Abstract
Background
Effective, safe, and affordable antivirals are needed for COVID-19. Several lines of research suggest that tenofovir may be effective against COVID-19, but no large-scale human studies with appropriate adjustment for comorbidities have been conducted.
Methods
We studied HIV-positive individuals on antiretroviral therapy (ART) in 2020 at 69 HIV clinics in Spain. We collected data on sociodemographics, ART, CD4-cell count, HIV-RNA viral-load, comorbidities and the following outcomes: laboratory-confirmed SARS-CoV-2 infection, COVID-19 hospitalization, intensive care unit (ICU) admission and death. We compared the 48-week risks for individuals receiving tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC), tenofovir alafenamide (TAF)/ FTC, abacavir (ABC)/lamivudine (3TC), and other regimes. All estimates were adjusted for clinical and sociodemographic characteristics via inverse probability weighting.
Results
Of 51,558 eligible individuals, 39.6% were on TAF/FTC, 11.9% on TDF/FTC, 26.6% on ABC/3TC, 21.8% on other regimes. There were 2,402 documented SARS-CoV-2 infections (425 hospitalizations, 45 ICU admissions, 37 deaths). Compared with TAF/FTC, the estimated risk ratios (RR) (95% CI) of hospitalization were 0.66 (0.43, 0.91) for TDF/FTC and 1.29 (1.02, 1.58) for ABC/3TC, the RRs of ICU admission were 0.28 (0.11, 0.90) for TDF/FTC and 1.39 (0.70, 2.80) for ABC/3TC, and the RRs of death were 0.37 (0.23, 1.90) for TDF/FTC and 2.02 (0.88–6.12) for ABC/3TC. The corresponding RRs of hospitalization for TDF/FTC were 0.49 (0.24, 0.81) in individuals ≥50 years and 1.15 (0.59, 1.93) in younger individuals.
Discussion
Compared with other antiretrovirals, TDF/FTC lowers COVID-19 severity among HIV-positive individuals with virological control. This protective effect may be restricted to individuals aged 50 years and older.
Introduction
Much research has focused on the repurposing of antivirals for the treatment and prevention of severe COVID-19. Remdesivir, originally developed against the Ebola virus, and molnupiravir, originally developed against the influenza virus, are now used to reduce the risk of hospitalization in high-risk individuals with recently diagnosed COVID-19 (1–5). More research is needed to determine whether tenofovir, an affordable oral drug with a proven safety record, also prevents severe COVID-19.
Among HIV-positive individuals, two observational studies found lower risk of COVID-19 hospitalization (6) and COVID-19 mortality (6–7) among users of tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) than among users of other antiretroviral regimes. Another observational study found a lower risk of severe COVID-19 in patients with chronic hepatitis B virus (HBV) infection treated with TDF/FTC than among those treated with entecavir (8). More recently, a large study in male U.S. veterans with HIV has reported that the risk of COVID-19-related hospitalization for TDF/FTC was less than half the risk for other antiretrovirals (9)). Careful adjustment for clinical characteristics, including those associated with risk of severe COVID-19 (e.g., renal disease), had little impact on the the association between TDF/FTC and lower risk of severe COVID-19. However, this study included only men with an average age of 59 years.
These findings suggest that TDF/FTC might be used as pre-exposure prophylaxis or early treatment of COVID-19 (10–11). This would be especially important for immunosuppressed patients for whom vaccines have suboptimal effectiveness and for individuals for which safety concerns arise with other drugs.
Here we report the findings from a nationwide cohort study of TDF/FTC and COVID-19 outcomes among men and women of all ages with HIV and on antiretroviral therapy.
Methods
Study population
Individuals with HIV in Spain receive care at specialized hospital outpatient clinics. The CoVIHd Collaboration (Covid-19 in HIV-positive individuals in Spain) includes HIV-positive individuals who were receiving antiretroviral therapy at the HIV clinics of 87 Spanish hospitals between January 1 and December 31, 2020. All clinics collected information on individuals with a history of SARS-CoV-2 infection, but this analysis is restricted to the 69 clinics that collected information on HIV-positive individuals with and without a history of SARS-CoV-2 infection. These 69 clinics serve approximately 44% of all persons on antiretroviral therapy with virological suppression in Spain (12).
Hospitals transmitted de-identified data to the coordinating center at the Institute of Health Carlos III in Madrid via a secure web-based application specifically designed for this purpose. For each individual, data included sociodemographic characteristics, dates and composition of all antiretroviral therapy regimes received during the study period, latest CD4 cell count and HIV RNA measurements before a COVID-19 diagnosis, comorbidities (from medical records, see Appendix 2), and date of laboratory-confirmed documented diagnosis of SARS-CoV-2 infection defined as positive results from a polymerase chain reaction (PCR) test (or, in a minority of cases, a SARS-CoV-2 antigen test or antibody test), following the Ministry of Health protocols ( 13). The ascertainment of hospitalizations due to COVID-19, intensive care unit (ICU) admissions due to COVID-19, and deaths from COVID-19 was complete, but no protocol was in place to systematically screen for asymptomatic infections and mild cases of COVID-19.
Eligibility criteria and follow-up
We included HIV-positive individuals aged 18 years or older who on February 1, 2020 had not received a diagnosis of SARS-CoV-2 infection and were on antiretroviral therapy, and who had virologically suppression (HIV RNA less than 50 copies/ml) in 2020. Virological suppression is an indicator of adequate adherence to antiretroviral therapy. For each individual, follow-up started on February 1 and ended on December 31, 2020. The goal was to emulate a (hypothetical) target trial in which individuals are randomly assigned to a particular nucleos(t)ide reverse transcriptase inhibitor (NRTI) combination before the start of SARS-CoV-2 transmission in their communities.
Antiretroviral therapy regimes
We classified antiretroviral therapy regimes according to their NRTI combination into 4 categories: tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC), tenofovir alafenamide (TAF)/ FTC, abacavir (ABC)/lamivudine (3TC), or other drug regimens excluding TDF, TAF and ABC. Most of the other drugs category were dual therapies including only one NRTI (3TC) (Appendix Table 1). We also studied regimens with three drugs according to the third drug class used along with the NRTI combination: integrase inhibitor, protease inhibitor, or nonnucleoside reverse transcriptase inhibitor (NNRTI).
Outcomes
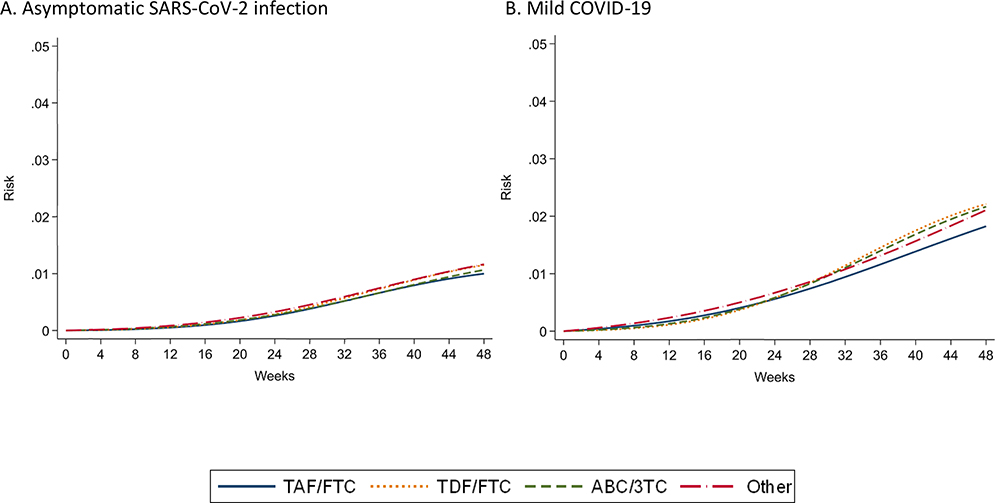
The outcomes of interest were any documented laboratory-confirmed diagnosis of SARS-CoV-2 infection and progressively more severe subsets of COVID-19: hospitalization due to COVID-19, ICU admission due to COVID-19, and death due to COVID-19. In supplemental analyses, we also considered documented asymptomatic SARS-CoV-2 infections and mild COVID-19 that did not require hospitalization.
Statistical Analysis
We calculated the 48-week risk (cumulative incidence) and 95% confidence interval (CI) for each outcome by NRTI combination. We estimated the risks using a pooled logistic model with indicators for NRTI combination (3 indicators, with TAF/FTC as the reference group), week of follow-up (linear and quadratic terms), and product terms between NRTI combination indicators and week of follow-up. To adjust for baseline prognostic factors, we used inverse probability (IP) weighting. To estimate the denominator of the weights we fit a multinomial logistic model for the four NRTI combinations with covariates: age (in years, linear and quadratic terms), sex (male, female), transmission category (heterosexual, homo/bisexual, injecting drug use, other), country of origin (Spain, other), CD4 (<350, 350–500, >500 cells/mm3), and indicators for hypertension, diabetes, chronic renal disease, cardiovascular disease, and treatment with immunosuppressants or corticosteroids. We compared the risks via risk differences and risk ratios and used a nonparametric percentile-based bootstrap with 500 samples to obtain 95% CIs.
To compare the risks by the non-NRTI drug in the antiretroviral regime, we fit a similar model with indicators for NNRTI, protease inhibitor, and integrase inhibitor. We conducted subgroup analyses by age group (<50, ≥50 years) and sex for documented SARS-CoV-2 infections, and COVID-19 hospitalization.
We also conducted several sensitivity analyses. To evaluate the potential impact of treatment changes regimes, we conducted an analysis in which individuals were censored if/when they switch from their baseline antiretroviral regime to another regime. To evaluate the potential impact of the choice of inverse probability weighting as the method to adjust for confounding, we repeated the analyses with adjustment via standardization and also estimated adjusted hazard ratios via a Cox regression model. To assess the impact of measured confounding due to comorbidities and other factors, we repeated the analysis with no adjustment at all. All analyses were conducted with Stata, version 15.0 (StataCorp).
