Abstract
Purpose
Endometriosis (EMS) is confirmed pathophysiologically to be an estrogen-dependent disease, similar to endometrial hyperplasia/cancer and breast cancer. Epidemiological and biological data on endometriosis might explain links between endometriosis and these cancers. We sought to identify the differences in the risk of endometrial cancer and breast cancer between women with and women without endometriosis.
Methods
We searched PubMed, EMBASE, the Cochrane Library, and four Chinese databases (CNKI, VIP, WanFang, CBM) to identify relevant studies published online between January 2011 and March 2021. In our meta-analysis, we used the Newcastle–Ottawa Scale (NOS) to evaluate the design and quality of all studies, and we calculated the pooled risk ratio (RR) using the random model. The Q test and I2 were used to evaluate the degree of heterogeneity of eligible studies. We used funnel plots and Begg’s and Egger’s tests to assess publication bias.
Results
Of the 1369 articles, we finally included 14 cohort studies and seven case–control studies. Data from large cohort and case–control studies indicate that women with endometriosis had an increased risk of both endometrial cancer [RR, 1.662; 95% CI, (1.148–2.407)] and breast cancer [RR, 1.082; 95% CI, (1.001–1.169)].
Conclusion
Endometriosis can increase the risk of endometrial cancer and breast cancer, and women with endometriosis are recommended to receive routine screening in long-term management.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12905-022-02028-x.
Keywords: Endometriosis, Endometrial cancer, Breast cancer, Risk, Meta-analysis
Introduction
Endometriosis (EMS) is a common inflammatory condition defined as endometrial-like tissues found outside of the uterus, mainly in the pelvic area (such as the ovaries, ligaments and peritoneum). Three well-recognized subtypes are named superficial endometriosis (SUP), ovarian endometrioma (OMA), and deep infiltrating endometriosis (DIE) [1]. Pelvic pain and infertility are the two main symptoms that severely impact women’s lives [2]. Because the current diagnosis of EMS requires surgical visualization and the confirmation of pathological results [3], the measurement of the incidence and prevalence of endometriosis is complicated, and estimates vary widely among different studies. Based on the prevalence of pelvic pain and infertility in the general population, the estimated population prevalence of endometriosis is approximately 10% [4, 5] and is higher in symptomatic women [6].
Although endometriosis is a benign gynecological disease, the pattern of its growth is similar to that of malignant disease [7]. The ectopic endometrium, similar to the normal endometrium, has the same reaction to hormones. Abnormal endometrium can adhere and implant into the peritoneum and then proliferate abundantly, which can also lead to invasion of surrounding tissues, such as the bladder and rectum. In addition, the abnormal endometrium shows great power to protect itself from destruction by the immune system [8]. Since first reported by Sampson in 1925 that EMS was associated with malignant tumors, an increasing number of studies have tried to find an association between EMS and cancer. EMS and several malignant tumors have some common risk factors, such as menstrual and reproductive history, cigarette smoking, diet, and environmental exposures [5]; beyond that, some of the treatments for endometriosis, such as physicotherapeutics and medication, also increase the risk of several cancer types [9–11], and Bhyan showed evidence that endometriosis may have shared genetic mechanisms with women’s cancers detected by integrated bioinformatic analysis [12]. Endometriosis is histologically typical and atypical; atypical endometriosis is regarded as the premalignant precursor and has the potential for direct malignant transformation [13–15]. Endometriosis leads to systematic changes, including chronic inflammation, an aberrant immune response or an aberrant milieu, which increases the risk of distal cancer [16].
