This cohort study used Surveillance, Epidemiology, and End Results Program data to assess trends in the incidence and mortality of larynx cancer in the US from 1986 through 2018.
Key Points
Question
Are trends in incidence and mortality of larynx cancer changing in the US?
Findings
In this cohort study of 40 850 US patients with larynx cancer from 1986 to 2018, both incidence and mortality of larynx cancer decreased, but incidence decreased more than mortality. The incidence of localized disease decreased more than the incidence of regional and distant disease.
Meaning
This study showed that an increasing number of patients with larynx cancer are presenting with more advanced disease, thus leading to an increase in the overall case-fatality rate.
Abstract
Importance
Larynx cancer is associated with considerable morbidity for patients and has a high mortality rate. Historical analyses showed that the incidence of larynx cancer was decreasing but the mortality was not similarly improving.
Objective
To assess whether incidence and mortality trends in larynx cancer in the US have improved.
Design, Setting, and Participants
This cohort study used population-based data from the Surveillance, Epidemiology, and End Results Program database for patients older than 18 years who were diagnosed with laryngeal cancer between January 1, 1986, and December 31, 2018. Data were analyzed from May 1, 2021, to May 31, 2022.
Main Outcomes and Measures
The main outcomes were incidence and mortality of larynx cancer by sex, subsite, and patterns of surgical treatment.
Results
Among 40 850 US patients with larynx cancer diagnosed from 1986 to 2018 (80.4% male), the incidence of larynx cancer decreased 55% from 5.00 per 100 000 people (95% CI, 4.70-5.32 per 100 000 people) to 2.26 per 100 000 people (95% CI, 2.11-2.42 per 100 000 people). During the same period, mortality decreased only 43% from 1.59 per 100 000 people (95% CI, 1.53-1.64 per 100 000 people) to 0.89 per 100 000 people (95% CI, 0.86-0.92 per 100 000 people). This corresponds to a 25% relative increase in case-fatality rate. Examination by stage showed a decrease in the incidence of localized disease at diagnosis of 40% from 2.65 per 100 000 people (95% CI, 2.44-2.89 per 100 000 people) to 1.60 per 100 000 people (95% CI, 1.45-1.76 per 100 000 people) from 1986 to 2002 and of 45% from 2.15 per 100 000 people (95% CI, 1.98-2.34 per 100 000 people) to 1.19 per 100 000 people (95% CI, 1.08-1.31 per 100 000 people) from 2005 to 2018. Distribution of larynx cancer by subsite remained stable, with most cases affecting the glottis. The proportion of patients receiving surgery as their first course of treatment decreased regardless of stage at presentation.
Conclusions and Relevance
In this cohort study, between 1986 and 2018, the incidence of larynx cancer decreased in the US, primarily because of the decrease in the incidence of localized disease. Mortality did not decrease similarly, resulting in an increased case-fatality rate overall. Encouraging earlier referrals for cancer concern, focusing resources where larynx cancer rates remain highest, renewing attention to research on new biologic causes of different tumor biologic characteristics, and conducting trials to directly compare treatments may help reverse this trend.
Introduction
Larynx cancer is associated with high morbidity with lifelong adverse effects due to the speech and swallowing adverse effects of treatment, but it is relatively rare; the estimated number of new cases in 2022 is 12 470 (18.76% of all head and neck cancers; 0.65% of all cancers of all sites).1 The estimated number of deaths from larynx cancer in 2022 is 3820, and thus, there is high risk for mortality.1 Efforts to preserve life, the larynx, and its function have been the focus of most research trials in this area, and in 1991, The Department of Veterans Affairs (VA) Laryngeal Cancer Study Group reported the results of a study testing induction chemotherapy followed by definitive radiation for cases responding to chemotherapy, and laryngectomy followed by adjuvant radiation therapy for cases not responding to chemotherapy.2 The study demonstrated that the recurrence-free survival was comparable between the 2 groups. This watershed study led to changes in practice patterns for advanced laryngeal cancer. Historical analyses demonstrated that although the incidence of larynx cancer was decreasing, mortality was not similarly improving.3,4 Reasons for the change in the case-fatality rate were hypothesized to include changes in trends by sex, stage at presentation, subsite, and/or patterns of treatment, but there were not enough years of data to make firm conclusions.
We sought to evaluate whether incidence and mortality trends in larynx cancer in the US improved over approximately 30 years since these shifts in treatment occurred. The aim was to characterize current patterns and make recommendations for future investigations and actions.
Methods
Data Sources
This cohort study used incidence data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program database from January 1, 1986, to December 31, 2018.5 The SEER Program has collected and provided population-based data on cancer incidence, histologic type, and initial treatment in the US since 1975. Mortality data were from the Centers for Disease Control and Prevention’s National Vital Statistics System,6 which collects data on the underlying cause of death reported on death certificates filed by each state. Patients for whom incident cancers are captured in the SEER database are followed up until death regardless of their place of residence at the time of death. The US Department of Veterans Affairs institutional review board for northern New England and the Dartmouth Hitchcock institutional review board has deemed studies using deidentified, publicly available data (SEER data) to be exempt from human participant review and to not require informed consent in accordance with the Common Rule. Permission to access and use the SEER data was obtained through a data use agreement with the SEER Program. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
To calculate overall incidence and mortality trends, we used 2 different data sets. We started with 1986, which was 5 years before the landmark VA trial publication.2 For this period (1986-1991), we used the original SEER 9 registries, which are the only SEER registries with data going back to 1986; these data are from Connecticut; Hawaii; Iowa; New Mexico; Utah; San Francisco, California; Detroit, Michigan; Seattle, Washington; and Atlanta, Georgia. To maximize the case counts available for the remainder of the analysis years (1992-2018), we used SEER 13, which in addition to the original SEER 9 sites includes Alaska, rural Georgia, and San Jose and Los Angeles, California. For these estimates, we used US mortality data, with the cause of death being larynx cancer.
