Introduction
Although we often think of cells as small, simple building blocks of life, in fact cells are highly complex and can perform a startling variety of functions. In our bodies, cells are programmed by complex differentiation pathways, and are capable of responding to a bewildering range of chemical and physical signals. Free living single-celled organisms, such as bacteria or protists, have to cope with varying environments, locate prey and potential mates, escape from predators – all of the same tasks that a free living animal is faced with. When animals face complex behavioral challenges, they rely on their cognitive abilities – the ability to learn from experience, to analyze a situation and choose an appropriate course of action. This ability is essential for survival, and should, in principle, be a ubiquitous feature of all living things regardless of the complexity of organism.
What is learning?
Learning is an aspect of thought, and hence before we can explore whether cells can learn, we need to confront the question of whether it is appropriate to use cognitive concepts when talking about single cells. The definition of thought and cognition has preoccupied philosophers for centuries, and has continued to do so. Rather than engaging with this debate, the approach we take in this Primer is that a cell is considered “thinking” if it acts like it is thinking, that is, its behavior can be predicted by invoking a thought-like process. Daniel Dennett referred to this approach as “the intentional stance”, whereby we drop the question of thought and focus on intentionality, and grant an entity intentionality if its behavior can be usefully predicted by assuming it “knows what it wants to do”. For example, instead of asking if I know my cat is hungry, and I open a can of food, I can predict its subsequent behavior with 100% accuracy if I view it as an intentional system, whereas if I view it as a huge collection of neurons whose interactions need to be understood on a physical level, current understanding of neurobiology does not allow me to predict what it will do when it hears the can-opener. The intentional stance allows the prediction of behavior in a far more parsimonious way, and on this basis we have chosen this purely operational approach for dealing with animal and cell behavior.
A hallmark of thought, or cognition, is learning, by which the behavior of a system is influenced by prior experience. We will say that a cell is able to learn if it is capable of performing one or more observable behaviors, and the probability of performing one behavior versus another changes as a result of past experiences. Many types of learning have been identified and distinguished by experimental psychologists, for example habituation and classical conditioning. In this primer we will explore what is known about the ability of single cells to manifest learning, defined in purely operational terms.
Habituation in cells
The simplest form of learning, observed in essentially all animals in which it has been tested, is habituation. Habituation refers to a decrease in the probability of a response to a stimulus following repeated applications of the stimulus. The classic example of habituation is the gill withdrawal reflex of the sea slug. The sea slug’s gill is a delicate structure that can be easily damaged by predators. When something touches the siphon, the slug will pull in its gill to keep its safe. But if you touch the siphon again and again, the slug will learn to ignore it. Much the same phenomenon occurs in human beings who live near train tracks – a loud train going by no longer creates a startle response once one “gets used to it”. Habituation is considered one of the most primitive and ubiquitous forms of learning. As such, if cells can learn at all, we would expect them to at least show this type of learning.
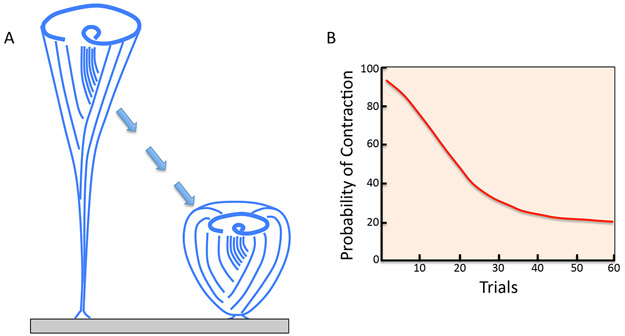
An unusually clear example of habituation in cells is provided by Stentor coeruleus, a giant ciliate that can be up to 1 mm long that lives in freshwater ponds. Stentor is a cone-shaped filter feeder that attaches to pond plants by its holdfast and then stretches out as it sucks algae and bacteria in through its oral apparatus. Stentor is so big that fish will try to eat it, and so it has evolved an escape mechanism whereby when anything touches the cell, it rapidly contracts into a ball (Figure 1A). This movement takes the bulk of cell body away from the line of attack, providing an effective defense. Once contracted, the cell needs to re-extend in order to feed. This whole process is energetically costly, hence a cell only wants to contract if it is really under attack. The problem is, a pond can be full of algae and other non-threatening objects that might casually bump into Stentor. How can it tell a true threat from a routine contact with a nearby plant, for example? The answer is habituation – when a Stentor cell is touched repeatedly with a constant level of force, for example if it was bumped again and again by an algal filament, it becomes less and less likely to contract (Figure 1B). This decreasing probability of response is the hallmark of habituation. A strong stimulus still triggers a full-speed contraction, arguing that the cell isn’t just getting tired (“motor fatigue”). Careful quantitative studies by Wood have shown that Stentor contraction displays many features of habituation seen in animals, most notably the fact that habituation takes place more rapidly for weaker stimuli than for strong stimuli. Once habituated, a Stentor cell retains its memory for at least several hours, but the biochemical basis of this memory remains unknown.
