Abstract
Despite remarkable advances over the past several decades, many therapeutic nanomaterials fail to overcome major in vivo delivery barriers. Controlling immunogenicity, optimizing biodistribution, and engineering environmental responsiveness are key outstanding delivery problems for most nanotherapeutics. However, notable exceptions exist including some lipid and polymeric nanoparticles, some virus-based nanoparticles, and nanoparticle vaccines where immunogenicity is desired. Self-assembling protein nanoparticles offer a powerful blend of modularity and precise designability to the field, and have the potential to solve many of the major barriers to delivery. In this review, we provide a brief overview of key designable features of protein nanoparticles and their implications for therapeutic delivery applications. We anticipate that protein nanoparticles will rapidly grow in their prevalence and impact as clinically relevant delivery platforms.
Introduction
Over the past 30 years, tremendous advances in nanoparticle platforms for therapeutic applications have been achieved.1,2 In 1995, the first cancer-treating nanoparticle was approved by the FDA: Doxil, a liposomal formulation of doxorubicin that significantly decreased cardiomyopathy caused by free doxorubicin.3,4 Less than three decades later, the first two mRNA vaccines were approved by the FDA: Pfizer-BioNtech’s Comirnaty and Moderna’s Spikevax, advanced lipid nanoparticle formulations that deliver synthetic mRNA to elicit highly protective immune responses against SARS-CoV-2.5,6 Despite these and many other advances, it is widely believed that nanotherapeutics have yet to achieve their full potential due to the inconsistent translation from in vitro results in cell culture to in vivo results in animal models, and preclinical results in animal models to clinical efficacy in humans.1,7,8 The clinical translation of nanotherapeutics is fundamentally limited by several biological barriers to delivery, manifesting as a much lower than expected clinical trial success rate (Figure 1).9 Successful therapeutic delivery systems must (1) efficiently localize to the body compartment or organ of interest (e.g., tumor), (2) localize to the cell type of interest (e.g., cancer cells), and (3) engage with the target cells in a therapeutic manner (e.g., kill cancer cells). Overcoming these sequential biological barriers requires precise engineering to endow nanoparticles with functionalities suited to their therapeutic application. Self-assembling protein nanoparticles are an emerging class of delivery vehicles with the potential to satisfy these requirements through directed evolution and rational design.
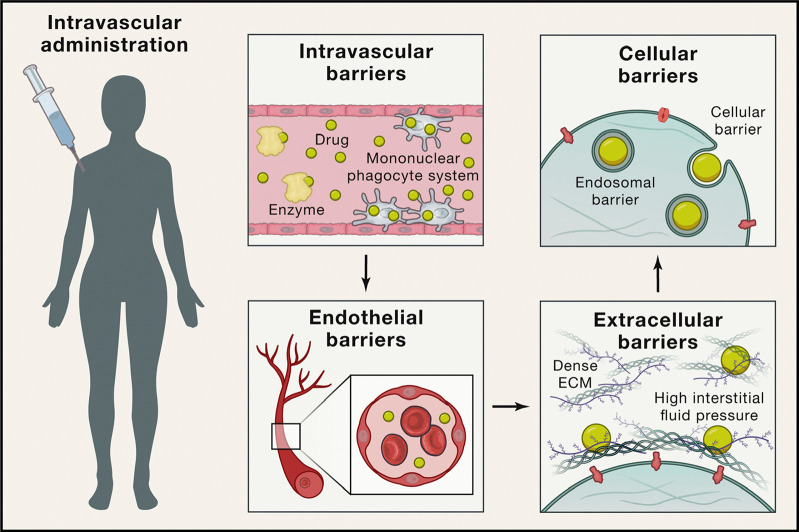
Figure 1.
Barriers to in vivo nanoparticle delivery following intravascular administration. For nanoparticles to successfully reach their targets, they must pass several barriers. This example uses intravascular administration as a case study; other routes of administration are reviewed elsewhere.10−17 Unless immune responses are specifically desired, the nanoparticles must evade recognition by the innate and adaptive immune system during circulation (e.g., the complement system, the mononuclear phagocytic system (MPS), and B cells). Once near the target organ, they must travel past endothelial cells and tight junctions, which line blood vessels. Then the nanoparticles must travel through extracellular matrices within the target tissue, and once they reach the target cells, must access their intended subcellular compartment for therapeutic payload delivery. Reprinted with permission.(8)
Here, we define self-assembling protein nanoparticles as atomically precise, bounded assemblies composed of symmetric protein oligomers forming three-dimensional shells with hollow interiors. The term “protein nanoparticles” refers to these assemblies unless otherwise noted. As discussed further in “Protein nanoparticle architecture and geometry,” these assemblies have rotational symmetry axes projected into all three dimensions. Examples of such assemblies include computationally designed protein nanoparticles, and natural, nonviral protein nanocontainers like ferritin.18−21 Although albumin and silk fibroin nanoparticles are also composed of proteins, they do not form atomically precise nanoparticles with defined symmetries.22,23 Viral vector and virus-derived particle engineering are also outside the scope of this review due to their distinct complexity. However, we will draw comparisons between many viral and nonviral protein assemblies, as virus-derived delivery systems are a major source of inspiration. Therapeutic applications of these other protein-based nanotherapeutics are thoroughly reviewed elsewhere.24−30
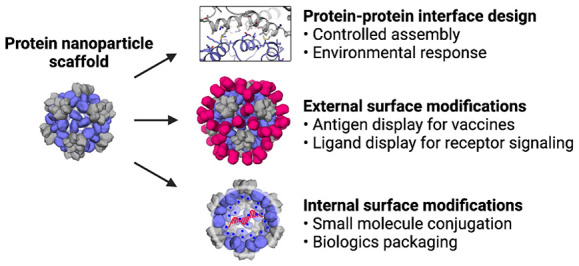
Part 1: Designable Features of Protein Nanoparticles
Introduction
Proteins are arguably the most information-rich molecules in the known universe. The information encoded in their amino acid sequences enables proteins to perform a remarkable variety of sophisticated functions that largely constitute the molecular basis of life. The greater the ability to harness this information for precise design, the more sophisticated and diverse the encodable structures can be.31,32 Computational protein design has become a widely used technique that helps manage the complexity of protein structures and energetics and, in conjunction with complementary approaches, can be used to develop functionalities that can be integrated into protein nanoparticles.33−36 The main features engineered for delivery purposes are physicochemical properties, environmental responsiveness, scaffold functionalization, and cargo encapsulation.37 In the following sections, we discuss how these features can be tuned and detail considerations for producing these materials at sufficient scale for clinical application.
Protein Nanoparticle Scaffold Design
To date, three primary approaches to designing and modifying protein nanoparticles have been explored in delivery applications: top-down modification of natural protein nanocontainers, bottom-up design of novel protein nanoparticles, and directed evolution of nanoparticle scaffolds to confer specific functions.
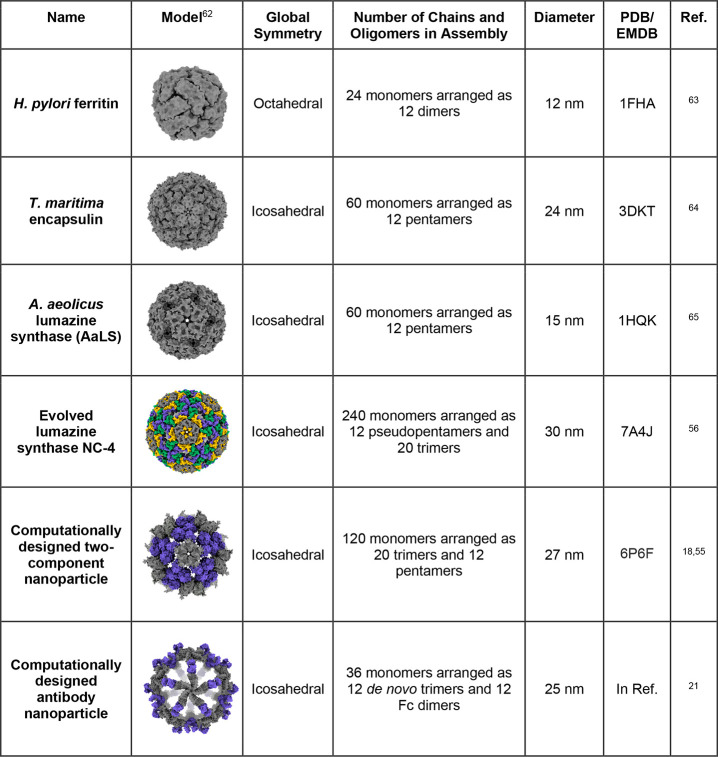
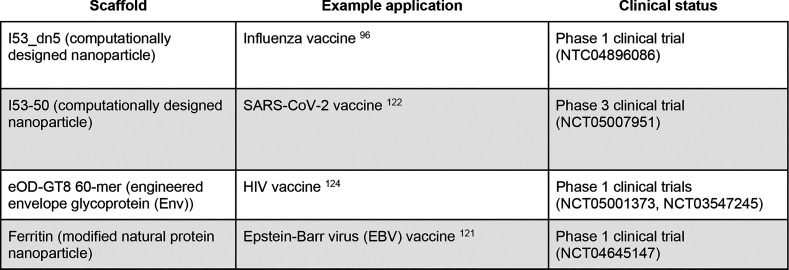
Currently, the most straightforward nanoparticle design method is top-down adaptation of an existing, naturally evolved viral capsid or cellular protein nanocontainer.38,39 Natural protein nanoparticles like ferritins, encapsulins, and lumazine synthases evolved over billions of years to serve highly specialized biological functions in specific environments, with many functionalities that would be virtually impossible to design using current methods.(Table 1).33,40−42 Natural protein nanoparticles can also be mutated or modified through the fusion of functional protein domains to confer additional properties. For example, Seo, Yoo, and Kim et al. reported a ferritin nanoparticle with a fused fibrinolytic domain to improve tumor accumulation of coadministered anticancer drugs.33 However, even modified natural protein nanocontainers are largely confined to a design space that is close to their initial states, making it challenging but not impossible to alter their fundamental structural and biochemical properties. Directed evolution, discussed below, has become a particularly powerful tool for modifying some of these underlying properties.
Table 1. Selected Examples of Non-Viral Protein Nanoparticle Platformsa.
Nanoparticle models were made using UCSF ChimeraX.62
Recent advances in protein engineering have enabled the bottom-up design of purpose-built protein nanoparticles, drastically expanding the protein nanoparticle design space. These methods typically apply principles of symmetry to arrange natural or de novo designed cyclic oligomers into protein nanoparticles through interface design or rigid genetic fusion, reviewed in Khmelinskaia, Wargacki, and King (2021).43−49 Padilla, Colovos, and Yeates et al. (2001) reported an early example of a self-assembling protein nanoparticle constructed using the rigid fusion of several oligomeric domains.48 Recently computational protein design has been applied to interface and rigid fusion methods enabling the design of increasingly complex protein nanoparticles with improving success rates.19,50,51 King and Baker et al. (2012) reported self-assembling protein nanoparticles with a variety of symmetries using computational protein interface design to arrange existing cyclic oligomers into the desired assemblies (Figure 2B).19 Many computational protein design methods currently require specific technical expertise and access to substantial computational resources. However, advances in machine learning are rapidly simplifying the protein design process and reducing the computational burden.52 This promises to make computational protein design a more widely accessible technique.
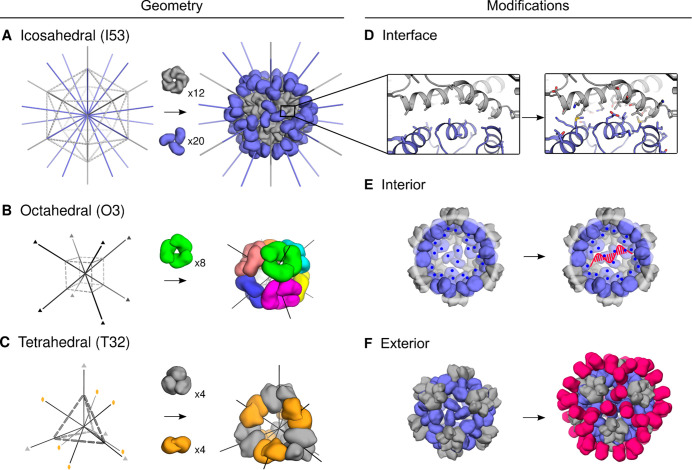
Figure 2.
