Abstract
Bovine respiratory disease (BRD) is the primary animal health concern facing feedlot producers. Many antimicrobial mitigation strategies are available, but few studies have compared feedlot performance during both the receiving and finishing periods following application of different antimicrobials used as metaphylaxis at arrival. The objective of this study was to compare antimicrobial metaphylaxis methods on clinical health and growth performance across both the receiving and finishing periods. A total of 238 multiple-sourced steers in two source blocks were used in a generalized complete block design. The four treatments included: 1) a negative control, 5 mL of sterile saline injected subcutaneously (CON); 2) subcutaneous administration of florfenicol at 40 mg/kg of BW (NUF); 3) subcutaneous administration of ceftiofur in the posterior aspect of the ear at 6.6 mg/kg of BW (EXC); and 4) subcutaneous administration of tulathromycin at 2.5 mg/kg of BW (DRA). The morbidity rate for the first treatment of BRD was decreased for the DRA and EXC treatments compared to CON and NUF (P < 0.01). Additionally, average daily gain (ADG), dry matter intake (DMI), and gain-to-feed (G:F) were greater (P ≤ 0.02) in the DRA treatment during the receiving period compared to all other treatments. The ADG was also greater (P < 0.05) for EXC than the CON treatment throughout the finishing period. Nonetheless, other growth performance variables did not differ among metaphylactic treatments during the finishing period (P ≥ 0.14). Likewise, no differences in carcass characteristics or liver abscess score were observed (P ≥ 0.18). All complete blood count (CBC) variables were affected by day (P ≤ 0.01) except mean corpuscular hemoglobin concentration (P = 0.29). Treatment × time interactions were observed for platelet count, white blood cell (WBC) count, monocyte count and percentage, and lymphocyte percentage (P ≤ 0.03). However, there were no observed hematological variables that differed among treatment (P ≥ 0.10). The results indicate that some commercially available antimicrobials labeled for metaphylactic use are more efficacious than others in decreasing morbidity rate.
Keywords: beef cattle, complete blood counts, growth performance, health outcomes, metaphylaxis
INTRODUCTION
Bovine respiratory disease (BRD) presents as one of the most common and costly illnesses within the cattle industry because of costs associated with antimicrobials, death loss, labor associated with treatment, and lost weight gain (Lofgreen, 1983; Gardner et al., 1999). High-risk cattle are more likely to contract BRD because of recent weaning, commingling of cattle from multiple sources, unknown vaccination history, and stress associated with the relocation process which includes transportation, new environment, and exposure to novel pathogens (Nickell and White, 2010; Dennis et al., 2020). Multiple bacterial pathogens contribute to BRD such as Pasteurella multocida, Histophilus somni, and most commonly Mannheimia haemolytica (Duff and Galyean, 2007; Woolums et al., 2018). The diverse factors that contribute to BRD make it a difficult disease to treat. One of the most effective combatants of BRD is preventing the cattle from developing the illness by providing a metaphylactic antibiotic at the time of arrival processing (Lofgreen, 1983). Metaphylaxis is the administration of long-acting antibiotics at arrival to cattle that may not have a chance to develop an appropriate immune response to control BRD (Urban-Chmiel and Grooms, 2012; Abell et al., 2017). Munoz et al. (2020) reported a decreased percentage of calves required treatment for BRD when given metaphylaxis (18.5% vs. 51.2%). Many commercially available metaphylactic antimicrobials act with differing efficacies, making the selection of medications challenging for commercial producers. Therefore, the objective of this study was to determine the effects of metaphylactic efficacy with differing antimicrobials on health outcomes and growth performance of high-risk beef cattle.
MATERIALS AND METHODS
Experimental procedures were approved by the Texas Tech University Institutional Animal Care and Use Committee (approval number 20039-04). The experiment was conducted from October 2020 to August 2021 at the Texas Tech University Burnett Center. The average observed temperature during the study period was 15.1 °C with a maximum of 42.2 °C and minimum of −17.7 °C. The total precipitation was 499.5 mm and average relative humidity was 44%. A chronology of key events (body weight [BW] measurement and blood collection) between blocks 1 and 2 is reported in Table 1.
Table 1.
Chronology of key events for high-risk steers in blocks 1 and 2.
| Item | Block 1 | Block 2 |
|---|---|---|
| Receiving period | ||
| Body weight measurement | Days -1, 0, 25, 38 | Days -1, 0, 33, 42 |
| Period 1 | Day 0 to 25 | Day 0 to 33 |
| Period 2 | Day 26 to 38 | Day 33 to 42 |
| Overall1 | Day 0 to 38 | Day 0 to 42 |
| Finishing period | ||
| Body weight measurement | Days 38, 252 | Days 42, 242 |
| Blood collection | ||
| Initial | Day 0 | Day 0 |
| Interim | Day 126 | Day 123 |
| Final | Day 252 | Day 242 |
1The length of the receiving period was not the same number of days for blocks 1 and 2. The cattle arrived on different days of the week and it was not possible for the receiving period to be an equal number of days between blocks 1 and 2.
Arrival Procedures
Crossbred steers (N = 238; arrival BW = 248 ± 9.5 kg) were sourced from multiple areas. Steers were blocked by arrival date with block 1 consisting of 123 steers purchased from an auction market in Dalhart, TX and shipped approximately 322 km arriving on October 22, 2020. Block 2 consisted of 115 steers purchased from an auction market in West Plains, MO and shipped approximately 1186 km arriving on December 2, 2020. After arrival, animals were placed into soil surface pens and allowed ad libitum access to water and long-stem grass hay and fed a receiving diet of approximately 65% concentrate at 1% of body weight (BW; Table 2). Within 24 h of arrival (day -1), steers were given an identification tag, individually weighed in a hydraulic squeeze chute calibrated with 454 kg of certified weigh cells before weighing with an accuracy of ±0.91 kg (Silencer, Moly Manufacturing, Lorraine, KS), and any bulls (N = 2) were identified, castrated, and not allocated to treatments since they could not be equally stratified among the four treatments. Additionally, all steers were vaccinated against bovine rhinotracheitis virus, bovine viral diarrhea virus (types 1 and 2), bovine parainfluenza-3 virus, and bovine respiratory syncytial virus (Vista 5 SQ; Merck Animal Health, Kenilworth, NJ), clostridial pathogens (Vision 8 with Spur; Merck Animal Health), and Mycoplasma bovis (Myco-B ONE DOSE; American Animal Health, Inc. Grand Prairie, TX). Steers also received ivermectin (Vetrimec pour-on; Vet One, Boise, ID) for treatment of internal and external parasites. Steers were sorted by BW within arrival block to experimental treatment assignments. Treatments were then applied randomly to pens. On day 0, BW was collected, and this BW was averaged with the day −1 BW to calculate initial BW.
Table 2.
Diet formulation and composition of receiving and finishing diets fed to high-risk beef steers1
| Item | Receiving diet | Finishing diet |
|---|---|---|
| Ingredient, % DM | ||
| Steam-flaked corn | 20.63 | 65.06 |
| Sweet bran | 53.46 | 20.39 |
| Chopped alfalfa | 19.92 | 8.06 |
| Yellow grease | 1.82 | 2.17 |
| Supplement2 | 2.05 | 2.01 |
| Limestone | 2.12 | 1.74 |
| Urea | - | 0.57 |
| Analyzed composition3 | ||
| Diet DM, % | 71.3 | 78.1 |
| Crude protein, % | 17.6 | 13.8 |
| Neutral detergent fiber, % | 32.3 | 16.6 |
| Acid detergent fiber, % | 15.3 | 7.4 |
| Total starch, % | 25.2 | 55.4 |
| Crude fat, % | 5.0 | 4.8 |
| Ca, % | 0.92 | 0.65 |
| P, % | 0.60 | 0.37 |
| NEm4, Mcal/kg | 1.87 | 2.12 |
| NEg4, Mcal/kg | 1.24 | 1.45 |
1Dry matter basis, except DM %.
2Supplement supplied 5.99% potassium chloride, 44.40% crude protein, 3.82% sodium, 8.34 mg/kg cobalt carbonate, 395.00 mg/kg copper sulfate, 408.00 mg/kg iron sulfate, 764 mg/kg manganous oxide, 2.92 mg/kg selenium, 2,490.00 mg/kg zinc sulfate, and 30 g/ton monensin sodium (Rumensin 90; Elanco Animal Health, Greenfield, IN) on a DM basis. Actual diet formulation based on weekly DM determinations.
3Analysis performed by Servi-Tech Laboratories, Amarillo, TX.
4NEm and NEg reported as tabular values (NASEM, 2016).
Experimental Treatments
Four treatments were used in a generalized complete block experimental design. The treatments consisted of the following: 1) a negative control, 5 mL of sterile saline injected subcutaneously (CON); 2) subcutaneous administration of florfenicol (Nuflor; Merck Animal Health) at 40 mg/kg of BW (NUF); 3) subcutaneous administration of ceftiofur in the posterior aspect of the ear (Excede; Zoetis, Parsippany, NJ) at 6.6 mg/kg of BW (EXC); and 4) subcutaneous administration of tulathromycin (Draxxin; Zoetis) at 2.5 mg/kg of BW (DRA). All antimicrobials were administered according to the label recommendations. A 3-day postmetaphylactic interval (PMI) was implemented for steers that received florfenicol, a 5-day PMI was implemented for steers that received ceftiofur, and a 7-day PMI was implemented for steers that received tulathromycin. Steers in the CON group did not have a PMI and were eligible for therapeutic treatment on day 0.
