Abstract
Background:
Human immunodeficiency virus (HIV) induces several metabolic derangements that contribute to cardiovascular disease (CVD), but it is unclear if HIV increases diabetes or hypertension risk. Refining longitudinal relationships between HIV-specific factors and CVD risk factors across different care settings may help inform CVD prevention among people with HIV (PWH).
Methods:
We tested the hypothesis that long-term higher cumulative viral load (viremia-copy-year) is associated with higher risk of diabetes mellitus (DM) and hypertension (HTN) by analyzing electronic records of PWH from two distinct health systems in Chicago (Northwestern Medicine and Howard Brown Health Care) receiving care in 2004-2019. We used joint longitudinal-survival models to assess multivariable-adjusted associations. Subgroup analysis per site were also conducted.
Results:
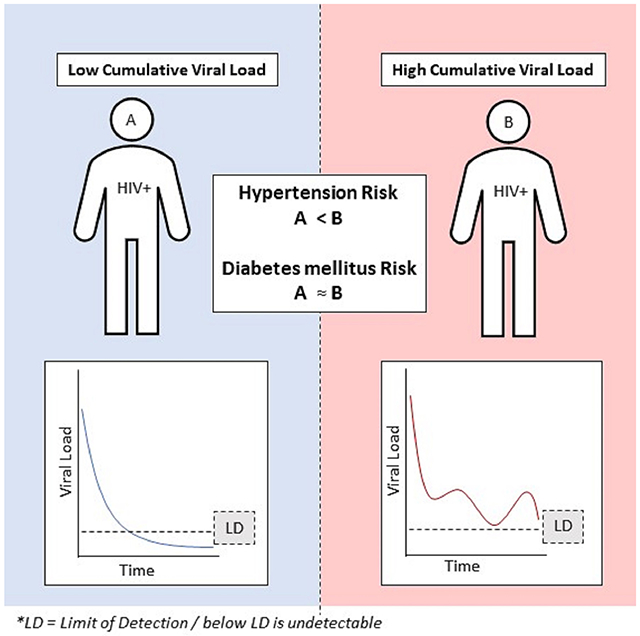
We observed 230 (3.0%) incident DM cases in 7,628 PWH without baseline DM and 496 (6.7%) HTN cases in 7,450 PWH without baseline HTN. Pooled analysis showed a direct association of viremia-copy-year with incident HTN (HR:1.20, 95%CI:1.14-1.26) but not with DM (HR:1.03, 95%CI:0.96-1.10). However, site-specific differences existed whereby the Northwestern-only analysis demonstrated a significant association of viremia-copy-year with HTN (HR:1.29, 95%CI:1.08-1.32). Additionally, higher social deprivation index (both sites) and diagnosis of mental health disorder (Howard Brown Health only) was associated with higher DM and HTN risk.
Conclusions:
Cumulative viral load may be associated with incident HTN among PWH. Associations of HIV control with CVD risk factors among PWH may differ by health care system context.
Keywords: epidemiology, human immunodeficiency virus, hypertension, diabetes mellitus, joint modeling
Graphical Abstract
I. Background
Cardiovascular disease is a leading cause of death among people with human immunodeficiency virus (HIV) on anti-retroviral treatment (ART).[1] Randomized trials demonstrated that early ART initiation and sustained viral suppression (through continuous ART use) significantly reduce the risk of cardiovascular disease (CVD).[2,3] Subsequent observational studies have supported this, with higher HIV viremia and lower CD4 count associating consistently with CVD events among people with HIV (PWH).[4]
While the reduction in CVD events through viral suppression is clear, studies investigating associations of viral suppression with intermediate CVD risk factors such as hypertension (HTN) and diabetes mellitus (DM) have yielded less consistent results. This is despite several plausible pathogenetic mechanisms involving chronic inflammation and immune dysfunction that arises from HIV.[5-8] Follow-up data from the Starting Timing of Antiretroviral Treatment trial demonstrated that the group with lower cumulative viral load did not experience significantly different risks of HTN and DM, as rates were comparable between the two treatment arms (early ART initiation vs deferred).[3] Meanwhile, more recent observational studies have found that PWH have significantly higher prevalence of HTN[5,9,10] and DM[11,12] compared to the control populations without HIV. However, this finding has not been consistently observed, especially for DM. [13]
Prior studies used only one-time measures of viral load (VL) (usually baseline) as the main predictor. This measure fails to account for the dynamic changes in VL during treatment and inadequately captures cumulative viral exposure.[14,15] Using cumulative VL, operationalized as viremia-copy-year, as a predictor can overcome this limitation and is useful for studying incident outcomes in PWH.[16,17] One study that applied this approach found that higher viremia-copy-years was associated with higher HTN risk, although limited data were available regarding exact timing of HTN.[18] Finally, none of the prior studies have directly compared association in risk across different health systems.
In this study, we analyzed associations of cumulative VL with risk of HTN and DM among PWH seen at two distinct urban healthcare systems – one a large academic tertiary care center and the other a community healthcare organization focused on lesbian, gay, bisexual, transgender, and queer (LGBTQ+) health.