The retrograde menstruation hypothesis, which is commonly accepted, posits that the mechanism of endometriosis is that eutopic endometrial tissues with molecular defects migrate retrogradely to the abdominal cavity mixed with blood, stick to the peritoneum, and proliferate aggressively, and that endometriosis (like endometrial cancer) can also be regulated by hormones. Therefore, the association between endometriosis and endometrial cancer seems to be noticed by researchers more easily, while breast cancer, which is the other common cancer among reproductive women, can also be influenced by hormone fluctuations. Endometriosis itself and before or after therapy may influence breast cancer directly or indirectly. Epidemiologically, several studies have clearly shown that endometriosis is a risk factor for ovarian cancer [13, 17, 18], but the impact on endometrial cancer and breast cancer is still controversial and even totally converse [18–25]. Several meta-analyses have been published in recent years on the association between endometriosis and cancer; based on 38 cohort studies or case–control studies published before October 24, 2019, Marina Kvaskoff estimated the summary relative risk to be SRR, 1.23; 95% CI, (0.97–1.57), which is not statistically significant, and SRR, 1.04; 95% CI, (1.00–1.09) for the relationship of endometriosis to endometrial cancer and breast cancer, respectively [16]. While this result is different from those of the prior meta-analyses, S. Gandini’s study, based on 32 studies published between 1989 and 2018, suggested that endometriosis confers an increased risk of endometrial cancer [SRR, 1.38, 95% CI (1.10–1.74)], while no association emerged for breast cancer [SRR, 1.04, 95% CI (0.99–1.09)] [26]. Whether endometriosis impacts the risk of these two cancers and the specific physiological and pathological mechanisms involved still need further investigation. Endometriosis, endometrial cancer, and breast cancer are all estrogen-related diseases. Our meta-analysis tried to find their association, and based on the current research fundamentals, we hypothesize that endometriosis can increase the risk of endometrial cancer and breast cancer. Currently, we have limited knowledge of endometriosis, and knowing its association with several cancer types can enhance our understanding of endometriosis pathophysiology, which may advance the treatment and clinical management of endometriosis. Therefore, we performed this meta-analysis to disentangle these intriguing and controversial issues.
Method
Search strategy
The reporting of this meta-analysis strictly followed the MOOSE checklist. We comprehensively searched for published relevant observational studies from the databases of PubMed, EMBASE, Cochrane Library, CNKI, VIP, WanFang, and CBM for the past 10 years (from 2011 to March 11, 2021). The search terms were the keywords combined with their corresponding MeSH terms (which are detailed in the supplementary files named “search strategy”). We also searched the references from selected publications to retrieve additional studies that were not identified through electronic searches.
Selection criteria and exclusion criteria
We included relevant studies that met the following criteria: (1) studies that examined endometriosis (which was diagnosed through self-reports, laparoscopy, surgery or other medical records) and endometrial cancer or breast cancer; (2) human studies and cohort or case–control studies; and (3) publications in which usable risk estimates, such as odds ratios (ORs), risk ratios (RRs), hazard ratios (HRs), and standard incidence ratios (SIRs) with 95% confidence intervals (CIs), were presented or necessary data were given for calculation. (4) If several studies were conducted in the same population, we would select the report with the most applicable estimates or the most recent report. However, we also excluded the following types of studies: (1) meta-analyses, reviews, case reports, editorials, and letters to the editor; (2) animal or cell experiments; (3) studies not published in English or Chinese; and (4) studies not meeting the inclusion criteria.
Data extraction
Based on the predetermined selection and exclusion criteria, two authors independently extracted data from each study using predesigned forms, and discrepancies were resolved by the third author. For each study, we independently extracted the first author’s name, year of publication, country, age at baseline of the study population, study design, follow-up time of the cohort studies, the sample size of the study, ascertainment of endometriosis and cancer cases, age at the diagnosis of endometriosis, adjustment factors and relative risk estimates with 95% CIs (we chose the model adjusted for the largest number of confounders when different crude and adjusted estimates were reported), and the method of information collection.
Quality assessment and risk of bias
The Newcastle–Ottawa Scale (NOS) was used to assess the quality of the included cohort and case–control studies [27]. The NOS is composed of three parameters of quality: the selection (four scores at most), comparability (two scores at most), and exposure for a case–control study or outcome for a cohort study (three scores at most). Currently, despite no standard criteria, a study with an NOS score ≥ 7 is considered a high-quality study [13]. Two authors independently evaluated the quality, and discrepancies were resolved by the third author.
Statistical analysis
We included in this meta-analysis studies reporting different measures of relative risk (RR): single-arm cohort studies (standardized incidence ratio), two-arm cohort studies (rate ratio), and case–control studies (odds ratio). Because the absolute risk of endometrial cancer and breast cancer is low, the three combined measurement methods yield similar relative risk (RR) estimates, and we combined all the RR estimates to ensure the comprehensiveness of the analysis and to enlarge the statistical effectiveness [13]. For the cohort study, when studies had two or more controls, we chose the general population cohort, and when studies had two or more experimental groups, we included the cohort with more populations during the data analysis. The Q test and I2 were used to evaluate the degree of heterogeneity of eligible studies. For the Q test, p > 0.10 was considered representative without statistical heterogeneity, data were interpreted using the fixed effect model, p < 0.10 indicated statistically significant heterogeneity, and the random effects model was chosen. For I2, the values of 0, 25, 50, and 75% correspond to no, low, moderate, and high heterogeneity, respectively [28]. We also conducted a subgroup analysis based on the information collection, study design, assessment of endometriosis and cancer, NOS score, and adjustment of confounding factors to evaluate potential sources of heterogeneity. Sensitivity analysis was conducted to evaluate the robustness of the results. We used funnel plots and Begg’s and Egger’s tests to assess publication bias. All statistical analyses were conducted by STATA software, version 16.0.