Figure 1. Habituation in Stentor coeruleus.
(A) Stentor cells stretch out into a cone shape to feed, using the cilia of their oral apparatus, at the end of the cone, to suck in food. While feeding, the cell is attached by its posterior holdfast to a substrate such as the leaf of a pond plant. When startled by a mechanical stimulation, the cell rapidly contracts towards its point of attachment, moving it away from a potential threat. (B) When a Stentor cell is tapped periodically with a uniform mechanical stimulus, it becomes less and less likely to contract, indicating that habituation has taken place.
A related species of Stentor, Stentor roeselii, has a more complex series of avoidance behavior in response to chemical stimuli such as carmine particles or mechanical poking, which have been extensively studied by Jennings (1902). In the first stage, the cell twists and bends, in apparently random direction, with the effect that the oral region is often steered away from the stimulus. In the second stage, if the stimulus persists, the cell switches from the bending response to a new response in which the direction of ciliary beating is transiently reversed, so as to blow away anything near the oral region. This reversal of beating occurs several times if the stimulus remains present. In the third stage, if stimulation continues, the cell contracts, just like Stentor coeruleus does. This sequence of three stages of responses is interesting as it speaks to the ability of a single cell to generate a nontrivial sequential behavior, suggesting that the cell can transition between at least three distinct behavioral states. What happens to these three responses if the stimulus is repeated? According to Jennings, the outcome differs between harmless mechanical stimuli and noxious chemical stimuli. For mechanical contact with a sufficiently small magnitude, the contraction response habituates, as in Stentor coeruleus, but the bending response does not, so that when touched lightly the cell continues to try to bend away, but does not contract. For toxic chemical stimuli, if the stimulus is repeated again and again, the cell stops the bending response and immediately triggers the contraction. In this case, it appears that the bending response has habituated and the contraction response has sensitized. This switching between behavioral modes in response to repeated application of different types of stimuli suggests a level of complex decision making that is normally associated with complex nervous systems, but it is important to remember that Stentor roeselii is still just a single cell!
