This ecological study examines the association between residential racial and economic segregation and cancer mortality at the county level in the US.
Key Points
Question
Is residential racial and economic segregation, measured by the Index of Concentration at the Extremes, associated with cancer mortality at the county level in the US?
Findings
In this ecological study of 3110 US counties, age-adjusted mortality rates were statistically significantly higher for the most deprived counties for all cancers combined and for 12 of 13 selected cancer sites compared with the most privileged counties, with the largest magnitude occurring with lung and bronchus cancer.
Meaning
This study’s findings suggest that residential racial and economic segregation may be associated with higher cancer mortality at the county level in the US.
Abstract
Importance
Residential segregation is a structural risk factor for poor cancer outcomes. Previous research examining the association of residential segregation with cancer outcomes was limited by older data, restricted geographic areas, and few cancer sites. To guide targeted interventions, a comprehensive evaluation of the association between segregation and cancer outcomes is needed.
Objective
To examine the association of residential racial and economic segregation with cancer mortality at the US county level for all cancers combined and for the 13 cancer types that represent the top 10 causes of cancer deaths in males or females.
Design, Setting, and Participants
This ecological study used county-level sociodemographic data from the 2015-2019 American Community Survey linked with 2015-2019 county-level mortality data. Data analysis was performed from September 2021 to April 2022.
Exposures
Residential racial and economic segregation measured by the Index of Concentration at the Extremes (ICE) and categorized into quintiles 1 (most deprived) through 5 (most privileged).
Main Outcomes and Measures
Age-adjusted cancer mortality was the outcome. Multilevel linear mixed modeling was used to calculate the adjusted mortality rate ratio (aRR).
Results
A total of 3110 counties were included. The age-adjusted mortality rates of all cancers combined were 179.8, 177.3, 167.6, 159.6, and 146.1 per 100 000 population (P < .001 for trend) for the 5 ICE categories (most deprived to least deprived), respectively. Compared with the least deprived counties, aRRs for all cancers combined were 1.22 (95% CI, 1.20-1.24) for the most deprived counties, followed by 1.17 (95% CI, 1.15-1.19), 1.10 (95% CI, 1.09-1.12), and 1.06 (95% CI, 1.04-1.08) for the other 3 quintiles, respectively (P < .001 for trend). Segregation was associated with increased mortality from 12 of 13 selected cancer sites, in which aRRs ranged from 1.06 (95% CI, 1.02-1.09) for brain and other nervous system cancer to 1.49 (95% CI, 1.43-1.54) for lung and bronchus cancer.
Conclusions and Relevance
The findings of this ecological study suggest that residential racial and economic segregation is associated with higher cancer mortality at the county level, highlighting opportunities for geographically targeted cancer prevention and control efforts.
Introduction
Residential segregation—geographic separation of predominantly poor and racial minority groups from privileged white populations1—is associated with adverse health outcomes in the US.2 Previous studies examining the association between residential segregation and cancer outcomes used older data3,4,5 or limited segregation measures6,7,8 and examined restricted geographic areas6,7,8,9,10,11,12 or few cancer sites (ie, breast,4,6 prostate,7 lung,3,9 and colorectal cancer10). In this ecological study, we investigated the association between county-level residential racial and economic segregation using the Index of Concentration at the Extremes (ICE), a well-validated metric of residential racial and economic segregation,13 and mortality rates for all cancer sites combined and the 13 cancer sites with the highest number of deaths among males or females in the US.
Methods
Data Source
We used the US Census Bureau’s 2015-2019 American Community Survey 5-year estimate data to calculate county-level ICE. We used the National Center for Health Statistics’ county-level 2015-2019 age-adjusted cancer mortality rate per 100 000 population data, which was exported from the SEER*Stat database. We also obtained county-level demographic characteristics and population size from the American Community Survey estimates and county poverty levels from the Small Area Income and Poverty Estimates Program.