For detailed analyses of incidence, mortality, and treatment patterns, we focused on the most recent data from 2005 to 2018 and used SEER 18, which in addition to the SEER 13 sites, includes greater California, Kentucky, Louisiana, New Jersey, and greater Georgia. For these estimates, we used incidence-based mortality data, with the cause of death being larynx cancer. The case-fatality rate was calculated by dividing the mortality rate by the incidence rate.
Definitions
Site and Subsites
To be included, cancers had to have their primary origin in the larynx, defined according to International Classification of Diseases for Oncology, Third Edition World Health Organization 2008 topographical codes C32.0 to C32.9. Larynx subsites were the glottis (C32.0), supraglottis (C32.1), subglottis (C32.2), and larynx not otherwise specified (NOS), which included laryngeal cartilage (C32.3), overlapping lesion of larynx (C32.8), and the specific code for larynx NOS (C32.9). Because larynx cancer is rare and cell sizes by subsite were small, we used SEER 18 to maximize the data available for the analysis of cancer by subsite. We provided data on the proportion of patients with larynx cancer by subsite from 1986 to 2016 in 5-year increments to minimize variability in the data and protect individuals from reidentification.
Stage at Diagnosis
Over the more than 40 years during which the SEER Program has been collating and reporting data on cancers in the US, staging systems have increased in sophistication and detail, and the SEER Program has created new variables to reflect these changes. Our analysis required that we combine 2 variables: SEER Historic Stage A (1973-2015) for the period from 1986 to 2003 and SEER Combined Summary Stage (2004 and after) for the period from 2004 to 2018. We used 2 approaches. When possible, we harmonized the 2 systems. When that was not possible, the differences were noted and interpreted in context in longitudinal analyses. In addition, during 2003 and 2004, because case reporting of larynx cancer stage transitioned from the old system to the new system, the data were not interpretable; thus, these 2 years of data were omitted from figures showing trends by stage over time. Details are given in the eMethods in the Supplement.
Treatment With Surgery
To characterize overall patterns of surgical treatment by stage, we used SEER 9 for the period from 1986 to 1991 and SEER 13 for the period from 1992 to 2016, examining 10-year comparisons. The SEER Program has 2 variables for this period: Site-Specific Surgery (1973-1997, with varying detail by year and site) for the period from 1986 to 1997 and RX Summ–Surg Prim Site (1998 and after) for the period from 1998 to 2016. Categories of treatment type were collapsed into a binary variable of no cancer-directed surgery of the primary site or cancer-directed surgery, which included local tumor destruction, local tumor excision, partial laryngectomy, total laryngectomy, and surgery NOS.
Statistical Analysis
Data were analyzed from May 1, 2021, to May 31, 2022. All calculations were estimated using age-adjusted rates per 100 000 people standardized to the US population in 2000. For each year’s estimate, 95% CIs were calculated by SEER Stat using the Tiwari method.7 Joinpoint regression fits trend data, such as cancer rates, to the simplest method (fewest number of infection points) that the data allow. Joinpoint regression is the approach used in National Cancer Institute publications reporting cancer trends. Joinpoint regression tests evaluate changes in rates over time (annual percent change [APC]), identify the number of significant joinpoints that most accurately describe the data, and allow for tests of statistical significance to be performed.8 The P value of each permutation test was estimated using Monte Carlo methods, and the overall asymptotic significance level was maintained through a Bonferroni adjustment. The APC was determined and deemed statistically significant if it was different from 0 at α = .05. Trends that are reported as increasing or decreasing refer to statistically significant increasing or decreasing trends estimated from the Joinpoint model. Non–statistically significant trends are referred to as stable.
Results
Case Distribution by Sex and Stage
The study included 40 850 US patients with larynx cancer diagnosed from 1986 to 2018. During this period, 80.4% of incident larynx cancers were diagnosed in men and 19.6% in women (Table). Most newly diagnosed larynx cancers (53.4%) were staged as localized, with proportions of 30.0%, 11.8%, and 4.7% for regional, distant, and unknown or unspecified stages, respectively.
Table. Distribution of Larynx Cancer Cases by Sex and Stage From 1986 to 2018a.
