Abstract
Recent data from the European Antimicrobial Resistance Surveillance Network (EARS-Net) show a large increase of +57% in Acinetobacter species bloodstream infections in the European Union and European Economic Area in the first years of the COVID-19 pandemic (2020–2021) compared with 2018–2019. Most were resistant to carbapenems, from intensive care units, and in countries with ≥ 50% carbapenem resistance in Acinetobacter spp. in 2018–2019. This highlights the requirement for reinforced Acinetobacter preparedness and infection prevention and control in Europe.
Keywords: Acinetobacter, bacteraemia, EU/EEA, COVID-19, pandemic
Bloodstream infections (BSIs) with Acinetobacter species commonly have poor outcomes, especially in intensive care unit (ICU) patients [1]. Acinetobacter spp. is intrinsically resistant to many antimicrobials, and additional acquired resistance further complicates the treatment of serious infections in already vulnerable patient groups. Recent data from the European Antimicrobial Resistance Surveillance Network (EARS-Net) show a large and statistically significant increase in reports of Acinetobacter spp. BSIs in the European Union (EU) and European Economic Area (EEA) during the period from 2017 to 2021 [2]. Most of this increase occurred in 2020 and 2021, the first years of the coronavirus disease (COVID-19) pandemic. Here we further explore this trend in a subset of data from laboratories that continuously reported data during that period.
Data description
Our data originate from qualitative routine antimicrobial susceptibility testing (AST) results of blood isolates collected by local clinical laboratories in national networks in EU/EEA countries. These results are reported annually by national centres to the European Centre for Disease Prevention and Control (ECDC), according to the EARS-Net reporting protocol [3]. In its analyses, EARS-Net only includes the first isolate per patient each year and for each bacterial species.
All EU countries, Iceland and Norway reported data to EARS-Net every year during the period 2017 to 2021 [2,4]. For this analysis, we restricted the dataset to BSIs with Acinetobacter spp. and to only those laboratories that reported carbapenem (imipenem and/or meropenem) antimicrobial susceptibility testing results for Acinetobacter spp. for every year in 2017 to 2021 (255 of 826 laboratories reporting, on average, per year). We made this restriction to limit bias from year-to-year changes in the number, hospital affiliation and type of reporting laboratories, and because not all countries can discriminate between laboratories that did not report and those that had no cases. The United Kingdom ceased reporting data to ECDC in 2020 when it withdrew from the EU and was hence not included. In addition, France was excluded because, following a major reorganisation of national surveillance in 2020, only a few laboratories were continuously identifiable. The Table presents data for the 28 included countries, with and without restriction to continuously reporting laboratories.
Table. Annual carbapenem-resistant Acinetobacter species bloodstream infections and carbapenem susceptibility testing results for Acinetobacter species bloodstream infections, in all laboratories that reported data to EARS-Net and in those that continuously reported data, EU/EEA countries, 2017–2021 (n = 31,242 infections).
| Carbapenem susceptibility test result | 2017 | 2018 | 2019 | 2020 | 2021 | Number of cases in 2020–2021 vs 2018–2019 |
Number of cases in 2020 vs average 2017–2019 |
Number of cases in 2021 vs average 2017–2019 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | % Change | p valuec | % Change | p valuec | % Change | p valuec | |
| All laboratories (annual mean = 826 laboratories)a | ||||||||||||||||
| R | 2,831 | 59.2 | 3,105 | 60.1 | 2,629 | 56.5 | 4,379 | 65.2 | 7,396 | 74.5 | +105.4 | <0.001d | +53.4 | <0.001d | +159.1 | <0.001d |
