Abstract
Objectives:
Personal protective equipment (PPE) is worn by health care providers (HCPs) to protect against hazardous exposures. Studies of HCPs performing critical resuscitation tasks in PPE have yielded mixed results and have not evaluated performance in care of children. We evaluated the impacts of PPE on timeliness or success of emergency procedures performed by pediatric HCPs.
Methods:
This prospective study was conducted at two tertiary children’s hospitals. For session 1, HCPs (MDs or RNs) wore normal attire; for session 2, they wore full-shroud PPE garb with 2 glove types: Ebola-level or chemical. During each session, they performed clinical tasks on a patient simulator: intubation, bag-valve mask ventilation, IV placement, push-pull fluid bolus, and defibrillation. Differences in completion time per task were compared.
Results:
There were no significant differences in MD completion time across sessions. For RNs, there was a significant difference between baseline and PPE sessions for both defibrillation and IV placement tasks. RNs were faster to defibrillate in Ebola PPE and slower when wearing chemical PPE (median difference = −3.5 vs. 2 seconds, respectively; p<0.01). RN IV placement took longer in Ebola and chemical PPE (5.5 vs. 42 seconds, respectively; p<0.01). After the PPE session, participants were significantly less likely to indicate that full-body PPE interfered with procedures, was claustrophobic, or slowed them down.
Conclusion:
PPE did not affect procedure timeliness or success on a simulated child, with the exception of IV placement. Further study is needed to investigate PPE’s impact on procedures performed in a clinical care context.
Keywords: personal protective equipment, procedural performance, resuscitation
INTRODUCTION
Personal protective equipment (PPE) is a term used to refer to barrier clothing, gloves, and/or headgear designed to protect an individual from a harmful exposure. Within the health care context, these exposures are typically from infectious diseases (e.g., Ebolavirus) or toxic materials (e.g., corrosives or nerve agents). The spectrum of PPE ranges from non-sterile gloves and paper facemasks to elaborate, full-body suits. The more complex PPE options are designed to provide a high level of protection but are bulky, limit tactile and auditory feedback, and restrict mobility. Even small effects on performance resulting from wearing PPE could have clinically relevant consequences for both individual and collective patient care.
While it seems intuitive that performing medical tasks that require psychomotor skill would be more difficult when wearing PPE, there are few published studies examining this. Existing literature has focused almost exclusively on simulated adult patients and on fundamental resuscitative tasks such as tracheal intubation and cardiopulmonary resuscitation,1–4 and we are unaware of studies examining the influence of PPE on procedural performance in pediatric patients. Children present additional challenges to procedural performance. For example, smaller body size, particularly smaller face and jaw size, limits the ability to create a seal for bag-mask ventilation; a smaller oral cavity impacts direct laryngoscopy; and smaller vessel size impacts the ability to place a venous catheter (IV).
Prompted by the 1995 Tokyo chemical weapon attack on a subway and the more recent 2015 Ebolaviral disease outbreak in West Africa, hospitals have focused on health care provider (HCP) preparedness for use of PPE during clinical care. Guidance on PPE practices have been provided by the US Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO).5,6 However, there are still significant knowledge gaps about how specific elements of care delivery may be impacted when providers must wear PPE while delivering patient care.
This prospective study examined the tradeoff between safety and procedural efficacy for HCPs (medical doctors [MDs] and registered nurses [RNs]) in key emergency procedures performed in children at two tertiary care centers. We hypothesized that PPE (the type used for Ebola patient care) would result in longer times to completion and lower rates of success for resuscitative tasks in simulated patients than normal attire for HCPs.
MATERIALS AND METHODS
Study Setting and Population
This study of hospital-based pediatric HCPs was approved by the local institutional review board at both tertiary care centers. There were 68 eligible HCP participants from the intensive care and emergency medicine and transport team provider pool. To be eligible, the participants (a) were able to perform the tasks to be studied as part of their scope of practice job responsibilities, (b) had received their institution’s PPE training, (c) were in their role for at least 1 year (as a surrogate marker of sufficient procedural competency), and (d) had no contraindication to wearing PPE.
