Key Points
Question
Which tumor types and/or molecular subtypes are associated with response to the combination of avelumab plus talazoparib?
Findings
This nonrandomized controlled trial including 223 patients found that avelumab plus talazoparib was safe and well tolerated. Objective responses were mostly observed in patients with BRCA-altered tumors, with limited activity seen in patients with non–BRCA-altered DNA damage response (DDR)–positive tumors; however, prolonged duration of response was observed in patients with triple-negative breast cancer; hormone receptor–positive, human epidermal growth factor receptor 2–negative, DDR-positive breast cancer; and BRCA-altered ovarian cancer.
Meaning
These findings suggest that treatment with avelumab plus talazoparib warrants further investigation in specific molecular and tumor subtypes in randomized clinical trials.
This nonrandomized controlled trial assesses responses associated with the combination of avelumab and talazoparib in patients with different tumor types and molecular subtypes.
Abstract
Importance
Preclinical data suggest that poly(ADP-ribose) polymerase (PARP) inhibitors have synergistic activity when combined with immune checkpoint inhibitors (ICIs); however, it is unknown which tumor types or molecular subtypes may benefit from this combination.
Objective
To investigate responses associated with the combination of avelumab and talazoparib in different tumor types and/or molecular subtypes.
Design, Setting, and Participants
In this phase 1b and 2 basket nonrandomized controlled trial, patients with advanced solid tumors were enrolled in the following cohorts: non–small cell lung cancer (NSCLC); DNA damage response (DDR)–positive NSCLC; triple-negative breast cancer (TNBC); hormone receptor–positive, human epidermal growth factor receptor 2 (ERBB2)–negative, DDR-positive breast cancer; recurrent, platinum-sensitive ovarian cancer (OC); recurrent, platinum-sensitive, BRCA1/2-altered OC; urothelial cancer; metastatic castration-resistant prostate cancer (mCRPC); DDR-positive mCRPC; and BRCA1/2- or ATM-altered solid tumors. Data were analyzed between June 17, 2021, and August 6, 2021.
Interventions
All patients in phases 1b and 2 received avelumab plus talazoparib.
Main Outcomes and Measures
The phase 1b primary end point was dose-limiting toxic effects. The phase 2 primary end point was objective response, measured as objective response rate (ORR). Secondary end points included safety, time to response, duration of response (DOR), progression-free survival, time to prostate-specific antigen progression and PSA response of 50% or greater (for mCRPC), cancer antigen 125 response (for OC), pharmacokinetics, immunogenicity, and biomarkers.
Results
A total of 223 patients (mean [SD] age, 63.2 [11.0] years; 117 [52.5%] men) were treated, including 12 patients in phase 1b and 211 patients in phase 2. The recommended phase 2 dose was avelumab 800 mg every 2 weeks plus talazoparib 1 mg once daily. In phase 2, the ORR was 18.2% (95% CI, 5.2%-40.3%) in patients with TNBC; 34.8% (95% CI, 16.4%-57.3%) in patients with HR-positive, ERBB2-negative, and DDR-positive BC; and 63.6% (95% CI, 30.8%-89.1%) in patients with platinum-sensitive, BRCA1/2-altered OC. Responses occurred more frequently in patients with BRCA1/2-altered tumors. Durable responses were observed in patients with TNBC (median [range] DOR, 11.1 [3.4-20.4] months); HR-positive, ERBB2-negative, and DDR-positive BC (median [range] DOR, 15.7 [3.9 to ≥20.6] months); and BRCA1/2-altered OC (median DOR not reached; range, 5.6 to ≥18.4 months). The most common grade 3 or greater treatment-related adverse events were anemia (75 patients [33.6%]), thrombocytopenia (48 patients [21.5%]), and neutropenia (31 patients [13.9%]).
Conclusions and Relevance
This nonrandomized controlled trial found that ORRs for avelumab plus talazoparib were comparable with those with PARP inhibitor or ICI monotherapy. Prolonged DOR in patients with TNBC; HR-positive, ERBB2-negative, and DDR-positive BC; and BRCA1/2-altered OC warrant further investigation in randomized clinical trials. These data highlight the importance of prospective patient selection in future studies of ICI and PARP-inhibitor combinations.
Trial Registration
ClinicalTrials.gov Identifier: NCT03330405
Introduction
Immune checkpoint inhibitors (ICIs) are effective as monotherapy or in combination with other agents in multiple solid tumors.1,2,3,4 Avelumab (an anti–programmed cell death 1 ligand 1 [PD-L1] antibody) is approved as monotherapy for treating metastatic Merkel cell carcinoma and locally advanced or metastatic urothelial carcinoma (UC) as first-line maintenance and second-line therapy and in combination with axitinib as first-line treatment of advanced renal cell carcinoma.5
Poly(ADP-ribose) polymerase (PARP) inhibitors are an effective treatment option for patients with tumors containing DNA damage response (DDR) alterations, such as BRCA1/2 (OMIM 113705 and OMIM 600185) alterations.6 Talazoparib, an oral PARP inhibitor, is approved for the treatment of deleterious or suspected deleterious germline BRCA1/2-altered, human epidermal growth factor receptor 2 (ERBB2)–negative, locally advanced or metastatic breast cancer (BC).7 In a phase 3 trial of patients with advanced BC and germline BRCA1/2 alterations, talazoparib significantly improved progression-free survival (PFS) vs chemotherapy8,9; talazoparib has also shown clinical activity in metastatic castration-resistant prostate cancer (mCRPC) and pancreatic cancer with germline or tumor BRCA1/2 alterations.10,11
Preclinical data and early-phase clinical trials suggest that PARP inhibitors have synergistic activity when administered in combination with ICIs.6,12,13,14 However, it is unknown which tumor types or molecular subtypes may benefit from this combination.
In this study, we report results from the JAVELIN PARP Medley trial that investigated the safety and efficacy of avelumab plus talazoparib in 10 prospectively defined tumor-selected and/or molecularly selected cohorts of patients, including those sensitive to PARP inhibition (BRCA-associated tumors and DDR-positive tumors) or those with ICI-sensitive tumors.
Methods
This nonrandomized controlled trial was conducted in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, the International Conference on Harmonization Guidelines for Good Clinical Practice, and the Declaration of Helsinki. All patients provided written informed consent before enrollment. The protocol was approved by the institutional review board or independent ethics committee at each participating center and is provided in Supplement 1. This study is registered on ClinicalTrials.gov (NCT03330405). This study is reported following the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline and Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.
Patients
Eligible patients (aged ≥18 years) had an Eastern Cooperative Oncology Group performance status of 0 or 1; histologically diagnosed, locally advanced (primary or recurrent) or metastatic solid tumor not amenable to treatment with curative intent; measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, with at least 1 measurable lesion (not required for patients with mCRPC); and adequate hematologic, kidney, and liver function. Complete eligibility criteria are detailed in the eAppendix in Supplement 2.
