Abstract
Affinity-purified rabbit polyclonal (PAB96-1) and mouse monoclonal (1G9-1C2) antibodies to synthetic H-DDDDDDD-OH, an antimicrobial anionic peptide (AP) originally isolated from ovine pulmonary surfactant, were prepared and used to assess the concentrations of AP-like molecules in human respiratory tract samples. In bronchoalveolar lavage fluids, concentrations of AP-like molecules measured by enzyme-linked immunosorbent assay were significantly lower in 13 patients with cystic fibrosis (CF) (mean ± standard deviation [SD], 0.78 ± 0.46 mM) than in 34 patients without CF (1.30 ± 0.66 mM) (P = 0.01). In pulmonary tissues of three patients without CF, very little antigen was stained in the apical cytoplasm of the bronchial and bronchiolar epithelium yet robust staining was seen in the alveolar epithelium. In pulmonary tissues of three patients with CF, robust staining of antigen was seen in the apical cytoplasm of the bronchial and bronchiolar epithelium yet no staining was seen in the alveolar epithelium. These results show that AP-like molecules are present in healthy human respiratory tract samples and differ in concentration and location of expression in patients with and without CF.
Patients with cystic fibrosis (CF) are predisposed to pulmonary infections, and this predisposition contributes to much of the morbidity and mortality associated with this highly prevalent inherited disease (6). One explanation for the increased susceptibility of these individuals to infection is a breakdown in local pulmonary innate immune mechanisms. For example, Smith et al. (14) demonstrated a striking correlation between the colonization of cultured airway epithelial cells in CF patients by Pseudomonas aeruginosa and a loss of endogenous epithelial cell antimicrobial activity. Subsequently, both the physiologic parameters leading to the loss of antimicrobial activity of airway epithelial cells in CF patients (e.g., the determination of NaCl concentration in airway lining fluid and its effect on antimicrobial activity) and the identification of all antimicrobial mechanisms in these tissues, cells, and secretions came under intense investigation. Some of these antimicrobial systems are just being defined (14), whereas others are known to involve cationic human β-defensins (8, 13), antimicrobial fragments of surfactant proteins (10), lysozyme (1), secretory leukoprotease inhibitor (9, 15), lactoferrin (16), and cathelicidins (2).
Three small, antimicrobial anionic peptides (AP), H-GADDDDD-OH, H-GDDDDDD-OH, and H-DDDDDDD-OH, are also present in ovine surfactant extracts (5), bronchoalveolar lavage (BAL) fluid, and airway epithelial cells (3). These peptides occur in millimolar concentrations (3), require zinc as a cofactor for antimicrobial activity (5), and are rapidly antimicrobial against both gram-positive and gram-negative organisms, including P. aeruginosa (4).
Since AP were detected in ovine BAL fluid by enzyme-linked immunosorbent assay (ELISA) and in ovine pulmonary epithelia by immunohistochemistry with affinity-purified polyclonal and monoclonal antibodies to H-DDDDDDD-OH (3), we were interested in whether these antibodies detect AP-like molecules in human BAL fluids and pulmonary epithelia. In this study, we show that AP-like molecules are detected in human BAL fluid and pulmonary epithelia and differ in concentration and location of expression in patients with and without CF.
Native BAL fluid from 47 patients (13 patients with CF and 34 patients without CF) and formalin-fixed pulmonary tissues from 6 patients (3 patients with CF and 3 patients without CF) were collected as approved by the Institutional Review Board, Department of Pediatrics, Allergy/Pulmonary Division, The University of Iowa Hospitals and Clinics. CF-unrelated BAL fluids were collected from healthy volunteers (n = 23) and patients with pulmonary infections (n = 6), asthma (n = 2), genetic disorders (n = 2), and alveolar proteinosis (n = 1). CF-unrelated pulmonary tissues (n = 3) were collected from patients who had died from nonpulmonary causes (i.e., head injuries from car accidents). They had all been maintained on ventilators transiently, and the lungs were deemed to be not suitable for transplant. Pulmonary pathology, typical of that in patients on ventilators, was present.