Steers were observed daily for symptoms of BRD and assigned a clinical illness score (0–4 severity scale) as described by Pillen et al. (2016). The eligibility of steers for therapeutic treatment was determined by ear tag identification and treatment record by a treatment-blinded investigator. Steers were considered to have a clinical case of BRD if they met the following criteria: 1) animal had a clinical illness score of 2 and a rectal temperature of ≥37.5 °C or 2) a clinical illness score of ≥3 regardless of rectal temperature. Rectal temperature was determined using a digital thermometer (GLA Agricultural Electronics, San Luis Obispo, CA). All steers that were individually removed from pen because of clinical illness symptoms were intravenously injected with 1 mL/45.4 kg of BW flunixin meglumine (Prevail, Vet One).
For the first therapeutic treatment steers received a subcutaneous injection of 12.5 mg/kg of BW of enrofloxacin (Baytril 100, Bayer Animal Health, Shawnee Mission, KS) and were assigned a 3-day posttreatment interval (PTI). After the expiration of the PTI, if steers again identified as a possible clinical case steers received tildipirosin at 4 mg/kg of BW (Zuprevo, Merck Animal Health) and were assigned a 7-day PTI. After expiration of the second PTI, if steers were again identified as a possible clinical case, steers received danofloxacin at 8 mg/kg of BW (Advocin, Zoetis). If steers continued to be symptomatic after their third treatment, they were classified as chronic and removed from the study after the receiving period (N = 2, 1 steer from CON and 1 steer from NUF). Steers treated once for BRD were considered BRD1, steers treated twice for BRD were considered BRD2, and steers treated three-times for BRD were considered BRD3. Two steers (both from the EXC treatment group) were treated for BRD in the finishing period (after day 46), all others were treated in the receiving period (before day 46). In addition, four steers died during the finishing period (1 steer from DRA [bloat], 1 steer from EXC [1 bloat, 1 injury], and 1 from NUF [bloat]).
Housing and Management
During the receiving period, steers were housed in partially shaded soil-surfaced pens with 57.6–63.4 m2 of pen space and 44.3–48.8 cm2 of linear bunk space per steer [Block 1 (N = 123 steers, 3 replications per treatment, 10–11 steers per pen); Block 2 (N = 115 steers, 3 replications per treatment, 10–11 steers per pen)]. All steers were fed the same receiving diet (Table 2), throughout the receiving period. On day 26, steers were revaccinated against bovine rhinotracheitis virus, bovine viral diarrhea virus (types 1 and 2), bovine parainfluenza-3 virus, and bovine respiratory syncytial virus (Vista 5 SQ; Merck Animal Health) and were implanted with 36 mg of zeranol (Ralgro Merck Animal Health).
On day 38 for block 1 and day 45 for block 2 after the completion of the receiving period, steers were sorted to partially slatted concrete-surfaced pens to begin the finishing period. The pens had 15.95 m2 of pen space and 60 cm of linear bunk space per animal. Previous metaphylactic treatments were maintained and pen mates were kept together. The steers in each pen during the receiving period were sorted by BW into pens within the same treatment for the finishing period. For example, steers in the DRA treatment during the receiving period remained in the DRA treatment for the finishing period. The pens of [Block 1 (N = 123 steers, 8 pens per treatment, 3–4 steers per pen); Block 2 (N = 115) steers, 8 pens per treatment, 3–4 steers per pen)].
Table 1 indicates the chronology of key events (body weight measurement and blood collection) between blocks 1 and 2. Body weights were collected in the morning before feeding on individual steers weighed to an accuracy of ±0.91 kg in a hydraulic squeeze chute calibrated with 454 kg of certified weigh cells before weighing (Silencer hydraulic squeeze chute; Moly Manufacturing, Lorraine, KS). Body weights were collected on days −1, 0, 25, 38, 126, and 252 for the steers in block 1 and days −1, 0, 33, 42, 123, and 242 for the steers in block 2. While the steers were restrained in the chute, blood was collected (as described subsequently) on days 0, 123 (block 1), 126 (block 2), and end (day 252 for block 1 and day 242 for block 2). The length of the receiving period was not the same number of days for blocks 1 and 2 because cattle arrived on different days of the week.
For complete blood count analyses (CBC), a 4-mL blood sample was collected via jugular-venipuncture into vacutainers containing EDTA for analyses of red blood cells, hemoglobin, hematocrit, platelets, white blood cells, neutrophils, lymphocytes, monocytes, eosinophils, and basophils. An automatic hemocytometer (ProCyte Dx Hematology Analyzer; IDEXX Laboratories, Inc., Westbrook, ME) was used to analyze CBC variables within 30–60 min of blood collection.
Throughout the study, steers had ad libitum access to water and feed. Feed bunks were evaluated daily at 0730 hours. Feed was delivered once daily at 0800 hours. The bunk management strategy was to allow less than 0.45 kg of orts at feeding. Diets were mixed in the feed mill (1.3-m3 Marion paddle mixer) and delivered via a tractor-pulled mixer (Rotomix 84-8 wagon mixer; Rotomix, Dodge City, KS) with a scale accuracy of ±0.454 kg.
During the finishing period, steers were transitioned to a finishing diet in a gradual four-step process of increasing concentrate feeding and using a 7–10-d adaptation to the new diet where steam-flaked corn was increased, and alfalfa hay and Sweet Bran were decreased. The finishing diet was based on steam-flaked corn and contained 30 g/ton monensin sodium (Rumensin 90, Elanco Animal Health, Greenfield, IN), but did not include tylosin phosphate. Diets were formulated to exceed nutrient requirements for growing and finishing steers (NASEM, 2016). Diet samples were collected three-times each week and composited. One-half of the weekly composite sample was used to determine dry matter (DM) using a 100 °C forced air oven over 24 h and used as the weekly DM factor to calculate dry matter intake (DMI). The second subsample of the weekly composite was used for chemical analysis of NDF, ADF, CP, fat, starch, Ca, and P (Servi-Tech Laboratories, Amarillo, TX).
Calculations
The ADG and G:F were calculated on a live BW basis. Average daily gain was calculated by subtracting the initial BW from the final BW, then divided by days on feed. The G:F was computed as the quotient of ADG divided by daily DMI. The carcass-adjusted data were calculated from the HCW divided by the overall average dressing percent.
The morbidity and mortality data in Table 3 were calculated on a pen basis. Variables from individual treatment records were averaged within pen. The morbidity for BRD 1, BRD 2, BRD 3, percent chronic, and respiratory mortality were summed by pen and divided by total number of steers in the pen during the receiving period. Days to the first therapeutic treatment and rectal temperatures were summarized from individual treatment records and averaged by pen. All data, with the exception of morbidity and mortality are reported with mortalities excluded.
Table 3.
Effect of metaphylaxis with a sterile saline (negative control), tulathromycin, ceftiofur, and florfenicol on health outcomes of high-risk, newly received beef steers1
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Item | CON | DRA | EXC | NUF | SEM2 | P-value |
| N, steers | 55 | 61 | 60 | 62 | - | - |
| N, pens | 6 | 6 | 6 | 6 | - | - |
| BRD13 % | 58.8a | 26.3b | 26.3b | 45.2a | 6.33 | <0.01 |
| BRD24 % | 29.3a | 3.3b | 8.44b | 14.5b | 4.25 | <0.01 |
| BRD35% | 7.4 | 0 | 1.7 | 1.7 | 2.54 | 0.20 |
| Chronic6 % | 1.9 | 0 | 0 | 1.6 | 1.26 | 0.57 |
| Respiratory mortality % | 1.8 | 1.6 | 1.7 | 0 | 1.55 | 0.80 |
| Days to | ||||||
| First treatment | 7c | 10b,c | 17a | 12b | 1.6 | <0.01 |
| Second treatment | 14 | 16 | 20 | 19 | 5.0 | 0.26 |
| Third treatment | 29 | - | 30 | 20 | - | 0.47 |
| Rectal temperature, °C | ||||||
| First treatment | 39.9 | 39.5 | 39.9 | 39.8 | 0.22 | 0.53 |
| Second treatment | 39.9 | 39.7 | 39.6 | 39.8 | 0.49 | 0.91 |
| Third treatment | 39.8 | - | - | 39.8 | - | 0.98 |
a,b,cWithin a row, means without a common superscript differ, P < 0.05.
1CON = no metaphylaxis at arrival; DRA = tulathromycin (Draxxin, Zoetis) administered as metaphylaxis at arrival; EXC = ceftiofur (Excede, Zoetis) administered as metaphylaxis at arrival; NUF = florfenicol (Nuflor, Merck) administered as metaphylaxis at arrival.
2Pooled standard error of least squares mean (N = 6 pens/mean).