Study Design and Treatment
JAVELIN PARP Medley is an open-label, multicenter, phase 1b and 2 basket trial of avelumab plus talazoparib in patients with prospectively selected, molecularly defined, locally advanced or metastatic solid tumors, including non–small-cell lung cancer (NSCLC); DDR-positive NSCLC; triple-negative BC (TNBC); hormone receptor (HR)–positive, ERBB2-negative, DDR-positive BC; recurrent platinum-sensitive ovarian cancer (OC); recurrent, platinum-sensitive, BRCA-altered OC; UC; mCRPC; DDR-positive mCRPC; and BRCA1/2- or ATM-altered (OMIM 607585) solid tumors. The NSCLC cohorts were recruited in parallel; however, the inclusion criteria for patients with DDR-positive NSCLC were revised in trial protocol amendment 3 on November 20, 2018 (Supplement 1). The inclusion criteria were revised such that patients with NSCLC were no longer required to have PD-L1–positive tumors and instead were required to have DDR-positive tumors and may have previously received prior anti–PD-L1 and anti–programmed cell death 1 (PD-1) treatment. The mCRPC cohorts were also run in parallel. The mCRPC cohort enrolled unselected patients with mCRPC with unknown DDR status at the time of enrollment, while the DDR-positive mCRPC cohort enrolled patients with previously known DDR-positive tumors at the time of enrollment. As the study used retrospective central testing for the presence of DDR alterations, it was possible that patients in the mCRPC cohort could have had DDR-positive tumors identified after enrollment.
In phase 1b, patients received avelumab 800 mg intravenously every 2 weeks plus talazoparib 1 mg orally once daily; talazoparib dose reductions to 0.75 or 0.5 mg or interruptions were allowed based on tolerability. In the phase 2 (dose-expansion) part, patients received the recommended phase 2 dose (RP2D) determined in the phase 1b. To mitigate infusion-related reactions, patients received antihistamine or acetaminophen prior to the first 4 avelumab infusions. Dose interruptions and reductions (talazoparib only) were permitted. In the event of a grade 3 or 4 adverse event (AE), avelumab or talazoparib administration was delayed until resolution to grade 1 or baseline (eAppendix in Supplement 2). Treatment was continued until disease progression, unacceptable toxic effects, or patient withdrawal. Patients with progressive disease who had ongoing clinical benefit could continue treatment at the treating physician’s discretion.
End Points and Assessments
The primary end point of the dose-finding part of phase 1b was first-cycle (28 days) dose-limiting toxic effects (DLTs; defined in the eAppendix in Supplement 2). In phase 2, the primary end point was confirmed objective response (OR; defined as best overall response of complete response [CR] or partial response [PR] per RECIST 1.1, confirmed by a second assessment ≥4 weeks after initial documentation). Secondary end points included safety, time to response, duration of response (DOR), PFS, time to prostate-specific antigen (PSA) progression and PSA response of 50% or greater (for mCRPC), cancer antigen 125 (CA-125) response (for OC), pharmacokinetics, immunogenicity, and biomarkers; definitions of end points are provided in the eAppendix in Supplement 2.
Blood samples for pharmacokinetics, pharmacodynamics, immunogenicity, and biomarker analyses were collected from all patients. In all patients, archival tumor tissue collected no more than 12 months before treatment was mandatory at screening (≤45 days before enrollment for the DDR-positive NSCLC; HR-positive, ERBB2-negative, and DDR-positive BC; and DDR-positive mCRPC cohorts). Optional fresh tumor biopsies were performed throughout treatment between day 15 of cycle 1 and day 1 of cycle 3. Biomarker methods are provided in the eAppendix, eTable 1, and eTable 2 in Supplement 2.
Statistical Analysis
In the dose-finding phase, DLTs were assessed in all patients who received at least 1 dose of study treatment and experienced a DLT during the first treatment cycle or completed the DLT observation period (first treatment cycle). Efficacy and safety were assessed in all patients who received at least 1 dose of study treatment. Avelumab pharmacokinetics and immunogenicity and talazoparib pharmacokinetics were analyzed in all patients who provided at least 1 sample for analysis.
An adaptive modified toxic effects probability interval design was used in phase 1b to identify the RP2D of talazoparib in combination with avelumab.15 Detailed information is provided in the trial protocol in Supplement 1 and eAppendix in Supplement 2.
Approximately 20 to 40 patients would be enrolled in each phase 2 cohort; detailed information is provided in the trial protocol in Supplement 1 and eAppendix in Supplement 2. Time to response was summarized using simple descriptive statistics. DOR and PFS were analyzed using the Kaplan-Meier method. We calculated 2-sided 95% CIs using the Clopper-Pearson method for ORR, and the Brookmeyer and Crowley method was used for DOR and PFS. No hypothesis was tested in this study. Biomarker data were analyzed using descriptive statistics. Analyses were conducted using SAS statistical software version 9.4 (SAS Institute). Data were analyzed between June 17, 2021, and August 6, 2021.
Results
Patients and Treatment
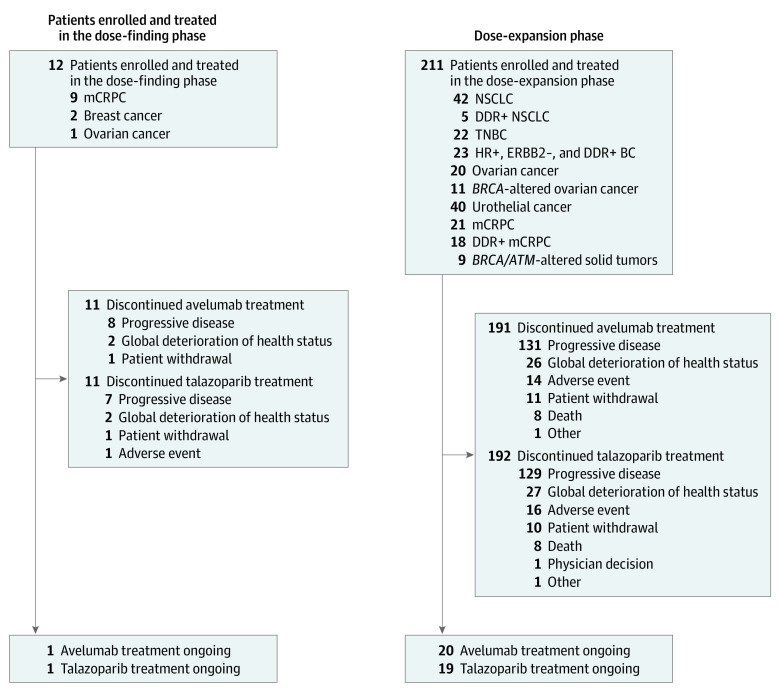
Between October 31, 2017, and November 7, 2019, a total of 223 patients (mean [SD] age, 63.2 [11.0] years; 117 [52.5%] men) were enrolled and treated, including 12 patients in phase 1b and 211 patients in phase 2 (Figure 1). Due to slow enrollment, the DDR-positive NSCLC and BRCA1/2- or ATM-altered solid tumor cohorts were stopped early. The data cutoff was September 21, 2020.
Figure 1. Trial Profile.
In total, 3 patients in the phase 1b portion experienced dose-limiting toxic effects leading to dose interruption. Of them, 2 patients (with thrombocytopenia) continued therapy following talazoparib dose reductions and recovery of platelet counts. Both patients subsequently discontinued from study treatment because of progressive disease; 1 patient discontinued 2 months after the start of treatment, and 1 withdrew from the study after 10 months of treatment. Only 1 patient permanently discontinued talazoparib because of neutropenia (after approximately 3 months); however, this patient continued to receive avelumab for a total of 5 months before discontinuing because of progressive disease. DDR indicates DNA damage repair; ERBB2, human epidermal growth factor receptor 2; HR, hormone receptor; mCRPC, metastatic castration-resistant prostate cancer; NSCLC, non–small-cell lung cancer; and TNBC, triple-negative breast cancer.