H-DDDDDDD-OH was synthesized by Multiple Peptide Systems (San Diego, Calif.) by using Merrifield resins and standard tert-butoxycarbonyl chemistry in combination with simultaneous multiple peptide synthesis as previously described (3, 4). Peptide quality and composition were assessed by analytical high-performance liquid chromatography, plasma desorption mass spectral analysis performed on a Biolon 20 Mass Analyzer, and amino acid analysis. Lyophilized peptide was 95 to 99% pure.
Affinity-purified rabbit polyclonal antibody (PAB96-1) and mouse monoclonal antibody (1G9-1C2) to H-DDDDDDD-OH were prepared, and their specificities and epitope binding sites were characterized in a competitive ELISA and with a SPOTs Epitope Mapping kit, respectively, as recently described (3). Both antibodies were specific for AP with C-terminal Asp residues (3). Epitopes that contained more than two C-terminal Asp residues were recognized by antibody 1G9-1C2 (at a concentration of 25 μg/ml only). Antibody PAB96-1, which was affinity purified on a gel coupled with H-DDDDDDD-OH, recognized only H-DDDDDDD-OH (at both 5- and 25-μg/ml concentrations).
The concentration of AP-like molecules in BAL fluid was assessed by direct ELISA performed as recently described (3) with both antibodies (PAB96-1 and 1G9-1C2). Native BAL fluid (100 μl) from each patient was incubated overnight at 26°C in high protein binding styrene plates (Immulon 4; Dynex Technologies, Inc., Chantilly, Va.). The positive control was synthetic peptide H-DDDDDDD-OH (1.0 to 0.063 mM) added to surfactant (1.0 mg/ml) from sheep 3149 (5), and the negative control was surfactant (1.0 mg/ml) from sheep 3149 without peptide. After 16 h, wells were blocked with gelatin blocking buffer. Either antibody PAB96-1 or antibody 1G9-1C2 (1.0 μg of antibody per ml of blocking buffer) was added and incubated for 1 h. The wells were washed, and bound antibody was detected with either peroxidase-labeled goat anti-mouse immunoglobulin G (IgG) (human serum-absorbed IgG, 1.0 μg per ml of blocking buffer) (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) or peroxidase-labeled goat anti-rabbit IgG (1.0 μg per ml of blocking buffer; Kirkegaard & Perry Laboratories, Inc.). Bound peroxidase-labeled antibody was detected with tetramethylbenzidine (TMB substrate and stop system; Kirkegaard & Perry Laboratories, Inc.). Concentrations of AP-like molecules in the BAL fluids were determined after extrapolation of their optical densities from those generated by a standard curve of synthetic peptide H-DDDDDDD-OH diluted within the range from 1.0 to 0.063 mM. Statistical differences among groups were determined by a one-way analysis of variance (SigmaStat 2.03; Jandel Scientific Corp., San Rafael, Calif.).
BAL fluid from patients with CF likely has additional protein that may compete with AP-like molecules for binding sites in the ELISA plates and thus give the appearance of artificially lower concentrations of AP-like molecules in these samples. To demonstrate the validity of the ELISA for quantitation of AP-like molecules, a representative set of BAL fluid samples (three samples from patients with CF and three samples from patients without CF) were diluted twofold. The ELISA was performed as described above. In all samples, a linear relationship was seen between the amount of protein in the BAL fluid and the optical density of the ELISA reaction mixture (mean ± standard deviation [SD], correlation coefficient, 0.80 ± 0.24). No significant differences were seen between the correlation coefficients for the groups. This shows that additional protein that may occur in the BAL fluid sample from a CF patient does not compete with AP-like molecules for binding sites in the ELISA plates or give the appearance of artificially lower concentrations of AP-like molecules in these samples.