II. Methods
A. Study Design and Data Source
We utilized data collected during the course of clinical care from electronic health data repositories of two healthcare systems in Chicago: Northwestern Medicine (NM) (2004-2019) and Howard Brown Health (HBH) (2012-2019). NM is a large academic health system in the greater Chicago area. HBH is a federally qualified health center with nine locations in Chicago with a focus on LGBTQ+, low-income, and uninsured populations. Data pulls were done by data analysts employed by the respective organizations. This work is part of studies cleared by the Northwestern Medicine and Howard Brown Health institutional review boards. Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the two review boards.
B. Eligibility criteria
For this analysis, we included data from all adults (≥18 years) with HIV with documented positive HIV test results and who had at least three VL tests from their first recorded VL test up to 2019. Depending on the outcome assessed, those that had the cardiometabolic condition of interest at baseline were excluded from the analysis (e.g., only those without DM at baseline were included in the incident DM analysis).
C. Main Predictor
The main exposure of interest is cumulative VL operationally measured as (log-)viremia-copy-year. To calculate this, repeated measures of VL were obtained from electronic health records. If reported as below the limit of detection, the value was replaced to be 20 copies/mL. Log-transformation was done to improve normality of VL.
D. Primary Outcomes and Case definition
Our two primary outcomes were incident HTN and incident DM and were determined using published EHR phenotypes based on diagnosis codes, medications, or labs.[19] The earliest time the person met any of the criteria was used as the date of diagnosis. Incident cases were those that appeared at least 90 days after the first HIV VL. (see Supplemental Methods)
E. Covariates
Demographic and clinical variables at baseline were extracted from the EHR. Baseline variables common to both sites (base set) include age at first VL, gender (male, not male; male from HBH include transgender male), race/ethnicity, insurance status, body mass index, baseline CD4 count (square-root transformed to improve normality), and year for first VL. We also adjusted for HTN at baseline in the DM analysis and DM at baseline in the HTN analysis.
Dataset specific covariates were also available which could be used for site-specific subgroup analyses involving social determinants of health (SDOH). NM had data on smoking status (assumed no record as non-smoking) and complete addresses which allowed linkage to the 2015 Social Deprivation Index (SDI) at the 9-digit zip code level.[20] The HBH dataset included sexual orientation, gender modality,[21] poverty status (≤100%, 100-≤200%, >200% federal poverty line (FPL)), housing status (permanent housing vs not), diagnosis of mental health disorder (binary), and substance use disorder (binary). HBH data was also linked to the ACS data, but due to data availability, at lower neighborhood precision of 5-digit zip code levels.
F. Statistical Analysis
To investigate the association of viremia-copy-year with incident DM/HTN we used joint longitudinal-survival modelling[22,23] implemented using the JMBayes package[24] in R4.1/RStudio.[25,26]
Briefly, this approach uses a longitudinal model to estimate VL over time. The model then calculates (log-)viremia-copy-year which is used as a time-varying exposure in the survival model. Joint modeling is the appropriate approach for incorporating a time-varying endogenous variable, such as viremia-copy-year, in survival analysis. Extended cox models, the usual approach, inadequately account for endogeneity and produce erroneous findings.[27] Joint models also allow the number and timing of repeated longitudinal measurements to vary across individuals making it suitable for EHR data.
For the pooled analysis, the longitudinal component modeled log-transformed VL over time and adjusted for site, age, gender, and year of first VL and the survival component adjusted for cumulative VL and a base set of variables which included site, age, gender, race & ethnicity, insurance status, body mass index (BMI), DM/HTN at baseline, CD4 count, and year of first VL (See Supplemental Methods). We compared the results of this pooled analysis to a Cox proportional hazards model adjusting for baseline VL instead of viremia-copy-year.
Also, we conducted site-specific subgroup analysis that examine any association between the event and SDOH while taking advantage of unique variables available to only one of the datasets. We decided to adjust for all theoretically relevant individual-level variables in the survival component. Since there were multiple collinear neigbhorhood-level variables available from the SDI dataset, we ran three different models: using SDI, using community poverty, and using proxies of segregation (%Black and %Hispanic population).
To account for missing data in our analyses, we generated 20 imputed data sets using multiple imputation by chained equations via the mice package.[28]. (see Supplemental Methods and Table S1 for missingness details).
III. Results
A. Cohort description
We included 7,628 PWH in the DM analysis and 7,450 in the HTN analysis, with ~70% of the samples coming from HBH (Table 1). Most (71%) of the pooled sample were male, 47% were non-Hispanic White and the average age at first VL was around 36 (SD: 13). While 50% of individuals in both cohorts had their first VL measurement at <100 copies/mL, this increased to 87% had VL <100 copies/mL by their most recent measurement. In the DM sample, 3% had HTN at baseline. Meanwhile, in the HTN sample, 7% had DM at baseline.
Table 1.
