Abstract
Background:
Angiotensin receptor-neprilysin inhibitor (ARNI) prescription in the United States remains suboptimal despite strong evidence for efficacy and value in heart failure with reduced ejection fraction. Factors responsible for under prescription are not completely understood. Economic limitations may play a disproportionate role in reduced access for some patients.
Methods:
This is an analysis of the Get with the Guidelines-Heart Failure registry, supplemented with data from the Distressed Community Index. Data were fit to a mixed-effects regression model to investigate clinical and socioeconomic factors associated with ARNI prescription at hospital discharge. Missing data were handled by multilevel multiple imputation.
Results:
Of the 136,144 patients included in analysis, 12.6% were prescribed an ARNI at discharge. The dominant determinants of ARNI prescription were ARNI use while inpatient (OR 72, 95%CI 58–89, p<0.001) and taking an ARNI prior to hospitalization (OR 9, 95%-CI 7–13, p<0.001). Having an ACEi/ARB/ARNI contraindication was associated with lower likelihood of ARNI prescription at discharge (OR 0.11, 95%-CI 0.10–0.12, p<0.001). Socioeconomic factors associated with lower likelihood of ARNI prescription included having no insurance (OR 0.60, 95%-CI 0.50–0.72, p<0.001) and living in a zip code identified as “distressed” (OR 0.81, 95%-CI 0.70–0.93, p=0.010). The rate of ARNI prescription is increasing with time (OR 2, 95%-CI 1.8–2.3, p<0.001 for patients discharged in 2020 as opposed to 2017), but the disparity in prescription rates between distressed and prosperous communities appears to be increasing.
Conclusions:
Multiple medical and socioeconomic factors contribute to low rates of ARNI prescription at hospital discharge. Potential targets for improving ARNI prescription rates include initiating ARNIs during hospitalization and aggressively addressing patients’ access barriers with the support of inpatient social services and pharmacists.
Background:
Heart failure (HF) affects over 6 million people in the United States and was associated with 43.6 billion United States dollars (USD) in health care costs in 2020.1,2 Approximately one half of patients with HF have reduced ejection fraction (HFrEF), defined as a left ventricular ejection fraction < 40%.
In large randomized clinical trials of patients with HFrEF, angiotensin receptor-neprilysin inhibitors (ARNIs) demonstrated a 20% reduction in cardiovascular death and hospitalizations compared to 10mg bid of enalapril, an active angiotensin converting enzyme inhibitor (ACEi) comparator.3 Modeling suggests that more than 30,000 deaths per year might be prevented by switching patients from an ACEi or angiotensin receptor blocker (ARB) to an ARNI.4 Accordingly, HF guidelines now recommend ARNI as first line therapy for HFrEF.5
Despite strong evidence, prescription rates for ARNIs have been low, 4–26% in studies performed in the last 5 years.6–8 Concerns of cost are likely tied to under-prescription, as despite multiple studies demonstrating ARNIs to be cost effective, the therapy is not covered by all insurance vendors, and out-of-pocket costs to patients can exceed 680USD per month without insurance.9,10 Contraindications for ARNIs are similar to ACEis and ARBs, and studies have showed similar rates of side effects and complications, so it is unlikely the low prescription rates are due primarily to medical reasons. Studies examining the association between hospital type and rates of ARNI prescription have found no relationship.11 Understanding the factors driving low rates of ARNI prescription is essential to developing strategies to increase use of this effective medication.
A growing body of evidence suggests that lower levels of economic well-being are associated with decreased prescription of effective medical therapy for HF.12 We hypothesized that with the substantial difference in up-front cost of ARNIs compared with ARB (just under 1400USD annual cost difference), lower economic well-being would be associated with decreased ARNI prescription.13 In this study we investigated the impact of clinical factors and community well-being on ARNI prescription at discharge from a HF hospitalization in the GWTG-HF registry, leveraging socioeconomic data from the Economic Innovation Group’s distressed community index.
Methods:
Study Design and Population
This is a retrospective study of adult patients enrolled in the Get with the Guidelines-Heart Failure (GWTG-HF) registry from 2017–2020. The GWTG-HF program prospectively enrolls patients hospitalized for new or worsening HF at participating hospitals and has been extensively described.14 These data are available from the American Heart Association upon reasonable request (www.heart.org/qualityresearch). Patients were included in this analysis if they had an ejection fraction < 40% at time of hospitalization and were alive at discharge from the hospital. Patients were excluded if they were discharged to hospice care and/or had a history of left ventricular assist device. Additionally, patients were excluded if they were missing data regarding prescription of an ARNI at discharge. Patients were not excluded from the study based on the presence of an ARNI contraindication as some patients with a contraindication received an ARNI at discharge despite this contraindication; instead we included the presence of a contraindication as an independent covariate in analysis. In patients with multiple hospitalizations, only the first hospitalization in the dataset was included in analysis.
The primary outcome measure was prescription of an ARNI at discharge from hospitalization. The primary independent variables of interest were those related to patient demographic and socioeconomic status. Socioeconomic status data included insurance status and the distressed community index (DCI).
DCI data are compiled and maintained by the Economic Innovation Group (website: eig.org/dci) and are available at from them for a small fee. The DCI dataset contains zip code-level socioeconomic data derived from the United States Census Bureau. The principal measure of interest from this dataset is the distress score, which ranks communities across 7 components (no high school diploma, housing vacancy rate, adults not working, poverty rate, median income ratio, change in employment, and change in establishments) to generate a score from 0–100, with 0 being the most prosperous and 100 being the most distressed. Based on this score, patients are split into quintiles based on their home zip code: prosperous, comfortable, mid-tier, at-risk, and distressed. The DCI dataset also designates zip codes as urban, suburban, small town, and rural. Data from the DCI dataset were merged with the GWTG-HF dataset on patient zip code. Previous studies have demonstrated that increasing DCI is associated with lower health care quality and worse health outcomes.15,16
Medical comorbidities, inpatient medications, discharge vitals, discharge serum laboratory measurements, contraindications to guideline-directed medical therapy (GDMT), and discharge prescriptions were included as covariates in the analysis.
Statistical Analyses
Baseline characteristics were summarized using median (interquartile range, IQR) when continuous and number (percent) when categorical. There was extensive data missingness, ranging from 0–70% missingness per variable. After pattern analysis, data were deemed to be “missing at random”, as opposed to “missing completely at random” and “missing not at random”. Patterns of missingness occurred primarily at the hospital level, with most hospitals systematically missing the same variables across all their patients. Additionally, data were found to be hierarchical in nature (patients within hospitals) based on unconditional mean modeling showing a large intraclass correlation coefficient. To take this into account, missing data were imputed via multilevel multiple imputation by fully conditional specification using the R package MICE (version 3.13.15), which has been extensively described.17–19 This particular analysis was complicated by a hierarchical data structure, so in addition to using traditional generalized linear modeling techniques in the imputation step, we leveraged random forest, a machine-learning decision tree analysis method.20,21
To investigate the impact of socioeconomic, demographic, and clinical determinants of ARNI prescription, we built an explanatory mixed effects logistic regression model following the 3-step procedure outlined by Sommet and Morselli.22 To facilitate interpretation of the model, continuous variables were grand mean centered. We proceeded to build a multilevel logistic regression model using the lme4 package (version 1.1.21).23
A complete case analysis (an analysis using records only with complete data so that no imputation is performed) was subsequently performed as a sensitivity analysis for the imputation analysis.
To investigate changes in practice over time, a post hoc analysis was performed to explore the major determinants of ARNI prescription based on the year of patients’ discharge. The data were stratified into two groups “early” versus “late” based on patients’ discharge year, with “early” being defined as patients discharged in 2017 or 2018 and “late” being defined as discharged in 2019 or 2020. Multilevel logistic regression models with identical independent variables were fit to these two patient populations. These models were compared to identify major changes in effect sizes of determinants of ARNI prescription.