3Percentage of steers treated for bovine respiratory disease (BRD) at least once.
4Percentage of steers treated for BRD at least twice.
5Percentage of steers treated for BRD at least three times.
6Percentage of steers treated for BRD four or more times.
Statistical Analysis
Data were analyzed as a generalized complete block design using PROC MIXED in SAS (SAS Institute Inc., Cary, NC). Growth performance was analyzed separately in the receiving period and finishing period because the cattle were re-penned after the completion of the receiving period. For growth performance during the receiving and finishing period, pen was the experimental unit, and the model included the fixed effect of metaphylactic treatment and the random effect of arrival block. Morbidity and mortality were analyzed as a binomial proportion using the GLIMMIX procedure of SAS. The CBC data were analyzed with repeated measures using animal as the experimental unit. The model included metaphylactic treatment, day, and the interaction of metaphylactic treatment × day. Animal within pen was the subject of the repeated measures and was included to control for any pen variation present when re-allotting cattle between the growing and finishing phase. Multiple covariance structures were tested, and the autoregressive 1 covariance structure resulted in the smallest Akaike and Schwarz Bayesian criteria and was considered the most appropriate for repeated measure analyses. Main effect means are presented for most CBC variables and in the presence of an interaction (P < 0.05) simple effect means are discussed in the text. For all variables, statistical significance was determined at P ≤ 0.05 and tendencies were noted at 0.05 < P ≤ 0.10.
RESULTS AND DISCUSSION
Morbidity and Mortality
Health outcomes during the receiving period are presented in Table 3. A greater (P < 0.01) percentage of steers were treated for BRD1 in the CON and NUF treatments than DRA and EXC. The lack of difference in BRD1 treatments among CON and NUF steers is similar to observations by Martín et al. (2007). Nonetheless, these results contrast with Catry et al. (2008), where metaphylactic florfenicol (NUF) produced fewer subsequent treatments for BRD than cattle that did not receive metaphylaxis. In addition, Gonzalez-Martin et al. (2011) reported no difference in BRD incidence among cattle receiving florfenicol (NUF) or tulathromycin (DRA) as metaphylaxis. The disparity between the current experiment and those of Gonzalez-Martin et al. (2011) and Catry et al. (2008) may be location, season, or type of pens, or the initial health status of the cattle. Similarly, Catry et al. (2008) and Gonzalez-Martin et al. (2011) used dairy calves and excluded any calves that appeared morbid during the experimental animal selection process. In the present study, no steers were excluded from the experiment based on arrival health status. Likewise, both studies began when a defined BRD outbreak occurred, rather than assigning metaphylactic treatment after feedlot arrival as in the present study.
In the present experiment, DRA decreased BRD1 by 42% compared to NUF (P = 0.04) and 55% compared to CON (P < 0.01), which is consistent with previous studies using high-risk cattle (Godinho et al., 2005; Kilgore et al., 2005; Nutsch et al., 2005; Rooney et al., 2005; Skogerboe et al., 2005; Tennant et al., 2014). Moreover, EXC decreased BRD2 by 55% compared to CON (P < 0.01), which aligns with previous studies (Hibbard et al. 2002a, 2002b; Encinias et al., 2006). As expected, a greater percentage of CON steers were treated for BRD2 than steers given antimicrobial metaphylaxis (P < 0.01), similar to reports by Godinho et al. (2005) where a greater proportion of cattle receiving no metaphylaxis required additional therapeutic treatment for BRD. The DRA treatment tended (P = 0.08) to decrease morbidity rate of BRD2 by 77% compared to NUF, and EXC did not differ (P ≥ 0.27) from NUF and DRA treatment groups. There was a tendency (P = 0.10) for CON steers to have a greater percentage of BRD3 treatments. Nevertheless, the percentage of chronic steers and respiratory mortality did not differ (P > 0.57).
The EXC group had the greatest days to first therapeutic treatment, whereas CON had the fewest (P < 0.01), with DRA and NUF being intermediate. There was no difference in days to second or third therapeutic treatment (P ≥ 0.26). These results could be confounded with the difference in PMI among metaphylactic antimicrobials used and design of the experiment thus results should be interpreted with caution. The DRA treatment had a 7-d PMI, the EXC treatment had a 5-d PMI, the NUF treatment had a 3-d PMI, and steers in the CON treatment could be therapeutically treated on day 0. Indeed, tulathromycin is known to be slowly metabolized in the body and have a prolonged activity in the lungs, and therefore it would be expected to have the greatest days to first therapeutic treatment (Evans, 2005), which was not the case in the present study. Ceftiofur is reported to have a half-life of 6 h, which is appreciably longer than most other cephalosporins in part because of the administration route in the postauricular aspect of the ear extending the duration of action (Merck, 2021). However, this does not explain the difference in days to first treat between the EXC and DRA treatment groups.
Rectal temperature did not differ (P > 0.53) among groups during any therapeutic treatment which is consistent with previously published literature (Godinho et al., 2005; Munoz et al., 2020). Nonetheless, Catry et al. (2008) reported rectal temperature was greater for calves given florfenicol than calves given no metaphylaxis. Rectal temperature is commonly used as a proxy for core body temperature to objectively diagnose illness and disease and is reportedly accurate in clinical diagnosis of BRD (Gonzalez-Martin et al., 2011).
Receiving Growth Performance
Growth performance during the receiving period is reported in Table 4. There were no differences in initial (P = 0.99) or final BW among treatments (P = 0.63). The literature surrounding the effect of metaphylaxis on BW within the receiving period is inconsistent. Our findings are supported by studies comparing tulathromycin and ceftiofur as metaphylaxis to a negative control that reported no difference in final BW (Godinho et al., 2005; Benton et al., 2008). In contrast, others have reported increased final BW during the receiving period when metaphylaxis is given (Catry et al., 2008; Munoz et al., 2020). The inconsistency of results could be explained by a different length of time in the receiving period [20 d in the Catry et al. (2008) study, and 56 d in the Munoz et al. (2020) study, compared to 38–45 d in the present study].
Table 4.
Effect of metaphylaxis with a sterile saline (negative control), tulathromycin, ceftiofur, and florfenicol on receiving growth performance of high-risk, newly received beef steers
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Item | CON | DRA | EXC | NUF | SEM2 | P-value |
| N, steers | 55 | 61 | 60 | 62 | - | - |
| N, pens | 6 | 6 | 6 | 6 | - | - |
| Initial body weight3, kg | 248 | 248 | 249 | 246 | 9.5 | 1.00 |
| Final body weight4, kg | 291 | 304 | 298 | 288 | 9.5 | 0.63 |
| Period 1 | ||||||
| ADG, kg | 0.75b | 1.20a | 0.81b | 0.45c | 0.201 | <0.01 |
| DMI, kg | 4.92b,c | 5.47a | 5.26a,b | 4.75c | 0.343 | <0.01 |
| DMI, % of BW | 1.91 | 2.07 | 2.02 | 1.88 | 0.122 | 0.08 |
| G:F | 0.151b | 0.218a | 0.151b | 0.095c | 0.0289 | <0.01 |
| Period 2 | ||||||
| ADG, kg | 1.60b | 1.69b | 1.82a,b | 2.15a | 0.185 | 0.05 |
| DMI, kg | 7.15 | 7.30 | 6.75 | 6.41 | 0.553 | 0.08 |
| DMI, % of BW | 2.55 | 2.49 | 2.37 | 2.36 | 0.159 | 0.28 |
| G:F | 0.226b | 0.233b | 0.279a,b | 0.336a | 0.0427 | <0.01 |
| Overall d 0 to 45 | ||||||
| ADG, kg | 1.04b.c | 1.36a | 1.17b | 1.00c | 0.055 | <0.01 |
| DMI, kg | 5.63a,b,c | 6.05a | 5.75a,b | 5.28c | 0.296 | 0.02 |
| DMI, % of BW | 2.10 | 2.20 | 2.11 | 1.99 | 0.111 | 0.14 |
| G:F | 0.185b | 0.224a | 0.206a,b | 0.190b | 0.0076 | <0.01 |
a,b,cWithin a row, means with different superscripts differ P < 0.05.
1CON = no metaphylaxis at arrival; DRA = tulathromycin (Draxxin, Zoetis) administered as metaphylaxis at arrival; EXC = ceftiofur (Excede, Zoetis) administered as metaphylaxis at arrival; NUF = florfenicol (Nuflor, Merck) administered as metaphylaxis at arrival. Period 1 was from day 1 to 38 and period 2 was from day 1 to 45.
2Standard error of least squares mean (N = 6 pens per mean).
3Average of BW on days -1 and 0.
4BW was shrunk 4%.
