Abstract
The number of cases of Candida auris infection or carriage and of countries reporting cases and outbreaks increased in the European Union and European Economic Area during 2020 and 2021. Eight countries reported 335 such cases in 2020 and 13 countries 655 cases in 2021. Five countries experienced outbreaks while one country reported regional endemicity. These findings highlight the need for adequate laboratory capacity and surveillance for early detection of C. auris and rapid implementation of control measures.
Keywords: Europe, healthcare-associated infections, fungal infections, multidrug-resistance, outbreak, surveillance, Candida
The European Centre for Disease Prevention and Control (ECDC) conducted two surveys collecting information on the epidemiological situation, laboratory capacity and preparedness for Candida auris in the European Union and European Economic Area (EU/EEA) for the periods 2013 to 2017 and January 2018 to May 2019 [1,2], but this information was not updated after the start of the COVID-19 pandemic. Attention to C. auris was raised again after a large outbreak affecting healthcare facilities in two regions in Italy [3], resulting in the initiation of a third C. auris survey in April 2022 to update the information on the epidemiological situation and control efforts for C. auris in the EU/EEA.
Survey on the epidemiological situation, laboratory capacity and preparedness for Candida auris
The national focal points for healthcare-associated infections and their alternates were invited to complete the third C. auris survey on 4 April 2022. This survey included 14 questions on the aggregated number of cases of C. auris infection or carriage (in the following called C. auris cases) and outbreaks reported per year in the period from June 2019 to December 2021 (with the option to also add retrospectively identified cases for the period from January 2013 to May 2019), on the national capacity for laboratory identification and on preparedness for C. auris. The questions were the same as in the previous two surveys but included an additional question on the epidemiological stage (described below).
Reported cases
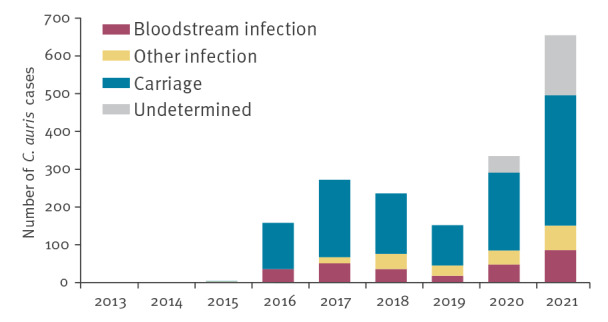
Replies to the survey were received from all 30 invited EU/EEA countries. Combining data from the three surveys, 1,812 C. auris cases were reported by 15 EU/EEA countries from 2013 to 2021. Case numbers by country and year are shown in Table 1. The number of reported cases nearly doubled between 2020 (335 cases reported by eight countries) and 2021 (655 cases reported by 13 countries) and were considerably higher than in previous years (Table 1, Figure 1). For most cases, carriage was reported (n = 1,146; 63.2%), while a bloodstream or another type of infection was reported for 277 (15.3%) and 186 (10.3%) cases, respectively. For the remaining 203 (11.2%) cases, no information on infection or carriage was available. Eleven EU/EEA countries had not detected any C. auris cases until 2021 and in four countries, information on C. auris cases was not available at national level (Table 1). In addition to the increase in the number of cases overall, the number of countries reporting C. auris cases increased, with a maximum of 13 countries reporting cases in 2021 (Table 1). Information on C. auris cases was collected for the period 2013 to 2021 in a standardised format. However, cases reported outside this period are mentioned in the footnotes to Table 1 if we believed that they represented a relevant change such as a new country affected or an earlier date of detection than previously known.