Overview of the Analytical Sample
| A. Diabetes Mellitus sample | ||||
|---|---|---|---|---|
| Overall | HBH | NM | p-value | |
| n | (n=7628) | (n=5346) | (n=2282) | |
| Age (mean (SD)) | 36.07 (12.85) | 33 (12.11) | 43.25 (11.60) | <0.001 |
| Male (%) | 5393 (70.7) | 3460 (64.7) | 1933 (84.7) | <0.001 |
| Race & ethnicity (%) | <0.001 | |||
| Black, non-Hispanic | 1964 (25.7) | 1232 (23.0) | 732 (32.1) | |
| Hispanic | 1196 (15.7) | 979 (18.3) | 217 (9.5) | |
| White, non-Hispanic | 3574 (46.9) | 2516 (47.1) | 1058 (46.4) | |
| Other* | 894 (11.7) | 619 (11.6) | 275 (12.1) | |
| Insurance category (%) | ||||
| Government | 1378 (18.1) | 839 (15.7) | 539 (23.6) | |
| Private | 2669 (35.0) | 1715 (32.1) | 954 (41.8) | |
| Other/uninsured | 3581 (46.9) | 2792 (52.2) | 789 (34.6) | |
| HTN at baseline (%) | 230 (3.0) | 74 (1.4) | 156 (6.8) | <0.001 |
| Body mass index | 27.09 (6.32) | 27.44 (6.67) | 26.59 (5.75) | <0.001 |
| CD4 (cells/mL) [MD [IQR]) | 462 [283, 668] | 601 [409, 788] | 435 [257, 628] | <0.001 |
| Viral load at baseline (MD [IQR]) | 81 [20, 26427] | 41 [20, 29667] | 161.5 [36, 20249] | <0.001 |
| <100 copies/mL on first VL (%) | 3901 (51.1) | 2875 (53.8) | 1026 (45.0) | <0.001 |
| <100 copies/mL on last VL (%) | 6629 (86.9) | 4676 (87.5) | 1953 (85.6) | 0.028 |
| Incident DM (%) | 230 (3.0) | 74 (1.4) | 156 (6.8) | <0.001 |
| Time to DM (MD [IQR]) | 3.16 [1.67, 6.25] | 2.69 [1.45, 5.14] | 5.02 [2.46, 7.64] | <0.001 |
| Total number of VL (MD [IQR]) | 9 [5, 15] | 7 [5, 12] | 15 [8, 23] | <0.001 |
| Time from First to Last VL (years, MD [IQR]) | 3.24 [1.70, 6.47] | 2.70 [1.45, 5.19] | 5.37 [2.65, 7.77] | <0.001 |
| B. Hypertension sample | ||||
| Overall | HBH | NM | p-value | |
| n | (n=7450) | (n=5318) | (n=2132) | |
| Age (mean (SD)) | 35.73 (12.70) | 32.89 (12.03) | 42.80 (11.53) | <0.001 |
| Male (%) | 5240 (70.3) | 3438 (64.6) | 1802 (84.5) | <0.001 |
| Race/etdnicity (%) | <0.001 | |||
| Black, non-Hispanic | 1892 (25.4) | 1214 (22.8) | 678 (31.8) | |
| Hispanic | 1196 (16.1) | 980 (18.4) | 216 (10.1) | |
| White, non-Hispanic | 3479 (46.7) | 2503 (47.1) | 976 (45.8) | |
| Other* | 883 (11.9) | 621 (11.7) | 262 (12.3) | |
| Insurance category (%) | ||||
| Government | 1319 (17.7) | 827 (15.6) | 492 (23.1) | |
| Private | 2575 (34.6) | 1698 (31.9) | 877 (41.1) | |
| Other/uninsured | 3556 (47.7) | 2793 (52.5) | 763 (35.8) | |
| DM at baseline (%) | 496 (6.7) | 136 (2.6) | 360 (16.9) | <0.001 |
| Body mass index | 26.98 (6.24) | 27.37 (6.61) | 26.37 (5.54) | <0.001 |
| CD4 (cells/mL) (MD [IQR]) | 458 [278, 666] | 596 [408, 790] | 429 [253, 619] | <0.001 |
| Viral load at baseline (median [IQR]) | 86.50 [20, 26938] | 40 [20, 29567] | 211 [42, 22804] | <0.001 |
| <100 copies/mL on first VL (%) | 3785 (50.8) | 2863 (53.8) | 922 (43.2) | <0.001 |
| <100 copies/mL on last VL (%) | 6460 (86.7) | 4649 (87.4) | 1811 (84.9) | 0.005 |
| Incident HTN (%) | 496 (6.7) | 136 (2.6) | 360 (16.9) | <0.001 |
| Time to HTN (MD [IQR]) | 3.05 [1.60, 6.01] | 2.69 [1.44, 5.15] | 4.53 [2.18, 7.42] | <0.001 |
| Total number of VL (MD [IQR]) | 9 [5, 15] | 7 [5, 12] | 14 [8, 23] | <0.001 |
| Time from First to Last VL (years, MD [IQR]) | 3.2 [1.68, 6.42] | 2.71 [1.45, 5.20] | 5.34 [2.54, 7.78] | <0.001 |
Notes:
Others include Asian, Pacific Islander, Multiracial, and Unspecified. DM – Diabetes mellitus, IQR – interquartile range, HBH – Howard Brown Health, HTN – hypertension, MD – median, NM – Northwestern Medicine. VL – viral load.
