Abstract
The technological evolution and widespread availability of wearables and handheld ECG devices capable of screening for atrial fibrillation (AF), and their promotion directly to consumers, has focused attention of healthcare professionals and patient organizations on consumer-led AF screening. In this Frontiers review, members of the AF-SCREEN International Collaboration provide a critical appraisal of this rapidly evolving field to increase awareness of the complexities and uncertainties surrounding consumer-led AF screening. Although there are numerous commercially available devices directly marketed to consumers for AF monitoring and identification of unrecognized AF, healthcare professional-led randomized controlled studies using multiple ECG recordings or continuous ECG monitoring to detect AF have failed to demonstrate a significant reduction in stroke. While it remains uncertain if consumer-led AF screening reduces stroke, it could increase early diagnosis of AF and facilitate an integrated approach, including appropriate anticoagulation, rate and/or rhythm management and risk factor modification, to reduce complications. Companies marketing AF screening devices should report the accuracy and performance of their products in high- and low-risk populations and avoid claims regarding clinical outcomes unless improvement is demonstrated in randomized clinical trials. Generally, the diagnostic yield of AF screening increases with the number, duration, and temporal dispersion of screening sessions, but the prognostic importance may be less than for AF detected by single-timepoint screening, which is largely permanent, persistent or high-burden paroxysmal AF. Consumer-initiated ECG recordings suggesting possible AF always require confirmation by a healthcare professional experienced in ECG reading, while suspicion of AF based on photoplethysmography must be confirmed with an ECG. Consumer-led AF screening is unlikely to be cost-effective for stroke prevention in the predominantly young, early adopters of this technology. Studies in older people at higher stroke risk are required to demonstrate both effectiveness and cost-effectiveness. The direct interaction between companies and consumers creates new regulatory gaps in relation to data privacy and the registration of consumer apps and devices. While several barriers for optimal use of consumer-led screening exist, results of large, ongoing trials, powered to detect clinical outcomes, are required before healthcare professionals should support widespread adoption of consumer-led AF screening.
Keywords: atrial fibrillation, screening, wearable devices
Section 1: Introduction
Atrial fibrillation (AF) is the most common clinically significant sustained arrhythmia and is associated with increased risk of stroke, heart failure, mortality, hospitalization, and cognitive decline.1, 2 Many AF episodes are asymptomatic, with stroke as the first manifestation of AF in at least 25% of AF-related strokes,1 which underlies the principle of screening for unknown AF to prevent stroke. For many years, healthcare professionals initiated screening for AF in different settings using pulse palpation and/or 12-lead ECG recordings.3, 4 European, Canadian and Australian AF guidelines recommend opportunistic pulse assessment for patients over 65 with a follow-up ECG, or 30-second ECG rhythm strip for an irregular pulse.1, 5, 6 However, pulse assessment is infrequently performed in routine primary care,7 and will mainly detect persistent or permanent AF.
Developments in technology allowing more intensive monitoring with intermittent ECG snapshots, or continuous ECG monitoring for extended periods with ECG patches, or implantable cardiac monitors (ICMs) and devices, will detect much more paroxysmal AF, often of shorter duration, which may represent lower AF burden with a relatively more benign prognosis.8 While episodes can be confirmed to be subclinical AF (SCAF), there is no agreement on cut-off for AF burden or episode duration that increases stroke risk. Notably, the US AF guidelines only mention, but do not make any recommendations for AF screening,9 while the US Preventive Services Task Force10 and other professional societies7 believe that there is insufficient evidence to determine whether systematic ECG screening for AF will do more good than harm, and recommend adequately powered randomized clinical trials to resolve this issue. This need was enunciated by AF-SCREEN in its 2017 white paper,7 and reaffirmed by the National Heart Lung and Blood Institute as a research priority for AF screening.8
Development and widespread availability of handheld ECG devices often linked to smartphones, and smart-watch/wearable ECG devices that rapidly acquire a medical quality rhythm strip, has fueled consumer interest, despite uncertainty over the value of systematic screening. Additionally, smartphone camera/flash photoplethysmography (PPG)-based rhythm monitoring devices and PPG-based smartwatch or fitness wearables have markedly extended the reach of screening, and enhanced feasibility of often nearly continuous AF screening without direction by physicians.11 Several studies have demonstrated the feasibility of AF screening using these tools in a variety of clinical settings, when directed by physicians, or health professionals3, 12–15 followed by three large studies of more intensive monitoring in large population groups in a non-clinical setting.16–18 Taking this new paradigm further, the consumer, rather than the healthcare professional, is now leading the effort to identify asymptomatic AF.19 We define this as consumer-led screening, because it is initiated without confirming the indication with a healthcare professional. The issue is that while the optimal screening intensity and burden of SCAF required to identify AF of prognostic significance remains unknown for physician-led screening, let alone consumer-led screening, many healthcare professionals are already seeing patients who have detected AF themselves with daily, weekly or quasi-continuous consumer-directed screening,20 and are uncertain of what the management response should be.21
In this Frontiers review, we summarize existing evidence and knowledge gaps on consumer-led AF screening, compiled by members of the AF-SCREEN International Collaboration. Key points do not represent guidelines or formal recommendations but rather provide consensus formulations on several aspects of this topic, including proposed protocols and pathways after AF is detected, as well as pertinent ethical, legal and privacy issues. These are intended to provide a better understanding of the complexities and uncertainties of consumer-led AF screening and support decision making in the real-world setting.
Section 2: WHO requirements and consumer-led screening for AF
The World Health Organisation (WHO) screening requirements published in 1968 are still valid, and must be fulfilled before screening for a given disease is adopted (Table 1).22 While screening for AF by healthcare professionals using pulse palpation and/or 12-lead ECG recordings may satisfy all the WHO criteria,3, 4 consumer-led screening for AF fulfils only some23 (Table 1). AF is an important health problem, and oral anticoagulants constitute an effective, accepted treatment to prevent stroke in patients at risk, with the important caveat that younger age groups owning wearables have a lower stroke risk.1 SCAF detected by wearables may also be considered a latent disease state, with growing evidence regarding risk factors and mechanisms underlying progression from latent to overt disease states.1, 24 However, appropriate care pathways for confirming the diagnosis and, if necessary, initiating appropriate treatment in individuals with positive findings have yet to be established. Currently, it is also unlikely that unselected screening for AF using wearables is cost-effective for stroke prevention, as many people who screen themselves will have a low stroke risk. In many cases, resultant healthcare utilization will prove unnecessary, the screening itself in those circumstances incurring additional cost and risk of extra tests without benefit. Finally, due to device costs and variable technological literacy, screening for AF using wearables may not be acceptable or available to the entire population and could accentuate health inequalities.
Table 1.
Does consumer-led screening for AF fulfil the WHO principles of early disease detection?22
| Principle | Consumer-led screening for AF* | |
|---|---|---|
| 1 | The condition sought should be an important health problem. | + |
| 2 | There should be an accepted treatment for patients with recognised disease. | (+) |
| 3 | Facilities for diagnosis and treatment should be available. | − |
| 4 | There should be a recognisable latent to early symptomatic stage. | + |
| 5 | There should be a suitable test or examination. | (+) |
| 6 | The test should be acceptable to the population. | (+) |
| 7 | The natural history of the condition, including development from latent to declared disease, should be adequately understood. | (+) |
| 8 | There should be an agreed policy on whom to treat as patients. | (+) |
| 9 | The cost of case finding (including diagnosis and treatment of patients diagnosed) should be economically balanced in relation to possible expenditure on medical care as a whole. | − |
| 10 | Case finding should be a continuing process and not a “once and for all” project. | (+) |
+ = does fulfil; (+) = does partly fulfil; - = does not fulfil
Principles 1,2, and 8 could be classified as negative (does not fulfil) for individuals at low risk of stroke.
Section 3: Technology used
The spectrum of consumer-led AF screening options range from intermittent rhythm checks to near-continuous rhythm monitoring, using ECG-based and PPG-based rhythm analysis technologies. Handheld ECG devices for intermittent rhythm checks are most well-studied for AF detection, and have been validated across various hospital, outpatient, and community settings.15, 25, 26 These devices deliver clinical-grade, primarily single-lead ECGs, but add costs when embedded into commercial smartwatches, or other wearables, or as add-on hardware/software (e.g. AliveCor KardiaMobile).
Rather than taking fingertip pulse, wrist-based wearables use PPG sensors to measure pulse at the back of the wrist, processing data frequently and passively during wear. It is important to recognize that although the PPG signal is continuously sampled by smartwatches, due to motion artifact, only a small minority of that PPG is of adequate quality to analyze for the presence of AF. In one study of simultaneous ECG and smartwatch PPG data in a controlled environment, designed to mimic real-world activity, only ~13% of all 30-second PPG samples were considered analyzable.27 Although PPG-based approaches for AF detection are comparable to ECG-based approaches for AF diagnosis when there is adequate signal quality,7 ultimately, an ECG is required to confirm AF.1, 7 Oscillometry-based algorithms that detect AF during automated blood pressure measurement have been shown to have high diagnostic accuracy.28
Although growing evidence suggests that consumer devices are accurate for AF detection in populations at high risk for the arrhythmia, few studies have evaluated their performance in low-risk populations. Furthermore, no randomized studies have shown that commercial technology-based AF screening reduces stroke or other AF complications.8 Even in high-risk older populations, the LOOP randomized trial using an implanted cardiac monitor did not demonstrate a reduction in stroke over 5 years.29 This was despite over 30% of participants having at least one episode of AF >6 minutes, and 85–90% receiving treatment with oral anticoagulants. Notably, there is an interaction of AF duration with comorbidities, such that the less severe the atrial alterations from comorbidities are, the more AF burden will be required to reach a high thromboembolic risk level.1 Moreover, the STROKESTOP randomized trial in people aged 75–76 using intermittent ECG recordings over 2 weeks, showed a reduction in the composite of ischemic stroke, death, hospitalization for bleeding, but no reduction in the specified secondary endpoint of ischemic stroke.30 It is therefore unlikely that screening, whether consumer-led or physician-initiated, will reduce stroke in young, low-risk populations. Nonetheless, whether consumer-led identification of AF at an earlier stage, with implementation of appropriate management including lifestyle modification, would delay progression to persistent AF and/or prevent heart failure and cognitive decline, remains to be determined.