Data handling, descriptive statistics, and modelling were performed in R version 3.6.0. Our R code and an annotated R Markdown file are available on GitHub at <https://jeffreyshowtran.github.io/files/tech_appendix.html>
Computational Details
IQVIA (Parsippany, New Jersey) served as the data collection and coordination center for the GWTG-HF registry. Each participating hospital received either human research approval to enroll cases without individual patient consent under the common rule, or a waiver of authorization and exemption from subsequent review by their institutional review board. Analyses were completed using the American Heart Association Precision Medicine Platform (https://precision.heart.org) on an r5.12xlarge AWS EC2 computing instance.
Results:
Population Characteristics
Of the 593,053 entries in the GWTG-HF registry, 206,145 met inclusion criteria. After removing duplicate hospitalizations and patients who met exclusion criteria, 136,144 patients remained in the dataset (Figure 1). 110,923 patients were missing one or more elements of data.
Figure 1.
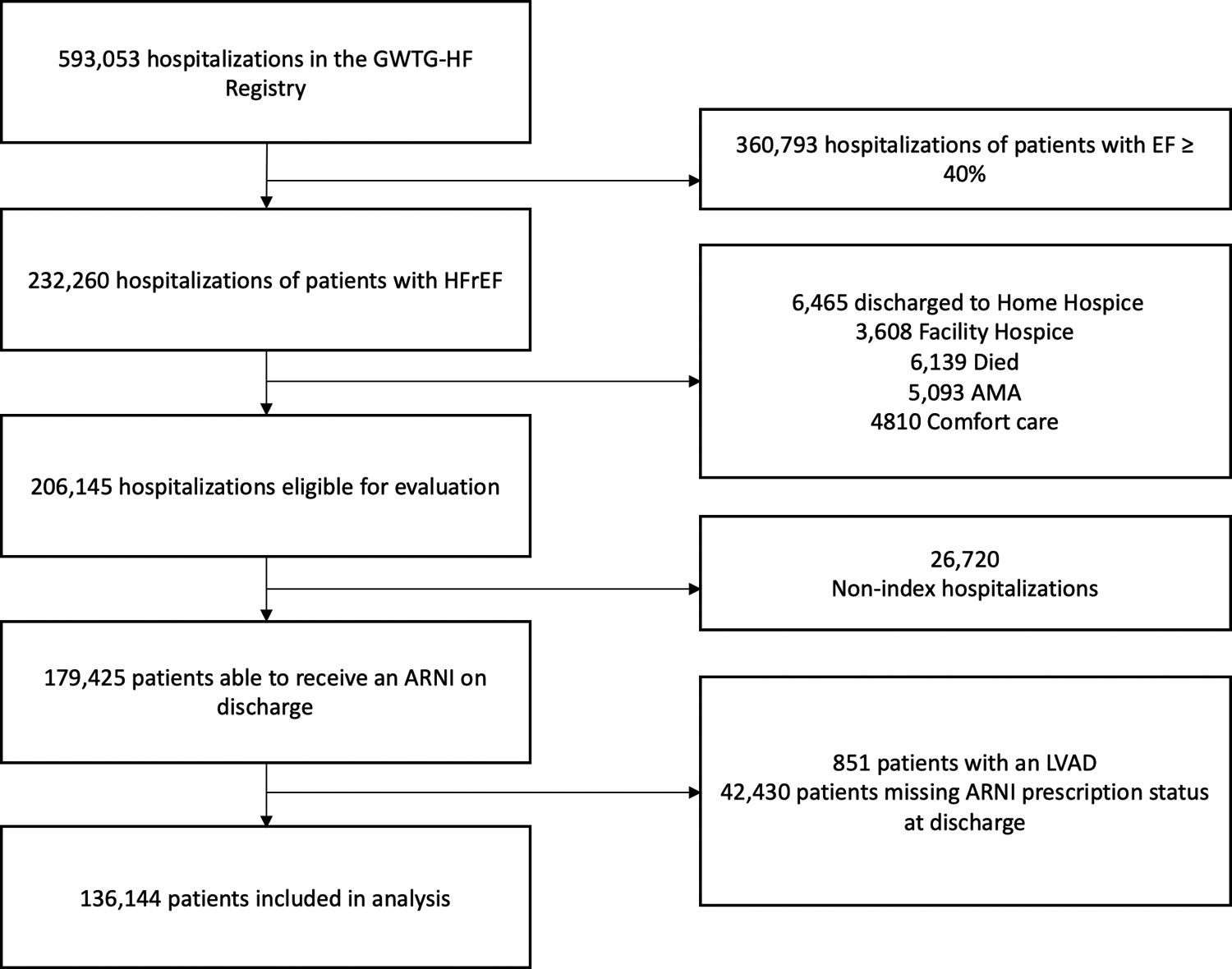
Consort Diagram of Analysis Population
Consort diagram showing sample extraction from the Get with the Guidelines-Heart Failure (GWTG-HF) registry. AMA: against medical advice; ARNI: angiotensin blocker-neprilysin inhibitor; EF: ejection fraction; HFrEF: heart failure with reduced ejection fraction; LVAD: left ventricular assist device.
The baseline characteristics and data missingness of the analyzed population are presented in Table 1. Frequencies reported for categorical data do not include missing data in the denominator. The median age of the study population was 68 (IQR 57–78) years. The majority of patients were male (66%) and Caucasian (57.7%). The median ejection fraction was 25% (IQR 20–33). Of patients with known insurance status, 6.4% were uninsured. There were more patients living in “distressed” communities versus “prosperous” communities based on the distressed community index (25.0 vs 16.3% of patients, respectively). 6.3% of patients were taking an ARNI prior to hospitalization. 10.9% of patients received an ARNI while hospitalized.
Table 1.
Descriptive Statistics
| ARNI (no) | ARNI (yes) | Total | ||
|---|---|---|---|---|
| N | 118,929 (87.4%) | 17,215 (12.6%) | 136,144 | |
| Demographics | ||||
| Age* | 68 (57,79) | 66 (56,76) | 68 (57,78) | |
| Missing | 0 | |||
| Sex | ||||
| Male | 78,546 (87.2%) | 11,498 (12.8%) | 90,044 (66.3%) | |
| Female | 40,105 (87.6%) | 5,673 (12.4%) | 45,778 (33.7%) | |
| Missing | 322 (0.2%) | |||
| Race | ||||
| Asian | 2,607 (90.8%) | 265 (9.2%) | 2,872 (2.1%) | |
| Black | 33,369 (86.2%) | 5,333 (13.8%) | 38,702 (28.4%) | |
| Hispanic | 10,154 (89.4%) | 1,209 (10.6%) | 11,363 (8.3%) | |
| Caucasian | 68,596 (87.4%) | 9,911 (12.6%) | 78,507 (57.7%) | |
| Other | 4,203 (89.4%) | 497 (10.6%) | 4,700 (3.5%) | |
| Missing | 0 | |||
| Socioeconomic Factors | ARNI (no) | ARNI (yes) | Total | |
| Distress Score | 57.1 (30.2, 80.0) | 57.4 (31.0, 79.6) | 57.1 (30.2, 79.9) | |
| Missing | 59,969 (44.0%) | |||
| Patient Distress Score Quintile | ||||
| Prosperous | 10,900 (87.8%) | 1,515 (12.2%) | 12,415 (16.3%) | |
| Comfortable | 11,659 (88.4%) | 1,536 (11.6%) | 13,195 (17.3%) | |
| Mid-Tier | 12,922 (87.3%) | 1,883 (12.7%) | 14,805 (19.4%) | |
| At-Risk | 14,617 (87.4%) | 2,115 (12.6%) | 16,732 (22.0%) | |
| Distressed | 16,728 (87.9%) | 2,300 (12.1%) | 19,028 (25.0%) | |
| Missing | 59,969 (44.0%) | |||
| ZIP Designation | ||||
| Urban | 22,258 (89.2%) | 2,683 (10.8%) | 24,941 (32.7%) | |
| Suburban | 23,415 (87.4%) | 3,379 (12.6%) | 26,794 (35.2%) | |
| Small Town | 11,503 (87.3%) | 1,669 (12.7%) | 13,172 (17.3%) | |
| Rural | 9,650 (85.6%) | 1,618 (14.4%) | 11,268 (14.8%) | |
| Missing | 59,969 (44.0%) | |||
| Insurance | ||||
| Medicaid | 22,149 (88.4%) | 2,913 (11.6%) | 25,062 (19.7%) | |
| Medicare | 49,574 (87.4%) | 7,130 (12.6%) | 56,704 (44.6%) | |
| Other | 31,866 (85.6%) | 5,376 (14.4%) | 37,242 (29.3%) | |
| None | 7,434 (92.1%) | 642 (7.9%) | 8,076 (6.4%) | |
| Missing | 9060 (6.7%) | |||
| Clinical Data | ARNI (no) | ARNI (yes) | Total | |
| Ejection Fraction† | 25 (20, 33) | 23 (19, 30) | 25 (20, 33) | |
| Missing | 0 | |||
| Serum Potassium at Discharge‡ | 4.0 (3.7, 4.3) | |||
| ≤5.0 | 34,743 (89.1%) | 4,231 (10.9%) | 38,974 (97.3%) | |
| >5.0 | 1,019 (93.1%) | 75 (6.9%) | 1,094 (2.7%) | |
| Missing | 96,076 (70.6%) | |||
| Serum Creatinine at Discharge§ | 1.3 (1.0, 1.8) | 1.2 (0.95, 1.5) | 1.3 (1.0, 1.7) | |