Results
Selection of articles
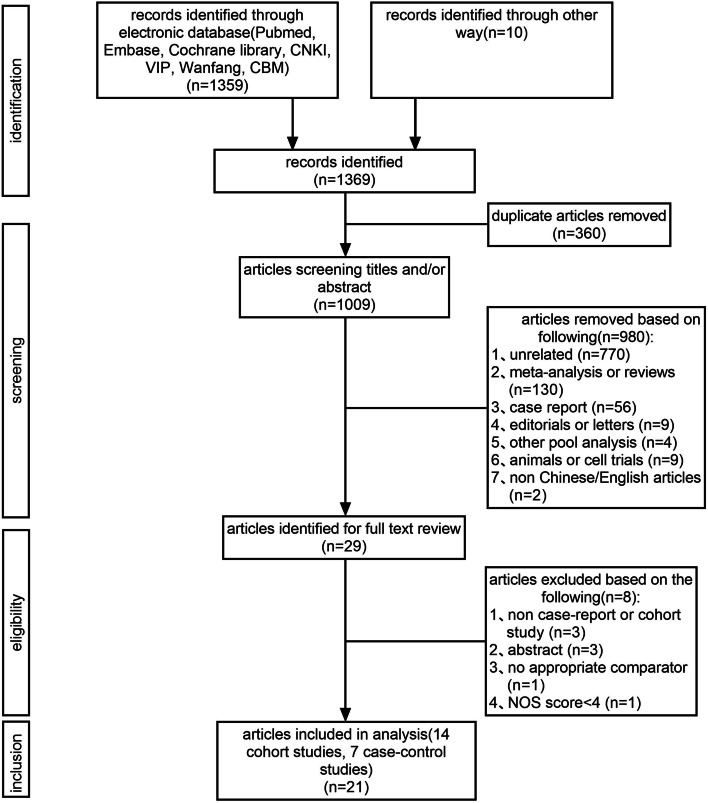
Two authors independently evaluated the eligibility of studies from the database according to the selection and exclusion criteria, and the third author resolved the disagreement between the two authors after discussion. As a result, a total of 1369 studies were identified. Subsequently, 360 duplicates and 980 unrelated articles were excluded after reviewing the titles and abstracts. Finally, a total of 29 full texts were further assessed, and 8 publications were excluded because they were non-case report or non-cohort studies (n = 3), consisted of only an abstract (n = 3), had no appropriate comparator (n = 1), or had a NOS score of < 4 (n = 1). As a result, 21 studies were included in this meta-analysis, including 14 cohort studies [19–22, 25, 29–37] and 7 case–control studies [23, 24, 38–42]. The flow chart of the study selection is presented in Fig. 1.
Fig. 1.
Flow chart for the selection of eligible studies
Characteristics of the included studies and quality assessment
A total of 21 articles were included in this analysis, and their characteristics are shown in Table 1. All articles were published between 2011 and 2021. Studies were conducted in America [21, 23, 30, 35, 36, 38], the UK [22, 25], China [29, 33, 34, 37, 39, 41], Korea [19], Denmark [20], Finland [31], Sweden [32], Germany [42], Puerto Rico [24], and Australia [40], with each of the latter seven countries having one study. Regarding the assessment of endometriosis, apart from studies including a clinical diagnosis made by the medical doctors during hospitalization or in the outpatient setting [19, 20], the ICD code of the disease [21, 29, 32–34, 37, 41], medical records from the hospital database or relevant medical documents [39], laparoscopy or surgery [22, 31, 35], and self-reports [23, 24, 30, 36, 38, 40, 42], the remaining study included both patient-reported and clinically reported information [25]. Other than three studies [20, 25, 31], the remaining 18 studies all adjusted several confounders when reporting RR estimates. Thirteen articles were related to endometrial cancer, and 16 articles involved breast cancer (Table 1).
