Abstract
The thalamus is known to be impaired in schizophrenia patients with auditory verbal hallucinations (AVHs). Abnormal filtering function of the thalamus has been found in schizophrenia patients with AVHs. However, a whole-structure approach has commonly been adopted when investigating thalamic dysconnectivity in patients with AVHs, and it remains unclear which thalamic nucleus is the critical structure underlying AVHs. Here, we investigated voxel-wise resting-state functional connectivity (rsFC) of the thalamic nucleus in drug-naïve patients with first-episode schizophrenia (FES) with AVHs. In addition, dynamic causal modeling was applied to compute effective connectivity and estimate causal relationships that could explain aberrant rsFC. Compared with the FES patients without AVH (NAVH) and normal controls, patients with AVHs had weaker rsFC of the bilateral medial pulvinar (PuM) nucleus-cerebellum. Moreover, compared with the normal control group, the AVH and NAVH groups had significantly stronger rsFC of the bilateral PuM nucleus-cerebral cortex, as well as weaker rsFC of the right medial geniculate nucleus-cerebral cortex. Compared with the NAVH and normal control groups, dynamic causal modeling revealed significantly stronger effective connectivity from the left PuM nucleus to the right inferior frontal gyrus in the AVH group. These findings indicate that the critical structure in the thalamus underlying AVHs is the PuM nucleus, and provide direct evidence that the cerebello-thalamo-cortical circuit is associated with AVHs.
Keywords: auditory verbal hallucinations, thalamic nucleus, cerebello-thalamo-cortical circuit, resting-state functional connectivity, effective connectivity
Introduction
Auditory verbal hallucinations (AVHs) are a prominent symptom of schizophrenia, and affect approximately 60%–80% of patients.1 AVHs are defined as hearing and perceiving voices in the absence of an external auditory stimulus. Based on the technology of functional magnetic resonance imaging, recent findings suggested that AVHs might be traced back to abnormally elevated resting-state activity in auditory cortex and other language-related cortex.2–5 Especially, the thalamus is involved in language production,6 a crucial node for brain physiology and part of functional and structural pathways relevant for schizophrenia patients with AVHs.7–12
As the filtering information station of the central nervous system, the thalamus is characterized by enhancing certain inputs but suppressing others in schizophrenia, showing impaired thalamic filtering of external speech from internal speech.13–15 Previous work has revealed that patients with thalamic damage can experience hallucinations.16–19 The thalamic medial pulvinar (PuM) nucleus and medial geniculate nucleus (MGN) have been associated with AVHs.11,12,20–22 In our previous study, one schizophrenia patient who could voluntarily control AVHs showed stronger connectivity from the left PuM nucleus to the left auditory cortex in the AVH state.11 Hypoactivation of the MGN and PuM nucleus occurred in poorly performing schizophrenia patients with AVHs during monitoring of self- and externally-generated speech.22 Hyperactivation of the thalamus at rest might inhibit the response to externally-generated speech. Abnormal glucose metabolism20 and iron deposition21 in the PuM nucleus have also been associated with hallucinations. However, the increase in D2 dopamine receptors (DRD2) in the MGN was found to decrease thalamo-cortical projections, and antipsychotics were able modulate the microRNA-processing gene Dgcr8 to decrease the DRD2 and further alleviate auditory hallucinations.12 These inconsistent findings mean that it remains unclear which specific thalamic nucleus is involved in abnormal functional connectivity in AVHs.
Previous studies have indirectly indicated that the thalamus is involved in the cerebello-thalamo-cortical circuit, which might be associated with AVHs, and have suggested that cerebello-thalamo-cortical connectivity could be a biomarker of psychosis prediction.23,24 Pinheiro et al25 reviewed and explored the role of the cerebello-thalamo-cortical circuit in AVHs. Hallucinations occurred following lesions within a structurally and functionally connected brain network, including the cerebellum, thalamus, and superior temporal gyrus (STG).26 Low frequency repetitive transcranial magnetic stimulation has been demonstrated to be effective in reducing AVHs through local inhibitory effects in the temporoparietal cortex and other connected cortical regions, including the STG, thalamus, and cerebellum.27 Moreover, the cerebellum returns connections to the temporal28 and frontal29 lobes via the thalamus.
Resting-state functional connectivity (rsFC) has been used to analyze abnormal thalamo-cerebellar and thalamo-cortical connectivity in schizophrenia patients with AVHs. Weaker rsFC between the thalamus and cerebellum,30 and stronger rsFC between the thalamus and ipsilateral auditory cortex,11 have been reported in schizophrenia patients with AVHs. Although rsFC analysis can identify altered connections between brain regions, rsFC does not indicate causal relationships between brain regions. Dynamic causal modeling (DCM) can characterize the causal relationships and information flow between brain regions based on rsFC, and has become a predominant method by which to quantify effective connectivity.31,32 Meanwhile, stronger effective connectivity from the left thalamus to the left auditory cortex has been found in patients with AVH.11,33 Although previous studies have provided evidence indicative of an abnormal filtering function of the thalamus in the case of AVHs, it is still unclear which thalamic nucleus is the critical structure underlying AVHs. A whole-structure approach has commonly been adopted when investigating thalamic dysconnectivity in AVHs.15,33,34 However, recent human neuroimaging indicates that there is a thalamic functional topography,35 and this information is missed when using whole-structure approaches. The anterior nucleus (AN) and the dorsomedial (DM) nucleus of the thalamus are known to be impaired in schizophrenia.36–38 Here, we investigated (1) voxel-wise rsFC between the thalamic nucleus (PuM nucleus, MGN, AN, and DM) and the whole brain; (2) region of interest (ROI)-wise effective connectivity using DCM analysis, according to the between-group differences in rsFC of the thalamic nucleus; (3) the relationship between thalamic connectivity and clinical measures in patients with AVHs. In this study, we found altered cerebello-thalamo-cortical connectivity in schizophrenia patients with AVHs.