Measures
Residential racial and economic segregation was measured by the ICE, which quantifies the extent to which an area’s residents are concentrated in extremes of racial and economic privilege (wealthy White people) and deprivation (poor Black people). Specifically, the ICE calculation includes 2 steps: (1) the difference between the number of White persons with more than the 80th percentile of household income and the number of Black persons with less than the 20th percentile of household income; and (2) the proportion of this difference represented among the total number of residents with race and income information available.14 The formula and a worked example of the ICE calculation are provided in the eAppendix in the Supplement. The ICE ranges from −1 to 1 and was categorized into quintiles, in which the first quintile represents the most deprived group and the fifth quintile represents the most privileged group.
The outcome variable was age-adjusted mortality rate at the county level for all cancers combined and for 13 cancer types representing the top 10 cancers in terms of mortality in males or females in the US15 (ie, cancers of lung and bronchus, colon and rectum, pancreas, and liver and intrahepatic bile duct; leukemia; cancers of the esophagus and urinary bladder; non-Hodgkin lymphoma; brain and other nervous system for both sexes; and prostate cancer for males and cancers of breast, ovary, and uterine corpus for females).
Covariates for the study included the proportion of the population in poverty (<10%, 10% to <20%, and ≥20%), metropolitan status (metropolitan counties, nonmetropolitan counties), and the proportion of the population with non-Hispanic White alone race (a continuous variable). These covariates are potential confounders because they are risk factors of cancer mortality and are associated with segregation (eTable 1 in the Supplement).
Statistical Analysis
Data analysis was performed from September 2021 to April 2022 and was conducted at the county level. We calculated the mean age-adjusted cancer mortality by ICE quintiles. In addition, we calculated the adjusted mortality rate ratio (aRR) of each quintile group compared with the fifth quintile group, with multilevel linear mixed modeling clustered at the state level, weighted by county population size, and adjusted for county demographic characteristics. Two-sided P < .05 was considered to be statistically significant. P value for trend was calculated by the same model with continuous ICE quintiles (1-5) as the exposure and mortality as the outcome. SAS, version 9.4 (SAS Institute Inc) was used for the statistical analyses.
Results
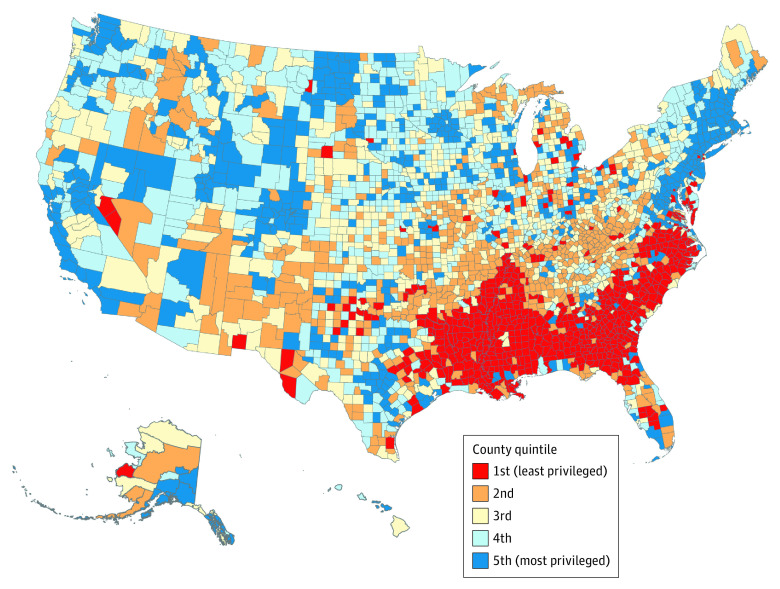
A total of 3110 counties were categorized into ICE quintiles (Figure 1). Compared with the most privileged (least segregated) counties (the fifth ICE quintile), deprived counties were more likely to be nonmetropolitan, included 20% or more of residents living below the poverty line, and had a lower proportion of non-Hispanic White people (all P < .001; eTable 1 in the Supplement). The age-adjusted mortality rates from all cancers combined were 179.8, 177.3, 167.6, 159.6, and 146.1 per 100 000 population for the first to the fifth ICE quintiles, respectively (P < .001 for trend) (Figure 2A; eTable 2 in the Supplement). The P value for trend was statistically significant for 12 of 13 specific cancer sites (eTable 2 and eFigure 1 in the Supplement).
Figure 1. Distribution of Residential Racial and Economic Segregation.