| Missing | 94,145 (69.2%) | |||
| Systolic Blood Pressure at Discharge | 117 (105, 131) | 113 (102, 127) | 116 (105, 131) | |
| ≥ 90 | 67,374 (87.9%) | 9,306 (12.1%) | 76,680 (98.0%) | |
| < 90 | 1,334 (83.5%) | 263 (16.5%) | 1,597 (2.0%) | |
| Missing | 57,867 (42.5%) | |||
| Heart Rate at Discharge | 78 (69, 88) | 77 (69, 88) | 78 (69, 88) | |
| ≥60 | 63,779 (87.6%) | 9041 (12.4%) | 72,820 (94.4%) | |
| <60 | 3740 (87.8%) | 520 (12.2%) | 4,260 (5.5%) | |
| Missing | 59,064 (43.4%) | |||
| Listed for Heart Transplant | ||||
| Yes | 118 (86.1%) | 19 (14.9%) | 137 (0.1%) | |
| No | 118,811 (87.4%) | 17,196 (12.6%) | 136,007 (99.9%) | |
| Missing | 0 | |||
| Taking an ARNI prior to hospitalization | ||||
| Yes | 632 (22.4%) | 2,189 (77.6%) | 2,821 (6.3%) | |
| No | 39,024 (93.5%) | 2,711 (6.5%) | 41,735 (93.7%) | |
| Missing | 91,588 (67.3%) | |||
| Has a contraindication to ACEi/ARB/ARNI | ||||
| Yes | 54,102 (99.2%) | 424 (0.8%) | 54,526 (45.1%) | |
| No | 53,858 (81.0%) | 12,638 (19.0%) | 66,496 (54.9%) | |
| Missing | 15,122 (11.1%) | |||
| Inpatient Medications | ARNI (no) | ARNI (yes) | Total | |
| Received ACEi or ARB as inpatient | ||||
| Yes | 42,615 (93.8%) | 2,821 (6.2%) | 45,436 (56.4%) | |
| No | 27,876 (79.5%) | 7,185 (20.5%) | 35,061 (43.6%) | |
| Missing | 55,647 (40.9%) | |||
| Received ARNI as inpatient | ||||
| Yes | 1,158 (13.2%) | 7,587 (86.7%) | 8,747 (10.9%) | |
| No | 69,333 (96.6%) | 2,417 (3.4%) | 71,750 (89.1%) | |
| Missing | 55,647 (40.9%) | |||
| Received any Inotrope Infusion as inpatient | ||||
| Yes | 4,498 (88.0%) | 611 (12.0%) | 5,109 (3.8%) | |
| No | 114,431 | 16,604 | 131,035 (96.2%) | |
| Missing | (87.3%) | (12.7%) | 0 | |
| Medical Comorbidities | ARNI (no) | ARNI (yes) | Total | |
| No Prior Medical History | ||||
| Yes | 3,098 (90.1%) | 342 (9.9%) | 3,440 (2.7%) | |
| No | 108,436 (87.3%) | 15,764 (12.7%) | 124,200 (97.3%) | |
| Missing | 8,504 (6.2%) | |||
| History of Congestive Heart Failure | ||||
| Yes | 82,700 (86.6%) | 12,820 (13.4%) | 95,520 (74.8%) | |
| No | 28,834 (89.8%) | 3,286 (10.2%) | 32,120 (25.2%) | |
| Missing | 8,504 (6.2%) | |||
| History of Chronic Kidney Disease | ||||
| Yes | 26,627 (91.1%) | 2,600 (8.9%) | 29,227 (22.9% | |
| No | 84,907 (86.3%) | 13,506 (13.7%) | 98,413 (77.1%) | |
| Missing | 8,504 (6.2%) | |||
| History of End-stage Renal Disease | ||||
| Yes | 4,495 (95.6%) | 209 (4.4%) | 4,704 (3.7%) | |
| No | 107,039 (87.1%) | 15,897 (12.9%) | 122,936 (96.3%) | |
| Missing | 8,504 (6.2%) | |||
| Discharge Medications/Planning | ARNI (no) | ARNI (yes) | Total | |
| Discharged with GDMT beta-blocker | ||||
| Yes | 98,215 (86.4%) | 15,469 (13.6%) | 113,684 (85.4%) | |
| No | 17,938 (92.3%) | 1,498 (7.7%) | 19,436 (14.6%) | |
| Missing | 3,024 (2.2%) | |||
| Discharged with ACEi or ARB | ||||
| Yes | 71,440 (96.8%) | 2,325 (3.2%) | 73,765 (54.4%) | |
| No | 47,294 (76.4%) | 14,572 (23.6%) | 61,866 (45.6%) | |
| Missing | 513 (0.4%) | |||
| Discharged with MRA | ||||
| Yes | 36,851 (82.5%) | 7,835 (17.5%) | 44,686 (34.7%) | |
| No | 75,930 (90.2%) | 8,262 (9.8%) | 84,192 (65.3%) | |
| Missing | 7,266 (5.3%) | |||
| Follow-up Visit Scheduled Prior to Discharge | ||||
| Yes | 106,280 (87.3%) | 15,450 (12.7%) | 121,730 (94.7%) | |
| No | 5,830 (86.2%) | 934 (13.8%) | 6,764 (5.3%) | |
| Missing | 7,650 (5.6%) | |||
| Discharged to Continued Care# | ||||
| Yes | 32,595 (90.1%) | 3,532 (9.8%) | 36,127 (57.7%) | |
| No | 22,945 (86.8%) | 3,504 (13.2%) | 26,449 (42.3%) | |
| Missing | 73,568 (54.0%) | |||
| Year of Discharge | ||||
| 2017 | 37,822 (91.9%) | 3,340 (8.1%) | 41,162 (30.2%) | |
| 2018 | 35,762 (88.6%) | 4,596 (11.4%) | 40,358 (29.6%) | |
| 2019 | 34,055 (83.6%) | 6,660 (16.4%) | 40,715 (29.9%) | |
| 2020 | 11,290 (81.2%) | 2,619 (18.8%) | 13,909 (10.2%) | |
| Hospital Characteristics | ARNI (no) | ARNI (yes) | Total | |
| Heart Transplant Center | ||||
| Yes | 14,073 (90.0%) | 1,564 (10.0%) | 15,637 (11.5%) | |
| No | 104,856 (87.0%) | 15,651 (13.0%) | 120,507 (88.5%) | |
| Missing | 0 | |||
| Academic Center|| | ||||
| Yes | 58,191 (87.8%) | 8,095 (12.2%) | 66,286 (48.7%) | |
| No | 60,738 (86.9%) | 9,120 (13.1%) | 69,858 (51.3%) | |
| Missing | 0 |
Descriptive data are presented for the total population and stratified over whether a patient was discharged with an ARNI or not. Data are presented as median (IQR) when continuous and number (percent) when categorical. Note that percentages are percent of the known data; missing data has been excluded from this calculation.