| Year | Cases, No. (%) | Total cases, No. | |||||
|---|---|---|---|---|---|---|---|
| Sex | Stage | ||||||
| Male | Female | Localized | Regional | Distant | Unknown or unspecified | ||
| 1986 | 855 (82.1) | 187 (17.9) | 557 (53.5) | 357 (34.3) | 56 (5.4) | 72 (6.9) | 1042 |
| 1987 | 868 (79.4) | 225 (20.6) | 532 (48.7) | 425 (38.9) | 62 (5.7) | 74 (6.8) | 1093 |
| 1988 | 889 (80.7) | 213 (19.3) | 586 (53.2) | 387 (35.1) | 59 (5.4) | 70 (6.4) | 1102 |
| 1989 | 842 (78.3) | 233 (21.7) | 579 (53.9) | 361 (33.6) | 72 (6.7) | 63 (5.9) | 1075 |
| 1990 | 880 (79.6) | 226 (20.4) | 589 (53.3) | 378 (34.2) | 76 (6.9) | 63 (5.7) | 1106 |
| 1991 | 824 (79.2) | 216 (20.8) | 557 (53.6) | 346 (33.3) | 66 (6.3) | 71 (6.8) | 1040 |
| 1992 | 1196 (81.3) | 275 (18.7) | 774 (52.6) | 489 (33.2) | 105 (7.1) | 103 (7.0) | 1471 |
| 1993 | 1090 (81.6) | 245 (18.4) | 717 (53.7) | 449 (33.6) | 79 (5.9) | 90 (6.7) | 1335 |
| 1994 | 1121 (78.3) | 311 (21.7) | 765 (53.4) | 501 (35.0) | 88 (6.1) | 78 (5.4) | 1432 |
| 1995 | 1130 (80.3) | 278 (19.7) | 779 (55.3) | 457 (32.5) | 104 (7.4) | 68 (4.8) | 1408 |
| 1996 | 1075 (78.5) | 295 (21.5) | 742 (54.2) | 473 (34.5) | 91 (6.6) | 64 (4.7) | 1370 |
| 1997 | 1077 (79.5) | 278 (20.5) | 717 (52.9) | 476 (35.1) | 78 (5.8) | 84 (6.2) | 1355 |
| 1998 | 1019 (78.4) | 281 (21.6) | 631 (48.5) | 526 (40.5) | 84 (6.5) | 59 (4.5) | 1300 |
| 1999 | 1060 (77.1) | 314 (22.9) | 678 (49.3) | 550 (40.0) | 105 (7.6) | 41 (3.0) | 1374 |
| 2000 | 1051 (79.9) | 264 (20.1) | 592 (45.0) | 573 (43.6) | 92 (7.0) | 58 (4.4) | 1315 |
| 2001 | 1014 (80.5) | 245 (19.5) | 622 (49.4) | 513 (40.7) | 87 (6.9) | 37 (2.9) | 1259 |
| 2002 | 1007 (81.0) | 236 (19.0) | 553 (44.5) | 569 (45.8) | 83 (6.7) | 38 (3.1) | 1243 |
| 2003 | 961 (80.9) | 227 (19.1) | 559 (47.1) | 510 (42.9) | 68 (5.7) | 51 (4.3) | 1188 |
| 2004 | 970 (78.8) | 261 (21.2) | 757 (61.5) | 232 (18.8) | 208 (16.9) | 34 (2.8) | 1231 |
| 2005 | 998 (80.4) | 243 (19.6) | 740 (59.6) | 254 (20.5) | 206 (16.6) | 41 (3.3) | 1241 |
| 2006 | 1012 (81.4) | 232 (18.6) | 733 (58.9) | 242 (19.5) | 225 (18.1) | 44 (3.5) | 1244 |
| 2007 | 1031 (81.1) | 240 (18.9) | 722 (56.8) | 255 (20.1) | 252 (19.8) | 42 (3.3) | 1271 |
| 2008 | 990 (80.6) | 238 (19.4) | 708 (57.7) | 245 (20.0) | 227 (18.5) | 48 (3.9) | 1228 |
| 2009 | 994 (82.8) | 207 (17.2) | 704 (58.6) | 241 (20.1) | 205 (17.1) | 51 (4.2) | 1201 |
| 2010 | 1063 (81.5) | 242 (18.5) | 740 (56.7) | 278 (21.3) | 235 (18.0) | 52 (4.0) | 1305 |
| 2011 | 1002 (81.4) | 229 (18.6) | 677 (55.0) | 263 (21.4) | 242 (19.7) | 49 (4.0) | 1231 |
| 2012 | 1045 (82.0) | 230 (18.0) | 725 (56.9) | 259 (20.3) | 249 (19.5) | 42 (3.3) | 1275 |
| 2013 | 1002 (82.3) | 216 (17.7) | 671 (55.1) | 257 (21.1) | 243 (20.0) | 47 (3.9) | 1218 |
| 2014 | 997 (81.1) | 233 (18.9) | 682 (55.4) | 257 (20.9) | 234 (19.0) | 57 (4.6) | 1230 |
| 2015 | 962 (81.3) | 222 (18.8) | 632 (53.4) | 260 (22.0) | 230 (19.4) | 62 (5.2) | 1184 |
| 2016 | 984 (82.1) | 215 (17.9) | 634 (52.9) | 296 (24.7) | 217 (18.1) | 52 (4.3) | 1199 |
| 2017 | 954 (80.1) | 237 (19.9) | 602 (50.5) | 313 (26.3) | 207 (17.4) | 69 (5.8) | 1191 |
| 2018 | 889 (81.3) | 204 (18.7) | 568 (52.0) | 283 (25.9) | 178 (16.3) | 64 (5.9) | 1093 |
Data used to estimate incidence and mortality trends. Data are from the Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat database, released April 2021, based on the November 2020 submission. For the period from 1986 to 1991, data are from SEER 9 research data; for the period from 1992 to 2018, data are from SEER 13 research data.5
Incidence and Mortality Trends From 1986 to 2018
Overall
Both the incidence and the mortality of larynx cancer decreased between 1986 and 2018 (Figure 1). The incidence of larynx cancer decreased 55% from 5.00 per 100 000 people (95% CI, 4.70-5.32 per 100 000 people) to 2.26 per 100 000 people (95% CI, 2.11-2.42 per 100 000 people). The APC was −2.4% (95% CI, −2.5% to −2.3%). However, mortality decreased only 43% from 1.59 per 100 000 people (95% CI, 1.53-1.64 per 100 000 people) to 0.89 per 100 000 people (95% CI, 0.86-0.92 per 100 000 people). The APC was stable at 0.07% (95% CI, −0.6% to 0.8%) between 1986 and 1993 and then decreased to −2.2% (95% CI, −2.3% to −2.2%) between 1993 and 2018. The earliest 5-year mean case-fatality rate between 1986 and 1990 was 31% (range, 31%-33%), and the most recent mean case-fatality rate between 2014 and 2018 was 37% (range, 36%-40%). Because incidence did not decrease to the same degree as mortality, there was a 25% relative increase in case-fatality rate.
