Abstract
Background and Hypothesis
Individuals at clinical high risk for psychosis (CHR-p) are less fit than nonclinical peers and show hippocampal abnormalities that relate to clinical symptoms. Exercise generates hippocampal neurogenesis that may ameliorate these hippocampal abnormalities and related cognitive/clinical symptoms. This study examines the impact of exercise on deficits in fitness, cognitive deficits, attenuated psychotic symptoms, hippocampal volumes, and hippocampal connectivity in individuals at CHR-p.
Study Design
In a randomized controlled trial, 32 individuals at CHR-p participated in either an exercise (n = 17) or waitlist (no exercise) (n = 15) condition. All participants were sedentary at use and absent of current antipsychotic medication, psychosis diagnoses, or a substance use disorder. The participants completed a series of fitness, cognitive tasks, clinical assessments, and an MRI session preintervention and postintervention. The exercise intervention included a high-intensity interval exercise (80% of VO2max) with 1-minute high-intensity intervals (95% of VO2max) every 10 minutes) protocol twice a week over 3 months.
Study Results
The exercise intervention was well tolerated (83.78% retention; 81.25% completion). The exercising CHR-p group showed that improved fitness (pre/post-d = 0.53), increased in cognitive performance (pre/post-d = 0.49), decrease in positive symptoms (pre/post-d = 1.12) compared with the waitlist group. Exercising individuals showed stable hippocampal volumes; waitlist CHR-p individuals showed 3.57% decreased hippocampal subfield volume. Exercising individuals showed that increased exercise-related hippocampal connectivity compared to the waitlist individuals.
Conclusions
The exercise intervention had excellent adherence, and there were clear signs of mechanism engagement. Taken together, evidence suggests that high-intensity exercise can be a beneficial therapeutic tool in the psychosis risk period.
Keywords: psychosis risk, hippocampus, cardiovascular fitness, cognitive deficits, positive symptoms
Introduction
Exercise may be a promising intervention for individuals across many stages of psychosis, including those at clinical high risk for psychosis (CHR-p).1–9 Psychosis is associated with poor physical health,1,6,10–12 which extends to those at CHR-p who are less active,13 less fit,14 and experience more barriers to exercise.10,15–19 Moderate to intense aerobic exercise engages neurogenesis and the production of brain-derived neurotrophic factors for the maintenance of healthy neurons in the hippocampus.1–9 This mechanism may be particularly impactful in CHR-p populations who have decreased hippocampal volumes20–22 and abnormal shape.23 These hippocampal abnormalities also may relate to clinical symptoms, cognitive symptoms, and severity in course of psychosis.24 Moderate to intense aerobic exercise interventions may combat these disease-driven hippocampal pathophysiology.4,13,20,25–27
Increased neurogenesis and plasticity2,25,26 in the hippocampus after exercise24 may address a broad range of hippocampal-mediated behaviors impacted in psychopathology23,25,28 In formal psychosis, hippocampal pathology has been well documented7,16,20,23,29–31 Pajonk et al showed that aerobic exercise increased hippocampal volume by 14% in schizophrenia patients and that this volume increase was proportional to the improvement in cardiovascular fitness (ie, VO2max), hippocampal-dependent cognition (ie, working memory), and total psychosis symptoms.28 Despite this evidence,28 meta-analyses of exercise interventions in psychosis32 show that there is frequently no increase in hippocampal volume in the exercise intervention group. Instead, these studies find a stable hippocampal volume in the exercise group and significant decreases in volume within the no exercise group.8,26,33 The latter suggests that exercise intervention may prevent pathogenic decreases in hippocampal volumes,28,32 and may be an effective early intervention for individuals with or at high-risk for psychosis.
The promise that exercise interventions show in psychosis disorders4,6,32 extends to individuals at CHR-p.7 Notably, even those individuals at CHR-p that do not convert to psychosis show elevated risk for poor physical and mental health outcomes (eg, lower cardiovascular fitness,14 depression,34,35 anxiety,35,36 sleep disturbance37–39), all of which may benefit from exercise intervention. Beyond these broad benefits individuals at CHR-p have decreased aerobic fitness,14 decreased hippocampal volume,20,23 and related cognitive deficits such as episodic memory dysfunction, specific mechanisms that are directly engaged addressed by exercise intervention.40–42 Mittal et al found that objectively assessed greater physical activity (ie, actigraphy) was related to larger gray matter volume and better occupational function in individuals at CHR-p at a single timepoint.13 With few exceptions,7,13,14 much of this research relies on retrospective, self-reported evaluations of fitness, which are often biased or inaccurate,14 and studies rarely manipulate fitness or activity level as an intervention in a lab setting.7 Indeed, randomized controlled trials have focused on psychosocial, pharmaceutical, or combined (ie, psychosocial with pharmaceutical) interventions for individuals at CHR-p.43 Despite recent initiatives greatly expanding comprehensive care for individuals in early psychosis,44 these programs are not widely available, are resource demanding, and experience problems with treatment engagement.45 Although there is some evidence that certain psychosocial and pharmaceutical treatments may reduce or delay conversion to psychosis,46 they do not improve cognition or negative symptoms.47,48 In contrast, exercise has shown benefits to both cognition and negative symptoms and could be a beneficial treatment target.2,5,49
In a supervised, open-label exercise intervention by Dean et al, CHR-p individuals who participated in aerobic exercise showed reduced positive symptoms and improvements in both hippocampal-occipital connectivity and cognitive function.7 In this study, individuals were assigned to exercise on a treadmill either twice a week or 3 times a week. Despite many areas of improvement (symptoms, cognition, hippocampal-occipital connectivity), there was no improvement in fitness (ie, VO2max). This study demonstrated the tolerability, feasibility, and benefits of exercise for individuals at CHR-p. However, this study lacked a waitlist control group for comparison. This methodological approach is not able to account for symptom remission, which is particularly important in CHR-p populations that experience an estimated 43%50–59%51 remission within a 12-month period. It is thus critical to examine the impact of exercise intervention in a randomized controlled trial.