Comparing the two systems, there were comparably higher proportion of male PWH and non-Hispanic Black PWH at NM than HBH in both cohorts. PWH at NM were also more likely to have private insurance, have comorbid DM or HTN at baseline, have higher baseline VL, and lower baseline CD4 counts. Additional summary statistics for site-specific variables can be found in Table S2. Most of the PWH in both cohorts reside in the northern neighborhoods (where many LGBTQ+-focused services are located). HTN and DM cases were detected throughout the city with some low sample south and western neighborhoods having cases almost as high as the high sample north neighborhoods. (Figure S1)
There were 230 DM and 496 HTN events during follow-up. The HBH subsample had lower unadjusted incidence rates than the NM subsample for both DM (74 (1.4%) vs 156 (6.8%) and HTN (136 (2.6%) vs 360 (16.9%)). The median time to event of the overall sample were 3.2 years for DM (Interquartile range (IQR):1.7-6.7) and 3.1 years for HTN (IQR:1.7-6.4) with longer median time to event for those in NM (DM: 5.4, IQR:2.7-7.8, HTN: 5.3, IQR:2.5-7.8) compared to HBH (DM: 2.7, IQR:1.4-5.2, HTN: 2.7, IQR:1.5-5.2).
The median time to event for incident DM cases (3.3, IQR:1.5-5.4) was comparable to median time to censoring for those who did not get diagnosed with DM (3.1, IQR:1.7-6.3). Those with incident DM had a lower number of VL tests during that period compared to non-cases (with DM: 6, IQR:3-11.8 vs no DM: 8, IQR:5-14). A similar pattern was seen in the HTN cohort with comparable lengths of follow-up (Time to HTN: 3.3, IQR:1.3-5.7 vs Time to censoring: 3.0, IQR:1.6-6.1) and lower VL tests in HTN cases (with HTN: 5, IQR:3-11 vs no HTN: 8, IQR: 5-13).
B. Pooled Analysis from Both sites
Using the pooled dataset, joint models adjusting for the base set variables available in both sites demonstrated that viremia-copy-year was not associated with incident DM (HR: 1.03, 95%CI: 0.96-1.10) but was associated with incident HTN (HR: 1.20, 95%CI: 1.14-1.26).(Table 2) Higher viremia-copy-year was associated with higher hazards of HTN. In contrast, sensitivity analysis with a standard Cox model that adjusts for the same base set covariates but used just baseline VL instead of viremia-copy-year showed no association of baseline VL with HTN nor DM. (Table S3)
Table 2.
Association of Cumulative Viral Load with Hazard for (A) Diabetes Mellitus and (B) Hypertension using Joint Model
| A. Diabetes Mellitus | |
|---|---|
| Variables | HR (95%CI) |
| Age at First Viral Load | 1.053 (1.050-1.06)* |
| Male gender | 1.24 (1.14-1.35)* |
| Race and ethnicity (ref: White) | |
| • Black | 2.00 (1.84-2.16)* |
| • Hispanic | 2.67 (2.36-3.03)* |
| • Other† | 1.52 (1.32-1.75)* |
| Has HTN at baseline | 0.93 (0.81-1.07) |
| Insurance (ref: public) | |
| • Private | 0.81 (0.74-0.89)* |
| • Uninsured/other | 0.92 (0.85-0.995)* |
| Body mass index at baseline | 1.07 (1.06-1.08)* |
| Seen at Northwestern Medicine | 1.33 (1.23-1.43)* |
| Year of First viral load (centered) | 0.94 (0.93-0.95)* |
| CD4 at baseline (square-root transform) | 0.997 (0.98-1.01) |
| (log−)Viremia-copy-year | 1.03 (0.96-1.10) |
| B. Hypertension | |
| Variables | HR (95%CI) |
| Age at First Viral Load | 1.049 (1.047-1.052)* |
| Male gender | 1.62 (1.49-1.76)* |
| Race and etdnicity (ref: White) | |
| • Black | 1.51 (1.41-1.61)* |
| • Hispanic | 1.17 (1.09-1.26)* |
| • Other† | 0.91 (0.82-0.999)* |
| Has DM at baseline | 1.17 (0.87-1.57)* |
| Insurance (ref: public) | |
| • Private | 0.62 (0.58-0.66)* |
| • Uninsured/other | 0.78 (0.74-0.83)* |
| Body mass index at baseline | 1.06 (1.05-1.07)* |
| Seen at Northwestern Medicine | 2.41 (2.25-2.59)* |
| Year of First viral load (centered) | 1.01 (1.004-1.02)* |
| CD4 at baseline (square-root transform) | 1.01 (1.004-1.02)* |
| (log−)Viremia-copy-year | 1.20 (1.14-1.26)* |
Notes:
pooled confidence interval does not cross one and is interpreted as having a significant association with the outcome.
Others include Asian, Pacific Islander, Multiracial, and Unspecified. Variables in italics are the additional variables for site-specific analysis. CI – confidence interval from pooled results of multiply imputed datasets (m=20), DM – Diabetes mellitus, HR – hazards ratio, HTN – hypertension.