Table 1. Reported cases of Candida auris infection or carriage, EU/EEA, 2013–2021 (n = 1,812).
| Country | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2013–2021 |
|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | 4 |
| Belgium | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 1 | 5 |
| Bulgaria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Croatia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cyprus | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA | NA | NA |
| Czechia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Denmark | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Estonia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Finland | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Francea | 0 | 0 | 2 | 1 | 1 | 0 | 3 | 4 | 4 | 15 |
| Germany | 0 | 0 | 2 | 0 | 5 | 2 | 3 | 5 | 10 | 27 |
| Greece | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 13 | 58 | 74 |
| Hungary | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Iceland | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ireland | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Italy | NA | NA | NA | NA | NA | NA | 1 | 49 | 242 | 292 |
| Latvia | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Liechtenstein | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Lithuania | NA | NA | NA | NA | NA | NA | NA | 0 | 0 | 0 |
| Luxembourg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| The Netherlands | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 5 |
| Norway | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 4 |
| Poland | NA | NA | NA | NA | NA | NA | 2 | 0 | 0 | 2 |
| Portugalb | NA | NA | NA | NA | NA | NA | NA | 0 | 0 | 0 |
| Romania | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Slovakia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Slovenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spain | 0 | 0 | 0 | 155 | 266 | 230 | 135 | 260 | 331 | 1,377 |
| Sweden | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| EU/EEA | 0 | 0 | 4 | 158 | 272 | 236 | 152 | 335 | 655 | 1,812 |
EEA: European Economic Area; EU: European Union; NA: information not available at national level.
a France reported one case retrospectively identified in 2007 which is not included in this table [18].
b Portugal reported one C. auris case for 2022 which is not included in this table.
Cells including one or more cases are coloured in grey for better visibility.
Figure 1.
Reported cases of Candida auris infection or carriage, EU/EEA, 2013–2021 (n = 1,812)a
EEA: European Economic Area; EU: European Union.
a Data reported by the United Kingdom until 2019 were excluded to ensure comparability over time by including the same set of countries. For this reason, the absolute case numbers differ from the number of cases reported for 2013–2019 in previous reports [1,2].
Information on the classification of cases of C. auris infection or carriage as imported or locally acquired was not available for 1,758 (97.0%) cases. Forty-four (2.4%) cases were reported as imported and 10 (0.6%) as locally acquired. A systematic analysis of the origin of imported cases was not possible due to scarce information. For the few cases with available information, countries in Africa (Egypt, Ethiopia, Kenya, South Africa), the Middle East (Iraq, Kuwait, United Arab Emirates) and Asia (India, Pakistan) were mentioned. Of note, there was also one cross-border transfer within the EU/EEA of a patient with C. auris originating from Spain.
Reported outbreaks and epidemiological stage of dissemination
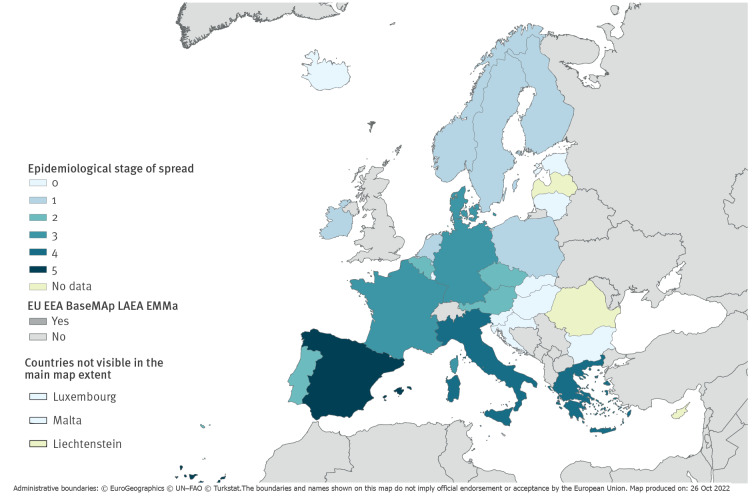
In the period 2019 to 2021, five countries (Denmark, France, Germany, Greece and Italy) reported 14 C. auris outbreaks defined as two or more cases with an epidemiological link, with 327 affected patients in total. The number of patients affected per outbreak ranged from two to 214 (Table 2). Inter-facility transmission occurred in eight outbreaks, and three outbreaks were reported as ongoing at the time of the survey (Table 2). The epidemiological stage of dissemination of C. auris was determined based on the respondents’ assessment in analogy to an epidemiological staging methodology that was previously developed and used for multidrug-resistant bacteria such as carbapenemase-producing Enterobacterales and carbapenem-resistant Acinetobacter baumannii [4,5]. Six countries reported that only imported C. auris cases had been detected (stage 1), four countries reported sporadic cases that were locally acquired or of unknown origin (stage 2), three countries reported sporadic outbreaks without or with only limited inter-facility spread (stage 3), two countries reported outbreaks with verified or plausible inter-facility spread (stage 4), and one country reported regional endemicity (stage 5) (Figure 2). This staging provides a snapshot of the epidemiological situation at the time of the survey and may not be indicative of the extent of future dissemination of C. auris within countries, especially within countries in stages 1–3 with currently few cases.