Study Design and Protocol
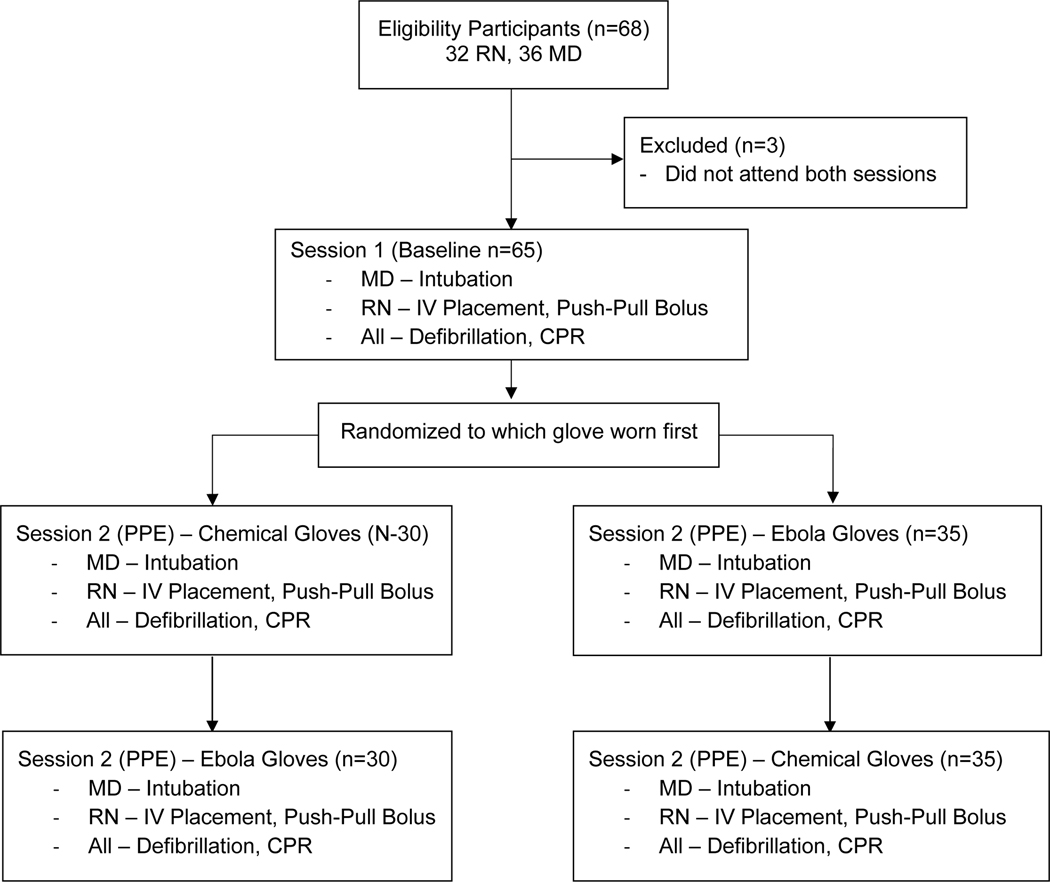
In this pre-post, single-arm study, participants served as their own controls. We chose this approach to limit the impact of interpersonal differences in performance speed or skill. Each participant attended two simulation laboratory sessions separated by a minimum of two weeks. During both sessions, each participant performed the same set of tasks in the same sequence. During session 1, participants wore normal attire (e.g., street clothes). During session 2, participants wore institutionally approved, full-body PPE including a shroud and a powered air-purifying respirator—consistent with recommendations for care of Ebola patients (hereafter referred to as Ebola-level).6 In addition, during the PPE session (session #2), participants performed all tasks twice to evaluate the difference between two PPE types: (a) PPE with nitrile (general hospital, non-sterile) gloves and (b) PPE with 12-mil chemical hazard class gloves (hereafter referred to as “chemical”). The glove order was randomized. The study flow is detailed in Figure 1. Session order was not randomized as we wanted participants to demonstrate performance with PPE exposure first. All study sessions were conducted using a 5-year-old child simulation manikin (Laerdal Medical, Wappingers Falls, NY).
Figure 1.
Study Flow Diagram
Tasks
The specific pediatric-oriented clinical tasks varied by provider type (MD and RN) and were chosen for inclusion based on their clinical importance and their ability to be assessed in a simulated setting. In all cases, timing was measured using a stopwatch in real time. Success of each task (Y/N) was based on predetermined criteria. Task process and outcome metrics are listed below.
