Abstract
The aims of this study were to investigate the effects of supplemental N-acetyl-l-cysteine (NAC) on chronic heat stress-induced oxidative stress and inflammation in the ovaries of growing pullets. A total of 120, 12-wk-old, Hy-Line Brown hens were randomly separated into 4 groups with 6 replicates of 5 birds in each group for 21 d. The 4 treatments were as follows: the CON group and CN group were supplemented with basal diet or basal diet with 1 g/kg NAC, respectively; and the HS group and HSN group were heat-stressed groups supplemented with basal diet or basal diet with 1 g/kg NAC, respectively. The results indicated that the ovaries suffered pathological damage due to chronic heat stress and that NAC effectively ameliorated these changes. Compared with the HS group, antioxidant enzyme activities (including SOD, GSH-Px, CAT, and T-AOC) were enhanced, while the MDA contents and the expression levels of HSP70 were decreased in the HSN group. In addition, NAC upregulated the expression levels of HO-1, SOD2, and GST by upregulating the activity of Nrf2 at different time points to mitigate oxidative stress caused by heat exposure. Simultaneously, NAC attenuated chronic heat stress-induced NF-κB pathway activation and decreased the expression levels of the proinflammatory cytokines IL-8, IL-18, TNF-α, IKK-α, and IFN-γ. Cumulatively, our results indicated that NAC could ameliorate chronic heat stress-induced ovarian damage by upregulating the antioxidative capacity and reducing the secretion of proinflammatory cytokines.
Key words: NAC, chronic heat stress, ovary, oxidative stress, inflammation
INTRODUCTION
The worldwide consumption of poultry eggs has increased significantly, contributing to a healthy diet rich in proteins, vitamins, and minerals. Simultaneously, the greenhouse effect is increasing the pressure on poultry farming (Yi et al., 2016). Owing to the presence of feathers, high metabolic body heat and a lack of sweat glands, poultry species are vulnerable to thermal stress, especially laying hens (Zhu et al., 2019). When the ambient temperature exceeds the thermoneutral zone (19–22°C for laying hens), poultry are considered to be heat-stressed. Heat stress could disturb their physiological dynamic balance and hinder their normal growth and development, even inducing sudden mortality in serious cases, resulting in huge economic losses (Pawar et al., 2016). Generally, heat stress disorder directly or indirectly affects the ovaries and affects follicle development, leading to ovarian dysfunction (Roth, 2020). Nevertheless, the mechanism of ovarian damage caused by heat stress has not been fully elucidated thus far. Hence, it is of great significance to research methods to prevent the pathogenesis of ovarian heat stress in growing pullets.
Previous reports have indicated that markedly upregulating several groups of proteins, such as heat shock proteins (HSPs) and antioxidant enzymes, plays key role in heat tolerance (Mays et al., 2019; Bai and Wang, 2020). HSPs are one of the most typical reaction products of animals during exposure to elevated thermal stress. Furthermore, as a key stress protein in the HSP family triggered by disparate environmental stressors, HSP70 is mainly upregulated by thermal damage and it can relieve post-heat stress disorder (Deane and Brown, 2018; Mays et al., 2019). In fact, concomitant with the change in HSP70, antioxidant enzymes (such as superoxide dismutase [SOD] and glutathione peroxidase [GSH-Px]) in the antioxidant defense system play an important role in maintaining the normal function of animals exposed to heat stress (Hu et al., 2020). Normally, there is a balance between antioxidant and pro-oxidant species, but it can be compromised by thermal stress, which means the stress protection of the antioxidant defense system has certain limitations. Under these circumstances, the body's normal regulatory functions are lost and ultimately this results in damage to normal tissues or cells (Videla and Valenzuela, 2022). Nuclear factor erythrocyte associated factor 2 (Nrf2) is the main regulator of oxidative stress induced by thermal damage (Zhao et al., 2018). Nrf2 plays a crucial role in protection against oxidative stress by inducing the expression of cytoprotective genes, such as heme oxygenase-1 (HO-1) and glutathione-S-transferases (GST) (Miao et al., 2020). Wang et al. (2019) revealed that heat stress induced oxidative stress in ovarian granulosa cells (GCs) and enhanced Nrf2 and HO-1 expression levels in primary GC cultures, which may, at the same time, preclude the production of reactive oxygen species (ROS) and trigger the antioxidant response of GCs to mitigate the damage.
Most notably, oxidative stress can promote activation of the transcription factor nuclear factor kappa B (NF-κB), which is essential in inflammatory cascades (Zhivotovsky and Kaminskyy, 2013). NF-κB is widely distributed in various tissues and cells, and its activation is important for downstream inflammatory mediators such as IL-8, TNF-α, and IFN-γ, regulating the occurrence of the inflammatory response (Huang et al., 2013; Park and Hong, 2016). Previous studies have indicated that heat stress can activate the NF-κB signaling pathway and cause inflammatory responses in different organs (such as the ovary, hepatic, and gut) (Ozdemir et al., 2017; Koch et al., 2019; Chen et al., 2020). When the ovaries of growing pullets become inflamed, oogenesis, oocyte maturation, fertilization, and embryo development are negatively affected, which results in a huge economic loss to the poultry industry worldwide. Inhibition of the NF-κB-mediated inflammatory response may therefore ensure and improve the treatment of oxidative damage caused by heat stress (Bao et al., 2012). In summary, activation of the NF-κB signaling pathway is an important regulatory mechanism when heat stress-induced inflammation and oxidative stress occur simultaneously in the ovary.
Recently, drug treatment strategies by supplying exogenous antioxidants have been increasingly developed and applied to the poultry industry to enhance their resistance to the thermal environment (Liu et al., 2016; Yi et al., 2016). Among these antioxidants, N-acetyl-l-cysteine (NAC) has high economic efficiency and has been shown to have great potential in overcoming from the harmful effects of oxidative stress and inflammatory responses in a variety of diseases (de Andrade et al., 2015). NAC mitigates oxidative stress by scavenging free radicals or triggering the catalytic activity of antioxidant systems such as SOD or GSH-Px (Nitescu et al., 2006). Dash et al. (2020) demonstrated that supplementing with NAC had antioxidant and anti-inflammatory effects on rat ovaries. They found that dietary NAC supplementation reversed the normal activity of antioxidant enzymes in the ovaries of arsenite fed rats, sustained normal utero-ovarian histomorphology, and inhibited the upregulation of the inflammatory markers TNF-α and NF-κB. Hu et al. (2020) found that provisioning NAC during in vitro maturation could protect oocytes from heat stress-induced ROS accumulation and apoptosis. Based on our previous results, it appears that NAC could ameliorate heat stress-induced oxidative stress and inflammation in the hypothalamus of hens (Zhao et al., 2021). Notably, evidence that NAC mitigates ovarian damage induced by chronic heat stress in poultry has not yet been provided. Based on the antioxidant and anti-inflammatory properties of NAC, we aimed to investigate its effects on chronic heat stress-induced ovarian injury in growing pullets. These results are expected to provide an identifiable qualification for drug screening for the treatment and prevention of ovarian damage caused by chronic heat stress.