Dose-Finding Phase
Of 12 patients in phase 1b, 9 (75.0%) had mCRPC, 2 (16.7%) had TNBC, and 1 (8.3%) had OC. Baseline characteristics are shown in Table 1. In total, 3 patients (25.0%) experienced DLTs, including 2 (16.7%) with grade 3 thrombocytopenia and 1 (8.3%) with grade 3 neutropenia. This led to interruption of both study drugs and, in 1 patient with neutropenia, permanent withdrawal of talazoparib. The RP2Ds were determined to be avelumab 800 mg intravenously every 2 weeks and talazoparib 1 mg orally once daily. One patient with advanced OC had a CR that was ongoing at data cutoff, with a DOR of 31.9 months, and 1 patient with mCRPC had a PR (Table 2).
Table 1. Baseline Characteristics of Patients in the Dose-Finding Phase and Dose-Expansion Cohorts.
| Characteristic | Patients, No. (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose-finding phase (n = 12) | NSCLC (n = 42) | DDR+ NSCLC (n = 5) | TNBC (n = 22) | HR+, ERBB2−, DDR+ BC (n = 23) | OC (n = 20) | BRCA-alt OC (n = 11) | UC (n = 40) | mCRPC (n = 21) | DDR+ mCRPC (n = 18) | BRCA/ATM alt (n = 9)a | |
| Age, y | |||||||||||
| <65 | 6 (50.0) | 15 (35.7) | 4 (80.0) | 15 (68.2) | 17 (73.9) | 12 (60.0) | 7 (63.6) | 16 (40.0) | 8 (38.1) | 3 (16.7) | 5 (55.6) |
| ≥65 | 6 (50.0) | 27 (64.3) | 1 (20.0) | 7 (31.8) | 6 (26.1) | 8 (40.0) | 4 (36.4) | 24 (60.0) | 13 (61.9) | 15 (83.3) | 4 (44.4) |
| Median (IQR) | 63.5 (55-70) | 67.5 (61-73) | 63.0 (59-64) | 56.5 (48-67) | 51.0 (42-66) | 62.5 (56-68) | 59.0 (56-70) | 66.5 (57-72) | 65.0 (57-67) | 70.5 (68-79) | 62.0 (59-69) |
| Sex | |||||||||||
| Women | 3 (25.0) | 9 (21.4) | 2 (40.0) | 22 (100) | 22 (95.7) | 20 (100) | 11 (100) | 14 (35.0) | 0 | 0 | 3 (33.3) |
| Men | 9 (75.0) | 33 (78.6) | 3 (60.0) | 0 | 1 (4.3) | 0 | 0 | 26 (65.0) | 21 (100) | 18 (100) | 6 (66.7) |
| ECOG PS | |||||||||||
| 0 | 4 (33.3) | 5 (11.9) | 1 (20.0) | 12 (54.5) | 12 (52.2) | 9 (45.0) | 8 (72.7) | 16 (40.0) | 4 (19.0) | 6 (33.3) | 1 (11.1) |
| 1 | 8 (66.7) | 37 (88.1) | 4 (80.0) | 10 (45.5) | 11 (47.8) | 11 (55.0) | 3 (27.3) | 24 (60.0) | 17 (81.0) | 12 (66.7) | 8 (88.9) |
| PD-L1 statusb | |||||||||||
| High | NA | 3 (7.1) | 1 (20.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Low | NA | 8 (19.0) | 1 (20.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Positive | NA | 0 | 0 | 8 (36.4) | 3 (13.0) | 5 (25.0) | 5 (45.5) | 13 (32.5) | 1 (4.8) | 2 (11.1) | 0 |
| Negative | NA | 22 (52.4) | 2 (40.0) | 6 (27.3) | 16 (69.6) | 13 (65.0) | 4 (36.4) | 19 (47.5) | 12 (57.1) | 12 (66.7) | 3 (33.3) |
| Unknownc | NA | 9 (21.4) | 1 (20.0) | 8 (36.4) | 4 (17.4) | 2 (10.0) | 2 (18.2) | 8 (20.0) | 8 (38.1) | 4 (22.2) | 6 (66.7) |
| DDR statusd | |||||||||||
| Positive | NA | 12 (28.6) | 3 (60.0) | 11 (50.0) | 19 (82.6) | 5 (25.0) | 10 (90.9) | 18 (45.0) | 7 (35.0) | 16 (88.9) | 8 (88.9) |
| Negative | NA | 30 (71.4) | 2 (40.0) | 11 (50.0) | 4 (17.4)e | 15 (75.0) | 1 (9.1) | 22 (55.0) | 13 (65.0) | 2 (11.1) | 1 (11.1) |
| TNM stage | |||||||||||
| III | 0 | 0 | 0 | 1 (4.5) | 1 (4.3) | 4 (20.0) | 3 (27.3) | 2 (5.0) | 0 | 0 | 1 (11.1) |
| IIIA | 0 | 3 (7.1) | 0 | 1 (4.5) | 0 | 0 | 0 | 3 (7.5) | 0 | 0 | 0 |
| IIIB | 0 | 6 (14.3) | 1 (20.0) | 0 | 0 | 2 (10.0) | 1 (9.1) | 2 (5.0) | 1 (4.8) | 0 | 0 |
| IV | 10 (83.3) | 33 (78.6) | 4 (80.0) | 20 (90.9) | 22 (95.7) | 13 (65.0) | 7 (63.6) | 33 (82.5) | 20 (95.2) | 18 (100) | 7 (77.8) |
| IVA | 1 (8.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IVB | 1 (8.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not reported | 0 | 0 | 0 | 0 | 0 | 1 (5.0) | 0 | 0 | 0 | 0 | 1 (11.1) |
| Prior anticancer therapies for advanced diseasef | |||||||||||
| 0 | 0 | 20 (47.6) | 2 (40.0) | 6 (27.3) | 3 (13.0) | 6 (30.0) | 5 (45.5) | 12 (30.0) | 0 | 0 | 0 |
| 1 | 1 (8.3) | 17 (40.5) | 2 (40.0) | 8 (36.4) | 6 (26.1) | 9 (45.0) | 5 (45.5) | 27 (67.5) | 1 (4.8) | 1 (5.6) | 5 (55.6) |
| 2 | 2 (16.7) | 4 (9.5) | 0 | 7 (31.8) | 3 (13.0) | 3 (15.0) | 1 (9.1) | 1 (2.5) | 3 (14.3) | 2 (11.1) | 0 |
| 3 | 1 (8.3) | 0 | 0 | 1 (4.5) | 6 (26.1) | 2 (10.0) | 0 | 0 | 4 (19.0) | 6 (33.3) | 0 |
| ≥4 | 8 (66.7) | 1 (2.4) | 1 (20.0) | 0 | 5 (21.7) | 0 | 0 | 0 | 13 (61.9) | 9 (50.0) | 4 (44.4) |
Abbreviations: −, negative; +, positive; alt, altered; BC, breast cancer; DDR, DNA damage repair deficient; ECOG PS, Eastern Cooperative Oncology Group performance status; ERBB2, human epidermal growth factor receptor 2; HR, hormone receptor; mCRPC, metastatic castration-resistant prostate cancer; NA, not available; NSCLC, non–small-cell lung cancer; OC, ovarian cancer; PD-L1, programmed cell death 1 ligand 1; TNBC, triple-negative BC; TNM, tumor, node, metastasis; UC, urothelial carcinoma.
Included patients with ampullary cancer, BC, CRPC, colon cancer, mCRPC, metastatic prostate cancer, pancreatic adenocarcinoma, pancreatic cancer, and pulmonary malignant neuroendocrine carcinoma (1 patient each). The site of primary tumor was not reported for 1 patient.
Obtained from central laboratory tests.
Pending data or missing sample.