Table 2. Candida auris outbreaks in EU/EEA countries detected in 2019–2021 (n = 14 outbreaks).
| Outbreak | Year the outbreak was detecteda | Number of cases |
Duration (weeks) | Inter-facility transmission |
Ongoing at time of survey |
|---|---|---|---|---|---|
| 1 | 2019 | 214 | 149 | Yes | Yes |
| 2 | 2020 | 50 | 80 | Yes | Yes |
| 3 | 2020 | 15 | 44.5 | Yes | No |
| 4 | 2020 | 11 | 49 | No | No |
| 5 | 2021 | 10 | 5 | Yes | No |
| 6 | 2021 | 5 | 28 | Yes | Yes |
| 7 | 2021 | 4 | 3.5 | Yes | No |
| 8 | 2021 | 4 | 4 | No | No |
| 9 | 2021 | 3 | 4 | Yes | No |
| 10 | 2021 | 3 | 11 | No | No |
| 11 | 2021 | 2 | 8 | No | No |
| 12 | 2021 | 2 | 8 | No | No |
| 13 | 2021 | 2 | 39 | Yes | No |
| 14 | 2021 | 2 | 9 | No | No |
| Total | 327 | NA | 8 yes | 3 yes | |
EEA: European Economic Area; EU: European Union; NA: not applicable.
a The data for year signify only the year when the outbreak was detected, while the number of cases is reported for the whole duration of the outbreak (see column ‘Duration’).
Figure 2.
Epidemiological stage of Candida auris spreada, assessment by survey respondents in EU/EEA countries, 2022 (n = 30 countries)
EEA: European Economic Area; EMMa: European Centre for Disease Prevention and Control Map Maker tool; EU: European Union; LAEA: Lambert azimuthal equal-area projection.
a Epidemiological stages of C. auris spread are defined as: Stage 0: No cases of C. auris infection or colonisation have been detected. Stage 1: Only imported cases of C. auris have been detected. Stage 2: Only sporadic cases of C. auris that were locally acquired or of unknown origin have been detected. Stage 3: Sporadic outbreaks of C. auris have occurred without or with only limited inter-facility spread. Stage 4: Multiple outbreaks of C. auris with verified or plausible inter-facility spread have occurred. Stage 5: C. auris is endemic in parts of the country (regional spread).
National surveillance, laboratory capacity and guidance
At the time of the survey, C. auris infection or carriage was notifiable in six of the 30 countries, prospective or retrospective surveillance was established in 12 countries, and 23 countries had a laboratory with reference capacity for identification and testing of C. auris. Twelve reference laboratories reported using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) for identification of C. auris, 10 used MALDI-TOF MS in combination with other methods such as D1/D2 or Internal transcribed spacer (ITS) sequencing, and one reference laboratory reported using ITS sequencing only. Guidance for laboratory testing and for infection prevention and control was reported as available in 17 and 15 countries, respectively. These numbers represent a small improvement in preparedness and response compared with 2019 [2].