Key points 1 and 2
-
1
Commercially available devices marketed directly to consumers for AF monitoring use ECG or PPG for rhythm analysis, and their algorithms identify unrecognized AF with variable accuracy.
-
2
Healthcare professional-led randomized controlled studies using multiple ECG recordings or continuous ECG monitoring have not yet shown a significant reduction in stroke. The effectiveness of consumer-led AF screening on AF outcomes including stroke remains unknown.
Section 4: Algorithms/AI used for AF diagnosis
As desire to detect AF moves to the consumer-led, device-driven preclinical setting, there is an increasing reliance on automated rhythm classification. The expectation is that AF diagnosis will be accurate enough that a clinician overread will not be necessary. Algorithms analyzing 12-lead ECGs that rely on a combination of P-wave morphology and heart rate irregularity perform well with a specificity of 99%.31 On the other hand, consumer devices rely on limited leads where P-waves can be undetectable, or on pulse detection using PPG and oscillometry, which are often hindered by noise.18, 27 Similarly, an irregular pulse can represent frequent atrial and ventricular ectopy, sinus arrhythmia and atrial rhythms with variable conduction. On the other hand, the presence of ventricular pacing or atrial flutter often lead to misclassification of AF as sinus rhythm.32 These issues will limit accuracy of AF algorithms in consumer-facing devices.
Consumer devices are often used by young, asymptomatic consumers,16, 17 lowering pretest AF probability. Therefore, algorithms must be highly specific, maintaining a low false positive rate, but sensitive enough that an acceptable proportion of episodes is identified. Sensitivity and specificity of AF algorithms can be adjusted by titrating the degree and duration of irregularity needed for classification. The Poincaré plot33 of each RR interval on the x-axis and the subsequent RR interval on the y-axis, is commonly used to quantify beat-to-beat irregularity (Figure 1A and 1B). Sensitivity and specificity can be adjusted by determining the degree of dispersion necessary to mark a plot as irregular.
Figure 1. Algorithms to Detect Atrial Fibrillation.
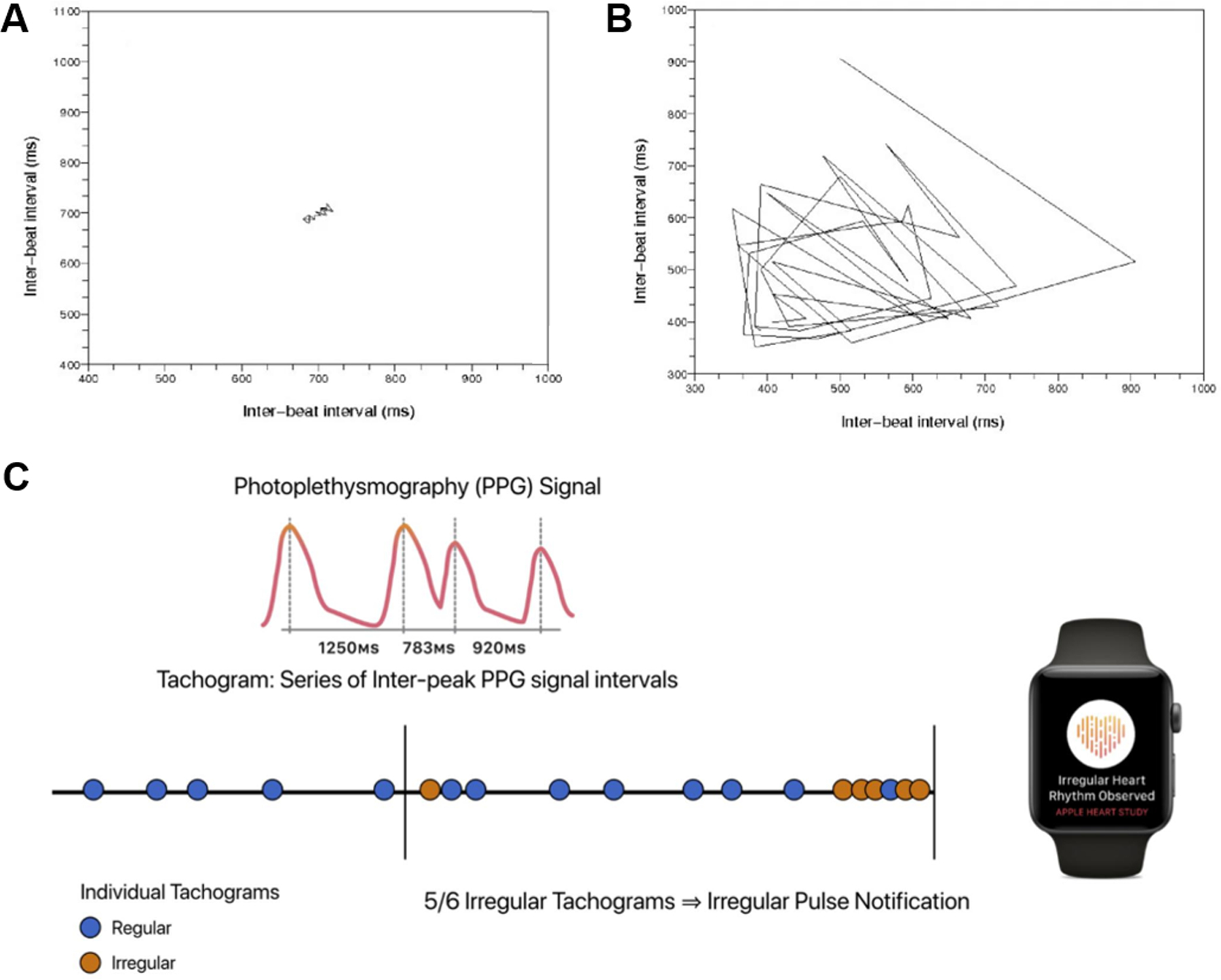
Depicted are Poincaré plots of (A) sinus rhythm and (B) atrial fibrillation, as well as (C) the algorithm used by the irregular pulse notification algorithm on the Apple Watch. One-minute pulse intervals are taken intermittently during rest and if an irregular pulse is detected, the pulse is sampled more frequently. If 5/6 pulse measurements are irregular, a possible AF alert is generated. Similar approaches have been taken by other manufacturers to increase specificity, some with greater stringency (e.g. Fitbit, which required 11 consecutive irregular overlapping 5 min tachograms, or >30 min irregular).
Algorithms that account for long monitoring periods by increasing the threshold of duration of pulse irregularity required to diagnose AF have been developed (Figure 1C).16 Short bouts of artefact or ectopic beats are unlikely to trigger an AF alarm, as are short bouts of AF, which are less likely to be clinically meaningful or associated with stroke.34 To further minimize false positives, accelerometers help ensure that consumers are not moving when an ECG or a pulse measurement is taken: in the extreme, this may limit diagnosis to sleep, which has been shown in the Fitbit Heart Study where 76% of first episodes of irregular heart rhythm were detected during sleep.18 In fact, as shown in the Fitbit Heart Study, limiting AF detection during periods of inactivity, maximizes the specificity of AF diagnosis, but only 7.5% of awake time had analyzable data.18 Rate limits are often programmed to optimize AF detection, though this will miss either slow or rapid AF with ventricular rates falling below or above the set threshold.35
Combinations of modalities, for example irregular pulse detection coupled with single-lead ECG confirmation, have been shown to improve accuracy.36 Additional ECG leads may also improve P-wave morphologic detection and help distinguish AF from other atrial ectopic rhythms, but are more difficult to use.37 Finally, as big data are collected on a growing consumer base, machine learning will improve performance of these algorithms, as has been done with clinical ECG patch devices.37, 38
Section 5: Density and Intensity of screening vs. prognostic significance of AF
Data from patients with pacemakers or defibrillators including atrial leads, or with ICMs, indicate that the incidence of asymptomatic SCAF lasting at least 5–6 minutes is approximately 30%.39–42 In addition, these studies showed that SCAF was an independent predictor of stroke and even increased mortality.43 There is a relationship between SCAF burden and stroke risk, with a greater risk seen among patients with episodes of many hours duration.44–46 However, the stroke risk for SCAF on an implanted device has been shown to be lower than with clinical AF, i.e. documented by an ECG.39, 47
Data from screening studies using smartphone-based, or handheld ECG devices suggest that diagnostic yield increases with intensity of AF screening (Figure 2),48 while a single measurement may not increase detection over usual care.49 On the other hand, AF detection rate is closely associated with AF burden.50, 51 In addition, the diagnostic yield increases with the temporal dispersion of screening, with more AF detected when the same monitoring duration is spread over several periods compared with a single period (e.g., three, temporally distinct 24-hour monitoring periods versus one continuous 72-hour monitoring period).52 Moreover, as demonstrated in simulations based on the LOOP and REVEAL-AF studies, a large burden of AF will be missed, if monitoring is not continuous.52, 53 Consequently, AF detected at a single time point likely means that the patient has a large AF burden (or persistent AF).