The current study is the first randomized controlled trial to examine the potential benefits of exercise interventions for individuals at CHR-p in terms of cardiovascular fitness (VO2max), hippocampal-dependent episodic memory, attenuated psychosis symptoms, and hippocampal subfield volumes. This study extends findings from an open-label, phase one study13,16 that demonstrated the potential benefits and feasibility of exercise interventions within a CHR-p group. First, this study reports important features of retention and feasibility in the methodological approach and the efficacy of the exercise intervention in improving fitness. Next, analyses investigate the impact of the exercise intervention on hippocampal-dependent episodic memory and attenuated psychotic symptoms with the expectation that treatment will improve cognitive and clinical symptoms. Then, randomized groups were compared in terms of hippocampal subfields volume changes (potential benefits of exercise and risk-related processes in the waitlist group) with the expectation that exercise will increase hippocampal volume. Finally, hippocampal-occipital connectivity was compared across groups over time to replicate and extend the open-label exercise study that observed increased exercise was associated with increased hippocampal-occipital connectivity.7
Methods
Overview
This trial was preregistered (https://clinicaltrials.gov/ct2/show/NCT02155699). All clinical interviewers, neuropsychological assessors, and neuroimaging research staff were blinded as to the status of participants until the study was concluded. Individuals were screened for eligibility; inclusion criteria included the presence of an attenuated psychosis syndrome and a current sedentary lifestyle. Participants were excluded for the current psychosis, antipsychotic prescription, or a substance use disorder. Eligible participants were then randomized into intervention conditions, which were balanced by gender, by a staff member that was excluded from all rating and data collecting activities. All participants completed VO2max, clinical symptom and diagnostic assessments, and an MRI session at the beginning and end of the study. After all pretrial assessments, individuals assigned to the exercise group completed 3 months of moderate to intense aerobic exercise.
Participants
Eligibility Criteria
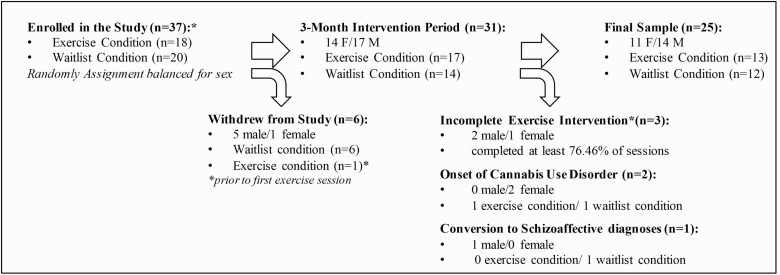
The inclusion criteria included the presence of an attenuated psychosis syndrome diagnosed with the Structured Interview for Psychosis-Risk Syndromes (SIPS)52 (see supplementary material) and current sedentary lifestyle. Consistent with past exercise interventions, the current study used individuals who were considered sedentary—meaning that they did not complete more than 60 min of maximal heart output per week for at least the past 6 months. Individuals were also excluded for onset of a substance use or psychosis spectrum diagnosis during the clinical trial and incomplete exercise sessions. The final sample was racially diverse including 44.4% White/Caucasian, 25.9% Black/African American, 11.1% Central/South American, 7.4% South Asian, 3.7% East Asian, and 7.4% Interracial. A full account of these exclusions is provided in figure 1 and supplementary materials. The conversion to psychosis rate among the waitlist group (11.8%) is similar to established rates of conversion (11% to 21.7%) for CHR-p individuals within a 12-month period; there were no conversions in the exercise group.53,54
Fig. 1.
Sample retention and exclusion over protocol.
Exercise Protocol
After the pretrial assessment, participants were randomized into the exercise or waitlist conditions. The exercise group participants completed a 3-month exercise treatment twice a week at moderate to intense levels of aerobic exercise (24 sessions over 12 weeks). To limit the perceived barriers to sessions,15 participants were provided with free transportation to the sessions, after the session access to healthy snacks and refreshments were provided. Participants received paid compensation after each session. All sessions were conducted as one-on-one sessions by the same exercise physiologist under the supervision of a physician to build rapport with participants and ensure consistency in the protocol. These high-intensity interval exercise sessions were tailored to the individual’s exercise fitness level based on the baseline VO2max assessment. For initial tolerance, the treadmill exercise intensity was set to elicit 55% of the participant’s VO2max which was increased to 80% gradually over the first 3 weeks. During the remainder of the trial (21 weeks), the 2 weekly, treadmill sessions were designed to elicit 80% of VO2max for the majority of the time with 1-min high-intensity intervals at 95% of VO2max, every 10 minutes for 3 repetitions for a total of 30 mins.32 This protocol was adapted from findings in the open-label pilot study, which suggested that fewer sessions and higher intensity of exercise were needed.7 After the exercise intervention, participants repeated the baseline visit again with separate study staff who were blinded to their exercise intervention status.
VO2max Assessment
VO2max was assessed via. a modified Balke protocol55 by an exercise physiologist under the supervision of a physician. In this modified Balke protocol, the treadmill speed was individualized in a procedure to elicit 70% of the age-predicted max heart rate and ratings of a “somewhat hard” perceived exertion (RPE). Then, the speed of the treadmill remained the same throughout the test, but the incline of the treadmill belt increased 2% every 2 min (or 2.5% for speeds 6 mph or greater) to exhaustion. Tests generally lasted 8–12 minutes to attain the recommended target for VO2max testing.56
Cognitive Assessment
Intelligence was assessed with the Wide Range Achievement Test (WRAT) reading subscale, which is considered to be a valid estimate of the premorbid intelligence quotient.57 Relational and Item-Specific Encoding Task58,59 was used to probe hippocampal-specific cognitive function,60 and recognition accuracy (d-prime) was used as the primary outcome variable (see supplementary information).
Clinical Assessments
The SIPS52 was administered to detect the presence of a prodromal syndrome for inclusion in the study as an individual at CHR-p and to formally assess attenuated negative and positive symptoms at both pretrial sessions and posttrial sessions. SCID was administered at pretrial to rule out formal psychosis and assess for other psychiatric disorders; the study sample included individuals with comorbid current diagnoses of depression, anxiety, and substance use disorders at similar rates previously reported in larger samples of CHR-p populations (see supplementary table S1).35 This interview was completed at baseline and at all follow-up visits to detect any diagnostic changes (eg, onset of SUD or conversion to psychosis), and at a one-year follow-up that assessed conversion to a psychosis disorder (see supplementary information).61,62
MRI Acquisition and Processing
Magnetic resonance imaging scans were acquired with a 3-Tesla Siemens Prisma magnetic resonance imaging scanner (Siemens Healthineers, Erlangen, Germany) at the Center for Translational Imaging and a standard 64-channel head coil. Structural images were collected with a T1-weighted 3D magnetization prepared rapid gradient multi-echo sequence (axial plane; TR = 2170 ms; TE = 1.69 ms; GRAPPA parallel factor = 2; 1 mm3 isomorphic voxels, FOV = 256 mm; flip angle = 7°, time = 13:46 minites). These data were analyzed using the automatic 7.1 FreeSurfer software.63 An automatic hippocampal subregion segmentation package (“segmentHA_T1”)20,64 identified and extracted hippocampal subfield volumes. Resting-state functional images were acquired with T2*-echo-planar imaging (TR = 0.55s; TE = 0.22s; FA = 47°; 2.0 × 2.0 × 2.0 mm voxel; 590 volumes). All data were preprocessed with FSL.v.6.0 (see supplementary information). ROI to ROI analyses (CONNv.20.b.) compared the conditions (exercise > waitlist) across timepoints (posttrial > pretrial) in terms of bilateral hippocampus-occipital lobe connectivity. The hippocampus and occipital lobe ROIs were chosen based on a hippocampus to whole brain connectivity study of exercise interventions in individuals at CHR-p7 in the phase one, pilot study upon which the current study is based.
