | 12 (46.2) | 9 (36.0) | 1 (3.8) | 4 (15.4) | 2 (12.5) | 0 | 0 | 5 (22.7) | 2 (4.9) |
| SD | 32 (20.1) | 26 (21.3) | 6 (16.2) | 9 (17.6) | 3 (11.5) | 6 (24.0) | 11 (42.3) | 4 (15.4) | 2 (12.5) | 1 (12.5) | 3 (30.0) | 2 (9.1) | 18 (43.9) |
| No CR, no PD | 10 (6.3) | 8 (6.6) | 2 (5.4) | 3 (5.9) | 2 (7.7) | 1 (4.0) | 2 (7.7) | 3 (11.5) | 0 | 0 | 1 (10.0) | 1 (4.5) | 4 (9.8) |
| PD | 55 (34.6) | 33 (27.0) | 22 (59.5) | 8 (15.7) | 3 (11.5) | 5 (20.0) | 3 (11.5) | 11 (42.3) | 11 (68.8) | 7 (87.5) | 4 (40.0) | 11 (50.0) | 14 (34.1) |
| NE | 20 (12.6) | 16 (13.1) | 4 (10.8) | 7 (13.7) | 4 (15.4) | 3 (12.0) | 5 (19.2) | 3 (11.5) | 1 (6.3) | 0 | 2 (20.0) | 2 (9.1) | 3 (7.3) |
| ORR, No. (%) [95% CI] | 42 (26.4) [19.7-34.0] | 39 (32.0) [23.8-41.0] | 3 (8.1) [1.7-21.9] | 24 (47.1) [32.9-61.5] | 14 (53.8) [33.4-73.4] | 10 (40.0) [21.1-61.3] | 5 (19.2) [6.6-39.4] | 5 (19.2) [6.6-39.4] | 2 (12.5) [1.6-38.3] | 0 [0.0-36.9] | 0 [0.0-30.8] | 6 (27.3) [10.7-50.2] | 2 (4.9) [0.6-16.5] |
Abbreviations: +, positive; −, negative; BICR, blinded independent central review; CR, complete response; FAS, full analysis set; HR, hormone receptor; mCRPC, metastatic castration-resistant prostate cancer; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease; TNBC, triple negative breast cancer; uLMS, uterine leiomyosarcoma.
Defined as breast, ovarian, prostate, and pancreatic cancers and uLMS.
Responders with breast cancer include patients with HR+/ERBB2− breast cancer and TNBC.
ORR in patients with mCRPC and measurable disease by BICR was 30.8%.
Responders with other tumors (with <5 patients) include 3 patients with uLMS (also included in the BRCA-dependent tumor types) and 1 patient each with uterine sarcoma, uterine carcinoma, and testicular cancer.
Figure 2. Efficacy Summary in the BRCA1/2 Cohort.