Figure 2. Trade-off between duration/intensity of screening for AF detection of AF, and AF stroke risk.
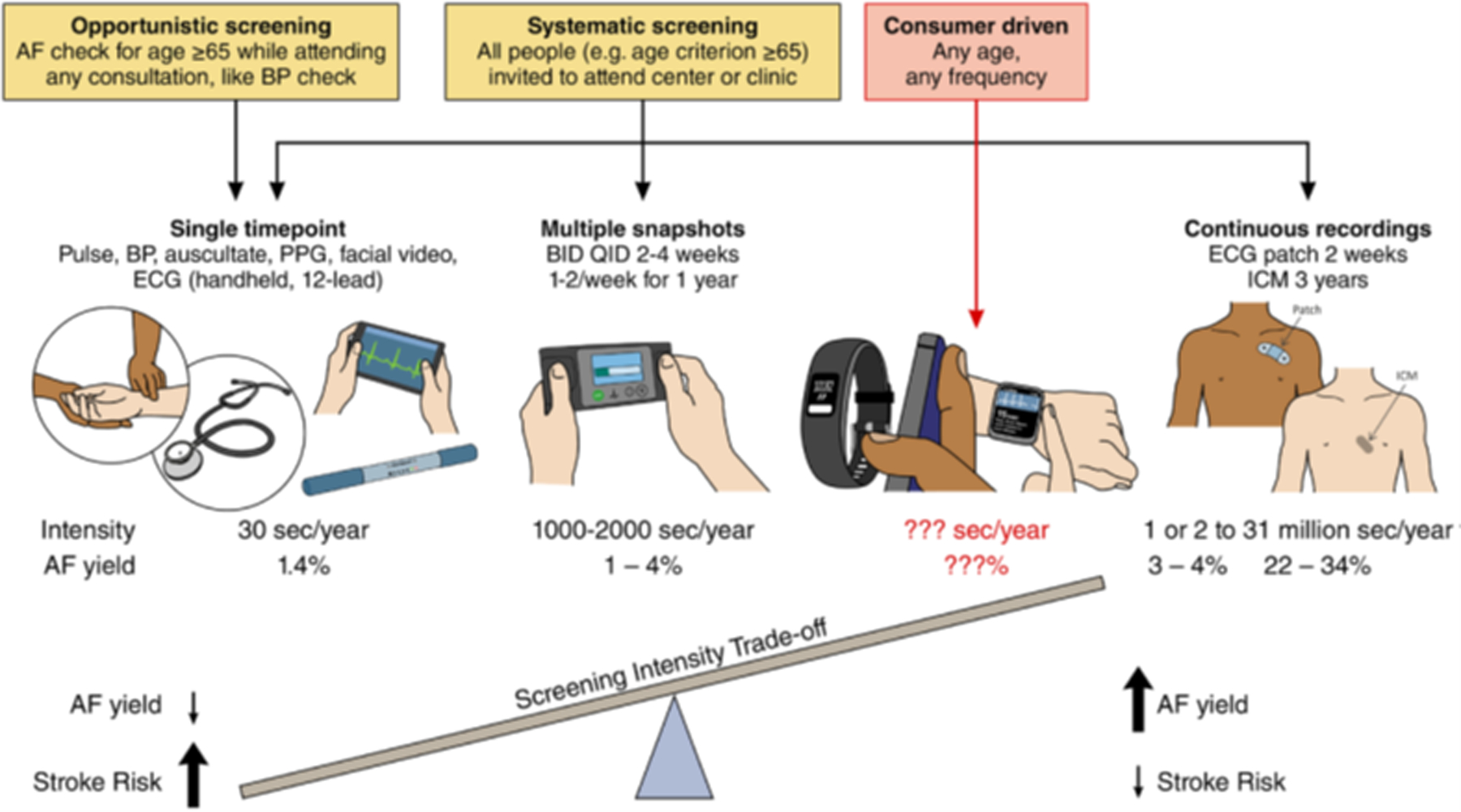
While increased screening intensity increases AF detection rates, it may also identify AF associated with low stroke risk. Nonetheless, the minimum AF burden at which the risk of stroke is sufficient to justify initiation of anticoagulation remains unclear. Therefore, the trade-off between increased detection of low-burden, possibly low-risk AF by continuous monitoring strategies, could be minimized by defining an intermittent monitoring strategy that would diminish the potential for missing individuals with a high burden, which in turn is associated with higher risk of stroke. BID indicates twice daily; BP, blood pressure; ICM, intracardiac monitor; PPG, Photoplethysmogram; and QID, 4 times a day. Modified from Benjamin et al.8
Two ongoing studies, NOAH-AFNET-6 and ARTESiA, will examine whether anticoagulation for SCAF of at least 6 minutes will decrease stroke or thromboembolic events.54, 55 More specifically for consumer-led screening, the ongoing Heartline Study (NCT04276441) will determine whether a smartwatch irregular rhythm notification algorithm/app and inbuilt ECG can reduce the risk of cardiovascular events. A summary of published and ongoing consumer-led AF screening studies is provided in Table 2.
Table 2.
Screening for AF using commercially available wearable devices
| Study/Year | Number of subjects | Device | Inclusion Criteria | AF detected by monitor | Comments |
|---|---|---|---|---|---|
| Apple Heart16 | 419,297 | Apple Watch | age ≥22, possession of Apple Watch | 0.52% received notification for irregular rhythm; AF confirmed in 34% | Citeless study Mean age 41 years PPV: 84% |
| Huawei study17 | 187,912 | wristband (Honor Band 4) or wristwatch (Huawei Watch GT) | Age ≥ 18 | 0.23% received a “suspected AF” notification; AF confirmed in 87% | Mean age 35 PPV: 92% |
| Verbrugge et al.90 | 12,328 | PPG only technology | Not specified | 1.1% diagnosed with possible AF | Participants were invided through an article in a local newspaper Mean age 49 |
| Heartline study (NCT04276441) | Apple Watch | Age ≥ 65 Possesion of iPhone 6s or later Medicare coverage |
Ongoing | Virtual study | |
| Fitbit Heart study18 | 455,699 | Fitbit | Age ≥ 22 No prior history of AF Fitbit account, with a compatible device paired |
0.07% received notification for irregular rhythm; AF confirmed in 32% | Median age 47 PPV: 98% AF alogirith operated only during periods of inactivity |
Key point 3:
-
3
The diagnostic yield of AF screening increases with the number, duration, and temporal dispersion of screening sessions, but the prognostic importance may be less than for AF detected by single-timepoint screening which is largely persistent or reflects high burden paroxysmal AF.
Section 6: Protocol or pathway after detection of AF by consumer-led screening (Figure 3)
Figure 3. Workflow when there is a suspicion of AF during consumer-led AF screening.
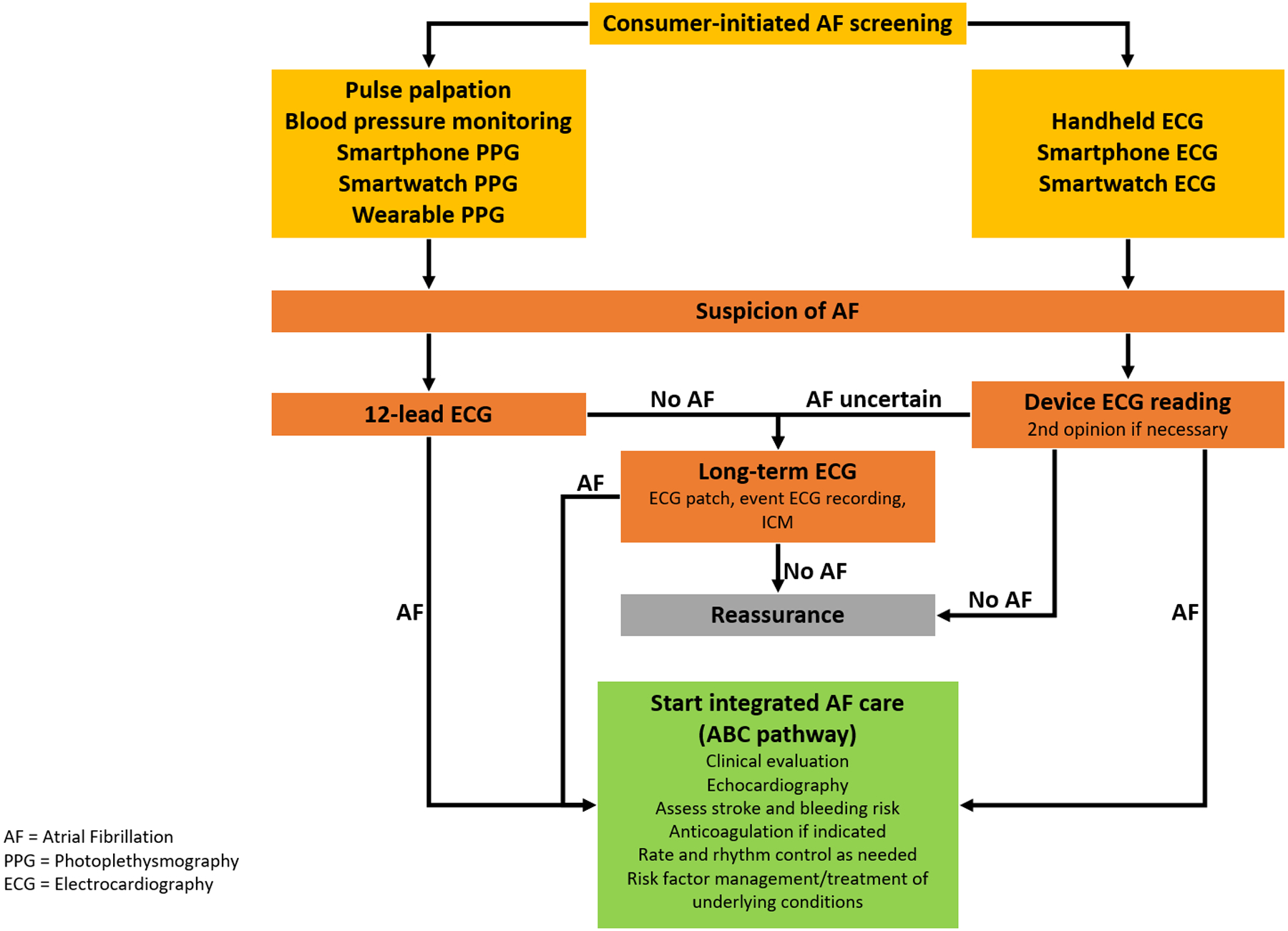
Consumer-initiated ECG recordings suggesting possible AF always require confirmation by a healthcare professional experienced in ECG reading, while suspicion of AF based on photoplethysmography must be confirmed with an ECG. If AF is confirmed, an integrated care approach, including appropriate anticoagulation, risk factor modification, and treatment of underlying cardiovascular co-morbidities, should be undertaken to reduce complications.