Bag-valve mask ventilation (BVM) – All participants were asked to perform BVM using a 0.5-L self-inflating bag and a preselected, appropriately sized mask. Participants had to assemble the self-inflating mask, attach the tubing to the wall oxygen regulator, and deliver three ventilations. Time to completion was defined as when the third ventilation (chest returned to baseline) was completed. Success was defined as three breaths. Participants had 5 minutes to complete the task. Direct observation was used, as it was found to be more accurate than the simulator’s built-in software recognition during pilot testing.
Endotracheal (ET) intubation – MD participants were asked to perform direct laryngoscopy using a 4.5-mm ET tube and a Miller 1 blade, placed at the head of the bed for each trial. Participants were instructed to stand at the head of the bed before starting. Time to completion was defined as when one ventilation (chest returned to baseline) was viewed to have been completed. At one site in which a video laryngoscope (C-MAC, Karl Storz, Tuttlingen, Germany) was available, a second outcome was assessed. Here, we recorded the time when the ET tube was inserted between the vocal cords using the C-MAC camera, which the participant could not see as the display was facing away. The recorded video feed was reviewed to determine procedural outcome. An attempt at tracheal intubation was defined as placement of a laryngoscope in the mouth of a manikin; a successful attempt was defined as insertion of the endotracheal tube into the trachea of the manikin prior to removal of the laryngoscope blade. Time to tube insertion was defined as the interval between being instructed to start and when the endotracheal tube tip was seen (by observation of the video feed) to pass through the glottic opening. Successful completion required intubation in no more than three attempts and ventilation within 3 minutes. As the other site did not have this equipment available, we collected only single-site data.
Defibrillation – All participants were asked to place defibrillation pads onto the manikin, turn on and set the defibrillator to 50 joules, and provide a single shock. Time was measured from instruction to begin to the discharge sound from the defibrillator. We defined success as delivering the shock. Each site used the defibrillator model that was available at their institution.
IV placement – RN participants were asked to place an IV in the arm of the Laerdal simulator. As we were interested in the mechanical steps of the process and not in the IV placement skill of the participant, we did not require catheter placement with the vein analog of the simulator’s arm. To successfully complete this task, the IV had to be placed, secured, and flushed within 3 minutes. Time was measured from instruction to begin to the IV flush.
Push-pull bolus – RN participants were asked to complete a 300-mL IV bolus using the push-pull technique and a three-way stopcock. We used a closed system in which IV fluid was pushed from the bag into a reservoir bag to simulate delivering fluid to a patient. Time to completion was measured from instruction to begin until the final syringe was pushed. Success was defined as completion of the bolus within 5 minutes.
The inter-rater reliability for the primary time to completion measures was assessed by timing the participant with two raters in a 10% sample of visits at each study site. Participants were asked to self-report their level of fatigue on a scale of 1 to 10 (with 1 being least fatigued and 10 being most fatigued) at the beginning and end of their session.
Two surveys were administered: a survey before the PPE session asked participants what difficulties they expected to experience regarding barriers related to PPE, and a second survey after the PPE session asked the same barrier questions. This survey consisted of 5-point anchored rating scales as well as multiple-choice items.
Data Analysis
Demographic characteristics were summarized with frequencies and percentages for categorical data and means and standard deviations for continuous data. The primary analysis was the effect of PPE on time to completion of a specific task. In addition, the median differences in time of performing each task with and without PPE were compared between two PPE types (Ebola PPE with non-sterile gloves and Ebola PPE with “chemical gloves) and by provider type (RN and MD) using two-sample median tests. Survey data were assessed using paired t-tests comparing session responses.7 Inter-rater reliability was assessed for agreement of raters at the PPE session using the intraclass correlation (ICC). All tests were two-sided; P<0.05 was considered to be statistically significant. Statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Sixty-eight eligible participants enrolled in the study, comprising 32 RNs and 36 MDs. A total of 65 participants (31 RNs and 34 MDs) attended both study sessions and were included in the final analysis. Participant demographics and reported experience are provided in Table 1. The mean (standard deviation) age was 44.6 (8.0) years for MDs and 38.1 (10.8) years for RNs. Altogether, 38.2% of MDs and 6.5% of RNs were male. More than half of RNs (54.8%) had previously worn PPE >10 times in their careers, whereas the majority (67.6%) of MDs reported ≤5 times. More than one-fourth of RNs self-reported their PPE experience level as “experienced” for both PPE types, whereas MDs reported they had little (63.6% in Ebola PPE and 78.8% in chemical PPE) or moderate (36.4% in Ebola PPE and 21.2% in chemical PPE) experience.