Older age, male gender, Black and Hispanic race and ethnicity, being part of the NM subgroup, and higher baseline BMI were associated with significantly higher hazards for both DM and HTN. Higher baseline CD4 count was associated with higher hazards for HTN, but not DM. Having private or other insurance/uninsured were associated with lower DM and HTN risk. (Table 2)
C. Northwestern Medicine only
In the NM-only analysis which adjusted for the base set variables, smoking status, and SDI score, there was an association between viremia-copy-year and HTN (HR: 1.29, 95%CI: 1.25-1.33) but not DM risk (HR: 1.05, 95%CI: 0.99-1.13). Like the pooled analyses, older age, male gender, Black race & ethnicity (vs white) and higher BMI were associated with significantly higher hazards for both outcomes. Hispanic and Other race and ethnicity was associated with higher DM but lower HTN risk. Higher CD4 score and history of smoking was also associated with higher HTN risk but not DM. Regarding neighborhood variables, higher SDI score, poverty rate, %Black population, and %Hispanic population were associated higher risk of DM and HTN. (Tables S4 and S5)
D. Howard Brown Health only
In the HBH-only analysis which adjusted for the base set variables and additional selected individual and neighborhood SDI score, there was no association between viremia-copy-year and the outcomes (DM: 1.01, 95%CI: 0.90-1.13; HTN: 0.98, 95%CI: 0.86-1.02).
Older age, higher BMI, Black or Hispanic race and ethnicity (vs white) and having public insurance (vs other/uninsured) were associated with higher risk of DM. In addition, transgender modality, having no access to permanent housing, higher income category, and having a mental health disorder were associated with increased risk of DM. Having HTN at baseline, have gay, lesbian, bisexual, or queer sexual orientation (vs heterosexual), and being uninsured (vs public) were associated with lower DM risk. Among neighborhood variables, higher SDI score and %Hispanic population were associated with higher DM risk. (Table S6)
Older age, higher BMI, non-white races and ethnicities, and having public insurance (vs other/uninsured) were associated with higher risk of HTN. Higher baseline CD4 count and male gender were also associated with higher HTN risk. Additional individual-level variables that were associated with higher risk include gay/lesbian sexual orientation (vs heterosexual), having a household income <100% FPL (vs >200% FPL), having a mental health disorder diagnosis, and having a substance use disorder diagnosis. Living in neighborhoods with higher SDI score or higher %Black or %Hispanic population were also associated with higher HTN risk. (Table S7)
IV. Discussion
In this paper, we observed heterogeneous associations of HIV viremia-copy-year with incident HTN, and no association with incident DM, in two distinct health care contexts. Given prior observational and mechanistic data, we anticipated that higher viremia-copy-year (poor viral suppression over time) would be associated with higher risk for DM and HTN. [6,29] However, we only observed this higher HTN risk with higher viremia-copy-year in PWH being seen at an academic tertiary care setting but not in a community care setting. Among PWH, certain social determinants of health were also identified as important correlates of HTN and DM risk within the two health systems.
The differential association we observed arise from multiple mechanisms related to differences in the two health system contexts. NM handles multimorbid or older individuals who were more likely be insured than HBH. Older PWH (as in the general population) tend to have higher rates of HTN.[9,10] Those at NM were also observed for longer periods than HBH. Thus, NM likely accrued more events that HBH and had higher power to detect associations. Additionally, there could also be differences in ascertainment with more frequent detection and screening in NM, an academic setting, compared to HBH, a primary care setting. Differences in provision of HIV care also translates to differences in achievement of viral suppression. HBH had better suppression rates than NM, facilitated by the robust social referral system at HBH, despite lower rate of insurance. Fewer individuals would then have higher viremia-copy-year at HBH, and this again would translate to fewer events and lower power to detect association, even if an association existed. Overall, these issues stress the need to conduct system-specific analyses to avoid masking heterogeneity and better inform guidance for local practice.
Our models also identified important predictors of cardiometabolic risk in these health systems. As expected, older age and higher BMI were associated with elevated risk of DM and, along with ever smoking, HTN .[5,30] Higher baseline CD4 count was also found to be associated with increased risk of HTN, even after adjusting for a potential mediation through cumulative VL. Prior research have shown that chronic inflammation is important in the pathogenesis of HTN in PWH.[5] While CD4 is an important component of these inflammation (and immune dysfunction) processes, findings of epidemiologic studies on CD4 seem to be mixed.[7,31-33] Decomposing the effects of time varying CD4 counts (or other immune cells), independent of direct viral damage, is beyond the scope of our analysis and future work on the topic is encouraged.
SDOH affect outcomes through multiple synergistic and antagonistic mechanisms.[34] One mechanism is that bias and structural barriers could prevent access to healthcare. For example, elevated HTN risk among Black PWH may stem from structural and interpersonal racism affecting preventive healthcare access and engagement.[34,35] These same factors, however, could also lead to less screening and underdiagnosis which may explain the observed lower HTN risk in uninsured.[36,37] Several SDOH produce chronic stress which influences metabolism and behaviors. Racial discrimination and having a mental health disorder could be sources of daily stress.[38,39] Additionally, having a mental health disorder which has been associated with social isolation and increased occurrence of traditional risk factors, both of which may increase HTN risk.[39,40] The combined result of different mechanisms, however, is hard to predict. We expected that LGBTQ+ would show higher risk because of minority stress and structural marginalizations.[41,42] However, LGB individuals had significantly lower risk of DM compared to heterosexual PWH. Explaining these surprising findings, especially how these variables interact with each other and the health system context (e.g., HBH have specific programs or focus towards LGBTQ+ and poor populations.) would be important for future work.