When consumer-led AF screening using an ECG-recording device suggests possible AF, this finding should always be confirmed by a healthcare professional with experience in ECG reading as recommended in guidelines,1 given the risk of false-positive ECG recordings. General/primary care practitioners will often be the first line of consultation in most cases with a suspicion of AF after consumer-led screening, although they may lack ECG experience/expertise,56 and interpretation may be more difficult for a single lead recording. The need for a second opinion in ECG reading in this setting is expected to be high, though electronic transmission is available to facilitate this. Many pathways alongside general practice can play an important role in the process, in particular noting the aternative locations in detecting AF as part of ‘know your pulse campaigns’ such as community pharmacies,57 although the same caveats of ECG confirmation pertain. Professional and patient organizations also have a central role in providing information and recommendations in the field, which may overcome the attrition of participants who received an irregular rhythm notification in the recent Fitbit Heart Study, but did not seek further medical attention.18
A suspicion of AF recorded on pulse-based devices requires confirmation by an ECG recording (Figure 3). If AF is persistent or permanent, a 12-lead ECG will be adequate, but if paroxysmal, continuous ECG monitoring for 1–2 weeks with an ECG patch or at least 48–96 hours of Holter monitoring is required. Frequent intermittent handheld ECGs over a few weeks may be an alternative.
Once the diagnosis of AF is made, further management requires a full history and physical examination and guideline-recommended evaluation and treatment. When appropriate, oral anticoagulant therapy should also be offered after considering AF-related stroke and bleeding risk. In most cases, the AF work-up can initially be managed by the general/primary care practitioner, including oral anticoagulant therapy ideally initiated at the time of definitive diagnosis, and not delayed, due to the higher risk of stroke soon after AF diagnosis.58, 59
Although extended consumer-led screening may identify individuals with low-burden AF not associated with increased risk of stroke,8 it is possible to use any AF episode identified as a trigger to implement lifestyle modifications, which have been shown to reverse the natural progression of AF and reduce AF recurrences, cardiovascular morbidity, and stroke,60–62 as shown in the mAFA-II trial of Mobile Health technology, albeit in an older population.63 Whether such lifestyle modifications in this younger population will translate into improved clinical outcomes remains to be determined. On the other hand, if a diagnosis of AF is not established through screening or after subsequent long-term monitoring, explanation and reassurance should be provided by the healthcare professional (Figure 3).
Key points 4 and 5:
-
4
A consumer-initiated ECG recording suggesting possible AF should always be confirmed by a healthcare professional with experience in ECG reading. If AF is suspected using a non-ECG screening method, this must be confirmed with an ECG before a definite AF diagnosis is made.
-
5
Consumer-led screening approaches could increase early diagnosis of AF and facilitate an integrated care approach, including appropriate anticoagulation, risk factor modification, and treatment of underlying cardiovascular co-morbidities, to reduce complications.
Section 7: Effectiveness of screening and economic burden
Modeling simulations based on the STROKESTOP trial suggest acceptable costs of €4,313 per quality adjusted life years gained among individuals 75 years and above screened twice daily for 2 weeks.64 Among Canadians aged ≥65 years, AF screening with a handheld ECG appears to lower lifetime costs.65 However, both analyses used modeled, rather than directly measured data, and both assessed system-driven, rather than consumer-led screening. Moreover, we do not know the lower limit of AF duration and/or burden that is associated with sufficient stroke risk to warrant anticoagulation. The short duration of many of the AF episodes in the LOOP study29 may partly explain the negative result as it pertains to stroke prevention, and provides an important caveat to AF detected by consumer-led screening, which may also be skewed towards shorter or infrequent episodes when performed semi-continuously.66 Most studies have concentrated on stroke, even though substantial morbidity and mortality related to AF is due to heart failure and dementia.67–69 If these could be reduced through AF screening, cost-effectiveness may be more favorable.
In the Apple and Huawei Heart Studies, a substantial proportion of those who received an irregular-pulse notification did not complete an examination to confirm AF.16, 17 Furthermore, among those who did complete follow-up, AF was not confirmed in 16% and 13%, respectively.16, 17 Similar findings were reported from the Fitbit Heart Study, where less than one fourth of the individuals having an irregular heart rhythm detected had a subsequent ECG monitoring with a patch device analyzed. In 32% of these cases, AF was confirmed, comprising 0.07% of the enrolled study population and 7.2% of those with an irregular heart rhythm detection, though the 98% PPV in those with simultaneous recordings was higher than reported in the Apple and Huawei Heart Studies (84% and 92%, respectively).18 The costs of managing false-positives which are higher in younger cohorts with low AF prevalence, could become substantial if consumer-led AF screening becomes widespread. Participants in the Apple, the Huawei and the Fitbit Heart Studies were young (mean age 41.6 and 35.4, and median age 47 years, respectively), raising concerns that extensive consumer-led screening in the younger population owning wearables could result in little advantage due to low stroke and AF incidence plus higher costs from further testing in false positive cases, and is therefore very unlikely to be cost-effective for stroke prevention. Moreover, there is an implicit risk of more aggressive referrals of these younger individuals with screen-detected AF for catheter ablation, even though the guidelines recommend catheter ablation to control symptoms.1, 2
Key point 6
-
6
Consumer-led screening for AF is unlikely to be cost-effective for stroke prevention in the current young adopters of the technology. Studies in older people at higher stroke risk are required to demonstrate both effectiveness and cost-effectiveness for stroke prevention.
Section 8: What should companies marketing devices be obliged to do?
Marketing claims by device companies should accurately reflect the research that has been conducted and disseminated to the clinical and scientific communities. Although this may be difficult to be enforced, strategies to acknowledge and reward companies that successfully adhere to these obligations may prove effective.
Ideally, post-market approval surveillance studies should be required by the FDA/EU for device manufacturers in real-world cohorts. However, these may not be practical because many of the companies that produce the most novel contributions may not have the resources to perform rigorous real-world evaluations. Therefore, as long as the company does not make marketing claims that extend beyond the evidence, there should not be any pre-specified requirements regarding the extent of the research that must be completed. Moreover, it should be highlighted that studies of new devices are typically conducted in carefully selected participants with high AF prevalence (e.g. pre- and post-cardioversion).70, 71 Although the positive predictive value is more relevant than sensitivity in the context of AF screening, it is important that companies report sensitivity, specificity, and positive predictive values (PPVs) in the population they were tested in, when describing screening test results, as well as how these metrics were derived, including cases excluded due to poor signal quality and/or poor adherence, so that clinicians and researchers can interpret results appropriately, and avoid an overly inflated impression of clinical utility and accuracy in real-world populations based on sensitivity and specificity alone.72 Particularly PPVs are not always reported in the respective publications and can be only modest, while the specificity is fairly high.73 In addition, predictive values should be reported not only for the AF prevalence in the particular research study, but should also be calculated given the expected prevalence among a well-defined group of individuals. For example, positive predictive value can be estimated for the general population and among those with particular AF risk factors, using prevalence previously reported in the literature.74 Positive and negative likelihood ratios, that are not influenced by disease prevalence, can also inform decision making.
Key points 7 and 8
-
7
Companies marketing AF screening devices should report the sensitivity, specificity, positive and negative predictive values of the current versions of their product and avoid any claims regarding quality of life, thromboembolism, stroke, or mortality unless a reduction in those outcomes is demonstrated in a prospective, randomized clinical trial.
-
8
Positive predictive values should be calculated and reported for the study population, the general population, and at least one well-defined population with AF risk factors.
Section 9: What is the healthcare providers’ view and what is new for health services?