Table 1.
Characteristics of cohort and case-control studies of the association between endometriosis and endometrial cancer or breast cancer
| Study, publication year and country | Study type | follow up time | Average age of baseline (years) | Sample size of the study | Endometriosis assessment | Age at diagnosis of endometriosis (years) | Cancer ascertainment | Adjusted RR(95%CI) | Adjustments | The way of information collection | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kyung Jin Eoh, 2021, Korean [19] | cohort study | NA | endometriosis cohort:40.4 ± 8.4 control:44.0 ± 17.0 | 263,273 | ICD code | ≥20 | ICD code | endometrial cancer: 4.59 (3.56–5.91) breast cancer: 1.44 (1.31–1.58) | Adjusted for age, insurance type, and comorbidities (diabetes, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, chronic kidney disease, liver cirrhosis, and heart) | database | 7 |
| Fanghua Shen, 2020, China [39] | case- control study | – | NA | 876 | medical records | all ages | medical records | endometrial cancer: 0.36 (0.094–1.381) | Adjusted by age at diagnosis and parity | database | 6 |
| Frida E. Lundberg, 2019, Sweden [32] | cohort study | control cohort: 23.4 yearsa infertile cohort: 25.8 yearsa | NA | 2,882,847 | ICD code | all ages | ICD code | endometrial cancer: 0.94 (0.76–1.17) breast cancer: 1.02 (0.96–1.07) | EC: Adjusted for age, calendar time, education level, country of birth, parity and age at first birth, salpingectomy and bilateral oophorectomy BC: Adjusted for age, calendar time, education level, country of birth, parity and age at first birth, salpingectomy, hysterectomy and bilateral oophorectomy | database | 6 |
| Hsing-Chi Hsu, 2019, China [37] | cohort study | NA | nurses cohort:34.0 ± 7.66 control:34.0 ± 7.74 | 178,870 | ICD code | > 20 | ICD code | breast cancer: 0.62 (0.22–1.78) | Adjust for study cohort, age, and comorbidities with significant crude HR (cradiovascular disease and diabetes mellitus) | database | 7 |
| Liisu Saavalainen, 2018, Finland [31] | cohort study | 16.8 yearsa | endometriosis cohort:36.4 ±? | 49,933 | surgery | 10–60 | ICD code | breast cancer: 0.99 (0.94–1.03) | – | database | 4 |
| Eric S. Surrey, 2018, American [21] | cohort study | NA | endometriosis cohort:36.5 ± 8.2 control:36.4 ± 8.4 | 134,805 | ICD code | 18–49 | ICD code | endometrial cancer: 2.4 (1.6–3.8) breast cancer: 1.4 (1.1–1.7) | Adjust for age, state, and insurance type. Models also controlled for 15 Charlson–Deyo comorbidities measured during year before index date | database | 8 |
| Carrie L Williams, 2018, UK [25] | cohort study | 8.8yearsa | endometriosis cohort:34.5 ± 4.8 | 225,786 | patient reported and clinic reported information | all ages | ICD code | endometrial cancer: 0.75 (0.35–1.43) breast cancer: 0.98 (0.86–1.12) | – | database | 4 |
| Chih-Ching Yeh, 2018. China [34] | cohort study | NA | NA | 120,582 | ICD code | all ages | ICD code | endometrial cancer: 1.89 (1.07–3.35) breast cancer:0.99 (0.80–1.23) | Adjusted for the birth year and geographicregion, occupation, urbanization, monthly income, comorbidity | database | 6 |
| Leslie V. Farland, 2016, American [35] | cohort study | 24 years | endometriosis cohort:35.6 ± 4.2 control:34.3 ± 4.7 | 16,325 | laparoscopy or laparotom | 25–42 | medical records | breast cancer: 0.96 (0.88–1.06) | Adjust for age, calendar time, family history of breast cancer, age at menarche, body mass index, body mass index at age 18 years, smoking, biopsy confirmed benign breast disease, alcohol intake, recent health seeking behavior, birth weight, parity +age at first birth, breastfeeding, oral contraceptive | questionaire | 7 |
| L Saraswat, 2018, UK [22] | cohort study | 29 years | endometriosis cohort:32.1 ± 7.3 general population cohort:32.1 ± 7.3 | 281,937 | laparoscopy or laparotom | all ages | ICD code | endometrial cancer: 1.14 (0.57–2.28) breast cancer: 1.28 (1.06–1.54) | Adjust for age, socio-economic status and duration of follow up | database | 6 |