Age is given in years.
Ejection fraction is presented in percent.
Serum potassium measurements are presented in mEq/L.
A history of chronic kidney disease is defined by the GWTG data dictionary as a serum creatinine >2mg/dL.
Academic centers were defined by the presence of resident post-graduate physicians.
For the variable “Discharged to Continued Care”, continued care is defined as home health care, skilled nursing facility, inpatient rehabilitation, intermediate care facility, long term acute care facility, or another acute care facility.
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin-receptor blocker; ARNI: angiotensin receptor-neprilysin inhibitor; GDMT: guideline-directed medical therapy; MRA: mineralocorticoid receptor angtagonist.
17,215 (12.6%) patients were prescribed an ARNI at the time of discharge. ARNI prescription rates increased yearly over the period of study, with 8.1% of patients prescribed an ARNI on discharge in 2017, 11.4% in 2018, 16.4% of patients in 2019, and 18.8% in the first half of 2020 (Table 2). In the population of 66,496 patients without documentation of an ARNI contraindication, the average rate of ARNI prescription over the study period was 19.0% (Table 3).
Table 2.
ARNI prescription rate by year in all patients
| Year | Prescribed an ARNI | Total Patients included in the study |
|---|---|---|
| 2017 | 3,340 (8.1%) | 41,162 |
| 2018 | 4,596 (11.4%) | 40,358 |
| 2019 | 6,660 (16.4%) | 40,715 |
| 2020 | 2,619 (18.8%) | 13,909 |
| Total | 17,215 (12.6%) | 136,144 |
Data were available only for the first six months of 2020. ARNI: angiotensin receptor-neprilysin inhibitor.
Table 3.
ARNI prescription rate by year in patients with no documented ARNI contraindication
| Year | Prescribed an ARNI | Patients with no documented ARNI contraindication |
|---|---|---|
| 2017 | 2,407 (11.4%) | 21,056 |
| 2018 | 3,303 (16.5%) | 20,043 |
| 2019 | 4,948 (26.2%) | 18,859 |
| 2020 | 1,980 (30.3%) | 6,538 |
| Total | 12,638 (19.0%) | 66,496 |
Angiotensin receptor-neprilysin inhibitor (ARNI) contraindications are only reported to the Get with the Guidelines-Heart Failure registry when they are present. As a result, no distinction can be made between a patient having no ARNI contraindications versus patients for whom a contraindication was not reported. 424 patients with an explicit contraindication to an ARNI received one anyways.
Nearly half (45.1%) of patients were documented as having a contraindication to ACEi, ARB, or ARNI (Tables 4, 5, and 6, respectively), the most frequently cited of which was “other medical reason” for ACEi and ARBs and “ACEi use within the last 36hrs” for ARNIs. The second most common contraindication to ARNI use was “patient reason”, which was not specified further. Of the 57,274 patients with an explicit ARNI contraindication, 424 were prescribed an ARNI at discharge. 47,294 patients did not receive an ACEI, ARB, or ARNI at discharge. Of these, 5,215 (11.0%) had no documented ACEi/ARB/ARNI contraindication. The ACEi/ARB/ARNI status of 195 patients is uncertain as they were not prescribed an ARNI, and discharge ACEi/ARB prescription status was also missing.
Table 4.
Reasons given for having a contraindication to ACEi
| Contraindication | No | Yes | ||
|---|---|---|---|---|
| Hypotension | 43,884 | 89.3% | 5,251 | 10.7% |
| Azotemia | 39,604 | 80.6% | 9,531 | 19.4% |
| Other medical reason | 17,742 | 36.1% | 31,393 | 63.9% |
| Patient reason | 44,089 | 89.7% | 5,046 | 10.3% |
| System reason | 48,639 | 99.0% | 496 | 1.0% |
Responses were available for 49,135 out of 136,144 patients (missing rate 63.9%). Percentages are calculated as a fraction of known responses. ACEi: Angiotensin-converting enzyme inhibitor.
Table 5.
Reasons given for having a contraindication to ARB
| Contraindication | No | Yes | ||
|---|---|---|---|---|
| Hypotension | 43,129 | 89.1% | 5,263 | 10.9% |
| Azotemia | 38,674 | 79.9% | 9,718 | 20.1% |
| Other medical reason | 17,506 | 36.2% | 30,886 | 63.8% |
| Patient reason | 44,190 | 91.3% | 4,202 | 8.7% |
| System reason | 47,734 | 98.6% | 658 | 1.4% |
Responses were available for 48,392 out of 136,144 patients. (missing rate 64.5%). Percentages are calculated as a fraction of known responses. ARB: angiotensin-receptor blocker.
Table 6.
Reasons given for having a contraindication to ARNI
| Contraindication | Count |
|---|---|
| ACEi use within the last 36hrs | 21,847 |
| Allergy | 997 |
| Hyperkalemia | 1,323 |
| Hypotension | 6,823 |
| Prohibitive renal dysfunction | 10,792 |
| Other medical reason | 2,571 |
| Patient reason | 12,139 |
| System reason | 782 |
| Total | 57,274 |
ARNI (angiotensin receptor-neprilysin inhibitor) contraindication data were provided only when a contraindication was present with no distinction between missing data and the absence of a contraindication.
Patients were treated at 560 unique sites, 11.5% of which were heart transplant centers and 48.7% academic centers based on the presence of resident physicians working at the site.
Predictors of ARNI Prescription at Hospital Discharge
Results of the mixed effects logistic regression model are shown in Table 7 and key findings are illustrated in figure 2. The intercept indicates that the baseline odds of being prescribed an ARNI were 0.10 (95%-CI 0.08–0.13, p<0.001) in patients with the baseline reference characteristics. For a comprehensive set of baseline characteristics, see the “comparison groups” in Table 7. Odds ratios presented in Table 7 are relative to this baseline profile.
Table 7.