| S/I | 1,950 | 40.8 | 2,063 | 39.9 | 2,028 | 43.5 | 2,333 | 34.8 | 2,528 | 25.5 | +18.8 | <0.001 | +15.9 | <0.001 | +25.5 | <0.001 |
| All | 4,781 | 100.0 | 5,168 | 100.0 | 4,657 | 100.0 | 6,712 | 100.0 | 9,924 | 100.0 | +69.3 | <0.001 | +37.9 | <0.001 | +103.8 | <0.001 |
| Continuously reporting laboratories (n = 255 laboratories)a,b | ||||||||||||||||
| R | 1,237 | 48.5 | 1,293 | 48.3 | 1,354 | 48.4 | 1,891 | 57.6 | 3,767 | 70.8 | +113.8 | <0.001d | +46.1 | <0.001d | +191.0 | <0.001d |
| R (Group 1 countriese) | 30 | 3.6 | 37 | 4.4 | 17 | 1.9 | 29 | 3.3 | 23 | 2.3 | −3.7 | 0.85 | +3.6 | 0.90 | −17.9 | 0.49 |
| R (Group 2 countriese) | 60 | 35.3 | 38 | 21.5 | 26 | 20.6 | 30 | 22.7 | 104 | 49.8 | +109.4 | <0.001d | −27.4 | 0.18 | +151,6 | <0.001d |
| R (Group 3 countriese) | 1,147 | 74.6 | 1,218 | 73.5 | 1,311 | 72.9 | 1,832 | 80.6 | 3,640 | 88.3 | +116.4 | <0.001d | +49.5 | <0.001d | +197.1 | <0.001d |
| S/I | 1,314 | 51.5 | 1,382 | 51.7 | 1,444 | 51.6 | 1,390 | 42.4 | 1,554 | 29.2 | +4.2 | 0.12 | +0.7 | 0.85 | +12.6 | 0.001 |
| S/I/R | 2,551 | 100.0 | 2,675 | 100.0 | 2,798 | 100.0 | 3,281 | 100.0 | 5,321 | 100.0 | +57.2 | <0.001 | +22.7 | <0.001 | +98.9 | <0.001 |
EARS-Net: European Antimicrobial Resistance Surveillance Network; EEA: European Economic Area; EU: European Union; I: susceptible, increased exposure; R: resistant; S: susceptible, standard dosing regimen.
a Data for France and the United Kingdom were excluded.
b Only includes EU/EEA laboratories that were identifiable as having reported ≥ 1 Acinetobacter spp. isolate with carbapenem susceptibility data every year in 2017–2021.
c Poisson regression model to assess the statistical significance of changes in the numbers of bloodstream infections.
d The chi-squared test comparing the percentage of carbapenem resistance in 2020–2021 vs 2018–2019 also had p < 0.001.
e The reporting countries were grouped according to the mean of their crude, national, annual percentage of Acinetobacter spp. resistance to carbapenems in 2018 and 2019. These were Group 1 (< 10% carbapenem resistance in 2018–2019): Austria, Belgium, Denmark, Estonia, Finland, Germany, Iceland, Ireland, Luxembourg, Malta, the Netherlands, Norway and Sweden; Group 2 (10% to < 50% carbapenem resistance in 2018–2019): Czechia, Portugal, and Slovenia; Group 3 (≥ 50% carbapenem resistance in 2018–2019): Bulgaria, Croatia, Cyprus, Greece, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Slovakia and Spain. Percentages in these rows refer to carbapenem-resistant isolates among all isolates analysed for the countries in a given group. The raw data for the group level are provided in the Supplement.
As the resistance percentages for Acinetobacter spp. varied substantially between EU/EEA countries [4], we grouped the countries according to their mean national annual carbapenem resistance percentage in 2018—2019. Countries in Group 1 had < 10% carbapenem resistance (n = 13; Austria, Belgium, Denmark, Estonia, Finland, Germany, Iceland, Ireland, Luxembourg, Malta, the Netherlands, Norway, Sweden), Group 2 had 10% to < 50% carbapenem resistance (n = 3: Czechia, Portugal, Slovenia) and Group 3 had ≥ 50% carbapenem resistance (n = 12: Bulgaria, Croatia, Cyprus, Greece, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Slovakia, Spain). When stratifying by patient ward type, we grouped the units as ‘ICU’ (adult and paediatric ICUs), ‘not ICU’ (all other ward types) and ‘unknown ward type’ (no information available on ward type).
We assessed the statistical significance of changes in the numbers of BSIs and in the percentage of carbapenem resistance comparing 2020–2021 with 2018–2019, using Stata Statistical Software (Release 15.1. College Station, TX: StataCorp LLC) for a Poisson regression model and a chi-squared test, respectively, with p values < 0.05 considered as significant.