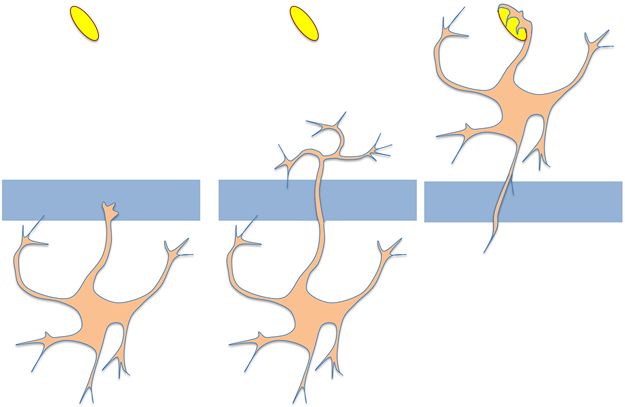
Habituation has also been demonstrated in the giant amoeba Physarum. A Physarum cell is a syncytium that can grow to the size of a dinner plate. Physarum moves over a surface in search of food, for example flakes of oatmeal. Physarum is repelled by quinine, and will tend not to move through regions of a Petri dish soaked in quinine. However, if a Physarum cell is separated from a food source by a strip of quinine-soaked agar, it will eventually take the chance and move across (Figure 2). If this experiment is repeated day after day, it is observed that the Physarum cell becomes increasingly likely to move across the quinine-soaked agar. This again reflects a type of habituation in that the cell has “learned” to ignore the chemically noxious region. Habituation is specific to the particular stimulus – if quinine-trained Physarum are moved to a new situation in which the agar bridge is soaked in caffeine, another noxious chemical, the habituated cell does not show any tendency to cross the caffeine bridge. The converse result is seen in cells habituated to cross a caffeine bridge – they learn to ignore the caffeine but still avoid quinine. This stimulus-specificity is important because it rules out motor fatigue as an alternative explanation. What makes the Physarum case even more remarkable is the fact that learning can be transferred by cell-cell fusion. Physarum cells will spontaneously fuse in culture, and when a habituated cell is fused to a fresh, untrained cell, it can confer the habituated memory to the resulting larger fused cell. It was observed that the pseudopod first crossing the aversive region was just as likely to come from the originally unhabituated cell as from the habituated one, suggesting that the “memory” had spread throughout the fused cell. Such experiments suggest that the basis for the memory might lie in a diffusible molecule of some sort as opposed to, for example, a membrane bound receptor, but ultimately this question depends on the relative timescales of memory transfer versus molecular diffusion. It is worth noting that in these experiments, the cell was “rewarded” with food for crossing the noxious patch, thus raising the formal possibility that the learning seen was actually a form of associative learning in which the cell learned to interpret the noxious chemical as signaling the presence of food. However there is no evidence for this and it seems that habituation is a far more parsimonious interpretation.
Figure 2. Habituation in Physarum.
The amoeba starts out on an agar petri dish in which one part of the plate contains a source of food (symbolized by the yellow oval to represent an oat flake, although in the actual experiment the oats were blended with agar and poured into the plate). As shown on the left, the cell was initially placed in a region of the dish separated from the food by a sector of agar containing a noxious chemical such as quinine or caffeine, symbolized by the gray rectangle. Physarum tends not to enter such regions, but eventually will do so, after which it finds the food patch and migrates to that part of the plate. If the cell is then taken off the dish and the entire experiment repeated, it will gradually take less time to move across the noxious patch and find the food.
Other forms of non-associative learning
Another type of learning observed in single cells is analogous to the “runway” learning paradigm in animals. In a runway experiment, an animal is placed at one end of a long hallway and has to run to the other end to either escape from the runway or to obtain a food reward. Learning in a runway model is measured by how fast the animal gets to the end after repeating the experiment multiple times. A unicellular version of the runway experiment was done with Stentor by placing single Stentor cells in a capillary tube and timing how long it takes the cells to escape from the tube. It has been reported that cells can escape from the tube more quickly following repeated training than cells that were first placed in the tube. This type of escape learning is not restricted to Stentor, but has also been reported in Paramecium. Subsequent studies showed the escape response, at least for Stentor, only becomes faster if the tube is vertically oriented. This would certainly constitute a form of learning, but here the big question is relevance – when does a cell need to escape from a vertically oriented tube? Such questions point to a need for further studies of protist behavior in their natural habitat in order to be able to interpret some of these laboratory experiments.