With smartphones and other wearables becoming ubiquitous in high- and even middle-income countries, physicians often have to deal with asymptomatic individuals who took a personal initiative of using “over-the-counter” wearable devices, which in turn raises questions related to confirmation of AF diagnosis, and subsequent physician interventions. A recent, anonymous, web-based survey including 588 healthcare professionals indicated that 60% are having to deal with people with detected AF as a result of screening with wearable devices at least occasionally, while 57% currently advise wearables/apps for AF detection in their patients, potentially for suggestive symptoms.20 Physicians may also have different perceptions of wearables in AF screening. They may be “innovators” or “early adopters”, potentially influced by innovation hype, but also “laggards” or “phobics”, who exert a strong resistance to adopt the technological innovation.75
Whatever the perceptions, the untoward consequences of consumer-initiated use of wearables are that AF detection will trigger an increasing number of contacts with various physicians (e.g. primary care, cardiologists, etc.), with need to perform ECGs, clinical evaluations, and other diagnostic tests before a final diagnosis can be confirmed (or refuted) and, thus, a decision on anticoagulation according to risk profile in case of AF can be made. Physicians and other healthcare professionals need to adapt to this evolving scenario, avoiding an opposing position. In a survey published in 2019 dealing with traditional ECG methods for AF screening in an ambulatory setting, not taking into account wearables, Dutch general practitioners reported that referral to a cardiologist after an AF diagnosis was not the rule, and in 83% of cases decision-making on treatment could occur without referral, suggesting the need for a better definition of criteria and methods for referral.76
Section 10: Legal, ethical and privacy issues
There is a range of important legal, ethical, and privacy issues that arise with devices used for consumer-led screening for AF (Figure 4). A difficult area is ownership and use of personal health data generated by use of consumer screening devices, which may not be sufficiently covered by existing regulatory regimes in many jurisdictions. Consumers have a legitimate expectation of privacy in relation to their health data.77 A key concern is that data could be accessed by advertisers, employers, and/or insurers, with potentially significant consequences.78
Figure 4. Legal and ethical issues with consumer-led AF screening.
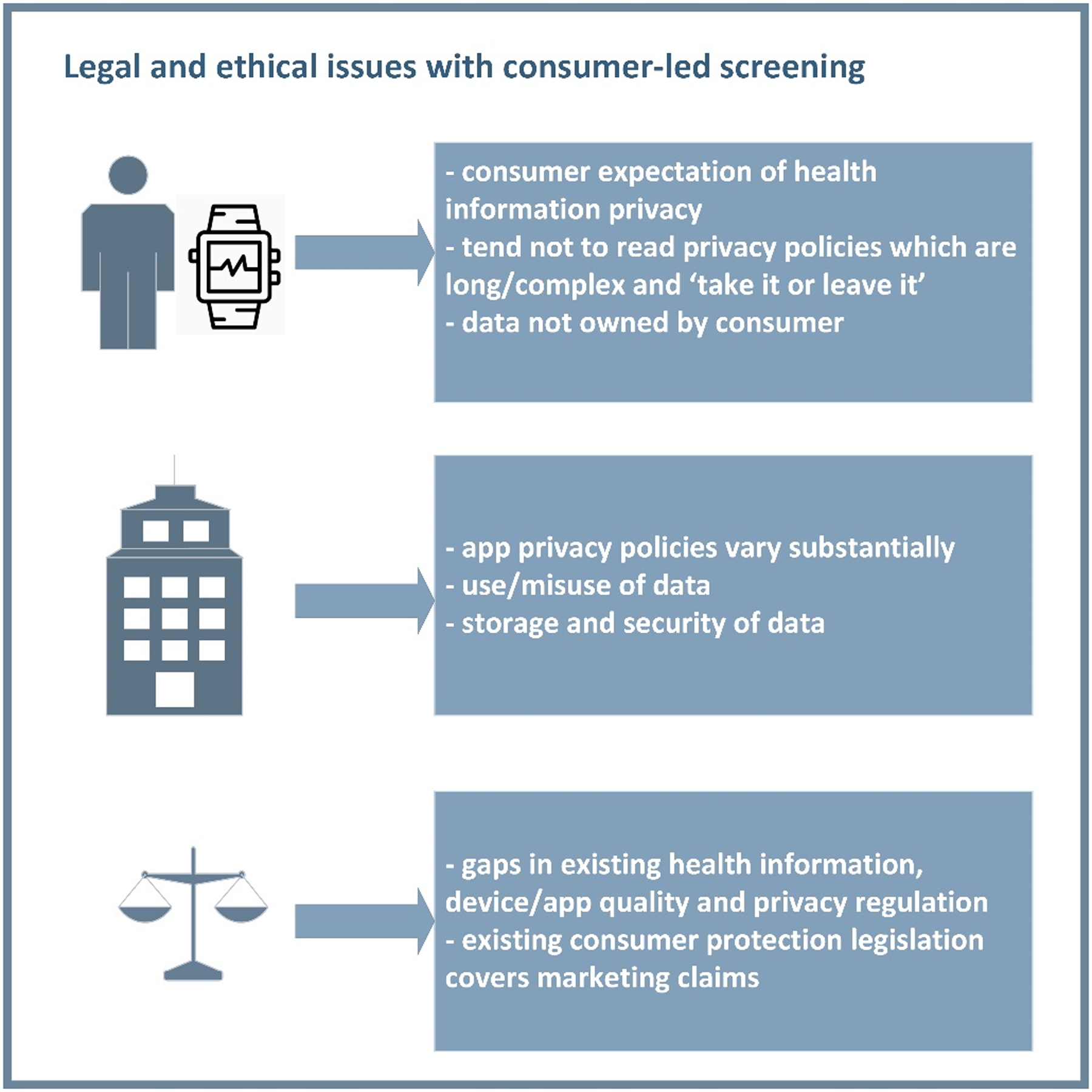
Important legal and ethical gaps in relation to data and registration of consumer screening apps and devices are shown.
It is important to note that the data generated are often not owned by consumers, who have little control or knowledge about how the information is used. Each app tends to have its own privacy and data use policy, with huge variability in data protection. Most privacy policies and user agreements are long ‘take it or leave it’ contracts, and are neither read nor understood by most consumers. Such documents may not be equivalent to traditional ‘informed consent’, especially related to potential use of artificial intelligence (AI) to interpret data.79
In the US, data from consumer apps are not generally covered by the Health Insurance Portability and Accountability Act (HIPAA), which regulates use and disclosure of health information.79 In most cases, the apps are not registered as medical devices by the FDA, although the FDA is working with a panel of technology companies to build an appropriate approval process for software as a medical device.79 In Europe, protection under the General Data Protection Regulation (GDPR) is much broader and provides appropriately strong protections for consumers regarding personal health data.79 In addition, there is a general requirement under existing consumer protection legislation in most countries not to make representations that are misleading or deceptive, and to have a reasonable basis for claims.79 Therefore, companies marketing apps and devices must adhere to these standards (which also must be enforced by regulators) and not advertise unproven benefits.
While there are some important legal and ethical issues, devices enabling consumer-led screening are here to stay. Therefore, the focus should be on adapting regulatory regimes to properly cover and enforce privacy, quality/safety, and consumer-protection aspects of consumer screening devices.80
Key point 9
-
9
There are important legal gaps in relation to data and registration of consumer screening apps and devices in many jurisdictions. Regulatory frameworks need to be updated to cover and enforce privacy, quality/safety, and consumer-protection aspects of consumer screening devices.
Section 11: What can we learn, harness for research?
The undoubtedly massive increase in the availability of “medically relevant” data derived by the consumer requires that healthcare professionals can use this information appropriately to the advantage of individual patients and the population at large. To achieve this, the technology which is used to collect or analyze data must be of medical grade, the raw or processed data must be fully validated in terms of its potential as a biomarker; it must be capable of integration with other healthcare information; and it must be linked to outcome data, to allow the full potential to be realized.
Consumer-led screening offers opportunities to link to national registries, particularly to evaluate factors associated with prevalent and incident AF, as well as AF-related complications (e.g. stroke, heart failure, dementia and death). This approach can be most easily employed in countries with nationwide national insurance data, for example Taiwan, South Korea, Denmark, Sweden.81 Linking consumer-derived data to national or insurance databases should allow their sole and added value to be assessed in terms of improving risk assessment for adverse outcomes associated with AF.
AI algorithms may allow instant summary and integration of multi-level information to predict the probability of prevalent/incident AF using historical ECG data. Such information can feed decision-support tools, and thus guide consumer-led self-initiated screening. In a recent example, AI applied to readily available features extracted from electronic health records and routine examinations of 12-lead ECGs in sinus rhythm, impressively predicted development of AF.82 Moreover, AI has been successfully applied to single-lead ECGs derived from wearable or handheld devices.83
Section 12: Consumer perspectives
By definition, consumers undergoing self-screening do not have known AF and their perspectives may only in part be extrapolated from studies involving patients with clinical AF. While patients with newly diagnosed AF frequently lack adequate AF information, education, or appropriate communication of its consequences by clinicians,84, 85 asymptomatic individuals with no history of AF may be even less likely to understand the condition, risks, and importance of screening. However, studies have shown that consumers using single-lead ECG devices, generally report high satisfaction. Specifically, those who have had AF detected expressed gratitude for its identification, while those who were told their ECG was normal were curious about the technology, but otherwise unconcerned.86
Another important challenge lies in the technology focus of contemporary consumer AF-screening devices. Older adults, who are at higher risk of AF and stroke, and could potentially benefit from screening, may experience challenges in the use of technology-based, AF-screening tools, including a lack of confidence in their technology self-efficacy87 and age-related decline in visual and fine motor skills. Providing training that includes step-by-step guidance and a manual significantly increases the successful use of technologies by older adults.88 The responsibility lies with manufacturers of these technologies and the healthcare professionals who recommend them to empower patients in using these tools appropriately and effectively. For example, the ongoing Heartline Study (NCT04276441) to determine the impact of AF screening on cardiovascular outcomes in older individuals, also includes a heart health engagement educational program.