MATERIALS AND METHODS
All animal care procedures and methods were approved by the Institutional Animal Care and Use Committee (Jiangxi Agricultural University, Nanchang, Jiangxi, China, JXAULL-2020-28).
Animals and Treatments
A total of 120, ten-wk-old, Hy-Line Brown growing pullets were purchased from a local commercial pullet farm (Guohua Co., Ltd., Nanchang, Jiangxi, China). The formal experiment began after 2 wk of acclimation that included a standard commercial diet and management. The basal diet was the commercial diet for young laying hens produced by Nanchang Zhengda Co., Ltd., China, and all growing pullets received the standard poultry ration according to the National Research Council (NRC).
At the age of 12 wk, they were randomly separated into 4 treatment groups in a 2 × 2 factorial trial scheme with two diets (basal diet or 1 g/kg NAC diet) and 2 temperatures (thermoneutral or heat stress). The thermoneutral and heat treatments were divided into separate rooms (Zhao et al., 2021). One room (22 ± 1°C) contained the CON group and the CN group, and another room (36 ± 1°C from 8 a.m. to 6 p.m. every day followed by 22 ± 1°C) contained the HS group and the HSN group. They were housed in a controlled environment, with 50 to 60% relative humidity and a lighting schedule of 12 h alternate light-dark cycles during the 3-wk experimental period.
Sample Collection
On days 7, 14, and 21 of the formal experiment, 10 pullets were randomly selected for cervical vertebrae dislocation from each subgroup. The ovarian tissues were quickly isolated in a sterile environment, cut into 1 cm3 cubes and then fixed in a 4% paraformaldehyde solution (BL539A, Biosharp, Beijing Labgic Technology Co., Ltd., China). The remaining ovarian tissues were collected and thoroughly washed with 0.9% normal saline (H10983065, Jiangxi Kelun Pharmaceutical Co., Ltd., China) to rinse away the blood and then separated into 2 parts. One was placed in freezing tubes, frozen in liquid nitrogen, and stored at −80°C (DW-86L388A, Haier, Suzhou Bozhao Scientific Instrument Co., Ltd., China) until used for determining the mRNA and protein expression levels. The other sample was stored at −20°C (BCD-290 W, Haier, Qingdao Haier Co., Ltd., China) for the detection of antioxidant indices.
Histopathological Examination
The isolated ovarian tissues were fixed in a 4% paraformaldehyde solution at room temperature for 24 h, dehydrated from a low concentration to a high concentration of alcohol (GB/T678-2002, XiLong Scientific, Xilong Scientific Co., Ltd., China) and then made transparent with xylene (GB/T16494-2013, XiLong Scientific, Xilong Scientific Co., Ltd.). Next, the samples were embedded in paraffin, sliced, and dewaxed. Hematoxylin and eosin (H&E) (60524ES60, Yeasen, Shanghai Yeasen Bio Technologies Co., Ltd., China) staining was performed to evaluate the effects of NAC on ovaries subjected to chronic heat stress. The results were examined and photographed under an optical microscope (7L41885, Olympus Corporation Tokyo, Japan).
Antioxidative Capacity
Antioxidant enzymes, including SOD (A001-3-2), GSH-Px (A005-1-2), catalase (CAT) (A007-1-1), total antioxidant capacity (T-AOC) (A015-2-1) and the content of malondialdehyde (MDA) (A003-1-2), were measured by diagnostic kits purchased from Nanjing Jiancheng Biotechnology Research Institute (Nanjing, China). All procedures were based on the manufacturer's instructions.
Analysis of Quantitative Real-Time PCR
Total RNA was extracted from 100 mg of frozen ovarian tissue samples using RNAiso Plus (9108, Takara, Japan). Thermo Scientific NanoDrop-2000 Spectrophotometers (ND 2000, Gene Co., Ltd., China) were used to detect the concentration and purity of the RNA. Then, the cDNA was removed with a One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (AT311-04, TransGen Biotech, China) and stored at −20°C. To amplify the cDNA fragments, primer pairs (Table 1) were used for qPCR. The reactions were carried out using a QuantStudio 7 Flex Real-Time PCR system (4485695, ABI Thermo Fisher Scientific, Waltham, MA) according to the instructions of the TransStart Tip Green qPCR SuperMix (AQ142-22, TransGen Biotech, China). The qPCR system included 5 μL Tip Green qPCR SuperMix (2 ×), 0.2 μL Forward primers, 0.2 μL Reverse primers, 0.2 μL Passive Referencing dye (50 ×), 1 μL cDNA templates and 3.4 μL water. The amplification included a denaturation step at 94°C for 30 s, followed by 40 cycles at 94°C for 5 s, 60°C for 30 s, and extension at 72°C for 30 s. Finally, the 2−ΔΔCt method was used to analyze the data and the results were displayed graphically with GraphPad Prism 8.0 (GraphPad Inc., La Jolla, CA).
Table 1.
Sequences of the primers used for the quantitative PCR analysis.