Trends in Acinetobacter species bloodstream infections from continuously reporting EU/EEA laboratories
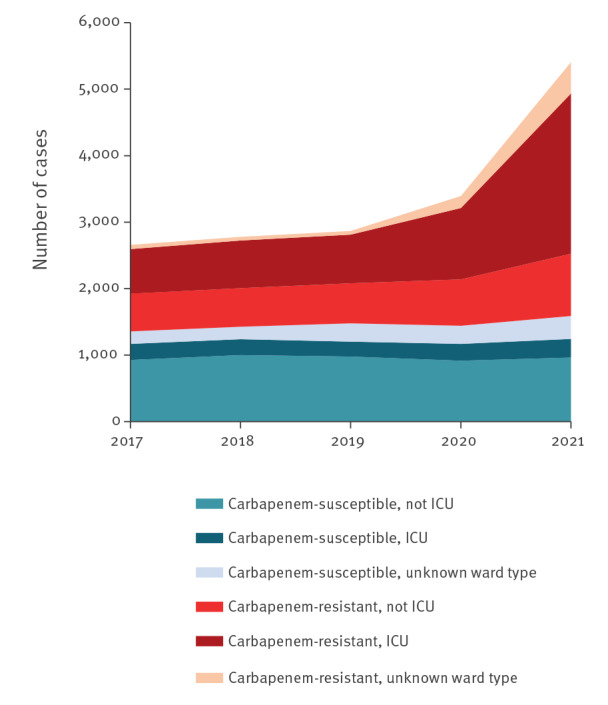
The total number of Acinetobacter spp. BSIs reported in 2020–2021 increased by + 57% compared with 2018–2019 (p < 0.001). Most of this increase was due to carbapenem-resistant Acinetobacter spp. BSIs, with the number of reports increasing by + 114% (p < 0.001), and the carbapenem resistance percentage increasing from 48.4% in 2018–2019 to 65.8% in 2020–2021 (p < 0.001) (Table). The number of carbapenem-resistant Acinetobacter spp. BSIs increased more among ICU patients (+ 144%) than non-ICU patients (+ 41%) (Figure 1). The small increase in the number of carbapenem-susceptible Acinetobacter spp. BSIs in 2020–2021 compared with 2018–2019 was not significant (p = 0.12).
Figure 1.
Acinetobacter species bloodstream infections reported by laboratories that continuously reported data to EARS-Net, by carbapenem susceptibility testing result and type of patient ward, EU/EEA, 2017–2021 (n = 16,626)
EARS-Net: European Antimicrobial Resistance Surveillance Network; EEA: European Economic Area; EU: European Union; ICU: intensive care unit.
Cases labelled carbapenem-susceptible include I (susceptible, increased exposure) and S (susceptible, standard dosing regimen).
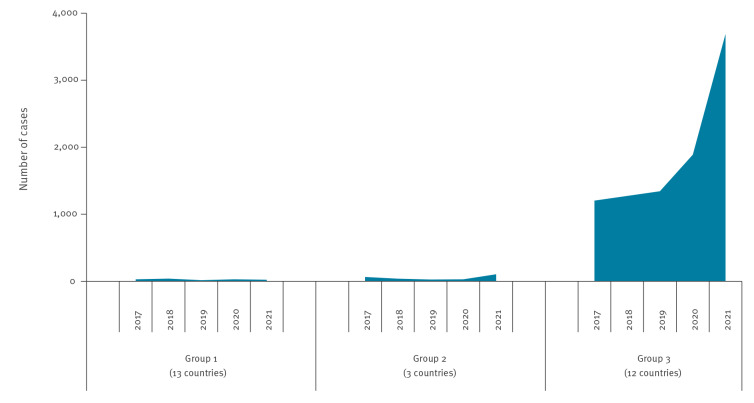
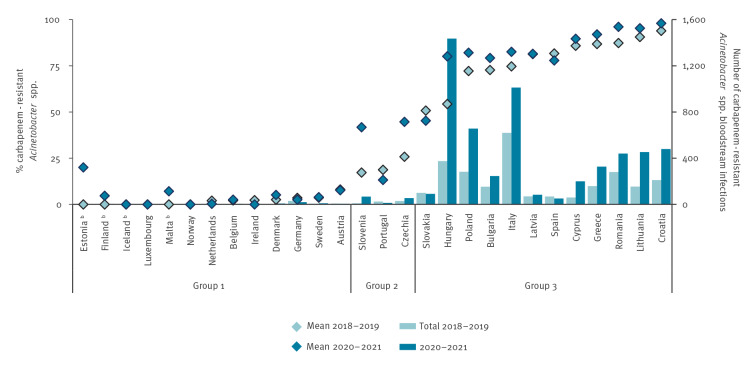
Countries in Group 3 (≥ 50% mean carbapenem resistance in Acinetobacter spp. in 2018–2019) experienced the most noticeable increases in the number of Acinetobacter spp. BSIs in 2020–2021. They had a statistically significant increase (p < 0.001) of + 116% in the number of reported cases in 2020–2021 (n = 5,472) compared with 2018–2019 (n = 2,529) (Table, Figure 2, Figure 3) [4]. In countries in Group 2, the increase was similar (+ 109%; p < 0.001), albeit with fewer reports per country (Table, Figure 3). Countries in Group 1 reported few cases (n = 52) in 2020–2021 and showed no significant change compared with 2018–2019 (n = 54; p = 0.85) (Table).