| Elizabeth M. Poole, 2017, America [36] | cohort study | 18 years | endometriosis cohort:44.5 ± 4.5b control:44.5 ± 4.6b | 107,721 | self-report | all ages | medical records | endometrial cancer: 0.74 (0.39–1.42) | Adjusted for BMI, parity, duration of post-menopausal hormones (by type), age at menopause, age at menarche, menstrual irregularity, infertility history, and duration of oral contraceptive use | questionaire | 7 |
| Julie Brøchner Mogensen, 2016, Denmark [20] | cohort study | 642,403 person-years | NA | 45,790 | clinical diagnoses | 25–49 | ICD code | endometrial cancer: 2.13 (1.77–2.55) breast cancer: 1.05 (1.00–1.11) | _ | database | 6 |
| Hann-Chin Yu, 2015, China [33] | cohort study | 10 years | _ | 139,392 | ICD code | all ages | ICD code | endometrial cancer: 2.83 (1.49–5.35) | Adjusted HRs were adjusted for patients’ age, urbanization level, monthly income, geographic region, hypertension, hyperlipidemia, obesity, and diabetes mellitus | database | 8 |
| Victor C. Kok, 2015, China [29] | cohort study | endometriosis cohort:9842 patients-years control cohort:36274 person-years | _ | 11,330 | ICD code | > 20 | ICD code | endometrial cancer: 4.05 (1.20–13.66) breast cancer: 1.15 (0.61–2.15) | Adjusted for age group, diabetes mellitus, chronic kidney disease, liver cirrhosis, rheumatoid arthritis, and use of medroxyprogesterone acetate, norethindrone acetate, danazol, and GnRH agonist | database | 8 |
| Stefanie Burghaus, 2015, German [42] | case- control study | – | endometrial cancer cases:65.6 ± 10.5 controls:60.9 ± 9.3 | 1305 | self-report | all ages | histopathology reports | endometrial cancer: 2.63 (1.28–5.41) | Adjusted for age, BMI, oral contraceptive use, pregnancies | questionnaire | 6 |
| Shu-Chun Chuang, 2015, China [41] | case- control study | – | – | 24,420 | ICD code | all ages | ICD code | breast cancer: 1.44 (1.15–1.80) | Adjusted for occupation, screen tests (never, once, and twice or above), and average ambulatory visit per year | database | 6 |
| Louise A. Brinton, 2014, American [30] | cohort study | 30.0 yearsa | _ | 12,193 | self-report | all ages | self-report and medical records | breast cancer: 1.12 (0.93–1.35) | Adjusted for study site, calendar year of first infertility evaluation and gravidity at first clinic visit | database and questionnaire | 4 |
| Jaime L. Matta, 2013, American [23] | case- control study | – | breast cancer cases:56.3 ±? controls:52.1 ±? | 991 | self-report | all ages | histopathology reports | breast cancer: 0.5 (0.30–0.90) | Adjusted by age, BMI, family history of breast cancer, menopause, alcohol use, smoking, multivitamin use, marital status, and saturated fat consumption | questionnaire | 7 |
| Luisa Morales, 2013, Puerto Rico [24] | case- control study | – | breast cancer cases:56.4 ± 12.6 controls:52.3 ± 12.5 | 1126 | self-report | all ages | histopathology reports | breast cancer: 0.61 (0.30–1.00) | Adjusted by age, BMI, family history of breast cancer, menopause, number of children, alcohol use, smoking, vitamin use | questionnaire | 4 |
| Ingrid J. Rowlands, 2011, Australia [40] | case- control study | – | endometrial cancer cases:61.29 ± 9.5 controls:60.83 ± 9.8 | 2938 | self-report | all ages | histopathology reports | endometrial cancer: 1.47 (1.00–2.17) | Adjusted for age (in years), age at menarche (in years), parous (no, yes), duration of OC use (never, b 60 months; ≥60 months), HRT use (≥ 3 months,< 3 months), smoking (ever, never), BMI (kg/m2) | questionnaire | 6 |
| Hazel B. Nichols, 2011, American [38] | case- control study | – | breast cancer cases:66.2 ± 7.4 controls:65.0 ± 7.5 | 10,046 | self-report | all ages | medical records | breast cancer: 0.99 (0.80–1.21) | Adjusted for age, US state, age at menarche, duration of oral contraceptive use, parity, age at first birth, age at menopause, postmenopausal hormone use, body mass index, mammography screening, and family history of breast cancer | telephone interview | 7 |
NA Information not available, RR Relative risk, CI Confidence interval;-a medium follow-up time;-b at midpoint of follow-up
The NOS scores included in this analysis ranged from 4 to 8 after we excluded the studies in which NOS scores were < 4. For cohort studies, 7 articles were of high quality, with an average score of 6.3. For case–control studies, 2 articles were of high quality, with an average score of 6.