| Genes | Accession number | Primer sequences (5′-3′) |
|---|---|---|
| GAPDH | NM_204305 | F: TGGCATCCAAGGAGTGAGC R: GGGGAGACAGAAGGGAACAG |
| HSP70 | NM_001006685.1 | F: CTGCTCTCATCAAGCGTAACACC R: AGCCCTCTCACCTTC ATACACC |
| Nrf2 | NM_205117.1 | F: CTGCCCAAAACTGCCGTA R: CAAATCTTGCTCCAGTTCCA |
| HO-1 | NM_205344.1 | F: GCTGAAGAAAATCGCCCAA R: ATCTCAAGGGCATTCATTCGG |
| GST | NM_001001776.1 | F: GGAAGCCATTTTAATGACAGA R: TCCTTTAAAAGCCTGTA GCAGA |
| SOD2 | XM_015285700.2 | F: TTTTCTCCTAAAGATGGCAAG R: CTTCCTGCTCATGGATCACAA |
| NF-κB | NM-205134 | F: TCAACGCAGGACCTAAAGACAT R: GCAGATAGCCAAGTTCAG GATG |
| TNFα | AY765397 | F: TGTGTATGTGCAGCAACCCGTAGT R: GGCATTGCAATTTGGACAGAAGT |
| IL-8 | NM-205018 | F: GGCTTGCTAGGGGAAATGA R: AGCTGACTCTGACTAGGAAACT GT |
| IFN-γ | NM_205149 | F: ACTGAGCCAGATTGTTTCGAT R: TCTTTCACCTTCTTCACGCCA T |
| IL-18 | NM_204608 | F: AGGTGAAATCTGGCAGTGGAAT R: ACCTGGACGCTGAATGCAA |
| IKK-α | NM_001012904.1 | F: ATGCACCTCACCCTCTTTCAT R: CTCTTCTCTTGCCTCCTGCAA |
Western Blotting
For the western blotting samples, 50 mg ovarian tissues were homogenized in 1 mL of ice-cold RIPA buffer (R0020, Solarbio Technology Co., Ltd., Beijing, China) containing 1 mmol/L PMSF and 1% phosphatase inhibitor (P1260, Solarbio Technology Co., Ltd., Beijing, China). After 30 minutes of lysing on ice, the extracts were centrifuged at a speed of 12,000 × g for 20 minutes at 4°C. Subsequently, the supernatant was collected, and the protein concentration was measured by a bicinchoninic Acid kit (PC0020, Solarbio Technology Co., Ltd., Beijing, China). Then, 40 μg of protein was subjected to 12% SDS-PAGE and transferred to nitrocellulose membranes. After blocking in 5% nonfat milk powder for 2 h at room temperature, the membranes were washed with TBST and then incubated overnight at 4°C with primary antibodies (1:1,000 dilution) against Nrf2, HO-1, NF-κB, and TNF-α from Wanleibio and GAPDH from Cell Signaling Technology. All of the antibodies were validated previously for use with chicken samples. After washing with TBST, the membranes were incubated with the secondary antibody (1: 2,500 dilution, Wanleibio, China) at room temperature for 1 h. Then, the membranes were washed three times for 10 minutes each. They were covered with ECL chemiluminescence reagents and exposed to a Bio-Rad ChemiDoc Touch imager (Bio-Rad ChemiDoc Touch, Bio-Rad, Hercules, CA). Finally, the gray values of the protein bands were analyzed by ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical Analysis
SPSS version 22.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 8.0 were used to perform the statistical analysis and generate the plots. ImageJ was used to annotate the images. The data were analyzed by ANOVA and the least significant difference (LSD) post-hoc test. All results are expressed as the mean ± SE. P < 0.05 was considered to be statistically significant, P < 0.01 was considered very significant, and P < 0.001 was considered extremely significant.
RESULTS
Effects of NAC on Chronic Heat Stress-Induced Histological Changes in the Ovaries
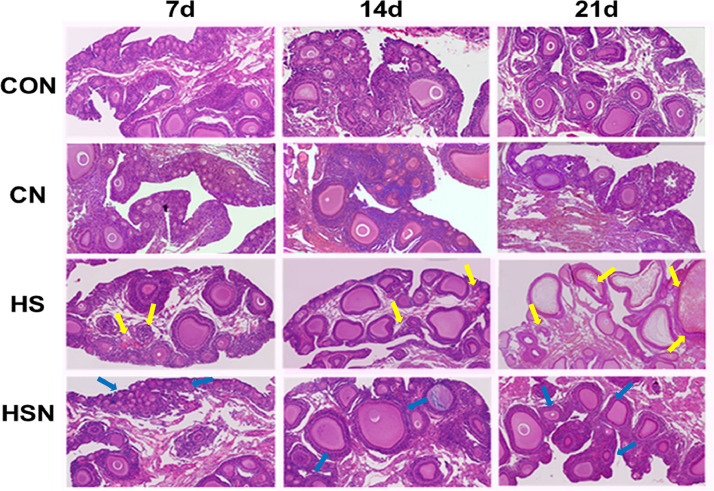
To observe the effects of NAC on chronic heat stress in the ovaries, histological images of the ovaries were taken on days 7, 14, and 21. As shown in Figure 1, in the ovarian cortex, the normal follicles in the CON group were clearly visible, regular in shape, and were in different sizes at different stages of development. With the increase in heat stress time, on days 7, 14, and 21, in the HS group, the follicles exhibited different degrees of stunted growth, an irregular form, ovarian interstitial hemorrhage, atresia follicles and loosely arranged granular cells (yellow arrows). These results indicated that ovary injury was associated with heat stress duration. Compared with the HS group, the HSN group had regular follicle morphology, with the original follicles and reduced ovarian interstitial hemorrhage (blue arrows). Supplementation with NAC can ameliorate the effects of the heat and restore the original structure of the ovaries. Here, we showed that no significant pathological changes were observed in the CN group.
Figure 1.
Results of H&E staining in ovarian tissues (40 ×). The sites of ovarian damage in the HS group are indicated by yellow arrows. The sites of follicular morphology in the HSN group are indicated by blue arrows.
Effects of NAC on the Antioxidative Capacity in the Ovaries of Growing Pullets
The effects of NAC on the antioxidative capacity in the ovaries of growing pullets are shown in Table 2. At the 3 time points (days 7, 14, and 21), MDA, CAT, SOD, GSH-Px, and T-AOC levels between the CON group and the CN group were not significantly different (P > 0.05). Compared with the CON group, the MDA content in the HS group was extremely significantly increased on days 7, 14 and 21 (P < 0.001). However, compared with the HS group, the MDA content of the HSN group was significantly decreased on day 7 (P < 0.05) and extremely significantly decreased on both days 14 and 21 (P < 0.001). As seen from the other results, in the HS group, the activities of CAT, SOD, GSH-Px, and T-AOC were significantly decreased at all time points. Under chronic heat stress, CAT and SOD were significantly decreased on days 7 (P < 0.01), 14 (P < 0.05), and 21 (P < 0.001). Meanwhile, the activities of GSH-Px and T-AOC were extremely significantly decreased on both days 14 and 21 (P < 0.001). Compared with the HS group, all of these activities were increased in the HSN group. On days 7 and 14, the activities of CAT and GSH-Px were significantly increased (P < 0.05), and CAT was very significantly increased on day 21 (P < 0.01). On days 7, 14, and 21, the activities of SOD were very significantly increased (P < 0.01). The activities of T-AOC were very significantly increased on days 14 and 21 (P < 0.01), but there was no significant difference on day 7 (P > 0.05).
Table 2.
Effects of NAC on antioxidative capacity in the ovaries.