Figure 2.
Bloodstream infections with carbapenem-resistant Acinetobacter species, reported by laboratories that continuously reported data to EARS-Net, by country groupa and year, EU/EEA, 2017–2021 (n = 9,542)
EARS-Net: European Antimicrobial Resistance Surveillance Network; EEA: European Economic Area; EU: European Union.
a The reporting countries were grouped according to the mean of their crude, national, annual percentage of Acinetobacter spp. resistance to carbapenems in 2018 and 2019. These were Group 1 (< 10% carbapenem resistance in 2018–2019): Austria, Belgium, Denmark, Estonia, Finland, Germany, Iceland, Ireland, Luxembourg, Malta, the Netherlands, Norway and Sweden; Group 2 (10% to < 50% carbapenem resistance in 2018–2019): Czechia, Portugal, and Slovenia; Group 3 (≥ 50% carbapenem resistance in 2018–2019): Bulgaria, Croatia, Cyprus, Greece, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Slovakia and Spain.
Figure 3.
Percentage and number of bloodstream infections with carbapenem-resistant Acinetobacter species from laboratories that continuously reported data to EARS-Net, by country groupa , EU/EEA, 2018–2019 vs 2020–2021 (n = 9,542)
EARS-Net: European Antimicrobial Resistance Surveillance Network; EEA: European Economic Area; EU: European Union.
a The reporting countries were grouped according to the mean of their crude, national, annual percentage of Acinetobacter spp. resistance to carbapenems in 2018 and 2019. These were Group 1 (< 10% carbapenem resistance in 2018–2019): Austria, Belgium, Denmark, Estonia, Finland, Germany, Iceland, Ireland, Luxembourg, Malta, the Netherlands, Norway and Sweden; Group 2 (10% to < 50% carbapenem resistance in 2018–2019): Czechia, Portugal, and Slovenia; Group 3 (≥ 50% carbapenem resistance in 2018–2019): Bulgaria, Croatia, Cyprus, Greece, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Slovakia and Spain.
b Reported < 25 Acinetobacter spp. isolates in both periods 2018–2019 and 2020–2021. As the data are restricted to laboratories that reported continuously in 2017–2021, the percentages and numbers for countries may differ from the dataset that included all laboratories (Table) [2,4].
For Latvia, the two datapoints for the mean overlap and only the dark symbol is visible.
Discussion
The observed trends for Acinetobacter spp. BSI in the EU/EEA are worrying because resistance to carbapenems causes a high burden of disease in vulnerable hospitalised patients [5-7]. Our findings suggest that countries where carbapenem-resistant Acinetobacter spp. were already well established before the COVID-19 pandemic (Group 3) had the biggest challenges in controlling further spread in 2020–2021.
Acinetobacter spp. is difficult to eradicate from the hospital environment, colonising hospital patients and staff and causing outbreaks, particularly in ICUs [1]. Several reports have identified Acinetobacter spp. as one of the most frequent causes of infectious complications in hospitalised patients with COVID-19 [8-10]. The observed increasing trends at EU/EEA level compared with the pre-pandemic situation [2,11,12] were probably driven by the profound impact of the COVID-19 pandemic on hospital care, which increased the number of patients at risk of Acinetobacter spp. BSI, and also by difficulties in applying infection prevention and control (IPC) measures. In 2020–2021, there were larger numbers of severely ill patients, many with severe pulmonary infection. High occupancy rates necessitated increased provision of ICU beds, often with staff who were overworked or less experienced [13,14]. Inappropriate application of contact precautions for COVID-19 patients, in particular suboptimal hand hygiene, as well as contamination and insufficient cleaning of the hospital environment, probably contributed to direct or indirect between-patient Acinetobacter spp. transmission [15-20]. Finally, reduced attention to antimicrobial stewardship, with resulting increased carbapenem use, may have contributed [21].