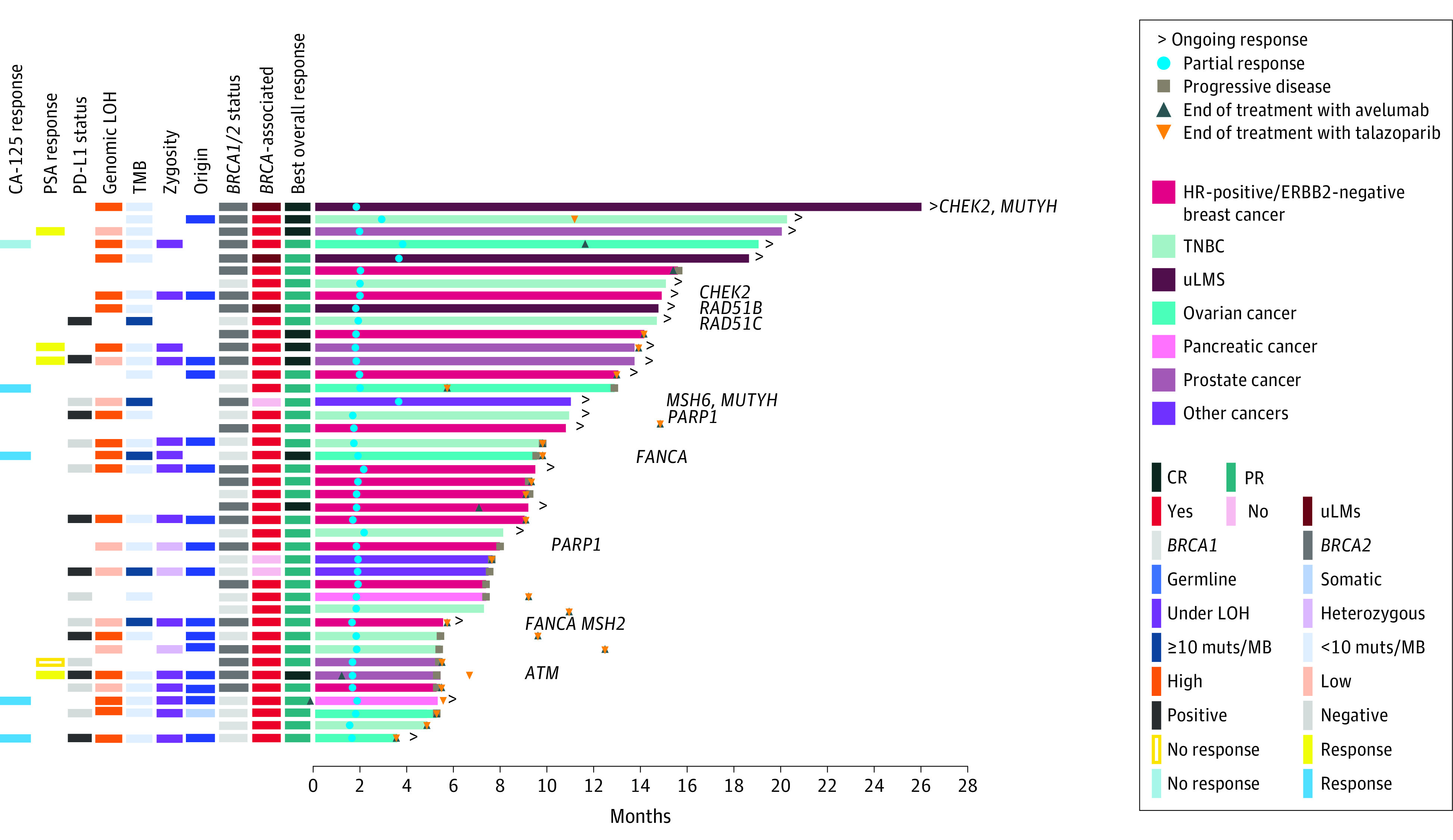
Time to and duration of response in the full analysis set per blinded independent central review, for all patients in the BRCA1/2 cohort and in BRCA-associated and non–BRCA-associated tumor types. Molecular analysis was based on results from central laboratories and supplemented by local laboratories when central results were not available. Patients with alterations in non-BRCA DNA damage response genes are indicated accordingly. CA-125 indicates cancer antigen 125; CR, complete response; HR, hormone receptor; LOH, loss of heterozygosity; mCRPC, metastatic castration-resistant prostate cancer; mut/MB, mutations per megabase; PD-L1, programmed cell death–ligand 1; PR, partial response; PSA, prostate-specific antigen; TMB, tumor mutational burden; TNBC, triple-negative breast cancer; uLMS, uterine leiomyosarcoma.