Predictors of ARNI Prescription at Hospital Discharge
| Odds Ratio | 95% Confidence Intervals | Lambda | p-value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age* | 0.988 | 0.985 | 0.991 | 0.256 | <0.001 |
| Sex (Comparison Group: Male) | |||||
| Female | 0.975 | 0.910 | 1.045 | 0.194 | 0.474 |
| Race (Comparison Group: Caucasian) | |||||
| Asian | 1.285 | 1.011 | 1.632 | 0.163 | 0.041 |
| Black | 1.115 | 1.013 | 1.227 | 0.277 | 0.026 |
| Hispanic | 1.264 | 1.096 | 1.460 | 0.235 | 0.001 |
| Other | 0.942 | 0.778 | 1.140 | 0.136 | 0.536 |
| Distress Score Quintile (Comparison Group: Prosperous) | |||||
| Comfortable | 0.962 | 0.844 | 1.069 | 0.472 | 0.559 |
| Mid-Tier | 0.920 | 0.813 | 1.042 | 0.417 | 0.192 |
| At-Risk | 0.880 | 0.769 | 1.007 | 0.404 | 0.064 |
| Distressed | 0.813 | 0.695 | 0.950 | 0.468 | 0.010 |
| Zip Designation (Comparison Group: Urban) | |||||
| Rural | 1.111 | 0.962 | 1.284 | 0441 | 0.153 |
| Small town | 1.054 | 0.915 | 1.284 | 0.368 | 0.469 |
| Suburban | 1.051 | 0.944 | 1.170 | 0.428 | 0.365 |
| Insurance (Comparison Group: Other Non-Medicare/Medicaid Insurance) | |||||
| Medicaid | 0.824 | 0.744 | 0.913 | 0.212 | <0.001 |
| Medicare | 0.968 | 0.890 | 1.051 | 0.198 | 0.437 |
| None | 0.597 | 0.496 | 0.717 | 0.354 | <0.001 |
| Ejection fraction† | 0.937 | 0.933 | 0.941 | 0.190 | <0.001 |
| Discharge Serum Creatinine* | 0.747 | 0.692 | 0.807 | 0.723 | <0.001 |
| Discharge Serum Potassium (Comparison Group: K≤5) | |||||
| K>5 | 0.887 | 0.679 | 1.158 | 0.562 | 0.379 |
| Discharge Systolic Blood Pressure (Comparison Group: sBP≥90) | |||||
| sBP<90 | 0.815 | 0.631 | 1.052 | 0.340 | 0.117 |
| Discharge Heart Rate (Comparison Group: HR≥60) | |||||
| HR<60 | 1.091 | 0.927 | 1.282 | 0.403 | 0.295 |
| No Prior Medical History (Comparison Group: No) | |||||
| Yes | 0.870 | 0.699 | 1.083 | 0.150 | 0.213 |
| History of Congestive Heart Failure (Comparison Group: No) | |||||
| Yes | 0.947 | 0.865 | 1.036 | 0.335 | 0.235 |
| History of Chronic Kidney Disease‡ (Comparison Group: No) | |||||
| Yes | 0.809 | 0.714 | 0.915 | 0.480 | 0.001 |
| History of End Stage Renal Disease (Comparison Group: No) | |||||
| Yes | 1.418 | 1.038 | 1.937 | 0.420 | 0.029 |
| Listed for Heart Transplant (Comparison Group: No) | |||||
| Yes | 0.485 | 0.160 | 1.473 | 0.056 | 0.202 |
| Taking ARNI Prior to Hospitalization (Comparison Group: No) | |||||
| Yes | 9.488 | 6.752 | 13.333 | 0.881 | <0.001 |
| Contraindication to ACEi/ARB/ARNI (Comparison Group: No) | |||||
| Yes | 0.113 | 0.104 | 0.124 | 0.347 | <0.001 |
| Received inpatient ARNI (Comparison Group: No) | |||||
| Yes | 72.091 | 58.301 | 89.142 | 0.846 | <0.001 |
| Received any inpatient inotrope (Comparison Group: No) | |||||
| Yes | 0.527 | 0.436 | 0.637 | 0.093 | <0.001 |
| Discharged with GDMT BB (Comparison Group: No) | |||||
| Yes | 1.818 | 1.634 | 2.022 | 0.237 | <0.001 |
| Discharged with ACEi or ARB (Comparison Group: No) | |||||
| Yes | 0.098 | 0.088 | 0.109 | 0.551 | <0.001 |
| Discharged with MRA (Comparison Group: No) | |||||
| Yes | 1.773 | 1.636 | 1.922 | 0.386 | <0.001 |
| Scheduled Follow Up on Discharge (Comparison Group: No) | |||||
| Yes | 1.172 | 1.001 | 1.373 | 0.261 | 0.050 |
| Discharged to Continued Care|| (Comparison Group: No) | |||||
| Yes | 0.771 | 0.703 | 0.846 | 0.516 | <0.001 |
| Year of Discharge (Comparison Group: Discharged in 2017) | |||||
| 2018 | 1.378 | 1.261 | 1.505 | 0.131 | <0.001 |
| 2019 | 1.831 | 1.678 | 1.999 | 0.138 | <0.001 |
| 2020 | 2.055 | 1.835 | 2.301 | 0.140 | <0.001 |
| Heart Transplant Center (Comparison Group: No) | |||||
| Yes | 0.555 | 0.347 | 0.887 | 0.023 | 0.014 |
| Academic Center§ (Comparison Group: No) | |||||
| Yes | 1.178 | 0.939 | 1.478 | 0.068 | 0.158 |
| Intercept | 0.100 | 0.075 | 0.134 | 0.298 | <0.001 |
Results obtained via mixed-effect logistic regression model with a random intercept assigned to hospital site. Missing data were handled by multilevel multiple imputation. Coefficients and 95% confidence intervals are presented as odds ratios. Unless otherwise indicated by an asterisk, odds ratios are relative to the defined comparison group. Variables denoted by a single asterisk (*) are continuous variables and indicate a change in the odds ratio per 1-unit change in the continuous variable, with the exception of (†) ejection fraction, where the estimate indicates the change in odds ratio per 5% change in ejection fraction. Lambda indicates the proportion of variation due to missing data.
All continuous variables have been grand mean centered, so the intercept can be interpreted as the odds of being prescribed an ARNI with all categorical variables assuming their baseline comparison group value and all continuous variables at their mean value. (‡) A history of chronic kidney disease is defined by the GWTG data dictionary as a serum creatinine >2mg/dL. (§) Academic centers were defined by the presence of resident post-graduate physicians. (||) For the variable “Discharged to Continued Care”, continued care is defined as home health care, skilled nursing facility, inpatient rehabilitation, intermediate care facility, long term acute care facility, or another acute care facility.
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin-receptor blocker; ARNI: angiotensin receptor-neprilysin inhibitor; BB: beta blocker; GDMT: guideline-directed medical therapy; HR: heart rate; K: potassium, MRA: mineralocorticoid receptor angtagonist; sBP: systolic blood pressure.
Figure 2.
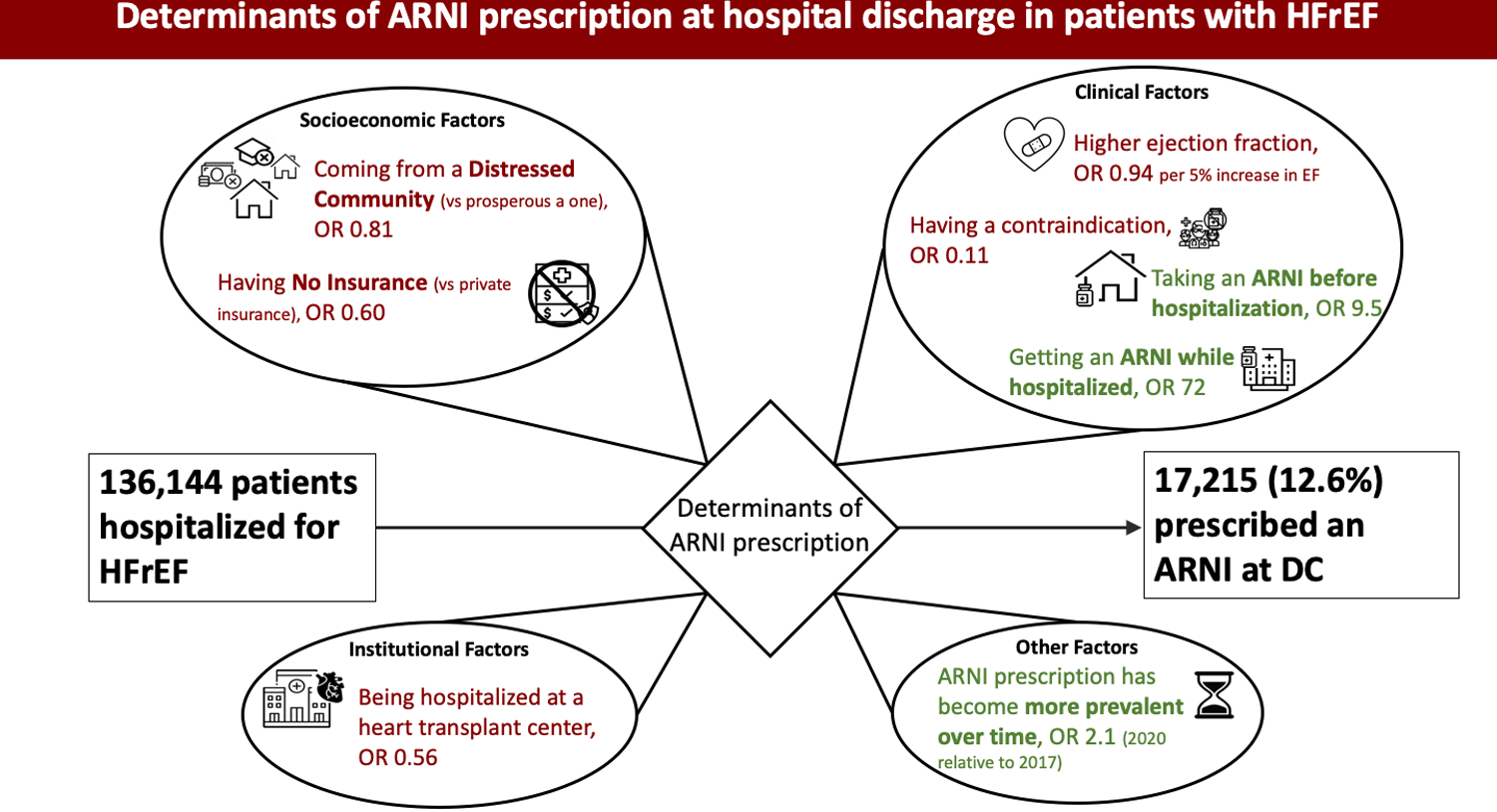
Illustration of Key Findings
Illustrated findings of the analysis exploring socioeconomic, clinical, and institutional factors that influence the prescription of angiotensin receptor-neprilysin inhibitors (ARNI) at discharge after hospitalization for heart failure with reduced ejection fraction (HFrEF). The driving determinants are primarily clinical factors, but socioeconomic factors and practice trends over time also play a role. OR: odds ratio.