Derived from baseline tumor, circulating tumor DNA, and germline DDR alteration data.
Based on a loss of heterozygosity score greater than the predefined cutoff, 3 patients had DDR+ tumors at enrollment but were subsequently considered DDR− because of the lack of a loss of heterozygosity score following a change in assay specifications; 2 patients were enrolled based on a local test result but received negative results centrally.
Includes regimens in the neoadjuvant, adjuvant, or advanced or metastatic setting. In patients with BC, disease was considered as advanced or metastatic if disease progression occurred during previous neoadjuvant or adjuvant cytotoxic therapy or within 6 months of the last treatment dose.
Table 2. Best Overall Response, Confirmed Objective Response, and Progression-Free Survival in the Dose-Finding Phase and Dose-Expansion Cohorts.
| Outcome | Patients, No. (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose-finding phase (n = 12) | NSCLC (n = 42) | DDR+ NSCLC (n = 5) | TNBC (n = 22) | HR+, ERBB2−, DDR+ BC (n = 23) | OC (n = 20) | BRCA-alt OC (n = 11) | UC (n = 40) | mCRPC (n = 21) | DDR+ mCRPC (n = 18) | BRCA/ATM alt (n = 9) | ||
| Best overall responsea | ||||||||||||
| CR | 1 (8.3) | 0 | 0 | 1 (4.5) | 1 (4.3) | 0 | 2 (18.2) | 1 (2.5) | 0 | 0 | 0 | |
| PR | 1 (8.3) | 7 (16.7) | 1 (20.0) | 3 (13.6) | 7 (30.4) | 4 (20.0) | 5 (45.5) | 5 (12.5) | 0 | 2 (11.1) | 1 (11.1) | |
| Stable disease | 3 (25.0) | 24 (57.1) | 1 (20.0) | 8 (36.4) | 8 (34.8) | 15 (75.0) | 4 (36.4) | 15 (37.5) | 7 (33.3) | 5 (27.8) | 1 (11.1) | |
| Non-CR or non-PD | 1 (8.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 (38.1) | 2 (11.1) | 0 | |
| PD | 5 (41.7) | 7 (16.7) | 3 (60.0) | 10 (45.5) | 7 (30.4) | 1 (5.0) | 0 | 14 (35).0 | 6 (28.6) | 6 (33.3) | 3 (33.3) | |
| Not evaluable | 1 (8.3) | 4 (9.5) | 0 | 0 | 0 | 0 | 0 | 5 (12.5) | 0 | 3 (16.7) | 4 (44.4) | |
| Patients with OR, % (95% CI) | 16.7 (2.1-48.4) | 16.7 (7.0-31.4)a | 20.0 (0.5-71.6)a | 18.2 (5.2-40.3)a | 34.8 (16.4-57.3)a | 20.0 (5.7-43.7)a | 63.6 (30.8-89.1)a | 15.0 (5.7-29.8)a | 0 (0-16.1)b | 11.1 (1.4-34.7)b | 11.1 (0.3-48.2)a,b | |
| Time to response, median (range), mo | NA (1.8-1.8)c | 3.7 (1.7-11.3) | 1.8 (1.8-1.8)d | 1.8 (1.6-2.0) | 1.9 (1.6-3.6) | 3.6 (1.7-17.9) | 1.7 (1.6-3.7) | 2.1 (1.4-5.9) | NA | NA (5.4-5.6)c | 1.8 (1.8-1.8)d | |
| Duration of response, median (95% CI), mo | NE (3.7-31.9)c | 17.5 (5.4-17.5) | NE (11.1)d | 11.1 (3.4-20.4) | 15.7 (3.9-NE) | 3.9 (3.7-5.5) | NR (5.6-NE) | NR | NA | NA (4.3-5.5)c | NE (9.13)d | |
| PFS, median (95% CI), monthsa | NA (NC) | 4.7 (3.7-7.4) | 1.9 (1.8-NE) | 3.6 (1.9-5.6) | 5.3 (2.0-12.8) | 7.2 (4.0-9.1) | NR (7.2-NE) | 3.6 (1.9-5.4) | 4.1 (1.9-NE) | 4.6 (1.7-9.8) | 1.8 (1.4-5.9) | |
| Probability of no disease progression, % (95% CI) | ||||||||||||
| At 4 mo | NA | 53.2 (36.2-67.5)a | 20.0 (0.8-58.2)a | 45.0 (23.9-64.1)a | 52.2 (30.5-70.0)a | 79.7 (54.5-91.9)a | 90.9 (50.8-98.7)a | 36.5 (21.6-51.6)a | 33.3 (7.8-62.3)b | 58.8 (32.0-78.1)b | 53.3 (26.3-74.4)a,b | |
| At 6 mo | NA | 42.0 (26.1-57.1)a | 20.0 (0.8-58.2)a | 20.0 (6.3-39.1)a | 33.8 (15.6-53.1)a | 53.1 (29.2-72.3)a | 90.9 (50.8-98.7)a | 25.0 (12.4-39.8)a | 16.7 (1.1-49.3)b | 35.3 (10.1-62.3)b | 40.0 (16.5-62.8)a,b | |
| At 8 mo | NA | 27.0 (137-42.1)a | 20.0 (0.8-58.2)a | 15.0 (3.8-33.3)a | 33.8 (15.6-53.1)a | 37.2 (16.7-57.8)a | 72.7 (37.1-90.3)a | 17.8 (7.1-32.5)a | 16.7 (1.1-49.3)b | 35.3 (10.1-62.3)b | 33.3 (12.2-56.4)a,b | |
| At 10 mo | NA | 18.0 (7.4-32.3)a | 20.0 (0.8-58.2)a | 15.0 (3.8-33.3)a | 33.8 (15.6-53.1)a | 26.6 (9.7-47.1)a | 63.6 (29.7-84.5)a | 17.8 (7.1-32.5)a | 16.7 (1.1-49.3)b | 35.3 (10.1-62.3)b | 26.7 (8.3-49.6)a,b | |
| At 12 mo | NA | 18.0 (7.4-32.3)a | 20.0 (0.8-58.2)a | 10.0 (1.7-27.1)a | 33.8 (15.6-53.1)a | 21.3 (6.6-41.3)a | 54.5 (22.9-78.0)a | 17.8 (7.1-32.5)a | NE (NE-NE) | 17.6 (1.2-50.4)b | 13.3 (2.2-34.6)a,b | |
| At 18 mo | NA | 10.8 (3.0-24.3)a | NE (NE-NE) | 5.0 (0.3-20.5)a | 18.8 (4.3-41.0)a | 15.9 (4.0-35.2)a | 54.5 (22.9-78.0)a | NE (NE-NE) | NE (NE-NE) | NE (NE-NE) | NE (NE-NE) | |
| Duration of treatment, median, (IQR) [range], mo | ||||||||||||
| Avelumab | 4.8 (2.4-7.7) [0.9-35.0] | 4.6 (2.8-9.2) [0.5-24.4] | 1.9 (1.8-10.6) [1.8-16.1] | 3.9 (2.0-6.6) [1.4-21.4] | 5.5 (2.7-13.8) [0.9-23.2] | 7.0 (5.5-9.8) [1.8-23.2] | 12.6 (9.2-21.2) [3.2-23.8] | 3.6 (1.8-8.7) [0.5-18.3] | 3.7 (1.8-4.1) [0.5-12.4] | 3.2 (1.8-9.9) [0.5-17.0] | 2.7 (1.1-5.9) [0.5-11.4] | |
| Talazoparib | 3.9 (1.7-7.6) [0.9-34.7] | 4.1 (2.1-7.8) [0.3-24.8] | 1.8 (1.8-10.5) [1.8-16.0] | 3.5 (1.9-6.6) [1.3-21.4] | 5.3 (2.3-13.8) [0.9-22.9] | 7.0 (5.5-10.9) [1.6-23.0] | 12.2 (9.2-21.1) [3.2-23.6] | 3.8 (1.6-7.9) [0.4-18.3] | 3.4 (1.9-4.1) [0.4-12.4] | 3.5 (1.8-9.9) [0.0-16.8] | 2.3 (0.9-6.0) [0.3-11.5] | |
Abbreviations: −, negative; +, positive; alt, altered; BC, breast cancer; CR, complete response; DDR, DNA damage repair deficient; ERBB2, human epidermal growth factor receptor 2; HR, hormone receptor; mCRPC, metastatic castration-resistant prostate cancer; NA, not available; NC, not calculable; NE, not estimable; NR, not reached; NSCLC, non–small-cell lung cancer; OC, ovarian cancer; OR, objective response; PD, progressive disease; PFS, progression-free survival; PR, partial response; TNBC, triple-negative BC; UC, urothelial carcinoma.