Table 1.
Demographic and Baseline Characteristics
| MD (N=34) | RN (N=31) | Total (N=65) | |
|---|---|---|---|
| Mean age (SD), years | 44.6 (8.0) | 38.1 (10.8) | 41.5 (9.9) |
| Male | 13 (38.2%) | 2 (6.5%) | 15 (23.1%) |
| Number of times PPE worn previously | |||
| 1–2 | 10 (29.4%) | 2 (6.5%) | 12 (18.5%) |
| 3–5 | 13 (38.2%) | 6 (19.4%) | 19 (29.2%) |
| 6–10 | 6 (17.6%) | 6 (19.4%) | 12 (18.5%) |
| >10 | 5 (14.7%) | 17 (54.8%) | 22 (33.8%) |
| Level of self-reported experience with PPE: Ebola-type | |||
| Little | 21 (63.6%) | 10 (32.3%) | 31 (48.4%) |
| Moderate | 12 (36.4%) | 13 (41.9%) | 25 (39.1%) |
| Experienced | 0 | 8 (25.8%) | 8 (12.5%) |
| Level of self-reported experience with PPE: Chemical-type | |||
| Little | 26 (78.8%) | 12 (40.0%) | 38 (60.3%) |
| Moderate | 7 (21.2%) | 10 (33.3%) | 17 (27.0%) |
| Experienced | 0 | 8 (26.7%) | 8 (12.7%) |
Data presented as n (%).
MD=medical doctor; PPE=personal protective equipment; RN=registered nurse, SD=standard deviation.
Performance of each task is reported in Table 2. There were no significant differences in completion time from session 1 (baseline) compared with session 2 for the MD group. For the RN group, there was a significant difference between baseline and PPE sessions for both defibrillation and IV placement tasks. RNs were faster to defibrillate in the Ebola PPE vs. session 1 and conversely slower when wearing chemical PPE (median difference = −3.5 vs. 2 seconds, respectively; both differences significant at p<0.01). IV placement took slightly longer in the Ebola PPE and appreciably longer in the “chemical” PPE (median difference = 5.5 vs. 42 seconds, respectively; p<.01).
Table 2.
Completion Time of Study Tasks
| Completion Time (sec) | Change in Completion Time from Baseline (sec) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Session 1 | Session 2 (Ebola) | Session 2 (Chemical) | Session 2 (Ebola) | Session 2 (Chemical) | |||||||
|
|
|
|
|
|
|||||||
| Task | N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | P-value |
| MD | |||||||||||
| Bag-mask ventilation | 34 | 15.5 (12–21) | 19 | 14 (11–20) | 15 | 16 (13–22) | 19 | −1 (−4–2) | 15 | 0 (−2–3) | 0.3073 |
| Endotracheal intubation – Chest rise | 34 | 34 (25–43) | 19 | 34 (31–42) | 15 | 35 (31–43) | 19 | 1 (−9–10) | 15 | −4 (−8–19) | 0.2876 |
| Endotracheal intubation – Glottic passage* | 20 | 18 (11–20.5) | 12 | 12 (9–17) | 9 | 12 (11–15) | 11 | −2 (−10–4) | 9 | −6 (−9−−2) | 0.6613 |
| Manual defibrillation | 34 | 40.5 (35–48) | 19 | 38 (32–52) | 15 | 41 (37–52) | 19 | 0 (−5–6) | 15 | 2 (−10–8) | 0.7258 |
| RN | |||||||||||
| Bag-mask ventilation | 31 | 18 (16–21) | 16 | 17 (15–18.5) | 15 | 20 (18–23) | 16 | −0.5 (−2.5–4) | 15 | 0 (−3–4) | 0.9556 |
| Manual defibrillation | 31 | 30 (25–43) | 16 | 28 (25–36.5) | 15 | 29 (25–48) | 16 | −3.5 (−7.5−−1) | 15 | 2 (−1–8) | 0.0026 |
| Intravascular catheter placement | 31 | 79 (62–100) | 16 | 82.5 (73.5–100.5) | 15 | 127 (89–160) | 16 | 5.5 (−4.5–24) | 15 | 42 (19–82) | 0.0081 |
| Fluid bolus (Push/Pull) | 31 | 132 (101–196) | 16 | 123 (102.5–187) | 15 | 118 (102–260) | 16 | 2.5 (−15–29.5) | 15 | −2 (−17–10) | 0.8551 |
Only assessed at one site. One participant’s completion time at baseline was missing.