In period 1, steers administered DRA had greater ADG than other treatments (P < 0.01). Steers given DRA had a 49% increase (P < 0.01) in overall ADG compared to the negative control. Purportedly, cattle experiencing active BRD have less ADG, thus because the DRA morbidity in the current experiment was least among treatments, it is plausible that this is the reason the ADG was greatest among treatments (Griffin, 1997; Gardner et al., 1999; Munoz et al., 2020). The NUF treatment had the least ADG within period 1 (P < 0.01). These data agree with Boyd et al. (2006), where lightweight calves were given a negative control, ceftiofur, or florfenicol as metaphylaxis on day 0. The ADG of calves treated with florfenicol was less than control and ceftiofur groups, which corresponded to an increase in BRD1 for steers given florfenicol vs. control or ceftiofur at arrival and is likely the reason ADG was less. In the present experiment, NUF had greater ADG in period 2 (P = 0.05) than CON and DRA but did not differ from EXC (P = 0.35). This is likely because of compensatory gain in response to a decreased ADG in period 1. The overall ADG for the receiving period was greatest for DRA, and least for the steers treated metaphylactically with NUF (P < 0.01), with CON and EXC being intermediate.
Period 1 DMI was greatest in DRA and EXC, and least in CON and NUF (P < 0.01). Florfenicol has been reported to temporarily reduce DMI of cattle (Boyd et al., 2006; Catry et al., 2008). In the current study, DRA tended (P = 0.08) to have a greater DMI than NUF in period 2. As expected, based on the results of periods 1 and2 combined, the overall DMI was greatest for DRA and least for NUF (P = 0.02). Our findings did not indicate that overall DMI during the receiving period for the CON treatment differed from steers that received metaphylaxis at arrival. In studies by Word et al. (2021) and Tennant et al. (2014), cattle receiving metaphylaxis did not have a greater DMI than the cattle administered a negative control. Conversely, others reported metaphylaxis increased DMI during the feedlot receiving period (Munoz et al., 2020).
There was a tendency (P = 0.08) for DMI as a percent of BW to differ in period 1. In addition, there was no difference in DMI as a percent of BW in period 2 or for the overall (P ≥ 0.14) receiving period, which suggests that even though a tendency for a difference was present early after feedlot arrival (period 1), the steers with decreased DMI compensated by the end of the receiving period.
The G:F in period 1 was greatest for DRA, least for NUF, with CON and EXC being intermediate and not differing (P < 0.01). Tulathromycin resulted in a greater G:F than CON, which was likewise observed by Munoz et al. (2020). Further, our finding that florfenicol resulted in the least G:F has been observed previously (Boyd et al., 2006). Others have reported that ceftiofur increased G:F over a negative control (Benton et al., 2008). In period 2, the G:F was greatest (P = 0.01) for the steers given NUF, perhaps because of compensatory gain after a decreased DMI and G:F in period 1. Likewise, EXC tended (P = 0.06) to differ from NUF; whereas CON and DRA had less G:F than NUF (P < 0.01) and did not differ in G:F during period 2. For the overall receiving period, G:F was greatest (P = 0.03) in steers that received DRA; whereby, CON, EXC and NUF did not differ (P ≥ 0.07). The increased G:F observed in the DRA treatment group in the present study is in contrast with the receiving period data reported by Gonzalez-Martin et al. (2011), though when G:F was evaluated over the entire receiving and finishing period no difference in G:F was reported.
Finishing Period Growth Performance and Carcass Characteristics
Growth performance during the finishing period is presented in Table 5. There was no difference among treatments in the initial BW (P = 0.33) or final BW (P = 0.22). Metaphylaxis at arrival generally does not change final BW before harvest (Gardner et al., 1999; Skogerboe et al., 2005; Booker et al., 2006; Word et al., 2021). Conversely, a study by Tennant et al. (2014) reported that final BW was greater in steers given tulathromycin as metaphylaxis at arrival compared to steers not given metaphylaxis. Although not statistically different, DRA and EXC both numerically increased final BW over CON and NUF; whereby an increase of 20 kg or greater in final BW is biologically relevant.
Table 5.
Effect of metaphylaxis with a sterile saline (negative control), tulathromycin, ceftiofur, and florfenicol on finishing growth performance of high-risk, steers
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Item | CON | DRA | EXC | NUF | SEM2 | P-value |
| N, steers | 55 | 61 | 60 | 62 | - | - |
| N, pens | 16 | 16 | 16 | 16 | - | - |
| Initial body weight3, kg | 294 | 306 | 299 | 288 | 7.03 | 0.33 |
| Final body weight3, kg | 581 | 602 | 607 | 587 | 14.9 | 0.22 |
| Overall finishing | ||||||
| ADG, kg | 1.41b | 1.46a,b | 1.52a | 1.48a,b | 0.030 | 0.05 |
| DMI, kg | 8.47 | 8.59 | 8.97 | 8.47 | 0.172 | 0.14 |
| DMI, % of BW | 1.94 | 1.89 | 1.99 | 1.94 | 0.031 | 0.23 |
| G:F | 0.167 | 0.171 | 0.170 | 0.175 | 0.0032 | 0.40 |
| Carcass-adjusted | ||||||
| Final BW4, kg | 586 | 606 | 606 | 590 | 13.6 | 0.20 |
| ADG, kg | 1.45b | 1.48a,b | 1.52a | 1.50a,b | 0.027 | 0.05 |
| DMI, % of BW | 1.93 | 1.88 | 1.98 | 1.93 | 0.028 | 0.24 |
| G:F | 0.171 | 0.172 | 0.169 | 0.175 | 0.031 | 0.40 |
a,b,cWithin a row, means with different superscripts differ P < 0.05.
1CON = no metaphylaxis at arrival; DRA = tulathromycin (Draxxin, Zoetis) administered as metaphylaxis at arrival; EXC = ceftiofur (Excede, Zoetis) administered as metaphylaxis at arrival; NUF = florfenicol (Nuflor, Merck) administered as metaphylaxis at arrival.
2Standard error of least squares mean (N = 6 pens per mean).
3All BW are shrunk by 4%.
4 Hot carcass weight divided by overall average DP (65.8%).
Over the entire finishing period, ADG was greatest for EXC, and least for CON (P = 0.05). In addition, the present study reported a 3.4% greater ADG between the DRA and CON treatment groups which is less than Munoz et al. (2020) reported, where metaphylactic tulathromycin resulted in a 15.8% greater ADG than a negative control. Previous literature on whether ADG during the finishing period is affected by arrival metaphylaxis is inconsistent. It has been commonly reported that metaphylaxis at arrival has no effect on ADG (Griffin, 1997; Gonzalez-Martin et al., 2011; Word et al., 2021). Additionally, a study by Tennant et al. (2014) reported cattle given tulathromycin as metaphylaxis had greater ADG than cattle given a negative control, which is in contrast to the present study where the CON and DRA treatments did not differ (P = 0.19) in ADG during the finishing period. The difference between these results could be explained by the overall decreased morbidity across the entire experimental period in both the steers given tulathromycin and the steers given a negative control in the Tennant et al. (2014) study. Furthermore, others have reported a greater ADG in cattle given tulathromycin as metaphylaxis than those given florfenicol (Rooney et al., 2005; Skogerboe et al., 2005), which is in contrast to the present study where DRA and NUF did not differ (P = 0.70) in overall ADG during the finishing period. Nonetheless, a study conducted by Gardner et al. (1999) reported cattle that exhibited clinical signs of BRD or those that had lung lesions at harvest had a decreased ADG than healthy cattle, which agrees with our finding that the EXC treatment had a greater ADG than the CON treatment.
There was no difference (P = 0.14) in DMI during the overall finishing period, which agrees with previous literature (Tennant et al., 2014; Word et al., 2021). Likewise, DMI as a percent of BW did not differ (P = 0.23) among treatment groups. There is some evidence that administering ceftiofur as metaphylaxis increases DMI in the finishing period. In a study by Booker et al. (2006), calves given ceftiofur as metaphylaxis had a greater DMI over the entire finishing period than those given tilmicosin phosphate (Booker et al., 2006).
The G:F among all treatments in the finishing period did not differ (P = 0.40). Our findings that metaphylaxis at arrival had no impact on G:F in the finishing period have been previously noted (Rooney et al., 2005; Booker et al., 2006; Gonzalez-Martin et al., 2011; Tennant et al., 2014; Word et al., 2021).
Carcass-adjusted ADG followed the same trend as ADG calculated from live BW where ADG was greatest for EXC and least for CON (P = 0.05; Table 5). No differences in carcass-adjusted growth performance, carcass characteristics, or liver score were observed (Table 6; P ≥ 0.12). These results are in agreement with prior terminal works that evaluated differing metaphylactic drugs and demonstrate that cattle treated for BRD multiple times are able to achieve similar compositional endpoints as nontreated contemporaries (Wilson et al., 2017; Word et al., 2021).
Table 6.