Designable features of nanoparticle scaffolds. Two main considerations when designing protein nanoparticle delivery systems are scaffold source and scaffold modifications. (A–C) Protein nanoparticle geometries commonly used for delivery applications have icosahedral, octahedral, tetrahedral, or dihedral symmetry.18,19,71 Notably, the octahedral nanoparticle in (B) is composed of eight identical trimer subunits, which are colored differently to help visually distinguish individual subunits in the context of the global structure. (D–F) Additional functional elements are designed into the nanoparticle through subunit interface, interior, and exterior modifications.25 (D) Interfaces between the trimer subunits (slate) and pentamer subunits (gray) were computationally designed. (E) Interior residues were mutated to hold a net positive charge, leading to electrostatic association of mRNA. (F) Additional protein domains can be displayed on the surface of existing nanoparticles. A, D: Reprinted with permission.(18) B: Reprinted with permission.(19) C: Reprinted with permission.(71)
Directed evolution is a powerful tool for refining existing protein nanoparticles.53 This method has been used for a variety of modifications ranging from improving nanoparticle biodistribution, packaging, and protection of nucleic acid to the sequestration of toxic enzymes.54−56 Notably, Tetter, Terasaka, and Steinauer et al. recently reported the design and directed evolution of an icosahedral nanocontainer into a self-mRNA encapsidating nanoparticle with a diameter almost twice as large as the native structure.56,57 Directed evolution experiments require both a selection assay that will drive designs exclusively toward a desired function and an initial protein nanoparticle that is reasonably evolutionarily close to that function. Choosing a selection assay and initial protein nanoparticle is a nuanced process that requires careful consideration, and these experiments can be labor intensive. Alternatively, a well-designed directed evolution experiment is a powerful tool that can be used to select for poorly understood features that would currently be impossible with rational design alone.55,56 As demonstrated in many viral vector gene delivery studies, a good selection assay can refine thousands to millions of highly diverse protein variants in a single experiment, making this a powerful complement to computational protein nanoparticle design.58−61
Protein Nanoparticle Architecture and Geometry
Nanoparticle geometry describes the arrangement of subunits composing a protein nanoparticle. All protein nanoparticles are constructed from many copies of an asymmetric unit that are arranged in a symmetric manner such that they form a closed, three-dimensional structure (Figure 2A–C). Tetrahedral, octahedral, icosahedral, and some dihedral symmetries are most often used to generate these closed structures.39,66,67 Cyclic homo-oligomers that can be incorporated into a protein nanoparticle usually need to be on a matched axis of symmetry. For example, a cyclic tetramer must be on the axis of 4-fold symmetry in an octahedrally symmetric nanoparticle and is not easily incorporated into tetrahedral and icosahedral nanoparticles. The symmetry group also determines the number of asymmetric units in a complete nanoparticle, and thus the valency of any ligands displayed on the surface of the nanoparticle. Though possible in other symmetry groups, icosahedral architectures are typically more easily designed to have large interior cavities and nonporous shells that can aid in cargo packaging and protection; the myriad naturally existing, icosahedral nanocontainers benefit from these advantages.42,63,64,68 While protein nanoparticle subunit size can vary widely, symmetry groups with larger numbers of asymmetric units will yield larger nanoparticles for a subunit of a given size.
The asymmetric unit is composed of one or more protein chains and at least two protein–protein interfaces oriented about axes of cyclic symmetry.69 An asymmetric unit may be made up of a single chain with two interfaces or have several chains with internal nonsymmetric protein–protein interfaces.18,70 As discussed further in “Manufacturing Protein Nanoparticles,” if an asymmetric unit is composed of two or more chains, the nanoparticle can in principle be assembled in vitro. The number of protein chains and their arrangement within the asymmetric unit determines how many unique fusion points (N and C termini) are available on the interior and exterior of the protein nanoparticle for the addition of functional domains.
Interface Design and Modifications
The high degree of cooperativity in protein nanoparticle assembly requires special consideration when designing protein nanoparticle interfaces.72−74 As with most protein–protein interface design, protein nanoparticle interface design has to date generally relied on hydrophobic packing of interface residues.43 However, protein nanoparticles assemble with a high degree of cooperativity, which results in efficient assembly of subunits with relatively weak interfaces but can result in kinetic trapping of partial or off-target assemblies if the intersubunit interface is too strong.75 Large hydrophobic interfaces between protein nanoparticle subunits can also interfere with soluble expression. Designing hydrophilic interfaces, as seen in many natural protein nanocontainers, could be one way to overcome this limitation.65
Protein nanoparticle interface design can be leveraged to encode environmental responsiveness and nanoparticle functionalization. Protein nanoparticle interfaces can be either scavenged from existing protein–protein interfaces and rigidly fused to a nanoparticle subunit or computationally designed from scratch. This flexibility can enable protein nanoparticles to directly scaffold natural proteins such as antibodies, thus functionalizing the nanoparticle.21 Additionally, protein nanoparticle interfaces can be selected or designed to allow for controlled assembly and disassembly of the protein nanoparticle under specific environmental conditions. Ionic strength, pH, and metal-dependent protein nanoparticles have been investigated for applications in both packaging and selective drug delivery.45,76,77
Interior Modifications
In addition to modifying protein subunit interfaces, useful features can be engineered into both the interior and exterior surfaces of nanoparticles through rational design or library selection.42,55−57 Interior modification approaches are largely inspired by viruses and virus-like particles.24,78,79 Three main strategies have been used to genetically modify protein nanoparticle interior and exterior surfaces: point mutation, genetic fusion, and loop insertion. Due to the symmetry and repetitive nature of self-assembling protein nanoparticles, small modifications are often unlikely to destabilize protein subunits, but can cause significant functional changes. Genetic modifications often enable chemical conjugations and post-translational modifications, providing opportunities for functionalization that benefit from years of research and development in both academia and industry.80−82
The interior nanoparticle surface is commonly modified to encapsulate specific materials (Figure 2E). It has been widely demonstrated that mutating interior-facing surface residues to hold a net charge facilitates electrostatic-mediated cargo encapsulation (e.g., encapsulation of supercharged fluorescent proteins or nucleic acids) (Figure 2E).18,55,83 Interior surface residues can also be mutated to include side chains capable of specific chemical reactions like copper-free click chemistry.84 Alternatively, larger domains like affinity peptides can be seamlessly integrated into the protein nanoparticle interior through genetic fusion. Genetic fusion of functional domains to the nanoparticle interior surface can either be bioactive themselves or enable specific cargo encapsulation such as mRNA and siRNA sequences.55,57,83,85
Exterior Modifications
Interactions between nanoparticles and their surrounding environments are primarily mediated by the exterior surfaces of the nanoparticles (Figure 2F).86,87 Two common goals of exterior modifications are (1) to alter the physicochemical properties of the scaffold surface and (2) to display a molecular recognition domain (e.g., a pathogen-derived antigen or receptor targeting domain). Similar to interior modification strategies, surface point mutations, chemical conjugations, genetic fusions, and loop insertions are commonly used to modify exterior surfaces.33,55,88−92 These methods have been used to increase in vivo circulation half-life and to incorporate post-translational modifications like glycosylation, which has been reported to increase germinal center delivery for vaccine applications.86,93 Surface modifications also have important implications for immunogenicity. “Stealth” coatings like polyethylene glycol (PEG) are often conjugated to nanoparticle surfaces to increase circulation time and reduce immunogenicity, but this strategy is clinically limited since many humans are sensitized to PEG.94
Displaying functional domains on nanoparticle surfaces controls specific biological interactions like targeted delivery to tumor vasculature or immune responses to pathogen-derived antigens in the context of nanoparticle vaccine delivery. As described above, the display of such domains is fundamentally linked to the symmetry and stoichiometry of the nanoparticle platform. Monomeric domains like tumor-targeting DARPins are symmetry-agnostic, while the display of multimeric antigens like influenza hemagglutinin usually requires fusion to a symmetry-matched nanoparticle component and sometimes facilitates antigen stabilization.41,95−98 A recent counterexample demonstrated the symmetry-agnostic display of influenza antigens on the surface of a VLP through SpyCatcher-SpyTag conjugation, an alternative to the genetic fusion approach, which has proven to be another robust and versatile technology for modifying nanoparticle exteriors.99
Manufacturing Protein Nanoparticles
Protein production efficiency impacts the cost and market distribution of a therapeutic, thereby determining its commercial viability.100,101 The main considerations in protein nanoparticle manufacturing are the dose, protein production, nanoparticle assembly, and purification. Vaccines typically require much smaller doses than other protein-based therapeutics such as monoclonal antibodies (micrograms vs hundreds of milligrams), making it easier to achieve commercially viable production efficiency.102−104 However, most of the clinical production of protein nanoparticle immunogens has occurred only in the past few years and there is still much to learn (e.g., National Clinical Trials (NTC) 05007951, 04896086, 03186781, 03814720, 04579250, 04784767, 04645147, 05001373, and 03547245 reported at clinicaltrials.gov).
Process development for manufacturing large-scale protein nanoparticle therapeutics must adhere to production efficiency requirements and current good manufacturing process (cGMP) standards.105,106 Fortunately, the ability to perform in vitro nanoparticle assembly offers several advantages for manufacturing multicomponent protein nanoparticles. In vitro assembly enables two (or more) standard recombinant biologics to be separately purified before nanoparticle assembly, benefiting from decades of research and development in manufacturing recombinant protein biologics like monoclonal antibodies.106,107 Assembling protein nanoparticles in vitro increases sample purity and gives engineers precise control over subunit composition and cargo encapsulation. While simple protein nanoparticle components can be successfully produced and purified in E. coli, many components require mammalian post-translational modifications and must be produced in eukaryotic cells.108,109 In vitro assembly also enables the generation of mosaic nanoparticles simply by mixing different component-antigen fusions together such that different antigens are codisplayed on the same nanoparticle scaffold. Phase I clinical trials are currently underway for mosaic influenza vaccines.96 Notably, SpyCatcher/SpyTag conjugation of antigens to nanoparticle scaffolds offers an alternative approach to in vitro mosaic assembly; mosaics generated using this method are anticipated to reach the clinic soon.110 While each therapeutic application will likely require unique process development, improving the manufacturing capabilities of protein nanoparticle immunogens will provide useful information to the entire field.
A distinguishing characteristic of protein nanoparticles is their complete genetic encodability. With the recent emergence of clinically validated techniques for delivering genetic information in vivo, this feature opens up a unique opportunity to deploy certain nanoparticle therapeutics and vaccines as nucleic acids.111−115 In concept, genetic delivery would streamline manufacturing and allow rapid prototyping and iteration in vivo. However, the inability to control or purify the translated protein(s) in vivo emphasizes the importance of optimizing the sequence of the nanoparticle therapeutic during design, as this will determine critical functional features such as expression level, monodispersity, and stability.
Part 1 Summary
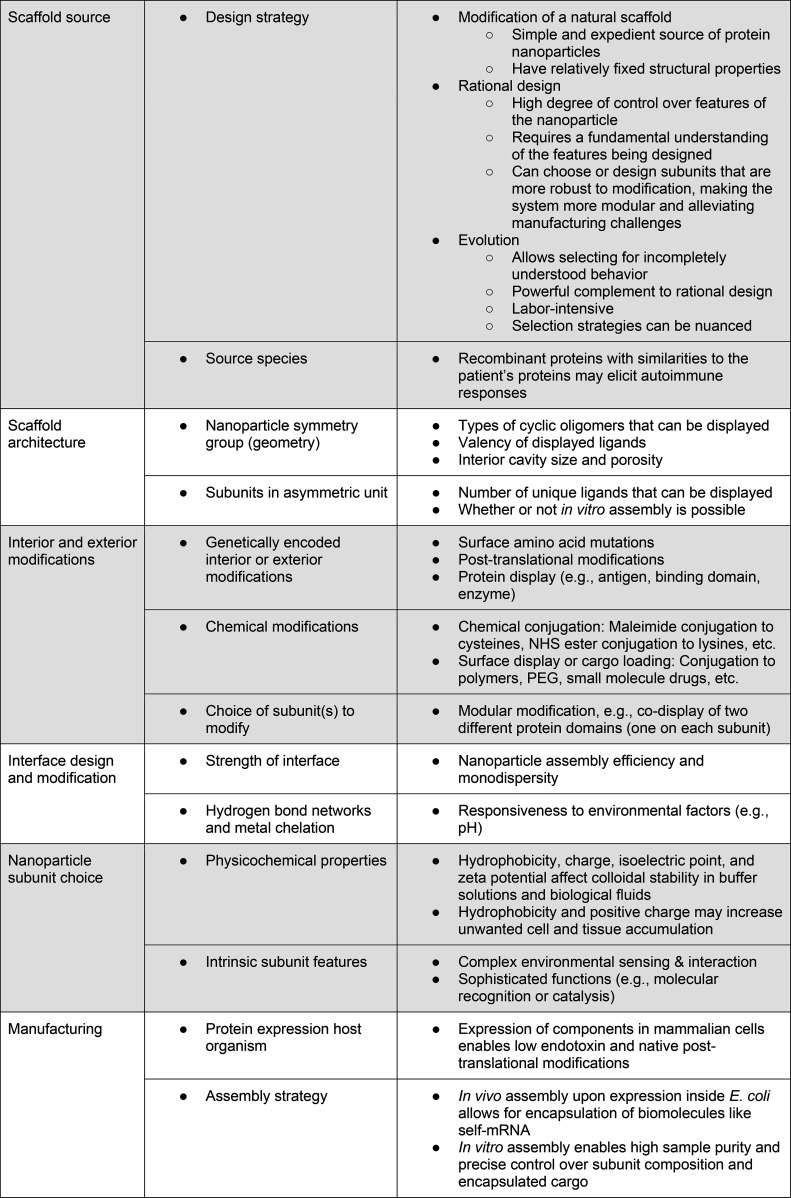
The key design variables for engineering self-assembling protein nanoparticles as therapeutics are summarized in Table 2. While computational design enables control over protein nanoparticle structure, purification and in vitro assembly methods enable control over the exact nanoparticle composition. As further discussed in “Therapeutic Applications of Protein Nanoparticles,” the nanoparticle characteristics combined with the delivery method critically influence the pharmacokinetics, biodistribution, and efficacy of the protein nanoparticle therapeutic.
Table 2. Key Variables Influencing the Potential Outcomes and Applications of Self-Assembling Protein Nanoparticles.