Segregation is measured by quintiles of the Index of Concentration at Extremes among US counties, 2015-2019.
Figure 2. Mean Age-Adjusted Mortality Rate and Adjusted Mortality Rate Ratios for All Cancers by Residential Segregation in US Counties, 2015-2019.
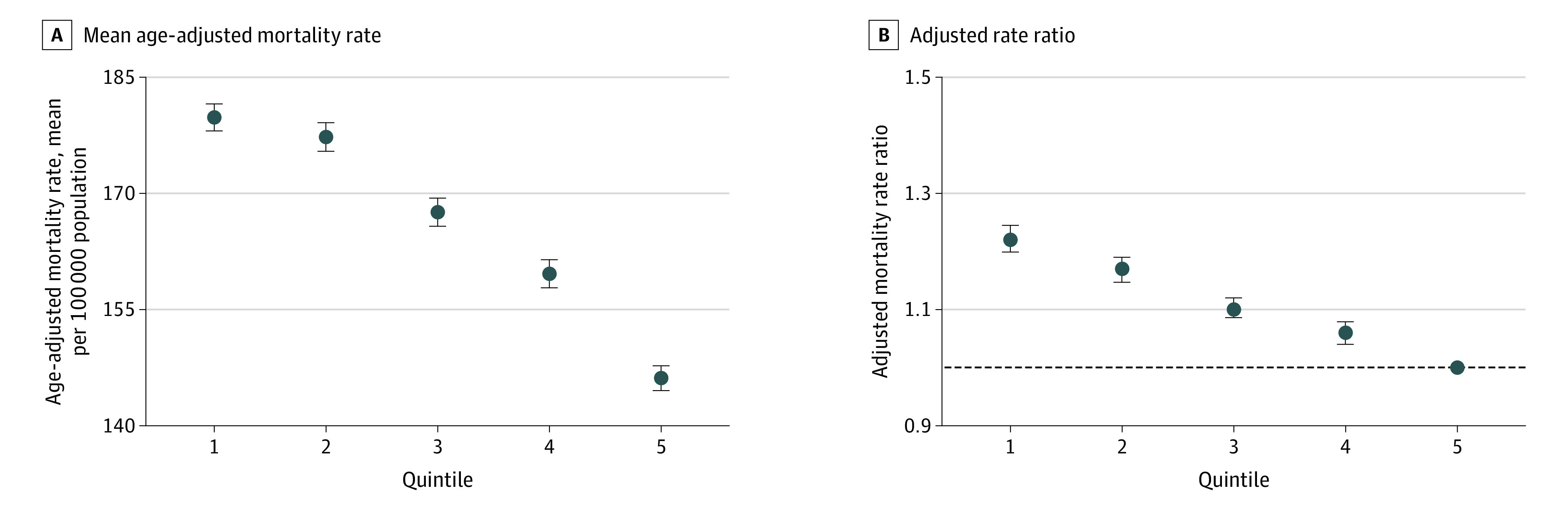
As measured by the Index of Concentration at the Extremes in US counties, 2015-2019, graphs show the mean age-adjusted mortality rate (A) and the adjusted mortality rate ratios (B), with the fifth quintile representing the most privileged counties and the first quintile representing the least privileged counties. The fifth quintile (horizontal dashed line) (B) was used as the reference group. Multivariable linear mixed models were clustered at the state level, weighted by county population size, and adjusted for metropolitan status, poverty level, and proportion of the population with non-Hispanic White alone race. Whiskers represent 95% CI.
The aRRs for all cancers combined and for the 13 cancer sites are shown in Figure 2 and eFigure 2 and eTable 3 in the Supplement. Compared with the fifth quintile (most privileged), the aRRs for all cancers combined for counties from the first to the fourth quintiles were 1.22 (95% CI, 1.20-1.24), 1.17 (95% CI, 1.15-1.19), 1.10 (95% CI, 1.09-1.12), and 1.06 (95% CI, 1.04-1.08), respectively (P < .001 for trend). Counties in the first ICE quintile had a statistically significantly higher aRR (ranging from 1.49 for lung and bronchus cancer to 1.06 for brain and other nervous system cancer) compared with counties in the fifth quintile for 12 of 13 cancer sites (except for ovarian cancer), with significant P values for trend for 12 of 13 cancer sites.