Discussion
Candida auris is an emerging fungal pathogen that has caused outbreaks of invasive healthcare-associated infections worldwide [6]. Candida auris is frequently resistant to fluconazole, and multidrug-resistant and even pandrug-resistant C. auris isolates have also been described, thus leaving very few treatment options [6-8]. In Europe, the UK and Spain were the first countries to report outbreaks [9]. This survey showed that the number of C. auris cases increased in the EU/EEA as did the number of countries reporting cases and outbreaks for 2020 and 2021. Before these years, the number of cases with C. auris was mainly driven by a large outbreak in one country and had decreased in 2018 and 2019 after a peak in 2017. This situation changed in 2020 and 2021 when additional countries started to experience outbreaks. The role played by the coronavirus disease (COVID-19) pandemic in this increase is difficult to ascertain. Restricted travel may have decreased the risk of importation of C. auris. However, difficult-to-control outbreaks of C. auris have been reported in units caring for COVID-19 patients worldwide [10-13]. At least two of the C. auris outbreaks described in this report involved COVID-19 patients or units dedicated to the care of COVID-19 patients: the outbreak in Germany involving two cases occurred in a COVID-19 intensive care unit (ICU) [14], and an outbreak in Italy was amplified after introduction of C. auris into a COVID-19 ICU [3,15].
The results of this survey show that cases and outbreaks with C. auris occurred in several EU/EEA countries within only few years after the first cases had been reported in the EU/EEA. There is now evidence of inter-facility spread of C. auris in two EU/EEA countries, and C. auris was assessed as endemic in at least one region in one country, with cases no longer occurring as part of circumscribed outbreaks. Equally worrisome is the fact that for four countries, information at national level on whether C. auris cases occurred within the country was not available, raising the possibility of undetected transmission and outbreaks in the EU/EEA. The high proportion of cases without information on importation or local acquisition even in countries with available information highlights the need to improve follow-up and surveillance. Cases without a clear link to hospitalisation abroad are an indication of local acquisition and may represent the tip of the iceberg of undetected transmission. The reported interregional spread as well as regional endemicity in one country show that C. auris is in the process of establishing itself as a healthcare-associated pathogen in the EU/EEA, similar to other countries such as the United States [16]. European-level surveillance therefore needs to improve with case definitions and standardised and regular case-based reporting.
Despite the increase in the number of cases and difficult-to-control outbreaks, there are also examples from EU/EEA countries where transmission of C. auris was contained with control measures after the occurrence of only few cases, for example in Denmark and Germany [14,17]. National surveillance, a mycology reference laboratory that provides reference testing to hospital laboratories as well as national guidance for laboratory testing and infection control are basic elements required for the control of C. auris. More detailed options for response are described in the latest ECDC rapid risk assessment published in February 2022 [3].
Conclusion
Local control of C. auris as soon as possible after introduction of a case to delay the establishment of C. auris in healthcare facilities will have a nationwide benefit for patients by reducing future healthcare-associated infections with C. auris. Control is more difficult to achieve once C. auris has spread within and between facilities or regions. It therefore continues to be of high importance that EU/EEA countries have adequate laboratory capacity and national surveillance for early detection of C. auris cases, and that measures to control and mitigate the consequences of its dissemination are rapidly implemented.
Ethical statement
All data were anonymised and collected in accordance with the European Parliament and Council decisions on the epidemiological surveillance and control of communicable disease in the European Community. Ethical approval and informed consent were thus not required.