Part 2: Therapeutic Applications of Protein Nanoparticles
Introduction
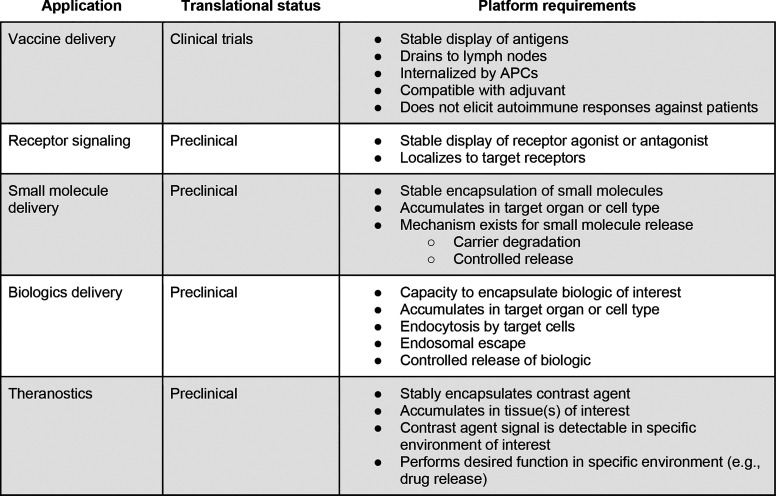
In this section, we discuss five common applications of protein nanotherapeutics, the key design criteria that must be considered for each, and their development status. We summarize this information in Tables 3 and 4.
Table 3. Examples of Self-Assembling Protein Nanoparticles in the Clinic.
Table 4. Platform Requirements for Therapeutic Applications of Protein Nanoparticles.
While many design criteria are unique to each application, several criteria are shared among all in vivo therapeutic applications. These criteria generally affect nanoparticle pharmacokinetics and biodistribution, which have been extensively studied using inorganic and polymeric nanoparticle platforms.10−17,116 The key physicochemical properties influencing these phenomena are shape, size, charge, hydrophobicity, rigidity, and specific molecular interactions. For reference, the physicochemical properties of protein nanoparticles are extremely diverse but are generally spherical, 10–30 nm in diameter, hold a net negative surface charge at physiological pH, have hydrophilic surfaces, and can be designed to include or exclude specific molecular interactions (e.g., display a binding domain to target a specific receptor). Regardless of delivery route, nanoparticles that are too hydrophobic, too positively charged, too large, or too small face significant delivery barriers such as aggregation, nonspecific cell uptake, liver and spleen accumulation, and kidney clearance (Figure 3). Nanoparticles ∼10–150 nm in diameter more efficiently travel from the delivery site to the target tissue (e.g., through blood, extracellular matrix, lymphatic system, or mucous membranes). Still, protein corona formation, immunogenicity, and clearance by the mononuclear phagocytic system (MPS) are critical barriers to most delivery systems.17,117,118 While certain overarching biodistribution phenomena like MPS clearance appear to be consistent between inorganic, polymeric, and protein nanoparticles, the surface chemistries of such nanoparticles are distinct. It is necessary to consider if, and how, findings in one class of materials (e.g., PLGA-based nanoparticles) translate to other classes of materials (e.g., self-assembling protein nanoparticles). Designing successful nanotherapeutic platforms is a balancing act between the design criteria, platform features, and fundamental delivery barriers.
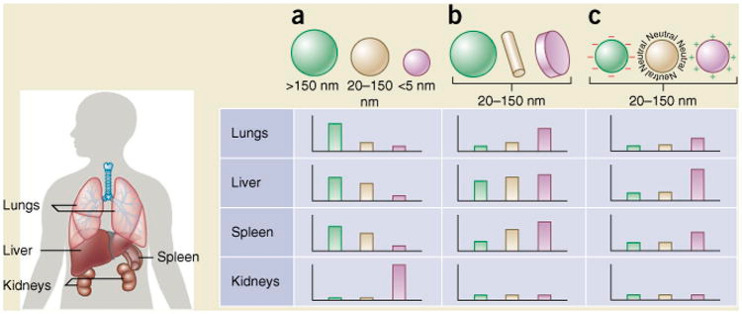
Figure 3.
Qualitative impacts of nanoparticle size, shape, and surface charge on biodistribution. The general effects of surface physicochemical properties on biodistribution were comprehensively reviewed by Blanco, Shen, & Ferrari and qualitatively graphed as relative accumulation in major mouse organs.10 Data were included from gold nanoparticles, liposomes, polymer micelles, zwitterionic nanoparticles, hydrogel nanoparticles, and more. (A) Nanoparticles greater than about 150 nm in diameter show increased accumulation in the lungs, liver, and spleen, while nanoparticles less than 5 nm in diameter show rapid renal clearance. (B) Spherical nanoparticles tend to have the least uptake by major clearance organs compared to cylindrical and discoidal nanoparticles. (C) Nanoparticles with positively charged surfaces show much higher nonspecific uptake than nanoparticles with negatively charged or neutral surfaces. Reprinted with permission.(10)
Vaccine Delivery
The goal of vaccines is to safely teach the immune system how to protect against infection or disease upon subsequent encounter with a pathogen. While traditional vaccines derived from whole pathogens provide effective prevention against many diseases, these are inappropriate or have fallen short for several diseases due to factors including safety considerations, insufficient immune stimulation, poor antigen stability, or engineering and manufacturing limitations.119 Protein nanoparticle immunogens, which deliver antigen in a repetitive array by genetic fusion or chemical or protein–peptide conjugation to nanoparticle scaffolds, aim to address many of these limitations and have been shown to elicit protective immune responses (Figure 4).97,98,110,120−126 This approach taps into multiple features of the immune system that have evolved to detect “particulate” pathogens featuring repetitive antigenic determinants. First, upon intramuscular injection, protein nanoparticles are the ideal shape and size to be taken up by peripheral or lymph node dendritic cells (spherical, ∼20–150 nm in diameter).11 Additionally, multivalent antigen display facilitates detection of pathogen-associated molecular patterns; protein nanoparticle-mediated B cell receptor cross-linking has been demonstrated to cause stronger immune responses than those elicited by soluble antigens.117,127,128 Although live-attenuated and inactivated vaccines also benefit from these immunological phenomena, self-assembling protein nanoparticles are widely considered safer since there is no infectious agent. Protein nanoparticles also allow the use of antigens that are engineered in ways that may be incompatible with viral or bacterial growth, such as prefusion-stabilized RSV F (Figure 4A).129,130 The engineered protein nanoparticle immunogen may possess, for example, increased stability, increasing immune exposure to the antigenic conformation or epitope against which an immune response is desired.98,131−133 Like VLP-based vaccines, the natural fit between the properties of self-assembling protein nanoparticles and vaccine delivery criteria is likely responsible for vaccines being the only application of protein nanoparticles that has advanced to the clinic to date—the delivery efficiency of vaccines is less fundamentally limited by immunogenicity compared to other therapeutic applications (Table 3).96,98,122,134−137 However, additional data on the development of antibody responses against nanoparticle scaffolds and their effects is needed.
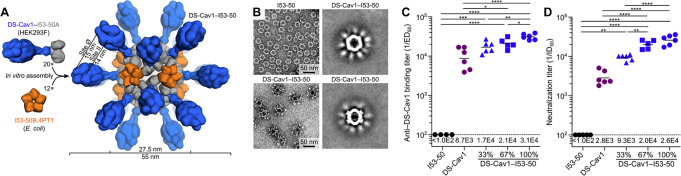
Figure 4.
The DS-Cav1-I53–50 nanoparticle immunogen. (A) A vaccine against respiratory syncytial virus (RSV) was engineered by outwardly displaying a trimeric RSV antigen (DS-Cav1) on the trimeric subunit of a computationally designed nanoparticle (I53–50). (B) Negative stain electron microscopy of I53–50 and DS-Cav1-I53–50 nanoparticles. Left: representative micrographs. Right: averages of nanoparticle micrographs. (C–D) Antibody binding titers ofDS-Cav-1-specific antibodies (C) or serum neutralizing antibodies (D) from mice immunized with bare nanoparticle (I53–50), free immunogen (DS-Cav1), or nanoparticle immunogens (DS-Cav1-I53–50) at valencies of 33%, 67%, or 100%. Each point represents and individual animal, geometric means are represented by horizontal lines and indicated at the bottom of the plots, and statistical significance is indicated: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. See original publication for more details. Reprinted with permission.(98)
Current vaccine delivery research with protein nanoparticle immunogens is focused on understanding the mechanisms that underlie their interactions with the immune system and developing new technological features that expand their capabilities.86,93,96,110,128,138−145 The modularity of self-assembling protein nanoparticles and increasingly precise methods for designing them are beginning to allow systematic testing of important features including nanoparticle architecture (e.g., icosahedral or tetrahedral scaffolds, which present antigen in distinct geometries), antigen identities and combinations (e.g., mosaic nanoparticle immunogens for broadly protective vaccines), post-translational modifications (e.g., glycosylation to increase germinal center accumulation), and surface modifications to tune immunogenicity or focus the immune response to specific sites of interest (e.g., molecular or steric shielding).51,86,93,96,110,124,146
Small Molecule Delivery
The hydrophobic nature of many small molecule drugs often results in poor solubility or nonspecific accumulation throughout the body, reducing therapeutic bioavailability, and increasing unwanted toxicities.147 Loading small molecules onto a carrier such as an antibody is an effective way to improve their biodistribution.148 However, the hydrophobic small molecule is often chemically conjugated to the surface of non-nanoparticle carriers, limiting drug loading capacity while also requiring careful optimization of drug-carrier ratio to maintain stability and desired biodistribution.149 Protein nanoparticle carriers possess an interior cavity for encapsulation of hydrophobic small molecules (Figure 3E). For example, ferritin nanoparticles have been engineered to encapsulate doxorubicin, cisplatin, and other small molecule therapeutics.20,41,88,150−155 Drug-loaded ferritin nanoparticles have not yet been clinically approved due to complexity in manufacturing and specific tissue targeting, but promising advancements are underway.
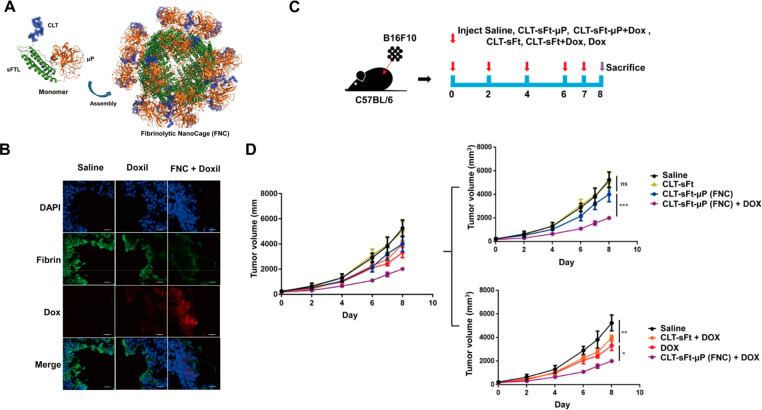
Recently, a ferritin nanoparticle was engineered to display a fibrinolytic domain that can dissolve blood clots in tumors. The coadministration of this ferritin nanocage (FNC) and liposomal doxorubicin (Doxil) lowered the risk of adverse blood clot-related events and increased Doxil tumor penetration in mice (Figure 5).33 The synergy between these two delivery systems enabled small molecule delivery (by Doxil) and extracellular biologics delivery (by fibrinolytic activity), highlighting the potential of combination therapies.
Figure 5.
Coadministration of liposomal doxorubicin (Doxil) with ferritin codisplaying clot-targeting and fibrinolytic domains. (A) The ferritin monomer was engineered to display a clot-targeting peptide (CLT) and fibrinolytic domain (μP), resulting in surface display on ferritin nanoparticles. (B) Fibrinolytic nanocages (FNC) coadministered with Doxil show increased tumor cell accumulation and decreased fibrin signal compared to saline or Doxil controls. (C–D) When delivered to tumor-bearing mice at the indicated dosing schedule (C), Doxil-FNC coadministration show increased tumor growth inhibition (purple) compared to mice treated with Doxil alone (red) and other controls (D). Reprinted with permission.(33)
Extracellular Biologics Delivery
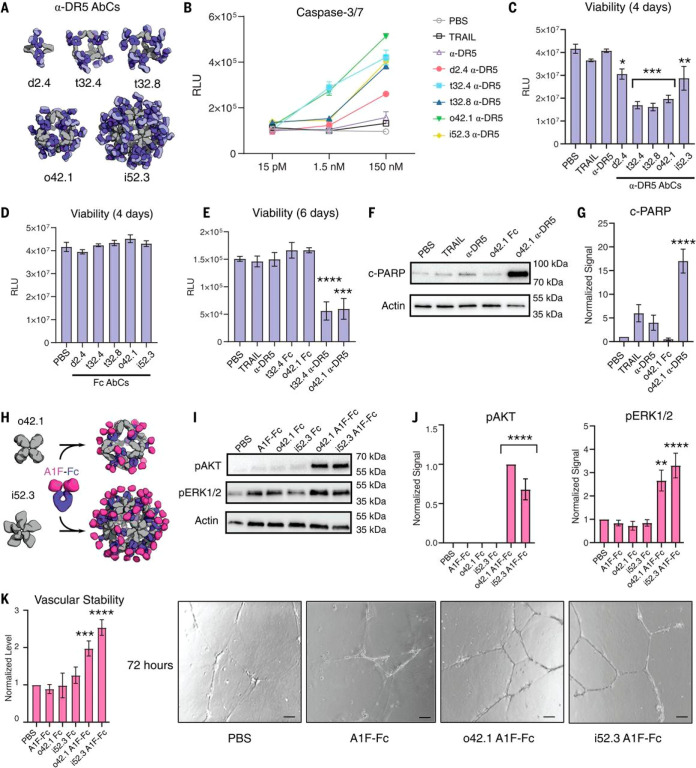
Targeted stimulation or inhibition of cell signaling receptors is the key mechanism of many therapeutics ranging from receptor tyrosine kinase inhibitors to bispecific antibodies. Clinical translation of many such therapeutics is hindered by challenges with ligand stability, pharmacokinetics, biodistribution, and hepatotoxicity.156−158 Displaying receptor agonists or antagonists on protein nanoparticles offers several opportunities to address these challenges: the (ant)agonist can be stabilized by scaffolding on a solid foundation, the signaling magnitude can be tuned through altering display valency and nanoparticle architecture, and the biodistribution profile can be improved through optimizing the physicochemical features of the nanoparticle.21,38,159−161 Inspired by receptor signaling applications based on VLPs, ferritin and computationally designed nanoparticles have recently been engineered to display a variety of therapeutic receptor signaling domains. Examples include interleukin-4 receptor (IL-4R)-targeting peptides to ameliorate asthma symptoms, antimesenchymal epithelial transition (MET) peptide pharmacophores to stimulate hepatocyte growth factor receptor, death receptor ligands (TRAIL) to kill tumor cells, and angiopoietin-agonizing antibodies to promote angiogenesis (Figure 6).21,38,159,162,163 These modular platforms offer exciting opportunities for research and development through systematically varying the nanoparticle features to interrogate and optimize biological responses.