The strongest predictors of ARNI prescription at discharge were inpatient ARNI use (OR 72, 95%-CI 58–89, p<0.001) and taking an ARNI prior to hospitalization (OR 9.5, 95%-CI 6.8–13, p<0.001). The strongest predictors against ARNI prescription at discharge were documented contraindication to an ACEi, ARB, or ARNI (OR 0.11, 95%-CI 0.10–0.12, p<0.001) and being prescribed an ACEi or ARB at discharge (OR 0.10, 95%-CI 0.09–0.11, p<0.001).
The likelihood of ARNI prescription increased steadily from 2017 to 2020, with odds ratios of 1.4 (95%-CI 1.3–1.5, p<0.001), 1.8 (95%-CI 1.7–2.0, p<0.001) and 2.1 (95%-CI 1.8–2.3, p<0.001) for patients discharged in 2018, 2019, and 2020 respectively, relative to patients discharged in 2017. Post hoc analysis demonstrated no significant changes in the major determinants of ARNI prescription by year of patient’s discharge.
Examination of demographic and socioeconomic factors associated with ARNI prescription showed an inverse association with age (OR 0.99 per 1-year increase in age, 95%-CI 0.99–0.99, p<0.001). Identifying as Asian, Black, or Hispanic (as opposed to identifying as Caucasian) was associated with an increased likelihood of ARNI prescription (Asian: OR 1.3, 95%-CI 1.0–1.6; Black: OR 1.1, 95%-CI 1.0–1.2; Hispanic OR 1.3, 95%-CI 1.1–1.5; p<0.05 for all three groups). Having no insurance or Medicaid insurance was associated with a significantly lower likelihood of ARNI prescription relative to having non-Medicare/Medicaid insurance (OR 0.60, 95%-CI 0.50–0.72 and OR 0.82, 95%-CI 0.74–0.91, respectively; p<0.001 for both groups). Living in a zip code identified as “distressed” based on the distressed community index was associated with a lower likelihood of ARNI prescription compared to living in a prosperous community (OR 0.81, 95%-CI 0.70–0.95, p=0.010).
Several clinical factors were associated with ARNI prescription. Increasing ejection fraction was associated with a decreasing likelihood of ARNI prescription (OR 0.937 per 5% increase in EF, 95%-CI 0.93–0.94 p<0.001). Higher serum creatinine was also associated with decreasing likelihood of ARNI prescription (OR 0.75 per 1mg/dL creatinine, 95%-CI 0.69–0.81s, p<0.001). Receiving an inotrope infusion while hospitalized was associated with a lower likelihood of ARNI prescription (OR 0.53, 95%-CI 0.44–0.64, p<0.001). A history of chronic kidney disease was associated with lower likelihood of ARNI prescription (OR 0.81, 95%-CI 0.71–0.92, p=0.001), but having end stage renal disease was associated with higher likelihood of ARNI prescription (OR 1.4, 95%-CI 1.0–1.9, p=0.029). Being discharged to continued care, defined as discharge with home health care, or to a skilled nursing facility, inpatient rehabilitation, intermediate care facility, long term acute care facility, or another acute care facility, was associated with lower likelihood of ARNI prescription (OR 0.77, 95%-CI 0.70–0.85, p<0.001). Having scheduled follow up on discharge was associated with an increased likelihood of ARNI prescription (OR 1.2, 95%-CI 1.0–1.4, p=0.050). Serum potassium > 5, systolic blood pressure < 90mmHg, and being listed for heart transplant were not significantly associated with the likelihood of ARNI prescription.
In terms of site characteristics, being hospitalized at a heart transplant center was associated with lower likelihood of ARNI prescription (OR 0.56, 95% CI 0.35–0.89, p=0.014). Being hospitalized at an academic facility was not significantly associated with likelihood of ARNI prescription (OR 1.2, 95% CI 0.94–1.5, p=0.16). The adjusted intraclass correlation coefficient for the model was 0.254, indicating 25% of the total variance observed in the model could be explained by unobserved differences between sites (e.g. physician practice styles, knowledge of the employed physician groups, aggressiveness of prescribers, etc.).
25,221 records (18.5%) were available for complete case analysis. Comparison of the complete case analysis model with the full model demonstrated highly similar effect estimates between the two models.
Predictors of ARNI Prescription Stratified by Year of Discharge
Results of the stratified analysis are demonstrated in Supplemental Table 1. In general, effect estimates remained consistent between the two stratified populations with a few notable exceptions; namely, the baseline odds of being prescribed an ARNI was higher in the late vs early population (OR 0.25, 95% CI 0.17–0.36 versus OR 0.08, 95% CI 0.05–0.12 for late vs early, respectively). Additionally, living in a zip code identified as “distressed” based on the distressed community index was associated with a significantly lower odds of being prescribed an ARNI in the late population but not in the early population (OR 0.76, 95% CI 0.62–0.93, p=0.009 versus OR 0.86, 95% CI 0.70–1.1, p-0.157 for late versus early, respectively).
Discussion:
In this large analysis of over 130,000 hospitalizations for heart failure in the GWTG-HF registry from 2017–2020, we found a low rate of ARNI prescription at hospital discharge (12.6%). In 66,496 patients in whom no ARNI contraindication was explicitly documented, the rate of ARNI prescription was similarly low at 19.0%. There was a statistically significant, steady increase in the rate of ARNI prescription from 2017 to 2020, from 8.1% in 2017 to 18.8% in the first 6 months of 2020 for the entire cohort. This increase in ARNI use may be due to increasing coverage by insurance agencies, broader awareness of ARNI’s beneficial effects among providers over time, or greater comfort with the medication as its use becomes more widespread and better understood. Overall, the major determinants of ARNI prescription did not change from 2017 to 2020, based on the results of our stratified analysis.
Our explanatory model suggests that the dominant clinical determinants of ARNI prescription at discharge are receipt of an ARNI while inpatient, taking an ARNI prior to hospitalization, and having no contraindications to an ACEi, ARB, or ARNI. The first two findings are consistent with previous studies of the GWTG-HF population, which have associated inpatient initiation or continuation of guideline-directed medical therapy with persistent use following hospitalization, as well as subsequent reductions in rehospitalization and mortality.24–27 These findings reinforce the importance of initiating or continuing optimum guideline-directed medical therapy, including ARNIs, during hospitalizations. In-hospital initiation of any medication allows safe initiation in a highly-monitored setting and should always be considered when a patient’s therapeutic regimen deviates from optimal guideline recommendations.
Patients with a contraindication to ACEi/ARB/ARNI constituted a large (45.1%) proportion of the population. The GWTG-HF registry contains extensive and unique data on contraindications to therapy, including reasons specific to each medication (see table 3 for more information). Previous studies of GWTG-HF data have largely excluded patients with therapy contraindications. We opted not to exclude these patients for three reasons: First, there were a small handful of patients (424) prescribed an ARNI at discharge despite documentation of a contraindication. Second, we felt that some contraindications may be modifiable rather than absolute, and hence represent a strategic target to improve prescription rates. Finally, contraindications to ARNI use included “patient reasons” and “system reasons”, neither of which are specified further, and these reasons constituted over 12,000 ARNI contraindications. We were concerned that patient and system reasons might include socioeconomic factors that were not otherwise captured other than perhaps in our insurance and distressed community index variables. However, it was ultimately not possible to test the impact of individual contraindications due to the inability to distinguish between patients who had no ARNI contraindications from those for whom the data was merely missing. To shed further light on this issue, further detail on contraindications may be helpful in future datasets, including explicit documentation of the absence of a contraindication. Nevertheless, medical contraindications such as hyperkalemia, azotemia, and prohibitive renal dysfunction made up the vast majority of contraindications. ARNI prescription rates may be improved by further research into the extent of renal dysfunction and electrolyte abnormalities associated with adverse events on ARNI initiation, hence truly constituting a contraindication, rather than subjective cutoffs.