This study was approved by the institutional review board at University Hospital Ramón y Cajal, Madrid, Spain.
Results
Of 51,558 eligible individuals (Figure 1), 39.6% were receiving TAF/FTC, 11.9% TDF/FTC, 26.6% ABC/3TC, and 21.8% other regimes (see Appendix Table 1 for a description of regimes in each category). The baseline characteristics of individuals in each of the four groups defined by NRTI combination are shown in Table 1. Individuals receiving TDF/FTC and TAF/FTC had similar age, sex, and CD4 cell counts, and were slightly younger than those receiving ABC/3TC or other regimes. The proportion of injecting drug users was slightly lower in persons on TAF/FTC than in the other groups. Individuals in the TDF/FTC group had a lower prevalence of hypertension, diabetes, and chronic renal disease than individuals in the other groups.
Figure 1. Flowchart of study population among HIV-positive individuals, CoVIHd Collaboration, Spain, February-December 2020.
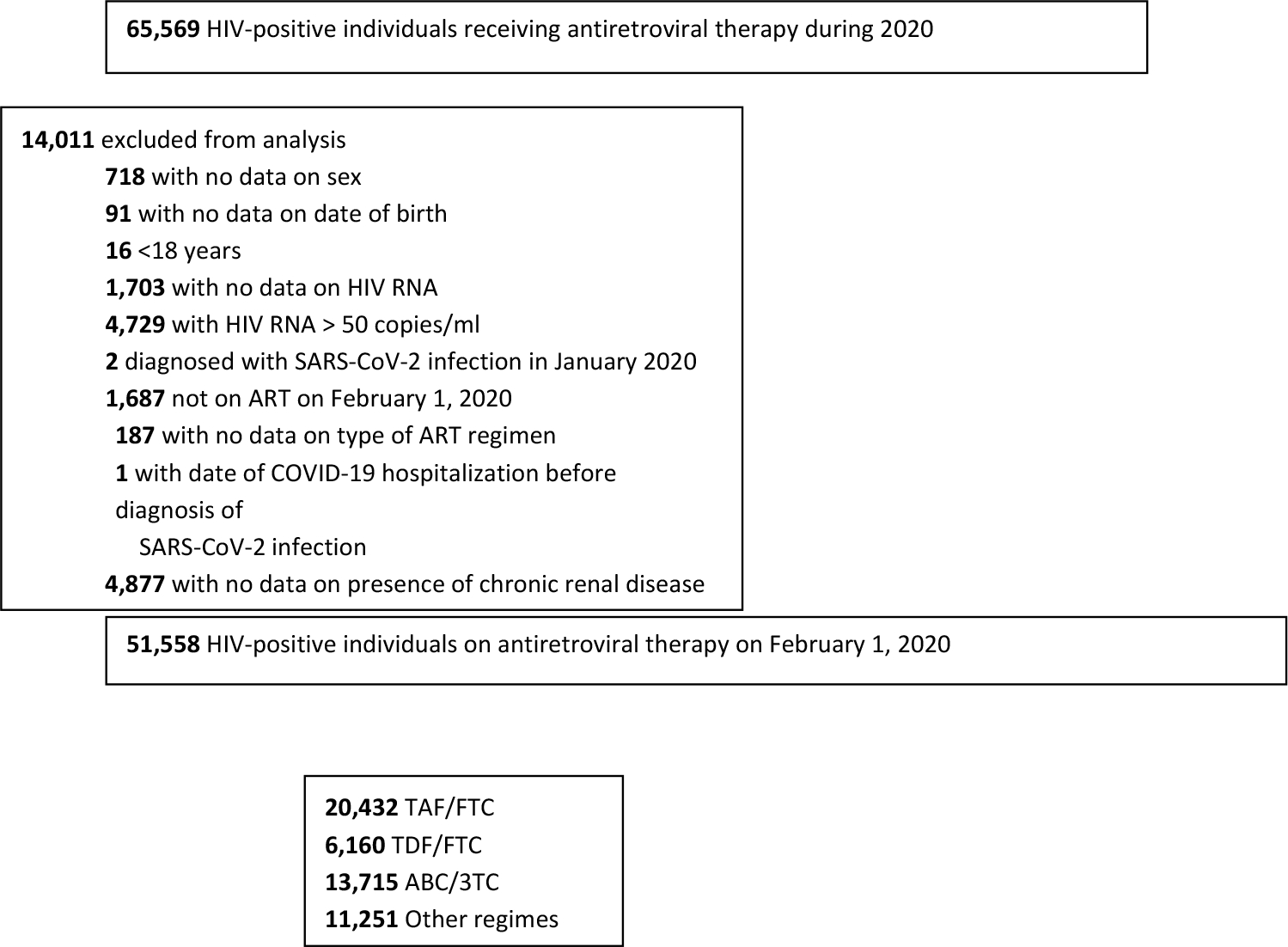
Table 1.
Baseline characteristics of 51,558 eligible individuals by NRTI combination in HIV-positive individuals, CoVIHd Collaboration, Spain, February-December 2020
| TAF/FTC N = 20,432 (39.6%) | TDF/FTC N = 6,160 (11.9%) | ABC/3TC N = 13,715 (26.6%) | Other regimes N = 11,251 (21.8%) | |
|---|---|---|---|---|
|
| ||||
| Sex [N (%)] | ||||
| Men | 16,527 (80.9) | 4,856 (78.8) | 10,797 (78.7) | 8,623 (76.6) |
| Women | 3,905 (19.1) | 1,304 (21.2) | 2,918 (21.3) | 2,628 (23.4) |
| Age, years [Median (IQR)] | 49 (39 – 56) | 48 (39 – 55) | 51 (41 – 57) | 53 (45 – 58) |
| Transmission category [N (%)] | ||||
| Heterosexual contact | 4,652 (22.8) | 1,463 (23.7) | 3,218 (23.5) | 2,726 (24.2) |
| Homo/bisexual contact | 8,939 (43.7) | 2,221 (36.1) | 4,859 (35.4) | 3,741 (33.2) |
| Injecting drug use | 3,501 (17.1) | 1,242 (20.2) | 2,665 (19.4) | 2,847 (25.3) |
| Other | 503 (2.5) | 147 (2.4) | 300 (2.2) | 328 (2.9) |
| Unknown | 2,837 (13.9) | 1,087 (17.6) | 2,673 (19.5) | 1,609 (14.3) |
| Country of origin [N (%)] | ||||
| Spain | 12,632 (61.8) | 3,836 (62.3) | 8,553 (62.4) | 8,077 (71.8) |
| Other | 4,689 (22.9) | 1,367 (22.2) | 2,325 (16.9) | 1,568 (13.9) |
| Unknown | 3,111 (15.2) | 957 (15.5) | 2,837 (20.7) | 1,606 (14.3) |
| CD4 cell count, cells/mm3 | ||||
| Median (IQR) | 704 (509–933) | 700 (511–929) | 746 (536–994) | 718 (520–948) |
| <350 | 2,059 (10.1) | 619 (10.0) | 1,179 (8.6) | 1,014 (9.0) |
| 350–500 | 2,775 (13.6) | 834 (13.5) | 1,735 (12.6) | 1,504 (13.4) |
| >500 | 15,414 (75.4) | 4,671 (75.8) | 10,718 (78.1) | 8,620 (76.6) |
| Unknown | 184 (0.9) | 36 (0.6) | 83 (0.6) | 113 (1.0) |
| Hypertension [N (%)] | ||||
| No | 16,804 (82.2) | 5,388 (87.5) | 10,897 (79.4) | 8,469 (75.3) |
| Yes | 3,091 (15.1) | 695 (11.3) | 2,589 (18.9) | 2,564 (22.8) |
| Unknown | 537 (2.6) | 77 (1.2) | 229 (1.7) | 218 (1.9) |
| Diabetes [N (%)] | ||||
| ȀNo | 18,490 (90.5) | 5,707 (92.6) | 12,217 (89.1) | 9,736 (86.5) |
| Yes | 1,486 (7.3) | 380 (6.2) | 1,302 (9.5) | 1,305 (11.6) |
| Unknown | 456 (2.2) | 73 (1.2) | 196 (1.4) | 210 (1.9) |
| Chronic renal disease [N (%)] | ||||
| No | 19,375 (94.8) | 5,952 (96.6) | 12,570 (91.6) | 10,028 (89.1) |
| Yes | 1,057 (5.2) | 208 (3.4) | 1,145 (8.3) | 1,223 (10.9) |
| Cardiovascular disease [N (%)] | ||||
| No | 16,628 (81.4) | 5,511 (89.5) | 11,759 (85.7) | 9,568 (85.0) |
| Yes | 1,051 (5.1) | 302 (4.9) | 773 (5.6) | 887 (7.9) |
| Unknown | 2,753 (13.5) | 347 (5.6) | 1,183 (8.6) | 796 (7.1) |
| Treatment with immunosuppressants or corticosteroids [N (%) | ||||
| No | 13,631 (66.7) | 4,313 (70.0) | 8,768 (63.9) | 7,805 (69.4) |
| Yes | 174 (0.8) | 78 (1.3) | 163 (1.2) | 142 (1.3) |
| Unknown | 6,627 (32.4) | 1,769 (28.7) | 4,784 (34.9) | 3,304 (29.4) |
During the 48-week follow-up, there were 2,402 documented SARS-CoV-2 infections (425 hospitalizations, 45 ICU admissions, and 37 deaths). Of the 1,955 SARS-CoV-2 infections with available information on disease severity, 539 were asymptomatic, 1037 had mild COVID-19, 298 moderate COVID-19 and 81 severe COVID-19. Figure 2 shows the estimated cumulative risks of documented SARS-CoV-2 infection, COVID-19 hospitalization, COVID-19 ICU admission, and COVID-19 death by NRTI combination. Appendix Figure 1 shows the risks of asymptomatic COVID-19 and mild COVID-19.
Figure 2. Estimated risks of COVID-19 outcomes by NRTI combination in HIV-positive individuals, *CoVIHd Collaboration, Spain, February-December 2020.