Figure 1. Incidence and Mortality of Larynx Cancer in the US From 1986 to 2018.
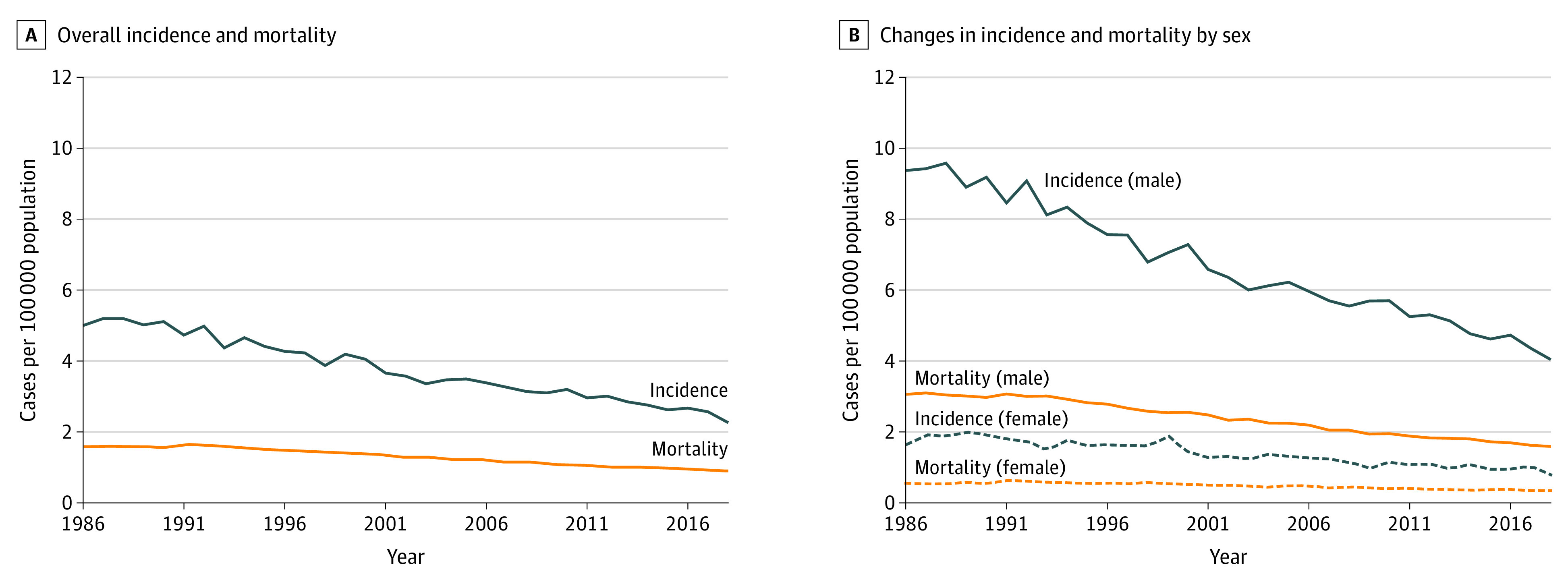
Incidence and mortality data are from the Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat database, released April 2021, based on the November 2020 submission from SEER 9 research data.5 Underlying mortality data were provided by the National Center for Health Statistics.6
Changes in Incidence by Sex
Between 1986 and 2018, the incidence of larynx cancer decreased among both men and women, but the decrease was greater among men than among women. Among men, new cases decreased 57% from 9.37 per 100 000 people (95% CI, 8.73-10.04 per 100 000 people) to 4.04 per 100 000 people (95% CI, 3.74-4.36 per 100 000 people). The APC was −2.5% (95% CI, −2.6% to −2.4%). Among women, new cases decreased 52% from 1.63 per 100 000 people (95% CI, 1.40-1.89 per 100 000 people) to 0.78 per 100 000 people (95% CI, 0.66-0.92 per 100 000 people). The APC was −2.4% (95% CI, −2.8% to −2.1%).
Changes in Mortality by Sex
Mortality due to larynx cancer also decreased among both men and women, with greater decreases among men than among women. Among men, mortality decreased 48% from 3.06 per 100 000 people (95% CI, 2.94-3.17 per 100 000 people) to 1.59 per 100 000 people (95% CI, 1.53-1.65 per 100 000 people). The APC was stable at −0.4% (95% CI, −1.0% to 0.2%) between 1986 and 1993 and then decreased to −2.5% (95% CI, −2.6% to −2.4%) between 1993 and 2018. This corresponds to a 21% increase in case-fatality rate among men. Among women, mortality decreased only 38% from 0.55 per 100 000 people (95% CI, 0.50-0.59 per 100 000 people) to 0.34 per 100 000 people (95% CI, 0.31-0.36 per 100 000 people). The APC was 2.3% (95% CI, 0.2%-4.5%) between 1986 and 1992 and then −2.2% (95% CI, −2.4% to −2.0%) between 1992 and 2018. This corresponds to a 29% increase in case-fatality rate among women.