| Item | Time (d) | Groups |
P value | |||
|---|---|---|---|---|---|---|
| CON | CN | HS | HSN | |||
| MDA content (nmol/mg prot) | 7 | 0.835±0.021 | 0.808±0.029 | 1.311±0.031***,# | 1.018±0.066* | <0.001 |
| 14 | 0.797±0.036 | 0.830±0.054 | 1.521±0.062***,### | 1.078±0.061⁎⁎ | <0.001 | |
| 21 | 0.942±0.045 | 0.868±0.066 | 1.598±0.053***,### | 1.078±0.051 | <0.001 | |
| CAT activity (U/mg prot) | 7 | 8.347±0.338 | 8.210±0.379 | 5.698±0.469**,# | 7.025±0.432* | 0.003 |
| 14 | 8.555±0.511 | 9.515±0.334 | 7.162±0.316*,# | 8.548±0.402 | 0.020 | |
| 21 | 9.687±0.299 | 9.509±0.369 | 4.967±0.207***,## | 7.821±0.521* | <0.001 | |
| SOD activity (U/mg prot) | 7 | 193.4±9.0 | 182.0±8.3 | 127.5±5.6***,## | 163.0±5.6⁎⁎ | 0.001 |
| 14 | 171.7±4.4 | 162.5±6.0 | 117.5±7.3**,## | 161.4±7.8 | <0.001 | |
| 21 | 174.2±5.2 | 185.3±8.4 | 108.5±4.6***,## | 156.1±7.2 | <0.001 | |
| GSH-Px activity (U/mg prot) | 7 | 257.4±11.8 | 254.1±10.3 | 158.4±10.0**,# | 200.8±8.4⁎⁎ | <0.001 |
| 14 | 213.0±8.7 | 231.1±11.4 | 129.6±11.7***,# | 171.2±11.5* | 0.001 | |
| 21 | 234.6±7.3 | 229.6±7.7 | 135.1±11.6***,# | 180.6±7.8⁎⁎ | <0.001 | |
| T-AOC activity (U/mg prot) | 7 | 2.291±0.082 | 2.366±0.086 | 1.683±0.044⁎⁎⁎ | 1.761±0.054⁎⁎⁎ | <0.001 |
| 14 | 2.173±0.143 | 2.110±0.105 | 1.349±0.075***,## | 1.860±0.078 | 0.001 | |
| 21 | 2.068±0.067 | 2.148±0.101 | 1.286±0.056***,## | 1.739±0.067* | <0.001 | |
Each value represents the mean ± SE of six growing pullets per group.
Abbreviations: CAT, catalase; CON, control group; CN, control+1 g/kg NAC group; GSH-Px, glutathione peroxidase; HS, chronic heat stress group; HSN, chronic heat stress +1 g/kg NAC group; MDA, malondialdehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
“*”Indicates a significant difference compared to the corresponding control: *P < 0.05, **P < 0.01., ***P < 0.001.
“#”Indicates a statistically significant difference between the corresponding groups: #P < 0.05; ##P < 0.01; ###P < 0.001.
The mRNA Expression Levels of HSP70 and Nrf2 Pathway-Related Antioxidant Genes in the Ovaries
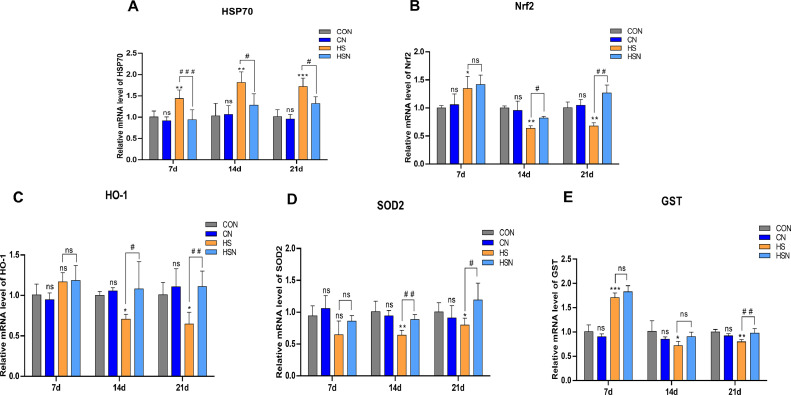
As shown in Figure 2, NAC regulated the mRNA expression levels of the HSP70 and Nrf2 pathway related antioxidant genes during heat stress-induced changes in the ovaries. In our experiment, all mRNA expression levels between the CON group and the CN group showed no significant changes (P > 0.05). In the HS group, the HSP70 level (Figure 2A) was increased in comparison with that in the CON group. The statistics showed that there were very significant increases on days 7 and 14 (P < 0.01) and extremely significant increase on day 21 (P < 0.001). At that time, HSP70 levels were decreased in the HSN group compared with the HS group. The calculations indicated that in the HSN group, there was an extremely significant decrease on day 7 (P < 0.001) and significant decrease on days 14 and 21 (P < 0.05).
Figure 2.
The mRNA expression levels of HSP70 and Nrf2 pathway related antioxidant genes in the ovaries. (A–E) The mRNA expression levels of HSP70, Nrf2, HO-1, SOD2, and GST.
Furthermore, we measured the mRNA expression levels of Nrf2 pathway-related antioxidant genes in the ovaries (Figures 2B–2E). Interestingly, compared with the CON group, the relative mRNA expression levels of Nrf2, HO-1, and GST in the HS group increased first and then decreased with an increasing duration of chronic heat stress, while SOD2 continued to decrease. The relative gene expression levels of Nrf2, GST, and SOD2 in the HSN group were increased during chronic heat stress in comparison with the HS group, while HO-1 decreased first and then increased. The statistics in the HSN group showed that the levels of Nrf2, SOD2, and HO-1 were significantly increased on days 14 and 21 (P < 0.05 or P < 0.01), but Nrf2 had no significant difference on day 7 (P >0.05). There was no significant difference in GST on days 7 and 14 (P > 0.05); however, it was very significantly increased on day 21 (P < 0.01). Meanwhile, the expression level of SOD2 was not significantly increased on day 7 (P > 0.05), and the expression level of HO-1 decreased significantly (P < 0.01).
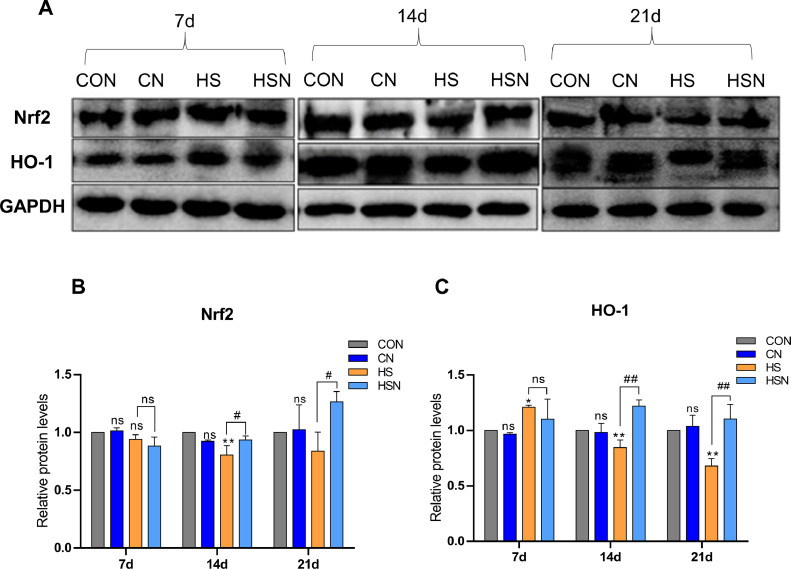
Effects of NAC on the Chronic Heat Stress-Induced Changes in the Protein Expression Levels of Nrf2 and HO-1 in the Ovaries
Nrf2 and HO-1 protein expression levels were assessed through western blotting to demonstrate the effects of NAC on chronic heat stress-induced changes in the ovaries (Figure 3). The protein expression levels of Nrf2 and HO-1 were not significantly different between the CON group and the CN group (P > 0.05). With an increasing duration of heat stress, the protein expression level of Nrf2 in the HS group showed a downward trend, while HO-1 showed a trend of first increasing and then decreasing. In contrast, Nrf2 and HO-1 in the HSN group increased with the duration of chronic heat stress in comparison with the HS group. The levels of Nrf2 were significantly increased on days 14 and 21 (P < 0.05), and the levels of HO-1 were very significantly increased on days 14 and 21 (P < 0.01).