If consumer-led screening is found effective in trials, equitable access, especially for those at high risk, is another consideration. These often costly devices are frequently unavailable in lower resource environments or to individuals without financial means88 and unlike traditional physician-initiated screening, patients may incur high out-of-pocket costs for the devices and any follow-up testing required. Currently, few insurance policies worldwide subsidize the costs of consumer-initiated AF-screening.89
As device data continues to be integrated into patient portals at some healthcare institutions,89 there are increased expectations from individuals for healthcare professionals receiving and managing data generated by consumer-led AF screening devices. Nonetheless, the impact of integration of such device data into electronic medical record systems on patient perspectives, as well as quality of life and outcomes, remains to be determined.
Key point 10
-
10
Barriers for the optimal use of consumer-led screening include consumer education, training, expectations, access to wearable devices, access to medical evaluation in case of AF detection, and costs
Conclusions
With the ubiquitous use of smartphones and wearables, physicians and other healthcare professionals are faced with a new paradigm, under which the consumer, rather than the physician, is leading the search for identifying asymptomatic AF. Consumer-led AF screening is already happening, encouraged by large tech companies motivated by sales from direct marketing, and many healthcare professionals commonly are asked to evaluate patients presenting with AF detected by wearable devices.20 Although there is a great potential for appropriate use of AF screening by consumers, there are also many caveats. Therefore, the benefits related to AF diagnosis, which might include implementation of risk factor modification in younger ages, or stroke prevention when risk is greater, should be weighed against the potential anxiety from AF diagnosis and/or false positive results. The usual response of initiation of oral anticoagulation after AF detection may not be appropriate for consumer-led screening. Importantly, there should be a pathway to referral to the general/primary care practitioner/cardiologist after diagnosis to ensure proper guideline-directed treatment. Given that the cost-effectiveness, and the effect of consumer-led AF screening on AF outcomes, including stroke, remain unknown, results of large, ongoing trials, powered to detect clinical outcomes, is required before healthcare professionals will support widespread adoption of consumer-led AF screening.
Acknowledgements
The AF-SCREEN International Collaboration, founded in September 2015, involves more than 180 physicians, nurses, allied health professionals, epidemiologists, health economists, and patient groups from 36 countries. Its major goal is to promote evidence generation and collection on screening for unknown or undertreated atrial fibrillation to reduce stroke and mortality worldwide. It supports the implementation of atrial fibrillation monitoring programs adapted to country-specific needs.
Sources of Funding
Within the past 3 years, AF-SCREEN received funding for holding its annual meetings from BMS/Pfizer Alliance, Bayer, Medtronic, iRhythm, Daiichi-Sankyo, Zenicor, Servier, Boehringer Ingelheim, Biotel, C-SPIN (Canadian Stroke Prevention Intervention Network), and Omron. The sponsors played no role in setting the agenda or the programs for the annual meetings and had no role in suggesting content, drafting, reviewing, or submitting this document for publication.
Abbreviations
- AF
atrial fibrillation
- ECG
electrocardiogram
- ICM
implantable cardiac monitor
- SCAF
subclinical atrial fibrillation
- PPG
photoplethysmography
- WHO
World Health Organisation
- AI
artificial intelligence
- PPV
positive predictive value
- FDA
Food and Drug Administration
- HIPAA
Health Insurance Portability and Accountability Act
- GDPR
General Data Protection Regulation
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL and Group ESCSD. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr., Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW and Members AATF. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–104. [DOI] [PubMed] [Google Scholar]
- 3.Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, Bennett AA, Briffa T, Bauman A, Martinez C, Wallenhorst C, Lau JK, Brieger DB, Sy RW and Freedman SB. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost. 2014;111:1167–76. [DOI] [PubMed] [Google Scholar]
- 4.Proietti M, Mairesse GH, Goethals P, Scavee C, Vijgen J, Blankoff I, Vandekerckhove Y, Lip GY and Belgian Heart Rhythm Week I. A population screening programme for atrial fibrillation: a report from the Belgian Heart Rhythm Week screening programme. Europace. 2016;18:1779–1786. [DOI] [PubMed] [Google Scholar]
- 5.Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, Cox JL, Dorian P, Gladstone DJ, Healey JS, Khairy P, Leblanc K, McMurtry MS, Mitchell LB, Nair GM, Nattel S, Parkash R, Pilote L, Sandhu RK, Sarrazin JF, Sharma M, Skanes AC, Talajic M, Tsang TSM, Verma A, Verma S, Whitlock R, Wyse DG, Macle L and Members of the Secondary P. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2020;36:1847–1948. [DOI] [PubMed] [Google Scholar]
- 6.Group NCAFGW, Brieger D, Amerena J, Attia J, Bajorek B, Chan KH, Connell C, Freedman B, Ferguson C, Hall T, Haqqani H, Hendriks J, Hespe C, Hung J, Kalman JM, Sanders P, Worthington J, Yan TD and Zwar N. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Diagnosis and Management of Atrial Fibrillation 2018. Heart Lung Circ. 2018;27:1209–1266. [DOI] [PubMed] [Google Scholar]
- 7.Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J, Albert CM, Anderson CS, Antoniou S, Benjamin EJ, Boriani G, Brachmann J, Brandes A, Chao TF, Conen D, Engdahl J, Fauchier L, Fitzmaurice DA, Friberg L, Gersh BJ, Gladstone DJ, Glotzer TV, Gwynne K, Hankey GJ, Harbison J, Hillis GS, Hills MT, Kamel H, Kirchhof P, Kowey PR, Krieger D, Lee VWY, Levin LA, Lip GYH, Lobban T, Lowres N, Mairesse GH, Martinez C, Neubeck L, Orchard J, Piccini JP, Poppe K, Potpara TS, Puererfellner H, Rienstra M, Sandhu RK, Schnabel RB, Siu CW, Steinhubl S, Svendsen JH, Svennberg E, Themistoclakis S, Tieleman RG, Turakhia MP, Tveit A, Uittenbogaart SB, Van Gelder IC, Verma A, Wachter R, Yan BP and Collaborators AF-S. Screening for Atrial Fibrillation: A Report of the AF-SCREEN International Collaboration. Circulation. 2017;135:1851–1867. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Go AS, Desvigne-Nickens P, Anderson CD, Casadei B, Chen LY, Crijns H, Freedman B, Hills MT, Healey JS, Kamel H, Kim DY, Link MS, Lopes RD, Lubitz SA, McManus DD, Noseworthy PA, Perez MV, Piccini JP, Schnabel RB, Singer DE, Tieleman RG, Turakhia MP, Van Gelder IC, Cooper LS and Al-Khatib SM. Research Priorities in Atrial Fibrillation Screening: A Report From a National Heart, Lung, and Blood Institute Virtual Workshop. Circulation. 2021;143:372–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM and Yancy CW. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 10.Force USPST, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Epling JW Jr., Kubik M, Li L, Ogedegbe G, Pbert L, Silverstein M, Stevermer J, Tseng CW and Wong JB. Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327:360–367. [DOI] [PubMed] [Google Scholar]
- 11.Ding EY, Marcus GM and McManus DD. Emerging Technologies for Identifying Atrial Fibrillation. Circ Res. 2020;127:128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan NY and Choy CC. Screening for atrial fibrillation in 13 122 Hong Kong citizens with smartphone electrocardiogram. Heart. 2017;103:24–31. [DOI] [PubMed] [Google Scholar]
- 13.Engdahl J, Andersson L, Mirskaya M and Rosenqvist M. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation. 2013;127:930–7. [DOI] [PubMed] [Google Scholar]
- 14.Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C and Gravenor MB. Assessment of Remote Heart Rhythm Sampling Using the AliveCor Heart Monitor to Screen for Atrial Fibrillation: The REHEARSE-AF Study. Circulation. 2017;136:1784–1794. [DOI] [PubMed] [Google Scholar]
- 15.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V and Rosenqvist M. Mass Screening for Untreated Atrial Fibrillation: The STROKESTOP Study. Circulation. 2015;131:2176–84. [DOI] [PubMed] [Google Scholar]
- 16.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M, Turakhia MP and Apple Heart Study I. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, Yan L, Xing Y, Shi H, Li S, Liu Y, Liu F, Feng M, Chen Y, Lip GYH and Investigators MI. Mobile Photoplethysmographic Technology to Detect Atrial Fibrillation. J Am Coll Cardiol. 2019;74:2365–2375. [DOI] [PubMed] [Google Scholar]
- 18.Lubitz SA, Faranesh AZ, Selvaggi C, AS J, McManus DD, Singer DE, Pagoto S, McConnell MV, Pantelopoulos A and Foulkes AS. Detection of Atrial Fibrillation in a Large Population using Wearable Devices: the Fitbit Heart Study. Circulation. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus GM. The Apple Watch can detect atrial fibrillation: so what now? Nat Rev Cardiol. 2020;17:135–136. [DOI] [PubMed] [Google Scholar]