Pulmonary tissues (from three patients with CF and three patients without CF) were sectioned onto ProbeOn Plus microscope slides (Fisher Scientific, Pittsburgh, Pa.) and processed for immunocytochemical detection of AP-like molecules. Briefly, paraffin was removed with xylene and the sections were rehydrated in 100% ethanol–90% ethanol–70% ethanol and rinsed in ultrapure water. The sections were then incubated in a gelatin blocking buffer containing 50% normal goat serum, washed in gelatin blocking buffer, washed again in 50% normal goat blocking serum, and incubated with antibody 1G9-1C2 (2.0 μg/ml) overnight at 4°C. Sections were then washed in gelatin blocking buffer, incubated with biotinylated goat anti-mouse antibody (Biogenex, San Ramon, Calif.), washed in buffer, and incubated in streptavidin-alkaline phosphatase for 30 min at room temperature. The color was developed with New Fuchsin (Biogenex). Sections were then counterstained with hematoxylin for 1 min (Shannon Lipshaw), dehydrated, infiltrated, and mounted.
The immunohistochemical procedure was optimized after a series of trials. Control tissue sections were processed as described above but without antibody 1G9-1C2 or with antibody 1G9-1C2 preincubated with 0.5 mM H-DDDDDDD-OH. In both of these controls, staining specific to H-DDDDDDD-OH could not be detected. Tissue sections were also incubated with trypsin for 15 or 30 min or heated in a microwave for 10 min in either pH 6.0 citrate buffer (Biogenex) or Tris buffer (pH 10.0) to further expose H-DDDDDDD-OH in cells. None of these treatments dramatically increased staining specific to H-DDDDDDD-OH. Tissue sections were also incubated in a variety of blocking buffer solutions, including those that contained fish gelatin, 10, 50, and 100% normal goat serum, fish gelatin with either 10, 50, and 100% normal goat serum, or a commercial blocker (Powerblock; Biogenex). Gelatin blocking buffer containing 50% normal goat serum was chosen and was found to enhance specificity as well as reduce nonspecific background and nuclear staining.
AP-like molecules were detected with antibody 1G9-1C2, and the concentrations were significantly lower in the 13 patients with CF (mean ± SD, 0.78 ± 0.46 mM) than in the 34 patients without CF (mean ± SD, 1.30 ± 0.66 mM) (P = 0.01). AP-like molecules were also detected with antibody PAB96-1, and the concentrations were similar to those detected with 1G9-1C2 and also significantly different between groups (P < 0.001).
Lesions were present in all six pulmonary tissues. Patients without CF had mild suppurative bronchopneumonia, suppurative bronchopneumonia, or bronchial ectasia with loss of cilia. Patients with CF had multifocal chronic lymphocytic bronchitis or severe diffuse chronic-active bronchopneumonia.