Figure 3.
Protein expression levels of Nrf2 and HO-1 in the ovaries. (A) The western blotting results of Nrf2 and HO-1. (B, C) Quantification of Nrf2 and HO-1.
The mRNA Expression Levels of Inflammatory Genes Associated With the NF-κB Pathway in the Ovaries
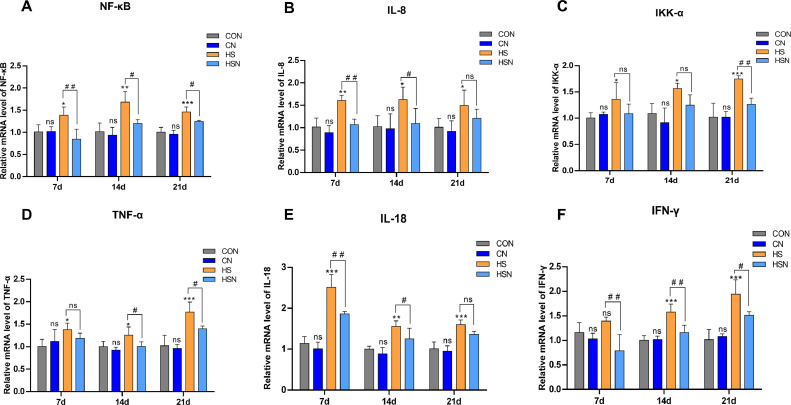
Figure 4 shows the effects of NAC on the mRNA expression levels of inflammatory genes associated with the NF-κB pathway during chronic heat stress-induced changes in the ovaries. The relative mRNA expression levels of NF-κB, IL-8, IKK-α, TNF-α, IL-18, and IFN-γ between the CON group and the CN group were not significantly different throughout the trial (P > 0.05). Their levels in the HS group were increased in comparison with the CON group. Our analysis demonstrated that chronic heat stress caused inflammatory damage to the ovaries of growing pullets. In contrast, the expression of all of these genes in the HSN group was decreased in comparison with the HS group. From our results, it appears that on day 7, the levels of NF-κB, IL-18, IL-8, and IFN-γ were very significantly decreased (P < 0.01). The levels of IL-8 and IL-18 on day 21 were decreased but without significance (P > 0.05). Otherwise, there were no significant differences in IKK-α on days 7 and 14 (P > 0.05).
Figure 4.
The mRNA expression levels of inflammatory genes associated with the NF-κB pathway in the ovaries. (A–F) The mRNA expression levels of NF-κB, IL-8, IKKα, TNF-α, IL-18, and IFN-γ.
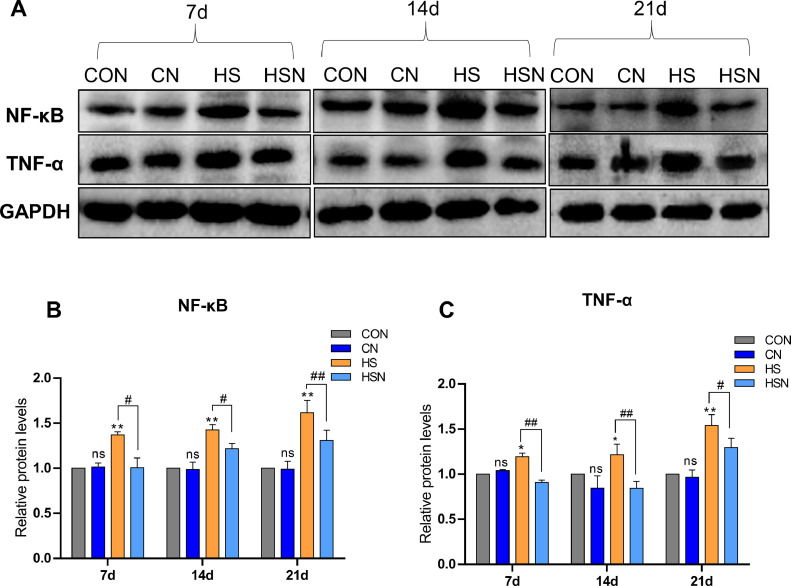
Effects of NAC on the Chronic Heat Stress-Induced Changes in the Protein Expression Levels of NF-κB and TNF-α in the Ovaries
The protein expression levels of NF-κB and TNF-α were verified to demonstrate the effects of NAC on the heat stress-induced changes in the ovaries (Figure 5). The protein expression levels of NF-κB and TNF-α were not significantly different between the CON group and the CN group (P > 0.05). In the HS group, the protein expression levels of NF-κB and TNF-α showed an increasing trend with an increasing duration of chronic heat stress in comparison with the CON group on days 7, 14, and 21 (P < 0.05 or P < 0.01). In contrast, after supplementation with NAC, the expression levels of NF-κB and TNF-α were significantly decreased at all three time points in comparison with the HS group (P < 0.05 or P < 0.01).
Figure 5.
Protein expression levels of NF-κB and TNF-α in the ovaries. (A) The western blotting results of NF-κB and TNF-α. (B, C) Quantification of NF-κB and TNF-α.
DISCUSSION
For laying hens, encountering constant hot temperatures during peak egg production can have serious effects on their performance and egg production traits (Ma et al., 2021). Post heat stress disorder disrupts the normal egg-laying process (Orhan et al., 2013; Pawar et al., 2016). Nevertheless, exactly “how” the effects of heat stress are associated with functional changes in the ovaries of growing pullets remains unknown. It is clearly related to whether long-lasting heat stress can hinder ovarian development and dysfunction during the prelaying period, which impairs their laying performance in the future (Gan et al., 2018). Following this notion, in our 3-wk trial, we investigated the mechanisms by which chronic heat stress impacts ovarian performance in growing pullets and found some corresponding protective measures. From the results obtained, the ovaries of growing pullets under chronic heat stress showed obvious damage, such as ovarian interstitial hemorrhage, follicular atresia, and a loose arrangement of granulosa cells. Combined with the previous research results, these results suggest that heat stress could cause ovarian damage in laying hens at different periods (Rozenboim et al., 2007; Cheng et al., 2018). Interestingly, NAC treatment effectively mitigated these pathological alterations in the ovaries and protected them from oxidative stress and inflammation caused by chronic heat stress.