Analytical Strategy
All analyses were conducted using a mixed-effects model approach, where the individual effects were accounted for as random effects and variables were nested within time (pretrial, posttrial). In the hippocampal analyses, total substructures were examined first, but descriptions of changes in main structures and subfields are provided for transparency (see supplementary information).
Results
Adherence and Tolerability of Intervention
This study retained 83.78% of the participants enrolled for a total of 17 in the exercise condition, and 14 in the final waitlist condition for a total of 31 participants (45.16% female) with complete data. Six individuals withdrew from the study prior to the completion of the protocol. Of the individuals who withdrew from the study, 83.33% were assigned to the waitlist condition. Only one individual assigned to the exercise group withdrew and did so prior to completing any exercise sessions (relative risk of treatment condition drop out [RR] = 0.30). Among exercising CHR-p individuals, 81.25% of the sample completed all 24 exercise sessions. Among the exercising CHR-p individuals that completed partial sessions (n = 3), 76.36% of sessions were attended (M = 18.3 sessions; SD = 3.78). Individuals with onset of new and significant smoking behavior (of any substance) that may negatively impact cardiovascular health and mechanisms to be examined during the randomized control trial period were excluded from analyses (n = 2 onset of cannabis use disorder, figure 1).
Final Sample Description
Our sample included 25 participants (46.88% female). Despite nearly half of the sample being female, there was a significant sex difference in the random assignment to exercise or waitlist such that 84.6% of the waitlist was female and 15.4% of the exercise group, χ2 = 9.00, P = .003. There were no significant differences in age, t(23) = 1.47, P = .16. The exercise and waitlist groups did not differ in terms of VO2max, t(23) = 0.73, P = .47, at pretrial. There was no group difference in pretrial symptoms (p’s < .35), cognitive tasks (P’s < .15), or hippocampal volume (P = .28).
Health Metrics (VO2max)
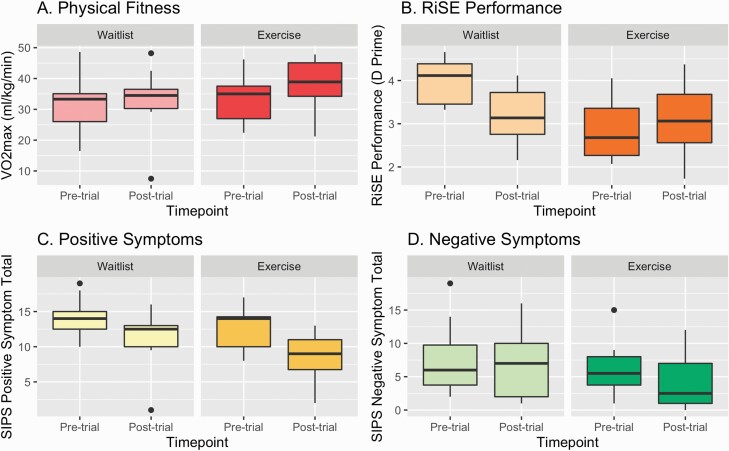
There was a significant exercise condition by time interaction in VO2max, F(1,25) = 4.81, P = .038, figure 1A. The exercise group showed an increased VO2max over the exercise intervention that was larger than the waitlist group. There were no significant main effects of exercise condition or time (P’s > .51). Groups significantly differed posttrial (figure 2A and table 1).
Fig. 2.
Mean VO2max, RiSE performance, and clinical symptoms by randomized control condition over time.
Table 1.
Demographic and Clinical Group Comparisons at Baseline
| Exercise | Waitlist | Statistics | |||
|---|---|---|---|---|---|
| Sex* | 11 female | 2 male | 3 female | 10 female | χ2 = 9.00, P = .003 |
| Mean | SD | Mean | SD | Cohen’s d | |
| Age | 21.15 | 1.72 | 21.57 | 1.78 | −0.24 |
| Positive symptoms | 12.08 | 3.52 | 13.5 | 3.9 | −0.38 |
| Negative symptoms | 5.54 | 3.87 | 7.33 | 5.45 | −0.38 |
*There was a significant difference in sex between groups which was modeled as a covariate in subsequent analyses; no other significant differences were present among the baseline variables P’s < .16.
Relational and Item-Specific Encoding Task
There was a significant exercise condition by time interaction in accuracy on the Relational and Item-Specific Encoding (RiSE) task, F(1,13) = 14.81, P = .0027 (figure 3), such that the exercise group increased recollection accuracy and the waitlist group decreased recollection accuracy (table 1). There was also a significant main effect of exercise condition group (F(1,11) = 8.60, P = .014) and time (F(1,11) =14.23, P = .0031). The waitlist group started out higher than the exercise group and stayed higher at posttrial (d = 0.55).
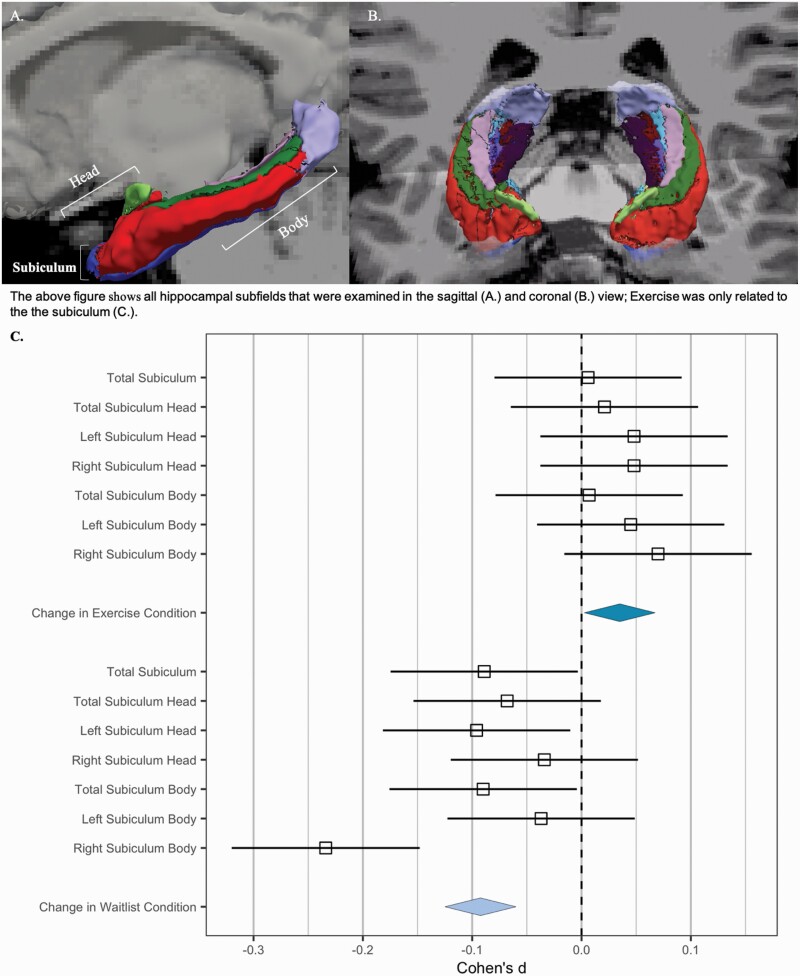
Fig. 3.