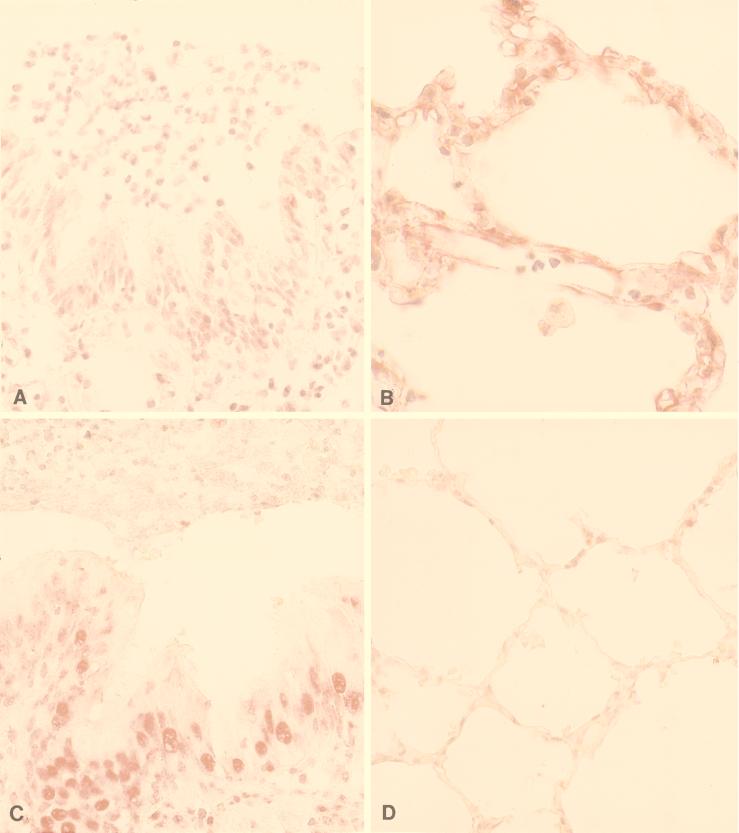
Immunohistochemical staining of pulmonary tissues with antibody 1G9-1C2 differed between groups (Fig. 1). For patients without CF, very little antigen was stained in the apical cytoplasm of the bronchial and bronchiolar epithelium (Fig. 1A) yet robust staining was seen in the alveolar epithelium (these findings were observed in all of three tissue sections; 60 to 90% of cells in the alveolar epithelium were stained; Fig. 1B). A similar pattern of nuclear staining was also seen: from 10 to 30% of bronchial and bronchiolar epithelial cell nuclei were stained (Fig. 1A), and 60 to 90% of alveolar epithelial cell nuclei were stained (Fig. 1B).
FIG. 1.
Immunohistochemical staining of pulmonary tissues from patients without CF and patients with CF. (A) In patients without CF, antibody 1G9-1C2 to H-DDDDDDD-OH stained very little antigen in the apical cytoplasm of the bronchial and bronchiolar epithelium and in the cytoplasm of pulmonary endothelial cells and sporadic alveolar macrophages (in all of three tissues examined). From 10 to 30% of bronchial and bronchiolar epithelial cell nuclei were also stained. (B) In patients without CF, robust cytoplasmic staining of alveolar type I epithelium was seen (in all of three tissue sections examined; 60 to 90% of cells were stained). From 60 to 90% of alveolar epithelial cell nuclei were also stained. (C) In patients with CF, moderate staining of antigen was seen in the apical cytoplasm of the bronchial and bronchiolar epithelium (in all of three tissue sections examined; 60 to 90% of cells were stained). From 60 to 90% of bronchial and bronchiolar epithelial cell nuclei were also stained. (D) In patients with CF, no cytoplasmic staining of alveolar type I epithelium was seen (in all of three tissue sections examined; only 10 to 30% of the cells were stained). Less than 10% of alveolar epithelial cell nuclei were stained.
In patients with CF, moderate staining of antigen was seen in the apical cytoplasm of the bronchial and bronchiolar epithelium (Fig. 1C) yet no staining was seen in the alveolar epithelium (these findings were observed in all of three tissue sections; only 10 to 30% of alveolar epithelial cells were stained; Fig. 1D). In addition, tissues from these patients had peribronchial and peribronchiolar lymphocytic infiltrates containing some stained lymphocytes. Neutrophils or suppurative exudate did not stain. Again, a similar pattern of nuclear staining was seen. From 60 to 90% of bronchial and bronchiolar epithelial cell nuclei were stained (Fig. 1C), and <10% of alveolar epithelial cell nuclei were stained (Fig. 1D).