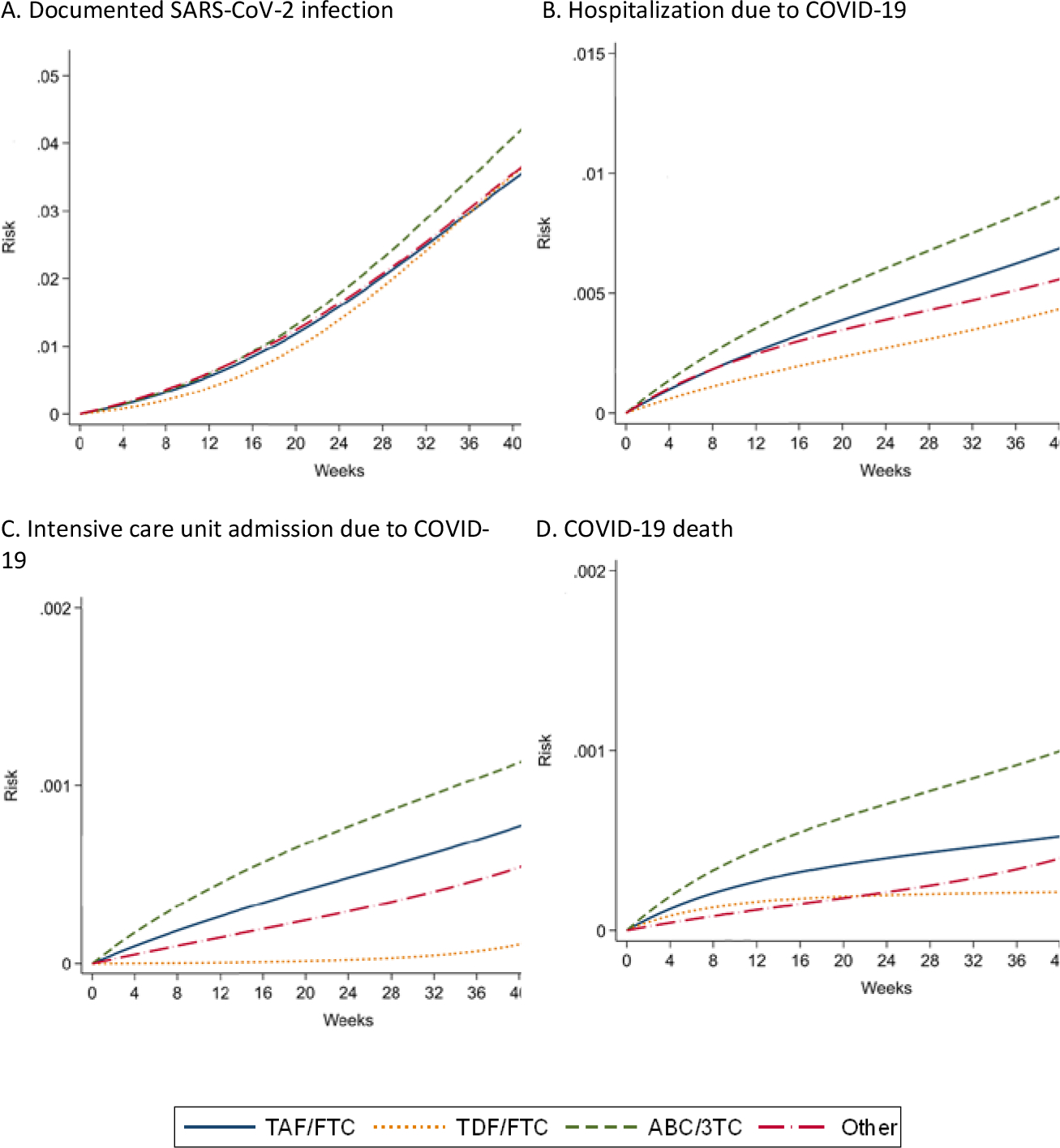
* Adjusted via inverse probability weighting for age (in years, linear and quadratic terms), sex (male, female), transmission category (heterosexual, homo/bisexual, injecting drug use, other), country of origin (Spain, other), CD4 (<350, 350-500, >500 cells/mm3), and hypertension, diabetes, chronic renal disease, cardiovascular disease, and treatment with immunosuppressants or corticosteroids.
Table 2 shows the estimated 48-week risks of each outcome by NRTI combination. The estimated risk (95% CI) of documented SARS-CoV-2 infection was 4.3 % (4.1, 4.6) for TAF/FTC, 4.5% (3.9, 5.0) for TDF/FTC, and 5.2% (4.8, 5.6) for ABC/3TC. The estimated risks of COVID-19 hospitalization, ICU and death were lowest for TDF/FTC and highest for ABC/3TC (Table 2). Compared with TAF/FTC, the estimated risk ratio (95% CI) of COVID-19 hospitalization was 0.66 (0.43, 0.91) for TDF/FTC, 1.29 (1.02, 1.58) for ABC/3TC, and 0.81 (0.62, 1.05) for others; the estimated risk ratio (95% CI) of COVID-19 ICU admission was 0.28 (0.11, 0.90) for TDF/FTC, 1.39 (0.70, 2.80) for ABC/3TC, and 0.76 (0.23, 1.77) for others; and the estimated risk ratio (95% CI) of COVID-19 death was 0.37 (0.23, 1.90) for TDF/FTC, 2.02 (0.88–6.12) for ABC/3TC, and 0.99 (0.34, 2.61) for others (Table 2). Compared with TAF/FTC, the estimated risk ratios (95% CI) of asymptomatic SARS-CoV-2 infection and mild COVID-19 were greater than 1 for TDF/FTC and ABC/3TC. (Appendix Table 2).
Table 2.
Estimated 48-week risks, risk differences and risk ratios of COVID-19 outcomes by NRTI combination in HIV-positive individuals,* CoVIHd Collaboration, Spain, February-December 2020
| Documented SARS-CoV-2 infection | Hospitalization due to COVID-19 | ICU admission due to COVID-19 | COVID-19 death | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| No. events | Risks (95%CI), % | Risk Differences (95% CI), % | Risk Ratios (95% CI) | No. events | Risks (95%CI), % | Risk Differences (95% CI), % | Risk Ratios (95% CI) | No. events | Risks (95% CI), % | Risk Differences (95% CI), % | Risk Ratios (95% CI) | No. events | Risks (95% CI), % | Risk Differences (95% CI), % | Risk Ratios (95% CI) | |
| TAF/FTC | 923 | 4.3 (4.1, 4.6) | 0 | 1.00 | 157 | 0.8 (0.7, 1.0) | 0 | 1.00 | 17 | 0.09 (0.05,0.14) | 0 | 1.00 | 9 | 0.06 (0.02, 0.11) | 0 | 1.00 |
| TDF/FTC | 300 | 4.5 (3.9, 5.0) | 0.16 (−0.48, 0.69) | 1.04 (0.89, 1.17) | 35 | 0.5 (0.4, 0.7) | −0.28 (−0.52, −0.08) | 0.66 (0.43, 0.91) | 2 | 0.03 (0.01,0.08) | −0.07 (−0.12,−0.004) | 0.28 (0.11, 0.90) | 1 | 0.02 (0.02, 0.09) | −0.04 (−0.07, 0.03) | 0.37 (0.23, 1.90) |
| ABC/3TC | 687 | 5.2 (4.8, 5.6) | 0.89 (0.40, 1.34) | 1.21 (1.09, 1.33) | 147 | 1.1 (0.9, 1.2) | 0.24 (0.02, 0.45) | 1.29 (1.02, 1.58) | 18 | 0.13 (0.08,0.19) | 0.04 (−0.03,0.12) | 1.39 (0.70, 2.80) | 18 | 0.12 (0.07, 0.18) | 0.06 (−0.01, 0.12) | 2.02 (0.88, 6.12) |
| Other regimes | 492 | 4.6 (4.1, 5.0) | 0.24 (−0.27, 0.77) | 1.06 (0.94, 1.18) | 86 | 0.7 (0.5, 0.8) | −0.16 (−0.34,0.04) | 0.81 (0.62, 1.05) | 8 | 0.07 (0.02,0.12) | −0.02 (−0.09,0.04) | 0.76 (0.23, 1.77) | 9 | 0.06 (0.02, 0.11) | 0 (−0.06, 0.06) | 0.99 (0.34, 2.61) |
Adjusted via inverse probability weighting for age (in years, linear and quadratic terms), sex (male, female), transmission category (heterosexual, homo/bisexual, injecting drug use, other), country of origin (Spain, other), CD4 (<350, 350–500, >500 cells/mm3), and hypertension, diabetes, chronic renal disease, cardiovascular disease, and treatment with immunosuppressants or corticosteroids.
Compared with TAF/FTC, the 48-week risk difference of hospitalizations per 1000 persons was −2.8 (95% CI −5.2 to −0.8) for TDF/FTC (Table 2). That is, the estimated number needed to treat with TDF/FTC vs. TAF/FTC during the study period would be 357 (192 to 1250) to prevent one hospitalization. The estimates were similar in sensitivity analyses that censored at treatment switching (Appendix Table 3), that adjusted for confounding via standardization (Appendix Table 4) or a Cox model (Appendix Table 5), and that did not adjust for any covariates (Appendix Table 6). The risk of COVID-19 hospitalization was similar across the three classes of third drug (Appendix Table 7).
Compared with TAF/FTC, the estimated risk ratio (95% CI) of COVID-19 hospitalization for TDF/FTC was 0.49 (0.24, 0.81) in individuals aged ≥50 years and 1.15 (0.59, 1.93) in younger individuals (Table 3). The corresponding risk ratio was similar in men and women (Appendix Table 8).
Table 3.
