ABSTRACT
This cross-sectional observational study that describes the epidemiological data of the first year of the COVID-19 pandemic in the Mato Grosso do Sul State, aimed to demonstrate the differences between indigenous and non-indigenous populations, characterize confirmed cases of COVID-19 according to risk factors related to ethnicity, comorbidities and their evolution and to verify the challenges in facing the disease in Brazil. SIVEP-Gripe and E-SUS-VE, a nationwide surveillance database in Brazil, from March 2020 to March 2021 in Mato Grosso do Sul state, were used to compare survivors and non-survivors from indigenous and non-indigenous populations and the epidemiological incidence curves of these populations. A total of 176,478, including 5,299 indigenous people, were confirmed. Among the indigenous population, 52.5% (confidence interval [CI] 51.2-53.9) were women, 38% (CI 36.7-39.4) were 20-39 years old, 56.7% were diagnosed by rapid antibody tests, 12.3% (CI 95%:11.5-13.2) had at least one comorbidity, and 5.3% (CI 95%:4.7–5.9) were hospitalized. In the non-indigenous patients, 56.8% were confirmed using RT-PCR, 4.4% (CI 95%:4.3-4.5) had at least one comorbidity, and 8.0% (CI 95%:7.9-8.2) were hospitalized. The majority of non-survivors were ≥60 years old (65.1% indigenous vs. 74.1% non-indigenous). The mortality in indigenous people was more than three times higher (11% vs. 2.9%). Indigenous people had a lower proportion of RT-PCR diagnoses; deaths were more frequent in younger patients and were less likely to be admitted to hospital. Mass vaccination may have controlled the incidence and mortality associated with COVID-19 in this population during the period of increased viral circulation.
Keywords: Health of indigenous peoples, Epidemiology, Coronavirus infections, COVID-19
INTRODUCTION
Indigenous populations in Brazil experience a disproportionate burden of disease due to geographical and socioeconomic factors and poorer social determinants of health than non-indigenous people 1 . The coronavirus disease 2019 (COVID-19) pandemic has raised great concerns about the increased risk of mortality in indigenous people. The spread of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in indigenous communities has exposed a failing health system, involving challenges with access to health services, social distancing, effective communication, and social support 2 - 4 . Data on the conduct and effects of pandemics on indigenous people are scarce. Thus, assessing the impact of this disease on this population is urgently needed.
The Mato Grosso do Sul State, located in the south of the Central-West region of Brazil, is home to the second largest indigenous population in the country. Bordering Paraguay and Bolivia, with a population of approximately 2.8 million inhabitants and great ethnic diversity, the state presents major challenges for health surveillance. Indigenous villages are located close to urban areas, where social and commercial relations with non-indigenous people can expedite the spread of COVID-19 in indigenous territories. Therefore, it is expected that the epidemiology of the disease in indigenous and non-indigenous people will follow similar patterns owing to their geographical proximity and unrestricted contact 4 .
Brazil has one of the worst responses to the COVID-19 pandemic worldwide 5 - 7 . Non-pharmaceutical interventions, such as testing all suspected cases, physical distancing measures, income support for households affected by COVID-19, and associated interventions (especially for more vulnerable populations as indigenous), have not been widely adopted 8 - 10 . In the period up to March 2021, the cumulative incidence rate of COVID-19 in Brazil was 6,117.2 cases per 100,000 inhabitants, and the cumulative mortality rate was 155.9 deaths per 100,000 inhabitants. In the same period, the incidence rate in the indigenous population was 515.9 per 100,000 inhabitants, and the mortality rate was 11.1 per 100,000 inhabitants.
Owing to a shortage of vaccines, the immunization plan proposed by the Ministry of Health of Brazil consists of four phases based on priority groups, with indigenous people living in villages included in phase one (indigenous people living in cities were not included). At this phase, two doses of the CoronaVac vaccine (Sinovac Life Sciences, Beijing, China) have been offered to all indigenous adults over 18 years of age since January 2021.