It is of interest that hypotension and hyperkalemia on discharge assessment were not significantly associated with decreased rates of ARNI prescription at discharge, but these effects must be interpreted in the presence of the ACEI/ARB/ARNI contraindication covariate. For example, the effect of hypotension was 0.815 with a p-value of 0.117; however, this effect size is interpreted in the absence of a contraindication to ACEI/ARB/ARNI, which includes clinically significant hypotension. So, the population to whom the 0.815 odds ratio applies is the population with a discharge blood pressure less than 90mmHg, but without clinically significant hypotension as assessed by the treating medical team. It should also be noted that discharge laboratory and vital signs values had the greatest degree of missingness, and this high degree of missing data increases the standard error (uncertainty) around the estimates.
Our analysis adds new information about the impact of socioeconomic factors on prescription of ARNIs at discharge. Having no insurance or Medicaid insurance as opposed to having private insurance, and living in a distressed community as opposed to living in a prosperous community were independently associated with lower likelihood of ARNI use at time of discharge. These findings support the growing body of evidence that socioeconomic strain impacts medical decision-making during hospitalization and through time of discharge, contributing to perpetuation of health disparities. Notably, the disparity in prescription rates between distressed and prosperous communities appear to be increasing over time rather than diminishing, based on the stratification analysis. This reinforces the urgent need to ensure patients from distressed communities receive optimum guideline-based care while hospitalized and on discharge. The best way to implement such change has not been demonstrated, but we suggest early and systematic use of hospital ancillary and support staff to address financial and insurance issues that may impact prescription of more expensive but more effective medications such as ARNIs. Encouragingly, no significant disparities were identified in ARNI prescription rates amongst ethnic minority groups, with Asians, Blacks, and Hispanics actually being more likely to receive an ARNI at discharge.
Our analysis suggests that being a transplant center is associated with lower likelihood of ARNI prescription independent of ARNI contraindications and discharge labs and vitals. This hospital characteristic was not explored in previous studies investigating associations between prescription practices and hospital characteristics.11 The lower likelihood of ARNI prescription at transplant centers may be due to the higher proportion of New York Heart Association class IV patients at these centers or higher expectations of outpatient follow up. In any case, strategies advocating ARNI prescription should be implemented hospital-wide regardless of center expertise.
Finally, a great deal of attention was spent on proper accounting for missing data. The most common methods of handling missing data include complete case analysis (also known as listwise deletion), mean imputation, and assigning missing data its own category, but use of any of these methods causes statistical models to become biased, which is to say that effect estimates can no longer be legitimately extrapolated to represent true effect size in the general population.19,28–30 In our multiple imputation analysis, we trained the computer on the potential distribution of missing values based on similar cases in the data. These distributions were then used to generate effect estimates. These estimates are considered unbiased (i.e., can be extrapolated to the general population), with uncertainty about missing values reflected in confidence intervals. Multiple imputation offers a powerful and statistically valid method of handling missing data, and this paper and its technical appendix demonstrate how multiple imputation can leverage machine learning and traditional generalized linear modelling to use available data as fully as possible, even in a hierarchical data structure. Although each multiple imputation procedure is unique to the scientific question being asked, the R markdown file we have made publicly available on GitHub lays out a step-by-step approach that we hope can offer some guidance to investigators pursuing studies with the GWTG-HF data and other large datasets.
Study Limitations:
The primary limitation of our study is the degree of missing data. Analyses leveraging multiple imputation provide estimates that ultimately converge on true effect sizes regardless of the volume of missingness, but this is not without a trade-off: uncertainty about the true value of the missing data is added into the calculation of standard errors. As a result, confidence intervals are widened and there is increased uncertainty about the true value of the effect size in the general population.
An additional study limitation includes lack of patient-level socioeconomic data in the GWTG registry. The DCI provides socioeconomic information at the zip code level only. Within that zip code, individuals will have variation in their true degree of economic well-being, which is not captured by the data available in the registry.
Finally, the GWTG-HF registry provides a wealth of information regarding contraindications to therapy and is a potentially valuable source in understanding why patients do not receive optimal therapy; however, the absence of an ARNI contraindication is not recorded within the GWTG-HF data so there is no way to determine whether a patient truly has no ARNI contraindications or if that patient’s contraindications were not reported. Having an ARNI contraindication was ultimately found to be a powerful negative predictor of ARNI prescription, but we were unable to determine which contraindications (see table 6) were the primary drivers of this effect.
Additionally, the reason patients were assigned contraindications to ARNIs could not be comprehensively investigated, as only admission and discharge laboratory and vitals assessments were provided. For example, patients listed as having a contraindication to an ARNI due to renal dysfunction or hypotension might not have significantly aberrant values at time of discharge, but may have had concerning values during hospitalization. These inpatient data were unavailable, so we were not able to clearly understand why some patients were designated as having contraindications to ARNIs. In addition, the most commonly cited contraindication in the data was “other medical reason” about which no additional data are available.
Conclusion:
The overall rate of ARNI prescription in the GWTG-HF registry from 2017–2020 was low at 12.6%, with inpatient administration of an ARNI and taking an ARNI prior to hospitalization being strong positive predictors of ARNI prescription at discharge. While the overall rate of ARNI prescription increased over the study duration, the disparity in prescription rates between patients from distressed versus prosperous communities became more pronounced over time. Even in this population of patients hospitalized for heart failure who are a high risk for morbidity and health care costs, socioeconomic status appears to drive disparities in medical care. This reinforces the urgent need to ensure patients from distressed communities receive optimum guideline-based care while hospitalized and on discharge.
Supplementary Material
Supplemental Table 1. Predictors of ARNI Prescription at Hospital Discharge Stratified by Early vs Late
Clinical Perspective:
What’s new?
Receiving an ARNI while inpatient and taking an ARNI prior to hospitalization are powerful predictors of receiving an ARNI at discharge; while this pattern has been described in the prescription practices of other guideline-directed medical therapy for heart failure, this is the largest study to show this pattern with ARNIs.
In addition, this study demonstrates that having no medical insurance and living in the lowest quintile of economic prosperity, as measured by the distressed community index, are associated with a decreased likelihood of ARNI prescription at hospital discharge. These disparities appear to be increasing with time.
What are the clinical implications?
Initiating an ARNI during hospitalization may improve prescription rates. Additionally, ARNI therapy should be initiated in the outpatient setting whenever possible, as this is associated with inpatient use and continuation at discharge.
Increasing inpatient administration of ARNI may be particularly effective for patients living in distressed communities and those without insurance. The availability of inpatient resources, such as social services and pharmacists, may make this a more effective strategy than outpatient prescription in these populations.
Acknowledgements
This project was developed through the Heart Failure Data Challenge, using the Get With The Guidelines® (GWTG) Heart Failure Registry data to target research related to heart failure and social/structural determinants of health. The data challenge was hosted by the American Heart Association (AHA) and the Association of Black Cardiologists (ABC). The American Heart Association Precision Medicine Platform (https://precision.heart.org/) was used for data analysis.
Sources of Funding:
Research reported in this publication was supported by a National Institute of Health T32 training grant (grant number: T32HL007249). The GWTG-HF program is provided by the American Heart Association. GWTG-HF is sponsored, in part, by Novartis, Boehringer Ingelheim, Boehringer Ingelheim and Eli Lilly Diabetes Alliance, Novo Nordisk, Sanofi, AstraZeneca, Bayer, Tylenol and Alnylam Pharmaceuticals.