Outcomes
Endometrial cancer
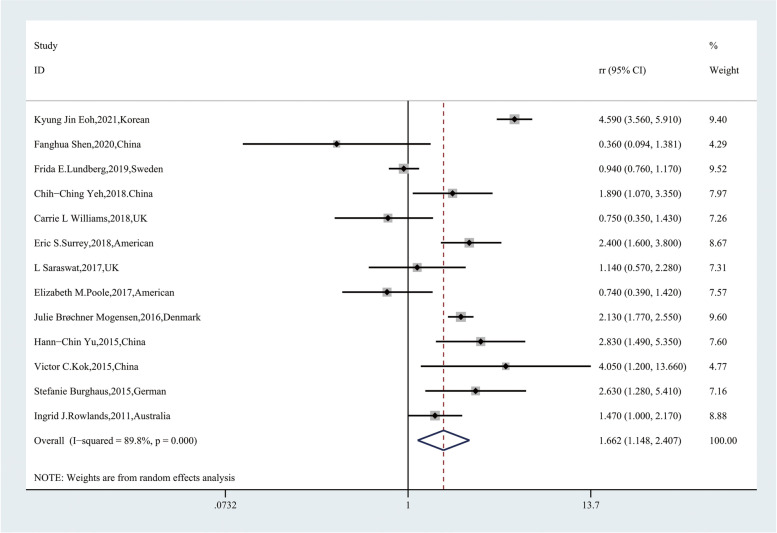
Thirteen articles were included in evaluating the risk relationship between endometriosis and endometrial cancer. In these 13 studies, endometriosis was associated with a significantly increased risk of endometrial cancer [RR, 1.662; 95% CI, (1.148–2.407)] (Fig. 2), while we found high heterogeneity (Q = 118.10, P = 0.000; I2 = 89.8%). We also performed a subgroup analysis to identify the cause of heterogeneity. In the group stratified by the ascertainment of endometrial cancer, ascertainment based on histopathology reports (P = 0.163; I2 = 48.5) and other methods (P = 0.344; I2 = 0.0) showed low heterogeneity (Table 2), which suggested that the method of identification of endometrial cancer may be one of the sources of heterogeneity.
Fig. 2.
Forest plot of the 13 included studies evaluating the association between endometriosis and endometrial cancer
Table 2.
Summary relative risks and 95%CI for the association between endometriosis and endometrial cancer by study characteristics
| Subgroup | No.of studies | Pooled RR(95%CI) | Heterogeneity | ||
|---|---|---|---|---|---|
| Random effect | Fixed effect | P | I2(%) | ||
| The way of information collection | |||||
| questionnaire | 3 | 1.403 (0.762,2.584) | 1.402 (1.036,1.895) | 0.034 | 70.3 |
| database | 10 | 1.743 (1.119,2.716) | 1.921 (1.721,2.143) | 0.000 | 91.6 |
| Study design | |||||
| Case-control studies | 3 | 1.358 (0.622,2.963)) | 1.526 (1.096,2.124) | 0.036 | 70.0 |
| Cohort studies | 10 | 1.755 (1.139,2.705) | 1.890 (1.696,2.107) | 0.000 | 91.8 |
| Endometriosis assessment | |||||
| Self-report | 3 | 1.403 (0.762,2.584) | 1.470 (1.000,2.170) | 0.034 | 70.3 |
| surgery | 1 | 1.140 (0.570,2.280) | 1.140 (0.570,2.280) | – | – |
| ICD | 5 | 2.389 (1.184,4.820) | 1.953 (1.692,2.254) | 0.000 | 94.500 |