This cross-sectional observational study that describes the epidemiological data of the first year of the COVID-19 pandemic in the Mato Grosso do Sul State, aimed to demonstrate the differences between indigenous and non-indigenous populations, characterize confirmed cases of COVID-19 according to risk factors related to ethnicity, comorbidities and their evolution and to verify the challenges in facing the disease in Brazil
MATERIALS AND METHODS
Study setting and population
We performed a cross-sectional observational study of COVID-19 in indigenous and non-indigenous patients from Mato Grosso do Sul State in the Central-West Region of Brazil, with a population of approximately 2.8 million people. It has the second largest indigenous population in Brazil, consisting of 80,841 people. The main ethnicities of this population include Guarani-Kaiowa, Terena, and Guarani-Nhandeva, representing 96% of the indigenous population of the state 11 .
We did a retrospective analysis between March 1, 2020, and March 31, 2021, of all COVID-19 cases registered in the Influenza Epidemiological Surveillance Information System, SIVEP-Gripe (Sistema de Informacao de Vigilancia Epidemiologica da Gripe), and E-SUS Epidemiological Surveillance (E-SUS Vigilancia Epidemiologica - E-SUS-VE), a nationwide surveillance databases used to monitor COVID-19 infections in Brazil. We accessed the data through the Health Department of the Mato Grosso do Sul State, which checks notifications daily, excludes incomplete and duplicate data, and provides anonymized organized data.
All cases who had their ethnicity recorded in the dataset were included in this study. Cases confirmed by laboratory or clinical criteria, as defined by the Ministry of Health, were selected 12 . This study was approved by the National Research Ethics Commission and the National Research Ethics Commission of the affiliated institution (Nº 4.311.712). Patients with laboratory confirmation for SARS-CoV-2 screening (reverse transcriptase-polymerase chain reaction [RT-PCR] or antigen screening) or immunological testing through antibody screening (before the vaccination period) were considered confirmed cases.
Data collection and study variables
COVID-19 incidence by municipality was calculated as the ratio between the absolute number of cases and the resident population in the municipality multiplied by 100,000. Data on the resident population by municipality correspond to the estimates by the Federal Accounts Court (TCU) for 2020, based on census data from the Brazilian Institute of Geography and Statistics in 2010 13 . The indigenous population estimated by municipality was based on the Special Secretary of Indigenous Health population data 11 .
Demographic data (ethnicity, sex, age, and city of residence), laboratory test results (RT-PCR and rapid tests), comorbidities, patient hospitalization, and clinical outcomes were evaluated. Data from indigenous and non-indigenous populations were evaluated separately.
Statistical analysis
Descriptive analysis included frequency analysis (%) and 95% confidence intervals for categorical variables, and the chi-square test was used to compare differences between groups where appropriate. The R Statistic (version 4.0, Windows, Microsoft, USA) was used for all the calculation. Spatial distribution was analyzed and processed in the GIS environment (QGIS version 3.8 Open Source, Geospatial Foundation Project), with meshes and vector points that considered municipalities with confirmed cases and incidence higher than 5,000 cases per 100,000 inhabitants in the indigenous population. The epidemic curve of COVID-19 cases was obtained based on the absolute daily variation of the new cases, 7-day moving average, and cumulative cases during the period. The digital mesh of the 79 municipalities was obtained from the Brazilian Institute of Geography and Statistics 14 .