We also found the different measures neighborhood conditions were associated with both DM and HTN risk. These indicators maybe capturing lower neighborhood investments and availability of health or social support services in that can help maintain good health.[20,43] Importantly, the lack of significant associations for SDOH or neighborhood variables does not necessarily mean a lack of association with the outcome. Our inclusion of potential mediator variables (e.g., body-mass index) in the models could produce null findings.[44]
Our paper addresses an important gap in the literature by proper handling of temporality in the modeling, by adjusting for cumulative exposures instead of just baseline VL, and by using large samples from two distinct health systems. However, our approach does have limitations. Some are related to modeling. Joint models are susceptible to biased estimates from misspecification.[45] This was mitigated by adding flexibility through polynomial terms in the longitudinal model. Also, current software can handle only one time-varying exposure so we are unable to explore the role of time-varying CD4 or ART regimens on risk. We can’t confirm prior findings about ART classes and DM/HTN risk.[5,6] Further, given the diversity of ART regimens and switching, our sample is unlikely to be sufficient to detect associations with adequate precision.
Several limitations are related to using EHR which carries inherent risks of misclassification and informed presence bias. We attempted to mitigate risks by using previously published phenotyping rules and including only those with sufficient system engagement.[46,47] Relatedly, the HTN definition didn’t use blood pressure so we can’t account for changes due to the new ACC/AHA definition or instrument-related variation in blood pressure measurement.[48] We also have an incomplete data capture. Individuals could be lost to follow-up, and be diagnosed outside included health systems. We may be underestimating cumulative exposure especially for individuals diagnosed before entry to our database. Due to lack of de-duplication, there is also the possibility of counting the same individual twice. In addition, there were differences in availability and specification of SDOH variables, which could explain the different associations found in the system-specific analysis. Finally, most of the data reflects the care of PWH in Chicago during the era of universal ART which may not reflect context in other areas in the US, much less the globe. Thus, replication of our analysis in other health systems or using research cohorts for HIV is recommended to confirm our findings and explore unanswered related questions.
Perspectives
Lowering cumulative VL exposure through anti-retroviral treatment directly reduces cardiovascular disease risk but may also indirectly reduce this risk through reduction of hypertension risk in PWH, especially older HIV populations. The differences in association between two health systems point to the need for system-specific analyses to better understand risk of patient under care and guide offered preventive services. In addition, our analysis pointed out the potential importance of mental health disorders and neighborhood conditions as correlates of DM and HTN risk. Future work on mechanisms how these SDOH variables translate to increased risk to guide development of interventions are needed. Overall, preventing HTN and DM among PWH requires more than just controlling HIV but also addressing behavioral and social factors, at individual and systemic levels.
Conclusion
Cumulative viral load maybe associated with risk of hypertension but not diabetes mellitus in PWH in certain health settings. Traditional risk factors and social determinants of health also play a role in the incidence of these conditions in the population. Future work to test replicability of these findings in other settings is recommended.
Supplementary Material
VII. Novelty and Relevance:
1. What is new?
Higher cumulative viral load was associated with increased risk of hypertension but not diabetes among people with HIV (PWH) at an academic health system but not in a network of primary care centers in Chicago.
Social factors, like neighborhood conditions, that affect healthcare access and stress was associated with hypertension and diabetes risk.
2. What is relevant?
Higher cumulative viral load may be associated with increased risk of hypertension but not diabetes.
Preventing hypertension and diabetes in PWH may require addressing social factors.
3. Clinical/Pathophysiological Implications
Our work raises the possibility that HIV viremia – and related tissue-specific and vascular immune/inflammatory dysregulation– affect pathophysiology underlying hypertension to a greater extent than for diabetes.
V. Sources of Funding and Disclosures
ASR was supported by the AHA Predoctoral Fellowship (825793). JS was partially supported by NHLBI R01HL158963. Data pull funding for NM was through MF (AHA 16FTF31200010) for NM. The HBH data pull was possible with support from the Third Coast Center for AIDS Research, an NIH funded center (P30 AI117943). This research was supported in part through the computational resources and staff contributions provided for the Quest high performance computing facility at Northwestern which is jointly supported by the Office of the Provost, the Office for Research, and Northwestern University Information Technology.
No other relevant disclosures.