Estimated 48-week risk, risk differences and risk ratios of COVID-19 outcomes by NRTI combination in HIV-positive individuals, stratified by age group, *CoVIHd Collaboration, Spain, February-December 2020
| Documented SARS-CoV-2 infection | Hospitalization due to COVID-19 | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. events | Risks (95% CI), % | Risk Differences (95%CI), % | Risk Ratios (95%CI) | No. events | Risks (95% CI), % | Risk Differences (95%CI), % | Risk Ratios (95%CI) | |
| <50 years | ||||||||
| TAF/FTC | 561 | 4.9 (4.5, 5.3) | 0 | 1.00 | 48 | 0.4 (0.3, 0.6) | 0 | 1.00 |
| TDF/FTC | 198 | 5.3 (4.6, 6.0) | 0.45 (−0.43, 1.25) | 1.09 (0.91, 1.27) | 18 | 0.5 (0.3, 0.8) | 0.06 (−0.21, 0.34) | 1.15 (0.59, 1.93) |
| ABC/3TC | 336 | 5.4 (4.8, 6.0) | 0.50 (−0.27, 1.21) | 1.10 (0.95, 1.26) | 29 | 0.5 (0.3, 0.6) | 0.02 (−0.22, 0.22) | 1.03 (0.59, 1.62) |
| Other regimes | 220 | 5.4 (4.7, 6.0) | 0.47 (−0.35, 1.23) | 1.10 (0.93, 1.26) | 17 | 0.4 (0.2, 0.6) | −0.04 (−0.26, 0.17) | 0.91 (0.49, 1.47) |
| ≥50 years | ||||||||
| TAF/FTC | 362 | 3.8 (3.4, 4.2) | 0 | 1.00 | 109 | 1.2 (1.0, 1.4) | 0 | 1.00 |
| TDF/FTC | 102 | 3.6 (2.9, 4.3) | −0.17 (−0.94, 0.64) | 0.96 (0.77, 1.18) | 17 | 0.6 (0.3, 0.9) | −0.61 (−1.03, −0.22) | 0.49 (0.24, 0.81) |
| ABC/3TC | 351 | 5.0 (4.6, 5.6) | 1.26 (0.62, 1.89) | 1.33 (1.15, 1.55) | 118 | 1.7 (1.4, 2.0) | 0.47 (0.11, 0.83) | 1.40 (1.08, 1.79) |
| Other regimes | 272 | 3.9 (3.4, 4.3) | 0.07 (−0.51, 0.64) | 1.02 (0.87, 1.18) | 69 | 0.9 (0.7, 1.1) | −0.29 (−0.62, 0.03) | 0.76 (0.54, 1.03) |
Adjusted via inverse probability weighting for age (in years, linear and quadratic terms), sex (male, female), transmission category (heterosexual, homo/bisexual, injecting drug use, other), country of origin (Spain, other), CD4 (<350, 350–500, >500 cells/mm3), and hypertension, diabetes, chronic renal disease, cardiovascular disease, and treatment with immunosuppressants or corticosteroids.
Compared with all NRTI combinations without TDF, the estimated risk ratios (95% CI) of COVID-19 hospitalization, ICU admission and death were 0.64 (0.42–0.89), 0.28 (0.11–0.84) and 0.29 (0.20–1.11), respectively, for TDF/FTC. In individuals aged ≥50 years, these risk ratios were 0.48 (0.24–0.76), 0.24 (0.18–0.88) and 0.22 (0.15–0.97).
Discussion
We studied over 50,000 HIV-positive individuals on antiretroviral therapy with adequate virological control in Spain during 2020, before the start of the SARS-CoV-2 vaccination campaign. The estimated risks of COVID-19 hospitalization and ICU admission were lower among individuals treated with TDF/FTC than among those treated with other antiretrovirals. The potential benefit of TDF/FTC appeared to be restricted to individuals over 50 years of age who have a higher risk of developing severe COVID-19. In this age group, the risk of COVID-19 hospitalization was about 50% lower for TDF/FTC compared with TAF/FTC, the most commonly used NRTI combination. The risk of death from COVID-19 was also lower for antiretroviral regimes based on TDF/FTC, but the estimates were very imprecise. In contrast, individuals on ABC/3TC had a higher risk of severe COVID-19 than those on other NRTI combinations. The estimated risks of documented infection and of mild infection are difficult to interpret because of incomplete ascertainment.
Our estimates are consistent with those from observational studies conducted in Spain (6,14,15), South Africa (7), and the United States (9). These studies, which preferentially included COVID-19 cases that were severe enough to be diagnosed, found a lower risk of severe COVID-19 among HIV-positive individuals on TDF/FTC compared with other NRTI combinations. The first study reported in Spain did not collect information on comorbidities, used reported population frequencies of antiretroviral use for non-cases (which resulted in a slight overestimation of the proportion of the population on TDF/FTC and an underestimation of the proportion on TAF/FTC), and did not restrict the analyses to persons with virological suppression (6). The present study improves upon it by including a large population of HIV-positive individuals with adequate antiretroviral control and adjusts for multiple comorbidities. Another study in Spain found a lower SARS-CoV-2 prevalence among TDF/FTC users than in TAF/FTC users (14).
A beneficial effect of TDF for prophylaxis or early treatment of COVID-19 is compatible with the results of two randomized trials in non-hospitalized patients. In France, a phase 2 trial in 60 outpatients with early COVID-19 found lower nasopharyngeal shedding of SARS-CoV-2 after initiation of TDF/FTC (16); in Spain and Latin America, the EPICOS trial could not rule out a beneficial effect of TDF/FTC as pre-exposure prophylaxis for COVID-19 among healthcare workers, but effect estimates were very imprecise because the target sample size was not met (17). In contrast, the PANCOVID trial in hospitalized patients with COVID-19 in Spain reported no beneficial effects of TDF/FTC compared with placebo (18). Note that temdesivir and molnupiravir also had minor or no effects in hospitalized patients (1–2,5), even though they prevented progression to severe COVID-19 in non-hospitalized patients (3,4). The EPICOS and PANCOVID trials were led by some of the authors of this report.
Our study has some limitations. First, residual confounding by yet to be identified factors cannot be excluded. However, such residual confounding seems unlikely because we adjusted for all known comorbidities that affect both antiretroviral treatment choice and COVID-19 severity and adjustment had little impact on our effect estimates. Therefore, the lowest risk of hospitalization in those receiving TDF/FTC cannot be easily explained by residual confounding. Second, we may have missed some mild (and asymptomatic) SARS-CoV-2 infections because of the lack of systematic testing. However, our main results concern severe outcomes (hospitalization, ICU admissions, and death) that are almost always detected by the health system This study does not allow to draw conclusions on SARS-CoV-2 infection. Third, missing data on comorbidities led to the exclusion of 22% of otherwise eligible individuals. However, estimates did not materially change in unadjusted analyses that included individuals with missing data on comorbidities. Fourth, even a large cohort like this one cannot provide precise estimates for the risks of infrequent events such as ICU admissions and deaths. Fifth, as in the vast majority of COVID-19 studies, data on exposure to the virus were not available. However, it is unlikely that persons on TDF/FTC were less likely to be exposed to SARS-CoV-2 than persons on other regimes.
A protective effect of TDF/FTC is biologically plausible. In silico studies suggest that all forms of tenofovir, like other nucleos(t)ide analogues, partly inhibit the SARS-CoV-2 RNA-dependent RNA-polymerase (RNAdRNAp) (19–21) and some, but not all, in vitro studies also suggest that tenofovir inhibits the RNAdRNAp (22–23). Because of the possible higher bioavailability of TDF than TAF in respiratory cells, TDF might result in greater inhibition of the SARS-CoV-2 RNApRNAp (24–28). In addition, tenofovir has been reported to have immunomodulatory effects (29–32) and animal models suggest that TDF/FTC increases nasopharyngeal SARS-CoV-2 clearance (33).
Compared with other drugs repurposed for COVID-19, TDF has several advantages. First, it has a solid safety track record in individuals with normal renal function (34–35), including pregnant women (36), and in fact is used routinely as pre-exposure prophylaxis for HIV infection. Second, it is administered orally and thus does not need to be administered in a healthcare facility. Third, it is an inexpensive generic drug that could be massively produced in many countries, including in settings with low COVID-19 vaccine coverage.
In summary, our findings suggest that treatment with TDF/FTC results in a lower severity of COVID-19 than treatment with other antiretrovirals among persons with HIV, especially those aged 50 years and older. A protective effect of TDF/FTC has clinical implications for persons with HIV, because TDF/FTC is an effective drug to control HIV infection in individuals without impaired renal function (37–38), and hepatitis B infection. A similar protection for HIV-negative individuals would be especially important for immunosuppressed patients for whom vaccines have suboptimal effectiveness. Confirmatory randomized trials of TDF/FTC for the prophylaxis and early treatment of COVID-19 are warranted.
Funding
This article is supported by grant R37AI102634 from the U.S. National Institutes of Health, and the Red Temática de Investigación Cooperativa en Sida (RD06/006, RD 12/0017/0018 and RD 16/0002/0006), grant COV20/01112 PROYECTOS DE INVESTIGACIÓN SOBRE EL SARS-COV-2 Y LA ENFERMEDAD COVID19 (Institute of Health Carlos III, Spain) and the Ministry of Health of Spain.
Role of funding source
The funding source had no role in the design of the study nor in its execution, analyses, interpretation of the data, or decision to submit results.
APPENDIX
Appendix Table 1.