Figure 6.
Computationally designed antibody cages (AbCs) activate apoptosis and angiogenic signaling pathways. (A and B) Caspase-3/7 is activated by AbCs formed with α-DR5 antibody (A), but not the free antibody, in RCC4 renal cancer cells (B). (C and D) α-DR5 AbCs (C), but not Fc AbC controls (D), reduce cell viability 4 days after treatment. (E) α-DR5 AbCs reduce viability 6 days after treatment. (F and G) o42.1 α-DR5 AbCs enhance PARP cleavage, a marker of apoptotic signaling; (G) is a quantification of (F) relative to PBS control. (H) The F-domain from angiopoietin-1 was fused to Fc (A1F-Fc) and assembled into octahedral (o42.1) and icosahedral (i52.3) AbCs. (I) Representative Western blots show that A1F-Fc AbCs, but not controls, increase pAKT and pERK1/2 signals. (J) Quantification of (I): pAKT quantification is normalized to o42.1 A1F-Fc signaling (no pAKT signal in the PBS control); pERK1/2 is normalized to PBS. (K) A1F-Fc AbCs increase vascular stability after 72 h. (Left) Quantification of vascular stability compared with PBS. (Right) Representative images; scale bars, 100 μm. All error bars represent means ± SEM; means were compared using analysis of variance and Dunnett posthoc tests (tables S8 and S9). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. Reprinted with permission.(21)
Intracellular Biologics Delivery
The goal of intracellular biologics delivery is to package macromolecular drugs, target specific cell types, and deliver these membrane-impermeable molecules to specific subcellular locations for therapeutic effect. Viral vectors, virus-like particles, and lipid nanoparticles have been extensively developed for these applications, seeing clinical use as genetic vaccines, ex vivo gene editing for CAR-T cell therapies, and in vivo gene therapies.111−115,164,165 However, expansion of these technologies to new clinical applications is challenging due to MPS clearance and neutralizing antibody responses.17,117,118
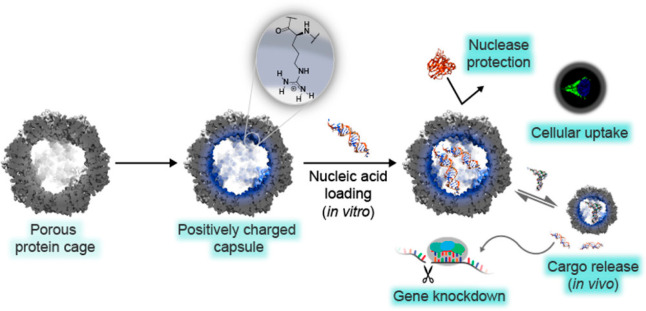
Designed protein nanoparticles are a comparatively nascent technology but have the potential to greatly expand the intracellular biologics delivery design space. Protein nanoparticles that deliver biologics such as siRNA to cultured cells through the endolysosomal pathway have been reported (Figure 7).166 Protein nanoparticles have also been reported with modifications that improve circulation half-life and target the nanoparticles to specific cells.55,167 For systemic, in vivo delivery, protein nanoparticles need to incorporate design features that provide stability during circulation with the ability to recognize, enter, and traffic within target cells. Viruses, VLPs, and some designed delivery vehicles achieve this by responding to the endosomal environment and switching states, satisfying the opposing needs of extracellular and endosomal activity.111,168−170 An exciting new space to explore with protein nanoparticles is engineering environmental responsive mechanisms, inspired by virus-derived particles.
Figure 7.
Computationally designed nanoparticle O3–33 was redesigned for siRNA delivery. The computationally designed octahedral protein nanoparticle O3–33 (porous protein cage) containing 144 hexahistidine tags on its surface was redesigned to have a positively charged interior to electrostatically associate with nucleic acid in vitro (positively charged capsule loaded with nucleic acid).19,166 The authors reported nuclease protection, uptake of the nanoparticles by HeLa cells, and subsequent cargo release leading to knockdown of intracellular GFP mRNA. The authors attributed successful endosomal escape to the hexahistidine tags. Reprinted with permission.(166)
Theranostics
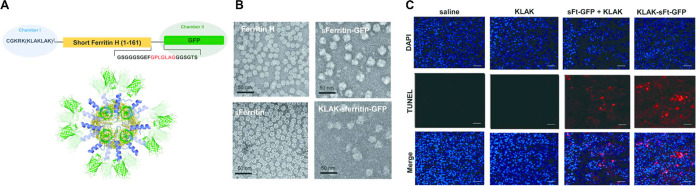
Combining minimally invasive diagnostic techniques with therapeutic delivery, “theranostics” conveniently synergizes the strengths of two medical procedures.26,83,171−173 Noninvasive imaging techniques like magnetic resonance imaging (MRI) are vital for diagnosing and treating disease, yet have low resolution at the cellular and molecular level. Protein nanoparticles can provide alternative optical properties and could be engineered to inform researchers or physicians about real-time cellular and molecular processes through environmental responsiveness.174,175 However, knowledge on protein nanoparticle pharmacokinetics and biodistribution is often lacking.176 The ability to switch between “imaging mode” (e.g., encapsulating gadolinium) and “therapeutic mode” (e.g., encapsulating a small molecule drug), or to simultaneously operate in both modes, offers an opportunity to more deeply probe the in vivo behavior of protein nanoparticles. Ferritin was recently used in this manner to deliver a cytotoxic peptide to tumors while simultaneously displaying green fluorescent protein (GFP) (Figure 8A–C).83 The option to operate in imaging mode, therapeutic mode, or both also offers a potential strategy for screening patients for therapeutic response before treatment.171 Theranostics could be especially impactful in the field of solid tumor delivery, where the heterogeneity within and between tumor types remains a medical challenge.177−179 However, like most other delivery applications, clinical translation of protein nanoparticle theranostics is still fundamentally limited by scaffold immunogenicity.
Figure 8.
Example of ferritin engineered as a theranostic by displaying a fluorescent protein and encapsulating a cytotoxic peptide. (A) Schematic for genetically fusing a cytotoxic peptide (chamber 1) and a fluorescent protein (chamber 2) to ferritin. (B) Electron micrographs of ferritin, ferritin displaying GFP, and ferritin fused to both GFP and a cytotoxic peptide (“KLAK” repeats). (C) Tumor cryosections from mice treated with a saline control, cytotoxic peptide (KLAK), ferritin-GFP and cytotoxic peptide (sFt-GFP + KLAK), or ferritin fused to both cytotoxic peptide and GFP (KLAK-sFt-GFP). Scale bars are 40 μm. Reprinted with permission.(83)
Remaining Challenges and Opportunities for Protein-Based Nanotherapeutics
Adaptive and innate immune responses are perhaps the largest challenge for the broad application of protein nanoparticle therapeutics.180,181 Preclinical and clinical experience with therapeutics and vaccines based on viral vectors has clearly established vector-neutralizing antibodies as a significant limitation. Antibody responses against the nanoparticle surface and MPS clearance would likely reduce therapeutic efficacy by preventing cargo delivery to the target tissues or cells.17,117,118 Altering physicochemical surface properties like lipid envelopes or glycosylation could significantly reduce such antibody responses. Although preliminary studies suggest that structural features such as aspect ratio and display of phagocytosis-preventing signaling molecules could reduce MPS clearance, new concepts and strategies will be needed to minimize the impact of immune responses against protein nanoparticle therapeutics.13−15,182 The growing body of data on protein nanoparticle vaccines—where immune responses are desirable instead of problematic—provides a valuable opportunity to learn how protein nanoparticles are perceived by the immune system.183,184 Lessons learned from these studies could be used to develop techniques for engineering immune evasion into protein nanoparticles.
Challenges in design and manufacturing hinder the development of efficacious protein nanotherapeutics. Although computational advances are broadening the accessibility of protein design, repurposing naturally existing protein nanocontainers and designing novel nanomaterials still require significant expertise and effort. Successfully designed protein nanotherapeutics must be manufactured from nucleic acid templates in biological systems, posing a challenge to meeting the demands of clinical production efficiency but offering opportunities for genetic delivery. Fortunately, molecular analysis and quality control of protein nanoparticles benefits greatly from the abundance of nanoscale analytical techniques. The monodisperse and atomically precise nature of protein nanoparticles enables straightforward and reliable use of analytical techniques, allowing for rapid design-build-test cycles.
There are many opportunities for meaningful advancement in the near future of protein nanoparticle engineering. Naturally occurring proteins and protein nanoparticles present innumerable examples of the sensitive and precise environmental responsiveness proteins can achieve; learning how to harness and engineer these kinds of responsiveness into protein nanoparticle therapeutics is a major opportunity for the field.111,170 Engineers are now able to selectively design and evolve nanoparticles with features such as larger interior cavities, nonspherical scaffolds, and specific responses to environmental cues of interest.33,46,56 Engineering and clinically translating self-assembling protein nanoparticles promises to be an exciting and abundant area of research over the next several years.
Acknowledgments
We would like to thank Chelsea Fries for comments on the manuscript. This work was supported by the National Institutes of Health (P50 AI150464 to N.P.K., 1R21CA232430-01 to S.H.P. and N.P.K., and 1R01CA257563 to S.H.P.), the National Science Foundation NSF (CHE 1629214 to N.P.K. and DGE-2140004 to C.R.), and the Audacious Project. Figures 2, 4, 5, and 8 were made using Inkscape.185 The TOC graphic was created with Biorender.com.
The authors declare the following competing financial interest(s): N.P.K. is a co-founder, shareholder, paid consultant, and chair of the scientific advisory board of Icosavax, Inc. The King lab has received an unrelated sponsored research agreement from Pfizer.
References
- Mitchell M. J.; Billingsley M. M.; Haley R. M.; Wechsler M. E.; Peppas N. A.; Langer R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov 2021, 20 (2), 101–124. 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. E.; Chen Z. G.; Shin D. M. Nanoparticle Therapeutics: An Emerging Treatment Modality for Cancer. Nat. Rev. Drug Discov 2008, 7 (9), 771–782. 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- Waterhouse D. N.; Tardi P. G.; Mayer L. D.; Bally M. B. A Comparison of Liposomal Formulations of Doxorubicin with Drug Administered in Free Form: Changing Toxicity Profiles. Drug Saf 2001, 24 (12), 903–920. 10.2165/00002018-200124120-00004. [DOI] [PubMed] [Google Scholar]
- Barenholz Y. Doxil®–the First FDA-Approved Nano-Drug: Lessons Learned. J. Controlled Release 2012, 160 (2), 117–134. 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Office of the Commissioner. FDA approves first COVID-19 vaccine https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed 2021 −11 −02).
- Office of the Commissioner. Coronavirus (COVID-19) update: FDA takes key action by approving second COVID-19 vaccine https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-key-action-approving-second-covid-19-vaccine (accessed 03/01/2022).