For context, in 2020–2021 compared with 2018–2019, the laboratories that continuously reported Acinetobacter spp. data to EARS-Net also reported more cases of BSI with Enterococcus faecium (+ 29%), E. faecalis (+ 16%), Pseudomonas aeruginosa (+ 8%), Klebsiella pneumoniae (+ 6%), but these differences were much less pronounced than for BSI with Acinetobacter spp. (+ 57%). Laboratories reported fewer cases of BSI with Streptococcus pneumoniae (− 47%), Escherichia coli (− 5%) and Staphylococcus aureus (− 1%). These differences probably depend on the epidemiological characteristics of the various pathogens. For example, S. pneumoniae and E. coli are more frequently transmitted in the community and in non-ICU hospital settings. During the COVID-19 pandemic, transmission of microorganisms in the community was affected by containment actions such as stay-at-home orders, physical distancing, hygiene measures and the use of face masks. This may have contributed to the sharp decline in typically community-acquired infections such as those caused by S. pneumoniae [22,23].
There were exceptions to the general trends by country group, indicating that the trends were not only explained by the pre-pandemic percentage of carbapenem resistance. For example, Portugal and Spain were outliers in their respective groups by reporting fewer Acinetobacter spp. BSIs in 2020–2021 than in 2018–2019, whereas Slovenia reported a larger increase in Acinetobacter spp. BSIs than other Group 2 countries.
Although reasons for the trends observed during the COVID-19 pandemic remain to be clarified, most factors that potentially favoured the increase in carbapenem-resistant Acinetobacter spp. infections, and in general multidrug-resistant microorganisms, are amenable to public health intervention. Options include rigorous adherence to hand hygiene, environmental cleaning, provision and appropriate use of personal protective equipment, appropriate training of healthcare staff, and promotion of antimicrobial stewardship programmes. While spread of carbapenem-resistant Acinetobacter spp. is difficult to control while established, recent evidence shows that Acinetobacter spp. outbreaks can be controlled through a bundle of measures including thorough environmental cleaning, even without ward closure [16,24]. Finally, any country with an increasing number of infections with carbapenem-resistant Acinetobacter spp. in 2020–2021, particularly those with comparatively moderate resistance percentages (e.g. 10% to < 50%, Group 2), should urgently ensure preparedness for the prevention and control of Acinetobacter spp. infections and outbreaks.
Conclusion
The large increase in carbapenem-resistant Acinetobacter spp. BSI in the EU/EEA during a time of great challenges for healthcare calls for reinforced application of the preparedness and response actions that we present above. Surveillance at local, national and EU/EEA levels will be vital to monitor whether this worrying development is halted or even reversed.
Ethical statement
This study only included anonymised surveillance data; therefore, ethical approval was not required.
Data availability statement
This manuscript presents a subset of data in an online database ‘the ECDC surveillance atlas of infectious diseases’ (ECDC Atlas) [4]. Although the ECDC Atlas contains the full dataset, users cannot generate the restricted dataset presented in our manuscript. Similarly, an upcoming ECDC publication, intended for publication on 17 November 2022 for European Antibiotic Awareness Day (EAAD; 18 November 2022), contains the full dataset, and a different restricted dataset. It is the ‘Antimicrobial resistance in the EU/EEA (EARS-Net) - Annual Epidemiological Report for 2021’ [2].
Acknowledgements
The authors acknowledge the work performed by the staff of the participating clinical microbiology laboratories and of the national healthcare services that provided data to EARS-Net.