RESULTS
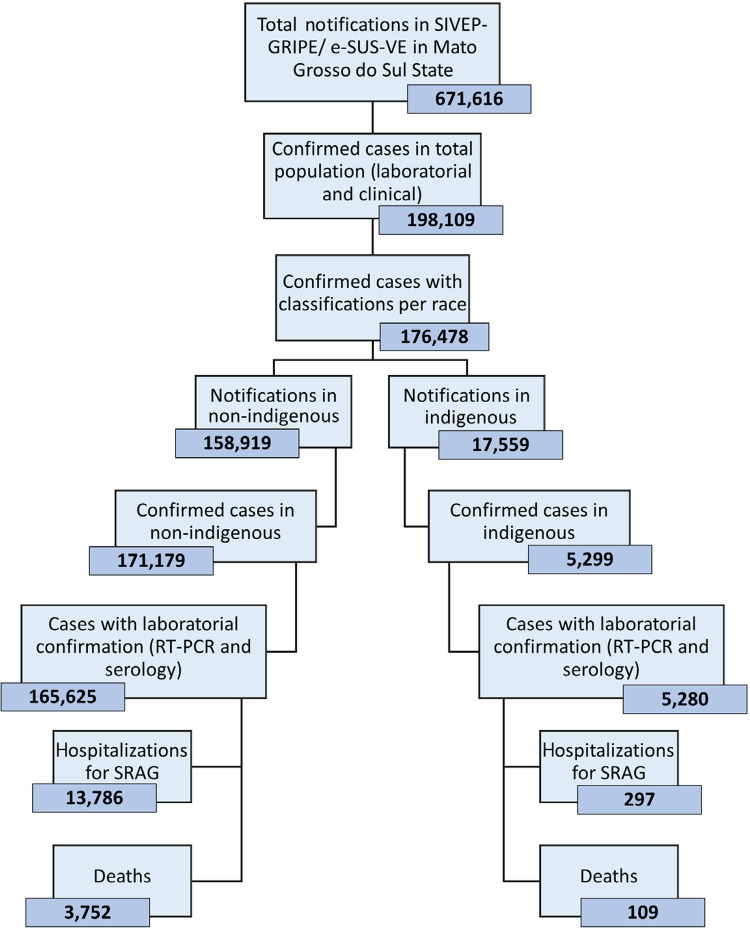
During the study period, 671,616 records were registered in the SIVEP-Gripe and e-SUS-VE datasets. Of these, 198,109 were COVID-19-confirmed cases and 176,478 had their ethnicity recorded, including 5,299 indigenous people ( Figure 1 ).
Figure 1. Flowchart of SIVEP-GRIPE/ e-SUS-VE data used in this study. SIVEP Gripe = Sistema de Informacao de Vigilancia Epidemiologica da Gripe; e-SUS-VE = E-SUS Vigilancia Epidemiologica.
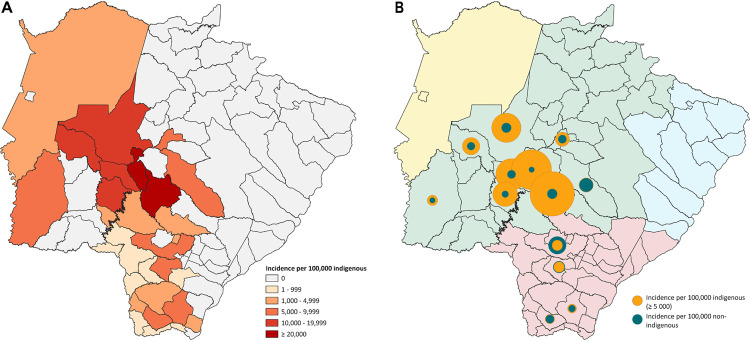
The global incidence per 100,000 inhabitants was similar between the indigenous and non-indigenous populations (6617 and 6543, respectively) ( Figure 2A ). However, in the north central regions of the state, the incidence in some cities was up to seven times higher among indigenous people, such as Anastacio (18,290 vs. 4,884), Aquidauana (17,085 vs. 5,731), Dois Irmao do Buriti (23,261 vs. 3,322), and Sidrolandia (25,897 vs. 5,832) ( Figure 2 ).
Figure 2. Distribution of COVID-19 cases: A) Proportional spatial distribution of incidence due to COVID-19 in the indigenous and non-indigenous populations; B) Distribution of the incidence of COVID-19-confirmed cases in the indigenous population by municipality.