Discussion
Using contemporary national data, we found that county-level residential segregation was associated with higher mortality from all cancer sites combined and for 12 of 13 common cancer sites evaluated, ranging from 6% for brain tumors to 49% for lung cancer when comparing the most deprived counties with the most privileged counties. Furthermore, most cancer sites showed a significant dose-response association between greater segregation and higher mortality.
Our findings extend previous research for 5 cancer sites (breast, prostate, lung, colon and rectum, and ovary) from limited geographic regions. Lung cancer had the highest age-adjusted mortality rates and highest aRRs in counties with the most segregation, likely due to cumulative effects of higher incidence from exposure to risk factors such as smoking and air pollution, less early detection through screening and early evaluation of signs and symptoms, and worse survival due to limited access to quality cancer care associated with segregation. The only cancer site in our study without an association between segregation and mortality was ovarian cancer, a finding inconsistent with a previous study between census tract–level segregation and ovarian cancer survival in Florida.11 Previous research suggests that the ICE measured at smaller geographic areas such as census tracts had increased values for the association with cancer outcomes compared with the ICE measured at larger geographic areas,1,14 which may partly explain our null finding for ovarian cancer.
Strengths and Limitations
Strengths of our study include contemporary nationwide data, evaluation of all cancers combined and the most common causes of cancer death, and use of the ICE to measure residential segregation. The ICE outperforms other metrics of residential segregation because it captures the synergistic effect of both racial and economic segregation, shows the directionality from deprivation to privilege, and avoids the collinearity issue with race and income in the multivariable analyses.13 To our knowledge, our study is the first to assess the association between segregation measured by the ICE and cancer mortality comprehensively; however, it also has limitations. As mentioned, segregation at the county level may not be sensitive enough to detect the association with cancer mortality; future studies at more granular levels (eg, zip code or census tract) are warranted. Also, the ecological study design limits causal inference. The effects of life course segregation on cancer outcomes at the individual level merit further research with longitudinal data and rigorous study designs.
Conclusions
Taken together with previous evidence, the findings of this ecological study suggest that residential segregation incorporating both race and income polarization is associated with increased cancer mortality at the county level, highlighting opportunities for geographically targeted cancer prevention and control efforts. Future research needs to confirm this association using individual-level data and to explore the pathways between segregation and cancer outcomes to inform geographically targeted interventions for reducing the impact of segregation on health.
eAppendix. The Calculation of the Index of Concentration at the Extremes
eFigure 1. Average Age-Adjusted Cancer Mortality Rates by Quintiles of Residential Racial and Economic Segregation Measured by the Index of Concentration at the Extremes in U.S. Counties, 2015-2019
eFigure 2. Adjusted Cancer Mortality Rate Ratios by Quintiles of Residential Racial and Economic Segregation Measured by the Index of Concentration at the Extremes in U.S. Counties, 2015-2019
eTable 1. Characteristics of the U.S. Counties by Quintiles of Residential Racial and Economic Segregation Measured by the Index of Concentration at the Extremes, 2015-2019
eTable 2. Age-Adjusted Cancer Mortality Rate of the U.S. Counties by Quintiles of Residential Racial and Economic Segregation Measured by the Index of Concentration at the Extremes, 2015-2019
eTable 3. Adjusted Cancer Mortality Rate Ratio by Residential Racial and Economic Segregation Measured by the Index of Concentration at the Extremes in the U.S. Counties, 2015-2019
References
- 1.Landrine H, Corral I, Lee JGL, Efird JT, Hall MB, Bess JJ. Residential segregation and racial cancer disparities: a systematic review. J Racial Ethn Health Disparities. 2017;4(6):1195-1205. doi: 10.1007/s40615-016-0326-9 [DOI] [PubMed] [Google Scholar]