- 20.Boriani G, Schnabel RB, Healey JS, Lopes RD, Verbiest-van Gurp N, Lobban T, Camm JA and Freedman B. Consumer-led screening for atrial fibrillation using consumer-facing wearables, devices and apps: A survey of health care professionals by AF-SCREEN international collaboration. Eur J Intern Med. 2020;82:97–104. [DOI] [PubMed] [Google Scholar]
- 21.Ding EY, Svennberg E, Wurster C, Duncker D, Manninger M, Lubitz SA, Dickson E, Fitzgibbons TP, Akoum N, Al-Khatib SM, Attia ZI, Ghanbari H, Marrouche NF, Mendenhall GS, Peters NS, Tarakji KG, Turakhia M, Wan EY and McManus DD. Survey of current perspectives on consumer-available digital health devices for detecting atrial fibrillation. Cardiovasc Digit Health J. 2020;1:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson JMG and Jungner G. Principles and Practice of Screening for Disease. Who Chronicle. 1968;22:473–&. [Google Scholar]
- 23.Extramiana F and Steg PG. Atrial Fibrillation Screening: The Tools Are Ready, But Should We Do It? Circulation. 2022;145:955–958. [DOI] [PubMed] [Google Scholar]
- 24.Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim YH, Lip GY, Ma CS, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR, Nattel S and Document R. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18:1455–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau JK, Lowres N, Neubeck L, Brieger DB, Sy RW, Galloway CD, Albert DE and Freedman SB. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013;165:193–4. [DOI] [PubMed] [Google Scholar]
- 26.Lowres N, Mulcahy G, Gallagher R, Ben Freedman S, Marshman D, Kirkness A, Orchard J and Neubeck L. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg. 2016;50:44–51. [DOI] [PubMed] [Google Scholar]
- 27.Bashar SK, Han D, Hajeb-Mohammadalipour S, Ding E, Whitcomb C, McManus DD and Chon KH. Atrial Fibrillation Detection from Wrist Photoplethysmography Signals Using Smartwatches. Sci Rep. 2019;9:15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stergiou GS, Kyriakoulis KG, Stambolliu E, Destounis A, Karpettas N, Kalogeropoulos P and Kollias A. Blood pressure measurement in atrial fibrillation: review and meta-analysis of evidence on accuracy and clinical relevance. J Hypertens. 2019;37:2430–2441. [DOI] [PubMed] [Google Scholar]
- 29.Svendsen JH, Diederichsen SZ, Hojberg S, Krieger DW, Graff C, Kronborg C, Olesen MS, Nielsen JB, Holst AG, Brandes A, Haugan KJ and Kober L. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet. 2021;398:1507–1516. [DOI] [PubMed] [Google Scholar]
- 30.Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J and Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet. 2021;398:1498–1506. [DOI] [PubMed] [Google Scholar]
- 31.Taggar JS, Coleman T, Lewis S, Heneghan C and Jones M. Accuracy of methods for diagnosing atrial fibrillation using 12-lead ECG: A systematic review and meta-analysis. Int J Cardiol. 2015;184:175–83. [DOI] [PubMed] [Google Scholar]
- 32.Wegner FK, Kochhauser S, Ellermann C, Lange PS, Frommeyer G, Leitz P, Eckardt L and Dechering DG. Prospective blinded Evaluation of the smartphone-based AliveCor Kardia ECG monitor for Atrial Fibrillation detection: The PEAK-AF study. Eur J Intern Med. 2020;73:72–75. [DOI] [PubMed] [Google Scholar]
- 33.Park J, Lee S and Jeon M. Atrial fibrillation detection by heart rate variability in Poincare plot. Biomed Eng Online. 2009;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahajan R, Perera T, Elliott AD, Twomey DJ, Kumar S, Munwar DA, Khokhar KB, Thiyagarajah A, Middeldorp ME, Nalliah CJ, Hendriks JML, Kalman JM, Lau DH and Sanders P. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39:1407–1415. [DOI] [PubMed] [Google Scholar]
- 35.Haverkamp W, Butler J and Anker SD. Can we trust a smartwatch ECG? Potential and limitations. Eur J Heart Fail. 2021;23:850–853. [DOI] [PubMed] [Google Scholar]
- 36.Avram R, Ramsis M, Cristal AD, Nathan V, Zhu L, Kim J, Kuang J, Gao A, Vittinghoff E, Rohdin-Bibby L, Yogi S, Seremet E, Carp V, Badilini F, Pletcher MJ, Marcus GM, Mortara D and Olgin JE. Validation of an algorithm for continuous monitoring of atrial fibrillation using a consumer smartwatch. Heart Rhythm. 2021;18:1482–1490. [DOI] [PubMed] [Google Scholar]
- 37.Rajakariar K, Koshy AN, Sajeev JK, Nair S, Roberts L and Teh AW. Modified positioning of a smartphone based single-lead electrocardiogram device improves detection of atrial flutter. J Electrocardiol. 2018;51:884–888. [DOI] [PubMed] [Google Scholar]
- 38.Hannun AY, Rajpurkar P, Haghpanahi M, Tison GH, Bourn C, Turakhia MP and Ng AY. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH and Investigators A. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 40.Healey JS, Alings M, Ha A, Leong-Sit P, Birnie DH, de Graaf JJ, Freericks M, Verma A, Wang J, Leong D, Dokainish H, Philippon F, Barake W, McIntyre WF, Simek K, Hill MD, Mehta SR, Carlson M, Smeele F, Pandey AS, Connolly SJ and Investigators A-I. Subclinical Atrial Fibrillation in Older Patients. Circulation. 2017;136:1276–1283. [DOI] [PubMed] [Google Scholar]
- 41.Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, Wachter R, Pouliot E, Ziegler PD and Investigators RA. Incidence of Previously Undiagnosed Atrial Fibrillation Using Insertable Cardiac Monitors in a High-Risk Population: The REVEAL AF Study. JAMA Cardiol. 2017;2:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nasir JM, Pomeroy W, Marler A, Hann M, Baykaner T, Jones R, Stoll R, Hursey K, Meadows A, Walker J and Kindsvater S. Predicting Determinants of Atrial Fibrillation or Flutter for Therapy Elucidation in Patients at Risk for Thromboembolic Events (PREDATE AF) Study. Heart Rhythm. 2017;14:955–961. [DOI] [PubMed] [Google Scholar]
- 43.Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, Cook J, Paraschos A, Love J, Radoslovich G, Lee KL, Lamas GA and Investigators M. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107:1614–9. [DOI] [PubMed] [Google Scholar]
- 44.Van Gelder IC, Healey JS, Crijns H, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau CP, Morillo CA, Hobbelt AH, Rienstra M and Connolly SJ. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 45.Rahimi K. Subclinical atrial fibrillation in need of more assertive evidence. Eur Heart J. 2017;38:1345–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uittenbogaart SB, Lucassen WAM, van Etten-Jamaludin FS, de Groot JR and van Weert H. Burden of atrial high-rate episodes and risk of stroke: a systematic review. Europace. 2018;20:1420–1427. [DOI] [PubMed] [Google Scholar]
- 47.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW and Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. [DOI] [PubMed] [Google Scholar]
- 48.Arya A, Piorkowski C, Sommer P, Kottkamp H and Hindricks G. Clinical implications of various follow up strategies after catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2007;30:458–62. [DOI] [PubMed] [Google Scholar]
- 49.Lubitz SA, Atlas SJ, Ashburner JM, Lipsanopoulos ATT, Borowsky LH, Guan W, Khurshid S, Ellinor PT, Chang Y, McManus DD and Singer DE. Screening for Atrial Fibrillation in Older Adults at Primary Care Visits: VITAL-AF Randomized Controlled Trial. Circulation. 2022;145:946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Zhang J, Li HB, Chen YX, Yang B, Guo YT and Chen YD. Validation of Single Centre Pre-Mobile Atrial Fibrillation Apps for Continuous Monitoring of Atrial Fibrillation in a Real-World Setting: Pilot Cohort Study. J Med Internet Res. 2019;21:e14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quer G, Freedman B and Steinhubl SR. Screening for atrial fibrillation: predicted sensitivity of short, intermittent electrocardiogram recordings in an asymptomatic at-risk population. Europace. 2020;22:1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diederichsen SZ, Haugan KJ, Kronborg C, Graff C, Hojberg S, Kober L, Krieger D, Holst AG, Nielsen JB, Brandes A and Svendsen JH. Comprehensive Evaluation of Rhythm Monitoring Strategies in Screening for Atrial Fibrillation: Insights From Patients at Risk Monitored Long Term With an Implantable Loop Recorder. Circulation. 2020;141:1510–1522. [DOI] [PubMed] [Google Scholar]
- 53.Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, Elkind MSV, Ziegler PD, Kaplon RE, Sherfesee L, Wachter R and Investigators RA. Rhythm monitoring strategies in patients at high risk for atrial fibrillation and stroke: A comparative analysis from the REVEAL AF study. Am Heart J. 2020;219:128–136. [DOI] [PubMed] [Google Scholar]
- 54.Kirchhof P, Blank BF, Calvert M, Camm AJ, Chlouverakis G, Diener HC, Goette A, Huening A, Lip GYH, Simantirakis E and Vardas P. Probing oral anticoagulation in patients with atrial high rate episodes: Rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH-AFNET 6) trial. Am Heart J. 2017;190:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopes RD, Alings M, Connolly SJ, Beresh H, Granger CB, Mazuecos JB, Boriani G, Nielsen JC, Conen D, Hohnloser SH, Mairesse GH, Mabo P, Camm AJ and Healey JS. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137–145. [DOI] [PubMed] [Google Scholar]