Incidence Trends by Stage From 1986 to 2018
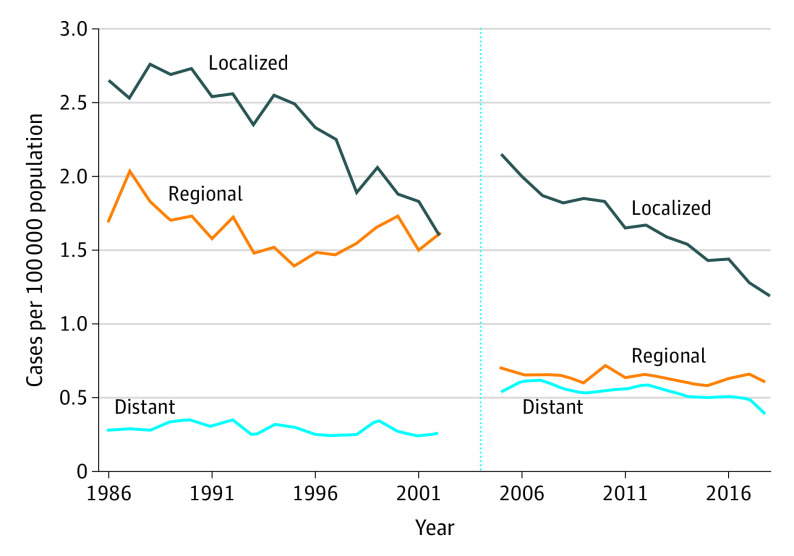
Figure 2 shows the main contribution to the overall decrease in the incidence of larynx cancer was from the decrease in incidence of localized disease at diagnosis. There were also shifts in incidence by stage attributable to changes in the staging system over time. Many cases previously staged as regional were subsequently staged as local, and some staged as regional shifted to being staged as distant. During 2003 and 2004, as case reporting of stage transitioned from the old system to the new system, the data were not interpretable; thus, these 2 years of data were omitted from Figure 2.
Figure 2. Incidence of Larynx Cancer by Stage From 1986 to 2018.
Data are from the Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat database, released April 2021, based on the November 2020 submission from SEER 9 research data.5 Data from 2003 and 2004 were omitted because of instability of estimates during the transition from the old to new staging system. The vertical dashed line demarcates the omitted data during the transition from one staging system to the next. The period to the left of the line is staging system Historic Stage A, and the period to the right of the line is Combined Summary Stage.
Incidence by Stage From 1986 to 2002
From 1986 to 2002, the incidence of larynx cancer decreased 40% for localized disease from 2.65 per 100 000 people (95% CI, 2.44-2.89 per 100 000 people) to 1.60 per 100 000 people (95% CI, 1.45-1.76 per 100 000 people); the APC was stable at −1.0% (95% CI, −2.3% to 0.3%) between 1986 and 1995 and then decreased significantly to −5.5% (95% CI, −7.4% to −3.5%) between 1995 and 2002. During the same period, the incidence of larynx cancer decreased 5% for regional disease from 1.70 per 100 000 people (95% CI, 1.53-1.89 per 100 000 people) to 1.61 per 100 000 people (95% CI, 1.46-1.77 per 100 000 people); the APC was −2.9% (95% CI, −4.7% to −1.0%) between 1986 and 1995 and stable at 1.8% (95% CI, −0.9% to 4.6%) between 1995 and 2002. The incidence decreased 7% for distant disease from 0.28 per 100 000 people (95% CI, 0.21-0.36 per 100 000 people) to 0.26 per 100 000 people (95% CI, 0.20-0.33 per 100 000 people). The APC was −1.2% (95% CI, −2.6% to 0.2%).
Incidence by Stage From 2005 to 2018
From 2005 to 2018, the incidence of larynx cancer decreased 45% for localized disease from 2.15 per 100 000 people (95% CI, 1.982.34 per 100 000 people) to 1.19 per 100 000 people (95% CI, 1.08-1.31 per 100 000 people); the APC decreased significantly to −3.4% (95% CI, −4.0% to −2.8%) between 2005 and 2016 and was −8.9% (95% CI, −18.1 to 1.2) between 2016 and 2018. During the same period, the Incidence decreased 16% for regional disease from 0.70 per 100 000 people (95% CI, 0.61-0.81 per 100 000 people) to 0.59 per 100 000 people (95% CI, 0.51-0.67 per 100 000 people) and the APC was −0.7% (95% CI, −1.5% to 0.1%). The incidence decreased 33% for distant disease from 0.54 per 100 000 people (95% CI, 0.46-0.64 per 100 000 people) to 0.36 per 100 000 people (95% CI, 0.31-0.43 per 100 000 people); the APC was stable at −1.0% (95% CI, −2.2% to 0.3%) between 2005 and 2016 and was −15.2% (95% CI, −31.0% to 4.3%) between 2016 and 2018. Incidence and mortality trends by sex and stage are shown in eFigure 1 in the Supplement.