Non-standard Abbreviations and Acronyms
- ACEi
angiotensin converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- ARNI
angiotensin receptor-neprilysin inhibitor
- CI
confidence interval
- DCI
distressed community index
- GDMT
guideline-directed medical therapy
- GWTG-HF
Get with the Guidelines-Heart Failure
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- IQR
interquartile range
- OR
odds ratio
- USD
United States dollars
Footnotes
Disclosures
None.
Works Cited:
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 4.Fonarow GC, Hernandez AF, Solomon SD, Yancy CW. Potential Mortality Reduction With Optimal Implementation of Angiotensin Receptor Neprilysin Inhibitor Therapy in Heart Failure. JAMA Cardiol. 2016;1:714–717. doi: 10.1001/jamacardio.2016.1724 [DOI] [PubMed] [Google Scholar]
- 5.Writing Committee M, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos G, Fonarow GC, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2016;134:e282–293. doi: 10.1161/CIR.0000000000000435 [DOI] [PubMed] [Google Scholar]
- 6.Luo N, Fonarow GC, Lippmann SJ, Mi X, Heidenreich PA, Yancy CW, Greiner MA, Hammill BG, Hardy NC, Turner SJ, et al. Early Adoption of Sacubitril/Valsartan for Patients With Heart Failure With Reduced Ejection Fraction: Insights From Get With the Guidelines-Heart Failure (GWTG-HF). JACC Heart Fail. 2017;5:305–309. doi: 10.1016/j.jchf.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 7.Mohanty AF, Levitan EB, King JB, Dodson JA, Vardeny O, Cook J, Herrick JS, He T, Patterson OV, Alba PR, et al. Sacubitril/Valsartan Initiation Among Veterans Who Are Renin-Angiotensin-Aldosterone System Inhibitor Naive With Heart Failure and Reduced Ejection Fraction. J Am Heart Assoc. 2021;10:e020474. doi: 10.1161/JAHA.120.020474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozaki AF, Krumholz HM, Mody FV, Tran TT, Le QT, Yokota M, Jackevicius CA. Prior Authorization, Copayments, and Utilization of Sacubitril/Valsartan in Medicare and Commercial Plans in Patients With Heart Failure With Reduced Ejection Fraction. Circ Cardiovasc Qual Outcomes. 2021;14:e007665. doi: 10.1161/CIRCOUTCOMES.120.007665 [DOI] [PubMed] [Google Scholar]
- 9.Sandhu AT, Ollendorf DA, Chapman RH, Pearson SD, Heidenreich PA. Cost-Effectiveness of Sacubitril-Valsartan in Patients With Heart Failure With Reduced Ejection Fraction. Ann Intern Med. 2016;165:681–689. doi: 10.7326/M16-0057 [DOI] [PubMed] [Google Scholar]
- 10.King JB, Shah RU, Bress AP, Nelson RE, Bellows BK. Cost-Effectiveness of Sacubitril-Valsartan Combination Therapy Compared With Enalapril for the Treatment of Heart Failure With Reduced Ejection Fraction. JACC Heart Fail. 2016;4:392–402. doi: 10.1016/j.jchf.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 11.Luo N, Lippmann SJ, Mentz RJ, Greiner MA, Hammill BG, Hardy NC, Laskey WK, Heidenreich PA, Chang CL, Hernandez AF, et al. Relationship Between Hospital Characteristics and Early Adoption of Angiotensin-Receptor/Neprilysin Inhibitor Among Eligible Patients Hospitalized for Heart Failure. J Am Heart Assoc. 2019;8:e010484. doi: 10.1161/JAHA.118.010484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung CL, Chao TF, Su CH, Liao JN, Sung KT, Yeh HI, Chiang CE. Income level and outcomes in patients with heart failure with universal health coverage. Heart. 2021;107:208–216. doi: 10.1136/heartjnl-2020-316793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeJong C, Kazi DS, Dudley RA, Chen R, Tseng CW. Assessment of National Coverage and Out-of-Pocket Costs for Sacubitril/Valsartan Under Medicare Part D. JAMA Cardiol. 2019;4:828–830. doi: 10.1001/jamacardio.2019.2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smaha LA, American Heart A. The American Heart Association Get With The Guidelines program. Am Heart J. 2004;148:S46–48. doi: 10.1016/j.ahj.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 15.Weeks WB, Ouayogode MHL, Weinstein JN. Association Between a Measure of Community Economic Distress and Medicare Patients’ Health Care Utilization, Quality, Outcomes, and Costs. J Gen Intern Med. 2018;33:1433–1435. doi: 10.1007/s11606-018-4478-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charles EJ, Mehaffey JH, Hawkins RB, Fonner CE, Yarboro LT, Quader MA, Kiser AC, Rich JB, Speir AM, Kron IL, et al. Socioeconomic Distressed Communities Index Predicts Risk-Adjusted Mortality After Cardiac Surgery. Ann Thorac Surg. 2019;107:1706–1712. doi: 10.1016/j.athoracsur.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sv Buuren. MICE: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45:1–67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 18.Sv Buuren. Flexible imputation of missing data. Second edition. ed. Boca Raton: CRC Press, Taylor & Francis Group; 2018. [Google Scholar]
- 19.Heymans MW, Eekhout I. Applied Missing Data Analysis with SPSS and (R)Studio. In: Amsterdam; 2019. [Google Scholar]
- 20.Doove LL VB S, & Dusseldorp E. Recursive partitioning for missing data imputation in the presence of interaction effects. Computational Statistics & Data Analysis. 2014;72:92–104. doi: 10.1016/j.csda.2013.10.025 [DOI] [Google Scholar]
- 21.Jaeger BC, Cantor R, Sthanam V, Xie R, Kirklin JK, Rudraraju R. Improving Outcome Predictions for Patients Receiving Mechanical Circulatory Support by Optimizing Imputation of Missing Values. Circ Cardiovasc Qual Outcomes. 2021;14:e007071. doi: 10.1161/CIRCOUTCOMES.120.007071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommet N, & Morselli D. Keep Calm and Learn Multilevel Logistic Modeling: A Simplified Three-Step Procedure Using Stata, R, Mplus, and SPSS. International Review of Social Psychology. 2017;30:203–218. doi: 10.5334/irsp.90 [DOI] [Google Scholar]
- 23.Bates D, Machler M, Bolker B, Walker S Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67:1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 24.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB, et al. Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE-HF program. J Am Coll Cardiol. 2008;52:190–199. doi: 10.1016/j.jacc.2008.03.048 [DOI] [PubMed] [Google Scholar]
- 25.Gilstrap LG, Fonarow GC, Desai AS, Liang L, Matsouaka R, DeVore AD, Smith EE, Heidenreich P, Hernandez AF, Yancy CW, et al. Initiation, Continuation, or Withdrawal of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers and Outcomes in Patients Hospitalized With Heart Failure With Reduced Ejection Fraction. J Am Heart Assoc. 2017;6:1–9. doi: 10.1161/JAHA.116.004675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez AF, Mi X, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Qualls LG, Peterson ED, Fonarow GC, Curtis LH. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA. 2012;308:2097–2107. doi: 10.1001/jama.2012.14795 [DOI] [PubMed] [Google Scholar]
- 27.Bhagat AA, Greene SJ, Vaduganathan M, Fonarow GC, Butler J. Initiation, Continuation, Switching, and Withdrawal of Heart Failure Medical Therapies During Hospitalization. JACC Heart Fail. 2019;7:1–12. doi: 10.1016/j.jchf.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roderick JAL, Donald BR. Statistical Analysis with Missing Data. 3rd ed. Wiley; 2019. [Google Scholar]
- 29.Enders CK. Multiple imputation as a flexible tool for missing data handling in clinical research. Behav Res Ther. 2017;98:4–18. doi: 10.1016/j.brat.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 30.Vach W, Blettner M. Biased estimation of the odds ratio in case-control studies due to the use of ad hoc methods of correcting for missing values for confounding variables. Am J Epidemiol. 1991;134:895–907. doi: 10.1093/oxfordjournals.aje.a116164 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Predictors of ARNI Prescription at Hospital Discharge Stratified by Early vs Late