Assessed by investigators per Response Evaluation Criteria in Solid Tumors version 1.1 for patients with BRCA1/2- or ATM-altered solid tumors without mCRPC.
Assessed by investigators per Response Evaluation Criteria in Solid Tumors version 1.1 and Prostate Cancer Working Group 3 patients with BRCA1/2- or ATM-altered solid tumors with mCRPC.
Raw values for the 2 responders in the cohort.
Raw values for 1 responder in the cohort.
Dose-Expansion Phase
Of 211 patients in phase 2, 20 patients (9.5%) were still receiving treatment at the data cutoff; the most common reason for treatment discontinuation of both avelumab and talazoparib was progressive disease (131 patients [62.1%]) (Figure 1; eTable 3 in Supplement 2). A total of 108 patients (51.2%) were men; the median (IQR) patient age was 65.0 (56.0-70.0) years. Baseline characteristics of each cohort are shown in Table 1, and duration of treatment is shown in eTable 3 in Supplement 2. Across all cohorts, the median (range) duration of treatment was 4.6 (0.46-24.4) months for avelumab and 4.4 (0.02-24.9) months for talazoparib.
Dose-Expansion Cohorts
The median DOR, median PFS, proportions of patients with a confirmed OR, and best percentage change in target lesion size from baseline by cohort are shown in Table 2, Figure 2, and eFigures 1 through 3 in Supplement 2. ORs and ORRs according to biomarker status are shown in eTables 4 and 5 in Supplement 2. CA-125 and PSA responses are shown in eTable 6 in Supplement 2, and biomarker status is shown in eTable 7 in Supplement 2.
Figure 2. Best Percentage Change in Size of Target Lesions Assessed by Investigators per RECIST v1.1 While Receiving Treatment in the Dose-Expansion Phase.
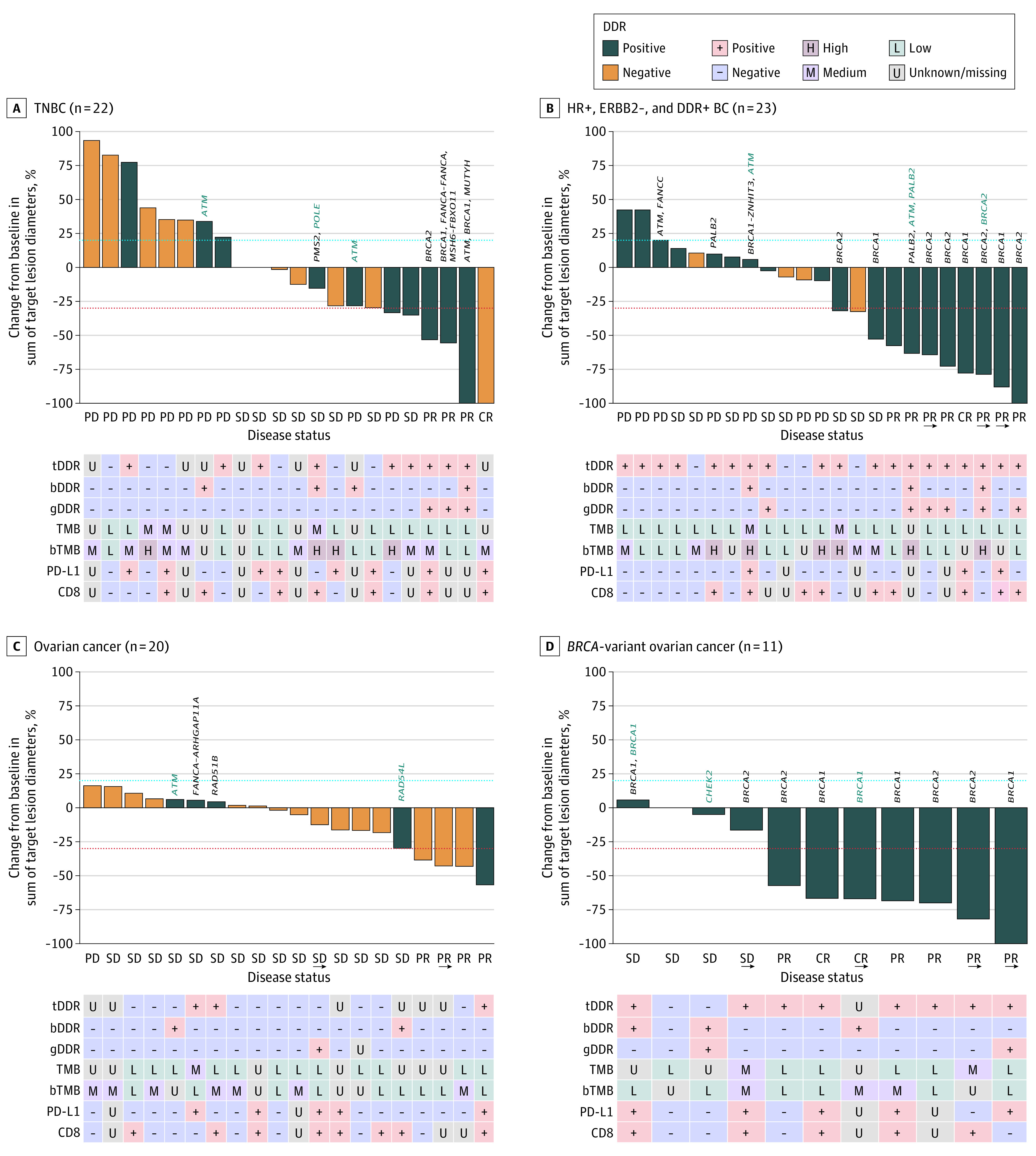
The blue dashed lines represent the threshold for progressive disease (PD), defined as an increase of at least 20% in target lesion diameter from baseline. The lower dashed lines represent the threshold for a partial response, defined as a decrease of at least 30% in target lesion diameter from baseline. In patients with DNA damage repair–positive (DDR+) status but for whom a DDR alteration is not specified, DDR status was confirmed by germline loss of heterozygosity (gLOH) score. Presence of a germline DDR+ alteration (gDDR) alone did not confirm DDR+ status. In the absence of positive solid tumor or circulating tumor DNA alteration results, detection of a known or likely deleterious germline variant suggested that a patient had a DDR+ tumor, provided that solid tumor and circulating tumor DNA results did not both suggest DDR-negative (DDR−). (Three patients were considered to have DDR+ tumors at enrollment, determined by a gLOH score above the predefined cutoff; however, their tumors were subsequently considered DDR− because of a change in gLOH assay specifications. Two patients were enrolled based on a local test result but received negative results centrally.) Arrows indicate ongoing treatment; bDDR, blood DDR; bTMB, blood tumor mutational burden; ERBB2, human epidermal growth factor receptor 2; HR, hormone receptor; PD-L1, programmed cell death 1 ligand 1; SD, stable disease; tDDR, tumor DDR. For some items, the genetic variation is written in vertical text above the bar, with black font indicating tDDR alteration and teal font, bDDR alteration.