Heat stress disrupts follicular development in poultry, triggering oxidative stress, and leading to increased follicular atresia (Yan et al., 2020). The balance of the antioxidant system of the body is compromised. Preliminary evidence suggests that Nrf2 is the main regulator of the body's response to oxidative damage and it can activate a variety of antioxidant enzymes to mitigate heat stress, such as SOD and GSH-Px (Malhotra et al., 2008; Akbarian et al., 2016). Dynamic changes in the activities of these enzymes depend on the intensity, severity and duration of the heat stress (Zhao et al., 2018). When the severity and duration of the heat stress surpass the dynamic balance of the body, excessive ROS are produced and the oxidative damage is alleviated (Wang et al., 2019; Hu et al., 2020). The activities of CAT, SOD, GSH-Px, and T-AOC in the ovaries of growing pullets were inhibited by chronic heat stress on days 7, 14, and 21. Our interpretation is that the intensity and duration of chronic heat stress on the ovaries of growing pullets exceeded the regulatory capacity of these antioxidant enzymes, and created an imbalance in the antioxidative-oxidative system that overwhelmed their intrinsic antioxidant defense. As a marker of lipid peroxidation damage, a higher MDA contents indicates, increased lipid peroxide accumulation and greater oxidative damage to tissue (Zhou et al., 2020). The concentrations of MDA were enhanced at all three time points in the HS group. From our results, it appears that the ovaries were strongly affected by lipid peroxidation damage during heat stress. Notably, a high level of HSP70 is related to the antioxidant defense ability of poultry under heat stress (Roushdy et al., 2018; Tang et al., 2018). From the results obtained, the mRNA expression levels of HSP70 in the ovaries were significantly increased under chronic heat stress. Hence, protective measures can be proposed by relying on the discoveries of these mechanisms to alleviate heat stress by the ovaries. As an ROS scavenger, NAC is known to increase the activities of SOD and GSH-Px in a thermal exposure environment by activating the Nrf2 signaling pathway, thus inhibiting the activities of MDA and HSP70 (Wang et al., 2018; Dash et al., 2020). When supplemented with NAC, the HSN group had increased CAT, SOD, GSH-Px, and T-AOC activity to different degrees on days 7, 14, and 21, and the inhibition of MDA and HSP70 increased at the same time. Thus, the NAC-activated Nrf2 signaling pathway plays an important role in mitigating heat stress to disrupt antioxidant-oxidant systems.
Incidentally, activation of the Nrf2 signaling pathway indicates that Nrf2 dissociates from Keap1 and then binds to the antioxidant response element ARE (Gao et al., 2020). The expression of downstream antioxidant proteins (such as HO-1) regulated by ARE and phase II detoxification enzymes (such as SOD2 and GST) usually occur together (Copple et al., 2008; Gao et al., 2020). When Japanese quails were exposed to cyclic chronic HS, they had reduced expression of Nrf2 and decreased antioxidant enzyme activities (Sahin et al., 2012; Orhan et al., 2013). Under our chronic heat stress model, the mRNA levels of Nrf2, HO-1, SOD2, and GST were significantly decreased on days 14 and 21, and the protein expression levels of Nrf2 and HO-1 were also significantly decreased. Most notably, on day 7, the mRNA levels of Nrf2, HO-1, and GST were significantly increased, and the protein expression levels of HO-1 were also significantly increased. It has been proposed that acute heat stress enhances the activities of antioxidant enzymes in the body, and then this decreases over time (Xie et al., 2015). We found that Nrf2 was still active on day 7. Meanwhile, Malhotra et al. (2008) mentioned that SOD2 expression is reduced when the body's antioxidant capacity is no longer sufficient to resist external thermal stimuli. In response to NAC supplementation, the activated Nrf2 signaling pathway upregulated the mRNA and protein expression levels of HO-1, SOD2 and GST, which are the downstream antioxidant genes of Nrf2. Therefore, we hypothesized that NAC under chronic heat stress could activate the Nrf2 signaling pathway and its downstream antioxidant genes to effectively improve the resistance of growing pullet ovaries to oxidative stress.
When oxidative stress occurs in animals, one of the transcription factors regulated by free radicals, NF-κB, can be activated, which results in the expression of related proinflammatory factors, TNF-α, IL-8, and IL-18, which then results in inflammation (Zhivotovsky and Kaminskyy, 2013). In addition, NF-κB-induced kinase (NIK) activates IKKα, which induces I-κBα ubiquitination and proteasome degradation under heat stress, resulting in NF-κB release and translocation to the nucleus, further stimulating NF-κB activation (Liu et al., 2018; Zhao et al., 2021). In response to inflammation, interferon γ (IFN-γ) is abnormally produced, which affects the body's immune function (Tang et al., 2021). At the same time, TNF-α and IFN-γ can induce the production of inducible nitric oxide synthase, which leads to the production of nitric oxide leading to a positive feedback cycle that exacerbates the inflammatory response damage (Jaiswal et al., 2000). Under chronic heat stress, the mRNA and protein expression levels of NF-κB and TNF-α were significantly increased to different degrees on days 7, 14, and 21. Consistently, the mRNA levels of IL-8, IKKα, IL-18, and IFN-γ were significantly increased. Gao et al. (2020) used quercetin to protect the livers of mice from inflammatory injury induced by high-intensity exercise stress, suggesting that inhibition of the NF-κB signaling pathway may play a role in its anti-inflammatory effects. Mohan et al. (2020) indicated that activation of the Nrf2 signaling pathway could enhance the activity of antioxidant enzymes and reduce the expression of inflammatory factors. In addition, NAC has been shown to have immunomodulatory effects and can downregulate levels of proinflammatory markers TNFα and IL-8 (Ahmed et al., 2013; Samuni et al., 2013). Based on these principles, we tested whether NAC regulated the NF-κB pathway in the ovaries of growing pullets at the mRNA and protein levels. Interestingly, the Nrf2 signaling pathway in the ovaries was activated, while both the mRNA and protein levels of NF-κB and its related proinflammatory factors were significantly decreased after NAC administration in the HSN group. Hence, we provide insights into the finding that NAC can inhibit the NF-κB signaling pathway under chronic heat stress in ovaries, which reduces the proinflammatory factor expression levels and mitigates the inflammatory response.
CONCLUSIONS
In conclusion, supplementing with NAC during chronic heat stress could alleviate the pathological changes in the ovaries of growing pullets. In addition, NAC protected ovaries from chronic heat stress-induced oxidative damage by regulating SOD, GSH-Px, CAT, and T-AOC activities, reducing the concentration of MDA and the level of HSP70, and activating the Nrf2 signaling pathway-related antioxidant genes HO-1, SOD2 and GST. Most notably, NAC inhibited the expression of the NF-κB signaling pathway-related proinflammatory factors IL-8, IL-18, TNF-α, IKK-α, and IFN-γ, thus reducing inflammatory damage and facilitating the development of the ovaries in growing pullets.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of Jiangxi Province [grant number 2017ACB20012], National Natural Science Foundation of China [grant number 32202880, 32060760], the Technology System of Modern Agricultural Poultry Industry of Jiangxi Province [grant number JXARS], and the earmarked fund for innovation team of Jiangxi Agricultural University [grant number JXAUCXTD006].