- 2.White K, Haas JS, Williams DR. Elucidating the role of place in health care disparities: the example of racial/ethnic residential segregation. Health Serv Res. 2012;47(3, pt 2):1278-1299. doi: 10.1111/j.1475-6773.2012.01410.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayanga AJ, Zeliadt SB, Backhus LM. Residential segregation and lung cancer mortality in the United States. JAMA Surg. 2013;148(1):37-42. doi: 10.1001/jamasurgery.2013.408 [DOI] [PubMed] [Google Scholar]
- 4.Haas JS, Earle CC, Orav JE, et al. Racial segregation and disparities in breast cancer care and mortality. Cancer. 2008;113(8):2166-2172. doi: 10.1002/cncr.23828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mobley LR, Kuo TM, Driscoll D, Clayton L, Anselin L. Heterogeneity in mammography use across the nation: separating evidence of disparities from the disproportionate effects of geography. Int J Health Geogr. 2008;7:32. doi: 10.1186/1476-072X-7-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pruitt SL, Lee SJ, Tiro JA, Xuan L, Ruiz JM, Inrig S. Residential racial segregation and mortality among black, white, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer. 2015;121(11):1845-1855. doi: 10.1002/cncr.29282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeChello LM, Gregorio DI, Samociuk H. Race-specific geography of prostate cancer incidence. Int J Health Geogr. 2006;5:59. doi: 10.1186/1476-072X-5-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell E, Kramer MR, Cooper HL, Thompson WW, Arriola KR. Residential racial composition, spatial access to care, and breast cancer mortality among women in Georgia. J Urban Health. 2011;88(6):1117-1129. doi: 10.1007/s11524-011-9612-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson AM, Johnson A, Hines RB, Bayakly R. The effects of residential segregation and neighborhood characteristics on surgery and survival in patients with early-stage non-small cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2016;25(5):750-758. doi: 10.1158/1055-9965.EPI-15-1126 [DOI] [PubMed] [Google Scholar]
- 10.Hao Y, Landrine H, Jemal A, et al. Race, neighbourhood characteristics and disparities in chemotherapy for colorectal cancer. J Epidemiol Community Health. 2011;65(3):211-217. doi: 10.1136/jech.2009.096008 [DOI] [PubMed] [Google Scholar]
- 11.Westrick AC, Bailey ZD, Schlumbrecht M, et al. Residential segregation and overall survival of women with epithelial ovarian cancer. Cancer. 2020;126(16):3698-3707. doi: 10.1002/cncr.32989 [DOI] [PubMed] [Google Scholar]
- 12.Krieger N, Kim R, Feldman J, Waterman PD. Using the Index of Concentration at the Extremes at multiple geographical levels to monitor health inequities in an era of growing spatial social polarization: Massachusetts, USA (2010-14). Int J Epidemiol. 2018;47(3):788-819. doi: 10.1093/ije/dyy004 [DOI] [PubMed] [Google Scholar]
- 13.Larrabee Sonderlund A, Charifson M, Schoenthaler A, Carson T, Williams NJ. Racialized economic segregation and health outcomes: a systematic review of studies that use the Index of Concentration at the Extremes for race, income, and their interaction. PLoS One. 2022;17(1):e0262962. doi: 10.1371/journal.pone.0262962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krieger N, Feldman JM, Kim R, Waterman PD. Cancer incidence and multilevel measures of residential economic and racial segregation for cancer registries. JNCI Cancer Spectr. 2018;2(1):pky009. doi: 10.1093/jncics/pky009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Cancer Society . Cancer Facts & Figures 2019. Accessed May 1, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. The Calculation of the Index of Concentration at the Extremes
eFigure 1. Average Age-Adjusted Cancer Mortality Rates by Quintiles of Residential Racial and Economic Segregation Measured by the Index of Concentration at the Extremes in U.S. Counties, 2015-2019
eFigure 2. Adjusted Cancer Mortality Rate Ratios by Quintiles of Residential Racial and Economic Segregation Measured by the Index of Concentration at the Extremes in U.S. Counties, 2015-2019
eTable 1. Characteristics of the U.S. Counties by Quintiles of Residential Racial and Economic Segregation Measured by the Index of Concentration at the Extremes, 2015-2019
eTable 2. Age-Adjusted Cancer Mortality Rate of the U.S. Counties by Quintiles of Residential Racial and Economic Segregation Measured by the Index of Concentration at the Extremes, 2015-2019
eTable 3. Adjusted Cancer Mortality Rate Ratio by Residential Racial and Economic Segregation Measured by the Index of Concentration at the Extremes in the U.S. Counties, 2015-2019