Supplementary Data
EARS-Net Study Group participants
Reinhild Strauss, Federal Ministry of Social Affairs, Health, Care and Consumer Protection, Vienna, Austria
Karl Mertens, Sciensano, Brussels; Belgium
Stefana Sabtcheva, National Oncology Centre - USHATO, Bulgaria
Arjana Tambic Andrasevic, University Hospital for Infectious Diseases, Zagreb, Croatia
Panagiota Maikanti, Nicosia General Hospital, Nicosia, Cyprus
Helena Žemličková, National Institute of Public Health, Prague, Czech Republic
Henrik Hasman, Referencelaboratoriet for antibiotikaresistens og Stafylokokker, Copenhagen, Denmark
Marina Ivanova, East-Tallinn Central Hospital Laboratory, Tallinn, Estonia
Kati Räisänen, Finnish Institute for Health and Welfare, Helsinki, Finland
Sylvie Maugat, Public Health France, Saint-Maurice, France
Ines Noll, Robert Koch Institute, Berlin, Germany
Kassiani Mellou, National Public Health Organization, Athens, Greece
Ákos Tóth, National Public Health Center, Hungary
Kristján Orri Helgason, Landspitali University Hospital of Iceland, Iceland
Stephen Murchan, Health Protection Surveillance Centre, Ireland
Giulia Errico, Istituto Superiore di Sanità, Italy
Ieva Voita, Centre for Disease Prevention and Control of Latvia, Riga, Latvia
Esther Walser-Domjan, Office of Public Health, Liechtenstein
Jolanta Miciulevičienė, National Public Health Surveillance Laboratory, Vilnius, Lithuania
Monique Perrin, Laboratoire national de Santé, Luxembourg
Elizabeth Anne Scicluna, Infection Control Unit, Mater Dei Hospital, Malta
Sjoukje H. S. Woudt, National Institute for Public Health and the Environment, the Netherlands
Ørjan Samuelsen, Norwegian National Advisory Unit on Detection of Antimicrobial Resistance; University Hospital of North Norway, Norway
Dorota Żabicka, National Medicines Institute, Poland
Manuela Caniça, National Institute of Health Dr. Ricardo Jorge, Portugal
Gabriel Adrian Popescu, Carol Davila University of Medicine and Pharmacy, Romania
Eva Schréterová, University Hospital of P.J.Šafárik Košice, Košice, Slovakia
Helena Ribič, National Institute of Public Health, Slovenia
Maria Belén Aracil García. Resistance Antibiotics Laboratory, National Center of Microbiology, Spain
Hanna Billström, The Public Health Agency of Sweden, Sweden
Conflict of interest: None declared.
Authors’ contributions: Conceptualisation and design of the study, and writing of the first draft: CG, DLM, HM, LDH, PK. Initial interpretation of the results and revision of the draft: CG, DLM, DP, HM, LDH, PK. The EARS-Net Study Group, CG, DLM, DP, HM, LDH and PK contributed to acquisition and analysis of the data and interpretation of results, subsequently critically reviewing the final manuscript.
References
- 1.European Centre for Disease Prevention and Control (ECDC). Rapid risk assessment: Carbapenem-resistant Acinetobacter baumannii in healthcare settings – 8 December 2016. Stockholm: ECDC, 2016 Available from: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-carbapenem-resistant-acinetobacter-baumannii-healthcare
- 2.European Centre for Disease Prevention and Control (ECDC). Antimicrobial resistance in the EU/EEA (EARS-Net) - Annual epidemiological report for 2021. Stockholm: ECDC; 2022. Available from: https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/report
- 3.European Centre for Disease Prevention and Control (ECDC). TESSy – The European Surveillance System – antimicrobial resistance (AMR) reporting protocol 2022 – European Antimicrobial Resistance Surveillance Network (EARS-Net) surveillance data for 2021. Stockholm: ECDC; 2022. Available from: https://www.ecdc.europa.eu/en/publications-data/ears-net-reporting-protocol-2022
- 4.European Centre for Disease Prevention and Control (ECDC). Surveillance atlas of infectious diseases. Stockholm: ECDC; 2022. Date accessed: 25 October 2022. Available from: https://www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases