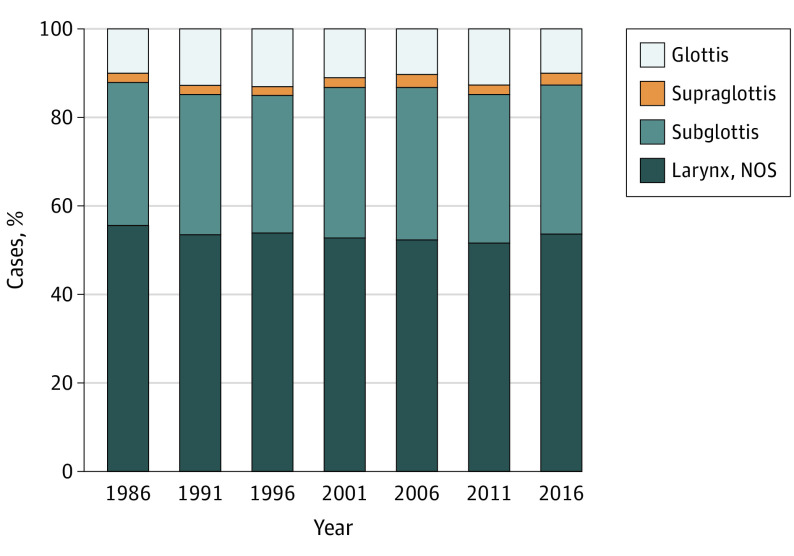
Incidence and Mortality Trends by Subsite
The distribution of larynx cancer by subsite remained relatively stable (Figure 3). Most cases affected the glottis, followed by supraglottis. The subglottis was the least common subsite. For all years from 1986 to 2016, 53.3% of cases affected the glottis; 33.0%, in the supraglottis; 1.5%, subglottis; and 12.1%, larynx NOS (includes overlapping lesion of the larynx and laryngeal cartilage). Details of trends over time are shown in eFigure 2 in the Supplement.
Figure 3. Proportion of Larynx Cancer Cases by Subsite From 1986 to 2016.
Data are from the Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat database, released April 2019, based on the November 2018 submission from SEER 18 research data.5 NOS indicates not otherwise specified (includes overlapping lesion of the larynx and laryngeal cartilage).
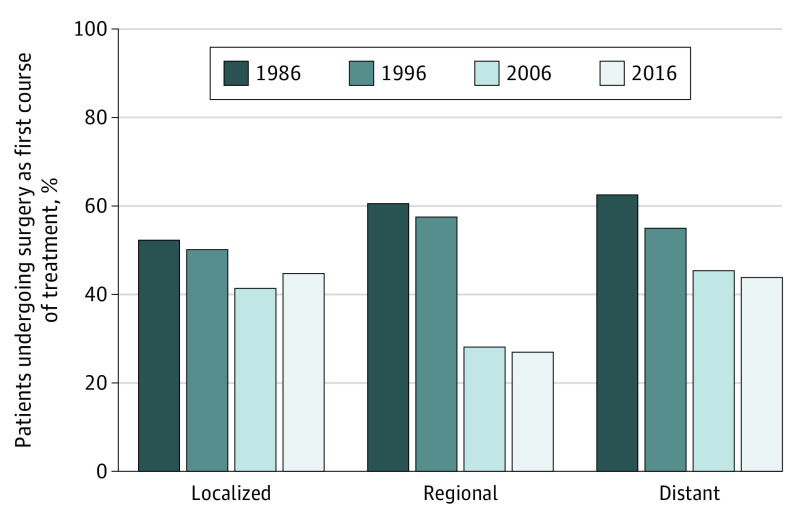
Surgical Treatment Trends by Stage
Between 1986 and 2016, the proportion of patients receiving surgery as their first course of treatment decreased regardless of stage at presentation (Figure 4). For localized disease, this proportion decreased 14% from 52.2% to 44.7%; for regional disease, it decreased 56% from 60.5% to 26.9%; and for distant disease, it decreased 30% from 62.5% to 43.7%.
Figure 4. Proportion of Patients With Larynx Cancer Treated With Surgery by Stage.
Data are from the Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat database, released April 2019, based on the November 2018 submission from SEER 18 research data.5 Data from 1986 and 1996 are from Historic Stage A, and data from 2006 and 2016 are from the Combined Summary Stage.
Surgical procedure data showed that the rates of total laryngectomy for any stage of cancer decreased 53% from 24.1% to 11.8% between 1988 to 2016. From 1988 to 1997, this rate decreased annually by 6.0% (95% CI, 4.2%-7.0%); between 1998 and 2008, the decrease accelerated slightly to 6.8% annually (95% CI, 4.8%-8.8%); and from 2008 to 2016, rates of laryngectomy remained stable, with an annual change of −1.3% (95% CI, −4.5% to 1.9%).
Discussion
This study showed that the incidence of larynx cancer decreased 55% between 1986 and 2018, but mortality only decreased 43% during the same period. Therefore, a patient diagnosed with larynx cancer in later years was more likely to die than they were in 1986, the case-fatality rate for larynx cancer increased 25%. The increase in case-fatality rate was associated with a decrease in people presenting with localized disease at the time of diagnosis, while the incidence of patients presenting with regional and distant disease remained stable by comparison. Since mortality among patients with localized disease was low, with more patients presenting with regional and distant disease, the overall mortality rate did not decrease at the same rate as the incidence decreased. Men were affected most by these trends because 81% of larynx cancers cases were diagnosed in men. The distribution of larynx cancer by subsite remained stable between 1986 and 2018, with the incidence rates of larynx cancer of all subtypes decreasing approximately equally. Therefore, changes in subsite distribution did not appear to have contributed to the increase in the case-fatality rate. Patients with supraglottis subsite had the highest incidence-based mortality compared with those who had other subtypes, with a similar proportion diagnosed in 2016 and 1986.