In the NSCLC cohort, the ORR was 16.7% (95% CI, 7.0%-31.4%) (Table 2). The median (range) DOR was 17.5 (5.4-17.5) months (eFigure 4 in Supplement 2). Responses were only observed in 1 patient with a DDR-positive tumor and 6 patients with DDR wild-type tumors and were not associated with PD-L1 expression (eTables 4 and 5 in Supplement 2). In the DDR-positive NSCLC cohort, 2 patients had DDR-negative tumors (enrolled under the original eligibility criteria), and 3 had DDR-positive tumors. Of 5 patients in this cohort, 1 had a confirmed OR (PR in a patient with high PD-L1 positivity, DDR-negative tumor, with response ongoing at data cutoff).
In the TNBC cohort, the ORR was 18.2% (95% CI, 5.2%-40.3%) and median (range) DOR was 11.1 (3.4-20.4) months. PRs were observed in patients with BRCA-altered tumors; the patient with a CR had a PD-L1-positive, DDR-negative tumor. In the HR-positive, ERBB2-negative, and DDR-positive BC cohort, the ORR was 34.8% (95% CI, 16.4%-57.3%), and responses were observed in 8 of 19 patients with DDR-positive tumors, including 6 patients with BRCA1/2-altered tumors. Median (range) DOR was 15.7 (3.9 to ≥20.6) months, and responses were ongoing in 3 patients with BRCA-altered tumors at the data cutoff.
In the platinum-sensitive, BRCA wild-type OC cohort, the ORR was 20.0% (95% CI, 5.7%-43.7%) and median (range) DOR was 3.9 (2.3-5.5) months. Of 4 patients who responded, 2 patients also had CA-125 responses (eTable 6 in Supplement 2). In the platinum-sensitive, BRCA-altered OC cohort, the ORR was 63.6% (95% CI, 30.8%-89.1%). In patients with centrally confirmed BRCA-altered tumors, the ORR was 70.0% (95% CI, 34.8%-93.3%). The median DOR was not reached, and the range was 5.6 to at least 18.4 months after a median follow-up of 16.1 (95% CI, 12.7-21.9) months, with responses ongoing in 4 patients at the data cutoff. Of 7 patients who responded, 6 patients also had CA-125 responses (eTable 6 in Supplement 2).
In the UC cohort, the ORR was 15.0% (95% CI, 5.7%-29.8%). ORRs were similar in patients with vs without prior platinum therapy (4 patients [14.3%] vs 2 patients [16.7%]). The median DOR was not reached, and the range was 3.9 to at least 14.7 months. The patient with a CR had a BRCA-altered, PD-L1–negative tumor, and CR was ongoing at the data cutoff.
In the mCRPC cohort, no confirmed ORs were reported; however, PSA responses were observed in 2 of 21 patients (95% CI, 1.2%-30.4%), both of whom had nonmeasurable disease. In the DDR-positive mCRPC cohort, the ORR was 11.1% (95% CI, 0.0%-16.1%) in patients with measurable disease. In the cohort with BRCA1/2- or ATM-altered solid tumors, the ORR was 11.1% (1 patient with a PR); DOR was 9.13 months, and median (range) PFS was 1.8 (1.5-5.9) months (Table 2).
Safety
The most common (experienced by ≥30% of patients) treatment-related AEs (TRAEs) of any grade were anemia (135 patients [60.5%]), thrombocytopenia (110 patients [49.3%]), neutropenia (77 patients [34.5%]), and fatigue (69 patients [30.9%]) (Table 3). Grade 3 or 4 TRAEs observed in more than 5% of patients were anemia (75 patients [33.6%]), thrombocytopenia (48 patients [21.5%]), and neutropenia (31 patients [13.9%]). Talazoparib dose reductions due to AEs (regardless of causality) occurred in 79 patients (34.5%) and were most frequently due to hematological toxic effects, including anemia (38 patients [17.0%]), thrombocytopenia (34 patients [15.2%]), and neutropenia (12 patients [5.4%]). TRAEs leading to discontinuation of any study drug occurred in 17 patients (7.6%). In the DDR-positive mCRPC cohort, 1 patient died due to acute respiratory syndrome that was, by the investigator, considered related to study treatment. A total of 29 patients (13.0%) had immune-related AEs of any grade; 8 patients (3.6%) had grade 3 or 4 immune-related AEs. There were 48 patients (21.5%) who experienced infusion-related reactions of any grade; 1 patient (<1%) experienced a grade 3 or 4 infusion related reaction. Results for pharmacokinetics and immunogenicity are given in the eAppendix and eFigure 5 in Supplement 2.
Table 3. Any-Grade Treatment-Related AEs Occurring in at Least 10% of Patients or Grade 3 or 4 Occurring in at Least 5% of Patients and All Immune-Related AEs and Infusion-Related Reactions in the Dose-Finding and Dose-Expansion Phases.
| Outcome | Patients, No. (%) (N = 223) | |
|---|---|---|
| Any grade AE | Grade 3-4 AE | |
| Treatment-related AEs | 208 (93.3) | 127 (57.0) |
| Anemiaa | 135 (60.5) | 75 (33.6) |
| Thrombocytopeniab | 110 (49.3) | 48 (21.5) |
| Neutropeniac | 77 (34.5) | 31 (13.9) |
| Fatigue | 69 (30.9) | 8 (3.6) |
| Nausea | 59 (26.5) | 1 (0.4) |
| Decreased appetite | 31 (13.9) | 1 (0.4) |
| White blood cell count decreased | 29 (13.0) | 4 (1.8) |
| Infusion-related reaction | 27 (12.1) | 1 (0.4) |
| Chills | 26 (11.7) | 0 |
| Diarrhea | 24 (11.8) | 4 (1.8) |
| Immune-related AEsd | 29 (13.0) | 8 (3.6) |
| Hypothyroidism | 12 (5.4) | 0 |
| Hyperthyroidism | 4 (1.4) | 0 |
| Blood thyroid-stimulating hormone increased | 1 (0.4) | 1 (0.4) |
| Rash | 4 (1.8) | 0 |
| Pruritus | 2 (0.9) | 0 |
| Rash maculopapular | 2 (0.9) | 0 |
| Colitis | 1 (0.4) | 1 (0.4) |
| Diarrhea | 1 (0.4) | 1 (0.4) |
| Pneumonitis | 2 (0.9) | 0 |
| Autoimmune neutropenia | 1 (0.4) | 1 (0.4) |
| Immune thrombocytopenia | 1 (0.4) | 1 (0.4) |
| Thrombocytopenia | 1 (0.4) | 1 (0.4) |
| Glucocorticoid deficiency | 1 (0.4) | 1 (0.4) |
| Hyperglycemia | 1 (0.4) | 1 (0.4) |
| Myocarditis | 1 (0.4) | 1 (0.4) |
| Uveitis | 1 (0.4) | 0 |
| Infusion-related reactionse | 48 (21.5) | 1 (0.4) |
| Any | 27 (12.1) | 1 (0.4) |
| Chills | 21 (9.4) | 0 |
| Pyrexia | 10 (4.5) | 0 |
| Flushing | 1 (0.4) | 0 |
Abbreviation: AE, adverse event.