| Other | 4 | 0.964 (0.347,2.677) | 1.937 (1.626,2.308) | 0.000 | 85.800 |
| Ascertainment of EC | |||||
| Histopathology reports | 2 | 1.810 (1.048,3.127) | 1.675 (1.191,2.356) | 0.163 | 48.5 |
| ICD | 9 | 1.922 (1.227,3.012) | 1.942 (1.740,2.168) | 0.000 | 92.1 |
| Other | 2 | 0.646 (0.361,1.157) | 0.646 (0.361,1.157) | 0.344 | 0.0 |
| NOS score | |||||
| < 7 | 8 | 1.336 (0.918,1.944) | 1.486 (1.316,1.678) | 0.000 | 84.0 |
| ≥ 7 | 5 | 2.457 (1.298,4.652) | 3.252 (2.678,3.951) | 0.000 | 86.4 |
| Adjustment for age | |||||
| Yes | 11 | 1.718 (1.071,2.756) | 1.782 (1.570,2.023) | 0.000 | 90.8 |
| No | 2 | 1.339 (0.484,3.701) | 1.994 (1.671,2.380) | 0.005 | 87.4 |
| Adjustment for BMI | |||||
| Yes | 3 | 1.403 (0.762,2.584) | 1.402 (1.036,1.895) | 0.034 | 70.3 |
| No | 10 | 1.743(1.119,2.716) | 1.921 (1.721,2.143) | 0.000 | 91.6 |
| Adjustment for oral contraceptive use history | |||||
| Yes | 4 | 1.645 (0.903,2.998) | 1.490 (1.112,1.998) | 0.023 | 68.4 |
| No | 9 | 1.644 (1.037,2.604) | 1.909 (1.710,2.131) | 0.000 | 92.5 |
| Adjustment for pregnancies | |||||
| Yes | 5 | 1.116 (0.727,1.713) | 1.056 (0.888,1.257) | 0.008 | 70.8 |
| No | 8 | 2.164 (1.470,3.185) | 2.506 (2.205,2.847) | 0.000 | 83.7 |
| Adjustment for smoking | |||||
| Yes | 1 | 1.470 (0.998,2.165) | 1.470 (0.998,2.165) | – | – |
| No | 12 | 1.677 (1.120,2.512) | 1.884 (1.693,2.097) | 0.000 | 90.6 |
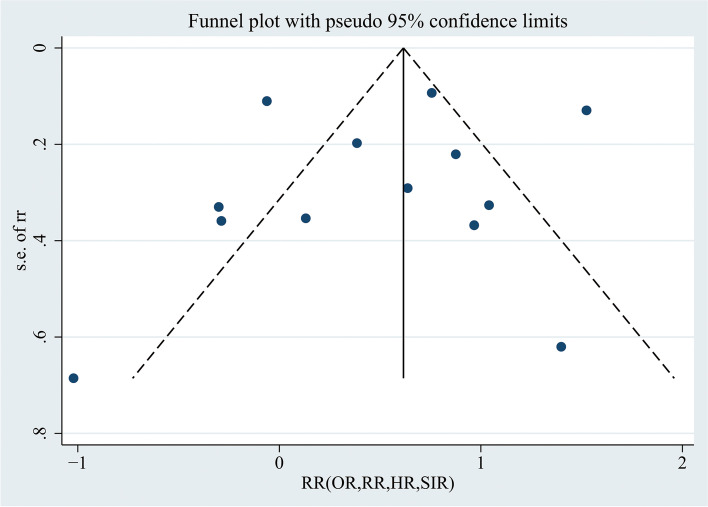
Publication bias was assessed by the funnel plot, as shown in Fig. 3. The funnel plot was visually symmetric. We used Begg’s and Egger’s tests to assess the symmetry of the funnel plot. The p values for Begg’s and Egger’s tests were p = 0.502 (> 0.05) and p = 0.629 (> 0.05), respectively, suggesting that there was no publication bias of the included studies.
Fig. 3.