Methods
Participants
This study randomly recruited 100 drug-naïve patients with first-episode schizophrenia (FES) and 50 age- and sex-matched normal controls (NCs) (Table 1). Schizophrenia diagnosis by a psychiatric specialist was made using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). The illness duration of all patients was less than 3 years, and the diapause was less than 6 months. Symptom severity of schizophrenia was assessed with the Positive and Negative Syndrome Scale (PANSS). Fifty patients reported experiencing AVHs within the past 4 weeks, most within the past week, while the other 50 patients reported no AVH (NAVH) in their lifetime or in the past 4 months. This was based on the PANSS scores at the time of screening, as well as detailed information regarding past symptomatology that was acquired in patient interviews and examination of the patients’ medical records. The severity of AVHs was assessed using the Auditory Hallucination Rating Scale (AHRS). Eleven patients reported that the voices appeared at least once a week; the other 39 patients reported hearing these voices at least once a day. Twelve patients reported that the voices continued for several seconds at a time; 26 patients reported voices lasting several minutes; 5 patients reported voices lasting more than an hour; and 7 patients reported that the voices could continue for several hours at a time. We collected PANSS data for 33 of the AVH patients and for all NAVH patients, and collected AHRS data for all AVH patients. All participants were right-handed. Exclusion criteria for all participants were as follows: (1) contraindications for MRI, (2) alcohol or drug abuse, and (3) severe physical disability and traumatic head injuries. NCs had no history of neurological or psychiatric illness. All subjects gave the informed consent, and this study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Table 1.
The Demographic and Clinical Data of Schizophrenia Patients, With and Without AVH, and Normal Controls
| AVH | NAVH | NC | F/X2/t Values | P Values | |
|---|---|---|---|---|---|
| Age (SD, n = 50) | 21.3 (7.7) | 21.3 (7.6) | 22.0 (7.7) | 0.139 | .871 |
| Sex (M/F, n = 50) | 24/26 | 25/25 | 24/26 | 0.053 | .974 |
| AHRS (SD, n = 50) | 23.86 (5.99) | — | — | — | — |
| PANSS (SD) (AVH: n = 33; NAVH: n = 50) | |||||
| PANSS total | 83.3 (14.6) | 82.6 (15.9) | — | 0.204 | .839 |
| PANSS positive | 20.3 (5.4) | 19.6 (6.2) | — | 0.537 | .593 |
| PANSS negative | 20.8 (4.8) | 21.0 (5.6) | — | −0.128 | .899 |
| PANSS general | 42.2 (7.5) | 42.0 (8.6) | — | 0.077 | .939 |
| PANSS hallucinations | 4.1 (1.5) | 2.4 (1.6) | — | 4.073 | .0002 |
| PANSS delusions | 4.7 (1.4) | 3.8 (1.7) | — | 1.966 | .056 |
Note: AHRS, auditory hallucination rating scale; AVH, auditory verbal hallucination; F, female; M, male; NAVH, without auditory verbal hallucination; NC, normal control; PANSS, positive and negative syndrome scale.
Data Acquisition
All subjects were scanned using a 3.0 T MRI scanner (Discovery MR750, GE, USA) with an 8-channel receiver array head coil. Head motion and scanner noise were reduced using foam paddings and earplugs. All participants were asked to remain alert with their eyes closed. We collected MRI data from all participants. Structural images were acquired using a 3D T1 BRAVO sequence with the following settings: repetition time (TR)/echo time (TE) = 8.2/3.2 ms, slice number = 188, slice thickness = 1 mm, slice gap = 0 mm, flip angle = 12°, field of view (FOV) = 25.6 × 25.6 cm2, number of averages = 1, matrix size = 256 × 256, voxel size = 1 × 1 × 1 mm3, scan time = 4.33 min. Functional images were acquired transversely with gradient spin echo planar imaging sequence with the following settings: TR/TE = 2000/30 ms, slice number = 32, slice thickness = 4 mm, slice gap = 0.5 mm, flip angle = 90º, FOV = 22 × 22 cm2, number of averages = 1, matrix size = 64 × 64, voxel size = 3.4375 × 3.4375 × 4 mm3. A total of 180 volumes were collected, resulting in a total scan time of 6 min. The patients in the AVH group reported that they experienced no hallucinations during scanning.