In the BRCA1/2 cohort, 119 patients had BRCA1/2-associated tumor types (defined as breast, ovarian, prostate, and pancreatic cancers) and 40 patients had non–BRCA1/2-associated cancer types.6 Within this BRCA1/2 cohort, the ORR was 30.3% (95% CI, 22.2%-39.3%) for BRCA1/2-associated tumor types vs 15.0% (95% CI, 5.7%-29.8%) for patients with non–BRCA1/2-associated tumor types, including 3 of 3 responses in patients with advanced uterine leiomyosarcoma (uLMS) (Table 2). In an exploratory analysis, we combined the patients with uLMS with the patients with BRCA1/2-associated tumor types to form 1 subset, defined collectively as BRCA1/2-dependent cancer types.6 In the BRCA1/2-dependent vs non–BRCA1/2-dependent groups (with 122 and 37 patients, respectively), ORRs were 32.0% (95% CI, 23.8%-41.0%) vs 8.1% (95% CI, 1.7%-21.9%), median DOR was 12.5 months (95% CI, 7.4 months to not estimable) vs 5.8 months (95% CI, 5.7 months to not estimable), and median PFS was 5.3 (95% CI, 3.7-7.3) months vs 1.9 (95% CI, 1.8-2.1) months, respectively (eFigure 1 in Supplement 2). The ORRs were 40.0% (95% CI, 30.1%-50.6%) vs 9.7% (95% CI, 2.0%-25.8%) in patients with BICR-assessed measurable disease, respectively (eTable 3 in Supplement 2).
Efficacy in the ATM Cohort
In June 2019, 41 patients had been rapidly enrolled and the interim analysis criteria had not yet been reached. At this time, clinical activity data for 19 patients followed up for 24 weeks was reviewed by the sponsor and the steering committee. The investigator-assessed ORR was 10.5% and was determined to be unlikely to surpass prespecified futility requirements. Further enrollment to this cohort was subsequently discontinued prior to the planned analysis. Final BICR-assessed ORR was 4.9% (Table 2).
Biomarker Analyses
Central tumor sequencing was performed in 134 patients enrolled in the BRCA1/2 cohort and 32 in the ATM cohort (eTable 4 in Supplement 2). The response rate was numerically higher for patients with germline vs somatic tumor alterations: 16 of 58 (27.6%; 95% CI, 16.7%-40.9%) vs 1 of 13 (7.7%; 95% CI, 0.2%-36.0%), respectively, although 95% CIs overlapped. We also investigated biallelic loss, TMB, whole-exome sequencing (WES) and whole-genome sequencing (WGS), and response to treatment. Results from these exploratory analyses are provided in eAppendix 3 and eFigures 2 and 3 in Supplement 2.
Safety
In total, 182 patients (98.1%) experienced at least 1 treatment-emergent adverse event (TEAE) (Table 3). The most common TEAEs were anemia (99 [49.5%]), nausea (93 [46.5%]), fatigue (66 [33.0%]), and thrombocytopenia (63 [31.5%]) (eTable 5 in Supplement 2). Treatment-related adverse events (TRAEs) of any grade occurred in 182 patients (91.0%), including grade 3 or greater TRAEs in 98 patients (49.0%) (Table 3). The most common grade 3 or greater TRAEs (≥5% of patients) were anemia (68 [34.0%]), thrombocytopenia (30 [15.0%]), and neutropenia (22 [11.0%]). TRAEs led to discontinuation of any study drug in 11 patients (5.5%) (eTable 6 in Supplement 2). The talazoparib dose was reduced because of TRAEs in 65 patients (32.5%) (eTable 6 in Supplement 2). No TRAEs resulted in death. Immune-related adverse events (irAEs) occurred in 25 patients (12.5%); grade 3 or greater irAEs occurred in 5 patients (2.5%) (eTable 6 in Supplement 2).
Table 3. Treatment-Related Adverse Eventsa.
| TRAE | Patients, No. (%) (n = 200) | |
|---|---|---|
| Any grade | Grade ≥3 | |
| Any | 182 (91.0) | 98 (49.0) |
| Anemiab | 92 (46.0) | 68 (34.0) |
| Nausea | 62 (31.0) | 1 (0.5) |
| Thrombocytopeniac | 58 (29.0) | 30 (15.0) |
| Fatigue | 45 (22.5) | 3 (1.5) |
| Neutropeniad | 40 (20.0) | 22 (11.0) |
| Diarrhea | 29 (14.5) | 0 |
| Asthenia | 22 (11.0) | 1 (0.5) |
| Decreased appetite | 20 (10.0) | 0 |
| Alopecia | 19 (9.5) | 0 |
| Vomiting | 19 (9.5) | 0 |
| Headache | 18 (9.0) | 1 (0.5) |
| ALT increased | 15 (7.5) | 3 (1.5) |
| AST increased | 15 (7.5) | 4 (2.0) |
| Infusion-related reactione | 41 (20.5) | 1 (0.5) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; TRAE, treatment-related adverse event.