List of Abbreviations and Acronyms
- ART
anti-retroviral treatment
- BMI
body mass index
- CVD
cardiovascular disease
- DM
diabetes mellitus
- HBH
Howard Brown Health
- HIV
human immunodeficiency virus
- HTN
hypertension
- LGBTQ+
lesbian, gay, bisexual, transgender, and queer
- NM
Northwestern Medicine
- PWH
people with HIV
- VL
viral load
- SDOH
social determinants of health
VI. References
- 1.Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So-Armah K, et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol [Internet]. 2016;117:214–20. doi: 10.1016/j.amjcard.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: Exploratory analyses from the SMART trial. Antivir Ther. 2008;13:177–87. [DOI] [PubMed] [Google Scholar]
- 3.Baker J V, Sharma S, Achhra AC, Bernardino JI, Bogner JR, Duprez D, et al. Changes in Cardiovascular Disease Risk Factors With Immediate Versus Deferred Antiretroviral Therapy Initiation Among HIV-Positive Participants in the START (Strategic Timing of Antiretroviral Treatment) Trial. J Am Heart Assoc. 2017;6:e004987. doi: 10.1161/JAHA.116.004987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living with HIV: A Scientific Statement from the American Heart Association. Circulation. 2019;140:e98–124. doi: 10.1161/CIR.0000000000000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahme SA, Bloomfield GS, Peck R. Hypertension in HIV-infected adults: Novel pathophysiologic mechanisms. Hypertension. 2018;72:44–55. doi: 10.1161/HYPERTENSIONAHA.118.10893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalra S, Agrawal N. Diabetes and HIV: Current understanding and future perspectives. Curr Diab Rep. 2013;13:419–27. doi: 10.1007/s11892-013-0369-9 [DOI] [PubMed] [Google Scholar]
- 7.Masenga SK, Hamooya BM, Nzala S, Kwenda G, Heimburger DC, Mutale W, et al. Patho-immune Mechanisms of Hypertension in HIV: a Systematic and Thematic Review. Curr Hypertens Rep. 2019;21. doi: 10.1007/s11906-019-0956-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noubissi EC, Katte JC, Sobngwi E. Diabetes and HIV. Curr Diab Rep. 2018;18. doi: 10.1007/s11892-018-1076-3 [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens [Internet]. 2017;11:530–40. doi: 10.1016/j.jash.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 10.Davis K, Perez-Guzman P, Hoyer A, Brinks R, Gregg E, Althoff KN, et al. Association between HIV infection and hypertension: a global systematic review and meta-analysis of cross-sectional studies. BMC Med. 2021;19. doi: 10.1186/s12916-021-01978-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–84. doi: 10.1001/archinte.165.10.1179 [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009-2010. BMJ Open Diabetes Res Care. 2017;5. doi: 10.1136/bmjdrc-2016-000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prioreschi A, Munthali RJ, Soepnel L, Goldstein JA, Micklesfield LK, Aronoff DM, et al. Incidence and prevalence of type 2 diabetes mellitus with HIV infection in Africa: A systematic review and meta-analysis. BMJ Open. 2017;7:1–11. doi: 10.1136/bmjopen-2016-013953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karamchand S, Leisegang R, Schomaker M, Maartens G, Walters L, Hislop M, et al. Risk factors for incident diabetes in a cohort taking first-line nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Med (United States). 2016;95:1–9. doi: 10.1097/MD.0000000000002844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okeke NL, Davy T, Eron JJ, Napravnik S. Hypertension among HIV-infected Patients in Clinical Care, 1996-2013. Clin Infect Dis. 2016;63:242–8. doi: 10.1093/cid/ciw223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron J, Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol. 2010;171:198–205. doi: 10.1093/aje/kwp347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mugavero MJ, Napravnik S, Cole SR, Eron JJ, Lau B, Crane HM, et al. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011;53:927–35. doi: 10.1093/cid/cir526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Chen X, Wijayabahu A, Zhou Z, Yu B, Spencer EC, et al. Cumulative HIV Viremia Copy-Years and Hypertension in People Living with HIV. Curr HIV Res. 2020;18:143–53. doi: 10.2174/1570162X18666200131122206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasada S, Rivera A, Nishtala A, Pawlowski AE, Sinha A, Bundy JD, et al. Differential associations of chronic inflammatory diseases with incident heart failure. JACC Hear Fail. 2020;8:489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res. 2013;48:539–59. doi: 10.1111/j.1475-6773.2012.01449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashley F. “Trans” Is My Gender Modality: a Modest Terminological Proposal. In: Erickson-Schroth L, editor. Trans Bodies, Trans Selves. 2nd ed. Oxford University Press; (in press); 2022. [Google Scholar]
- 22.Rizopoulus D. Joint Models for Longitudinal and Time-to-Event Data with Applications in R. CRC Press; 2012. [Google Scholar]
- 23.Rizopoulos D. An Introduction to the Joint Modeling of Longitudinal and Survival Data, with Applications in R. Slides [Internet]. 2017;1–235. doi: 10.1007/978-1-4757-3447-8_7 [DOI] [Google Scholar]
- 24.Rizopoulos D. The R package jmbayes for fitting joint models for longitudinal and time-to-event data using MCMC. J Stat Softw. 2016;72. doi: 10.18637/jss.v072.i07 [DOI] [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. [Google Scholar]
- 26.R Studio Team. RStudio: Integrated Development for R. Boston, MA: RStudio, Inc; 2019. [Google Scholar]