Our updated analysis showed that the trends observed in historical analyses of both SEER and National Cancer Database data continued until 2018 and were consistent with results from a recent analysis of the National Cancer Database.3,4,9 More recent analyses, focused on incidence patterns, have shown greater decreases in incidence in regions with high socioeconomic status compared with regions with low socioeconomic status and in urban regions compared with rural regions.10,11,12
Our analysis demonstrated that regardless of the stage at presentation, the proportion of patients undergoing surgery as their first course of treatment continued to decrease over time, as to our knowledge, first observed by Chen et al13 from 1985 to 2007. Some researchers have proposed that the move toward nonsurgical treatment may be adversely affecting outcomes.12,14 Our analysis of mortality by stage was limited by the small numbers available for analysis but suggests that mortality by stage has not been increasing, which would tend to favor no association of treatment changes with mortality. A change in survival time may be associated with treatment changes, but survival analyses were not the focus of our study. Survival times are associated with stage at diagnosis and can be misleading because people can appear to survive longer if they are diagnosed earlier or the cancer is upstaged as cancer imaging becomes more sensitive without any actual improvements in outcomes.13 Survival estimates are ideal for comparing cancer therapies but not progress in population health efforts, which was the aim of this analysis.14 We observed that rates of total laryngectomy decreased significantly, and previous studies also showed this trend in association with increased use of chemoradiation.13 One survival analysis using SEER data in combination with Medicare data to follow a cohort with T3 glottic laryngeal cancer over time suggested an association between surgical treatment and longer survival for patients receiving surgery as their first course of treatment.15 We did not do a detailed analysis of outcomes by treatment received as performed in the aforementioned study because our analysis used only SEER data, while the other studies used SEER data in combination with Medicare data (to more reliably obtain chemotherapy and radiation treatment data). The SEER Program only has reliable data about surgery as the first course of treatment; chemotherapy and radiation data are not complete.16 Furthermore, analyses of observational data by treatment received can be subject to confounding by indication; patients may have received one treatment instead of another for reasons not observable in the data, and this may also impact outcomes. For these reasons, the data use agreement for SEER treatment data states that analyses of SEER treatment data alone would not be supported when there is a comparison of outcomes by treatment received.
Implications
Our findings suggest that patients with larynx cancer are presenting more often with advanced disease than in the past. We hypothesize that possible reasons for more advanced presentation include differences in access to health care as demonstrated by Orosco et al,17 delays due to longer proton pump inhibitor trials for dysphonia, or changes in practice patterns of initial workup and referral orders by primary care physicians. With the decreasing incidence of larynx cancer, it is possible that the differential diagnosis practices in general medicine have shifted to underestimate the likelihood of larynx cancer in patients presenting with voice or swallow change. In a study of the presentation of head and neck cancers at primary health centers and local private practices, it was demonstrated that 20% of patients diagnosed with head and neck cancer after referral from a primary care physician were initially overlooked, with no specialty referral order placed or scheduled follow-up appointment made at the time of their initial visit.18 Larynx cancer in particular was associated with an increased risk of tumor-related death if it was overlooked at initial presentation.18 Early larynx cancer also lacks specific, visible, and palpable signs other than hoarseness, which may make it difficult for primary care clinicians to select patients for otolaryngology referrals.19 The American Academy of Family Physicians guidelines on dysphonia recommend laryngoscopy in patients with hoarseness for greater than 2 weeks with risk factors for or symptoms of dysplasia or carcinoma (ie, smoking, heavy alcohol use, long-standing gastroesophageal reflux disease, dysphagia, and hemoptysis) and that antibiotics, oral corticosteroids, and proton pump inhibitors should not be used for empirical treatment of hoarseness in the absence of a clear indication.20 Similarly, American Academy of Otolaryngology–Head and Neck Surgery clinical practice guidelines recommend that laryngoscopy be done if dysphonia fails to resolve or improve within 4 weeks or irrespective of duration if a serious underlying cause is suspected.21 Emphasizing laryngoscopy and avoidance of long courses of treatment with medication prior to referral without periodic re-evaluation may help encourage earlier presentation of larynx cancer and subsequent treatment at earlier stages. The findings demonstrating that rates of larynx cancer were relatively higher in rural regions and regions with lower socioeconomic status suggest that resources should be focused in these areas.
We found that fewer patients received surgery as a first course of treatment later in the study period than earlier in the period, but because the largest pattern change was the decrease in incidence of localized disease, stage at diagnosis was likely the largest contributor to the change in the case-fatality rate of larynx cancer. Nevertheless, there are likely to be concerns that treatment patterns are affecting case-fatality rates. In the future, to test hypotheses about how treatment approach affects survival and mortality, it may be helpful to update analyses of categories of cause-specific mortality among patients with larynx cancer compared with other cancers or causes of death among those receiving different larynx cancer treatments to test hypotheses regarding decreased survival due to, for example, lung disease–attributable chronic aspiration. Historical data demonstrated increases in non–cancer-related deaths (ie, pneumonia) among patients treated with chemoradiation, suggesting that even with laryngeal preservation, outcomes may be impacted by late toxic effects.20 This finding is in contrast to lower odds of late pneumonia among patients treated with initial surgery and postoperative radiation.22
Limitations
This study has limitations. The data used for the study are considered to be the best source of US population-based data, but for analyses covering long periods such as ours, the data set is a 10% to 28% representative sample of the US and estimates might vary slightly from the actual rates. When possible, we restricted analyses to more recent periods and used SEER 18, which captures 28% of the US population. Analyses by stage over time also present challenges because staging systems change over time and imaging has become more sensitive, which can lead to upstaging. To mitigate this, we used broad staging groups (localized, regional, and distant), which allowed good quality inferences about the trends in stage at diagnosis while minimizing the inaccuracies that can be introduced due to increased sensitivity of imaging over time. We also carefully examined changes in the staging systems and explained these changes to facilitate interpretation. In addition, SEER data have been shown to have a high rate of accuracy for the capture of surgery as the first course of treatment, but they are less complete for radiation and chemotherapy, making analyses by treatment received invalid. Therefore, we restricted our analyses to changes in rates of surgery over time.