TRAEs of any grade occurring in 10% or more of patients or grade 3 or greater in 5% or more of patients are shown. Adverse events are reported as preferred terms, and some are grouped according to hematologic cluster terms.
Anemia was defined as any event having the following preferred terms: anemia, hematocrit decreased, hemoglobin decreased, iron deficiency anemia, or red blood cell count decreased.
Thrombocytopenia was defined as any event having the following preferred terms: immune thrombocytopenia, platelet count decreased, or thrombocytopenia.
Neutropenia was defined as any event having the following preferred terms: autoimmune neutropenia, febrile neutropenia, neutropenia, or neutrophil count decreased.
Infusion-related reactions were identified based on a list of prespecified Medical Dictionary for Regulatory Activities Terminology preferred terms and time of onset and resolution of the events in relation to the avelumab infusions, regardless of investigator-assessed causality.
Discussion
To our knowledge, this is the first trial to assess the combination of PARP and PD-L1 inhibitors as a pan-cancer tumor-agnostic treatment strategy in BRCA1/2- and ATM-altered cancers. Neither the BRCA1/2 nor ATM cohort met the prespecified target ORR of 40%, indicating that a pan-cancer tumor-agnostic approach with this combination is not an optimal clinical strategy. Clinical activity within the BRCA1/2 cohort was mainly observed in patients with BRCA1/2-associated tumor types (ovarian, breast, prostate, and pancreatic cancers) and uLMS; limited benefit was seen in patients with non–BRCA1/2-associated tumor types. These data are consistent with a recent large clinicogenomic analysis, which indicated that BRCA1/2 alterations have pleiotropic effects that are tumor-lineage dependent, while most BRCA1/2 alterations in non–BRCA-associated cancers may be unrelated to tumor pathogenesis and unlikely to be therapeutically actionable.6 Further studies are required to determine the underlying mechanisms that mediate differences in tolerance to defects in homologous recombination across various tumor lineages. Our findings indicate that rather than a tumor-agnostic drug development strategy used with biomarkers such as NTRK fusions18 and microsatellite instability,19 future clinical trial approaches with PARP and anti–PD-1/PD-L1 inhibitor combinations should be focused on BRCA-associated tumors.
A notable exception in the non–BRCA1/2-associated tumors is uterine cancer, which comprised 5 of 6 patients who responded to therapy, none with the more common endometrioid histology. All 3 patients with uLMS had prolonged responses ongoing at the data cutoff. In contrast to BRCA1/2-associated tumor types, typified by their frequency of germline BRCA1/2 alterations, patients with uLMS harbored somatic BRCA2 homozygous deletions associated with a high gLOH phenotype. The prolonged clinical benefit may be attributable to the inability of tumors bearing BRCA large homozygous deletions to develop reversion alterations, a known mechanism of acquired resistance to PARP inhibitors.6 Consistent with this hypothesis, patients with BRCA1/2 homozygous deletions have been reported as extraordinary responders to PARP inhibitors.10,20,21,22,23,24 Somatic biallelic BRCA2 loss is present in 6.5% of all uterine sarcomas, and our data suggest that this tumor type may be BRCA dependent.6
When the BRCA1/2 cohort was further explored for clinical activity by tumor type, the overall efficacy of avelumab plus talazoparib remained generally consistent with previous PARP inhibitor monotherapy and/or in combination with ICIs. The confirmed response rates in breast, ovarian, and pancreatic cancers were comparable with prior studies,8,11,25,26,27,28 notwithstanding that these studies typically did not use central independent review of ORR and/or frequently included unconfirmed responses.