Carcass characteristics and liver score of finishing beef steers administered metaphylaxis with tulathromycin, ceftiofur, florfenicol, or given no metaphylaxis at feedlot arrival
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Item | CON | DRA | EXC | NUF | SEM | P-value |
| Hot carcass weight, kg | 385 | 399 | 398 | 388 | 10.7 | 0.44 |
| DP2, % | 65.78 | 65.75 | 65.69 | 65.73 | 0.434 | 0.99 |
| Marbling score3 | 553 | 520 | 532 | 545 | 18.1 | 0.45 |
| Fat thickness, cm | 1.74 | 1.77 | 1.74 | 1.75 | 0.105 | 0.99 |
| Longissimus dorsi area, cm sq | 90.41 | 91.64 | 92.83 | 90.25 | 2.454 | 0.66 |
| Calculated YG | 3.46 | 3.54 | 3.45 | 3.49 | 0.166 | 0.98 |
| EBF4, % | 31.82 | 31.85 | 31.75 | 31.85 | 0.670 | 0.99 |
| AFBW5, kg | 549 | 568 | 570 | 551 | 14.8 | 0.18 |
| Choice or greater, % | 82.4 | 87.8 | 86.5 | 92.2 | 4.70 | 0.51 |
| Abscessed livers, % | 17.7 | 12.7 | 15.6 | 14.1 | 9.76 | 0.93 |
1CON = no metaphylaxis at arrival; DRA = tulathromycin (Draxxin, Zoetis) administered as metaphylaxis at arrival; EXC = ceftiofur (Excede, Zoetis) administered as metaphylaxis at arrival; NUF = florfenicol (Nuflor, Merck) administered as metaphylaxis at arrival.
2Calculated as hot carcass weight divided by final shrunk live BW.
3Leading digit indicates score and following digits indicate degree of marbling within score; 5 = modest.
4Empty body fat, %. Estimated using equations of Guiroy et al. (2001).
5Final shrunk BW adjusted to equivalent 28% EBF using equations of Tylutki et al. (1994).
Complete Blood Count
The least squares means for initial, interim, and final CBC variables by day are presented in Table 7. All variables were affected by day (P < 0.01) except for mean corpuscular hemoglobin concentration (P = 0.29).
Table 7.
Day effects of complete blood count for high-risk beef steers given metaphylaxis with a negative control, tulathromycin, ceftiofur, and florfenicol at arrival
| Day1 | |||||
|---|---|---|---|---|---|
| Item | Initial | Interim | Final | SEM2 | P-value |
| Red blood cells, M/µL | 10.13a | 9.97a | 9.13b | 0.067 | <0.01 |
| Hemoglobin, g/dL | 13.24c | 14.64b | 14.91a | 0.083 | <0.01 |
| Hematocrit, % | 39.74c | 43.97b | 44.67a | 0.271 | <0.01 |
| Mean corpuscular volume, fL | 39.49c | 44.15b | 49.14a | 0.286 | <0.01 |
| Mean corpuscular hemoglobin, pg | 13.19c | 14.70b | 16.40a | 0.093 | <0.01 |
| Mean corpuscular hemoglobin concentration, g/dL | 33.43 | 33.21 | 33.40 | 0.127 | 0.29 |
| Reticulocyte, K/µL | 0.62b | 1.78a | 1.82a | 0.146 | <0.01 |
| Reticulocyte, % | <0.01b | 0.01a | 0.01a | 0.003 | 0.01 |
1Initial = day 0 for blocks 1 and 2, interim = day 126 for block 1, day 123 for block 2, final = day 252 for group 1, day 242 for group 2.
2Largest standard error of least squares means.
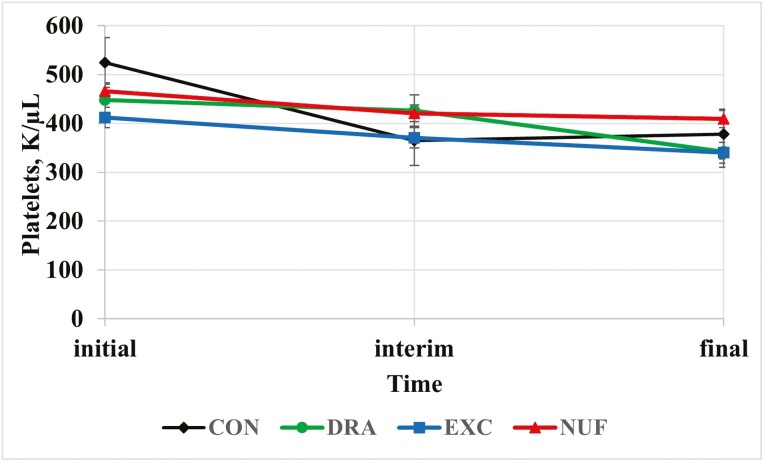
There was a treatment × time interaction for platelet count (P = 0.02; Figure 1). Steers administered CON had greater concentrations of platelets than DRA or EXC at the initial measurement (P < 0.01), though at this time metaphylactic treatment had yet to be administered and the reason for the difference in baseline is unknown. Platelets decreased in the steers administered DRA as metaphylaxis between interim and final (P < 0.01). Platelets are responsible for blood clotting and high platelet count can indicate inflammation, which is plausible as platelet count was greatest for all treatments at the initial time point, when stress and inflammation were likely increased as a result of the stress of marketing and transportation. To mediate inflammation, platelets exert chemotaxis on leukocytes (Klinger, 1997). In the present study, WBC were elevated and could explain why platelets were greatest initially in correlation with the greater morbidity rate, though this does not offer an explanation as to why the CON treatment had greater platelet count than DRA or EXC. Furthermore, excessive platelet count is uncommon and likely a secondary condition observed with stress (Roland et al., 2014), which steers were experiencing at the initial measurement. The literature about the significance of platelets as an immune response to BRD is inconsistent. Platelets have often been reported as poor predictors of BRD (Richeson et al., 2013; Moisá et al., 2019). In a study by Fontenot (2015), platelets were reported to decrease with the number of times calves were treated for BRD.
Figure 1.
Treatment by time interaction for platelet count (K/µL; P = 0.02). CON = no metaphylaxis at arrival; DRA = tulathromycin (Draxxin, Zoetis) administered as metaphylaxis at arrival; EXC = ceftiofur (Excede, Zoetis) administered as metaphylaxis at arrival; NUF = florfenicol (Nuflor, Merck) administered as metaphylaxis at arrival. Initial = day 0, interim = day 126 for block 1 and day 110 for block 2, and final = day 252 for block 1 and day 242 for block 2.
An elevated red blood cell (RBC) count can indicate lung disease, inflammation, or dehydration; conditions which are routinely associated with high-risk calves at feedlot arrival. Likewise, elevated RBC count can indicate a greater risk of BRD diagnosis (Richeson et al., 2013). In the present study, RBC count was decreased at the final compared to initial and interim measurements (P < 0.01); however, all values observed during the study were within normal ranges for bovine (Merck, 2021).
Hemoglobin concentration, hematocrit, mean corpuscular volume, and mean corpuscular hemoglobin increased (P < 0.01) across all time points, where initial was least, interim was intermediate, and final was greatest with no abnormal values observed (Merck, 2021). Likewise, reticulocyte count and percentage increased from initial to interim (P ≤ 0.01). These RBC-related measurements are all closely related and the pathogenic state causing an abnormal value of one variable is often related to the same of another. For example, decreased hemoglobin at feedlot entry has been correlated to decreased incidence of BRD treatment (Fontenot, 2015). Closely related to RBC and hemoglobin, hematocrit is the ratio of RBC, leukocytes, and platelets to total blood volume (Pagana et al., 2015). Hematocrit is often used as a proxy to indicate dehydration. Overall, it is important to note that hematological ranges fluctuate with age and the time effects in which differentials change but remain within a normal range could be confounded with age and the length of time in between sample collections (Roland et al., 2014).
The least squares means for metaphylactic treatment effects on CBC variables are presented in Table 8. Metaphylactic treatment had no effect (P ≥ 0.17) on any blood variables throughout the study. This is likely confounded by sample time, as treatment had not been administered when the first sample was collected, and steers morbidity was nonexistent at the other two sample points. If blood had been collected at times when steers were experiencing the greatest morbidity after metaphylaxis treatment was administered, the results could be very different.
Table 8.
Treatment effects of complete blood count for high-risk beef steers given metaphylaxis with a negative control, tulathromycin, ceftiofur, and florfenicol at arrival
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Item | CON | DRA | EXC | NUF | SEM2 | P-value |
| Red blood cells, M/µL | 9.74 | 9.77 | 9.74 | 9.72 | 0.102 | 0.99 |
| Hemoglobin, g/dL | 14.29 | 14.24 | 14.21 | 14.32 | 0.121 | 0.90 |
| Hematocrit, % | 42.80 | 42.59 | 42.74 | 43.05 | 0.403 | 0.86 |
| Mean corpuscular volume, fL | 44.22 | 43.78 | 44.22 | 44.81 | 0.382 | 0.25 |
| Mean corpuscular hemoglobin, pg | 14.77 | 14.65 | 14.71 | 14.92 | 0.123 | 0.39 |
| Mean corpuscular hemoglobin concentration, g/dL | 33.41 | 33.50 | 33.13 | 33.35 | 0.151 | 0.31 |
| Reticulocyte, K/µL | 1.28 | 1.30 | 1.35 | 1.71 | 0.167 | 0.17 |
| Reticulocyte, % | 0.004 | 0.007 | 0.009 | 0.010 | 0.0036 | 0.67 |
1CON = no metaphylaxis at arrival; DRA = tulathromycin (Draxxin, Zoetis) administered as metaphylaxis at arrival; EXC = ceftiofur (Excede, Zoetis) administered as metaphylaxis at arrival; NUF = florfenicol (Nuflor, Merck) administered as metaphylaxis at arrival.