Profile of COVID-19 cases
In the indigenous population, 52.5% (CI 95%:51.2-53.9) of COVID-19-confirmed cases were women, and 38% (CI 95%:36.7-39.4) were aged between 20 and 39 years, with an average age of 32 years. Regarding diagnostic tests, 56.7% of indigenous individuals were confirmed by rapid antibody tests, whereas in non-indigenous individuals, 56.8% were confirmed by RT-PCR. Among indigenous people, 12.3% (CI 95%:11.5-13.2) had at least one comorbidity, whereas 4.4% (CI 95%:4.3-4.5) of non-indigenous people had at least one comorbidity. In the indigenous population, 5.3% (CI 95%:4.7-5.9) of the cases were hospitalized, while it was 8.0% (CI 95%:7.9-8.2) in the non-indigenous population. The case fatality rates were 2% (CI 95%:1.7-2.5) and 2.2% (CI 95%:2.1-2.3) in the indigenous and non-indigenous populations, respectively ( Table 1 ).
Table 1. Demographics, baseline characteristics, and clinical outcomes of the indigenous and non-indigenous patients with COVID-19 March 2020 to March 2021 in Mato Grosso do Sul State.
| Indigenous (N=5.299) | % | CI | Non-Indigenous (N=171.179) | % | CI | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 2.515 | 47.5 | 46.1 – 48.8 | 79.587 | 46.5 | 46.3 – 46.3 |
| Female | 2.784 | 52.5 | 51.2 – 53.9 | 91.592 | 53.5 | 53.3 – 53.7 |
| Age | ||||||
| 0–19 | 1.328 | 25.1 | 23.1 – 26.3 | 17.198 | 10.0 | 9.9 – 10.2 |
| 20–39 | 2.015 | 38.0 | 36.7 – 39.4 | 74.052 | 43.3 | 43.0 – 43.5 |
| 40–59 | 1.312 | 24.8 | 23.6 – 25.9 | 56.676 | 33.1 | 32.9 – 33.3 |
| >=60 | 644 | 12.2 | 11.3 – 13.1 | 23.253 | 13.6 | 13.4 – 13.7 |
| Laboratorial testing | ||||||
| IgM/IgG rapid tests | 3.003 | 56.7 | 55.3 – 58.0 | 68.399 | 40.0 | 39.7 – 40.2 |
| RT-PCR | 2.272 | 42.9 | 41.5 – 44.2 | 97.226 | 56.8 | 56.6 – 57.1 |
| Not informed | 24 | 0.5 | 0.3 – 0.7 | 5.534 | 3.2 | 3.2 - 3.3 |
| Comorbidities | ||||||
| Only one | 653 | 12.3 | 11.5 – 13.2 | 7.502 | 4.4 | 4.3 – 4.5 |
| Two | 132 | 2.5 | 2.1 – 3.0 | 1.855 | 1.1 | 1.0 – 1.2 |
| More than two | 65 | 1.2 | 1.0 – 1.6 | 1.098 | 0.64 | 0.60 – 0.68 |
| Not informed | 4.447 | 84 | 83.0 – 85.0 | 160.676 | 93.9 | 93.7 – 94.0 |
| Hospital admission | 281 | 5.3 | 4.7 – 5.9 | 13.786 | 8.0 | 7.9 – 8.2 |
| Died | 109 | 2.0 | 1.7– 2.5 | 3.752 | 2.2 | 2.1 -2.3 |
RT-PCR = reverse-transcriptase polymerase chain reaction.
Profile of non-survivors for COVID-19
Regarding indigenous non-survivors, 56.9% of the deaths occurred among men, 65.1% were ≥60 years old, and 14.7% had at least one comorbidity. Furthermore, indigenous people had a higher prevalence of diabetes mellitus (31.8% vs. 24.6%) and chronic kidney disease (6.8% vs. 3.5%). In the non-indigenous population, 54.1% were men, 74.1% were aged ≥60 years, and 30% had at least one comorbidity ( Table 2 ). The most prevalent comorbidities were cardiovascular diseases (29.4%), diabetes mellitus (24.1%), and obesity (8%). Non-indigenous people had a higher prevalence of cardiovascular disease (24.6% vs. 18.5%) and obesity (8% vs. 6.5%). The number of deaths in a non-hospital setting was more than three times higher among indigenous people (11% vs. 2.9%) ( Table 2 ), with the most prevalent comorbidities being cardiovascular disease (29.4%), diabetes mellitus (24.1%), and obesity (8%). Indigenous individuals had a higher proportion of diabetes mellitus (31.8% vs. 24.6%) and chronic kidney disease (6.8% vs. 3.5%). Cardiovascular disease (24.6% vs. 18.5%) and obesity (8% vs. 6.5%) were more prevalent among non-indigenous populations.