- 5. Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mestrovic T, Robles Aguilar G, Swetschinski LR, Ikuta KS, Gray AP, Davis Weaver N, et al. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis. Lancet Public Health. 2022;7(11):e897-913. 10.1016/S2468-2667(22)00225-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56-66. 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS One. 2021;16(5):e0251170. 10.1371/journal.pone.0251170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Toole RF. The interface between COVID-19 and bacterial healthcare-associated infections. Clin Microbiol Infect. 2021;27(12):1772-6. 10.1016/j.cmi.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Protonotariou E, Mantzana P, Meletis G, Tychala A, Kassomenaki A, Vasilaki O, et al. Microbiological characteristics of bacteremias among COVID-19 hospitalized patients in a tertiary referral hospital in Northern Greece during the second epidemic wave. FEMS Microbes. 2021;2:xtab021. 10.1093/femsmc/xtab021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lötsch F, Albiger B, Monnet DL, Struelens MJ, Seifert H, Kohlenberg A, et al. Epidemiological situation, laboratory capacity and preparedness for carbapenem-resistant Acinetobacter baumannii in Europe, 2019. Euro Surveill. 2020;25(45):2001735. 10.2807/1560-7917.ES.2020.25.45.2001735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lepape A, Jean A, De Waele J, Friggeri A, Savey A, Vanhems P, et al. European intensive care physicians’ experience of infections due to antibiotic-resistant bacteria. Antimicrob Resist Infect Control. 2020;9(1):1. 10.1186/s13756-019-0662-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leo CG, Sabina S, Tumolo MR, Bodini A, Ponzini G, Sabato E, et al. Burnout among healthcare workers in the COVID 19 era: A review of the existing literature. Front Public Health. 2021;9:750529. 10.3389/fpubh.2021.750529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sexton JB, Adair KC, Proulx J, Profit J, Cui X, Bae J, et al. Emotional exhaustion among US health care workers before and during the COVID-19 pandemic, 2019-2021. JAMA Netw Open. 2022;5(9):e2232748. 10.1001/jamanetworkopen.2022.32748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meda M, Gentry V, Reidy P, Garner D. Unintended consequences of long-sleeved gowns in a critical care setting during the COVID-19 pandemic. J Hosp Infect. 2020;106(3):605-9. 10.1016/j.jhin.2020.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perez S, Innes GK, Walters MS, Mehr J, Arias J, Greeley R, et al. Increase in hospital-acquired carbapenem-resistant Acinetobacter baumannii infection and colonization in an acute care hospital during a surge in COVID-19 admissions - New Jersey, February-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(48):1827-31. 10.15585/mmwr.mm6948e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tanne JH. Covid-19: Antimicrobial resistance rose dangerously in US during pandemic, CDC says. BMJ. 2022;378:o1755. . 10.1136/bmj.o1755 [DOI] [PubMed] [Google Scholar]
- 18. Thoma R, Seneghini M, Seiffert SN, Vuichard Gysin D, Scanferla G, Haller S, et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob Resist Infect Control. 2022;11(1):12. 10.1186/s13756-022-01052-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomczyk S, Taylor A, Brown A, de Kraker MEA, El-Saed A, Alshamrani M, et al. Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: a global survey. J Antimicrob Chemother. 2021;76(11):3045-58. . 10.1093/jac/dkab300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United States Centres for Disease Control and Prevention (US CDC). COVID-19: U.S. impact on antimicrobial resistance, special report 2022. Atlanta: CDC; 2022. Available from: https://stacks.cdc.gov/view/cdc/117915
- 21. Langford BJ, So M, Raybardhan S, Leung V, Soucy JR, Westwood D, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27(4):520-31. 10.1016/j.cmi.2020.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brueggemann AB, Jansen van Rensburg MJ, Shaw D, McCarthy ND, Jolley KA, Maiden MCJ, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health. 2021;3(6):e360-70. 10.1016/S2589-7500(21)00077-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rybak A, Levy C, Angoulvant F, Auvrignon A, Gembara P, Danis K, et al. Association of nonpharmaceutical interventions during the COVID-19 pandemic with invasive pneumococcal disease, pneumococcal carriage, and respiratory viral infections among children in France. JAMA Netw Open. 2022;5(6):e2218959. 10.1001/jamanetworkopen.2022.18959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meschiari M, Lòpez-Lozano JM, Di Pilato V, Gimenez-Esparza C, Vecchi E, Bacca E, et al. A five-component infection control bundle to permanently eliminate a carbapenem-resistant Acinetobacter baumannii spreading in an intensive care unit. Antimicrob Resist Infect Control. 2021;10(1):123. 10.1186/s13756-021-00990-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.