Data Processing
Data preprocessing information is provided in the Supplementary materials. The seeds for the PuM nucleus, MGN, AD, and MD of the bilateral thalami were selected using the automated anatomical labeling 3 (AAL3) template39 to calculate rsFC (figure 1A and Supplementary figure 1A). For each individual subject, a seed-to-voxel rsFC analysis was carried out to compute the correlation maps between the seed and voxel at resting-state, using movement parameters, white matter, and cerebrospinal fluid signals as nuisance regressors. The global signal was not regressed out as has been recently suggested when processing functional data from patients with schizophrenia.40 After that, Z-transformed connectivity maps were obtained and compared between groups using one-way analysis of variance (ANOVA), with age, sex, and mean framewise displacement (FD) as covariates. Then, post hoc comparisons using Bonferroni’s test were performed, and pair-wise different Z-value maps corresponding to between-group rsFC differences were computed. The statistically significant threshold was set at voxel-wise P < .001, cluster-wise P < .05, and the minimum cluster size of 37 voxels after Gaussian random field (GRF) correction.
Fig. 1.
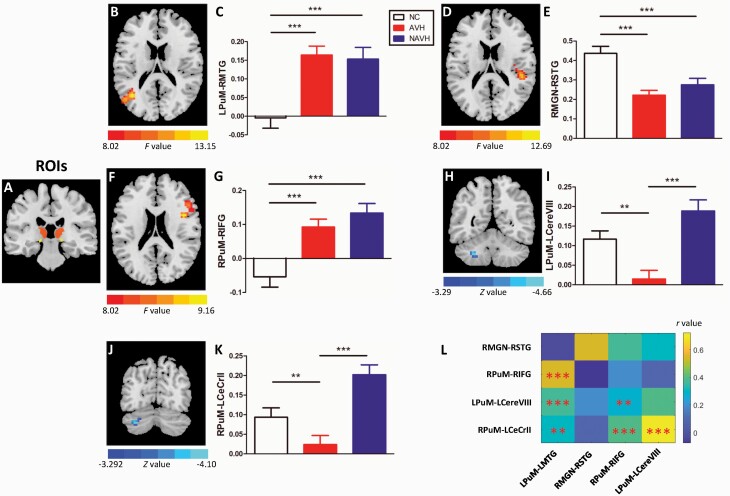
Resting-state functional connectivity of the PuM nucleus/MGN. (A) Regions of interest (ROIs) selection. (B–G) Compared with normal controls (NCs), schizophrenia patients with auditory verbal hallucinations (AVHs), and without auditory verbal hallucination (NAVH), had significantly stronger resting-state functional connectivity (rsFC) of the left medial pulvinar (LPuM) nucleus-left middle temporal gyrus (LMTG), and right PuM (RPuM) nucleus-right inferior frontal gyrus (RIFG), and weaker rsFC of the right medial geniculate nucleus (RMGN)-right superior temporal gyrus (RSTG). (H–K) Moreover, compared with the NAVH and NC groups, the AVH group had significantly weaker rsFC of the LPuM nucleus-left cerebellum VIII (LCereVIII) and RPuM nucleus-left cerebellum crus II (LCeCrII). (L) Correlation matrix between the rsFC of the thalamic nucleus-cerebral cortex and the thalamic nucleus-cerebellum. **P < .01, ***P < .001.
To evaluate the relationship between one altered rsFC and another altered rsFC, the correlation analysis was also calculated for all participants and each group. Given that we identified 5 significantly altered connections, Bonferroni’s test was used to correct for multiple comparisons to find significant correlations in (5 × 5 − 5) ÷ 2 = 10 connections.
Dynamic Causal Modeling
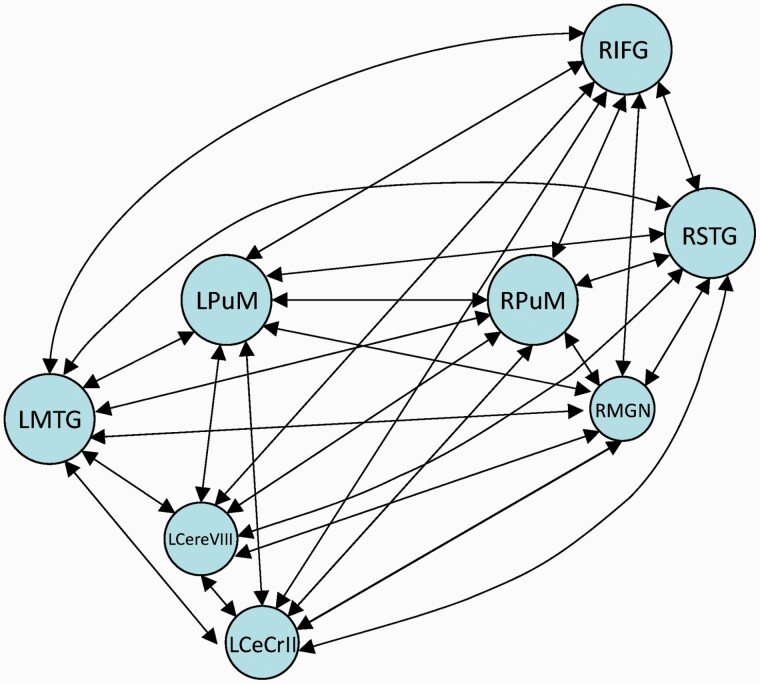
The spectral DCM analyses were performed using DCM12, which is based on SPM12. According to the between-group differences in rsFC of the thalamic nucleus (PuM nucleus or MGN), we selected eight areas as ROIs, including the bilateral PuM nuclei, right MGN, left middle temporal gyrus (MTG), right STG, right inferior frontal gyrus (IFG), left cerebellum VIII, and left cerebellum crus II (figures 1 and 2). Time series were extracted from the eight ROIs. First, a general linear model was set up in SPM12 with cosine basis functions from 1/128 Hz to 0.1 Hz as effects of interest, and movement parameters, white matter signals, and cerebrospinal fluid signals as nuisance regressors.31,41 The full model was constructed with the eight ROIs as nodes. Bilateral connections between nodes were made, which resulted in 64 connections, including the self-connection of each node (figure 2). We only report connection coefficients with a posterior probability >.95. The connection coefficients for each group were assessed using one-sample Wilcoxon-Signed-Rank test and compared with the value 0, whereby an index value of significantly <0 indicates negative effective connectivity and an index value of significantly >0 indicates positive effective connectivity. To determine if there were significant between-group differences in the connection coefficients, we performed Kruskal–Wallis’ H test, then Dunn’s test as the post hoc test.