2Largest standard error of least squares means.
The least squares means for initial, interim, and final day effects on WBC differentials are presented in Table 9. All variables were affected by day (P < 0.01), but nonetheless all observed values were within normal reference ranges. Neutrophil count was greater at the initial time point and decreased at the interim and final measurements. According to Pagana et al. (2015), a greater neutrophil count can indicate inflammation, infection, or stress, and they are particularly implicated in the pathogenesis of inflammatory respiratory tract diseases (Earley et al., 2016), which could explain why the observed neutrophil count was greater initially among treatments. The lymphocyte and eosinophil concentrations decreased from interim to final (P < 0.01) while basophil count decreased from initial to interim (P < 0.01) and eosinophil percentage increased (P < 0.01) from initial to interim.
Table 9.
Day effects of complete blood count for high-risk beef steers given metaphylaxis with a negative control, tulathromycin, ceftiofur, and florfenicol at arrival
| Day1 | |||||
|---|---|---|---|---|---|
| Item | Initial | Interim | Final | SEM2 | P-value |
| Neutrophils, K/uL | 5.11a | 2.99b | 2.88b | 0.205 | <0.01 |
| Lymphocytes, K/uL | 6.43a | 6.48a | 5.12b | 0.116 | <0.01 |
| Eosinophils, K/uL | 0.22b | 0.27b | 0.44a | 0.025 | <0.01 |
| Neutrophils, % | 38.38a | 25.48c | 30.12b | 0.700 | <0.01 |
| Eosinophils, % | 1.68c | 2.42b | 4.58a | 0.204 | <0.01 |
| Basophil, % | 0.05a | 0.02b | 0.03b | 0.004 | <0.01 |
| Neutrophil:lymphocyte ratio | 0.82a | 0.46c | 0.60b | 0.027 | <0.01 |
1Initial = day 0 for blocks 1 and 2, interim = day 126 for block 1, day 123 for block 2, final = day 252 for group 1, day 242 for group 2.
2Largest standard error of least squares means.
The neutrophil:lymphocyte ratio decreased (P < 0.01) from initial to interim and increased (P < 0.01) from interim to final, though it was within normal ranges. Lymphocytes are the most abundant subpopulation of WBC in bovines, therefore a ratio greater than 1 is an indicator of stress (Roland et al., 2014). Similar to RBC and their differentials, changes in WBC and their differentials that do not change outside of normal ranges are likely because of age and are confounded with sampling times.
The metaphylactic treatment effects on WBC differentials are presented in Table 10. There was a tendency (P = 0.10) for EXC to have a greater neutrophil count than NUF, though EXC did not differ (P ≥ 0.06) from DRA or CON. There was no treatment effect (P ≥ 0.12) on lymphocyte concentration, eosinophil concentration and percentage, neutrophil percentage, basophil percentage, and neutrophil:lymphocyte ratio. Additionally, these differentials were all within normal ranges (Merck, 2021).
Table 10.
Treatment effects of complete blood count for high-risk beef steers given metaphylaxis with a negative control, tulathromycin, ceftiofur, and florfenicol at arrival
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Item | CON | DRA | EXC | NUF | SEM2 | P-value |
| Neutrophils, K/uL | 3.50 | 3.62 | 4.12 | 3.39 | 0.234 | 0.10 |
| Lymphocytes, K/uL | 5.70 | 6.07 | 6.13 | 6.14 | 0.151 | 0.12 |
| Eosinophils, K/uL | 0.29 | 0.31 | 0.34 | 0.30 | 0.031 | 0.77 |
| Neutrophils, % | 31.02 | 31.87 | 32.19 | 30.23 | 0.854 | 0.30 |
| Eosinophils, % | 2.91 | 2.92 | 2.97 | 2.77 | 0.247 | 0.51 |
| Basophil, % | 0.03 | 0.03 | 0.04 | 0.04 | 0.004 | 0.13 |
| Neutrophil:Lymphocyte ratio | 0.63 | 0.62 | 0.67 | 0.59 | 0.032 | 0.27 |
1CON = no metaphylaxis at arrival; DRA = tulathromycin (Draxxin, Zoetis) administered as metaphylaxis at arrival; EXC = ceftiofur (Excede, Zoetis) administered as metaphylaxis at arrival; NUF = florfenicol (Nuflor, Merck) administered as metaphylaxis at arrival.
2Largest standard error of least squares means.
Lindholm-Perry et al. (2018) reported that neutrophils were greater in morbid cattle compared to those that were healthy or asymptomatic. It is unclear why the EXC treatment would have greater concentrations of neutrophils than the NUF treatment when the morbidity rate in the NUF treatment group was greater. It is important to note that neutrophils have been reported to be poor predictors of BRD (Richeson et al., 2013), suggesting the treatment differences are better explained by the BRD incidence rates of the antibiotics used as metaphylaxis than as a result of immune response to BRD.
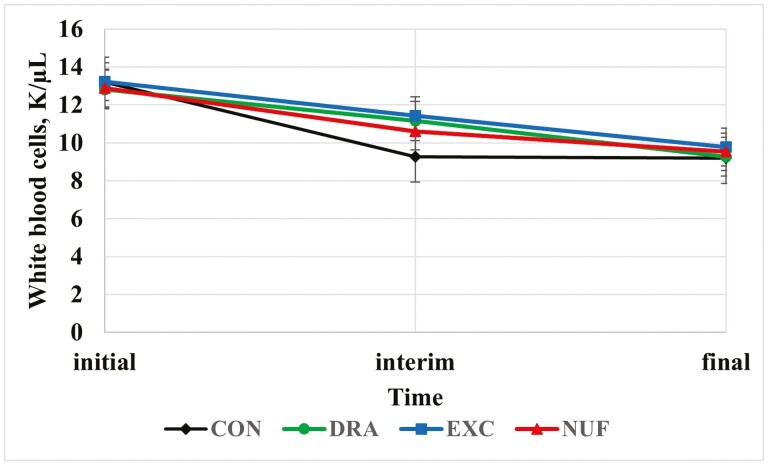
A treatment × time interaction was observed for WBC counts (P < 0.01; Figure 2). The CON treatment had the least WBC at the interim measurement compared to DRA, EXC, and NUF (P ≤ 0.01). Steers in the EXC and NUF treatment group had a decrease in WBC across all time points (P ≤ 0.01) compared to CON and DRA treatments. A greater WBC concentration can be indicative of infection, inflammation, or stress (Pagana et al., 2015). The greatest WBC count on day 0 is expected as calves had recently undergone transport and handling stress as well as a greater morbidity rate than the other two 2 time points.
Figure 2.
Treatment by time interaction for white blood cell count (K/µL; P < 0.01). CON = no metaphylaxis at arrival; DRA = tulathromycin (Draxxin, Zoetis) administered as metaphylaxis at arrival; EXC = ceftiofur (Excede, Zoetis) administered as metaphylaxis at arrival; NUF = florfenicol (Nuflor, Merck) administered as metaphylaxis at arrival. Initial = day 0, interim = day 126 for block 1 and day 110 for block 2, and final = day 252 for block 1 and day 242 for block 2.
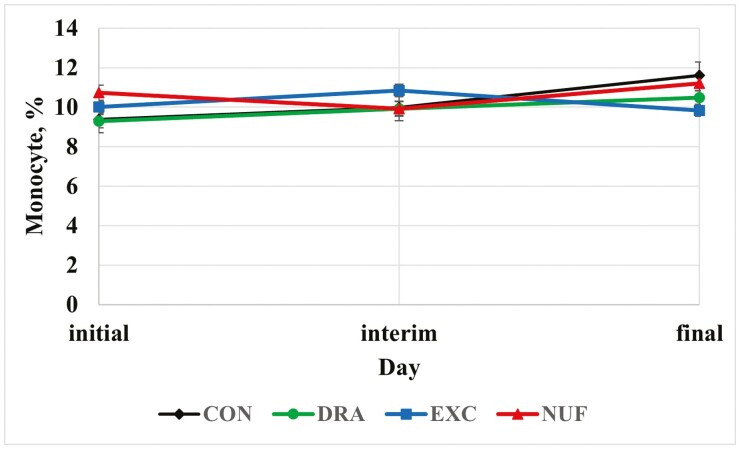
There was a treatment × time interaction for monocyte concentrations (P < 0.01; Figure 3). At the interim measurement, steers administered CON, DRA, and NUF had lesser monocyte concentrations than EXC (P ≤ 0.01). Likely, NUF and CON had a greater monocyte percentage as a result of the greater morbidity rates compared to the other treatments. Like neutrophils, monocytes are phagocytic, and a greater monocyte count or percentage can be indicative of inflammation or viral infection (Roland et al., 2014; Pagana et al., 2015). Conversely, Lindholm-Perry et al. (2018) reported calves with a low monocyte count at weaning were more likely to develop BRD.
Figure 3.