A final example of non-associative learning by single cells is the demonstration that Physarum can anticipate periodic stimuli. The speed of movement of a Physarum amoeba varies as a function of environmental conditions, with warm moist conditions favoring movement compared with cold dry conditions. When a moving cell is suddenly exposed to colder, drier air, it slows down, and then speeds up again when the previous warm moist air is restored. When Physarum cells were exposed to periodic bouts of cold dry air once per hour, the cells slowed down during each cold pulse, and then sped up again after normal conditions were restored. After three rounds, the cells were then kept at constant warm, moist conditions. Despite the fact that conditions were no longer varying, almost half of all cells slowed down again one hour after the last exposure, and some showed a second or even a third slowdown at regular intervals of an hour. The same effect was obtained using different periods of stimulation, again producing one or more slowdown events at regular intervals corresponding to the interval of the stimulation. These results show that a single cell can learn and anticipate when a stimulus is expected based on periodic exposures. It is worth noting that this behavior differs from the circadian cycle not only in its much shorter period but more important in the fact that cell movement was not periodic prior to the stimulation, so it isn’t simply that the period stimulation entrains a free running cellular oscillator. Somehow, the cell is remembering a period, and then using that memory to control its future behavior. This fits the definition of learning as the alteration of future decisions based on past experience.
Associative learning by cells
Habituation is a type of learning, but it is considered the simplest type of learning in that it only relates to a single stimulus. More advanced types of learning are associative in nature, meaning that they involve the linkage of two or more stimuli. The most well known example is “classical conditioning” as exemplified by Pavlov’s dogs. Classical conditioning involves a behavior or response, such as salivation in dogs, and two stimuli, one of which (the smell of food) naturally creates a given response, and the other of which (hearing a bell) normally does not. By repeated application of both stimuli together, an animal will, over time, generate the response in the presence of the conditioned stimulus. Classical conditioning shows that an animal can learn to relate two stimuli to each other, hence the name associative learning.
Classical conditioning has been reported in the ciliate Paramecium. Paramecium cells swim using an array of cilia, and can reverse the direction of the ciliary motion to escape from harmful stimuli such as electrical shocks. Sound waves such as musical tones generated by a speaker do not normally generate the escape response. lt has been reported that when Paramecium cells were exposed to electric shock immediately following a 500 Hz tone, after three or more application training cycles, the cells would begin to trigger their escape movement in response to the tone alone. This striking result, published in 1972, does not seem to have been further explored.
Beyond protists
Most studies of learning in cells have been conducted in free-living single-celled organisms (protists), primarily for the reason that the large size and dramatic behaviors of such cells lend themselves to observational studies of learning. Compared with mammalian cells in culture, the rapid responses of protists to stimuli make experiments much easier and allow short-term learning effects to be analyzed. Moreover, protists must survive in a highly variable world full of predators and prey, a far more complex and unpredictable environment than the well-regulated milieu of the body. To do so, they will need to be able to sense and respond to a wide range of stimuli and inputs, and adapt to widely varying local conditions. It is thus not surprising that protists would be under evolutionary pressure to learn and adapt. But what about in metazoans? Is learning a general property of all cells, even in a multicellular context?
Animal behavior and learning is driven by the nervous system, a large and complex array of cells that communicate with each other. Nervous systems, as their name implies, are inherently multicellular objects. However, if one drills down deep enough, we find that in many cases it is still individual cells that are doing the actual learning. The classical paradigm for memory, long term potentiation (LTP), takes place in individual cells. The best studied example of animal habituation, the gill withdrawal reflect of Aplysia, can ultimately be traced back to changes in the response characteristics of individual neurons. There is thus a case to be made that even in a highly connected nervous system, learning is taking place in individual cells.
Besides the nervous system, the other obvious place to look for learning is in the immune system. An experiment to look for classical conditioning in macrophages exploited the fact that bacterial lipopolysaccharide (LPS), an indicator of bacterial infection, triggers the release of interleukin 6 (IL-6). Macrophages in culture were treated periodically with LPS along with streptomycin, a chemical that normally does not trigger IL-6 release, and then the ability of streptomycin to trigger IL-6 release by itself was tested. In these experiments, no evidence for associative learning was found. It has, however, been shown that macrophages show habituation to LPS, so even though associative learning has not been seen, clearly non-associative learning, in the form of habituation, occurs within immune cells.
How do cells learn?