Fig. 2.
The full model was investigated using dynamic causal modeling. DCM, dynamic causal modeling; LCereVIII, left cerebellum VIII; LCeCrII, left cerebellum crus II; LMTG, left middle temporal gyrus; LPuM, left medial pulvinar; RIFG, right inferior frontal gyrus; RMGN, right medial geniculate nucleus; RPuM, right medial pulvinar; RSTG, right superior temporal gyrus.
Correlation Analysis
To investigate the relationship between altered connectivity and symptom severity, we performed correlation analysis between significant rsFC and effective connectivity with clinical measures in the AVH and NAVH groups. Multiple comparisons were corrected using the Bonferroni method (P < .05/50 = .001).
Results
Demographic and Clinical Data
No significant between-group difference in age or sex were found, and no significant between-group difference in PANSS total, positive, negative, general, or delusion scores between the AVH and NAVH groups, except for hallucination scores (Table 1).
Resting-State Functional Connectivity of the Thalamic Nucleus
The one-way ANOVA revealed significant between-group differences in rsFC of the left PuM nucleus-left MTG (figure 1B), right MGN-right STG (figure 1D), and right PuM nucleus-right IFG (figure 1F) (voxel level P < .001, cluster level P < .05, GRF-corrected; Table 2). We extracted the rsFC values of the above connections for each group, and performed a one-way ANOVA followed by post hoc comparisons using Bonferroni’s test. Compared with the NC group, both the AVH and NAVH groups showed significantly stronger rsFC of the left PuM nucleus-left MTG (figure 1C) and right PuM nucleus-right IFG (figure 1G), as well as weaker rsFC of the right MGN-right STG (figure 1E) (P < .001, Bonferroni-corrected). The rsFC of the left PuM nucleus-left MTG showed a trend towards a correlation with the AHRS scores in the AVH group (r = 0.266; P = .062). The rsFC of the right PuM nucleus-right IFG was positively correlated with the severity of positive symptoms in the NAVH group (r = 0.317; P = .025). However, this significance did not remain after Bonferroni correction (P < .05/50 = .001).
Table 2.
Between-Group Differences in the Resting-State Functional Connectivity of the PuM Nucleus or MGN
| Between-group Differences | Regions | Cluster Size (Voxels) | Peak MNI Coordinate | Peak F/Z Values | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| AVH/NAVH > NC | LPuM-RMTG | 125 | −39 | −60 | 18 | 13.158 |
| RPuM-RIFG | 131 | 42 | 6 | 21 | 13.243 | |
| AVH/NAVH < NC | RMGN-RSTG | 75 | 48 | −30 | 18 | 13.690 |
| AVH < NAVH | LPuM-LCereVIII | 73 | −27 | −54 | −42 | −4.664 |
| RPuM-LCeCrII | 75 | −33 | −72 | −36 | −4.100 | |
Note: AVH, auditory verbal hallucination; LCeCrII, left cerebellum crus II; LCereVIII, left cerebellum VIII; LMTG, left middle temporal gyrus; LPuM, left medial pulvinar; MNI, Montreal Neurological Institute; NAVH, without auditory verbal hallucination; NC, normal control; RIFG, right inferior frontal gyrus; RMGN, right medial geniculate nucleus; RPuM, right medial pulvinar; RSTG, right superior temporal gyrus.
Moreover, compared with the NAVH group, the AVH group had weaker rsFC of the left PuM nucleus-left cerebellum VIII and right PuM nucleus-left cerebellum crus II (voxel level P < .001, cluster level P < .05, GRF-corrected; Table 2, figure 1H and J). We also extracted the rsFC values of the above connections for each group and performed a one-way ANOVA followed by post hoc comparisons with Bonferroni’s test. Compared with the NAVH (P < .001, Bonferroni-corrected) and NC (P < .01, Bonferroni-corrected) groups, the AVH group showed significantly weaker rsFC of the left PuM nucleus-left cerebellum VIII (figure 1I) and right PuM nucleus-left cerebellum crus II (figure 1K).