DISCLOSURES
We declare that there are no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2022.102274.
Appendix. Supplementary materials
REFERENCES
- Ahmed M., Lamia M., Hend M., Abd E. High dose N-acetyl cysteine improves inflammatory response and outcome in patients with COPD exacerbations. Egypt. J. Chest Dis. Tu. 2013;62:51–57. [Google Scholar]
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Biotechnol. Sci. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Z., Wang M. Warmer temperature increases mercury toxicity in a marine copepod. Ecotoxicol. Environ. Saf. 2020;201 doi: 10.1016/j.ecoenv.2020.110861. [DOI] [PubMed] [Google Scholar]
- Bao B., Thakur A., Li Y., Ahmad A., Azmi A., Banerjee S., Kong D., Ali S., Lum L., Sarkar F. The immunological contribution of NF-κB within the tumor microenvironment: a potential protective role of zinc as an anti-tumor agent. BBA-Biomembranes. 2012;1825:160–172. doi: 10.1016/j.bbcan.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Cheng Y., Wen C., Zhou Y. Protective effects of dietary mannan oligosaccharide on heat stress-induced hepatic damage in broilers. Environ. Pollut. R. Sci. 2020;27:29000–29008. doi: 10.1007/s11356-020-09212-2. [DOI] [PubMed] [Google Scholar]
- Cheng C.Y., Tu W.L., Chen C.J., Chan H.L., Chen C.F., Chen H.H., Tang P.C., Lee Y.P., Chen S.E., Huang S.Y. Functional genomics study of acute heat stress response in the small yellow follicles of layer-type chickens. Sci. Rep. 2018;8:1320. doi: 10.1038/s41598-017-18335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copple I.M., Goldring C.E., Jenkins R.E., Chia A.J., Randle L.E., Hayes J.D., Kitteringham N.R., Park B.K. The hepatotoxic metabolite of acetaminophen directly activates the Keap1-Nrf2 cell defense system. Hepatology. 2008;48:1292–1301. doi: 10.1002/hep.22472. [DOI] [PubMed] [Google Scholar]
- Dash M., Dey A., Chattopadhyay S. Mitigation of arsenic driven utero-ovarian malfunction and changes of apoptotic gene expression by dietary NAC. Ecotox. Environ. Safe. 2020;199 doi: 10.1016/j.ecoenv.2020.110675. [DOI] [PubMed] [Google Scholar]
- de Andrade K.Q., Moura F.A., dos Santos J.M., de Araujo O.R., de Farias Santos J.C., Goulart M.O. Oxidative stress and inflammation in hepatic diseases: therapeutic possibilities of N-Acetylcysteine. Int. J. Mol. Sci. 2015;16:30269–30308. doi: 10.3390/ijms161226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane C.A.S., Brown I.R. Knockdown of heat shock proteins HSPA6 (Hsp70B') and HSPA1A (Hsp70-1) sensitizes differentiated human neuronal cells to cellular stress. Neurochem. Res. 2018;43:340–350. doi: 10.1007/s11064-017-2429-z. [DOI] [PubMed] [Google Scholar]
- Gan L., Fan H., Nie W., Guo Y. Ascorbic acid synthesis and transportation capacity in old laying hens and the effects of dietary supplementation with ascorbic acid. J. Anim. Sci. Biotechnol. 2018;9:71. doi: 10.1186/s40104-018-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., Liu Y., Jiang C., Liu L., Li J., Li D., Guo X., Wang Z., Yang Y., Liu L., Yao P., Tang Y. Intensive running enhances NF-κB activity in the mice liver and the intervention effects of quercetin. Nutrients. 2020;12:2770. doi: 10.3390/nu12092770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Li X., Wang Y., Cao Y., Yao D., Sun L., Qin L., Qiu H., Zhan X. Adipocyte-derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. Acta Physiol. (Oxford, England) 2020;228:e13339. doi: 10.1111/apha.13339. [DOI] [PubMed] [Google Scholar]
- Hu H., Dai S., Li J., Wen A., Bai X. Glutamine improves heat stress-induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2-related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult. Sci. 2020;99:1454–1461. doi: 10.1016/j.psj.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Cheng L., Wang X., Luo G., Zhao T., Tian J., An L. N-acetyl-l-cysteine protects porcine oocytes undergoing meiotic resumption from heat stress. Reprod. Toxicol. 2020;91:27–34. doi: 10.1016/j.reprotox.2019.10.006. [DOI] [PubMed] [Google Scholar]
- Huang W., Xu L., Zhou X., Gao C., Yang M., Chen G., Zhu J., Jiang L., Gan H., Gou F., Feng H., Peng J., Xu Y. High glucose induces activation of NF-κB inflammatory signaling through IκBα sumoylation in rat mesangial cells. Biochem. Bioph. Res. Co. 2013;438:568–574. doi: 10.1016/j.bbrc.2013.07.065. [DOI] [PubMed] [Google Scholar]
- Jaiswal M., LaRusso N.F., Burgart L.J., Gores G.J. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- Koch F., Thom U., Albrecht E., Weikard R., Nolte W., Kuhla B., Kuehn C. Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc. Natl. Acad. Sci. U. S. A. 2019;116:10333–10338. doi: 10.1073/pnas.1820130116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Yu H., Baiyun R., Lu J. Protective effects of dietary luteolin against mercuric chloride-induced lung injury in mice: Involvement of AKT/Nrf2 and NF-kappa B pathways. Food Chem. Toxicol. 2018;113:296–302. doi: 10.1016/j.fct.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Liu L., Fu C., Yan M., Xie H., Li S., Yu Q., He S., He J. Resveratrol modulates intestinal morphology and HSP70/90, NF-kappaB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016;7:1329–1338. doi: 10.1039/c5fo01338k. [DOI] [PubMed] [Google Scholar]
- Ma Y., Shi Y., Wu Q., Ma W. Dietary arsenic supplementation induces oxidative stress by suppressing nuclear factor erythroid 2-related factor 2 in the livers and kidneys of laying hens. Poult. Sci. 2021;100:982–992. doi: 10.1016/j.psj.2020.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra D., Thimmulappa R., Navas-Acien A., Sandford A., Elliott M., Singh A., Chen L., Zhuang X., Hogg J., Pare P., Tuder R., Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J. Resp. Crit. Care. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mays C., Armijo E., Morales R., Kramm C., Flores A., Tiwari A., Bian J., Telling G., Pandita T., Hunt C., Soto C. Prion disease is accelerated in mice lacking stress-induced heat shock protein 70 (HSP70) J. Biol. Chem. 2019;294:13619–13628. doi: 10.1074/jbc.RA118.006186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Q., Si X., Xie Y., Chen L., Liu Z., Liu L., Tang X., Zhang H. Effects of acute heat stress at different ambient temperature on hepatic redox status in broilers. Poult. Sci. 2020;99:4113–4122. doi: 10.1016/j.psj.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan T., Narasimhan K.K.S., Ravi D.B., Velusamy P., Chandrasekar N., Chakrapani L.N., Srinivasan A., Karthikeyan P., Kannan P., Tamilarasan B., Johnson T., Kalaiselvan P., Periandavan K. Role of Nrf2 dysfunction in the pathogenesis of diabetic nephropathy: therapeutic prospect of epigallocatechin-3-gallate. Free Radic. Biol. Med. 2020;160:227–238. doi: 10.1016/j.freeradbiomed.2020.07.037. [DOI] [PubMed] [Google Scholar]