P-value was computed using two-sample median test.
IQR=interquartile range; MD=medical doctor; RN=registered nurse.
All participants were able to meet the defined standards for BVM, ET intubation, and defibrillation in both study sessions. For the fluid push task, two RNs did not meet the standard for sessions 1 and 2, and one additional RN did not meet the standard for session 2 only. For the IV placement task, all met the standard for session 1 and three did not meet the standard for session 2. Inter-rater reliability was high (ICC >0.85) for all tasks except IV placement (ICC=0.45). Participant-reported fatigue did not change significantly from the beginning to the end of either session.
In the survey regarding concerns about PPE and procedural efficacy (Table 3) administered before the PPE session, the participants agreed that PPE would interfere with procedures and slow procedures, were neutral on the impact on focus, did not feel that PPE was claustrophobic, and felt they were prepared to don PPE. After the PPE session, participants were significantly more likely to agree that PPE with non-sterile gloves impacted procedures (mean score change=0.5, p<.01) but were less likely to agree that PPE interfered with procedures (−0.6, p<.0001), was claustrophobic (−0.4, p=0.02), or slowed them (−0.4, p<.01). After the PPE session, they also felt more positively than before regarding their preparedness to don PPE (0.5, p<.001).
Table 3.
Change in Attitudes Before and After PPE Session
| Attitude Score, mean (SD) | ||||
|---|---|---|---|---|
| Question | Session 1 (N=65) | Session 2 (N=65) | Change (N=65) | P-value |
| Non-sterile gloves interfere with procedures | 1.8 (0.9) | 2.3 (1.0) | 0.5 (1.1) | 0.0014 |
| Full body PPE suits interfere with procedures | 4.1 (0.6) | 3.4 (1.0) | −0.6 (1.0) | <.0001 |
| PPE makes it hard for me to focus on my procedure | 3.0 (1.0) | 3.0 (1.2) | 0.0 (1.1) | 0.8292 |
| PPE is claustrophobic | 2.8 (1.1) | 2.4 (1.2) | −0.4 (1.2) | 0.0197 |
| Slower performing procedures in full-body PPE | 4.2 (0.6) | 3.8 (1.0) | −0.4 (1.1) | 0.0038 |
| Prepared to appropriately don Ebola-type gear | 3.6 (1.3) | 4.1 (0.8) | 0.5 (1.0) | 0.0002 |
Attitude score: 1=Strongly Disagree, 2=Disagree, 3=Neither Agree nor Disagree, 4=Agree, 5=Strongly Agree.
P-value was computed using paired t-test.
PPE=personal protective equipment; SD=standard deviation.
DISCUSSION
We found no evidence that the PPE we tested meaningfully impacted performance for most emergent procedures performed by HCPs on a simulated 5-year-old child with the exception of time to IV placement in the thicker, more cumbersome (“chemical”) of the two glove types tested. While there were statistically significant differences in time for RN defibrillation and for IV placement in Ebola gloves, these were under 5 seconds, which would be expected to have little clinical impact.
We chose tasks that are important and representative of skills that an HCP may be required to perform in the care of an exposed or infectious child in a hospital setting. We specifically did not limit our task choices based on a specific diagnosis (i.e., Ebola). In this study, we sought to generalize beyond a single etiology requiring PPE.
Fatigue
Interestingly, participants did not report more fatigue after completing procedures in whole-body PPE gear compared to baseline. While none of the procedures appear to be aerobically demanding (in contrast to activities such as chest compressions), they did require concentration and manual dexterity. The total time in PPE was <1 hour, limiting our ability to comment on fatigue in a setting where PPE is worn for longer durations.
Participant Feedback
Of the participant feedback overall, it is worth noting that the group did not report PPE to be claustrophobic before exposure to PPE during the study and reported it to be even less claustrophobic after exposure. The presumption that HCPs view PPE as claustrophobic may not be supported. This is consistent with previous work by Udayasiri et al., who found that only a minority of adult emergency providers found PPE to be claustrophobic.3 Less surprisingly, participants reported that PPE was perceived to affect procedure efficiency. However, they felt less strongly about this barrier after the PPE session, suggesting that this experience moderated their concerns.