Overall, AP-like molecules were detected in human BAL fluid, demonstrating the presence of peptides with a composition similar to H-DDDDDDD-OH, an antimicrobial AP originally isolated from ovine pulmonary surfactant (3, 5). This was not an unexpected finding as others have reported similar results. In the 1980’s, antimicrobial AP-like molecules were partially isolated from mouse, rabbit, and human BAL fluid (11, 12). Biochemical characterization of the partially purified extracts demonstrated that the antimicrobial activity was heat stable and trypsin sensitive and that this activity was reversed by incubation with the cation chelator EDTA. A rough estimate of peptide molecular size was 3.4 kDa. A similar molecule was isolated from human BAL fluid (7). Reverse-phase high-performance liquid chromatography fractionation led to partial purification of an antibacterial peptide which behaved as an anionic peptide with a molecular size of <10 kDa and whose activity was heat stable, Zn dependent, and inhibited by both EDTA and phosphate.
The concentration of AP-like molecules was significantly lower in patients with CF than in those without CF. This is an interesting finding and suggests a physiologic deficiency in peptide concentration which may predispose patients with CF to respiratory infections. This finding is contrary to the situation proposed for other antimicrobial substances that are present in similar amounts in patients with and without CF yet are inactivated in the increased NaCl content environment in the airway lining fluid of CF patients (14).
AP-like antigen was also detected in the respiratory epithelia of patients with and without CF; this finding is similar to the immunohistochemical results seen in sheep pulmonary tissues (3). In that study, antibodies PAB96-1 and 1G9-1C2 identified antigen in the apical cytoplasm of the bronchial and bronchiolar epithelia and in the cytoplasm of pulmonary endothelial cells and sporadic alveolar macrophages (3). Goblet cells and epithelial cells of pulmonary alveoli were not stained. In this study, patients without and with CF had inverse staining patterns of antigen in pulmonary tissues with these same antibodies. For example, patients without CF had very little stained antigen in the bronchial and bronchiolar epithelium yet a significant amount of stained antigen in the alveolar epithelium. In contrast, patients with CF had a significant amount of stained antigen in the bronchial and bronchiolar epithelium yet no stained antigen in the alveolar epithelium. Whether this difference is a result of persistent respiratory bacterial infection or an abnormality associated with the CF phenotype that increases the susceptibility of the respiratory tract to bacterial infection is not yet known.
The origin of AP-like molecules is not yet known. In an ovine model, AP molecules are synthesized in airway epithelium, released into airway surface fluid, and disseminated throughout the respiratory tract. Based on their known surfactant-binding properties they could then become associated with the alveolar epithelium. In patients without CF, AP-like molecules may have been released from their sites of synthesis and disseminated throughout the respiratory tract. In CF patients, there may be a block in release of the AP-like molecules from their original sites of synthesis and hence an accumulation in the respiratory tree but none in the distal alveoli. Perhaps the abnormal airway fluid composition in CF contributes to this lack of secretion or lack of trafficking throughout the respiratory tract. Cytoplasmic staining of alveolar wall cells could also contribute to the increase in staining found in the alveoli. It could be that the difference in distribution of staining is a result of production of two or more AP-like molecules: one preferentially produced by epithelial cells and another produced by alveolar wall cells.
AP-like molecules may occur individually or as part of a larger protein zymogen, and a number of observations are consistent with this hypothesis. First, the amino acid sequence of the ovine AP, H-DDDDDDD-OH, is similar to those of the charge-neutralizing activation peptides of group I serine proteases (e.g., human trypsinogen activation peptide, which has the sequence H-VDDDDK-OH). Synthetic trypsinogen activation peptide and other similar fragments have antimicrobial activity (4). Second, antibodies PAB96-1 and 1G9-1C2 recognize several larger proteins (range, 25.7 to 53.7 kDa) in Western blots of solubilized tracheal epithelial cells and lung homogenates (3). Third, a degenerate oligonucleotide sequence of ovine AP and AP-like molecules used to probe a Southern blot of human DNA identified strong hybridizing bands (all <4 kb), also suggesting that AP is part of a much larger gene product (data not shown). Finally, a number of AP-like sequences have been found in other cellular and nuclear proteins. The signal detected in nuclei may be based on multiple genes known to be expressed in the nuclei, well characterized in humans, which encode products that contain AP-like peptide sequences including nucleophosmin, TFIID transcription factor, and topoisomerase II. Although this may help explain the specificity of the epithelial cell nuclear staining, it does not explain the differences in epithelial staining seen between the groups.