Composite term that includes anemia, hematocrit decreased, hemoglobin decreased, red blood cell count decreased, and iron deficiency anemia.
Composite term that includes thrombocytopenia, platelet count decreased, and immune thrombocytopenia.
Composite term that includes neutropenia, febrile neutropenia, neutrophil count decreased, and autoimmune neutropenia.
Immune-related adverse events are a subset of all AEs classified as immune related based on reported term and medical review.
Infusion-related reactions are a subset of all AEs classified as infusion-related reactions based on the reported term and timing of onset and resolution in relation to the avelumab infusion.
Discussion
This nonrandomized controlled trial is the largest and most histologically diverse basket trial, to our knowledge, to assess the safety and clinical efficacy of a PARP inhibitor and PD-1 and PD-L1 inhibitor combination in patients with prospectively defined subgroups with known biomarker (BRCA1/2 and DDR) statuses. To identify molecular subtypes that may respond to avelumab and talazoparib, we used a panel of 34 genes to define DDR status. While alterations in genes implicated in homologous recombination are associated with sensitization to PARP inhibition, a large analysis by Hsiehchen et al16 reported that such alterations also are associated with benefit to ICIs independent of tumor mutation burden and tumor type, thus complicating attempts to attribute benefit to either of the respective single agents.
Avelumab 800 mg every 2 weeks plus talazoparib 1 mg once daily was generally well tolerated, and no new safety signals were identified compared with both drugs given as monotherapy. Toxic effects were manageable with dose modifications, and the proportion of patients who discontinued study treatment due to TRAEs was low.
In patients with NSCLC and UC, and with the caveat of cross-trial comparison, the clinical activity was similar to that seen in previous studies of ICI monotherapy.17,18,19,20,21,22 These cohorts included at least 1 prior line of therapy for advanced disease in more than 50% of patients, low or negative PD-L1 expression, and low tumor mutational burden (where known) in most patients. In the NSCLC cohort, responses were observed in three-fourths of patients with high PD-L1 expression. However, DDR-positive or BRCA-altered tumors were not associated with significantly increased antitumor activity in either the NSCLC cohort or the UC cohort, suggesting that DDR alterations, including some BRCA alterations, did not increase sensitivity to the combination. As previously reported, BRCA1/2 alterations are an indispensable founding event for certain cancers; however, in other tumors, they appear to be biologically neutral.23
Disease control was achieved in all patients with confirmed BRCA-altered, platinum-sensitive OC; despite the small sample size, the findings are comparable with reports of patients treated with PARP-inhibitor monotherapy24 or PARP inhibitor plus ICI combination therapy.13 With the caveat of cross-trial comparison, the median DOR among patients with BRCA-altered, platinum-sensitive OC compared favorably with that reported with olaparib monotherapy in a similar population.24,25 This is also supported by the observation that 55% of patients were alive and progression free at 18 months after treatment start, suggesting potentially improved efficacy compared with that seen with olaparib monotherapy (median PFS, 12.7 months in a study by Liu et al26 and 13.2 months in the SOLO3 trial24). Overall, and with the limitations of small sample size and nonrandomized trial design, these results suggest improved response durability and PFS in patients with platinum-sensitive, BRCA1/2-altered OC.27 Lower ORRs were observed in patients with platinum-sensitive, unselected OC, since most patients (75%) in this cohort had DDR-negative disease; in patients with DDR-positive disease, only 1 had a BRCA-altered tumor. The difference in clinical outcomes between biomarker-unselected and biomarker-selected OC cohorts highlights the importance of appropriate patient selection using biomarkers associated with response to optimize patient benefit, even with combination approaches.
In the advanced BC cohorts (ie, TNBC and HR-positive, ERBB2-negative, and DDR-positive BC), clinical activity was primarily observed in patients with BRCA-altered tumors. While the number of patients with BRCA-altered tumors was a small proportion of the total population (3 of 22 patients with TNBC; 9 of 19 patients with HR-positive, ERBB2-negative, and DDR-positive BC), most responses were observed in patients with BRCA- altered tumors (3 of 4 patients with TNBC; 6 of 8 patients with HR-positive, ERBB2-negative, and DDR-positive BC). Despite the small sample size, the response rate in patients with BRCA-altered tumors (3 of 3 patients with TNBC; 6 of 9 patients with HR-positive, ERBB2-negative, and DDR-positive BC) was comparable with the phase 3 EMBRACA and phase 2 ABRAZO studies of talazoparib monotherapy in patients with germline BRCA-altered, advanced BC and the combination study of niraparib and pembrolizumab in patients with advanced or metastatic TNBC.8,14,28 The median DORs in patients with advanced TNBC (11.1 months) and HR-positive, ERBB2-negative, and DDR-positive BC (15.7 months) compare favorably with the DOR in the EMBRACA study (median DOR, 5.4 months), suggesting improved response durability with combination treatment.
The clinical activity of avelumab plus talazoparib in unselected patients with mCRPC and DDR-positive mCRPC was limited. In the mCRPC cohort, most patients (62%) had DDR-negative tumors, while in the DDR-positive mCRPC cohort, most patients with DDR-positive tumors (14 of 16 patients) had non–BRCA-altered tumors, and the efficacy was consistent with other studies of patients with mCRPC and BRCA wild-type tumors treated with PARP-inhibitor monotherapy.11,29,30 Overall, these data suggest that clinical benefit was limited for other DDR alterations, and the addition of ICIs did not extend clinical benefit to patients with mCRPC with BRCA1/2 wild-type tumors.
In several cohorts, no clear association of PD-L1 status, tumor mutational burden, CD8+ T-cell level, or DDR status with antitumor response was observed. However, in other cohorts (eg, DDR-positive mCRPC), the limited sample sizes and/or imbalances in distribution precluded these formal assessments.
Limitations
This study has some limitations. Several of the patient groups have small numbers, which prevents us from drawing definitive conclusions. Furthermore, some of the analyses were retrospective and exploratory. Additionally, due to single-arm study design, the response rates for the combination of avelumab and talazoparib were compared with historical data for avelumab or talazoparib monotherapy in similar patient populations.
Conclusions
In this nonrandomized controlled trial, the combination of avelumab and talazoparib was generally well tolerated. The addition of talazoparib was not associated with significantly extending the clinical activity of avelumab in patients with DDR-positive NSCLC and unselected NSCLC or UC. In patients with PARP inhibitor–sensitive tumor types, most responses were observed in patients with BRCA-altered tumors, and activity was limited in patients with BRCA wild-type, DDR-positive tumors. A potential reason for this may have been receipt of prior chemotherapy, including prior platinum, which could have affected the response of BRCA wild-type DDR-positive tumors to talazoparib due to cross-resistance mechanisms, such as restoration of HR. Furthermore, a limitation of these data is that the association of response with individual BRCA wild-type DDR genes could not be assessed due to small sample sizes. Selection of patients via DDR status could guide future therapeutic strategies, eg, use of other DDR inhibitors, in combination with ICIs, perhaps tailored to specific tumor types.
Overall, these data highlight the importance of prospective patient selection in future studies of ICI and PARP inhibitors or other DDR inhibitor combinations. The addition of avelumab did not extend DOR across all tumor types; however, potential clinical benefit was observed in patients with TNBC, HR-positive, ERBB2-negative, and DDR-positive BC, and BRCA-altered OC, warranting further investigation in the context of an optimized patient selection strategy and randomized clinical trials.