We found some significant correlations between above connections in each group (Supplementary tables 1–3) and across all participants (Supplementary table 4, figure 1L). For example, the connection of the left PuM nucleus-left cerebellum VIII was significantly correlated with the connection of the left PuM nucleus-left MTG within each group. Slight differences were existed among three groups. In general, the AVH group showed weaker correlations between connections.
We found stronger and weaker rsFC of the AN or DM nucleus with the cerebral cortex and subcortex in the AVH and NAVH groups compared with that in the NC group, but no significant difference for the AN or DM nucleus connectivity between the AVH and NAVH groups (Supplementary materials, Supplementary table 5, and Supplementary figure 1).
Effective Connectivity of the Thalamic Nucleus
The full model analyzed in this study is shown in figure 2, and the effective connectivity values are shown in Supplementary table 6. In the single-group analysis (one-sample Wilcoxon-Signed-Rank test), we found significant strong negative effective connectivity for the self-connection of each node and other positive/negative effective connectivity (figure 3) for each group (Supplementary materials, Supplementary table 6).
Fig. 3.
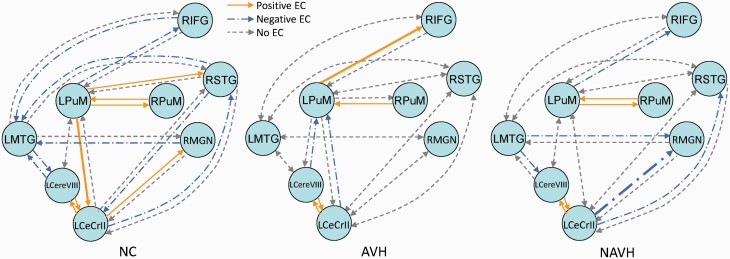
The DCM model at the group level. The solid and long-dash dotted lines represent significant nonzero values revealed from single-group analysis. The solid lines represent significant positive effective connectivity, and the long-dash dotted lines represent significant negative effective connectivity. The dash lines indicate that the connectivity value was not a significant nonzero value. The bold lines indicate significant differences between groups. AVH, auditory verbal hallucination; DCM, dynamic causal modeling; LCereVIII, left cerebellum VIII; LCeCrII, left cerebellum crus II; LMTG, left middle temporal gyrus; LPuM, left medial pulvinar; NAVH, without auditory verbal hallucination; NC, normal control; RIFG, right inferior frontal gyrus; RMGN, right medial geniculate nucleus; RPuM, right medial pulvinar; RSTG, right superior temporal gyrus.
The between-group comparisons (Kruskal–Wallis’ H test) showed significant between-group differences in effective connectivity from the left PuM nucleus to the right IFG, from the left PuM nucleus to the left cerebellum crus II, and from the left cerebellum crus II to the right MGN (P = .004; P = .039; P = .017). Dunn’s post hoc test revealed significantly stronger effective connectivity from the left PuM nucleus to the right IFG in the AVH group, compared with the NAVH and NC groups (P = .025; P = .007), and weaker effective connectivity from the left PuM nucleus to the left cerebellum crus II, and from the left cerebellum crus II to the right MGN, in the NAVH group compared with the NC group (P = .034; P = .020; figure 3). No significant correlation was found between altered effective connectivity and symptom severity in the AVH or NAVH groups.
Discussion
In this study, we characterized rsFC and effective connectivity of the thalamic nucleus, and found that the critical structure in the thalamus underlying AVHs was the PuM nucleus. Our results also showed aberrant cerebello-thalamo-cortical connectivity in FES patients with AVHs. Compared with the NAVH and NC groups, the AVH group showed significantly weaker rsFC of the left PuM nucleus-left cerebellum VIII and right PuM nucleus-left cerebellum crus II. Compared with the NC group, both the AVH and NAVH groups showed significantly stronger rsFC of the left PuM nucleus-left MTG and right PuM nucleus-right IFG, as well as weaker rsFC of the right MGN-right STG. Significantly stronger effective connectivity from the left PuM nucleus to the right IFG was found in the AVH group compared with the NAVH and NC groups.
These findings indicate that abnormal thalamic connectivity was mainly found in the PuM nucleus and partly in the MGN in patients with AVHs. More studies have linked AVHs to the PuM nucleus,11,20,21 rather than the MGN.12 The pulvinar nucleus is closely related to the visual system, as well as the auditory system.42 Indeed, the pulvinar nucleus is densely connected to cortical areas involved in multisensory perception. In terms of the anatomical structure, the inferior, lateral, and medial parts of the pulvinar nucleus are all densely connected with the visual system, and the inferior and medial parts of the pulvinar nucleus are also connected with the auditory system; in terms of its functional role, the inferior, lateral, and medial parts of the pulvinar nucleus are all heavily involved in processing auditory and visual information.43 Qin and Yu42 have also reported that the pulvinar nucleus is a multimodal thalamic nucleus.