Hippocampal subiculum subfields by randomized control condition over time: (A) All hippocampal subfields that were examined in the sagittal and (B) Coronal (upper panel) view; exercise was only related to the subiculum (graph in lower panel) (C) And is displayed above (upper panel).
Clinical Symptoms
There was a significant exercise condition group by time interaction, F(1,25) = 5.31, P = .03 (table 1, figure 2B); the exercise group showed a decrease in positive symptoms over the exercise intervention that was larger than the waitlist group. Negative symptoms were not significantly changed by the exercise intervention condition by time, F(1,25) = 0.33, P = .57 (table 1 and figure 2C).
Hippocampal Subvolumes
There was no significant exercise condition group by time interaction in hippocampal total subvolumes (F’s = 2.61–90.14; P’s = .12–.71). However, there were significant main effect of the exercise condition group in the subiculum (F(19) = 6.56, P = .02), such that the exercise group had larger subiculum volumes that remained larger (table 2). Within the subiculum, there was a general pattern of decreases in volume in the waitlist group, and stable volume in the exercise group showed (figure 3). The right subiculum body showed a significant main effect of group (F(18) = 11.80, P = .003), and moderate, insignificant effects of time (F(18) = 3.57, P = .07), and group by time interaction (F(18) = 3.12, P = .09).
Table 2.
Mean Fitness, Cognitive, Clinical Symptoms, Hippocampal Subfield Volumes, and Connectivity by Randomized Control Condition and Timepoint
| Exercise | Waitlist | |||||
|---|---|---|---|---|---|---|
| Pretrial | Posttrial | Change (d) | Pretrial | Posttrial | Change (d) | |
| VO2max | 33.92 (7.4) | 38.2 (7.8) | 0.54 | 31.48 (8.87) | 32.97 (10.18) | 0.16 |
| BMI | 25.5 (2.65) | 25.11 (2.82) | 25.01 (7.81) | 25.73 (7.24) | ||
| RiSE task | 2.75 (0.45) | 3.00 (0.57) | 0.49 | 3.73 (0.44) | 3.13 (0.56) | −0.083 |
| WRAT | 105.83 (13.38) | 114.83 (13.64) | 114.8 (14.97) | 110.4 (12.26) | ||
| Positive symptoms | 12.08 (3.52) | 8 (3.46) | 1.12 | 13.50 (3.90) | 11.38 (3.82) | 0.55 |
| Negative symptoms | 5.54 (3.87) | 4.38 (3.84) | 0.31 | 7.33 (5.45) | 6.67 (4.68) | 0.13 |
| Hippocampal subfields (mm3) | ||||||
| Total subiculum | 889.184 (111.674) | 889.868 (111.375) | 0.006 | 772.823 (108.34) | 762.393 (126.12) | −0.089 |
| Head | 393.723 (54.263) | 394.896 (55.394) | 0.021 | 346.718 (58.485) | 342.567 (62.993) | −0.068 |
| Left | 196.268 (25.966) | 197.508 (26.01) | 0.048 | 177.011 (31.148) | 173.907 (33.563) | −0.096 |
| Right | 197.456 (30.647) | 197.388 (31.974) | 0.048 | 169.707 (29.142) | 168.659 (31.903) | −0.034 |
| Body | 495.461 (70.247) | 494.972 (70.351) | 0.007 | 426.105 (68.505) | 419.826 (70.448) | −0.09 |
| Left | 251.337 (41.901) | 253.196 (40.779) | 0.045 | 221.768 (44.178) | 223.293 (38.355) | −0.037 |
| Right | 241.776 (33.678) | 244.123 (33.742) | 0.07 | 204.336 (30.57) | 196.534 (35.879) | −0.234 |
| Hippocampal-occipital connectivity (b-values) | 0.15 (0.11) | 0.07 (0.12) | 0.67 | 0.06 (0.15) | 0.11 (0.18) | −0.29 |
Hippocampal Connectivity
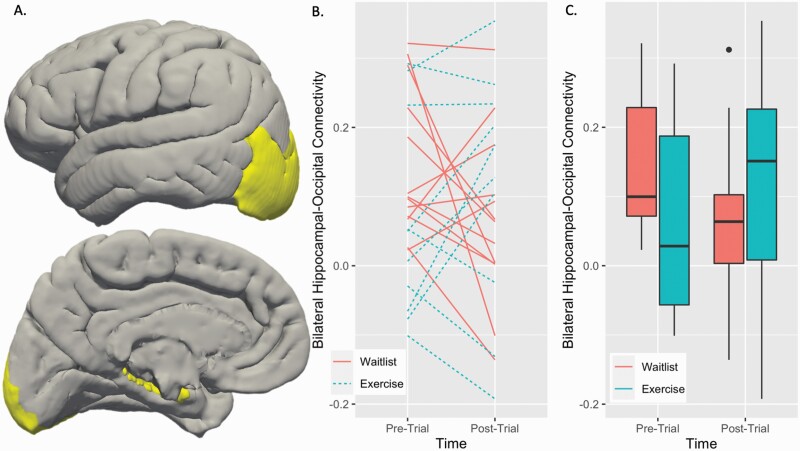
There was a significant exercise condition group by time interaction in hippocampal-occipital connectivity (figure 4), such that exercise was associated with increased hippocampal-occipital connectivity, F(1,21) = 4.67, P-FDR = 0.042, such that connectivity increased in the exercise group and decreased in the waitlist group (table 1).
Fig. 4.
Hippocampal resting-state connectivity by randomized control condition over time: (A) Hippocampal and Occipital regions of interest entered into the connectivity model displayed in MNI 125 2 mm space, (B) slopes in connectivity values for each CHR-p participant at each timepoint, (C) randomized control condition group averages and distributions in connectivity over time.