Trial Protocol and Statistical Analysis Plan
eAppendix. Supplementary Data
eTable 1. PD-L1 Scoring By Indication
eTable 2. Rationale for DDR Gene Testing
eTable 3. Treatment Disposition of Patients in the Dose-Finding and Dose-Expansion Cohorts
eTable 4. Objective Response Rate by Investigators Per RECIST 1.1 According to Biomarker Status in the Dose-Expansion Cohorts
eTable 5. Objective Response Assessed by the Investigator Per RECIST 1.1 by Alteration Status in the Dose-Expansion BC Cohorts
eTable 6. Summary of CA-125 and PSA Response in the Dose-Expansion Cohorts
eTable 7. Summary of Biomarker Status of Patients in the Dose-Expansion Cohorts
eFigure 1. Best Percentage Change in Size of Target Lesions Among Patients Receiving Treatment Assessed by Investigators Per RECIST v1.1 in the Dose-Expansion Phase
eFigure 2. BOR in Patients in the Dose-Expansion Phase
eFigure 3. Percentage Change in Sum of Diameters of Target Lesions From Baseline
eFigure 4. Time to and Duration of Response by Investigators Per Response Evaluation Criteria in Solid Tumors Version 1.1
eFigure 5. Avelumab Steady-State Exposures Following 800 mg Intravenous Infusion Every 2 Weeks Coadministered With Oral Talazoparib 1 mg Once Daily vs Avelumab 10 mg/kg Every 2 Weeks Monotherapy
Data Sharing Statement
Reference
- 1.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115. doi: 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi: 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 3.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374-1385. doi: 10.1016/S1470-2045(16)30364-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burtness B, Harrington KJ, Greil R, et al. ; KEYNOTE-048 Investigators . Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928. doi: 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 5.EMD Serono . Bavencio (avelumab) prescribing information. Accessed October 11, 2022. https://www.emdserono.com/us-en/pi/bavencio-pi.pdf
- 6.Vikas P, Borcherding N, Chennamadhavuni A, Garje R. Therapeutic potential of combining PARP inhibitor and immunotherapy in solid tumors. Front Oncol. 2020;10:570. doi: 10.3389/fonc.2020.00570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfizer . Talzenna (talazoparib) prescribing information. Accessed October 11, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?id=11046
- 8.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753-763. doi: 10.1056/NEJMoa1802905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litton JK, Hurvitz SA, Mina LA, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. 2020;31(11):1526-1535. doi: 10.1016/j.annonc.2020.08.2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bono J, Ramanathan RK, Mina L, et al. Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov. 2017;7(6):620-629. doi: 10.1158/2159-8290.CD-16-1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Bono JS, Mehra N, Scagliotti GV, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol. 2021;22(9):1250-1264. doi: 10.1016/S1470-2045(21)00376-4 [DOI] [PubMed] [Google Scholar]
- 12.Li A, Yi M, Qin S, Chu Q, Luo S, Wu K. Prospects for combining immune checkpoint blockade with PARP inhibition. J Hematol Oncol. 2019;12(1):98. doi: 10.1186/s13045-019-0784-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domchek SM, Postel-Vinay S, Im SA, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020;21(9):1155-1164. doi: 10.1016/S1470-2045(20)30324-7 [DOI] [PubMed] [Google Scholar]
- 14.Vinayak S, Tolaney SM, Schwartzberg L, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 2019;5(8):1132-1140. doi: 10.1001/jamaoncol.2019.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji Y, Wang SJ. Modified toxicity probability interval design: a safer and more reliable method than the 3 + 3 design for practical phase I trials. J Clin Oncol. 2013;31(14):1785-1791. doi: 10.1200/JCO.2012.45.7903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiehchen D, Hsieh A, Samstein RM, et al. DNA repair gene mutations as predictors of immune checkpoint inhibitor response beyond tumor mutation burden. Cell Rep Med. 2020;1(3):100034. doi: 10.1016/j.xcrm.2020.100034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non–small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19(1):51-64. doi: 10.1016/S1470-2045(17)30900-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ready NE, Ott PA, Hellmann MD, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J Thorac Oncol. 2020;15(3):426-435. doi: 10.1016/j.jtho.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 21.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non–small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 22.Bellmunt J, de Wit R, Vaughn DJ, et al. ; KEYNOTE-045 Investigators . Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026. doi: 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571(7766):576-579. doi: 10.1038/s41586-019-1382-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penson RT, Valencia RV, Cibula D, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38(11):1164-1174. doi: 10.1200/JCO.19.02745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matulonis UA, Penson RT, Domchek SM, et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: a multistudy analysis of response rates and safety. Ann Oncol. 2016;27(6):1013-1019. doi: 10.1093/annonc/mdw133 [DOI] [PubMed] [Google Scholar]
- 26.Liu JF, Brady MF, Matulonis UA, et al. A phase III study comparing single-agent olaparib or the combination of cediranib and olaparib to standard platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer. J Clin Oncol. 2020;38(15_suppl):6003. doi: 10.1200/JCO.2020.38.15_suppl.6003 [DOI] [Google Scholar]
- 27.Ding L, Kim HJ, Wang Q, et al. PARP inhibition elicits STING-dependent antitumor immunity in Brca1-deficient ovarian cancer. Cell Rep. 2018;25(11):2972-2980.e5. doi: 10.1016/j.celrep.2018.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner NC, Telli ML, Rugo HS, et al. ; ABRAZO Study Group . A phase II study of talazoparib after platinum or cytotoxic nonplatinum regimens in patients with advanced breast cancer and germline BRCA1/2 mutations (ABRAZO). Clin Cancer Res. 2019;25(9):2717-2724. doi: 10.1158/1078-0432.CCR-18-1891 [DOI] [PubMed] [Google Scholar]
- 29.Abida W, Campbell D, Patnaik A, et al. Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: analysis from the phase II TRITON2 study. Clin Cancer Res. 2020;26(11):2487-2496. doi: 10.1158/1078-0432.CCR-20-0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi: 10.1056/NEJMoa1911440 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix. Supplementary Data
eTable 1. PD-L1 Scoring By Indication
eTable 2. Rationale for DDR Gene Testing
eTable 3. Treatment Disposition of Patients in the Dose-Finding and Dose-Expansion Cohorts
eTable 4. Objective Response Rate by Investigators Per RECIST 1.1 According to Biomarker Status in the Dose-Expansion Cohorts
eTable 5. Objective Response Assessed by the Investigator Per RECIST 1.1 by Alteration Status in the Dose-Expansion BC Cohorts
eTable 6. Summary of CA-125 and PSA Response in the Dose-Expansion Cohorts
eTable 7. Summary of Biomarker Status of Patients in the Dose-Expansion Cohorts
eFigure 1. Best Percentage Change in Size of Target Lesions Among Patients Receiving Treatment Assessed by Investigators Per RECIST v1.1 in the Dose-Expansion Phase
eFigure 2. BOR in Patients in the Dose-Expansion Phase
eFigure 3. Percentage Change in Sum of Diameters of Target Lesions From Baseline
eFigure 4. Time to and Duration of Response by Investigators Per Response Evaluation Criteria in Solid Tumors Version 1.1
eFigure 5. Avelumab Steady-State Exposures Following 800 mg Intravenous Infusion Every 2 Weeks Coadministered With Oral Talazoparib 1 mg Once Daily vs Avelumab 10 mg/kg Every 2 Weeks Monotherapy
Data Sharing Statement