Treatment by time interaction for monocyte percentage (P < 0.01). CON = no metaphylaxis at arrival; DRA = tulathromycin (Draxxin, Zoetis) administered as metaphylaxis at arrival; EXC = ceftiofur (Excede, Zoetis) administered as metaphylaxis at arrival; NUF = florfenicol (Nuflor, Merck) administered as metaphylaxis at arrival. Initial = day 0, interim = day 126 for block 1 and day 110 for block 2, and final = day 252 for block 1 and day 242 for block 2.
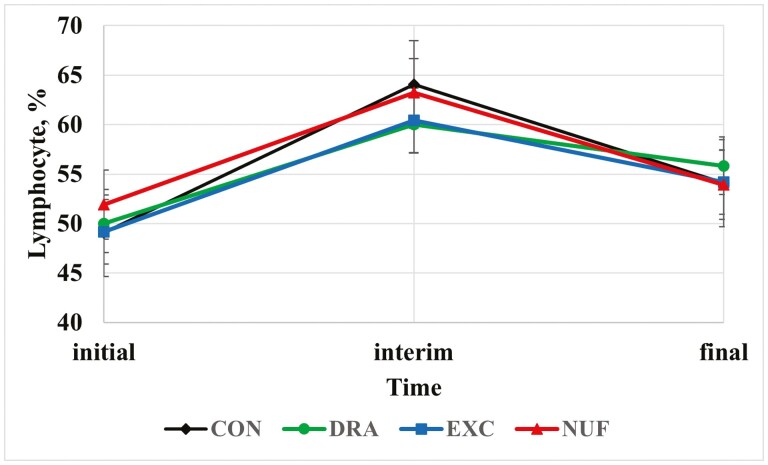
There was a treatment × time interaction for percentage of lymphocytes (P = 0.03; Figure 4). The CON group had a greater interim lymphocyte percentage than both DRA and EXC (P ≤ 0.04). Lymphocytes are the largest proportion of WBC in cattle, though they decrease with age (Roland et al., 2014). In a study conducted by Moisá et al. (2019), a negative association was reported between lymphocyte concentration and BRD. Nevertheless, it should be noted that in other literature lymphocytes have been reported to be poor predictors of BRD based on a low area under the curve (Richeson et al., 2013).
Figure 4.
Treatment by time interaction for lymphocyte percentage (P = 0.03. CON = no metaphylaxis at arrival; DRA = tulathromycin (Draxxin, Zoetis) administered as metaphylaxis at arrival; EXC = ceftiofur (Excede, Zoetis) administered as metaphylaxis at arrival; NUF = florfenicol (Nuflor, Merck) administered as metaphylaxis at arrival. Initial = day 0, interim = day 126 for block 1 and day 110 for block 2, and final = d 252 for block 1 and day 242 for block 2.
CONCLUSION
The results of this study indicate administering metaphylaxis after arrival with either tulathromycin or ceftiofur can decrease the morbidity rate of high-risk calves. Administering florfenicol as metaphylaxis after arrival increased cattle treated for BRD once by 23.1% vs. a negative control where steers did not receive metaphylaxis. During the receiving period, the ADG of steers administered tulathromycin was greatest and DMI and G:F was greatest among those administered tulathromycin and ceftiofur. Tulathromycin increased ADG during the growing phase and ceftiofur had the greatest long-term increase in ADG vs. CON, but did not differ from other metaphylactic antimicrobials used. Bovine respiratory disease remains a complex and multifactorial health challenge in high-risk cattle, but producers can improve the health outcomes of their cattle with several commercially available antimicrobials administered as metaphylaxis that decrease morbidity and mortality and subsequently improve growth performance.
Acknowledgment
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer. This work was supported by Mitigating Antimicrobial Resistance, grant number 2020-68015-30857, project accession 1022218 from the USDA National Institute of Food and Agriculture and was funded in part by the Beef Checkoff. Any opinions, findings, conclusions, or recommendations expressed in this presentation are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
Contributor Information
Carley M Coppin, Department of Animal and Food Science, Texas Tech University, Lubbock, TX, 79409.
Taylor M Smock, Department of Animal and Food Science, Texas Tech University, Lubbock, TX, 79409.
Cory L Helmuth, Department of Animal and Food Science, Texas Tech University, Lubbock, TX, 79409.
Jeff L Manahan, Department of Animal and Food Science, Texas Tech University, Lubbock, TX, 79409.
Nathan S Long, Department of Animal and Food Science, Texas Tech University, Lubbock, TX, 79409.
Ashley A Hoffman, Department of Animal and Food Science, Texas Tech University, Lubbock, TX, 79409.
Jeffrey A Carroll, USDA-ARS, Livestock Issues Research Unit, Lubbock, TX 79403.
Paul R Broadway, USDA-ARS, Livestock Issues Research Unit, Lubbock, TX 79403.
Nicole C Burdick Sanchez, USDA-ARS, Livestock Issues Research Unit, Lubbock, TX 79403.
James E Wells, USDA-ARS, U.S. Meat Animal Research Center, Clay Center, NE 68933.
Samodha C Fernando, Department of Animal Science, University of Nebraska-Lincoln, Lincoln, NE 68583.
Kristin E Hales, Department of Animal and Food Science, Texas Tech University, Lubbock, TX, 79409.
Conflict of interest statement
None declared.
LITERATURE CITED
- Abell, K. M., Theurer M. E., Larson R. L., White B. J., and Apley M.. . 2017. A mixed treatment comparison meta-analysis of metaphylaxis treatments for bovine respiratory disease in beef cattle. J. Anim. Sci. 95:626–635. doi: 10.2527/jas.2016.1062 [DOI] [PubMed] [Google Scholar]
- Benton, J. R., Erickson G. E., Klopfenstein T. J., Luebbe M. K., and Smith D. R.. . 2008. Effect of exceed administered to calves at arrival in the feedlot on performance and respiratory disease. Nebraska Beef Cattle Report—[accessed January 18, 2022]https://digitalcommons.unl.edu/animalscinbcr/37/.
- Booker, C. W., Schunicht O. C., Guichon P. T., Jim G. K., Wildman B. K., Pittman T. J., and Perrett T.. . 2006. An evaluation of the metaphylactic effect of ceftiofur crystalline free acid in feedlot calves. Vet. Ther. 7:257–274. [PubMed] [Google Scholar]
- Boyd, M. E., Bowers A. M., Engelken T. J., Bryson W. L., and Moseley W. M.. . 2006. Feed intake response of feedlot cattle following single-dose treatment of ceftiofur crystalline free acid sterile suspension of florfenicol. Bovine Pract. 40:46–50. doi: 10.21423/bovine-vol40no1p46-50 [DOI] [Google Scholar]
- Catry, B., Duchateau L., Van de Ven J., Laevens H., Opsomer G., Haesebrouck F., and de Kruif A. J.. . 2008. Efficacy of metaphylactic florfenicol therapy during natural outbreaks of bovine respiratory disease. Vet. Pharm. Ther. 31:479–487. doi: 10.1111/j.1365-2885.2008.00981.x [DOI] [PubMed] [Google Scholar]
- Dennis, E. J., Schroeder T. C., and Renter D. G.. . 2020. Net return distributions when metaphylaxis is used to control bovine respiratory disease in high health-risk cattle. Transl. Anim. Sci 4:1091–1102. doi: 10.1093/tas/txaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff, G. C., and Galyean M. L.. . 2007. Board-invited review: recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. doi: 10.2527/jas.2006-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley, B., Sporer K. B., and Gupta S.. . 2016. Invited review: relationship between cattle transport, immunity and respiratory disease. Animal 11:486–492. doi: 10.1017/S1751731116001622 [DOI] [PubMed] [Google Scholar]
- Encinias, M., Walker D., Murdock C., and Reeves L.. . 2006. Effects of prophylactic administration of ceftiofur crystalline free acid on health and performance of newly received beef calves. Proceedings. Western Section American Society of Animal Science; 57:160–163—[ Accessed January 18, 2022] https://www.asas.org/sections/western-section/publications. [Google Scholar]
- Evans, N. A. 2005. Tulathromycin: an overview of a new triamilide antibiotic for livestock respiratory disease. Vet. Ther. 6:83–95. [PubMed] [Google Scholar]
- Fontenot, L. 2015. Hematological variables as predictors of bovine respiratory disease in newly received cattle fed in confinement [master’s dissertation]. Canyon (TX): West Texas A&M University. [Google Scholar]
- Gardner, B. A., Dolezal H. G., Bryant L. K., Owens F. N., and Smith R. A.. . 1999. Health of finishing steers: effects on performance, carcass traits, and meat tenderness. J. Anim. Sci. 77:3168–3175. doi: 10.2527/1999.77123168x [DOI] [PubMed] [Google Scholar]
- Godinho, K. S., Wolf R. M., Sherington J., Rowan T. G., Sunderland S. J., and Evans N. A.. . 2005. Efficacy of tulathromycin in the treatment and prevention of natural outbreaks of bovine respiratory disease in European cattle. Vet. Ther. 6:122–135. [PubMed] [Google Scholar]
- Gonzalez-Martin, J., Elvira L., Lopez M. C., Villalobos N. P., Lopez-Guerrero E. C., and Astiz S. J. L. S.. . 2011. Reducing antibiotic use: selective metaphylaxis with florfenicol in commercial feedlots. Livest. Sci 141:173–181. doi: 10.1016/j.livsci.2011.05.016 [DOI] [Google Scholar]