- Paunovska K.; Sago C. D.; Monaco C. M.; Hudson W. H.; Castro M. G.; Rudoltz T. G.; Kalathoor S.; Vanover D. A.; Santangelo P. J.; Ahmed R.; et al. A Direct Comparison of in Vitro and in Vivo Nucleic Acid Delivery Mediated by Hundreds of Nanoparticles Reveals a Weak Correlation. Nano Lett. 2018, 18 (3), 2148–2157. 10.1021/acs.nanolett.8b00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.; Ukidve A.; Kim J.; Mitragotri S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181 (1), 151–167. 10.1016/j.cell.2020.02.001. [DOI] [PubMed] [Google Scholar]
- Bae Y. H.; Park K. Advanced Drug Delivery 2020 and beyond: Perspectives on the Future. Adv. Drug Delivery Rev. 2020, 158, 4–16. 10.1016/j.addr.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco E.; Shen H.; Ferrari M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33 (9), 941–951. 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S. T.; Swartz M. A.; Hubbell J. A. Targeting Dendritic Cells with Biomaterials: Developing the next Generation of Vaccines. Trends Immunol 2006, 27 (12), 573–579. 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Qi R.; Wang Y.; Bruno P. M.; Xiao H.; Yu Y.; Li T.; Lauffer S.; Wei W.; Chen Q.; Kang X.; et al. Nanoparticle Conjugates of a Highly Potent Toxin Enhance Safety and Circumvent Platinum Resistance in Ovarian Cancer. Nat. Commun. 2017, 8 (1), 2166. 10.1038/s41467-017-02390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Wang Y.; Ran F.; Cui Y.; Liu C.; Zhao Q.; Gao Y.; Wang D.; Wang S. A Comparison between Sphere and Rod Nanoparticles Regarding Their in Vivo Biological Behavior and Pharmacokinetics. Sci. Rep 2017, 7 (1), 4131. 10.1038/s41598-017-03834-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton S. E. A.; Ropp P. A.; Pohlhaus P. D.; Luft J. C.; Madden V. J.; Napier M. E.; DeSimone J. M. The Effect of Particle Design on Cellular Internalization Pathways. Proc. Natl. Acad. Sci. U. S. A 2008, 105 (33), 11613–11618. 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W.; Kim B. Y. S.; Rutka J. T.; Chan W. C. W. Nanoparticle-Mediated Cellular Response Is Size-Dependent. Nat. Nanotechnol 2008, 3 (3), 145–150. 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- Madathiparambil Visalakshan R.; González García L. E.; Benzigar M. R.; Ghazaryan A.; Simon J.; Mierczynska-Vasilev A.; Michl T. D.; Vinu A.; Mailänder V.; Morsbach S.; et al. The Influence of Nanoparticle Shape on Protein Corona Formation. Small 2020, 16 (25), 2000285. 10.1002/smll.202000285. [DOI] [PubMed] [Google Scholar]
- Lazarovits J.; Chen Y. Y.; Sykes E. A.; Chan W. C. W. Nanoparticle-Blood Interactions: The Implications on Solid Tumour Targeting. Chem. Commun. 2015, 51 (14), 2756–2767. 10.1039/C4CC07644C. [DOI] [PubMed] [Google Scholar]
- Bale J. B.; Gonen S.; Liu Y.; Sheffler W.; Ellis D.; Thomas C.; Cascio D.; Yeates T. O.; Gonen T.; King N. P.; et al. Accurate Design of Megadalton-Scale Two-Component Icosahedral Protein Complexes. Science 2016, 353 (6297), 389–394. 10.1126/science.aaf8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N. P.; Sheffler W.; Sawaya M. R.; Vollmar B. S.; Sumida J. P.; André I.; Gonen T.; Yeates T. O.; Baker D. Computational Design of Self-Assembling Protein Nanomaterials with Atomic Level Accuracy. Science 2012, 336 (6085), 1171–1174. 10.1126/science.1219364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.; Hong Y.; Gong Y.; Zheng S.; Xie D.. Bioengineered Ferritin Nanocarriers for Cancer Therapy Int. J. Mol. Sci.. 2021, 22 ( (13), ). 7023. 10.3390/ijms22137023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divine R.; Dang H. V.; Ueda G.; Fallas J. A.; Vulovic I.; Sheffler W.; Saini S.; Zhao Y. T.; Raj I. X.; Morawski P. A.. et al. Designed Proteins Assemble Antibodies into Modular Nanocages bioRxiv 2021. 10.1126/science.abd9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowinsky E. K.; Donehower R. C. Paclitaxel (taxol). N. Engl. J. Med. 1995, 332 (15), 1004–1014. 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- Pham D. T.; Tiyaboonchai W. Fibroin Nanoparticles: A Promising Drug Delivery System. Drug Deliv 2020, 27 (1), 431–448. 10.1080/10717544.2020.1736208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. J.; Guenther C. M.; Suh J. Adeno-Associated Virus (AAV) Vectors: Rational Design Strategies for Capsid Engineering. Curr. Opin Biomed Eng. 2018, 7, 58–63. 10.1016/j.cobme.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas T.; Young M. Viruses: Making Friends with Old Foes. Science 2006, 312 (5775), 873–875. 10.1126/science.1123223. [DOI] [PubMed] [Google Scholar]
- Schwarz B.; Douglas T. Development of Virus-like Particles for Diagnostic and Prophylactic Biomedical Applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2015, 7 (5), 722–735. 10.1002/wnan.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz N. F.; Lim S.; Sainsbury F. Protein Cages and Virus-like Particles: From Fundamental Insight to Biomimetic Therapeutics. Biomater Sci. 2020, 8 (10), 2771–2777. 10.1039/D0BM00159G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y.; Yang T.; Yang S.; Yang M.; Mao C. Protein Nanoparticles Directed Cancer Imaging and Therapy. Nano Converg 2022, 9 (1), 2. 10.1186/s40580-021-00293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi N.; Quevedo D. F.; Gregory J. V.; Lahann J. Emerging Methods in Therapeutics Using Multifunctional Nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2020, 12 (4), e1625. 10.1002/wnan.1625. [DOI] [PubMed] [Google Scholar]
- Wagner H. J.; Weber W.; Fussenegger M. Synthetic Biology: Emerging Concepts to Design and Advance Adeno-Associated Viral Vectors for Gene Therapy. Adv. Sci. 2021, 8 (9), 2004018. 10.1002/advs.202004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.-S.; Boyken S. E.; Baker D. The Coming of Age of de Novo Protein Design. Nature 2016, 537 (7620), 320–327. 10.1038/nature19946. [DOI] [PubMed] [Google Scholar]
- Jumper J.; Evans R.; Pritzel A.; Green T.; Figurnov M.; Ronneberger O.; Tunyasuvunakool K.; Bates R.; Zidek A.; Potapenko A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596 (7873), 583–589. 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J.; Do Yoo J.; Kim M.; Shim G.; Oh Y.-K.; Park R.-W.; Lee B.; Kim I.-S.; Kim S.. Fibrinolytic Nanocages Dissolve Clots in the Tumor Microenvironment, Improving the Distribution and Therapeutic Efficacy of Anticancer Drugs Exp. Mol. Med.. 2021. 53 (10), 1592. 10.1038/s12276-021-00688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan R. A.; Boyken S. E.; Ng A. H.; Samson J. A.; Dods G.; Westbrook A. M.; Nguyen T. H.; Lajoie M. J.; Chen Z.; Berger S.; et al. De Novo Design of Bioactive Protein Switches. Nature 2019, 572 (7768), 205–210. 10.1038/s41586-019-1432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie M. J.; Boyken S. E.; Salter A. I.; Bruffey J.; Rajan A.; Langan R. A.; Olshefsky A.; Muhunthan V.; Bick M. J.; Gewe M.; et al. Designed Protein Logic to Target Cells with Precise Combinations of Surface Antigens. Science 2020, 369 (6511), 1637–1643. 10.1126/science.aba6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyken S. E.; Benhaim M. A.; Busch F.; Jia M.; Bick M. J.; Choi H.; Klima J. C.; Chen Z.; Walkey C.; Mileant A.; et al. De Novo Design of Tunable, pH-Driven Conformational Changes. Science 2019, 364 (6441), 658–664. 10.1126/science.aav7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk T.; Đorđević S.; Conejos-Sánchez I.; Vicent M. J. Therapeutic Potential of Polypeptide-Based Conjugates: Rational Design and Analytical Tools That Can Boost Clinical Translation. Adv. Drug Delivery Rev. 2020, 160, 136–169. 10.1016/j.addr.2020.10.007. [DOI] [PubMed] [Google Scholar]
- Yoo J. D.; Bae S. M.; Seo J.; Jeon I. S.; Vadevoo S. M. P.; Kim S.-Y.; Kim I.-S.; Lee B.; Kim S. Designed Ferritin Nanocages Displaying Trimeric TRAIL and Tumor-Targeting Peptides Confer Superior Anti-Tumor Efficacy. Sci. Rep 2020, 10 (1), 19997. 10.1038/s41598-020-77095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome L. H.; Kickhoefer V. A. Development of the Vault Particle as a Platform Technology. ACS Nano 2013, 7 (2), 889–902. 10.1021/nn3052082. [DOI] [PubMed] [Google Scholar]
- Yeates T. O.; Liu Y.; Laniado J. The Design of Symmetric Protein Nanomaterials Comes of Age in Theory and Practice. Curr. Opin. Struct. Biol. 2016, 39, 134–143. 10.1016/j.sbi.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Van de Steen A.; Khalife R.; Colant N.; Mustafa Khan H.; Deveikis M.; Charalambous S.; Robinson C. M.; Dabas R.; Esteban Serna S.; Catana D. A.; et al. Bioengineering Bacterial Encapsulin Nanocompartments as Targeted Drug Delivery System. Synth. Syst. Biotechnol. 2021, 6 (3), 231–241. 10.1016/j.synbio.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma Y.; Edwardson T. G. W.; Hilvert D. Tailoring Lumazine Synthase Assemblies for Bionanotechnology. Chem. Soc. Rev. 2018, 47 (10), 3543–3557. 10.1039/C8CS00154E. [DOI] [PubMed] [Google Scholar]
- Khmelinskaia A.; Wargacki A.; King N. P. Structure-Based Design of Novel Polyhedral Protein Nanomaterials. Curr. Opin. Microbiol 2021, 61, 51–57. 10.1016/j.mib.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malay A. D.; Miyazaki N.; Biela A.; Chakraborti S.; Majsterkiewicz K.; Stupka I.; Kaplan C. S.; Kowalczyk A.; Piette B. M. A. G.; Hochberg G. K. A.; et al. An Ultra-Stable Gold-Coordinated Protein Cage Displaying Reversible Assembly. Nature 2019, 569 (7756), 438–442. 10.1038/s41586-019-1185-4. [DOI] [PubMed] [Google Scholar]
- Churchfield L. A.; Tezcan F. A. Design and Construction of Functional Supramolecular Metalloprotein Assemblies. Acc. Chem. Res. 2019, 52 (2), 345–355. 10.1021/acs.accounts.8b00617. [DOI] [PubMed] [Google Scholar]
- Ballister E. R.; Lai A. H.; Zuckermann R. N.; Cheng Y.; Mougous J. D. In Vitro Self-Assembly of Tailorable Nanotubes from a Simple Protein Building Block. Proc. Natl. Acad. Sci. U. S. A 2008, 105 (10), 3733–3738. 10.1073/pnas.0712247105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laniado J.; Meador K.; Yeates T. O.. A Fragment-Based Protein Interface Design Algorithm for Symmetric Assemblies Protein Eng. Des. Sel. 2021, 34 10.1093/protein/gzab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J. E.; Colovos C.; Yeates T. O. Nanohedra: Using Symmetry to Design Self Assembling Protein Cages, Layers, Crystals, and Filaments. Proc. Natl. Acad. Sci. U. S. A 2001, 98 (5), 2217–2221. 10.1073/pnas.041614998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon K. A.; Nguyen V. N.; Morgan C.; Yeates T. O. Design and Characterization of an Icosahedral Protein Cage Formed by a Double-Fusion Protein Containing Three Distinct Symmetry Elements. ACS Synth. Biol. 2020, 9 (3), 517–524. 10.1021/acssynbio.9b00392. [DOI] [PubMed] [Google Scholar]
- Vulovic I.; Yao Q.; Park Y.-J.; Courbet A.; Norris A.; Busch F.; Sahasrabuddhe A.; Merten H.; Sahtoe D. D.; Ueda G.. et al. Generation of Ordered Protein Assemblies Using Rigid Three-Body Fusion Proc. Natl. Acad. Sci. U. S. A. 2021, 118 ( (23), ) 10.1073/pnas.2015037118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda G.; Antanasijevic A.; Fallas J. A.; Sheffler W.; Copps J.; Ellis D.; Hutchinson G. B.; Moyer A.; Yasmeen A.; Tsybovsky Y.. et al. Tailored Design of Protein Nanoparticle Scaffolds for Multivalent Presentation of Viral Glycoprotein Antigens Elife 2020, 9 10.7554/eLife.57659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anishchenko I.; Pellock S. J.; Chidyausiku T. M.; Ramelot T. A.; Ovchinnikov S.; Hao J.; Bafna K.; Norn C.; Kang A.; Bera A. K.; et al. De Novo Protein Design by Deep Network Hallucination. Nature 2021, 600 (7889), 547–552. 10.1038/s41586-021-04184-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer M. S.; Liu D. R. Methods for the Directed Evolution of Proteins. Nat. Rev. Genet 2015, 16 (7), 379–394. 10.1038/nrg3927. [DOI] [PubMed] [Google Scholar]
- Wörsdörfer B.; Woycechowsky K. J.; Hilvert D. Directed Evolution of a Protein Container. Science 2011, 331 (6017), 589–592. 10.1126/science.1199081. [DOI] [PubMed] [Google Scholar]