We also explored whether biallelic BRCA1/2 loss or high gLOH scores were associated with efficacy. In patients with BRCA1/2-dependent tumors, the response rate was similar for tumors with BRCA alterations under LOH vs heterozygous tumors (29.8% vs 22.2%) and high gLOH vs low gLOH tumors (36.4% vs 31.6%). These results are consistent with prior reports suggesting that BRCA1/2 zygosity and gLOH may not correlate with outcomes in BRCA1/2-altered, BRCA1/2-dependent tumors treated with PARP inhibitors.6,29 In ovarian cancer, several studies have validated homologous recombination deficient positivity and/or high gLOH as a biomarker predictive of response to PARP inhibition, with 2 US Food and Drug Administration–approved companion diagnostics for this indication.30 However, it is important to note that this testing was used to identify the subset of patients with BRCA1/2 wild-type tumors more likely to benefit from PARP inhibitors, and BRCA1/2 alteration status remains the strongest predictor of response.25,31,32,33,34 Furthermore, in the BRCA1/2 cohort, the ORR was numerically higher for germline vs somatic mutations (27.6% and 7.7%, respectively), although 95% CIs overlapped. This may partly reflect the higher rate of germline alterations in BRCA1/2-dependent tumors, likely due to intrinsic tumor characteristics and pre-enrichment due to increased germline testing in patients with these cancer types.
Response to therapy in the BRCA1/2 cohort was numerically higher for patients with TMB of 10 or more mutations per megabase (mut/Mb) vs TMB of less than 10 mut/Mb tumors, which is consistent with data from KEYNOTE-158, which identified TMB of 10 or more mut/Mb as a predictive biomarker for response to PD-1/PD-L1 blockade.35 However, few BRCA1/2-associated tumors were included. Although limited by small numbers of participants, responses in high TMB, non–BRCA-associated tumors (Figure 2) suggest that PD-L1 blockade is contributing to efficacy and support TMB as a potential predictive biomarker in this population. Patients enrolled in the ATM cohort had limited clinical benefit, leading to early closure of this cohort. The lack of efficacy observed in the ATM cohort has important implications for the development of PARP inhibitors.
Limitations
Study limitations include the single-group study design, which makes assessment of response rates for the combination challenging without comparing with historical data for avelumab or talazoparib monotherapy in similar patient populations. In addition, many of the subanalyses were retrospective and exploratory and often limited by small numbers of patients, which prevents us from drawing definitive conclusions.
Conclusions
In this nonrandomized controlled trial, we evaluated BRCA1/2- and ATM-altered cancers in a pan-cancer tumor-agnostic study of patients with a range of solid tumors, including rare cancers such as uLMS. The combination of avelumab and talazoparib was well tolerated, with no new safety signals identified. In BRCA1/2-altered cancers, efficacy for the combination was mainly observed in BRCA1/2-associated tumor types (ovarian, breast, prostate, and pancreatic) and uLMS and was comparable with that reported for PARP inhibitor monotherapy.
Trial Protocol
eAppendix 1. Definitions of Select Terms and End Points
eAppendix 2. Supplementary Methods
eAppendix 3. Supplementary Results
eTable 1. Patient Disposition and Treatment Exposure
eTable 2. Best Overall Response and Confirmed Objective Response by Investigator
eTable 3. Best Overall Response and Confirmed Objective Response by BICR With Measurable Disease in BRCA-Dependent Tumors and in the BRCA1/2 Cohort in Tumor Types With at Least 5 Patients
eTable 4. Summary of Genetic/Genomic Biomarker Status (Central Laboratory Testing)
eTable 5. Summary of TEAEs (Any Grade Occurring in ≥10% of Patients or Grade ≥3 Occurring in ≥5% of Patients)
eTable 6. Treatment-Related AEs, IRRs, and irAEs
eFigure 1. PFS for All Patients in the BRCA1/2 Cohort and in BRCA-Dependent and non–BRCA-Dependent Tumor Types
eFigure 2. Molecular Analysis of a Subset of Patients Treated at Memorial Sloan Kettering Cancer Center
eFigure 3. Additional Molecular Analysis of a Subset of Patients
eFigure 4. Best Percentage Change from Baseline per BICR
eReferences.