Symptoms at 1-year Follow-up
There were no differences between groups at 12-month follow-up in terms of total positive symptoms (exercise: M = 8.89, SD = 4.26; waitlist: M = 9.0, SD = 2.93). Negative symptoms (exercise: M = 4.57, SD = 4.89; waitlist: M = 7.5, SD = 5.45) did not significantly differ (t(13) = 1.09, P = .29) but showed a moderate effect size (d = 0.56).
Discussion
A 3-month period of regular and intense exercise shows the potential for promoting health, reducing positive symptoms, and attenuating pathophysiology associated with CHR-p.20 These findings also extend and confirm previous findings from an open-label, phase one pilot study of exercise in individuals at CHR-p that also found exercise related to improvements in cognition, clinical symptoms, and brain features.7 Critically, exercise interventions may be particularly useful in addressing the associated vulnerability and consequences of progressive hippocampal pathology characteristic of psychosis risk.20,23,29,65 The exercise intervention showed signs of maintenance of subiculum volume in the exercise group and a decreasing subiculum volume in the waitlist.4 Finally, exercise involves minimal risk and interference as an additive treatment alongside traditional early interventions6 yet has positive implications for mental health.66
The exercise intervention was well tolerated and effective for individuals at CHR-p; individuals engaged in the exercise intervention were 33% less probably to drop out compared with the waitlist group, which is lower than typical dropout rates reported for psychotherapy (RR = 1.55), pharmacological (RR = 1.59), and nutritional (RR = 1.49) interventions or in individuals with psychosis undergoing a similar protocol (1.13).28,67,68 Indeed, no individuals withdrew from the study during exercise interventions, and all participants completed at least 76.46% of the exercise sessions. Although more research is needed on the study components that impact adherence, addressing known perceived barriers to exercise may have aided in the retention of participants.15 For example, providing transportation to address accessibility and one-on-one sessions with the same exercise physiologist to reduce self-perception barriers.15 This study adds to a growing literature demonstrating that exercise interventions are both feasible and well tolerated in individuals at clinical high-risk for psychosis.6,7,69,70 These findings highlight the importance of examining exercise intervention in the early risk phase of psychopathology. Indeed, exercise interventions may be particularly beneficial in individuals at CHR-p due to high adherence/engagement and fewer comorbid physical diseases than individuals with chronic psychosis.
Cardiovascular fitness was significantly improved by the exercise intervention. The improvement in fitness (12.62% increase in VO2 max) is similar to the 5%–18% improvement of fitness seen in the psychosis literature,2,3,28 addressing the deficits in physiological fitness reported in extant literature.14 Meta-analyses of exercise intervention in psychosis suggest that the benefits of exercise are dose-dependent; this validation may indicate a sufficient dose to improve fitness.32
Episodic memory was improved, ie, recognition accuracy,58,59 in the exercising CHR-p group. In contrast, individuals on the waitlist started with higher accuracy scores that decreased posttrial; though it remained higher than the exercising group, even when accounting for stable estimates of intelligence.57 Notably, the study design had no initial balancing for cognition. This lack of initial cognitive balance resulted in group differences71,72 may underestimate cognitive gains made by exercising CHR-p individuals. The observed cognitive gains are consistent with the open-label pilot study7,16 and a larger psychosis literature.1,2,4,5
Exercising CHR-p individuals showed a decrease in attenuated positive symptoms compared with the waitlist group. Although both groups showed some reduction in positive symptoms (statistical regression), the improvement was more significant in the exercising CHR-p group compared to the waitlist group. This finding is consistent with previous literature, which found exercise to be effective in decreasing positive symptoms in individuals at CHR-p7 and with a psychosis diagnosis.4,73 Exercise interventions may be an excellent companion treatment to reduce positive symptoms.74 Indeed, the exercise intervention showed a similar effect (d = 1.12) to reported effect sizes for psychosocial treatments (d = 0.89–1.12)75 and medication with psychosocial combinations (d = 1.03-1.18).76
Exercising CHR-p individuals did not show significant increases in hippocampal subfields (0.97% increase); however, they also did not show the decrease in hippocampal volume that was observed in the waitlist condition (3.97% decrease). This decrease in volume has been previously observed in independent samples of individuals across the psychosis risk spectrum at a single timepoint20 and in individuals with psychosis in the waitlist groups (3%–17% decrease) in other exercise intervention studies.8,28,33 Similarly, many studies report no relationship between exercise8,33 or physical activity13 and hippocampal volume in individuals along the psychosis spectrum. These findings are consistent with neural diathesis-stress models of psychosis, which suggest that cascading mechanisms of stress30 may result in a loss in gray matter volume77 in regions like the hippocampus that are particularly sensitive to stress and central to psychosis deficits.31
Hippocampal-occipital functional connectivity was significantly increased in the exercise group compared to the waitlist group. Although the current sample size was small, it is notable that the exercise group showed a moderate effect size (d = 0.64), while the waitlist group showed a small decrease in connectivity over the same time period (d = −0.29). Additionally, this finding partially replicates and extends the open-label pilot study7 which found that hippocampal-occipital connectivity increased after exercise in a whole brain, exploratory analyses. These findings may suggest that aerobic exercise may address hippocampal dysconnectivity associated with psychosis risk.27,29
The present study had many strengths, but there are also several opportunities for growth in future studies. It is a notable limitation that the current sample size is small; however, the current sample size (17 exercise, 14 waitlist) is comparable or larger than extant randomized control studies in psychosis (sample size per group: 8–17).1,2,28,78–81 CHR-p individuals make up a heterogenous population82–84; future studies should consider heterogeneity in clinical and cognitive symptoms in larger samples. Negative symptoms did not reduce in the exercise intervention as expected,7,13,32,49,73 but the negative symptoms were relatively low in all conditions. Future studies would also benefit from within-session exercise fidelity data and expanded cognitive batteries. Finally, there are several benefits of exercise not examined by the current study, including increases to brain-derived neurotrophic factors and other neurotrophins, serotonin, and quality of sleep, as well as reductions in negative factors, such as decreasing inflammation and cortisol, among other metabolic impacts. Recent reports suggest that among individuals with psychosis spectrum disorders enhancement of aerobic fitness may extend aerobic exercise’s benefits beyond cognition to affective and social functioning as well.85 Future longitudinal research is needed to determine whether sustaining exercise promotes long-term gains and mitigation of symptom progression.