Table 2. Characteristics of the COVID-19-confirmed 2019 cases reported in the SIVEP-Gripe database who died by March 2020 to March 2021 in Mato Grosso do Sul State.
| Indigenous (N=109) | % | CI | Non-Indigenous (N=3,752) | % | CI | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 62 | 56.9 | 47.5 – 65.8 | 2.030 | 54.1 | 52.51 - 55.69 |
| Female | 47 | 43.1 | 34.2 – 52.6 | 1.722 | 45.9 | 44.31 – 47.49 |
| Age | ||||||
| 0-19 | 0 | 0 | 5 | 0.2 | 0.10 – 0.31 | |
| 20-39 | 9 | 8.3 | 4.4 – 14.9 | 140 | 3.7 | 3.17 – 4.39 |
| 40-59 | 29 | 26.6 | 19.2 – 35.6 | 826 | 22.0 | 20.72 – 23.37 |
| >=60 | 71 | 65.1 | 55.8 – 73.4 | 2.781 | 74.1 | 72.69 – 75.50 |
| Comorbidities | ||||||
| Only one | 16 | 14.7 | 9.2 – 22.5 | 1.127 | 30.0 | 28.6 – 31.5 |
| Two | 1 | 0.9 | 0.2 – 5.0 | 350 | 9.3 | 8.4 – 10.3 |
| More than two | 1 | 0.9 | 0.2 – 5.0 | 211 | 5.7 | 4.9 – 6.4 |
| Not informed | 91 | 83.5 | 75.4 – 89.3 | 2.064 | 55.0 | 53.4 – 56.6 |
| Hospital admission | ||||||
| Yes | 96 | 88.1 | 80.7 – 92.90 | 3.621 | 96.5 | 95.87 – 97.05 |
| No | 12 | 11.0 | 6.4 – 18.3 | 107 | 2.9 | 2.37 – 3.43 |
| Not informed | 1 | 0.9 | 0.16 – 5.0 | 19 | 0.6 | 0.32 – 0.79 |
SIVEP Gripe = Sistema de Informacao de Vigilancia Epidemiologica da Gripe.
Cumulative cases
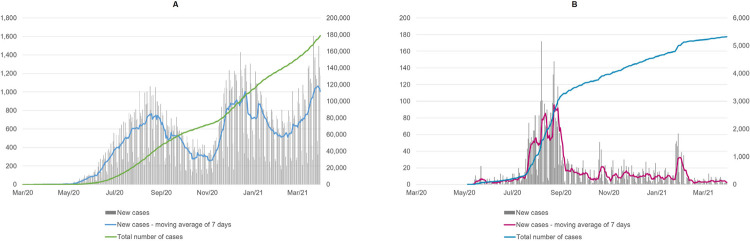
The curves for indigenous and non-indigenous populations exhibited heterogeneous behavior. The highest incidence in indigenous people occurred from July to September 2020, with no further increase in incidence during that period. The acceleration (highest period) of the curve in non-indigenous people was from November 2020 to March 2021, and was not accompanied by any increase in the incidence, making it relatively stable ( Figure 3 ).
Figure 3. Total number of daily recordings of confirmed new COVID-19 cases for one-year, cumulative cases, and moving average of 7 days in the non-indigenous (A), and indigenous (B) population from the Mato Grosso do Sul State.