- Griffin, D. 1997. Economic impact associated with respiratory disease in beef cattle. Vet. Clin. N. Am. Food Anim. Pract. 13:367–377. doi: 10.1016/s0749-0720(15)30302-9 [DOI] [PubMed] [Google Scholar]
- Guiroy, P. J., Fox D. G., Tedeschi L. O., Baker M. J., and Cravey M. D.. . 2001. Predicting individual feed requirements of cattle fed in groups. J. Anim. Sci. 79:1983–1995. doi: 10.2527/2001.7981983x [DOI] [PubMed] [Google Scholar]
- Hibbard, B., Robb E. J., S. T.Chester, Jr., Dame K. J., Boucher J. F., and Alaniz G. R.. . 2002a. Dose determination and confirmation of a long-acting formulation of ceftiofur (ceftiofur crystalline free acid) administered subcutaneously for the treatment of bovine respiratory disease. J. Vet. Pharm. Ther. 25:175–180. doi: 10.1046/j.1365-2885.2002.00403.x [DOI] [PubMed] [Google Scholar]
- Hibbard, B., Robb E. J., S. T.Chester, Jr., Dame K. J., Moseley W. W., and Bryson W. L.. . 2002b. Dose determination and confirmation for ceftiofur crystalline-free acid administered in the posterior aspect of the ear for control and treatment of bovine respiratory disease. Vet. Ther. 3:22-30. [PubMed] [Google Scholar]
- Kilgore, W. R., Spensley M. S., Sun F., Nutsch R. G., Rooney K. A., and Skogerboe T. L.. . 2005. Clinical effectiveness of tulathromycin, a novel triamilide antimicrobial, for the control of respiratory disease in cattle at high risk for developing bovine respiratory disease. Vet. Ther. 6:136–142. [PubMed] [Google Scholar]
- Klinger, M. H. F. 1997. Platelets and inflammation. Anat. Embryol. 196:1–11. doi: 10.1007/s004290050075 [DOI] [PubMed] [Google Scholar]
- Lindholm-Perry, A. K., Kuehn L. A., McDaneld T. G., Miles J. R., Workman A. M., Chitko-McKown C. G., and Keele J. W.. . 2018. Complete blood count data and leukocyte expression of cytokine genes and cytokine receptor genes associated with bovine respiratory disease in calves. BMC Res. Notes 11:786–786. doi: 10.1186/s13104-018-3900-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgreen, G. P. 1983. Mass medication in reducing shipping fever-bovine respiratory disease complex in highly stressed calves. J. Anim. Sci. 56:529–536. doi: 10.2527/jas1983.563529x [DOI] [PubMed] [Google Scholar]
- Martín, G. J. V., Partida E. L., Villalobos P. N., López C. M., López-Guerrero C. E., and Blanco A. S.. . 2007. Evaluation of mass and selective metaphylaxis medication with florfenicol at feedlot entry as a tool against bovine respiratory disease under commercial conditions in Spain. Cattle Pract 15:309–311. [Google Scholar]
- Merck. 2021. Merck Veterinary Manual. Whitehouse Station, NJ: Merck & Co., Inc.—[accessed January 18, 2022] www.merckvetmanual.com. [Google Scholar]
- Moisá, S. J., Aly S. S., Lehenbauer T. W., Love W. J., Rossitto P. V., Van Eenennaam A. L., Trombetta S. C., Bortoluzzi E. M., and Hulbert L. E.. . 2019. Association of plasma haptoglobin concentration and other biomarkers with bovine respiratory disease status in pre-weaned dairy calves. J. Vet. Diagn. 31:40–46. doi: 10.1177/1040638718807242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz, V. I., Samuelson K. L., Tomczak D. J., Seiver H. A., Smock T. M., and Richeson J. T.. . 2020. Comparative efficacy of metaphylaxis with tulathromycin and pentavalent modified-live virus vaccination in high-risk, newly received feedlot cattle. Appl. Anim. Sci. 36:799–807. doi: 10.15232/aas.2020-02054 [DOI] [Google Scholar]
- NASEM. 2016. The National Academics of Sciences Engineering and Medicine Nutrient requirements of beef cattle. 8th rev. ed. Washington, DC: Natl. Acad. Press. [Google Scholar]
- Nickell, J. S., and White B. J.. . 2010. Metaphylactic antimicrobial therapy for bovine respiratory disease in stocker and feedlot cattle. Vet. Clin. N. Am. Food Anim. Pract. 26:285–301. doi: 10.1016/j.cvfa.2010.04.006 [DOI] [PubMed] [Google Scholar]
- Nutsch, R. G., Skogerboe T. L., Rooney K. A., Weigel D. J., Gajewski K., and Lechtenberg K. F.. . 2005. Comparative efficacy of tulathromycin, tilmicosin, and florfenicol in the treatment of bovine respiratory disease in stocker cattle. Vet. Ther. 6:167–179. [PubMed] [Google Scholar]
- Pagana, K. D., Pagana T. J., and Pagana T. N.. . 2015. Mosby’s diagnostic and laboratory test reference. 12th ed. St. Louis, MO: Elsevier. [Google Scholar]
- Pillen, J. L., Pinedo P. J., Ives S. E., Covey T. L., Naikare H. K., and Richeson J. T.. . 2016. Alteration of activity variables relative to clinical diagnosis of bovine respiratory disease in newly received feed lot cattle. Bovine Pract. 50:1–8. doi: 10.21423/bovine-vol50no1p1-8 [DOI] [Google Scholar]
- Richeson, J. T., Pinedo P. J., Kegley E. B., Powell J. G., Gadberry M. S., Beck P. A., and Falkenberg S. M.. . 2013. Association of hematologic variables and castration status at the time of arrival at a research facility with the risk of bovine respiratory disease in beef calves. J. Am. Vet. Med. Assoc. 243:1035–1041. doi: 10.2460/javma.243.7.1035 [DOI] [PubMed] [Google Scholar]
- Roland, L., Drillich M., and Iwersen M.. . 2014. Hematology as a diagnostic tool in bovine medicine. J. Vet. Diagn. 26:592–598. doi: 10.1177/1040638714546490 [DOI] [PubMed] [Google Scholar]
- Rooney, K. A., Nutsch R. G., Skogerboe T. L., Weigel D. J., Gajewski K., and Kilgore W. R.. . 2005. Efficacy of tulathromycin compared with tilmicosin and florfenicol for the control of respiratory disease in cattle at high risk of developing bovine respiratory disease. Vet. Ther. 6:154–166. [PubMed] [Google Scholar]
- Skogerboe, T. L., Rooney K. A., Nutsch R. G., Weigel D. J., Gajewski K., and Kilgore W. R.. . 2005. Comparative efficacy of tulathromycin versus florfenicol and tilmicosin against undifferentiated bovine respiratory disease in feedlot cattle. Vet. Ther. 6:180–196. [PubMed] [Google Scholar]
- Tennant, T. C., Ives S. E., Harper L. B., Renter D. G., and Lawrence T. E.. . 2014. Comparison of tulathromycin and tilmicosin on the prevalence and severity of bovine respiratory disease in feedlot cattle in association with feedlot performance, carcass characteristics, and economic factors. J. Anim. Sci. 92:5203–5213. doi: 10.2527/jas.2014-7814 [DOI] [PubMed] [Google Scholar]
- Tylutki, T. P., Fox D. G., and Anrique R. G.. . 1994. Predicting net energy and protein requirements for growth of implanted and nonimplanted heifers and steers and nonimplanted bulls varying in body size. J. Anim. Sci. 72:1806–1813. doi: 10.2527/1994.7271806x [DOI] [PubMed] [Google Scholar]
- Urban-Chmiel, R., and Grooms D.. . 2012. Prevention and control of bovine respiratory disease. J. Livest. Sci 3:27–36. [Google Scholar]
- Wilson, B. K., Step D. L., Maxwell C. L., Gifford C. A., Richards C. J., and Krehbiel C. R.. . 2017. Effect of bovine respiratory disease during the receiving period on steer finishing performance, efficiency, carcass characteristics, and lung scores. Prof. Anim. Sci. 33:24–36. doi: 10.15232/pas.2016-01554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolums, A. R., Karisch B. B., Frye J. G., Epperson W., Smith D. R., J.Blanton, Jr., Austin F., Kaplan R., Hiott L., Woodley T., . et al. 2018. Multidrug resistant Mannheimia haemolytica isolated from high-risk beef stocker cattle after antimicrobial metaphylaxis and treatment for bovine respiratory disease. Vet. Microbiol. 221:143-152. doi: 10.1016/j.vetmic.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Word, A. B., Ellis G. B., Holland B. P., Streeter M. N., and Hutcheson J. P.. . 2021. Effects of antimicrobial metaphylaxis using no antimicrobial, tilmicosin, or tildipirosin and 2 different days on feed on the health and growth performance of lightweight beef steer calves originating from Mexico. Appl. Anim. Sci. 37:207–216. doi: 10.15232/aas.2020-02117 [DOI] [Google Scholar]