Description of antiretroviral regimes in each NRTI combination in HIV-positive individuals, CoVIHd Collaboration, Spain, February-December 2020
| TAF/FTC N = 20,432 | TDF/FTC N = 6,160 | ABC/3TC N = 13,715 | Other N = 11,251 | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| BIC/TAF/FTC | 7,517 (37·1) | EFV/TDF/FTC | 2,186 (35·5) | DTG/ABC/3TC | 9,204 (67·1) | DTG/3TC | 3,832 (34·1) |
| EVG/COBI/TAF/FTC | 3,937 (19·4) | RPV/TDF/FTC | 1,340 (21·7) | NVP+ABC/3TC | 1,083 (7·9) | DTG/RPV | 1,747 (15·5) |
| RPV/TAF/FTC | 3,695 (18.2) | RAL+TDF/FTC | 692 (11.2) | RPV+ABC/3TC | 1,043 (7.6) | bDRV | 1,688 (15.0) |
| DRV/COBI/TAF/FTC | 3,210 (15.8) | bDRV+TDF/FTC | 624 (10.1) | RAL+ABC/3TC | 961 (7.0) | bDRV+3TC | 1,200 (10.7) |
| RAL+TAF/FTC | 744 (3.7) | DTG+TDF/FTC | 619 (10.0) | bDRV+ABC/3TC | 560 (4.1) | bDRV+DTG | 796 (7.1) |
| DTG+TAF/FTC | 682 (3.4) | NVP+TDF/FTC | 325 (5.3) | EFV+ABC/3TC | 554 (4.0) | bDRV+RAL | 520 (4.6) |
| NVP+TAF/FTC | 396 (1.9) | EVG/COBI/TDF/FTC | 140 (2.3) | ATVr+ABC/3TC | 108 (0.8) | bDRV+RPV | 221 (2.0) |
| EFV+TAF/FTC | 91 (0.4) | bATV+TDF/FTC | 86 (1.4) | ETV+ABC/3TC | 87 (0.6) | bDRV+ETV | 144 (1.3) |
| Other | 160 (0.8) | LPVr+TDF/FTC | 50 (0.8) | Other | 115 (0.8) | RAL+ETV | 143 (1.3) |
| ETV+TDF/FTC | 42 (0.7) | DTG | 136 (1.2) | ||||
| Other | 56 (0.9) | CAB+RPV | 126 (1.1) | ||||
| RAL+3TC | 112 (1.0) | ||||||
| Other | 586 (5.2) | ||||||
ABC, abacavir; BIC, Bictegravir; bATV, Atazanavir boosted; bDRV, Darunavir boosted; CAB, Cabotegravir; COBI, Cobicistat; DRV, Darunavir; DTG, Dolutegravir; EFV, Efavirenz; EVG, Elvitegravir; ETV, Etravirine; FTC, Emtricitabine; LPVr, Lopinavir/ritonavir; RAL, RaltegravirNVP, Nevirapine; RPV, Rilpivirine; TAF, Tenofovir alafenamide; 3TC, Lamivudine.
Appendix Table 2.
Estimated 48-week risks, risk differences and risk ratios of asymptomatic SARS-CoV-2 infection and mild COVID-19 by NRTI combination in HIV-positive individuals,* CoVIHd Collaboration, Spain, February-December 2020
| Asymptomatic SARS-CoV-2 infection | Mild COVID-19 | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. events | Risks (95% CI), % | Risk Differences (95%CI), % | Risk Ratios (95%CI) | No. events | Risks (95% CI), % | Risk Differences (95%CI), % | Risk Ratios (95%CI) | |
| TAF/FTC | 206 | 1.00 (0.87, 1.12) | 0 | 1.00 | 389 | 1.82 (1.64, 2.01) | 0 | 1.00 |
| TDF/FTC | 77 | 1.15 (0.91, 1.45) | 0.15 (−0.13, 0.44) | 1.15 (0.87, 1.46) | 155 | 2.21 (1.82, 2.58) | 0.39 (−0.07, 0.76) | 1.21 (0.97, 1.43) |
| ABC/3TC | 133 | 1.06 (0.89, 1.26) | 0.07 (−0.16, 0.29) | 1.07 (0.85, 1.31) | 274 | 2.16 (1.91, 2.42) | 0.34 (0.01, 0.65) | 1.19 (1.00, 1.38) |
| Other regimes | 123 | 1.16 (0.96, 1.38) | 0.17 (−0.06, 0.41) | 1.17 (0.95, 1.44) | 219 | 2.10 (1.84, 2.42) | 0.28 (−0.04, 0.64) | 1.15 (0.98, 1.37) |
Adjusted via inverse probability weighting for age (in years, linear and quadratic terms), sex (male, female), transmission category (heterosexual, homo/bisexual, injecting drug use, other), country of origin (Spain, other), CD4 (<350, 350–500, >500 cells/mm3), and hypertension, diabetes, chronic renal disease, cardiovascular disease, and treatment with immunosuppressants or corticosteroids.
Appendix Table 3.
Per-protocol analysis: Estimated 48-week risk differences and risk ratios of COVID-19 outcomes by NRTI combination in HIV-positive individuals,* CoVIHd Collaboration, Spain, February-December 2020
| Documented SARS-CoV-2 infection | Hospitalization due to COVID-19 | ICU admission due to COVID-19 | COVID-19 death | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Risk Differences (95% CI), % | Risk Ratios (95% CI) | Risk Differences (95% CI), % | Risk Ratios (95% CI) | Risk Differences (95% CI), % | Risk Ratios (95% CI) | Risk Differences (95% CI), % | Risk Ratios (95% CI) | |
| TAF/FTC | 0 | 1.00 | 0 | 1.00 | 0 | 1.00 | 0 | 1.00 |
| TDF/FTC | 0.10 (−0.57, 0.68) | 1.02 (0.88, 1.16) | −0.27 (−0.54, −0.04) | 0.68 (0.43, 0.95) | −0.06 (−0.12, 0.01) | 0.33 (0.12, 1.07) | −0.04 (−0.08, 0.03) | 0.37 (0.23, 1.93) |
| ABC/3TC | 0.87 (0.36, 1.37) | 1.19 (1.08, 1.32) | 0.22 (−0.003, 0.45) | 1.26 (1.00, 1.58) | 0.04 (−0.04, 0.13) | 1.44 (0.64, 2.94) | 0.04 (−0.03, 0.10) | 1.61 (0.68, 4.44) |
| Other regimes | 0.56 (0.04, 1.12) | 1.13 (1.01, 1.26) | −0.13 (−0.33, 0.07) | 0.85 (0.64, 1.10) | −0.01 (−0.09, 0.06) | 0.86 (0.25, 2.08) | 0.004 (−0.06,0.07) | 1.07 (0.36, 2.75) |
Adjusted via inverse probability weighting for age (in years, linear and quadratic terms), sex (male, female), transmission category (heterosexual, homo/bisexual, injecting drug use, other), country of origin (Spain, other), CD4 (<350, 350–500, >500 cells/mm3), and hypertension, diabetes, chronic renal disease, cardiovascular disease, and treatment with immunosuppressants or corticosteroids.
Appendix Table 4.
Standardized 48-week risk differences and risk ratios of COVID-19 outcomes by NRTI combination in HIV-positive individuals,* CoVIHd Collaboration, Spain, February-December 2020
| Documented SARS-CoV-2 infection | Hospitalization due to COVID-19 | ICU admission due to COVID-19 | COVID-19 death | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Risk Differences (95% CI), % | Risk Ratios (95% CI) | Risk Differences (95% CI), % | Risk Ratios (95% CI) | Risk Differences (95% CI), % | Risk Ratios (95% CI) | Risk Differences (95% CI), % | Risk Ratios (95% CI) | |
| TAF/FTC | 0 | 1.00 | 0 | 1.00 | 0 | 1.00 | 0 | 1.00 |
| TDF/FTC | 0.34 (−0.28, 0.89) | 1.08 (0.94, 1.21) | −0.25 (−0.52,0.003) | 0.73 (0.47, 1.00) | −0.06 (−0.12, 0.02) | 0.40 (0.15, 1.26) | −0.03 (−0.06, 0.04) | 0.45 (0.28, 2.14) |
| ABC/3TC | 0.87 (0.40, 1.32) | 1.20 (1.09, 1.32) | 0.28 (0.03, 0.49) | 1.30 (1.03, 1.61) | 0.05 (−0.03, 0.14) | 1.51 (0.77, 3.04) | 0.07 (0.01, 0.13) | 2.23 (1.10, 5.77) |
| Other regimes | 0.21 (−0.29, 0.72) | 1.05 (0.93, 1.17) | −0.15 (−0.35, 0.06) | 0.83 (0.64, 1.07) | −0.02 (−0.10, 0.05) | 0.75 (0.24, 1.75) | 0.003 (−0.04,0.06) | 1.06 (0.40, 2.65) |
Adjusted via standardization for age (in years, linear and quadratic terms), sex (male, female), transmission category (heterosexual, homo/bisexual, injecting drug use, other), country of origin (Spain, other), CD4 (<350, 350–500, >500 cells/mm3), and hypertension, diabetes, chronic renal disease, cardiovascular disease, and treatment with immunosuppressants or corticosteroids.
Appendix Table 5.
Estimated hazard ratios of COVID-19 outcomes by NRTI combination in HIV-positive individuals,* CoVIHd Collaboration, Spain, February-December 2020
| Documented SARS-CoV-2 infection | Hospitalization due to COVID-19 | ICU admission due to COVID-19 | COVID-19 death | |
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | ||||
| TAF/FTC | 1.00 | 1.00 | 1.00 | 1.00 |
| TDF/FTC | 1.08 (0.93, 1.21) | 0.73 (0.47, 1.00) | 0.40 (0, 1.26) | 0.44 (0, 1.98) |
| ABC/3TC | 1.21 (1.09, 1.33) | 1.31 (1.03, 1.62) | 1.51 (0.77, 3.18) | 2.25 (1.09, 5.60) |
| Other regimes | 1.05 (0.93, 1.18) | 0.83 (0.63, 1.07) | 0.75 (0.24, 1.83) | 1.06 (0.41, 2.69) |
Adjusted from Cox model with covariates age (in years, linear and quadratic terms), sex (male, female), transmission category (heterosexual, homo/bisexual, injecting drug use, other), country of origin (Spain, other), CD4 (<350, 350–500, >500 cells/mm3), and hypertension, diabetes, chronic renal disease, cardiovascular disease, and treatment with immunosuppressants or corticosteroids.
Appendix Table 6.