DISCUSSION
The COVID-19 pandemic has highlighted social vulnerability and inadequate healthcare services in Brazilian indigenous communities, with high incidence and mortality rates in these populations, and we evaluated these vulnerabilities within the second largest indigenous population in Brazil. Our data demonstrated the evolution of the COVID-19 pandemic in its first year among ethnically distinct populations in Mato Grosso do Sul State, Brazil, which is home to the second largest indigenous population in the country. Although the global incidence of the disease per 100,000 inhabitants was similar, we found significant regional variations in the incidence and epidemiological curves between the indigenous and non-indigenous populations.
Our data showed a heterogeneous distribution of incidence and mortality among indigenous people in the state. We hypothesized that the healthcare characteristics differ for each region. Furthermore, there may be differences in the characteristics of indigenous populations in the northern and southern regions. Indigenous people have more individuals under 15 years old and fewer elderly individuals than the overall population 11 . This may partially explain why a greater number of younger indigenous patients tested positive.
The pandemic may disproportionately affect people from minority ethnic communities including indigenous populations 15 - 17 . Indigenous people had more missing data regarding comorbidities and hospitalizations. Therefore, improving the quality and completeness of the data across health and administrative datasets is essential for building a complete picture of ethnic disparities.
In our study, the indigenous cases had a lower proportion of diagnostic tests by RT-PCR, and the non-survivors were less likely to be admitted to a hospital compared to the non-indigenous population. We also identified three times more deaths in a non-hospital setting among indigenous people (11% vs. 2.9%). These disparities may also be reflected in other determinants, such as access to healthcare. Socioeconomic determinants are directly related to access to COVID-19 tests, which may explain the difference in testing between indigenous and non-indigenous people 18 , 19 .
The Brazilian COVID-19 epidemic highlights the non-equitable access to COVID-19 diagnostics as a factor that potentially contributes to the sustained spread of the virus. The lack of investment in RT-PCR, geographical barriers, and ease of access using point-of-care tests can justify the use of rapid antibody tests, even though the gold standard for diagnosing COVID-19 is RT-PCR 18 . Although antibody-based tests are appropriate for surveillance studies, they cannot be used to identify acute cases (transmitters) 20 .
Some studies have shown that COVID-19-associated hospitalization rates have been higher among men than among women 21 , 22 , with the former having higher mortality rates 23 , 24 . Similar to our findings, although the number of women diagnosed with COVID-19 was higher, more men died from the disease in both populations studied. The case fatality rate of COVID-19 was 2.05 and 2.19 per 100,000 people for the indigenous and non-indigenous populations, respectively. Previous data have shown the worst disease lethality in indigenous people 3 , 25 , 26 , though this may have changed due to the decrease in the number of cases and deaths after vaccination. In addition, our assessment covered indigenous peoples from remote and urban areas, failing to differentiate indicators related to spatial localization.
Brazilian states, including Mato Grosso do Sul, saw a further significant increase in cases of COVID-19 as of December 2020, which was termed the “second wave.” The new Brazilian SARS-CoV-2 variant, “Gamma,” has quickly become predominant in the country and was thought to be a large factor behind a massive second wave that has brought the country’s death toll to the second highest in the world 27 . It was hypothesized that the SARS-CoV-2 variants circulating in the second wave might have higher inherent transmissibility than the pre-existing variants 28 , 29 .
Surprisingly, in our analyses, the indigenous population did not experience a significant increase in the number of cases and deaths when the non-indigenous population experienced the worst point of the pandemic. We hypothesized that mass vaccination of indigenous adults protected the population in time to face the second wave, given that no new non-pharmacological measures were implemented during that period.
In terms of vaccination rate, 70% of indigenous people received the first dose, and almost 53% had received the second dose (4 weeks between doses), as of March 2021. A recent study has shown that the CoronaVac vaccine is effective against the gamma variant 30 . Our study reinforces that the number of new infections can be controlled in a population with a vaccine coverage rate, even in a scenario of high circulation of a new variant of concern, gamma.
CONCLUSION
Despite the limitations intrinsic to the descriptive studies of secondary data, which may not reflect the reality in its entirety, we believe that this work fulfilled its purpose of generating hypotheses that should be confirmed with other methodological designs. In addition, the data included all patients who declared themselves to be indigenous, and not just those who resided in indigenous territories.