Limitations
This study involved providers performing discrete procedures not in the context of normal patient care, which may reduce generalizability in exchange for study protocol consideration to reduce variability in recording time to events. Some of the procedures we studied may not be performed in the setting of hemorrhagic fevers, primarily related to concerns of HCP exposure (e.g., BVM).6 However, these same procedures might be appropriate for other conditions in which similar PPE garb would be worn. The decision to have baseline (street garb) be the first session may have masked a learning effect of repeating the procedures over the two sessions. The lower rater agreement for IV placement may reflect that this procedure was by far the longest task to complete and the defined end point (securing the IV) was the most challenging part of the task for participants. Lastly, our sample was a convenience sample of hospital staff from two tertiary care pediatric programs and thus may not be representative of the larger population of providers.
Conclusion
Overall, we found no evidence that the PPE we tested impacted timeliness or procedural success for most emergency procedures performed by pediatric HCPs, with the exception of IV placement. Participants, in general, had a more positive attitude regarding performing procedures in PPE garb after wearing PPE in the study. Our data suggest that the procedures we studied can be performed for children in PPE garb without clinically relevant delays, excluding IV placement. As a next step, research is needed to investigate procedures done within a clinical workflow.
Financial support:
This work was supported under contract HHSN275201000003I from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. (Order number for TO41: HHSN27500041.) The Best Pharmaceuticals for Children Act - Pediatric Trials Network is an alliance of clinical research sites cooperating in the design and conduct of pediatric clinical trials. The Network provided financial and logistical support for the research presented herein under the above contract. The Network is coordinated by the Duke Clinical Research Institute, with data analysis support from the Emmes Corporation.
Grant money for commercial research
CH reports grant money to Duke Clinical Research Institute to conduct research conceived and sponsored by Purdue Pharma LLP.
Footnotes
Portions of this work were presented at the following meeting: Pediatric Academic Societies Meeting: Toronto, Canada. May 2018. The use of PPE during pediatric resuscitation: which tasks are most affected? The results of a survey during a simulation trial. Good G, Donoghue AJ, Kou M, Eiger C, Siegel D, Nash M, Henretig F, Hornik CP, Stacks H, Kochman A, Gosnell L, Sharma G, Lewandowski A, Krug S, Adler M.
Consulting for commercial interests, including advisory board work
CH has received funding personally from Sarfez Pharma for consulting.
Conflicts of interest:
MDA, SK, CE, GLG, MK, MN, FMH, LG, JYC, JD, GS, DS, and AJD report no conflict of interest.
References
- 1.Chen J, Lu KZ, Yi B, Chen Y. Chest compression with personal protective equipment during cardiopulmonary resuscitation: A randomized crossover simulation study. Medicine (Baltimore). 2016;95:e3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenland KB, Tsui D, Goodyear P, et al. Personal protection equipment for biological hazards: does it affect tracheal intubation performance? Resuscitation. 2007;74:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Udayasiri R, Knott J, McD Taylor D, et al. Emergency department staff can effectively resuscitate in level C personal protective equipment. Emerg Med Australas. 2007;19:113–121. [DOI] [PubMed] [Google Scholar]
- 4.Garner A, Laurence H, Lee A. Practicality of performing medical procedures in chemical protective ensembles. Emerg Med Australas. 2004;16:108–113. [DOI] [PubMed] [Google Scholar]
- 5.Seigel J, Rhinehart E, Jackson M, et al. ; the Healthcare Infection Control Practices Advisory Committee. 2007. guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. Available at: https://www.cdc.gov/hai/pdfs/Isolation2007.pdf. Accessed Feb 27, 2019. [DOI] [PMC free article] [PubMed]
- 6.World Health Organization. Interim infection prevention and control guidance for care of patients with suspected or confirmed filovirus haemorrhagic fever in health-care settings, with focus on ebola. Available at: http://www.euro.who.int/__data/assets/pdf_file/0005/268772/Interim-Infection-Prevention-and-Control-Guidance-for-Care-of-Patients-with-Suspected-or-Confirmed-Filovirus-Haemorrhagic-Fever-in-Health-Care-Settings,-with-Focus-on-Ebola-Eng.pdf.
- 7.Norman G. Likert scales, levels of measurement and the “laws” of statistics. Adv Health Sci Educ Theory Pract. 2010;15:625–632. [DOI] [PubMed] [Google Scholar]