We found stronger connectivity from the PuM nucleus to the IFG in patients with AVHs. The abnormal activity of the pulvinar nucleus might impair speech selection and filtering in patients with AVHs. Lesions of the pulvinar nucleus have been reported to lead disruption in speech selection.18 Froesel et al43 proposed several roles of the pulvinar nucleus, including sensory attention, selection, distractor filtering, and perception. Moreover, we found weaker connectivity between the PuM nucleus and cerebellum in patients with AVHs. The connection from the cerebellum to the thalamus reportedly affects the comparison of expected and actual sensory feedback.25 Stronger brain activity of the bilateral PuM nuclei has been found in the AVH state in the schizophrenia patient with AVHs.11 However, hypoactivation of the PuM nucleus has been reported in schizophrenia patients with AVHs during monitoring of self- and externally-generated speech.22 These findings also highlight the gateway function of the pulvinar nucleus, whereby hyperactivation of the PuM nucleus at rest might inhibit activation for externally-generated speech.
Our findings directly indicated aberrant cerebello-thalamo-cortical connectivity in FES patients with AVHs. Our findings offer direct support for the hypothesis that the cerebello-thalamo-cortical circuit is associated with AVHs, and support the forward model of AVHs25 that erratic prediction of sensory consequences in voice and sound production is linked to an aberrant cerebello-thalamo-cortical circuit. Others have also proposed that AVHs result from mechanistic changes in the forward model of the motor system.44 Voice perception is thought to engage an internal forward model that generates predictions, and voice-selective prediction alterations have been found in nonclinical voice hearers.45 The forward model predicts the sensory consequences of an action given the current state and motor command.46 When preparing for or imaging speaking, the cerebellum receives inputs from the cortex, which is thought to perform the predictive computation that underlies the forward model.47 When speaking, the auditory system relies on sensory feedback to identify one’s own and others’ voices. The comparison of expected and actual sensory feedback is thought to be processed in the cerebellum via the thalamus.46 Pinheiro et al25 proposed that cerebellar circuitry might play a central role in the forward model, and error signals following altered sensory feedback may allow the cerebellum to issue a feedback command via the thalamus to premotor areas, resulting in decreased sensory suppression and increased attention to self-generated feedback. Defective self-monitoring during identifying the source of one’s own or others’ speech has been proposed in AVHs,48 which is also associated with abnormal activation of the thalamus.22 Therefore, the above hypotheses partly involve the filtering function of the thalamus that also supports the resting-state hypothesis of AVHs. Northoff and Qin3 proposed a resting-state hypothesis of AVHs, and indicated that since the internally auditory stimulus was derived from memory-based auditory experiences of previous external stimuli, hyperactivity of the default-mode network in AVHs might modulate auditory cortex activity and result in the internally-generated auditory stimulus being treated as an externally-generated one.
We found that the rsFC and effective connectivity might be independent and complementary measures of connections. On the one hand, rsFC is the consistency of temporal activity between two different brain regions, and effective connectivity is the effect of one brain area on another. Therefore, there can be rsFC between two brain regions, not necessarily effective connectivity. Similarly, when rsFC is abnormal, effective connectivity is not necessarily abnormal, which might explain why significantly different rsFC was not directly related to effective connectivity in this study. On the other hand, effective connectivity could be simply considered as functional connectivity with directional information, and we can use abnormal rsFC as the research hypothesis of effective connectivity. Therefore, we combined these two types of connectivity and found that the two measures were complementary. Weaker rsFC of the left PuM nucleus-left cerebellum VIII might be caused by negative information flow from the left cerebellum VIII to the left PuM, and stronger rsFC of the right PuM-right IFG might be modulated by the left PuM in the AVH group. Negative effective connectivity with the left MTG disappeared and the left PuM was activated in the AVH group, which might explain the stronger rsFC of the left PuM nucleus-left MTG. The connections between the right MGN and right STG seem be modulated by the cerebellum crus II in the NC group, but not in the AVH or NAVH groups, which explain the weaker rsFC of the right MGN-right STG in the AVH and NAVH groups. Rehme et al49 also found strong interactions between the two connectivity analyses.
In conclusion, our findings indicate that the critical structure in the thalamus underlying AVHs is the PuM nucleus, and provide direct evidence that the cerebello-thalamo-cortical circuit is associated with AVHs.
Supplementary Material
Acknowledgments
We thank the clinicians who involved in recruiting and assessing the subjects. The authors declare no conflicts of interest.
Contributor Information
Yarui Wei, Department of Magnetic Resonance Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China.
Kangkang Xue, Department of Magnetic Resonance Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China.
Meng Yang, Department of Magnetic Resonance Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China.
Huan Wang, Hefei National Laboratory for Physical Sciences at Microscale, School of Life Science, University of Science and Technology of China, Hefei 230027, China.
Jingli Chen, Department of Magnetic Resonance Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China.
Shaoqiang Han, Department of Magnetic Resonance Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China.
Xiaoxiao Wang, Hefei National Lab for Physical Sciences at the Microscale and Centers for Biomedical Engineering, University of Science and Technology of China, Hefei 230027, China.