- 56.Mant J, Fitzmaurice DA, Hobbs FD, Jowett S, Murray ET, Holder R, Davies M and Lip GY. Accuracy of diagnosing atrial fibrillation on electrocardiogram by primary care practitioners and interpretative diagnostic software: analysis of data from screening for atrial fibrillation in the elderly (SAFE) trial. BMJ. 2007;335:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.da Costa FA, Mala-Ladova K, Lee V, Tous S, Papastergiou J, Griffiths D, Chaumais MC, Hersberger KE, Viola R, Paulino E, Lobban T, Neubeck L, Freedman B and Antoniou S. Awareness campaigns of atrial fibrillation as an opportunity for early detection by pharmacists: an international cross-sectional study. J Thromb Thrombolysis. 2020;49:606–617. [DOI] [PubMed] [Google Scholar]
- 58.Martinez C, Wallenhorst C, Rietbrock S and Freedman B. Ischemic Stroke and Transient Ischemic Attack Risk Following Vitamin K Antagonist Cessation in Newly Diagnosed Atrial Fibrillation: A Cohort Study. J Am Heart Assoc. 2020;9:e014376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singer DE, Ziegler PD, Koehler JL, Sarkar S and Passman RS. Temporal Association Between Episodes of Atrial Fibrillation and Risk of Ischemic Stroke. JAMA Cardiol. 2021;6:1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garnvik LE, Malmo V, Janszky I, Ellekjaer H, Wisloff U, Loennechen JP and Nes BM. Physical activity, cardiorespiratory fitness, and cardiovascular outcomes in individuals with atrial fibrillation: the HUNT study. Eur Heart J. 2020;41:1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH and Sanders P. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol. 2015;65:2159–69. [DOI] [PubMed] [Google Scholar]
- 62.Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, Prabhu S, Stub D, Azzopardi S, Vizi D, Wong G, Nalliah C, Sugumar H, Wong M, Kotschet E, Kaye D, Taylor AJ and Kistler PM. Alcohol Abstinence in Drinkers with Atrial Fibrillation. N Engl J Med. 2020;382:20–28. [DOI] [PubMed] [Google Scholar]
- 63.Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, Wen J, Xing Y, Wu F, Xia Y, Liu T, Wu F, Liang Z, Liu F, Zhao Y, Li R, Li X, Zhang L, Guo J, Burnside G, Chen Y, Lip GYH and m AFAIITI. Mobile Health Technology to Improve Care for Patients With Atrial Fibrillation. J Am Coll Cardiol. 2020;75:1523–1534. [DOI] [PubMed] [Google Scholar]
- 64.Aronsson M, Svennberg E, Rosenqvist M, Engdahl J, Al-Khalili F, Friberg L, Frykman-Kull V and Levin LA. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace. 2015;17:1023–9. [DOI] [PubMed] [Google Scholar]
- 65.Tarride JE, Quinn FR, Blackhouse G, Sandhu RK, Burke N, Gladstone DJ, Ivers NM, Dolovich L, Thornton A, Nakamya J, Ramasundarahettige C, Frydrych PA, Henein S, Ng K, Congdon V, Birtwhistle RV, Ward R and Healey JS. Is Screening for Atrial Fibrillation in Canadian Family Practices Cost-Effective in Patients 65 Years and Older? Can J Cardiol. 2018;34:1522–1525. [DOI] [PubMed] [Google Scholar]
- 66.Freedman B and Lowres N. High-intensity atrial fibrillation screening to prevent stroke. Lancet. 2021;398:1465–1467. [DOI] [PubMed] [Google Scholar]
- 67.Friberg L and Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J. 2018;39:453–460. [DOI] [PubMed] [Google Scholar]
- 68.Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J, Commerford P, Jansky P, Avezum A, Sigamani A, Damasceno A, Reilly P, Grinvalds A, Nakamya J, Aje A, Almahmeed W, Moriarty A, Wallentin L, Yusuf S, Connolly SJ, Registry R-LAF and Cohort Study I. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet. 2016;388:1161–9. [DOI] [PubMed] [Google Scholar]
- 69.Rivard L, Friberg L, Conen D, Healey JS, Berge T, Boriani G, Brandes A, Calkins H, Camm AJ, Yee Chen L, Lluis Clua Espuny J, Collins R, Connolly S, Dagres N, Elkind MSV, Engdahl J, Field TS, Gersh BJ, Glotzer TV, Hankey GJ, Harbison JA, Haeusler KG, Hills MT, Johnson LSB, Joung B, Khairy P, Kirchhof P, Krieger D, Lip GYH, Lochen ML, Madhavan M, Mairesse GH, Montaner J, Ntaios G, Quinn TJ, Rienstra M, Rosenqvist M, Sandhu RK, Smyth B, Schnabel RB, Stavrakis S, Themistoclakis S, Van Gelder IC, Wang JG and Freedman B. Atrial Fibrillation and Dementia: A Report From the AF-SCREEN International Collaboration. Circulation. 2022;145:392–409. [DOI] [PubMed] [Google Scholar]
- 70.Chen E, Jiang J, Su R, Gao M, Zhu S, Zhou J and Huo Y. A new smart wristband equipped with an artificial intelligence algorithm to detect atrial fibrillation. Heart Rhythm. 2020;17:847–853. [DOI] [PubMed] [Google Scholar]
- 71.Tison GH, Sanchez JM, Ballinger B, Singh A, Olgin JE, Pletcher MJ, Vittinghoff E, Lee ES, Fan SM, Gladstone RA, Mikell C, Sohoni N, Hsieh J and Marcus GM. Passive Detection of Atrial Fibrillation Using a Commercially Available Smartwatch. JAMA Cardiol. 2018;3:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sensitivity Trevethan R., Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front Public Health. 2017;5:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan PH, Wong CK, Pun L, Wong YF, Wong MM, Chu DW and Siu CW. Head-to-Head Comparison of the AliveCor Heart Monitor and Microlife WatchBP Office AFIB for Atrial Fibrillation Screening in a Primary Care Setting. Circulation. 2017;135:110–112. [DOI] [PubMed] [Google Scholar]
- 74.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 75.Rogers EM. Diffusion of innovations. 5th ed. ed. New York, N.Y.: Simon & Schuster; 2003. [Google Scholar]
- 76.Verbiest-van Gurp N, van Mil D, van Kesteren HAM, Knottnerus JA and Stoffers H. How do Dutch general practitioners detect and diagnose atrial fibrillation? Results of an online case vignette study. BMC Fam Pract. 2019;20:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campion EW and Jarcho JA. Watched by Apple. N Engl J Med. 2019;381:1964–1965. [DOI] [PubMed] [Google Scholar]
- 78.Orchard JJ, Neubeck L, Orchard JW, Puranik R, Raju H, Freedman B, La Gerche A and Semsarian C. ECG-based cardiac screening programs: Legal, ethical, and logistical considerations. Heart Rhythm. 2019;16:1584–1591. [DOI] [PubMed] [Google Scholar]
- 79.Gerke S, Minssen T and Cohen G. Ethical and legal challenges of artificial intelligence-driven healthcare. Artif Intell Healthcare. 2020;12:295–336. [Google Scholar]
- 80.Segura Anaya LH, Alsadoon A, Costadopoulos N and Prasad PWC. Ethical Implications of User Perceptions of Wearable Devices. Sci Eng Ethics. 2018;24:1–28. [DOI] [PubMed] [Google Scholar]
- 81.Torp-Pedersen C, Goette A, Nielsen PB, Potpara T, Fauchier L, John Camm A, Arbelo E, Boriani G, Skjoeth F, Rumsfeld J, Masoudi F, Guo Y, Joung B, Refaat MM, Kim YH, Albert CM, Piccini J, Avezum A, Lip GYH and External R. ‘Real-world’ observational studies in arrhythmia research: data sources, methodology, and interpretation. A position document from European Heart Rhythm Association (EHRA), endorsed by Heart Rhythm Society (HRS), Asia-Pacific HRS (APHRS), and Latin America HRS (LAHRS). Europace. 2020;22:831–832. [DOI] [PubMed] [Google Scholar]
- 82.Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, Carter RE, Yao X, Rabinstein AA, Erickson BJ, Kapa S and Friedman PA. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394:861–867. [DOI] [PubMed] [Google Scholar]
- 83.Clifford GD, Liu C, Moody B, Lehman LH, Silva I, Li Q, Johnson AE and Mark RG. AF Classification from a Short Single Lead ECG Recording: the PhysioNet/Computing in Cardiology Challenge 2017. Comput Cardiol 2017;44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rush KL, Burton L, Van Der Merwe F, Hatt L and Galloway C. Atrial fibrillation care in rural communities: a mixed methods study of physician and patient perspectives. BMC Fam Pract. 2019;20:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thrysoee L, Stromberg A, Brandes A and Hendriks JM. Management of newly diagnosed atrial fibrillation in an outpatient clinic setting-patient’s perspectives and experiences. J Clin Nurs. 2018;27:601–611. [DOI] [PubMed] [Google Scholar]
- 86.Orchard J, Freedman SB, Lowres N, Peiris D and Neubeck L. iPhone ECG screening by practice nurses and receptionists for atrial fibrillation in general practice: the GP-SEARCH qualitative pilot study. Aust Fam Physician. 2014;43:315–9. [PubMed] [Google Scholar]
- 87.Marquie JC and Huet N. Age differences in feeling-of-knowing and confidence judgements as a function of knowledge domain. Psychol Aging. 2000;15:451–60. [DOI] [PubMed] [Google Scholar]
- 88.Barnard Y, Bradley MD, Hodgson F and Lloyd AD. Learning to use new technologies by older adults: Perceived difficulties, experimentation behaviour and usability. Comput Human Behavior. 2013;29:1715–1724. [Google Scholar]
- 89.Dinh-Le C, Chuang R, Chokshi S and Mann D. Wearable Health Technology and Electronic Health Record Integration: Scoping Review and Future Directions. JMIR Mhealth Uhealth. 2019;7:e12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verbrugge FH, Proesmans T, Vijgen J, Mullens W, Rivero-Ayerza M, Van Herendael H, Vandervoort P and Nuyens D. Atrial fibrillation screening with photo-plethysmography through a smartphone camera. Europace. 2019;21:1167–1175. [DOI] [PubMed] [Google Scholar]