Unadjusted 48-week risk differences and risk ratios of COVID-19 outcomes by NRTI combination in HIV-positive individuals, CoVIHd Collaboration, Spain, February-December 2020
| Documented SARS-CoV-2 infection | Hospitalization due to COVID-19 | ICU admission due to COVID-19 | COVID-19 death | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Risk Differences (95% CI), % | Risk Ratios (95% CI) | Risk Differences (95% CI), % | Risk Ratios (95% CI) | Risk Differences (95% CI), % | Risk Ratios (95% CI) | Risk Differences (95% CI), % | Risk Ratios (95% CI) | |
| TAF/FTC | 0 | 1.00 | 0 | 1.00 | 0 | 1.00 | 0 | 1.00 |
| TDF/FTC | 0.35 (−0.29, 0.92) | 1.08 (0.94, 1.21) | −0.20 (−0.44, 0.01) | 0.74 (0.48, 1.01) | −0.05 (−0.10, 0.01) | 0.39 (0.14, 1.21) | −0.03 (−0.06, 0.02) | 0.37 (0.22, 1.71) |
| ABC/3TC | 0.49 (0.02, 0.95) | 1.11 (1.00, 1.22) | 0.30 (0.07, 0.50) | 1.39 (1.09, 1.73) | 0.05 (−0.02, 0.12) | 1.58 (0.83, 3.16) | 0.09 (0.03, 0.15) | 2.98 (1.48, 8.62) |
| Other regimes | −0.14 (−0.64, 0.37) | 0.97 (0.86, 1.08) | −0.0.004 (−0.20, 0.18) | 0.99 (0.77, 1.28) | −0.01 (−0.07, 0.05) | 0.85 (0.29, 1.81) | 0.04 (−0.02, 0.10) | 1.82 (0.66, 4.58) |
Appendix Table 7.
Estimated 48-week risks, risk differences and risk ratios of COVID-19 outcomes by third antiretroviral drug in HIV-positive individuals,* CoVIHd Collaboration, Spain, February-December 2020
| Documented SARS-CoV-2 infection | Hospitalization due to COVID-19 | ICU admission due to COVID-19 | COVID-19 death | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| No. events | Risks (95%CI), % | Risk Differences (95% CI), % | Risk Ratios (95% CI) | No. events | Risks (95%CI), % | Risk Differences (95% CI), % | Risk Ratios (95% CI) | No. events | Risks (95% CI), % | Risk Differences (95% CI), % | Risk Ratios (95% CI) | No. events | Risks (95% CI), % | Risk Differences (95% CI), % | Risk Ratios (95% CI) | |
| II | 1,140 | 4.6 (4.3, 4.9) | 0 | 1.00 | 214 | 0.8 (0.7, 1.0) | 0 | 1.00 | 23 | 0.08 (0.05,0.12) | 0 | 1.00 | 12 | 0.04 (0.02,0.07) | 0 | 1.00 |
| PI | 231 | 5.0 (4.2, 5.7) | 0.35 (−0.48,1.19) | 1.08 (0.90, 1.27) | 42 | 1.0 (0.6, 1.4) | 0.12 (−0.28,0.56) | 1.14 (0.70, 1.71) | 4 | 0.08 (0.02,0.17) | −0.007 (−0.07,0.08) | 0.92 (0.20, 2.22) | 4 | 0.15 (0.03,0.36) | 0.11 (−0.02,0.31) | 3.69 (0.62,10.77) |
| NNRTI | 533 | 5.2 (4.7, 5.7) | 0.62 (0.03, 1.21) | 1.13 (1.01, 1.28) | 83 | 0.9 (0.7, 1.1) | 0.04 (−0.20,0.29) | 1.04 (0.77, 1.37) | 10 | 0.16 (0.06,0.30) | 0.08 (−0.02,0.22) | 2.00 (0.74, 4.25) | 12 | 0.19 (0.09,0.32) | 0.15 (0.04, 0.28) | 4.47 (1.68,11.73) |
II: Integrase inhibitor, PI: protease inhibitor, NNRTI: non-nucleoside reverse-transcriptase inhibitor
Adjusted via inverse probability weighting for NRTI combination (TAF/FTC, TDF/FTC, ABC/3TC), age (in years, linear and quadratic terms), sex (male, female), transmission category (heterosexual, homo/bisexual, injecting drug use, other), country of origin (Spain, other), CD4 (<350, 350–500, >500 cells/mm3), and hypertension, diabetes, chronic renal disease, cardiovascular disease, and treatment with immunosuppressants or corticosteroids.
Appendix Table 8.
Estimated 48-week risks, risk differences and risk ratios of COVID-19 outcomes by NRTI combination in HIV-positive individuals, stratified by sex,* CoVIHd Collaboration, Spain, February-December 2020
| Documented SARS-CoV-2 infection | Hospitalization due to COVID-19 | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. events | Risks (95% CI), % | Risk Differences (95%CI), % | Risk Ratios (95%CI) | No. events | Risks (95% CI), % | Risk Differences (95%CI), % | Risk Ratios (95%CI) | |
| Men | ||||||||
| TAF/FTC | 734 | 4.3 (4.0, 4.6) | 0 | 1.00 | 126 | 0.8 (0.7, 1.0) | 0 | 1.00 |
| TDF/FTC | 228 | 4.3 (3.8, 4.9) | 0.08 (−0.51, 0.72) | 1.02 (0.88, 1.18) | 26 | 0.5 (0.3, 0.8) | −0.29 (−0.56, 0.01) | 0.64 (0.40, 1.01) |
| ABC/3TC | 554 | 5.3 (4.9, 5.8) | 1.08 (0.52, 1.60) | 1.25 (1.12, 1.39) | 123 | 1.1 (0.9, 1.3) | 0.30 (0.06, 0.55) | 1.36 (1.06, 1.75) |
| Other regimes | 385 | 4.7 (4.2, 5.1) | 0.40 (−0.15, 0.99) | 1.09 (0.97, 1.24) | 71 | 0.7 (0.6, 0.9) | −0.11 (−0.32, 0.13) | 0.86 (0.64, 1.18) |
| Women | ||||||||
| TAF/FTC | 189 | 4.6 (4.0, 5.3) | 0 | 1.00 | 31 | 0.8 (0.6, 1.2) | 0 | 1.00 |
| TDF/FTC | 72 | 5.0 (3.9, 6.2) | 0.45 (−0.86, 1.68) | 1.10 (0.83, 1.41) | 9 | 0.6 (0.2, 1.1) | −0.23 (−0.70, 0.27) | 0.73 (0.27, 1.45) |
| ABC/3TC | 133 | 4.8 (3.9, 5.6) | 0.19 (−0.83, 1.11) | 1.04 (0.83, 1.26) | 24 | 0.8 (0.5, 1.2) | 0.005 (−0.44, 0.40) | 1.01 (0.58, 1.61) |
| Other regimes | 107 | 4.2 (3.5, 5.0) | −0.33 (−1.34, 0.66) | 0.93 (0.72, 1.16) | 15 | 0.5 (0.3, 0.8) | −0.33 (−0.72, 0.06) | 0.61 (0.28, 1.10) |
Adjusted via inverse probability weighting for age (in years, linear and quadratic terms), transmission category (heterosexual, homo/bisexual, injecting drug use, other), country of origin (Spain, other), CD4 (<350, 350–500, >500 cells/mm3), and hypertension, diabetes, chronic renal disease, cardiovascular disease, and treatment with immunosuppressants or corticosteroids.
Appendix Figure 1. Estimated risks of asymptomatic SARS-CoV-2 infection and mild COVID-19 by NRTI combination in HIV-positive individuals,* CoVIHd Collaboration, Spain, February-December 2020.
* Adjusted via inverse probability weighting for age (in years, linear and quadratic terms), sex (male, female), transmission category (heterosexual, homo/bisexual, injecting drug use, other), country of origin (Spain, other), CD4 (<350, 350-500, >500 cells/mm3), and hypertension, diabetes, chronic renal disease, cardiovascular disease, and treatment with immunosuppressants or corticosteroids.
Appendix 1: Investigators of the CoVIHd Collaboration in Spain
Principal investigators (PI) and co-investigators of the participating hospitals by Spanish region
Andalucía
Hospital Virgen del Rocío: PIs: LF. López Cortés, N. Espinosa. Co-investigators: S. Llaves, C. Roca, M.Herrero, C.Sotomayor, MJ.Rodriguez Hernandez, JM.Cisneros.
Hospital Universitario Virgen de la Victoria: PIs: J. Santos, R.Palacios. Co-investigators: C. Gómez-Ayerve, I.Pérez-Hernández, A.Prolo, M.Villalobos, M.López-Jódar, I.Viciana.
Hospital Costa del Sol: PIs: J. Olalla, A del Arco Jiménez. Co-investigators: J Pérez Stachowski, JM García de Lomas, MD Martín Escalante, J García Alegría, MA Onieva, F Fernández Sánchez.
Hospital Virgen de las Nieves: PIs: C. Hidalgo Tenorio, J.Pasquau. Co-investigators: C. García, S. Sequera.
Hospital Universitario de Valme: PIs: J. Macías. Co-investigators: P. Rincón, M. Fernández.
Hospital Universitario Juan Ramón Jiménez: PIs: D. Merino Co-investigators: L. Corpa, M. Raffo, A.Jimenez, M.Franco,
Hospital Regional Universitario de Málaga: PIs: M.Castaño, M. Delgado.
Hospital Universitario Reina Sofía: PIs: A. Rivero, A. Rivero-Juarez.
Hospital Universitario Torrecárdenas: PIs: A. Collado.
Hospital Universitario de Puerto Real: PIs: A. Romero.
Aragón
Hospital Clínico Universitario Lozano Blesa: PIs: I. Sanjoaquín. Co-investigators: M. Gimeno, JM. Vinuesa, S. Letona, MJ. Crusells.
Hospital General San Jorge: PIs: M. Egido. Co-investigators: T. Omiste.
Hospital Miguel Servet: PIs: R. García, M. Forga
Canarias
Hospital Insular de Las Palmas: PIs: JL. Pérez-Arellano, C. Lavilla. Co-investigators: L. Suárez-Hormiga, L. López-Delgado, A. Granados, M. Hernandez-Cabrera, E. Pisos, N. Jaén.
Hospital Universitario de Canarias: PIs: JL Gómez Sirvent, MR.Alemán. Co-investigators: MM.Alonso, R.Pelazas, D.García Rosado, AM.López-Lirola, I. Hernández.