Regarding clinical data, comorbidities in cases of SARS-CoV-2 do not specify the type or degree of disease, as in cases of cardiovascular disease or kidney disease. In addition, we highlighted the disparities between ethnic minorities in their access to healthcare and the heterogeneous distribution between different municipalities. The indigenous population had a lower average age and fewer comorbidities, but a similar mortality rate as the non-indigenous population. The possible protective effect of the vaccine maintained a stable incidence rate during the worst moments of the pandemic in the state.
The control of the disease in a region with ethnic diversity and different access to healthcare services, with areas bordering two countries with different coping strategies, as well as the lack of coordination of the federal government for COVID-19 control, demonstrates the challenges that Brazilian states face. Our findings can be extrapolated to other regions and help to improve institutional health policies.
Footnotes
FUNDING: This work was funded by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq grant Nº 401727/2020-3), Secretaria do Estado de Saude do Mato Grosso do Sul and Universidade Federal da Grande Dourados. LAO and SS received a research grant from CNPq. Sponsors didn’t take part in data collection, analysis and interpretation nor in manuscript writing.
REFERENCES
- 1.Santos AP, Mazzeti CM, Franco MC, Santos NL, Conde WL, Leite MS, et al. Estado nutricional e condições ambientais e de saúde de crianças Pataxó, Minas Gerais, Brasil. Cad Saude Publica. 2018;34:e00165817. doi: 10.1590/0102-311X00165817. [DOI] [PubMed] [Google Scholar]
- 2.Santos RV, Pontes AL, Coimbra CE., Jr A “total social fact”: COVID-19 and indigenous peoples in Brazil. Cad Saude Publica. 2020;36:e00268220. doi: 10.1590/0102-311X00268220. [DOI] [PubMed] [Google Scholar]
- 3.Cupertino GA, Cupertino MC, Gomes AP, Braga LM, Siqueira-Batista R. COVID-19 and Brazilian indigenous populations. Am J Trop Med Hyg. 2020;103:609–612. doi: 10.4269/ajtmh.20-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simionatto S, Barbosa M, Marchioro SB. COVID-19 in Brazilian indigenous people: a new threat to old problems. Rev Soc Bras Med Trop. 2020;53:e20200476. doi: 10.1590/0037-8682-0476-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferigato S, Fernandez M, Amorim M, Ambrogi I, Fernandes LM, Pacheco R. The Brazilian government’s mistakes in responding to the COVID-19 pandemic. 1636Lancet. 2020;396 doi: 10.1016/S0140-6736(20)32164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos VS, Souza Araújo AA, Oliveira JR, Quintans-Júnior LJ, Martins-Filho PR. COVID-19 mortality among indigenous people in Brazil: a nationwide register-based study. J Public Health (Oxf.) 2021;43:e250–e251. doi: 10.1093/pubmed/fdaa176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ventura D, Aith F, Reis R. The catastrophic Brazilian response to covid-19 may amount to a crime against humanity. Thebmjopinion. Weblog. [cited 2022 Sep 22]. https://blogs.bmj.com/bmj/2021/04/05/the-catastrophic-brazilian-response-to-covid-19-may-amount-to-a-crime-against-humanity/
- 8.Boschiero MN, Palamim CV, Ortega MM, Mauch RM, Marson FA. One year of coronavirus disease 2019 (COVID-19) in Brazil: a political and social overview. 44Ann Glob Health. 2021;87 doi: 10.5334/aogh.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candido DS, Claro IM, De Jesus JG, Souza WM, Moreira FR, Dellicour S, et al. Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science. 2020;369:1255–1260. doi: 10.1126/science.abd2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortega F, Orsini M. Governing COVID-19 without government in Brazil: ignorance, neoliberal authoritarianism, and the collapse of public health leadership. Glob Public Health. 2020;15:1257–1277. doi: 10.1080/17441692.2020.1795223. [DOI] [PubMed] [Google Scholar]
- 11.Brasil. Ministério da Saúde. Secretaria Especial de Saúde Indígena. Distrito Sanitário Especial Indígena do Mato Grosso do Sul . Plano de contingência COVID-19/DSEI-MS. Campo Grande: Ministério da Saúde; 2020. [cited 2022 Sep 22]. http://ds.saudeindigena.icict.fiocruz.br/handle/bvs/1820 . [Google Scholar]
- 12.Brasil. Ministério da Saúde . Guia de vigilância epidemiológica: emergência de saúde pública de importância nacional pela doença pelo coronavírus 2019: COVID-19. Brasília: Ministério da Saúde; 2021. [cited 2022 Sep 22]. https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/guias-e-manuais/2021/guia-de-vigilancia-epidemiologica-covid-19-3.pdf/view . [Google Scholar]
- 13.Instituto Brasileiro de Geografia e Estatística Brasil: Mato Grosso do Sul: panorama. [cited 2022 Sep 22]. https://cidades.ibge.gov.br/brasil/ms/panorama .