- Butterfield G. L.; Lajoie M. J.; Gustafson H. H.; Sellers D. L.; Nattermann U.; Ellis D.; Bale J. B.; Ke S.; Lenz G. H.; Yehdego A.; et al. Evolution of a Designed Protein Assembly Encapsulating Its Own RNA Genome. Nature 2017, 552 (7685), 415–420. 10.1038/nature25157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetter S.; Terasaka N.; Steinauer A.; Bingham R. J.; Clark S.; Scott A. J. P.; Patel N.; Leibundgut M.; Wroblewski E.; Ban N.; et al. Evolution of a Virus-like Architecture and Packaging Mechanism in a Repurposed Bacterial Protein. Science 2021, 372 (6547), 1220–1224. 10.1126/science.abg2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaka N.; Azuma Y.; Hilvert D. Laboratory Evolution of Virus-like Nucleocapsids from Nonviral Protein Cages. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (21), 5432–5437. 10.1073/pnas.1800527115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala D. S.; Sun S.; Santiago-Ortiz J. L.; Shapiro M. G.; Romero P. A.; Schaffer D. V. In Vivo Selection of a Computationally Designed SCHEMA AAV Library Yields a Novel Variant for Infection of Adult Neural Stem Cells in the SVZ. Mol. Ther 2018, 26 (1), 304–319. 10.1016/j.ymthe.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman B. E.; Pravdo P. L.; Simpson B. P.; Kumar S. R.; Chan K. Y.; Banerjee A.; Wu W.-L.; Yang B.; Huber N.; Pasca S. P.; Gradinaru V. Cre-Dependent Selection Yields AAV Variants for Widespread Gene Transfer to the Adult Brain. Nat. Biotechnol. 2016, 34 (2), 204–209. 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson H. H.; Olshefsky A.; Sylvestre M.; Sellers D. L.; Pun S. H. Current State of in Vivo Panning Technologies: Designing Specificity and Affinity into the Future of Drug Targeting. Adv. Drug Delivery Rev. 2018, 130, 39–49. 10.1016/j.addr.2018.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabebordbar M.; Lagerborg K. A.; Stanton A.; King E. M.; Ye S.; Tellez L.; Krunnfusz A.; Tavakoli S.; Widrick J. J.; Messemer K. A.; et al. Directed Evolution of a Family of AAV Capsid Variants Enabling Potent Muscle-Directed Gene Delivery across Species. Cell 2021, 184 (19), 4919–4938. 10.1016/j.cell.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E. F.; Goddard T. D.; Huang C. C.; Meng E. C.; Couch G. S.; Croll T. I.; Morris J. H.; Ferrin T. E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30 (1), 70–82. 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. M.; Artymiuk P. J.; Yewdall S. J.; Smith J. M. A.; Livingstone J. C.; Treffry A.; Luzzago A.; Levi S.; Arosio P.; Cesareni G.; et al. Solving the Structure of Human H Ferritin by Genetically Engineering Intermolecular Crystal Contacts. Nature 1991, 349 (6309), 541–544. 10.1038/349541a0. [DOI] [PubMed] [Google Scholar]
- Sutter M.; Boehringer D.; Gutmann S.; Günther S.; Prangishvili D.; Loessner M. J.; Stetter K. O.; Weber-Ban E.; Ban N. Structural Basis of Enzyme Encapsulation into a Bacterial Nanocompartment. Nat. Struct. Mol. Biol. 2008, 15 (9), 939–947. 10.1038/nsmb.1473. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Meining W.; Fischer M.; Bacher A.; Ladenstein R. X-Ray Structure Analysis and Crystallographic Refinement of Lumazine Synthase from the Hyperthermophile Aquifex Aeolicus at 1.6 A Resolution: Determinants of Thermostability Revealed from Structural Comparisons. J. Mol. Biol. 2001, 306 (5), 1099–1114. 10.1006/jmbi.2000.4435. [DOI] [PubMed] [Google Scholar]
- Yeates T. O. Geometric Principles for Designing Highly Symmetric Self-Assembling Protein Nanomaterials. Annu. Rev. Biophys 2017, 46, 23–42. 10.1146/annurev-biophys-070816-033928. [DOI] [PubMed] [Google Scholar]
- Laniado J.; Yeates T. O. A Complete Rule Set for Designing Symmetry Combination Materials from Protein Molecules. Proc. Natl. Acad. Sci. U. S. A 2020, 117 (50), 31817–31823. 10.1073/pnas.2015183117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenstein R.; Meyer B.; Huber R.; Labischinski H.; Bartels K.; Bartunik H. D.; Bachmann L.; Ludwig H. C.; Bacher A. Heavy Riboflavin Synthase from Bacillus Subtilis. Particle Dimensions, Crystal Packing and Molecular Symmetry. J. Mol. Biol. 1986, 187 (1), 87–100. 10.1016/0022-2836(86)90408-0. [DOI] [PubMed] [Google Scholar]
- Wukovitz S. W.; Yeates T. O. Why Protein Crystals Favour Some Space-Groups over Others. Nat. Struct. Biol. 1995, 2 (12), 1062–1067. 10.1038/nsb1295-1062. [DOI] [PubMed] [Google Scholar]
- Hsia Y.; Bale J. B.; Gonen S.; Shi D.; Sheffler W.; Fong K. K.; Nattermann U.; Xu C.; Huang P.-S.; Ravichandran R.; et al. Corrigendum: Design of a Hyperstable 60-Subunit Protein Icosahedron. Nature 2016, 540 (7631), 150. 10.1038/nature20108. [DOI] [PubMed] [Google Scholar]
- King N. P.; Bale J. B.; Sheffler W.; McNamara D. E.; Gonen S.; Gonen T.; Yeates T. O.; Baker D. Accurate Design of Co-Assembling Multi-Component Protein Nanomaterials. Nature 2014, 510 (7503), 103–108. 10.1038/nature13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargacki A. J.; Wörner T. P.; van de Waterbeemd M.; Ellis D.; Heck A. J. R.; King N. P. Complete and Cooperative in Vitro Assembly of Computationally Designed Self-Assembling Protein Nanomaterials. Nat. Commun. 2021, 12 (1), 883. 10.1038/s41467-021-21251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnick A. Are Weak Protein-Protein Interactions the General Rule in Capsid Assembly?. Virology 2003, 315 (2), 269–274. 10.1016/S0042-6822(03)00586-5. [DOI] [PubMed] [Google Scholar]
- Deeds E. J.; Bachman J. A.; Fontana W. Optimizing Ring Assembly Reveals the Strength of Weak Interactions. Proc. Natl. Acad. Sci. U. S. A 2012, 109 (7), 2348–2353. 10.1073/pnas.1113095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan M. F.; Chandler D. Dynamic Pathways for Viral Capsid Assembly. Biophys. J. 2006, 91 (1), 42–54. 10.1529/biophysj.105.076851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag B. K.; Liu C.; Haritos V. S.; He L. Understanding the Interplay between Self-Assembling Peptides and Solution Ions for Tunable Protein Nanoparticle Formation. ACS Nano 2018, 12 (7), 6956–6967. 10.1021/acsnano.8b02381. [DOI] [PubMed] [Google Scholar]
- Kim M.; Rho Y.; Jin K. S.; Ahn B.; Jung S.; Kim H.; Ree M. pH-Dependent Structures of Ferritin and Apoferritin in Solution: Disassembly and Reassembly. Biomacromolecules 2011, 12 (5), 1629–1640. 10.1021/bm200026v. [DOI] [PubMed] [Google Scholar]
- Sharma J.; Uchida M.; Miettinen H. M.; Douglas T. Modular Interior Loading and Exterior Decoration of a Virus-like Particle. Nanoscale 2017, 9 (29), 10420–10430. 10.1039/C7NR03018E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy K.; Selivanovitch E.; Luque D.; Lee B.; Edwards E.; Castón J. R.; Douglas T. Cargo Retention inside P22 Virus-Like Particles. Biomacromolecules 2018, 19 (9), 3738–3746. 10.1021/acs.biomac.8b00867. [DOI] [PubMed] [Google Scholar]
- Hermanson G. T.Bioconjugate Techniques; Academic Press: Cambridge, MA, 2013. [Google Scholar]
- Drachman J. G.; Senter P. D. Antibody-Drug Conjugates: The Chemistry behind Empowering Antibodies to Fight Cancer. Hematology Am. Soc. Hematol. Educ. Program 2013, 2013, 306–310. 10.1182/asheducation-2013.1.306. [DOI] [PubMed] [Google Scholar]
- Baskin J. M.; Prescher J. A.; Laughlin S. T.; Agard N. J.; Chang P. V.; Miller I. A.; Lo A.; Codelli J. A.; Bertozzi C. R. Copper-Free Click Chemistry for Dynamic in Vivo Imaging. Proc. Natl. Acad. Sci. U. S. A 2007, 104 (43), 16793–16797. 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.; Kim G. S.; Seo J.; Gowri Rangaswamy G.; So I.-S.; Park R.-W.; Lee B.-H.; Kim I.-S. Double-Chambered Ferritin Platform: Dual-Function Payloads of Cytotoxic Peptides and Fluorescent Protein. Biomacromolecules 2016, 17 (1), 12–19. 10.1021/acs.biomac.5b01134. [DOI] [PubMed] [Google Scholar]
- Wang Y.-H.; Jian M.-L.; Chen P.-J.; Tsou J.-C.; Truong L. P.; Wang Y.-S. Ferritin Conjugates With Multiple Clickable Amino Acids Encoded by C-Terminal Engineered Pyrrolysyl-tRNA Synthetase. Front Chem. 2021, 9, 779976. 10.3389/fchem.2021.779976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.-M.; Choi S.-H.; Jeon H.; Kim I.-S.; Ahn H. J. Chimeric Capsid Protein as a Nanocarrier for siRNA Delivery: Stability and Cellular Uptake of Encapsulated siRNA. ACS Nano 2011, 5 (11), 8690–8699. 10.1021/nn202597c. [DOI] [PubMed] [Google Scholar]
- Tokatlian T.; Read B. J.; Jones C. A.; Kulp D. W.; Menis S.; Chang J. Y. H.; Steichen J. M.; Kumari S.; Allen J. D.; Dane E. L.; et al. Innate Immune Recognition of Glycans Targets HIV Nanoparticle Immunogens to Germinal Centers. Science 2019, 363 (6427), 649–654. 10.1126/science.aat9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer C. D.; Jumeaux C.; Gupta B.; Stevens M. M. Peptide and Protein Nanoparticle Conjugates: Versatile Platforms for Biomedical Applications. Chem. Soc. Rev. 2018, 47 (10), 3574–3620. 10.1039/C7CS00877E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H.; Lee J.; Min J.; Kang S. Developing Genetically Engineered Encapsulin Protein Cage Nanoparticles as a Targeted Delivery Nanoplatform. Biomacromolecules 2014, 15 (10), 3794–3801. 10.1021/bm501066m. [DOI] [PubMed] [Google Scholar]
- Choi H.; Eom S.; Kim H.-U.; Bae Y.; Jung H. S.; Kang S. Load and Display: Engineering Encapsulin as a Modular Nanoplatform for Protein-Cargo Encapsulation and Protein-Ligand Decoration Using Split Intein and SpyTag/SpyCatcher. Biomacromolecules 2021, 22 (7), 3028–3039. 10.1021/acs.biomac.1c00481. [DOI] [PubMed] [Google Scholar]
- Wang L.; Xing D.; Le Van A.; Jerse A. E.; Wang S. Structure-Based Design of Ferritin Nanoparticle Immunogens Displaying Antigenic Loops of Neisseria Gonorrhoeae. FEBS Open Bio 2017, 7 (8), 1196–1207. 10.1002/2211-5463.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H.; Lee J.; Kim H.; Heo S.; Min J.; Kang S. Genetically Engineering Encapsulin Protein Cage Nanoparticle as a SCC-7 Cell Targeting Optical Nanoprobe. Biomater Res. 2014, 18 (1), 21. 10.1186/2055-7124-18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel-Souzy S.; Hamelmann N. M.; Zarzuela-Pura S.; Paulusse J. M. J.; Cornelissen J. J. L. M. Introduction of Surface Loops as a Tool for Encapsulin Functionalization. Biomacromolecules 2021, 22 (12), 5234–5242. 10.1021/acs.biomac.1c01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read B. J.; Won L.; Kraft J. C.; Sappington I.; Aung A.; Wu S.; Bals J.; Chen C.; Lee K. K.; Lingwood D.; et al. Mannose-Binding Lectin and Complement Mediate Follicular Localization and Enhanced Immunogenicity of Diverse Protein Nanoparticle Immunogens. Cell Rep 2022, 38 (2), 110217. 10.1016/j.celrep.2021.110217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk J. S.; Xu Q.; Kim N.; Hanes J.; Ensign L. M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Delivery Rev. 2016, 99, 28–51. 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahnd C.; Wyler E.; Schwenk J. M.; Steiner D.; Lawrence M. C.; McKern N. M.; Pecorari F.; Ward C. W.; Joos T. O.; Plückthun A. A Designed Ankyrin Repeat Protein Evolved to Picomolar Affinity to Her2. J. Mol. Biol. 2007, 369 (4), 1015–1028. 10.1016/j.jmb.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Boyoglu-Barnum S.; Ellis D.; Gillespie R. A.; Hutchinson G. B.; Park Y.-J.; Moin S. M.; Acton O. J.; Ravichandran R.; Murphy M.; Pettie D.; et al. Quadrivalent Influenza Nanoparticle Vaccines Induce Broad Protection. Nature 2021, 592 (7855), 623–628. 10.1038/s41586-021-03365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo M.; Wei C.-J.; Yassine H. M.; McTamney P. M.; Boyington J. C.; Whittle J. R. R.; Rao S. S.; Kong W.-P.; Wang L.; Nabel G. J. Self-Assembling Influenza Nanoparticle Vaccines Elicit Broadly Neutralizing H1N1 Antibodies. Nature 2013, 499 (7456), 102–106. 10.1038/nature12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcandalli J.; Fiala B.; Ols S.; Perotti M.; de van der Schueren W.; Snijder J.; Hodge E.; Benhaim M.; Ravichandran R.; Carter L.; et al. Induction of Potent Neutralizing Antibody Responses by a Designed Protein Nanoparticle Vaccine for Respiratory Syncytial Virus. Cell 2019, 176 (6), 1420–1431. 10.1016/j.cell.2019.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahikainen R.; Rijal P.; Tan T. K.; Wu H.-J.; Andersson A.-M. C.; Barrett J. R.; Bowden T. A.; Draper S. J.; Townsend A. R.; Howarth M. Overcoming Symmetry Mismatch in Vaccine Nanoassembly through Spontaneous Amidation. Angew. Chem., Int. Ed. Engl. 2021, 60 (1), 321–330. 10.1002/anie.202009663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E.UPDATED: J&J’s Doxil shortage to last until at least end of 2014 https://www.fiercepharma.com/m-a/updated-j-j-s-doxil-shortage-to-last-until-at-least-end-of-2014 (accessed 2022 −01 −19).