These findings represent preliminary evidence in a larger critical body of future work in exercise interventions in individuals at CHR-p. Future research should examine exercise intervention in terms of longitudinal aims to fully investigate long-term gains and continued engagement in fitness behaviors as well as the influence on clinical course, each of which involves unique modeling challenges. For continued fitness engagement, it may be necessary for the intervention to be more accessible for long-term engagement (eg, mobile gaming system2,86 or in the context of established community health facilities). Additionally, there may be unaddressed barriers to long-term fitness engagement including factors such as medications, life events, medical issues, personality/self-efficacy, resources, culture, and more.15,87 Insights from future exercise intervention research would also help address potential long-term adherence issues while making provisions to model other possible disease mechanisms that exercise may be influencing.88–91 It will also be important for future studies to build wider scale interventions with larger samples able to examine heterogeneity in symptoms and response (ideally including diversity in culture and potentially international sites). The conclusions from these future studies would be critical for designing intermediary RCT trials that may further optimize and solidify implementation for clinical and community translation.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Contributor Information
Katherine S F Damme, Department of Psychology, Northwestern University, Evanston, IL, USA; Institute for Innovations in Developmental Sciences (DevSci), Northwestern University, Evanston and Chicago, IL, USA.
Tina Gupta, Department of Psychology, Northwestern University, Evanston, IL, USA.
Ivanka Ristanovic, Department of Psychology, Northwestern University, Evanston, IL, USA; Institute for Innovations in Developmental Sciences (DevSci), Northwestern University, Evanston and Chicago, IL, USA.
David Kimhy, Department of Psychology, Northwestern University, Evanston, IL, USA; Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, USA; MIRECC, The James J. Peters VA Medical Center, Bronx, NY, USA.
Angela D Bryan, Department of Psychology and Neuroscience, University of Colorado, Boulder, CO, USA; Institute for Neuroscience, University of Colorado, Boulder, CO, USA.
Vijay A Mittal, Department of Psychology, Northwestern University, Evanston, IL, USA; Institute for Innovations in Developmental Sciences (DevSci), Northwestern University, Evanston and Chicago, IL, USA; Institute for Cognitive Science, University of Colorado, Boulder, CO, USA; Department of Psychiatry, Northwestern University, Chicago, IL, USA; Medical Social Sciences, Northwestern University, Chicago, IL, USA; Institute for Policy Research (IPR), Northwestern University, Chicago, IL, USA.
Funding
This work was supported by the National Institutes of Mental Health (Grant R01MH094650, R01MH103231, R01MH112545 to V.A.M.; R21/R33MH103231 to V.A.M. and A.D.B.; R01 MH110623; R21 MH126357 to D.K.; T32MH126368 to K.S.F.D.).
References
- 1. Kimhy D, Vakhrusheva J, Bartels MN, et al. Aerobic fitness and body mass index in individuals with schizophrenia: implications for neurocognition and daily functioning. Psychiatry Res. 2014;220(3):784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kimhy D, Vakhrusheva J, Bartels MN, et al. The impact of aerobic exercise on brain-derived neurotrophic factor and neurocognition in individuals with schizophrenia: a single-blind, randomized clinical trial. Schizophr Bull. 2015;41(4):859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armstrong HF, Bartels MN, Paslavski O, et al. The impact of aerobic exercise training on cardiopulmonary functioning in individuals with schizophrenia. Schizophr Res. 2016;173(1):116–117. [DOI] [PubMed] [Google Scholar]
- 4. Vakhrusheva J, Marino B, Stroup TS, Kimhy D.. Aerobic exercise in people with schizophrenia: neural and neurocognitive benefits. Curr Behav Neurosci Rep. 2016;3(2):165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ospina LH, Wall M, Jarskog LF, et al. Improving Cognition via Exercise (ICE): study protocol for a multi-site, parallel-group, single-blind, randomized clinical trial examining the efficacy of aerobic exercise to improve neurocognition, daily functioning, and biomarkers of cognitive change in individuals with schizophrenia. J Psychiatr Brain Sci. 2019:4:e190020. doi: 10.20900/jpbs.20190020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mittal VA, Vargas T, Juston Osborne K, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dean DJ, Bryan AD, Newberry R, Gupta T, Carol E, Mittal VA.. A supervised exercise intervention for youth at risk for psychosis: an open-label pilot study. J Clin Psychiatry. 2017;78(9):e1167–e1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scheewe TW, Takken T, Kahn RS, Cahn W, Backx FJG.. Effects of exercise therapy on cardiorespiratory fitness in patients with schizophrenia. Med Sci Sports Exerc. 2012;44(10):1834–1842. [DOI] [PubMed] [Google Scholar]
- 9. Firth J, Schuch F, Mittal VA.. Using exercise to protect physical and mental health in youth at risk for psychosis. Res Psychother. 2020;23(1):433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koivukangas J, Tammelin T, Kaakinen M, et al. Physical activity and fitness in adolescents at risk for psychosis within the Northern Finland 1986 Birth Cohort. Schizophr Res. 2010;116(2):152–158. [DOI] [PubMed] [Google Scholar]
- 11. Vancampfort D, De Hert M, Myin-Germeys I, et al. Lower cardiorespiratory fitness is associated with more time spent sedentary in first episode psychosis: a pilot study. Psychiatry Res. 2017;253:13–17. [DOI] [PubMed] [Google Scholar]
- 12. Shah P, Iwata Y, Caravaggio F, et al. Alterations in body mass index and waist-to-hip ratio in never and minimally treated patients with psychosis: a systematic review and meta-analysis. Schizophr Res. 2019;208:420–429. [DOI] [PubMed] [Google Scholar]
- 13. Mittal VA, Gupta T, Orr JM, et al. Physical activity level and medial temporal health in youth at ultra high-risk for psychosis. J Abnorm Psychol. 2013;122(4):1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Damme KSF, Sloan RP, Bartels MN, et al. Psychosis risk individuals show poor fitness and discrepancies with objective and subjective measures. Sci Rep. 2021;11(1):9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newberry RE, Dean DJ, Sayyah MD, Mittal VA.. What prevents youth at clinical high risk for psychosis from engaging in physical activity? An examination of the barriers to physical activity. Schizophr Res. 2018;201:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mittal V, Dean D, Gupta T, Bryan A.. Aerobic exercise intervention for clinical high-risk youth improves cognitive and hippocampal abnormalities. Schizophr Bull. 2017;43(Suppl 1):S168. [Google Scholar]
- 17. Deighton S, Addington J.. Exercise practices in individuals at clinical high risk of developing psychosis. Early Interv Psychiatry. 2015;9(4):284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hodgekins J, French P, Birchwood M, et al. Comparing time use in individuals at different stages of psychosis and a non-clinical comparison group. Schizophr Res. 2015;161(2–3):188–193. [DOI] [PubMed] [Google Scholar]