The Candida auris survey collaborative group includes the following national experts
Birgit Willinger (Austria), Katrien Lagrou (Belgium), Ivva Philipova (Bulgaria), Ana Budimir (Croatia), Linos Hadjihannas, Markella Marcou (Cyprus), Lucie Bareková, Jan Kubele (Czech Republic), Maiken Cavling Arendrup (Denmark), Liidia Dotsenko (Estonia), Laura Lindholm, Outi Lyytikäinen (Finland), Marie Desnos-Ollivier, Françoise Dromer (France), Jane Hecht, Oliver Kurzai (Germany), Lida Politi, Georgia Vrioni (Greece), Ágnes Hajdu, Katalin Kiss (Hungary), Ólafur Guðlaugsson (Iceland), Susanna Frost (Ireland), Michela Sabbatucci (Italy), Ieva Voita (Latvia), Esther Walser-Domjan (Liechtenstein), Rolanda Valintėlienė (Lithuania), Alexandre Mzabi, Monique Perrin (Luxembourg), Rodianne Abela (Malta), Daan W. Notermans, Paul E. Verweij (Netherlands), Oliver Kacelnik, Miriam Sare (Norway), Katarzyna Dzierżanowska-Fangrat (Poland), Ana Lebre, José Artur Paiva (Portugal), Gabriel Adrian Popescu, Roxana Serban (Romania), Slavka Litvová, Mária Štefkovičová (Slovakia), Mojca Serdt, Rok Tomazin (Slovenia), Ana Alastruey-Izquierdo (Spain) and Erja Chryssanthou (Sweden).
Conflict of interest: K. L. received consultancy fees from MRM Health, MSD and Gilead, speaker fees from FUJIFILM WAKO, Pfizer and Gilead and a service fee from Thermo fisher Scientific and TECOmedical.
M.C.A. has, over the past 5 years, received research grants/contract work (paid to the SSI) from Amplyx, Basilea, Cidara, F2G, Gilead, Novabiotics and Scynexis, and speaker honoraria (personal fee) from Astellas, Chiesi, Gilead, MSD, and SEGES. She is the current chairman of the EUCAST-AFST.
P.E.V. received research grants from F2G and Gilead Sciences (institution contracted for research grants), honoraria for lectures from F2G, Gilead Sciences and Pfizer (paid to institution) and has participated on advisory boards for F2G and Mundipharma (payment to institution) in the past 36 months.
J. A. P. has, in the last five years, given talks and consulted for MSD, Pfizer, Gilead, Astra-Zeneca, AOP Orphan Pharmaceuticals, Cepheid, Jansen.
A. A. I. has, over the past 5 years, received honoraria for educational talks on behalf of Gilead and Pfizer.
Authors’ contributions: A. Kohlenberg, D.L. Monnet, D. Plachouras: design and implementation of the survey, compilation of information received from individual countries and preparation of European overview, drafting and reviewing the manuscript.
Candida auris survey collaborative group: compilation and analysis of national data from laboratories and surveillance databases, validation of national data included in the manuscript and review of the manuscript.
References
- 1. Kohlenberg A, Struelens MJ, Monnet DL, Plachouras D, Candida auris survey collaborative group . Candida auris: epidemiological situation, laboratory capacity and preparedness in European Union and European Economic Area countries, 2013 to 2017. Euro Surveill. 2018;23(13). 10.2807/1560-7917.ES.2018.23.13.18-00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Plachouras D, Lötsch F, Kohlenberg A, Monnet DL, Candida auris survey collaborative group . Candida auris: epidemiological situation, laboratory capacity and preparedness in the European Union and European Economic Area*, January 2018 to May 2019. Euro Surveill. 2020;25(12). 10.2807/1560-7917.ES.2020.25.12.2000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control (ECDC). Candida auris outbreak in healthcare facilities in northern Italy, 2019-2021. Rapid risk assessment. Stockholm: ECDC; 2022. Available from: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-candida-auris-outbreak-healthcare-facilities-northern-italy