Data Sharing Statement
References
- 1.Pilié PG, Gay CM, Byers LA, O’Connor MJ, Yap TA. PARP inhibitors: extending benefit beyond BRCA-mutant cancers. Clin Cancer Res. 2019;25(13):3759-3771. doi: 10.1158/1078-0432.CCR-18-0968 [DOI] [PubMed] [Google Scholar]
- 2.Riaz N, Blecua P, Lim RS, et al. Pan-cancer analysis of bi-allelic alterations in homologous recombination DNA repair genes. Nat Commun. 2017;8(1):857. doi: 10.1038/s41467-017-00921-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chudasama P, Mughal SS, Sanders MA, et al. Integrative genomic and transcriptomic analysis of leiomyosarcoma. Nat Commun. 2018;9(1):144. doi: 10.1038/s41467-017-02602-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi J, Manzano A, Dong W, et al. Integrated mutational landscape analysis of uterine leiomyosarcomas. Proc Natl Acad Sci U S A. 2021;118(15):e2025182118. doi: 10.1073/pnas.2025182118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hensley ML, Chavan SS, Solit DB, et al. Genomic landscape of uterine sarcomas defined through prospective clinical sequencing. Clin Cancer Res. 2020;26(14):3881-3888. doi: 10.1158/1078-0432.CCR-19-3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571(7766):576-579. doi: 10.1038/s41586-019-1382-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587-13598. doi: 10.18632/oncotarget.7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domchek SM, Postel-Vinay S, Im SA, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020;21(9):1155-1164. doi: 10.1016/S1470-2045(20)30324-7 [DOI] [PubMed] [Google Scholar]
- 9.Vinayak S, Tolaney SM, Schwartzberg L, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 2019;5(8):1132-1140. doi: 10.1001/jamaoncol.2019.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin KK, Harrell MI, Oza AM, et al. BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2019;9(2):210-219. doi: 10.1158/2159-8290.CD-18-0715 [DOI] [PubMed] [Google Scholar]
- 11.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753-763. doi: 10.1056/NEJMoa1802905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner NC, Telli ML, Rugo HS, et al. ; ABRAZO Study Group . A phase II study of talazoparib after platinum or cytotoxic nonplatinum regimens in patients with advanced breast cancer and germline BRCA1/2 mutations (ABRAZO). Clin Cancer Res. 2019;25(9):2717-2724. doi: 10.1158/1078-0432.CCR-18-1891 [DOI] [PubMed] [Google Scholar]
- 13.de Bono JS, Mehra N, Scagliotti GV, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol. 2021;22(9):1250-1264. doi: 10.1016/S1470-2045(21)00376-4 [DOI] [PubMed] [Google Scholar]
- 14.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355(6330):1152-1158. doi: 10.1126/science.aam7344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115. doi: 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374-1385. doi: 10.1016/S1470-2045(16)30364-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-1230. doi: 10.1056/NEJMoa2002788 [DOI] [PubMed] [Google Scholar]
- 18.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731-739. doi: 10.1056/NEJMoa1714448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber LJ, Sandhu S, Chen L, et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229(3):422-429. doi: 10.1002/path.4140 [DOI] [PubMed] [Google Scholar]
- 21.Weigelt B, Comino-Méndez I, de Bruijn I, et al. Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian cancer. Clin Cancer Res. 2017;23(21):6708-6720. doi: 10.1158/1078-0432.CCR-17-0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quigley D, Alumkal JJ, Wyatt AW, et al. Analysis of circulating cell-free DNA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov. 2017;7(9):999-1005. doi: 10.1158/2159-8290.CD-17-0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Necchi A, Raggi D, Giannatempo P, et al. Exceptional response to olaparib in BRCA2-altered urothelial carcinoma after PD-L1 inhibitor and chemotherapy failure. Eur J Cancer. 2018;96:128-130. doi: 10.1016/j.ejca.2018.03.021 [DOI] [PubMed] [Google Scholar]