Cantabria
Hospital Universitario Marqués de Valdecilla: PIs: MC. Fariñas, F. Arnaiz de las Revillas
Co-investigators: C. González-Rico, A. Illaro; J. Calvo, ME. Cano, M. Gutierrez- Cuadra, C. Armiñanzas.
Castilla y León
Hospital Universitario de Burgos: PIs: C. Navarro-San Francisco. Co-investigators: M Fernandez Regueras
Hospital Clínico Universitario de Valladolid: PIs: C. Dueñas. Co-investigators: E. Tapia, S. Gutiérrez, G. Zapico, L. Rodríguez.
Hospital Río Hortega de Valladolid: PIs: J. Gómez. Co-investigators: M.Cobos, M. González, AM. Corcho, J. Abadía.
Hospital Universitario de León: PIs: JM. Guerra.
Complejo Asistencial Universitario de Palencia: PIs: JJ. Sánchez. Co-investigators: C. Sanchez, Y. Morán.
Complejo Asistencial Ntra. Sra. de Sonsoles de ávila: PIs: MA. Garcinuño. Co-investigators: C. Grande, AC. Antolí, M.Pedromingo,JM.Barragán-Casas.
Hospital General de Segovia: PIs: EM.Ferreira. Co-investigators: P.Bachiller, AM. Carrero, S.Muñóz, JM Alonso de los Santos.
Castilla La Mancha
Complejo Hospitalario de Toledo: PIs: F. Cuadra. Co-investigators: J. Largo, MA. Sepulveda.
Hospital General La Mancha Centro: PIs: JR. Barberá. Co-investigators: E. Arroyo.
Hospital Virgen de la Luz: PIs: P. Geijo
Hospital General Universitario de Albacete: PIs: E. Martínez.
Cataluña
Hospital Clínic Barcelona: PIs E. Martínez, JL. Blanco, E. de Lazzari. Co-investigators: JM.Miró, J.Mallolas, M.Laguno, M.Martínez-Rebollar, B.Torres, A.Iniciarte, A.González Cordón, L. de la Mora.
Hospital Universitari Vall d’Hebron: PIs: A. Curran, V. Falcó. Co-investigators: JN. García, J. Burgos, J. Navarro, P. Suanzes, M. Sanchiz, I. Rodriguez.
Hospital Universitari de Bellvitge: PIs: A. Imaz, D. Podzamczer. Co-investigators: S. Scévola, P. Prieto, A. Silva, M. Saumoy, J. Tiraboschi.
Hospital Santa María: PIs E. González. Co-investigators: N. Abdulghani, R. Sola, T. Comella, L. Gutierrez.
Hospital Universitari Mútua Terrassa: PIs: D. Dalmau. Co-investigators: M. Cairó, M. Martinez, R. Font, X.Martínez-Lacasa
Consorci Corporació Sanitària Parc Taulí de Sabadell: PIs G. Navarro. Co-investigators: S. Calzado, M. Navarro, B Lopez, MC Navarro.
Parc Sanitari Sant Joan de Déu: PIs: V. Diaz-Brito. Co-investigators: A. Delicado, M. Sanmartí, E. Moreno, F. Medina.
Hospital Universitari Joan XXIII de Tarragona: PIs: F. Vidal. Co-investigators: E. Yeregui, A. Marti, A. Rull, J. Peraire
Consorcio Sanitario del Maresme (Hospital de Mataró): PIs L. Force. Co-investigators: P. Barrufet, L.Arbones, L.Albiach.
Hospital Dr. Josep Trueta: PIs A. Oller. Co-investigators: X. Salgado, M. Lora.
Consorcio Hospitalario de Vic: PIs I. Vilaró. Co-investigators: MJ. Martínez.
Consorci Sanitari de Terrasa:PIs M. Aranda. Co-investigators: R. Solé, M. Roca.
Hospital de Viladecans: PIs A. Lérida. Co-investigators: M. Muelas, A. Figueras, L. Escolano, N.Carrasco Fons.
Hospital Sant Jaume de Calella: PIs O. del Río. Co-investigators: S. Valero.
Hospital de Mollet: PIs P. Vázquez. Co-investigators: JM. Tricas, M. Priegue.
Fundació Salut Empordà (Figueres): PIs J. Cucurull. Co-investigators: S. Vega, JA. Damián.
Hospital de Palamós: PIs: M.Hortos. Co-investigators: A. Masabeu
Hospital de Tortosa Verge de la Cinta: PIs: A. Orti. Co-investigators: E. Chamarro, C.Escrig
Hospital General de Granollers: PIs: E. Deig.
Comunidad de Madrid
Hospital Universitario LA PAZ- H. Carlos III: PIs: JR. Arribas, R.Montejano, J.Cadiñanos.
Co-investigators: C. Busca, R. Micán, R.de Miguel-Buckley, JI.Bernardino, M.Montes, J.González-García, L.Ramos, L.Martín-Carbonero.
Hospital Fundación Jiménez Díaz: PIs: A. Cabello, M.Górgolas, A.Herrero. Co-investigators: B.álvarez álvarez, L.Prieto Pérez, I.Carrillo Acosta, A.Al-Hayani, R.Fernández Doblas, R.Téllez, S.Heili-Frades, JM. Milicua.
Hospital Universitario Ramón y Cajal: PIs: S. Moreno, MJ. Pérez, S. del Campo. Co-investigators:, JA. Pérez, P. Vizcarra, J. Martínez Sanz, M. Sánchez Conde, MJ. Vivancos, AM. Moreno, S.Serrano Villar, JL.Casado.
Hospital General Universitario Gregorio Marañón: PIs: J Berenguer, JC. López. Co-investigators: T. Aldamiz.
Hospital 12 de Octubre: PIs: D. Rial, O. Bisbal. Co-investigators: F. Pulido, R. Rubio, M. de Lagarde, A. Pinto, R. Font, O. Arce.
Hospital Universitario de La Princesa: PIs: I. de los Santos, J. Sanz. Co-investigators: LJ. García Fraile, A. Espiño, A. Sancha, M. Ciudad, A. Bautista, A. Gutiérrez.
Hospital Infanta Leonor-Vallecas: PIs: P. Ryan, G.Cuevas. Co-investigators: J.Troya, M.Matarranz, M.Torres Macho, J.Pardo-Guimera, J.Valencia, E.Jiménez.
Hospital Universitario Fundación Alcorcón: PIs:M. Velasco. Co-investigators: L. Moreno, R. Hervás, O. Martín, JE. Losa.
Hospital Universitario Príncipe de Asturias: PIs: J. Sanz Moreno. Co-investigators: M. Novella, C. Hernández, JA. Arranz, V Sampériz.
Hospital Universitario Rey Juan Carlos: PIs: S. Nistal.
Hospital General de Villalba: PIs: J. Hernández.
Hospital Universitario Infanta Elena: PIs: M. Rubio. Co-investigators A. Jiménez.
Hospital Quirón Salud: PIs: D.Carnevali. Co-investigators: J. Aguareles, A. Roda, P Guisado.
Hospital Universitario Infanta Sofía: PIs: I. Suárez.
Hospital Clínico San Carlos: PIs: J. Vergas, V. Estrada.
Hospital Puerta de Hierro: PIs: A. Díaz DE Santiago, S. de la Fuente.
Hospital Severo Ochoa-Leganés: PIs: M. Cervero
Hospital de Getafe: PIs: Sergio Rodriguez
Comunidad Foral de Navarra
Complejo Hospitalario de Navarra: PIs: M. Rivero, E.Moreno. Co-investigators: A. Arrondo, M Gracia, C. Ibero, J. Repáraz
Comunidad Valenciana
Hospital Clínico Universitario de Valencia: PIs: MJ. Galindo, R. Ferrando. Co-investigators: AI. De Gracia, M. García, N. Gómez, M. Martínez.
Hospital La Fé: PIs: M. Tasias, M. Montero. Co-investigators: M. Salavert, S. Cuéllar, E. Calbuig, M. Blanes, J. Fernández.
Hospital General Universitario de Valencia: PIs: M García Deltoro, N Gómez Muñoz. Co-investigators: M Martínez Roma, M García Rodríguez, C Ricart Olmos, JI Gutiérrez Salcedo, JI Mateo González, V Abril López de Medrano.
Hospital General Universitario de Elche: PIs: F. Gutiérrez. Co-investigators: M. Masiá, S. Padilla, J. García, C. Robledano.
Hospital Marina Baixa: PIs: C. Amador
Hospital Universitario de la Plana: PIs: I. Pedrola. Co-investigators: A. Blasco L. Fandos, M. Arnal.
Hospital General Universitario de Alicante: PI: X. Portilla.
Hospital Arnau de Vilanova-Lliria: PIs: J. Flores, C. Tortaiada.
Hospital Peset: M. Madrazo, A. Artero.
Galicia
Hospital álvaro Cunqueiro: PIs: G. Pousada, A. Ocampo. Co-investigators: M. Crespo, A. Pérez, C.Miralles.
Complejo Hospitalario Universitario de Ferrol: PIs: A. Mariño. Co-investigators: N. Valcarce, H.Alvarez, L. Vilariño, JF. García.
Hospital de Santiago de Compostela: A. Antela, E. Losada.
Islas Baleares
Hospital Universitari Son Espases: M. Riera, F. Alberti. Co-investigators: AM. Santos.
La Rioja
Hospital San Pedro – CIBIR: PIs: JR. Blanco. Co-investigators: V. Ibarra, J. Gallardo, JA. Oteo, L. Pérez.
País Vasco
Hospital de Donostia: PIs: JA. Iribarren MA, Von Wichmann. Co-investigators: X.Camino, MJ.Bustinduy, H.Azkune, M.Ibarguren, MA.Goenaga, I.Alvarez Rodriguez.
Hospital de Basurto: PIs: M.de la Peña, I Lombide. Co-investigators: M.López, J. de Miguel, V.Polo, S.Ibarra, OI.Ferrero, J.Muñóz.
Hospital Universitario Araba: PIs: JJ. Portu.