- Nitescu N., Ricksten S.E., Marcussen N., Haraldsson B., Nilsson U., Basu S., Guron G. N-acetylcysteine attenuates kidney injury in rats subjected to renal ischaemia-reperfusion. Nephrol. Dial. Transplant. 2006;21:1240–1247. doi: 10.1093/ndt/gfk032. [DOI] [PubMed] [Google Scholar]
- Orhan C., Tuzcu M., Gencoglu H., Sahin N., Hayirli A., Sahin K. Epigallocatechin-3-gallate exerts protective effects against heat stress through modulating stress-responsive transcription factors in poultry. Br. Poult. Sci. 2013;54:447–453. doi: 10.1080/00071668.2013.806787. [DOI] [PubMed] [Google Scholar]
- Ozdemir O., Tuzcu M., Sahin N., Orhan C., Tuzcu Z., Sahin K. Organic chromium modifies the expression of orexin and glucose transporters of ovarian in heat-stressed laying hens. Cell. Mol. Biol. 2017;63:93–98. doi: 10.14715/cmb/2017.63.10.15. [DOI] [PubMed] [Google Scholar]
- Park M., Hong J. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5:15. doi: 10.3390/cells5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar S.S., Sajjanar B., Lonkar V.D., Kurade N.P., Bal S.K. Assessing and mitigating the impact of heat stress in poultry. Adv. Anim. Vet. Sci. 2016;4:6. [Google Scholar]
- Roth Z. Reproductive physiology and endocrinology responses of cows exposed to environmental heat stress - experiences from the past and lessons for the present. Theriogenology. 2020;155:150–156. doi: 10.1016/j.theriogenology.2020.05.040. [DOI] [PubMed] [Google Scholar]
- Roushdy E.M., Zaglool A.W., El-Tarabany M.S. Effects of chronic thermal stress on growth performance, carcass traits, antioxidant indices and the expression of HSP70, growth hormone and superoxide dismutase genes in two broiler strains. J. Therm. Biol. 2018;74:337–343. doi: 10.1016/j.jtherbio.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Tako E., Gal-Garber O., Proudman J.A., Uni Z. The effect of heat stress on ovarian function of laying hens. Poult. Sci. 2007;86:1760–1765. doi: 10.1093/ps/86.8.1760. [DOI] [PubMed] [Google Scholar]
- Sahin K., Orhan C., Tuzcu Z., Tuzcu M., Sahin N. Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food Chem. Toxicol. 2012;50:4035–4041. doi: 10.1016/j.fct.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Samuni Y., Goldstein S., Dean O.M., Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta. 2013;1830:4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Tang L.P., Liu Y.L., Ding K.N., Hou X.J., He Y.M. Chai Hu oral liquid enhances the immune functions of both spleen and bursa of Fabricius in heat-stressed broilers through strengthening TLR4-TBK1 signaling pathway. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Yin B., Xu J., Bao E. Rosemary reduces heat stress by inducing CRYAB and HSP70 expression in broiler chickens. Oxid. Med. Cell Longev. 2018;2018 doi: 10.1155/2018/7014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videla L.A., Valenzuela R. Perspectives in liver redox imbalance: toxicological and pharmacological aspects underlying iron overloading, nonalcoholic fatty liver disease, and thyroid hormone action. Biofactors. 2022;48:400–415. doi: 10.1002/biof.1797. [DOI] [PubMed] [Google Scholar]
- Wang X., Liu J., Zhang X., Zhao S., Zou K., Xie J., Wang X., Liu C., Wang J., Wang Y. Seabuckthorn berry polysaccharide extracts protect against acetaminophen induced hepatotoxicity in mice via activating the Nrf-2/HO-1-SOD-2 signaling pathway. Phytomedicine. 2018;38:90–97. doi: 10.1016/j.phymed.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang C., Elsheikh N.A.H., Li C., Yang F., Wang G., Li L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging (Albany NY) 2019;11:5535–5547. doi: 10.18632/aging.102136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Tang L., Lu L., Zhang L., Lin X., Liu H., Odle J., Luo X. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poult. Sci. 2015;94:1635–1644. doi: 10.3382/ps/pev105. [DOI] [PubMed] [Google Scholar]
- Yan J., Deng D., Wu Y., Wu K., Qu J., Li F. Catalpol protects rat ovarian granulosa cells against oxidative stress and apoptosis through modulating the PI3K/Akt/mTOR signaling pathway. Biosci. Rep. 2020;40 doi: 10.1042/BSR20194032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D., Hou Y., Tan L., Liao M., Xie J., Wang L., Ding B., Yang Y., Gong J. N-acetylcysteine improves the growth performance and intestinal function in the heat-stressed broilers. Anim. Feed Sci. Tech. 2016;220:83–92. [Google Scholar]
- Zhao Q., Zhou L., Liu J., Du X., Asad M.A., Huang F., Pan G., Cheng F. Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant. Physiol. Biochem. 2018;122:90–101. doi: 10.1016/j.plaphy.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhuang Y., Shi Y., Xu Z., Zhou C., Guo L., Liu P., Wu C., Hu R., Hu G., Guo X., Xu L. Effects of N-acetyl-l-cysteine on heat stress-induced oxidative stress and inflammation in the hypothalamus of hens. J. Therm. Biol. 2021;98 doi: 10.1016/j.jtherbio.2021.102927. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B., Kaminskyy V.O. Free radicals in cross talk between autophagy and apoptosis. Antioxid. Redox Sign. 2013;21:86–102. doi: 10.1089/ars.2013.5746. [DOI] [PubMed] [Google Scholar]
- Zhou X., Zhang Z., Liu X., Wu D., Ding Y., Li G., Wu Y. Typical reactive carbonyl compounds in food products: formation, influence on food quality, and detection methods. Compr. Rev. Food. Sci. F. 2020;19:503–529. doi: 10.1111/1541-4337.12535. [DOI] [PubMed] [Google Scholar]
- Zhu L., Liao R., Wu N., Zhu G., Yang C. Heat stress mediates changes in fecal microbiome and functional pathways of laying hens. Appl. Microbiol. Biot. 2019;103:461–472. doi: 10.1007/s00253-018-9465-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.