In summary, these observations form the foundation for further work to isolate and characterize AP-like molecules present in the human airway to assess their role in innate pulmonary defense. Whether there is a genetic or physiologic basis for differences in AP-like molecule concentrations in patients with CF or whether changes in concentration affect the susceptibility of the respiratory tract to pulmonary pathogens will have to be determined.
Acknowledgments
This research was supported by the Cystic Fibrosis Foundation (BROGDE97Z0).
REFERENCES
- 1.Arima H, Ibrahim H R, Kinoshita T, Kato A. Bactericidal action of lysozymes attached with various sizes of hydrophobic peptides to the C-terminal using genetic modification. FEBS Lett. 1997;415:114–118. doi: 10.1016/s0014-5793(97)01071-5. [DOI] [PubMed] [Google Scholar]
- 2.Bals R, Wang X R, Zasloff M, Wilson J M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brogden K A, Ackermann M, Huttner K M. Detection of anionic antimicrobial peptides in ovine bronchoalveolar lavage fluid and respiratory epithelium. Infect Immun. 1998;66:5948–5954. doi: 10.1128/iai.66.12.5948-5954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brogden K A, Ackermann M, Huttner K M. Small, anionic, and charge-neutralizing propeptide fragments of zymogens are antimicrobial. Antimicrob Agents Chemother. 1997;41:1615–1617. doi: 10.1128/aac.41.7.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brogden K A, De Lucca A J, Bland J, Elliott S. Isolation of an ovine pulmonary surfactant-associated anionic peptide bactericidal for Pasteurella haemolytica. Proc Natl Acad Sci USA. 1996;93:412–416. doi: 10.1073/pnas.93.1.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis P B, Drumm M, Konstan M W. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 7.Ellison R T, III, Boose D, LaForce F M. Isolation of an antibacterial peptide from human lung lavage fluid. J Infect Dis. 1985;151:1123–1129. doi: 10.1093/infdis/151.6.1123. [DOI] [PubMed] [Google Scholar]
- 8.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 9.Hiemstra P S, Maassen R J, Stolk J, Heinzel-Wieland R, Steffens G J, Dijkman J H. Antibacterial activity of antileukoprotease. Infect Immun. 1996;64:4520–4524. doi: 10.1128/iai.64.11.4520-4524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaser M R, Skouteris G G. Inhibition of bacterial growth by synthetic SP-B1-78 peptides. Peptides. 1997;18:1441–1444. doi: 10.1016/s0196-9781(97)00211-8. [DOI] [PubMed] [Google Scholar]
- 11.LaForce F M, Boose D S. Effect of zinc and phosphate on an antibacterial peptide isolated from lung lavage. Infect Immun. 1984;45:692–696. doi: 10.1128/iai.45.3.692-696.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaForce F M, Boose D S. Sublethal damage of Escherichia coli by lung lavage. Am Rev Respir Dis. 1981;124:733–737. doi: 10.1164/arrd.1981.124.6.733. [DOI] [PubMed] [Google Scholar]
- 13.McCray P B, Jr, Bentley L. Human airway epithelia express a β-defensin. Am J Respir Cell Mol Biol. 1997;16:343–349. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- 14.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 15.Tomee J F C, Hiemstra P S, Heinzel-Wieland R, Kauffman H F. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J Infect Dis. 1997;176:740–747. doi: 10.1086/514098. [DOI] [PubMed] [Google Scholar]
- 16.Tomita M, Takase M, Wakabayashi H, Bellamy W. Antimicrobial peptides of lactoferrin. Adv Exp Med Biol. 1994;357:209–218. doi: 10.1007/978-1-4615-2548-6_20. [DOI] [PubMed] [Google Scholar]