- Sethuraman N.; Stadheim T. A. Challenges in Therapeutic Glycoprotein Production. Curr. Opin. Biotechnol 2006, 17 (4), 341–346. 10.1016/j.copbio.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Kelley B. Industrialization of mAb Production Technology: The Bioprocessing Industry at a Crossroads. MAbs 2009, 1 (5), 443–452. 10.4161/mabs.1.5.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir N.; Streatfield S. J.; Yusibov V. Virus-like Particles as a Highly Efficient Vaccine Platform: Diversity of Targets and Production Systems and Advances in Clinical Development. Vaccine 2012, 31 (1), 58–83. 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N.; Thatte U.; Kshirsagar N.; Leav B.; Molrine D.; Cheslock P.; Kapre S. V.; Kulkarni P. S. SII RMab author group. Safety and Pharmacokinetics of a Human Monoclonal Antibody to Rabies Virus: A Randomized, Dose-Escalation Phase 1 Study in Adults. Vaccine 2012, 30 (50), 7315–7320. 10.1016/j.vaccine.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Wu L.; Su S.; Liu F.; Xu T.; Wang X.; Huang Y.; Sun X.; Ge X.; Chen T.; Liu H.; et al. Removal of the Tag from His-Tagged ILYd4, a Human CD59 Inhibitor, Significantly Improves Its Physical Properties and Its Activity. Curr. Pharm. Des 2012, 18 (27), 4187–4196. 10.2174/138161212802430486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz J.; Wurm F. M. Recombinant Proteins for Industrial versus Pharmaceutical Purposes: A Review of Process and Pricing. Processes 2019, 7 (8), 476. 10.3390/pr7080476. [DOI] [Google Scholar]
- Jiang H.; Horwitz A. A.; Wright C.; Tai A.; Znameroski E. A.; Tsegaye Y.; Warbington H.; Bower B. S.; Alves C.; Co C.; et al. Challenging the Workhorse: Comparative Analysis of Eukaryotic Micro-Organisms for Expressing Monoclonal Antibodies. Biotechnol. Bioeng. 2019, 116 (6), 1449–1462. 10.1002/bit.26951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. R.; Love J. C. Alternative Hosts as the Missing Link for Equitable Therapeutic Protein Production. Nat. Biotechnol. 2021, 39 (4), 404–407. 10.1038/s41587-021-00884-w. [DOI] [PubMed] [Google Scholar]
- Matthews C. B.; Kuo A.; Love K. R.; Love J. C. Development of a General Defined Medium for Pichia Pastoris. Biotechnol. Bioeng. 2018, 115 (1), 103–113. 10.1002/bit.26440. [DOI] [PubMed] [Google Scholar]
- Cohen A. A.; Gnanapragasam P. N. P.; Lee Y. E.; Hoffman P. R.; Ou S.; Kakutani L. M.; Keeffe J. R.; Wu H.-J.; Howarth M.; West A. P.; et al. Mosaic Nanoparticles Elicit Cross-Reactive Immune Responses to Zoonotic Coronaviruses in Mice. Science 2021, 371 (6530), 735–741. 10.1126/science.abf6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.; Tai P. W. L.; Gao G. Adeno-Associated Virus Vector as a Platform for Gene Therapy Delivery. Nat. Rev. Drug Discov 2019, 18 (5), 358–378. 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulcha J. T.; Wang Y.; Ma H.; Tai P. W. L.; Gao G. Viral Vector Platforms within the Gene Therapy Landscape. Signal Transduct Target Ther 2021, 6 (1), 53. 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N.; Hogan M. J.; Weissman D. Recent Advances in mRNA Vaccine Technology. Curr. Opin. Immunol 2020, 65, 14–20. 10.1016/j.coi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Chokkalingam N.; Tello-Ruiz E.; Wise M. C.; Bah M. A.; Walker S.; Tursi N. J.; Fisher P. D.; Schultheis K.; Broderick K. E.; et al. A DNA-Launched Nanoparticle Vaccine Elicits CD8+ T-Cell Immunity to Promote In Vivo Tumor Control. Cancer Immunol Res. 2020, 8 (11), 1354–1364. 10.1158/2326-6066.CIR-20-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo M.; Porter E.; Zhang Y.; Silva M.; Li N.; Dobosh B.; Liguori A.; Skog P.; Landais E.; Menis S.; et al. Immunogenicity of RNA Replicons Encoding HIV Env Immunogens Designed for Self-Assembly into Nanoparticles. Mol. Ther 2019, 27 (12), 2080–2090. 10.1016/j.ymthe.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshyar N.; Gray S.; Han H.; Bao G. The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11 (6), 673–692. 10.2217/nnm.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot A. S.; Scott D. W. Immunogenicity of Protein Therapeutics. Trends Immunol 2007, 28 (11), 482–490. 10.1016/j.it.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Gustafson H. H.; Holt-Casper D.; Grainger D. W.; Ghandehari H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10 (4), 487–510. 10.1016/j.nantod.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries C. N.; Curvino E. J.; Chen J.-L.; Permar S. R.; Fouda G. G.; Collier J. H. Advances in Nanomaterial Vaccine Strategies to Address Infectious Diseases Impacting Global Health. Nat. Nanotechnol 2021, 16 (4), 1–14. 10.1038/s41565-020-0739-9. [DOI] [PubMed] [Google Scholar]
- Rodrigues M. Q.; Alves P. M.; Roldão A.. Functionalizing Ferritin Nanoparticles for Vaccine Development Pharmaceutics 2021, 13 ( (10), ) 10.3390/pharmaceutics13101621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo M.; Bu W.; Joyce M. G.; Meng G.; Whittle J. R.R.; Baxa U.; Yamamoto T.; Narpala S.; Todd J.-P.; Rao S. S.; et al. Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site. Cell 2015, 162 (5), 1090–1100. 10.1016/j.cell.2015.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A. C.; Fiala B.; Schafer A.; Wrenn S.; Pham M. N.; Murphy M.; Tse L. V.; Shehata L.; O’Connor M. A.; Chen C.; et al. Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2. Cell 2020, 183 (5), 1367–1382. 10.1016/j.cell.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antanasijevic A.; Ueda G.; Brouwer P. J. M.; Copps J.; Huang D.; Allen J. D.; Cottrell C. A.; Yasmeen A.; Sewall L. M.; Bontjer I.; et al. Structural and Functional Evaluation of de Novo-Designed, Two-Component Nanoparticle Carriers for HIV Env Trimer Immunogens. PLoS Pathog 2020, 16 (8), e1008665. 10.1371/journal.ppat.1008665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H.; Chen X.; Boyington J. C.; Cheng C.; Zhang Y.; Jafari A. J.; Stephens T.; Tsybovsky Y.; Kalyuzhniy O.; Zhao P.; et al. Glycan Masking Focuses Immune Responses to the HIV-1 CD4-Binding Site and Enhances Elicitation of VRC01-Class Precursor Antibodies. Immunity 2018, 49 (2), 301–311. 10.1016/j.immuni.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagoutte P.; Mignon C.; Stadthagen G.; Potisopon S.; Donnat S.; Mast J.; Lugari A.; Werle B. Simultaneous Surface Display and Cargo Loading of Encapsulin Nanocompartments and Their Use for Rational Vaccine Design. Vaccine 2018, 36 (25), 3622–3628. 10.1016/j.vaccine.2018.05.034. [DOI] [PubMed] [Google Scholar]
- Choi B.; Moon H.; Hong S. J.; Shin C.; Do Y.; Ryu S.; Kang S. Effective Delivery of Antigen-Encapsulin Nanoparticle Fusions to Dendritic Cells Leads to Antigen-Specific Cytotoxic T Cell Activation and Tumor Rejection. ACS Nano 2016, 10 (8), 7339–7350. 10.1021/acsnano.5b08084. [DOI] [PubMed] [Google Scholar]
- Bachmann M. F.; Jennings G. T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol 2010, 10 (11), 787–796. 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- Kato Y.; Abbott R. K.; Freeman B. L.; Haupt S.; Groschel B.; Silva M.; Menis S.; Irvine D. J.; Schief W. R.; Crotty S. Multifaceted Effects of Antigen Valency on B Cell Response Composition and Differentiation In Vivo. Immunity 2020, 53 (3), 548–563. 10.1016/j.immuni.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan J. S.; Chen M.; Joyce M. G.; Sastry M.; Stewart-Jones G. B. E.; Yang Y.; Zhang B.; Chen L.; Srivatsan S.; Zheng A.; et al. Structure-Based Design of a Fusion Glycoprotein Vaccine for Respiratory Syncytial Virus. Science 2013, 342 (6158), 592–598. 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhamedova M.; Wrapp D.; Shen C.-H.; Gilman M. S.A.; Ruckwardt T. J.; Schramm C. A.; Ault L.; Chang L.; Derrien-Colemyn A.; Lucas S. A.M.; et al. Vaccination with Prefusion-Stabilized Respiratory Syncytial Virus Fusion Protein Induces Genetically and Antigenically Diverse Antibody Responses. Immunity 2021, 54 (4), 769–780. 10.1016/j.immuni.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauthner M.; Havenar-Daughton C.; Sok D.; Nkolola J. P.; Bastidas R.; Boopathy A. V.; Carnathan D. G.; Chandrashekar A.; Cirelli K. M.; Cottrell C. A.; et al. Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity 2017, 46 (6), 1073–108. 10.1016/j.immuni.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli K. M.; Carnathan D. G.; Nogal B.; Martin J. T.; Rodriguez O. L.; Upadhyay A. A.; Enemuo C. A.; Gebru E. H.; Choe Y.; Viviano F.; et al. Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance. Cell 2020, 180 (1), 206. 10.1016/j.cell.2019.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesterhenn F.; Yang C.; Bonet J.; Cramer J. T.; Wen X.; Wang Y.; Chiang C.-I.; Abriata L. A.; Kucharska I.; Castoro G.. et al. De Novo Protein Design Enables the Precise Induction of RSV-Neutralizing Antibodies Science 2020, 368 ( (6492), ) 10.1126/science.aay5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q.; Potter C. S.; Carragher B.; Lander G.; Sworen J.; Towne V.; Abraham D.; Duncan P.; Washabaugh M. W.; Sitrin R. D. Characterization of Virus-like Particles in GARDASIL® by Cryo Transmission Electron Microscopy. Hum. Vaccin. Immunother 2014, 10 (3), 734–739. 10.4161/hv.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce M. G.; Wheatley A. K.; Thomas P. V.; Chuang G.-Y.; Soto C.; Bailer R. T.; Druz A.; Georgiev I. S.; Gillespie R. A.; Kanekiyo M.; et al. Vaccine-Induced Antibodies That Neutralize Group 1 and Group 2 Influenza A Viruses. Cell 2016, 166 (3), 609–623. 10.1016/j.cell.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldão A.; Mellado M. C. M.; Castilho L. R.; Carrondo M. J. T.; Alves P. M. Virus-like Particles in Vaccine Development. Expert Rev. Vaccines 2010, 9 (10), 1149–1176. 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- Jeong H.; Seong B. L. Exploiting Virus-like Particles as Innovative Vaccines against Emerging Viral Infections. J. Microbiol 2017, 55 (3), 220–230. 10.1007/s12275-017-7058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirleaf E. J.; Clark H. Report of the Independent Panel for Pandemic Preparedness and Response: Making COVID-19 the Last Pandemic. Lancet 2021, 398 (10295), 101–103. 10.1016/S0140-6736(21)01095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott R. K.; Lee J. H.; Menis S.; Skog P.; Rossi M.; Ota T.; Kulp D. W.; Bhullar D.; Kalyuzhniy O.; Havenar-Daughton C.; et al. Precursor Frequency and Affinity Determine B Cell Competitive Fitness in Germinal Centers, Tested with Germline-Targeting HIV Vaccine Immunogens. Immunity 2018, 48 (1), 133–146. 10.1016/j.immuni.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine J.; Julien J.-P.; Menis S.; Ota T.; Kalyuzhniy O.; McGuire A.; Sok D.; Huang P.-S.; MacPherson S.; Jones M.; et al. Rational HIV Immunogen Design to Target Specific Germline B Cell Receptors. Science 2013, 340 (6133), 711–716. 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine J. G.; Kulp D. W.; Havenar-Daughton C.; Sarkar A.; Briney B.; Sok D.; Sesterhenn F.; Ereno-Orbea J.; Kalyuzhniy O.; Deresa I.; et al. HIV-1 Broadly Neutralizing Antibody Precursor B Cells Revealed by Germline-Targeting Immunogen. Science 2016, 351 (6280), 1458–1463. 