Dear Editor,
We read with great interest the meta-analysis recently published by Cheema et al. in the Journal of Infection on the topic of efficacy and safety of fluvoxamine for the treatment of COVID-19 patients.1
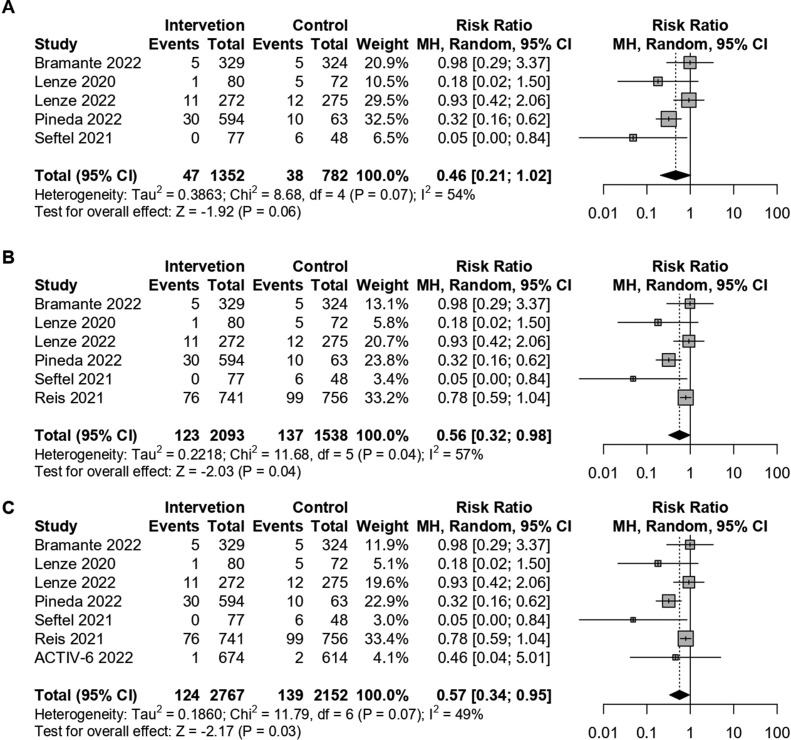
In their meta-analysis Cheema et al. concluded that fluvoxamine does not decrease the risk of hospitalisation in patients with COVID-19 (RR 0.46; 95% CI: 0.21–1.02; I2 = 54%; p = 0.06). This conclusion stands in contrast to conclusions of prior meta-analyses such as the meta-analysis published by Lee et al. in JAMA Network Open which found that fluvoxamine showed a high probability of being associated with reduced hospitalization in outpatients with COVID-19.2
Upon further inspection of the meta-analysis by Cheema et al., it seems that the authors had made an unfortunate mistake and failed to include results from the TOGETHER trial3 (marked as Reis 2021 in the original meta-analysis and kept as such in this reanalysis) in their hospitalisation outcome meta-analysis, recreated on Fig. 1 A. The TOGETHER trial was a placebo-controlled, randomised, adaptive platform trial conducted amongst 1497 high-risk symptomatic Brazilian COVID-19 outpatients, which found that fluvoxamine 100 mg twice daily for 10 days significantly reduced the need for hospitalisation defined as a composite outcome of either retention in a COVID-19 emergency setting or transfer to a tertiary hospital. Data regarding secondary outcomes such as mortality and hospitalisation rate is readily available in Table 3 of the TOGETHER study manuscript and Cheema et al. included results from the TOGETHER trial in their mortality outcome analysis but failed to do the same regarding the hospitalisation outcome.
Fig. 1.
Forest plot recreating the original meta-analysis by Cheema, H. A. et al.1 regarding the effect of fluvoxamine on COVID-19 outpatient hospitalisation risk outcome (Fig. 1A). Reanalysis of the hospitalisation outcome with inclusion of data from the TOGETHER trial (Reis, 2021) on Fig. 1B and with the additional inclusion of data from the recently published preprint of the ACTIV-6 study on Fig. 1C.
We have thus reanalysed the hospitalisation outcome with the data presented by Cheema et al., but with the inclusion of hospitalisation data from the TOGETHER trial, Fig. 1B. According to our reanalysis, fluvoxamine does statistically significantly reduce the risk of hospitalisation in COVID-19 outpatients (RR 0.56; 95% CI: 0.32–0.98; I2= 57%; p = 0.04). Additionally, we have also included results from the recently published preprint of the ACTIV-6 trial on fluvoxamine4 which included 1331 COVID-19 positive randomised outpatients and found no statistically significant benefit of fluvoxamine, although in a predominantly vaccinated population (67%) and using a lower dose of 50 mg twice daily. Nevertheless, when we included the hospitalisation outcome data from the ACTIV-6 trial in the meta-analysis, the results remained statistically significant in favour of fluvoxamine (RR= 0.57; 95% CI: 0.34–0.95; I2= 49%; p = 0.03), Fig. 1C.
In conclusion, the failure to include the TOGETHER trial in the hospitalisation risk outcome meta-analysis by Cheema et al. significantly impacted the results of their analysis and resulted in an erroneous conclusion. Based on our reanalysis, it appears fluvoxamine is associated with a statistically significant decrease in the risk of hospitalisation when given to COVID-19 outpatients.
Authors’ contributions
All authors participated equally in all parts of the manuscript.
Funding
No funding was received for this study.
Data disclosure statement
All analysed data is presented in the manuscript.
Declaration of Competing Interest
No conflicts of interest to declare.
References
- 1.Cheema H.A., et al. Efficacy and safety of fluvoxamine for the treatment of COVID-19 patients: a systematic review and meta-analysis. J Infect. 2022;1:1–11. doi: 10.1016/j.jinf.2022.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee T.C., et al. Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reis G., et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy, M.W. et al. Fluvoxamine for outpatient treatment of COVID-19: a decentralized, placebo-controlled, randomized, platform clinical trial. medRxiv 2022.10.17.22281178 (2022). doi: 10.1101/2022.10.17.22281178. [DOI]