Hong Li, Department of Psychiatry, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China.
Yong Zhang, Department of Magnetic Resonance Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China.
Xueqin Song, Department of Psychiatry, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China.
Jingliang Cheng, Department of Magnetic Resonance Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China.
Funding
This work was supported by Natural Science Foundation of China (81601467, 81871327); Medical Science and Technology Research Project of Henan Province (201701011).
References
- 1. Bauer SM, Schanda H, Karakula H, et al. Culture and the prevalence of hallucinations in schizophrenia. Compr Psychiatry. 2011;52(3):319–325. [DOI] [PubMed] [Google Scholar]
- 2. Hugdahl K, Sommer IE. Auditory verbal hallucinations in schizophrenia from a levels of explanation perspective. Schizophr Bull. 2018;44(2):234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Northoff G, Qin P. How can the brain’s resting state activity generate hallucinations? A ‘resting state hypothesis’ of auditory verbal hallucinations. Schizophr Res. 2011;127(1–3):202–214. [DOI] [PubMed] [Google Scholar]
- 4. Zweerings J, Hummel B, Keller M, et al. Neurofeedback of core language network nodes modulates connectivity with the default-mode network: a double-blind fMRI neurofeedback study on auditory verbal hallucinations. Neuroimage. 2019;189:533–542. [DOI] [PubMed] [Google Scholar]
- 5. Liu S, Wang H, Song M, et al. Linked 4-way multimodal brain differences in schizophrenia in a large Chinese Han population. Schizophr Bull. 2019;45(2):436–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruce C, Carroll WH. Role of the thalamus in language is it related to schizophrenic thought disorder. Schizophr Bull. 1987;13:605–621. [DOI] [PubMed] [Google Scholar]
- 7. Pergola G, Selvaggi P, Trizio S, Bertolino A, Blasi G. The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev. 2015;54:57–75. [DOI] [PubMed] [Google Scholar]
- 8. Silbersweig DA, Stern E, Frith C, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378(6553):176–179. [DOI] [PubMed] [Google Scholar]
- 9. Cui LBA, Liu L, Guo F, et al. Disturbed brain activity in resting-state networks of patients with first-episode schizophrenia with auditory verbal hallucinations: a cross-sectional functional MR imaging study. Radiology. 2017;283:809–818. [DOI] [PubMed] [Google Scholar]
- 10. Zhuo CJ, Wang CX, Song XQ, et al. A unified model of shared brain structural alterations in patients with different mental disorders who experience own-thought auditory verbal hallucinations-a pilot study. Brain Behav. 2020;10:e01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei Y, Wang X, Wang Y, et al. Functional magnetic resonance imaging in a single schizophrenia patient with voluntary control over auditory verbal hallucinations. Schizophr Res. 2020;215:465–466. [DOI] [PubMed] [Google Scholar]
- 12. Chun S, Westmoreland JJ, Bayazitov IT, et al. Specific disruption of thalamic inputs to the auditory cortex in schizophrenia models. Science. 2014;344(6188):1178–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Behrendt RP. Dysregulation of thalamic sensory “transmission” in schizophrenia: neurochemical vulnerability to hallucinations. J Psychopharmacol. 2006;20(3):356–372. [DOI] [PubMed] [Google Scholar]
- 14. Spalletta G, Piras F, Gravina P, Bello ML, Bernardini S, Caltagirone C. Glutathione S-transferase alpha 1 risk polymorphism and increased bilateral thalamus mean diffusivity in schizophrenia. Psychiatry Res. 2012;203(2–3):180–183. [DOI] [PubMed] [Google Scholar]
- 15. Cui LB, Liu L, Guo F, et al. Disturbed brain activity in resting-state networks of patients with first-episode schizophrenia with auditory verbal hallucinations: a cross-sectional functional MR imaging study. Radiology. 2017;283(3):810–819. [DOI] [PubMed] [Google Scholar]
- 16. Dutschke LL, Steinau S, Wiest R, Walther S. Brain tumor-associated psychosis and spirituality-a case report. Front Psychiatry. 2017;8:00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hori A, Ikeda K, Kosaka K, Shinohara S, Iizuka R. System degeneration of the thalamus. A clinico-neuropathological study. Arch Psychiatr Nervenkr (1970). 1981;231(1):71–80. [DOI] [PubMed] [Google Scholar]
- 18. Van Buren JM, Borke RC. Alterations in speech and the pulvinar. A serial section study of cerebrothalamic relationships in cases of acquired speech disorders. Brain. 1969;92(2):255–284. [DOI] [PubMed] [Google Scholar]
- 19. Feinberg I, Guazzelli M. Schizophrenia–a disorder of the corollary discharge systems that integrate the motor systems of thought with the sensory systems of consciousness. Br J Psychiatry. 1999;174:196–204. [DOI] [PubMed] [Google Scholar]
- 20. Kanemoto H, Kazui H, Adachi H, et al. Thalamic pulvinar metabolism, sleep disturbances, and hallucinations in dementia with Lewy bodies: positron emission tomography and actigraphy study. Int J Geriatr Psychiatry. 2020;35(8):934–943. [DOI] [PubMed] [Google Scholar]