10.1126/science.aad9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Z.; Wiehe K.; Saunders K. O.; Henderson R.; Cain D. W.; Parks R.; Martik D.; Mansouri K.; Edwards R. J.; Newman A.. et al. Ability of Nucleoside-Modified mRNA to Encode HIV-1 Envelope Trimer Nanoparticles bioRxiv 202238110514. 10.1016/j.celrep.2022.110514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise M. C.; Xu Z.; Tello-Ruiz E.; Beck C.; Trautz A.; Patel A.; Elliott S. T.C.; Chokkalingam N.; Kim S.; Kerkau M. G.; et al. In Vivo Delivery of Synthetic DNA-Encoded Antibodies Induces Broad HIV-1-Neutralizing Activity. J. Clin. Invest 2019, 130 (2), 827–837. 10.1172/JCI132779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo M.; Joyce M. G.; Gillespie R. A.; Gallagher J. R.; Andrews S. F.; Yassine H. M.; Wheatley A. K.; Fisher B. E.; Ambrozak D. R.; Creanga A.; et al. Mosaic Nanoparticle Display of Diverse Influenza Virus Hemagglutinins Elicits Broad B Cell Responses. Nat. Immunol 2019, 20 (3), 362–372. 10.1038/s41590-018-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A. C.; Miranda M. C.; Schafer A.; Pham M. N.; Greaney A.; Arunachalam P. S.; Navarro M.-J.; Tortorici M. A.; Rogers K.; O’Connor M. A.; et al. Elicitation of Broadly Protective Sarbecovirus Immunity by Receptor-Binding Domain Nanoparticle Vaccines. Cell 2021, 184 (21), 5432–5447. 10.1016/j.cell.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliepen K.; van Montfort T.; Melchers M.; Isik G.; Sanders R. W. Immunosilencing a Highly Immunogenic Protein Trimerization Domain. J. Biol. Chem. 2015, 290 (12), 7436–7442. 10.1074/jbc.M114.620534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla A. K.; Garg A.; Aggarwal D. Paclitaxel and Its Formulations. Int. J. Pharm. 2002, 235 (1–2), 179–192. 10.1016/S0378-5173(01)00986-3. [DOI] [PubMed] [Google Scholar]
- Horwitz S.; O'Connor O. A; Pro B.; Illidge T.; Fanale M.; Advani R.; Bartlett N. L; Christensen J. H.; Morschhauser F.; Domingo-Domenech E.; et al. Brentuximab Vedotin with Chemotherapy for CD30-Positive Peripheral T-Cell Lymphoma (ECHELON-2): A Global, Double-Blind, Randomised, Phase 3 Trial. Lancet 2019, 393 (10168), 229–240. 10.1016/S0140-6736(18)32984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblett K. J.; Senter P. D.; Chace D. F.; Sun M. M. C.; Lenox J.; Cerveny C. G.; Kissler K. M.; Bernhardt S. X.; Kopcha A. K.; Zabinski R. F.; et al. Effects of Drug Loading on the Antitumor Activity of a Monoclonal Antibody Drug Conjugate. Clin. Cancer Res. 2004, 10 (20), 7063–7070. 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]
- Khoshnejad M.; Greineder C. F.; Pulsipher K. W.; Villa C. H.; Altun B.; Pan D. C.; Tsourkas A.; Dmochowski I. J.; Muzykantov V. R. Ferritin Nanocages with Biologically Orthogonal Conjugation for Vascular Targeting and Imaging. Bioconjugate Chem. 2018, 29 (4), 1209–1218. 10.1021/acs.bioconjchem.8b00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M.; Fan K.; Zhou M.; Duan D.; Zheng J.; Yang D.; Feng J.; Yan X. H-Ferritin-Nanocaged Doxorubicin Nanoparticles Specifically Target and Kill Tumors with a Single-Dose Injection. Proc. Natl. Acad. Sci. U. S. A 2014, 111 (41), 14900–14905. 10.1073/pnas.1407808111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira J. C.; Perrine M.; Pericle F.; Cavallo F.. Virus-Like Particles as an Immunogenic Platform for Cancer Vaccines Viruses 2020, 12 ( (5), ) 10.3390/v12050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonen L.; van Hest J. C. M. Functionalization of Protein-Based Nanocages for Drug Delivery Applications. Nanoscale 2014, 6 (13), 7124–7141. 10.1039/C4NR00915K. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Banerjee M. A Smart Viral Vector for Targeted Delivery of Hydrophobic Drugs. Sci. Rep 2021, 11 (1), 7030. 10.1038/s41598-021-86198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwardson T. G. W.; Tetter S.; Hilvert D. Two-Tier Supramolecular Encapsulation of Small Molecules in a Protein Cage. Nat. Commun. 2020, 11 (1), 5410. 10.1038/s41467-020-19112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp N. P.; Gelderblom H.; Guchelaar H.-J. Clinical Pharmacokinetics of Tyrosine Kinase Inhibitors. Cancer Treat. Rev. 2009, 35 (8), 692–706. 10.1016/j.ctrv.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Thakur A.; Huang M.; Lum L. G. Bispecific Antibody Based Therapeutics: Strengths and Challenges. Blood Rev. 2018, 32 (4), 339–347. 10.1016/j.blre.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Lemke J.; von Karstedt S.; Zinngrebe J.; Walczak H. Getting TRAIL Back on Track for Cancer Therapy. Cell Death Differ. 2014, 21 (9), 1350–1364. 10.1038/cdd.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J. O.; Kim S.; Choi E.; Shin K.; Cha K.; So I.-S.; Kim S.-J.; Jun E.; Kim D.; Ahn H. J.; et al. Designed Nanocage Displaying Ligand-Specific Peptide Bunches for High Affinity and Biological Activity. ACS Nano 2013, 7 (9), 7462–7471. 10.1021/nn403184u. [DOI] [PubMed] [Google Scholar]
- Rujas E.; Kucharska I.; Tan Y. Z.; Benlekbir S.; Cui H.; Zhao T.; Wasney G. A.; Budylowski P.; Guvenc F.; Newton J. C.; et al. Multivalency Transforms SARS-CoV-2 Antibodies into Ultrapotent Neutralizers. Nat. Commun. 2021, 12 (1), 3661. 10.1038/s41467-021-23825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. T.; Fallas J. A; Saini S.; Ueda G.; Somasundaram L.; Zhou Z.; Xavier Raj I.; Xu C.; Carter L.; Wrenn S.; et al. F-Domain Valency Determines Outcome of Signaling through the Angiopoietin Pathway. EMBO Rep 2021, 22 (12), e53471. 10.15252/embr.202153471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarczyk S. J.; Sitaraman K.; Young H. A.; Hughes S. H.; Chatterjee D. K. Protein Delivery Using Engineered Virus-like Particles. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (41), 16998–17003. 10.1073/pnas.1101874108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y.; Terasaka N.; Sakai K.; Mihara E.; Wakabayashi R.; Matsumoto K.; Hilvert D.; Takagi J.; Suga H. De Novo Peptide Grafting to a Self-Assembling Nanocapsule Yields a Hepatocyte Growth Factor Receptor Agonist. iScience 2021, 24 (11), 103302. 10.1016/j.isci.2021.103302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. C.; Riddell S. R. Designing Chimeric Antigen Receptors to Effectively and Safely Target Tumors. Curr. Opin. Immunol 2015, 33, 9–15. 10.1016/j.coi.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyquem J.; Mansilla-Soto J.; Giavridis T.; van der Stegen S. J. C.; Hamieh M.; Cunanan K. M.; Odak A.; Gönen M.; Sadelain M. Targeting a CAR to the TRAC Locus with CRISPR/Cas9 Enhances Tumour Rejection. Nature 2017, 543 (7643), 113–117. 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwardson T. G. W.; Mori T.; Hilvert D. Rational Engineering of a Designed Protein Cage for siRNA Delivery. J. Am. Chem. Soc. 2018, 140 (33), 10439–10442. 10.1021/jacs.8b06442. [DOI] [PubMed] [Google Scholar]
- Levasseur M. D.; Mantri S.; Hayashi T.; Reichenbach M.; Hehn S.; Waeckerle-Men Y.; Johansen P.; Hilvert D. Cell-Specific Delivery Using an Engineered Protein Nanocage. ACS Chem. Biol. 2021, 16 (5), 838–843. 10.1021/acschembio.1c00007. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Yumul R. C.; Pun S. H. Virus-Inspired Polymer for Efficient In Vitro and In Vivo Gene Delivery. Angew. Chem., Int. Ed. Engl. 2016, 55 (39), 12013–12017. 10.1002/anie.201605958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.-Y.; Mao C.-Q.; Du X.-J.; Du J.-Z.; Wang F.; Wang J. Surface Charge Switchable Nanoparticles Based on Zwitterionic Polymer for Enhanced Drug Delivery to Tumor. Adv. Mater. 2012, 24 (40), 5476–5480. 10.1002/adma.201202296. [DOI] [PubMed] [Google Scholar]
- Banskota S.; Raguram A.; Suh S.; Du S. W.; Davis J. R.; Choi E. H.; Wang X.; Nielsen S. C.; Newby G. A.; Randolph P. B.. et al. Engineered Virus-like Particles for Efficient in Vivo Delivery of Therapeutic Proteins. Cell 2022 10.1016/j.cell.2021.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man F.; Lammers T.; de Rosales R. T. M. Imaging Nanomedicine-Based Drug Delivery: A Review of Clinical Studies. Mol. Imaging Biol. 2018, 20 (5), 683–695. 10.1007/s11307-018-1255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz D.; Vidal X.; Sunna A.; Care A. Bioengineering a Light-Responsive Encapsulin Nanoreactor: A Potential Tool for In Vitro Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13 (7), 7977–7986. 10.1021/acsami.0c21141. [DOI] [PubMed] [Google Scholar]
- Putri R. M.; Fredy J. W.; Cornelissen J. J. L. M.; Koay M. S. T.; Katsonis N. Labelling Bacterial Nanocages with Photo-Switchable Fluorophores. ChemPhysChem 2016, 17 (12), 1815–1818. 10.1002/cphc.201600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi A.; Ho G.; Kunth M.; Ling B.; Lakshmanan A.; Lu G.; Bourdeau R. W.; Schröder L.; Shapiro M. G. Recombinantly Expressed Gas Vesicles as Nanoscale Contrast Agents for Ultrasound and Hyperpolarized MRI. AIChE J. 2018, 64 (8), 2927–2933. 10.1002/aic.16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett K. M.; Shapiro E. M.; Sotak C. H.; Koretsky A. P. Controlled Aggregation of Ferritin to Modulate MRI Relaxivity. Biophys. J. 2008, 95 (1), 342–351. 10.1529/biophysj.107.116145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku T. S.; Lim S. Protein Nanoparticles in Molecular, Cellular, and Tissue Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2021, 13 (5), e1714. 10.1002/wnan.1714. [DOI] [PubMed] [Google Scholar]
- Geninatti Crich S.; Bussolati B.; Tei L.; Grange C.; Esposito G.; Lanzardo S.; Camussi G.; Aime S. Magnetic Resonance Visualization of Tumor Angiogenesis by Targeting Neural Cell Adhesion Molecules with the Highly Sensitive Gadolinium-Loaded Apoferritin Probe. Cancer Res. 2006, 66 (18), 9196–9201. 10.1158/0008-5472.CAN-06-1728. [DOI] [PubMed] [Google Scholar]
- Elzoghby A. O.; Hemasa A. L.; Freag M. S. Hybrid Protein-Inorganic Nanoparticles: From Tumor-Targeted Drug Delivery to Cancer Imaging. J. Controlled Release 2016, 243, 303–322. 10.1016/j.jconrel.2016.10.023. [DOI] [PubMed] [Google Scholar]
- Hwang M. P.; Lee J.-W.; Lee K. E.; Lee K. H. Think Modular: A Simple Apoferritin-Based Platform for the Multifaceted Detection of Pancreatic Cancer. ACS Nano 2013, 7 (9), 8167–8174. 10.1021/nn403465a. [DOI] [PubMed] [Google Scholar]
- Mingozzi F.; High K. A. Immune Responses to AAV Vectors: Overcoming Barriers to Successful Gene Therapy. Blood 2013, 122 (1), 23–36. 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino A. T.; Markusic D. M. Immune Response Mechanisms against AAV Vectors in Animal Models. Mol. Ther Methods Clin Dev 2020, 17, 198–208. 10.1016/j.omtm.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P. L.; Harada T.; Christian D. A.; Pantano D. A.; Tsai R. K.; Discher D. E. Minimal “Self” Peptides That Inhibit Phagocytic Clearance and Enhance Delivery of Nanoparticles. Science 2013, 339 (6122), 971–975. 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen B.; Tolia N. H. Protein-Based Antigen Presentation Platforms for Nanoparticle Vaccines. NPJ. Vaccines 2021, 6 (1), 70. 10.1038/s41541-021-00330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens M. B. RTS,S/AS01 Vaccine (MosquirixTM): An Overview. Hum. Vaccin. Immunother 2020, 16 (3), 480–489. 10.1080/21645515.2019.1669415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkscape Project, 2020. Inkscape, Available at: https://inkscape.org.