- 4. Lötsch F, Albiger B, Monnet DL, Struelens MJ, Seifert H, Kohlenberg A, et al. Epidemiological situation, laboratory capacity and preparedness for carbapenem-resistant Acinetobacter baumannii in Europe, 2019. Euro Surveill. 2020;25(45). 10.2807/1560-7917.ES.2020.25.45.2001735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brolund A, Lagerqvist N, Byfors S, Struelens MJ, Monnet DL, Albiger B, et al. Worsening epidemiological situation of carbapenemase-producing Enterobacteriaceae in Europe, assessment by national experts from 37 countries, July 2018. Euro Surveill. 2019;24(9). 10.2807/1560-7917.ES.2019.24.9.1900123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jeffery-Smith A, Taori SK, Schelenz S, Jeffery K, Johnson EM, Borman A, et al. Candida auris: a review of the literature. Clin Microbiol Rev. 2017;31(1):e00029-17. . Available from: https://www.ncbi.nlm.nih.gov/pubmed/29142078 10.1128/CMR.00029-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacobs SE, Jacobs JL, Dennis EK, Taimur S, Rana M, Patel D, et al. Candida auris pan-drug-resistant to four classes of antifungal agents. Antimicrob Agents Chemother. 2022;66(7):e0005322. 10.1128/aac.00053-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyman M, Forsberg K, Reuben J, Dang T, Free R, Seagle EE, et al. Notes from the field: transmission of pan-resistant and echinocandin-resistant Candida auris in health care facilities - Texas and the District of Columbia, January-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(29):1022-3. 10.15585/mmwr.mm7029a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control (ECDC). Candida auris in healthcare-settings – Europe. First Update. Stockholm: ECDC; 2018. Available from: https://www.ecdc.europa.eu/sites/portal/files/documents/RRA-Candida-auris-European-Union-countries.pdf
- 10. Prestel C, Anderson E, Forsberg K, Lyman M, de Perio MA, Kuhar D, et al. Candida auris outbreak in a COVID-19 specialty care unit - Florida, July-August 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):56-7. 10.15585/mmwr.mm7002e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan American Health Organization (PAHO)/World Health Organization (WHO). Epidemiological alert: Candida auris outbreaks in health care services in the context of the COVID-19 pandemic. Washington, DC: PAHO/WHO; 2021. Available from: https://iris.paho.org/bitstream/handle/10665.2/53377/EpiUpdate6February2021_eng.pdf?sequence=1&isAllowed=y
- 12. Allaw F, Kara Zahreddine N, Ibrahim A, Tannous J, Taleb H, Bizri AR, et al. First Candida auris outbreak during a COVID-19 pandemic in a tertiary-care center in Lebanon. Pathogens. 2021;10(2):157. 10.3390/pathogens10020157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nobrega de Almeida J, Jr, Brandão IB, Francisco EC, de Almeida SLR, de Oliveira Dias P, Pereira FM, et al. Axillary Digital Thermometers uplifted a multidrug-susceptible Candida auris outbreak among COVID-19 patients in Brazil. Mycoses. 2021;64(9):1062-72. 10.1111/myc.13320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hinrichs C, Wiese-Posselt M, Graf B, Geffers C, Weikert B, Enghard P, et al. Successful control of Candida auris transmission in a German COVID-19 intensive care unit. Mycoses. 2022;65(6):643-9. 10.1111/myc.13443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Pilato V, Codda G, Ball L, Giacobbe DR, Willison E, Mikulska M, et al. Molecular epidemiological investigation of a nosocomial cluster of C. auris: evidence of recent emergence in Italy and ease of transmission during the COVID-19 pandemic. J Fungi (Basel). 2021;7(2):140. 10.3390/jof7020140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC). Tracking Candida auris. Atlanta: CDC. [Accessed: 26 Oct 2022]. Available from: https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html
- 17. Theut M, Antsupova V, Andreasen AS, Buhl D, Midttun M, Knudsen JD, et al. [The first two cases of Candida auris in Denmark]. Ugeskr Laeger. 2022;184(16):V10210768. [PubMed] [Google Scholar]
- 18. Desnos-Ollivier M, Fekkar A, Bretagne S. Earliest case of Candida auris infection imported in 2007 in Europe from India prior to the 2009 description in Japan. J Mycol Med. 2021;31(3):101139. 10.1016/j.mycmed.2021.101139 [DOI] [PubMed] [Google Scholar]