- 21. Tuite PJ, Provias JP, Lang AE. Atypical dopa responsive parkinsonism in a patient with megalencephaly, midbrain Lewy body disease, and some pathological features of Hallervorden-Spatz disease. J Neurol Neurosurg Psychiatry. 1996;61(5):523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumari V, Fannon D, Ffytche DH, et al. Functional MRI of verbal self-monitoring in schizophrenia: performance and illness-specific effects. Schizophr Bull. 2010;36(4):740–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao H, Chén OY, Chung Y, et al. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun. 2018;9(1):3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bernard JA, Orr JM, Mittal VA. Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. Neuroimage Clin. 2017;14:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pinheiro AP, Schwartze M, Kotz SA. Cerebellar circuitry and auditory verbal hallucinations: an integrative synthesis and perspective. Neurosci Biobehav Rev. 2020;118:485–503. [DOI] [PubMed] [Google Scholar]
- 26. Kim NY, Hsu J, Talmasov D, et al. Lesions causing hallucinations localize to one common brain network. Mol Psychiatry. 2021;26(4):1299–1309. [DOI] [PubMed] [Google Scholar]
- 27. Tracy DK, O’Daly O, Joyce DW, et al. An evoked auditory response fMRI study of the effects of rTMS on putative AVH pathways in healthy volunteers. Neuropsychologia. 2010;48(1):270–277. [DOI] [PubMed] [Google Scholar]
- 28. Pastor MA, Artieda J, Arbizu J, Marti-Climent JM, Peñuelas I, Masdeu JC. Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. J Neurosci. 2002;22(23):10501–10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7(7):511–522. [DOI] [PubMed] [Google Scholar]
- 30. Clos M, Diederen KM, Meijering AL, Sommer IE, Eickhoff SB. Aberrant connectivity of areas for decoding degraded speech in patients with auditory verbal hallucinations. Brain Struct Funct. 2014;219(2):581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friston KJ, Kahan J, Biswal B, Razi A. A DCM for resting state fMRI. Neuroimage. 2014;94:396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mao CP, Chen FR, Huo JH, et al. Altered resting-state functional connectivity and effective connectivity of the habenula in irritable bowel syndrome: a cross-sectional and machine learning study. Hum Brain Mapp. 2020;41(13):3655–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li B, Cui LB, Xi YB, et al. Abnormal effective connectivity in the brain is involved in auditory verbal hallucinations in schizophrenia. Neurosci Bull. 2017;33(3):281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao Z, Li X, Feng G, et al. Altered effective connectivity in the default network of the brains of first-episode, drug-naïve schizophrenia patients with auditory verbal hallucinations. Front Hum Neurosci. 2018;12:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20(5):1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry. 2001;158(4):618–624. [DOI] [PubMed] [Google Scholar]
- 37. Thermenos HW, Seidman LJ, Breiter H, et al. Functional magnetic resonance imaging during auditory verbal working memory in nonpsychotic relatives of persons with schizophrenia: a pilot study. Biol Psychiatry. 2004;55(5):490–500. [DOI] [PubMed] [Google Scholar]
- 38. Sui J, Pearlson GD, Du Y, et al. In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biol Psychiatry. 2015;78(11):794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rolls ET, Huang CC, Lin CP, Feng J, Joliot M. Automated anatomical labelling atlas 3. Neuroimage. 2020;206:116189. [DOI] [PubMed] [Google Scholar]
- 40. Yang GJ, Murray JD, Glasser M, et al. Altered global signal topography in schizophrenia. Cereb Cortex. 2017;27(11):5156–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fridgeirsson EA, Figee M, Luigjes J, et al. Deep brain stimulation modulates directional limbic connectivity in obsessive-compulsive disorder. Brain. 2020;143(5):1603–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qin W, Yu C. Neural pathways conveying novisual information to the visual cortex. Neural Plast. 2013;2013:864920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Froesel M, Cappe C, Ben Hamed S. A multisensory perspective onto primate pulvinar functions. Neurosci Biobehav Rev. 2021;125:231–243. [DOI] [PubMed] [Google Scholar]
- 44. Frith C, Rees G, Friston K. Psychosis and the experience of self. Brain systems underlying self-monitoring. Ann N Y Acad Sci. 1998;843:170–178. [DOI] [PubMed] [Google Scholar]
- 45. Pinheiro AP, Schwartze M, Kotz SA. Voice-selective prediction alterations in nonclinical voice hearers. Sci Rep. 2018;8(1):14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. [DOI] [PubMed] [Google Scholar]
- 47. Tanaka H, Ishikawa T, Lee J, Kakei S. The cerebro-cerebellum as a locus of forward model: a review. Front Syst Neurosci. 2020;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johns LC, Rossell S, Frith C, et al. Verbal self-monitoring and auditory verbal hallucinations in patients with schizophrenia. Psychol Med. 2001;31(4):705–715. [DOI] [PubMed] [Google Scholar]
- 49. Rehme AK, Eickhoff SB, Grefkes C. State-dependent differences between functional and effective connectivity of the human cortical motor system. Neuroimage. 2013;67:237–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.