Conclusions
This population-based cohort study found that between 1986 and 2018, the incidence of larynx cancer decreased in the US, and this was associated with a decrease in the number of people presenting with localized disease at the time of diagnosis. Mortality did not decrease similarly, resulting in an increased case-fatality rate overall. Encouraging earlier referrals for cancer concern, focusing resources in locations where larynx cancer rates remain high, renewing attention to research looking for potential new biologic causes resulting in different tumor biologic characteristics, and conducting trials to directly compare treatments are suggested to help reverse this trend.
eMethods.
eFigure 1. Incidence & Incidence-Based Mortality of Larynx Cancer by Stage & Sex 2005–2018
eFigure 2. Incidence & Incidence-Based Mortality of Larynx Cancer by Subsite 1986–2018
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Wolf GT, Fisher SG, Hong WK, et al. ; Department of Veterans Affairs Laryngeal Cancer Study Group . Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324(24):1685-1690. doi: 10.1056/NEJM199106133242402 [DOI] [PubMed] [Google Scholar]
- 3.Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 Pt 2)(suppl 111):1-13. doi: 10.1097/01.mlg.0000236095.97947.26 [DOI] [PubMed] [Google Scholar]
- 4.Davies L, Welch HG. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135(3):451-457. doi: 10.1016/j.otohns.2006.01.029 [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Institute . Surveillance, Epidemiology, and End Results Program. Accessed September 15, 2022. http://www.seer.cancer.gov
- 6.Centers for Disease Control and Prevention . National Center for Health Statistics. Accessed September 15, 2022. https://www.cdc.gov/nchs
- 7.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547-569. doi: 10.1177/0962280206070621 [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335-351. doi: [DOI] [PubMed] [Google Scholar]
- 9.Li MM, Zhao S, Eskander A, et al. Stage migration and survival trends in laryngeal cancer. Ann Surg Oncol. 2021;28(12):7300-7309. doi: 10.1245/s10434-021-10318-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang JA, Lango MN. Diverging incidence trends for larynx and tonsil cancer in low socioeconomic regions of the US. Oral Oncol. 2019;91:65-68. doi: 10.1016/j.oraloncology.2019.02.024 [DOI] [PubMed] [Google Scholar]
- 11.Zuniga SA, Lango MN. Effect of rural and urban geography on larynx cancer incidence and survival. Laryngoscope. 2018;128(8):1874-1880. doi: 10.1002/lary.27042 [DOI] [PubMed] [Google Scholar]
- 12.Pagedar NA, Kahl AR, Tasche KK, et al. Incidence trends for upper aerodigestive tract cancers in rural United States counties. Head Neck. 2019;41(8):2619-2624. doi: 10.1002/hed.25736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen AY, Fedewa S, Zhu J. Temporal trends in the treatment of early- and advanced-stage laryngeal cancer in the United States, 1985-2007. Arch Otolaryngol Head Neck Surg. 2011;137(10):1017-1024. doi: 10.1001/archoto.2011.171 [DOI] [PubMed] [Google Scholar]
- 14.Welch HG, Schwartz LM, Woloshin S. Are increasing 5-year survival rates evidence of success against cancer? JAMA. 2000;283(22):2975-2978. doi: 10.1001/jama.283.22.2975 [DOI] [PubMed] [Google Scholar]
- 15.Al-Gilani M, Skillington SA, Kallogjeri D, Haughey B, Piccirillo JF. Surgical vs nonsurgical treatment modalities for T3 glottic squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142(10):940-946. doi: 10.1001/jamaoto.2016.1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with Medicare Claims. Published 2014. Accessed September 10, 2022. http://www.lww-medicalcare.com [DOI] [PMC free article] [PubMed]
- 17.Voora RS, Kotha NV, Kumar A, et al. Association of race and health care system with disease stage and survival in veterans with larynx cancer. Cancer. 2021;127(15):2705-2713. doi: 10.1002/cncr.33557 [DOI] [PubMed] [Google Scholar]
- 18.Alho OP, Teppo H, Mäntyselkä P, Kantola S. Head and neck cancer in primary care: presenting symptoms and the effect of delayed diagnosis of cancer cases. CMAJ. 2006;174(6):779-784. doi: 10.1503/cmaj.050623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shephard EA, Parkinson MAL, Hamilton WT. Recognising laryngeal cancer in primary care: a large case-control study using electronic records. Br J Gen Pract. 2019;69(679):e127-e133. doi: 10.3399/bjgp19X700997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.House SA, Fisher EL. Hoarseness in adults. Am Fam Physician. 2017;96(11):720-728. [PubMed] [Google Scholar]
- 21.Stachler RJ, Francis DO, Schwartz SR, et al. Clinical practice guideline: hoarseness (dysphonia) (update). otolaryngology–head and neck surgery (United States). 2018;158(1_suppl):S1-S42. [DOI] [PubMed]
- 22.Gourin CG, Starmer HM, Herbert RJ, et al. Short- and long-term outcomes of laryngeal cancer care in the elderly. Laryngoscope. 2015;125(4):924-933. doi: 10.1002/lary.25012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Incidence & Incidence-Based Mortality of Larynx Cancer by Stage & Sex 2005–2018
eFigure 2. Incidence & Incidence-Based Mortality of Larynx Cancer by Subsite 1986–2018