- 14.Instituto Brasileiro de Geografia e Estatística Mapas. [cited 2022 Sep 22]. https://portaldemapas.ibge.gov.br/portal.php#homepage .
- 15.Baqui P, Bica I, Marra V, Ercole A, van Der Schaar M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Glob Health. 2020;8:e1018-26. doi: 10.1016/S2214-109X(20)30285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathur R, Rentsch CT, Morton CE, Hulme WJ, Schultze A, MacKenna B, et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. 2021;397:1711–1724. doi: 10.1016/S0140-6736(21)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polidoro M, Mendonça FA, Meneghel SN, Alves-Brito A, Gonçalves M, Bairros F, et al. Territories under siege: risks of the decimation of indigenous and Quilombolas peoples in the context of COVID-19 in South Brazil. J Racial Ethn Health Disparities. 2021;8:1119–1129. doi: 10.1007/s40615-020-00868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 19.Cobre AF, Böger B, Fachi MM, Vilhena RO, Domingos EL, Tonin FS, et al. Risk factors associated with delay in diagnosis and mortality in patients with COVID-19 in the city of Rio de Janeiro, Brazil. Cien Saude Colet. 2020;25(Suppl 2):4131–4140. doi: 10.1590/1413-812320202510.2.26882020. [DOI] [PubMed] [Google Scholar]
- 20.Kameda K, Barbeitas MM, Caetano R, Löwy I, Oliveira AC, Corrêa MC, et al. Testing COVID-19 in Brazil: fragmented efforts and challenges to expand diagnostic capacity at the Brazilian Unified National Health System. Cad Saude Publica. 2021;37:e00277420. doi: 10.1590/0102-311X00277420. [DOI] [PubMed] [Google Scholar]
- 21.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. m1966BMJ. 2020;369 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. m1091BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fellows M, Paye V, Alencar A, Nicácio M, Castro I, Coelho ME, et al. Under-reporting of COVID-19 cases among indigenous peoples in Brazil: a new expression of old inequalities. 638359Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.638359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jardim TD, Dias IM, Grande AJ, O’Keeffe M, Dazzan P, Harding S. COVID-19 experience among Brasil’s indigenous people. Rev Assoc Med Bras. 2020;66:861–863. doi: 10.1590/1806-9282.66.7.861. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization Coronavirus (COVID-19) dashboard. [cited 2022 Sep 22]. https://covid19.who.int .
- 28.Sabino EC, Buss LF, Carvalho MP, Prete CA, Jr., Crispim MA, Fraiji NA, et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397:452–455. doi: 10.1016/S0140-6736(21)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faria NR, Mellan TA, Whittaker C, Claro IM, Candido DS, Mishra S, et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372:815–821. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hitchings MD, Ranzani OT, Torres MS, Oliveira SB, Almiron M, Said R, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: a test-negative case-control study; Lancet Reg Health Am; ;. 2021. 100025. [DOI] [PMC free article] [PubMed] [Google Scholar]