- 27.Papageorgiou G, Mauff K, Tomer A, Rizopoulos D. An overview of joint modeling of time-to-event and longitudinal outcomes. Annu Rev Stat Its Appl. 2019;6:223–40. doi: 10.1146/annurev-statistics-030718-105048 [DOI] [Google Scholar]
- 28.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 29.Hsue PY. Mechanisms of Cardiovascular Disease in the Setting of HIV Infection. Can J Cardiol [Internet]. 2019;35:238–48. doi: 10.1016/j.cjca.2018.12.024 [DOI] [PubMed] [Google Scholar]
- 30.Sarkar S, Brown TT. Diabetes in People with HIV. Curr Diab Rep. 2021;21. doi: 10.1007/s11892-021-01382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Socio GV, Ricci E, Maggi P, Parruti G, Pucci G, Di Biagio A, et al. Prevalence, awareness, treatment, and control rate of hypertension in HIV-infected patients: The HIV-HY study. Am J Hypertens. 2014;27:222–8. doi: 10.1093/ajh/hpt182 [DOI] [PubMed] [Google Scholar]
- 32.Dimala CA, Kadia BM, Kemah BL, Tindong M, Choukem SP. Association between CD4 Cell Count and Blood Pressure and Its Variation with Body Mass Index Categories in HIV-Infected Patients. Int J Hypertens. 2018;2018. doi: 10.1155/2018/1691474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manner IW, Trøseid M, Oektedalen O, Baekken M, Os I. Low Nadir CD4 Cell Count Predicts Sustained Hypertension in HIV-Infected Individuals. J Clin Hypertens. 2013;15:101–6. doi: 10.1111/jch.12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieger N. Theories for social epidemiology in the 21st century: an ecosocial perspective. Int J Epidemiol. 2001;30:668–77. [DOI] [PubMed] [Google Scholar]
- 35.Caceres BA, Streed CG, Corliss HL, Lloyd-Jones DM, Matthews PA, Mukherjee M, et al. Assessing and Addressing Cardiovascular Health in LGBTQ Adults: A Scientific Statement From the American Heart Association. Circulation. 2020;1–12. doi: 10.1161/cir.0000000000000914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayanian JZ, Zaslavsky AM, Weissman JS, Schneider EC, Ginsburg JA. Undiagnosed Hypertension and Hypercholesterolemia among Uninsured and Insured Adults in the Third National Health and Nutrition Examination Survey. Am J Public Health. 2003;93:2051–4. doi: 10.2105/AJPH.93.12.2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huguet N, Larson A, Angier H, Marino M, Green BB, Moreno L, et al. Rates of Undiagnosed Hypertension and Diagnosed Hypertension without Anti-hypertensive Medication following the Affordable Care Act. Am J Hypertens. 2021;34:989–98. doi: 10.1093/ajh/hpab069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beatty Moody DL, Chang YF, Pantesco EJ, Darden TM, Lewis TT, Brown C, et al. Everyday discrimination prospectively predicts blood pressure across 10 years in racially/ethnically diverse midlife women: Study of women’s health across the nation. Ann Behav Med. 2019;53:608–20. doi: 10.1093/abm/kay069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuffee Y, Ogedegbe C, Williams NJ, Ogedegbe G, Schoenthaler A. Psychosocial Risk Factors for Hypertension: an Update of the Literature. Curr Hypertens Rep. 2014;16. doi: 10.1007/s11906-014-0483-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen BE, Edmondson D, Kronish IM. State of the art review: Depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. 2015;28:1295–302. doi: 10.1093/ajh/hpv047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caceres BA, Streed CG, Corliss HL, Lloyd-Jones DM, Matthews PA, Mukherjee M, et al. Assessing and Addressing Cardiovascular Health in LGBTQ Adults: A Scientific Statement From the American Heart Association. Circulation. 2020;142:e321–32. doi: 10.1161/CIR.0000000000000914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Streed CG, Beach LB, Caceres BA, Dowshen NL, Moreau KL, Mukherjee M, et al. Assessing and Addressing Cardiovascular Health in People Who Are Transgender and Gender Diverse: A Scientific Statement from the American Heart Association. Circulation. 2021;136–48. doi: 10.1161/CIR.0000000000001003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellowski JA, Kalichman SC, Matthews KA, Adler N. A pandemic of the poor: social disadvantage and the US HIV epidemic. Am Psychol. 2013;68:197–209. doi: 10.1037/a0032694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan SM, Peters ES, Trapido EJ, Oral E, Scribner RA, Rung AL. Assessing mediation of behavioral and stress pathways in the association between neighborhood environments and obesity outcomes. Prev Med Reports [Internet]. 2016;4:248–55. doi: 10.1016/j.pmedr.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arisido MW, Antolini L, Bernasconi DP, Valsecchi MG, Rebora P. Joint model robustness compared with the time-varying covariate Cox model to evaluate the association between a longitudinal marker and a time-to-event endpoint. BMC Med Res Methodol. 2019;19:1–13. doi: 10.1186/s12874-019-0873-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldstein BA, Bhavsar NA, Phelan M, Pencina MJ. Controlling for informed presence bias due to the number of health encounters in an electronic health record. Am J Epidemiol. 2016;184:847–55. doi: 10.1093/aje/kww112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasada S, Rivera A, Nishtala A, Pawlowski AE, Sinha A, Bundy JD, et al. Differential Associations of Chronic Inflammatory Diseases With Incident Heart Failure. JACC Hear Fail. 2020;1–10. doi: 10.1016/j.jchf.2019.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carey RM, Whelton PK. Evidence for the universal blood pressure goal of <130/80 mm hg is strong: Controversies in hypertension - Pro side of the argument. Hypertension. 2020;76:1384–90. doi: 10.1161/HYPERTENSIONAHA.120.14647 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