- 19. Damme KSF, Schiffman J, Ellman LM, Mittal VA.. Sensorimotor and Activity Psychosis-Risk (SMAP-R) scale: an exploration of scale structure with replication and validation. Schizophr Bull. 2021;47(2):332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vargas T, Dean DJ, Osborne KJ, et al. Hippocampal subregions across the psychosis spectrum. Schizophr Bull. 2018;44(5):1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Erp TGM, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okada N, Fukunaga M, Yamashita F, et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry. 2016;21(10):1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dean DJ, Orr JM, Bernard JA, et al. Hippocampal shape abnormalities predict symptom progression in neuroleptic-free youth at ultrahigh risk for psychosis. Schizophr Bull. 2016;42(1):161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. Neuroimage. 2018;166:230–238. [DOI] [PubMed] [Google Scholar]
- 25. Kandola A, Hendrikse J, Lucassen PJ, Yücel M.. Aerobic exercise as a tool to improve hippocampal plasticity and function in humans: practical implications for mental health treatment. Front Hum Neurosci. 2016;10:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maurus I, Hasan A, Röh A, et al. Neurobiological effects of aerobic exercise, with a focus on patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2019;269(5):499–515. [DOI] [PubMed] [Google Scholar]
- 27. Mittal VA, Walker EF.. Minor physical anomalies and vulnerability in prodromal youth. Schizophr Res. 2011;129(2–3):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pajonk FG, Wobrock T, Gruber O, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67(2):133–143. [DOI] [PubMed] [Google Scholar]
- 29. Damme KSF, Ristanovic I, Vargas T, Mittal VA.. Timing of menarche and abnormal hippocampal connectivity in youth at clinical-high risk for psychosis. Psychoneuroendocrinology. 2020;117:104672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corcoran C, Walker E, Huot R, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29(4):671–692. [DOI] [PubMed] [Google Scholar]
- 31. Walker EF, Diforio D.. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104(4):667–685. [DOI] [PubMed] [Google Scholar]
- 32. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malchow B, Keeser D, Keller K, et al. Effects of endurance training on brain structures in chronic schizophrenia patients and healthy controls. Schizophr Res. 2016;173(3):182–191. [DOI] [PubMed] [Google Scholar]
- 34. Hui C, Morcillo C, Russo DA, et al. Psychiatric morbidity, functioning and quality of life in young people at clinical high risk for psychosis. Schizophr Res. 2013;148(1):175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Addington J, Cornblatt BA, Cadenhead KS, et al. At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168(8):800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McAusland L, Buchy L, Cadenhead KS, et al. Anxiety in youth at clinical high risk for psychosis. Early Interven Psychiatry. 2017;11(6):480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lunsford-Avery JR, Gonçalves B da SB, Brietzke E, et al. Adolescents at clinical-high risk for psychosis: circadian rhythm disturbances predict worsened prognosis at 1-year follow-up. Schizophr Res. 2017;189:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zaks N, Velikonja T, Parvaz MA, et al. Sleep disturbance in individuals at clinical high risk for psychosis. Schizophr Bull. 2022;48(1):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poe SL, Brucato G, Bruno N, et al. Sleep disturbances in individuals at clinical high risk for psychosis. Psychiatry Res. 2017;249:240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carrión RE, McLaughlin D, Goldberg TE, et al. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2013;70(11):1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bora E, Lin A, Wood SJ, Yung AR, McGorry PD, Pantelis C.. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr Scand. 2014;130(1):1–15. [DOI] [PubMed] [Google Scholar]
- 42. Addington J, Barbato M.. The role of cognitive functioning in the outcome of those at clinical high risk for developing psychosis. Epidemiol Psychiatr Sci. 2012;21(4):335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Addington J, Addington D, Abidi S, Raedler T, Remington G.. Canadian treatment guidelines for individuals at clinical high risk of psychosis. Can J Psychiatry. 2017;62(9):656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kane JM, Schooler NR, Marcy P, et al. The RAISE early treatment program for first-episode psychosis: background, rationale, and study design. J Clin Psychiatry. 2015;76(3):24016590–24016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kline ER, Johnson KA, Szmulewicz A, et al. “Real-world” first-episode psychosis care in Massachusetts: Lessons learned from a pilot implementation of harmonized data collection. Early Interv Psychiatry. 2021. doi: 10.1111/eip.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Formica MJC, Phillips LJ, Hartmann JA, et al. Has improved treatment contributed to the declining rate of transition to psychosis in ultra-high-risk cohorts? Schizophr Res. 2020;243:274–284. [DOI] [PubMed] [Google Scholar]
- 47. Addington J, Devoe DJ, Santesteban-Echarri O.. Multidisciplinary treatment for individuals at clinical high risk of developing psychosis. Curr Treat Options Psychiatry. 2019;6(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Niendam TA, Bearden CE, Johnson JK, et al. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr Res. 2006;84(1):100–111. [DOI] [PubMed] [Google Scholar]
- 49. Kurebayashi Y, Mori K, Otaki J.. Effects of mild-intensity physical exercise on neurocognition in inpatients with schizophrenia: a pilot randomized controlled trial. Perspect Psychiatr Care. doi: 10.1111/ppc.12896 [DOI] [PubMed] [Google Scholar]
- 50. Michel C, Ruhrmann S, Schimmelmann BG, Klosterkötter J, Schultze-Lutter F.. Course of clinical high-risk states for psychosis beyond conversion. Eur Arch Psychiatry Clin Neurosci. 2018;268(1):39–48. [DOI] [PubMed] [Google Scholar]
- 51. Simon AE, Umbricht D.. High remission rates from an initial ultra-high risk state for psychosis. Schizophr Res. 2010;116(2):168–172. [DOI] [PubMed] [Google Scholar]
- 52. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. [DOI] [PubMed] [Google Scholar]
- 53. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ruhrmann S, Schultze-Lutter F, Salokangas RKR, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241–251. [DOI] [PubMed] [Google Scholar]
- 55. Balke B, Ware RW.. The Present Status of Physical Fitness in the Air Force. SCHOOL OF AVIATION MEDICINE RANDOLPH AFB TX; 1959. Accessed February 20, 2020. https://apps.dtic.mil/docs/citations/ADA036235.
- 56. Hollenberg M, Ngo LH, Turner D, Tager IB.. Treadmill exercise testing in an epidemiologic study of elderly subjects. J Gerontol A Biol Sci Med Sci. 1998;53A(4):B259–B267. [DOI] [PubMed] [Google Scholar]
- 57. Gladsjo JA, Heaton RK, Palmer BW, Taylor MJ, Jeste DV.. Use of oral reading to estimate premorbid intellectual and neuropsychological functioning. J Int Neuropsychol Soc. 1999;5(3):247–254. [DOI] [PubMed] [Google Scholar]
- 58. Murray LJ, Ranganath C.. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci. 2007;27(20):5515–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ragland JD, Ranganath C, Barch DM, et al. Relational and Item-Specific Encoding (RISE): task development and psychometric characteristics. Schizophr Bull. 2012;38(1):114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ragland JD, Ranganath C, Harms MP, et al. Functional and neuroanatomic specificity of episodic memory dysfunction in schizophrenia: a functional magnetic resonance imaging study of the relational and item-specific encoding task. JAMA Psychiatry. 2015;72(9):909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gibbon M, Spitzer RL, Benjamin LS, First MB.. Structured Clinical Interview for DSM-5 (SCID-5). Published 1997. Accessed May 2, 2019. https://www.appi.org/products/structured-clinical-interview-for-dsm-5-scid-5.