- 24.Randall M, Burgess K, Buckingham L, Usha L. Exceptional response to olaparib in a patient with recurrent ovarian cancer and an entire BRCA1 germline gene deletion. J Natl Compr Canc Netw. 2020;18(3):223-228. doi: 10.6004/jnccn.2019.7378 [DOI] [PubMed] [Google Scholar]
- 25.Domchek SM, Aghajanian C, Shapira-Frommer R, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140(2):199-203. doi: 10.1016/j.ygyno.2015.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tung NM, Robson ME, Ventz S, et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020;38(36):4274-4282. doi: 10.1200/JCO.20.02151 [DOI] [PubMed] [Google Scholar]
- 27.Moore KN, Secord AA, Geller MA, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):636-648. doi: 10.1016/S1470-2045(19)30029-4 [DOI] [PubMed] [Google Scholar]
- 28.Shroff RT, Hendifar A, McWilliams RR, et al. Rucaparib monotherapy in patients with pancreatic cancer and a known deleterious BRCA mutation. JCO Precis Oncol. 2018;2018. doi: 10.1200/PO.17.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blum JL, Laird AD, Litton JK, et al. Determinants of response to talazoparib in patients with HER2-negative, germline BRCA1/2-mutated breast cancer. Clin Cancer Res. 2022;28(7):1383-1390. doi: 10.1158/1078-0432.CCR-21-2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arora S, Balasubramaniam S, Zhang H, et al. FDA approval summary: olaparib monotherapy or in combination with bevacizumab for the maintenance treatment of patients with advanced ovarian cancer. Oncologist. 2021;26(1):e164-e172. doi: 10.1002/onco.13551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray-Coquard I, Pautier P, Pignata S, et al. ; PAOLA-1 Investigators . Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416-2428. doi: 10.1056/NEJMoa1911361 [DOI] [PubMed] [Google Scholar]
- 32.Mirza MR, Monk BJ, Herrstedt J, et al. ; ENGOT-OV16/NOVA Investigators . Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164. doi: 10.1056/NEJMoa1611310 [DOI] [PubMed] [Google Scholar]
- 33.Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18(1):75-87. doi: 10.1016/S1470-2045(16)30559-9 [DOI] [PubMed] [Google Scholar]
- 34.Coleman RL, Oza AM, Lorusso D, et al. ; ARIEL3 investigators . Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949-1961. doi: 10.1016/S0140-6736(17)32440-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353-1365. doi: 10.1016/S1470-2045(20)30445-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Definitions of Select Terms and End Points
eAppendix 2. Supplementary Methods
eAppendix 3. Supplementary Results
eTable 1. Patient Disposition and Treatment Exposure
eTable 2. Best Overall Response and Confirmed Objective Response by Investigator
eTable 3. Best Overall Response and Confirmed Objective Response by BICR With Measurable Disease in BRCA-Dependent Tumors and in the BRCA1/2 Cohort in Tumor Types With at Least 5 Patients
eTable 4. Summary of Genetic/Genomic Biomarker Status (Central Laboratory Testing)
eTable 5. Summary of TEAEs (Any Grade Occurring in ≥10% of Patients or Grade ≥3 Occurring in ≥5% of Patients)
eTable 6. Treatment-Related AEs, IRRs, and irAEs
eFigure 1. PFS for All Patients in the BRCA1/2 Cohort and in BRCA-Dependent and non–BRCA-Dependent Tumor Types
eFigure 2. Molecular Analysis of a Subset of Patients Treated at Memorial Sloan Kettering Cancer Center
eFigure 3. Additional Molecular Analysis of a Subset of Patients
eFigure 4. Best Percentage Change from Baseline per BICR
eReferences.
Data Sharing Statement