The experimental observations of learning in cells raises the obvious question of mechanism. In a computer, the substrate of computation is a logic gate, while in multicellular brain, the substrate of computation is the neuron, but what about inside a single cell? Many proteins are regulated by multiple inputs, allowing them to act as logic gates. This is also true for promotors that are controlled by multiple activators and repressors. The complexity of signaling networks within a cell is staggering, and it is fair to say that we are still coming to grips with the computational capacity of such networks. Gene networks can emulate both logic gates and computational neural networks, and hence can, at least in principle, produce a very wide range of computations and learning abilities. For example, theoretical studies have revealed gene regulatory networks that are capable of associative learning.
A major challenge is how to bridge the gap in scales between molecular implementations of logic gates on the one hand, and behavior of whole cells on the other. One strategy is to apply molecular genetic methods to cells as they learn and behave, with the goal of identifying genes required for a particular form of learning or behavior. The other strategy is to identify computationally interesting molecular networks and find out how they are used in the context of cell behavior. In computer science, the same problem of a gap in scales exists in that it is almost impossible to jump from a gate-level description of a computer to an understanding of the computation it is performing. In computer science, the solution has been to interpose additional levels of description. For example, in many cases, a computation can be described in abstract terms such as a finite state machine, in which the computer is represented as a set of possible states and rules for transitioning between them. This same paradigm can be applied to cells, provided we have ways to classify cell states. Traditional molecular descriptors of cell state, such as RNA sequencing, are inherently destructive, meaning that it isn’t possible to determine the state of a cell without killing it, thus preventing us from directly observing state transitions. However, microscopy-based methods for inferring cell state are now becoming available, allowing state transitions to be recorded. It will be of great interest to see how such approaches can inform our understanding of learning and problem solving in cells.
Where can I find out more?
- Dennett D 1981. True believers: the intentional strategy and why it works. in “Scientific Explanation”, Heath AF, ed. Oxford University Press, Oxford. [Google Scholar]
- Thompson RF, Spencer WA. 1966. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psych. Rev 73, 16–43. [DOI] [PubMed] [Google Scholar]
- Wood DC. 1970. Parametric studies of the response decrement produced by mechanical stimuli in the protozoan, Stentor coeruleus. J. Neurobiol 1, 345–60. [DOI] [PubMed] [Google Scholar]
- Jennings HS. 1902. Studies on reactions to stimuli in unicellular organisms. IX. On the behavior of fixed infusoria (Stentor and Vorticella) with special reference to the modifiability of protozoan reactions. Am. J. Physiol 8, 23–60. [Google Scholar]
- Boisseau RP, Vogel D, Dussutour A. 2016. Habituation in non-neural organisms: evidence from slime moulds. Proc. R. Soc. B 283, 20160446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel D, Dussutour A. 2016. Direct transfer of learned behaviour via cell fusion in non-neural organisms. Proc. R. Soc. B 283, 20162382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet DA and Francis D. 1972. Learning in Stentor. J. Protozool 19. 484–487. [Google Scholar]
- Hinkle DJ, Wood DC. 1994. Is tube-escape learning by protozoa associative learning? Behavioral Neurosci. 108, 94–90. [DOI] [PubMed] [Google Scholar]
- Huber JC, Rucker WB, and McDiarmid CG. 1974. Retention of escape training and activity changes in single paramecia. Journal of Comparative and Physiological Psychology, 86, 258–266. [Google Scholar]
- Saigusa T, Tero A, Nakagaki T, Kuramoto Y. 2008. Amoebae anticipate periodic events. Phys. Rev. Lett 100, 018101. [DOI] [PubMed] [Google Scholar]
- Hennessey T, Rucker WB, McDiarmid CG. 1972. Classical conditioning in paramecia. Animal Learning & Behavior 7, 417–423. [Google Scholar]
- Nisonne G, Appelgren A, Axelsson J, Fredrikson M, Lekander M. 2011. Learning in a simple biological system: a study of classical conditioning of human macrophages in vitro. Behav. Brain Func 7, 47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando CT, Liekens AML, Bingle LEH, Beck C, Lenser T, Stekel DJ, Rowe JE. 2009. Molecular circuits for associative learning in single-celled organisms. J R. Soc. Interface 6, 463–9 [DOI] [PMC free article] [PubMed] [Google Scholar]