- 62. First MB, Williams JB.. Structured Clinical Interview for DSM-5: Research Version. Washington, DC: American Psychiatric Association; 2015. [Google Scholar]
- 63. Fischl B. FreeSurfer. NeuroImage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Soman S, Furst A, Kerchner G.. Hippocampal subfield analysis in MCI patients: comparing freesurfer on 3T MRI with hand segmentation on 7T data. Neurology. 2014;82(10 Supplement). Accessed October 26, 2021. https://n.neurology.org/content/82/10_Supplement/P6.224. [Google Scholar]
- 65. Bernard JA, Orr JM, Mittal VA.. Abnormal hippocampal-thalamic white matter tract development and positive symptom course in individuals at ultra-high risk for psychosis. npj Schizophr. 2015;1:15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mittal VA, Firth J, Kimhy D.. Combating the dangers of sedentary activity on child and adolescent mental health during the time of COVID-19. J Am Acad Child Adolesc Psychiatry. 2020;59(11):1197–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T.. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ. 2013;346:f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Woods SW, Breier A, Zipursky RB, et al. Randomized trial of olanzapine versus placebo in the symptomatic acute treatment of the schizophrenic prodrome. Biol Psychiatry. 2003;54(4):453–464. [DOI] [PubMed] [Google Scholar]
- 69. Firth J, Carney R, Elliott R, et al. Exercise as an intervention for first-episode psychosis: a feasibility study. Early Interv Psychiatry. 2018;12(3):307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lederman O, Ward PB, Rosenbaum S, et al. Stepping up early treatment for help-seeking youth with at-risk mental states: feasibility and acceptability of a real-world exercise program. Early Interv Psychiatry. 2019. doi: 10.1111/eip.12871. [DOI] [PubMed] [Google Scholar]
- 71. Green MJ, Girshkin L, Kremerskothen K, Watkeys O, Quidé Y.. A systematic review of studies reporting data-driven cognitive subtypes across the psychosis spectrum. Neuropsychol Rev. 2020;30(4):446–460. [DOI] [PubMed] [Google Scholar]
- 72. Weinberg D, Lenroot R, Jacomb I, et al. Cognitive subtypes of schizophrenia characterized by differential brain volumetric reductions and cognitive decline. JAMA Psychiatry. 2016;73(12):1251–1259. [DOI] [PubMed] [Google Scholar]
- 73. Dauwan M, Begemann MJH, Heringa SM, Sommer IE.. Exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2016;42(3):588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thompson E, Millman ZB, Okuzawa N, et al. Evidence-based early interventions for individuals at clinical high risk for psychosis: a review of treatment components. J Nerv Ment Dis. 2015;203(5):342–351. [DOI] [PubMed] [Google Scholar]
- 75. Radua J, Davies C, Fusar-Poli P.. Evaluation of variability in individual response to treatments in the clinical high-risk state for psychosis: a meta-analysis. Schizophr Res. 2021;227:20–27. [DOI] [PubMed] [Google Scholar]
- 76. Davies C, Radua J, Cipriani A, et al. Efficacy and acceptability of interventions for attenuated positive psychotic symptoms in individuals at clinical high risk of psychosis: a network meta-analysis. Front Psychiatry. 2018;9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Howes OD, McCutcheon R.. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry. 2017;7(2):e1024–e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Campos C, Mesquita F, Marques A, Trigueiro MJ, Orvalho V, Rocha NBF.. Feasibility and acceptability of an exergame intervention for schizophrenia. Psychol Sport Exerc. 2015;19:50–58. [Google Scholar]
- 79. Oertel-Knöchel V, Mehler P, Thiel C, et al. Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2014;264(7):589–604. [DOI] [PubMed] [Google Scholar]
- 80. Svatkova A, Mandl RCW, Scheewe TW, Cahn W, Kahn RS, Hulshoff Pol HE.. Physical exercise keeps the brain connected: biking increases white matter integrity in patients with schizophrenia and healthy controls. Schizophr Bull. 2015;41(4):869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nuechterlein KH, Ventura J, McEwen SC, Gretchen-Doorly D, Vinogradov S, Subotnik KL.. Enhancing cognitive training through aerobic exercise after a first schizophrenia episode: theoretical conception and pilot study. Schizophr Bull. 2016;42(Suppl 1):S44–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dean DJ, Walther S, Bernard JA, Mittal VA.. Motor clusters reveal differences in risk for psychosis, cognitive functioning, and thalamocortical connectivity: evidence for vulnerability subtypes. Clin Psychol Sci. 2018;6:721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gupta T, Cowan HR, Strauss GP, Walker EF, Mittal VA.. Deconstructing negative symptoms in individuals at clinical high-risk for psychosis: evidence for volitional and diminished emotionality subgroups that predict clinical presentation and functional outcome. Schizophr Bull. 2021;47(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mittal VA, Addington JM.. Embracing heterogeneity creates new opportunities for understanding and treating those at clinical-high risk for psychosis. Schizophr Res. 2021;227:1–3. [DOI] [PubMed] [Google Scholar]
- 85. Kimhy D, Tay C, Vakhrusheva J, et al. Enhancement of aerobic fitness improves social functioning in individuals with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2021;271(2):367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kimhy D, Khan S, Ayanrouh L, et al. Use of active-play video games to enhance aerobic fitness in schizophrenia: feasibility, safety, and adherence. Psychiatr Serv. 2016;67(2):240–243. [DOI] [PubMed] [Google Scholar]
- 87. Myers RS, Roth DL.. Perceived benefits of and barriers to exercise and stage of exercise adoption in young adults. Health Psychol. 1997;16(3):277–283. [DOI] [PubMed] [Google Scholar]
- 88. Petzinger GM, Fisher BE, Van Leeuwen JE, et al. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson’s disease. Mov Disord. 2010;25(S1):S141–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Petzinger GM, Holschneider DP, Fisher BE, et al. The effects of exercise on dopamine neurotransmission in Parkinson’s disease: targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast. 2015;1(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Petzinger GM, Fisher BE, Akopian G, et al. The role of exercise in facilitating basal ganglia function in Parkinson’s disease. Neurodegener Dis Manag. 2011;1(2):157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Paillard T, Rolland Y, Barreto P de S.. Protective effects of physical exercise in alzheimer’s disease and parkinson’s disease: a narrative review. J Clin Neurol. 2015;11(3):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.