Hospital Osi Barakaldo-Sestao: PIs: R. Silvariño.
Hospital Universitario de Cruces: PIs: J. Goikoetxea, E.Bereciartua.
Principado de Asturias
Hospital Universitario Central de Asturias: PIs: V. Asensi, M. Rivas-Carmenado.
Murcia
Hospital Clínico Universitario Virgen de la Arrixaca: PIs: C. Galera. Co-investigators: M. Fernandez, H. Albendín, A.Castillo, MA.Merlos.
Hospital General Universitario Reina Sofía: PIs: E. Bernal. Co-investigat
Appendix 2: Definition of comorbidities
High blood pressure defined as systolic and diastolic values 130 and 85–89
Diabetes Mellitus defined as
Fasting blood glucose ≥126 mg / dl or,
Blood glucose ≥200 mg / dl after oral glucose resistance test (2h) or,
HbA1c ≥6.5%
Chronic kidney disease defined as:
eGFR ≤ 60 mL / min for ≥ 3 months or,
Albumin / creatinine ratio> 100 mg / mmol.
Chronic cardiovascular disease defined as the presence of any of the following in the clinical history: ischemic heart disease (myocardial infarction, angina pectoris), cardiopulmonary disease, arrhythmias and heart failure, cerebrovascular disease (hemorrhage, stroke, embolism, thrombosis, cerebral apoplexy or stroke), iseases of the arteries (atherosclerosis, aneurysm, embolism and arterial thrombosis).
Immune disorder (other than HIV infection) defined as the presence of any immunosuppressant/corticosteroid therapy in the clinical history.
References
- 1.WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med 2021; 384:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 2020;383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med 2022; 386:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernal AJ, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med 2022;386:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arribas JR, Bhagani S, Lobo SM, Khaertynova I, Mateu L, et al. Randomized Trial of Molnupiravir or Placebo in Patients Hospitalized with Covid-19.NEJM Evidence 2021. DOI: 10.1056/EVIDoa2100044 [DOI] [PubMed] [Google Scholar]
- 6.Del Amo J, Polo R, Moreno S, Díaz A, Martínez E, Arribas JR, et al. Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy: A Cohort Study. Ann Intern Med 2020; 173:536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulle A, Davies M-A, Hussey H, Ismail M, Morden E, Vundle Z, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis 2021;73: e2005–e2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muñoz-Mateos B, Buti M, Fernandez I, Hernández M, Bernal V, Diaz F, et al. Tenofovir reduces the severity of COVID-19 infection in chronic hepatitis B patients. J Hepatol 2021;75: S746–7 [Google Scholar]
- 9.Li G, Park LS, Lodi S, Logan RW, Cartwright EJ, Aoun-Barakat L, Casas Romero JP, et al. Tenofovir disoproxil fumarate and COVID-19 outcomes in men with HIV. AIDS 2022. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeJong C, Spinelli MA, Okochi H, Gandhi M. Tenofovir-based PrEP for COVID-19: an untapped opportunity? AIDS 2021;35:1509–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Amo J, Polo R, Moreno S, Jarrín I, Hernán MA. SARS-CoV-2 infection and coronavirus disease 2019 severity in persons with HIV on antiretroviral treatment. AIDS 2022; 36:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centro Nacional de Epidemiología, Instituto de Salud Carlos III, Plan Nacional sobre el Sida, Dirección General de Salud Pública. Unidad de vigilancia del VIH, ITS y hepatitis. Actualización del Continuo de Atención del VIH en España, 2017–2019 [Internet]. 2020. Available from: https://www.mscbs.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/home.htm
- 13.Ministerio de Sanidad. Interpretación de las pruebas diagnósticas frente a la SARS-CoV-2 [Internet]. 2020. Available from: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/INTERPRETACION_DE_LAS_PRUEBAS.pdf
- 14.Berenguer J, Díez C, Martín-Vicente M, Micán R, Pérez-Elías MJ, García-Fraile LJ, et al. Prevalence and factors associated with SARS-CoV-2 seropositivity in the Spanish HIV Research Network Cohort. Clin Microbiol Infect 2021; 27:1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nomah DK, Reyes-Urueña J, Díaz Y, Moreno S, Aceiton J, Bruguera A, et al. Impact of tenofovir on SARS-CoV-2 infection and severe outcomes among people living with HIV: a propensity score-matched study. J Antimicrob Chemother 2022. Jun 9;dkac177. doi: 10.1093/jac/dkac177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parienti JJ, Prazuck T, Peyro-Saint-Paul L, Fournier A, Valentin C, Brucato S, et al. Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial. EClinicalMedicine 2021; 38:100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polo R, García-Albéniz X, Terán C, Morales M, Rial-Crestelo D, Garcinuño M, et al. Daily tenofovir disoproxil fumarate/emtricitabine and hydroxychloroquine for pre-exposure prophylaxis of COVID-19: a double-blind placebo controlled randomized trial in healthcare workers. Clin Microbiol Infect 2022. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velasco M, Montejano R, Sierra G, et al. for the PANCOVID Study Group. TDF/FTC for high-risk patients with COVID-19: The PANCOVID Randomized Clinical Trial. 29th Conference on Retroviruses and Opportunistic Infections, February 2022. Abstract 460. [Google Scholar]
- 19.Elfiky AA. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci 2020;253:117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanella I, Zizioli D, Castelli F, Quiros-Roldan E. Tenofovir, Another Inexpensive, Well-Known and Widely Available Old Drug Repurposed for SARS-COV-2 Infection. Pharmaceuticals 2021;14(5):454.34064831 [Google Scholar]
- 21.Copertino DCJ, Casado Lima BC, Duarte RRR, Powell TR, Ormsby CE, Wilkin T, et al. Antiretroviral drug activity and potential for pre-exposure prophylaxis against COVID-19 and HIV infection. J Biomol Struct Dyn 2021;1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clososki GC, Soldi RA, Silva RM da, Guaratini T, Lopes JNC, Pereira PRR, et al. Tenofovir Disoproxil Fumarate: New Chemical Developments and Encouraging in vitro Biological Results for SARS-CoV-2. J Braz Chem Soc 2020; 31:1552–6. [Google Scholar]
- 23.Chien M, Anderson TK, Jockusch S, Tao C, Li X, Kumar S, et al. Nucleotide Analogues as Inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID-19. J Proteome Res 2020; 19:4690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seifert SM, Chen X, Meditz AL, Castillo-Mancilla JR, Gardner EM, Predhomme JA, et al. Intracellular Tenofovir and Emtricitabine Anabolites in Genital, Rectal, and Blood Compartments from First Dose to Steady State. AIDS Res Hum Retroviruses 2016; 32:981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med 2011; 3:112re4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cottrell ML, Garrett KL, Prince HMA, Sykes C, Schauer A, Emerson CW, et al. Single-dose pharmacokinetics of tenofovir alafenamide and its active metabolite in the mucosal tissues. J Antimicrob Chemother 2017; 72:1731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Mascio M, Srinivasula S, Bhattacharjee A, Cheng L, Martiniova L, Herscovitch P, et al. Antiretroviral tissue kinetics: in vivo imaging using positron emission tomography. Antimicrob Agents Chemother 2009; 53:4086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Twigg HL, Schnizlein-Bick CT, Weiden M, Valentine F, Wheat J, Day RB, et al. Measurement of antiretroviral drugs in the lungs of HIV-infected patients. HIV Ther 2010; 4:247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melchjorsen J, Risør MW, Søgaard OS, O’Loughlin KL, Chow S, Paludan SR, et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. J Acquir Immune Defic Syndr 2011; 57:265–75. [DOI] [PubMed] [Google Scholar]
- 30.Zídek Z, Franková D, Holý A. Activation by 9-(R)-[2-(phosphonomethoxy) propyl]adenine of chemokine (RANTES, macrophage inflammatory protein 1alpha) and cytokine (tumor necrosis factor alpha, interleukin-10 [IL-10], IL-1beta) production. Antimicrob Agents Chemother 2001;45:3381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zídek Z, Potmesil P, Kmoníèková E, Holý A. Immunobiological activity of N-[2-(phosphonomethoxy)alkyl] derivatives of N6-substituted adenines, and 2,6-diaminopurines. Eur J Pharmacol 2003; 475:149–59. [DOI] [PubMed] [Google Scholar]
- 32.Kostecká P, Holý A, Farghali H, Zídek Z, Kmoníčková E. Differential effects of acyclic nucleoside phosphonates on nitric oxide and cytokines in rat hepatocytes and macrophages. Int Immunopharmacol 2012; 12:342–9. [DOI] [PubMed] [Google Scholar]
- 33.Park SJ, Yu KM, Kim YI, Kim SM, Kim EH, Kim SG, et al. Antiviral Efficacies of FDA-Approved Drugs against SARS-CoV-2 Infection in Ferrets. mBio 2020;11: e01114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pilkington V, Hill A, Hughes S, Nwokolo N, Pozniak A. How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP. J Virus Erad 2018;4(4):215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palacios R, Hidalgo C, Ríos MJ, Rivero A, Muñoz L, Lozano F, et al. Effectiveness and safety of simplification from tenofovir-lamivudine (TDF-3TC) to tenofovir-emtricitabine (TDF-FTC) co-formulation (Truvada) in virologically suppressed HIV-infected patients on HAART. Eur J Clin Microbiol Infect Dis 2009;28(4):399–402. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez-Diaz S, Bateman BT, Straub L, Zhu Y, Mogun H, Fischer M, et al. Safety of Tenofovir Disoproxil Fumarate (TDF) for Pregnant Women facing the COVID-19 Pandemic. Am J Epidemiol 2021; 190:2339–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, et al. Intramuscular AZD7442 (Tixagevimab– Cilgavimab) for Prevention of Covid-19. N Engl J Med 2022; 386:2188–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotton CN. Belt and Suspenders: Vaccines and Tixagevimab/Cilgavimab for Prevention of COVID-19 in Immunocompromised Patients. Ann Intern Med 2022; 175:892–894 [DOI] [PMC free article] [PubMed] [Google Scholar]