Funnel plot using data from the 13 studies evaluating the association between endometriosis and endometrial cancer
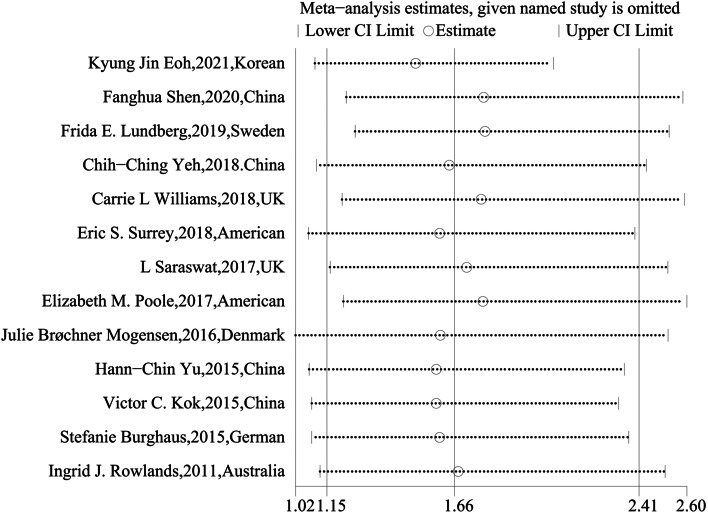
We used leave-one-out sensitivity analysis to evaluate whether any small study effect influenced the pooled effect size. As a result, no significant changes were observed in the sensitivity analysis (Fig. 4), suggesting that this meta-analysis is stable.
Fig. 4.
Sensitivity analysis of the 13 studies evaluating the association between endometriosis and endometrial cancer
Breast cancer
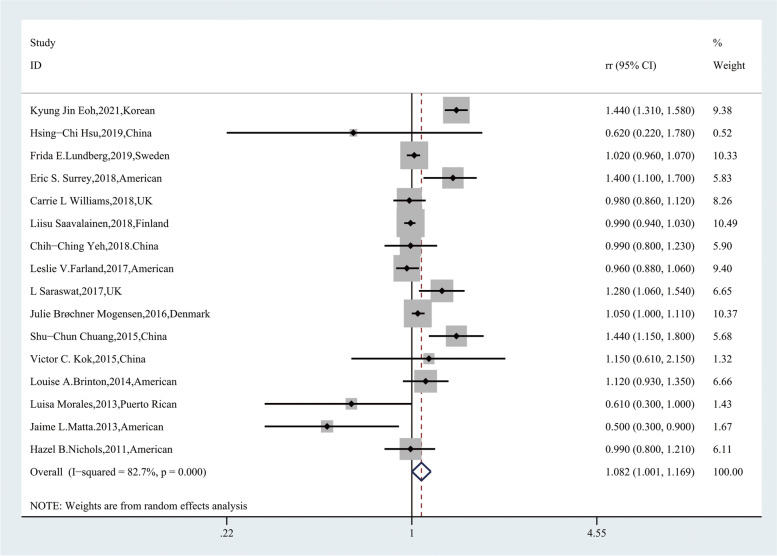
A total of 16 articles were included in evaluating the influence of endometriosis on breast cancer. In these 16 studies, endometriosis increased the risk of breast cancer [RR, 1.082; 95% CI, (1.001–1.169)] (Fig. 5), but we also found high heterogeneity within the group (Q = 86.62, P = 0.000; I2 = 82.7%). In the subgroup analysis, the group stratified by the ascertainment of breast cancer, ascertainment based on histopathology reports (P = 0.632; I2 = 0) and other methods (P = 0.349; I2 = 5.0) showed low heterogeneity. Studies adjusted for oral contraceptive use and pregnancies also showed low histopathology, which may have been the source of heterogeneity (Table 3).
Fig. 5.
Forest plot of the 16 included studies evaluating the association between endometriosis and breast cancer
Table 3.
Summary relative risks and 95%CI for the association between endometriosis and breast cancer by study characteristics
| Subgroup | No.of studies | Pooled RR(95%CI) | Heterogeneity | ||
|---|---|---|---|---|---|
| Random effect | Fixed effect | P | I2(%) | ||
| 16 | 1.082 (1.001,1.169) | 1.048 (1.022,1.074) | 0.000 | 82.7 | |
| The way of information collection | |||||
| questionnaire | 3 | 0.711 (0.450,1.124) | 0.933 (0.852,1.022) | 0.027 | 72.3 |
| database | 11 | 1.134 (1.037,1.241) | 1.058 (1.030,1.086) | 0.000 | 86.1 |
| both questionnaire and database | 1 | 1.120 (0.930,1.349) | 1.120 (0.930,1.350) | – | – |
| telephone interview | 1 | 0.990 (0.805,1.218) | 0.990 (0.805,1.219) | – | – |
| Study design | |||||
| Case-control studies | 4 | 0.877 (1.001,1.169) | 1.071 (0.929,1.234) | 0.000 | 83.3 |
| Cohort studies | 12 | 1.094 (1.011,1.183) | 1.047 (1.021,1.074) | 0.000 | 83.9 |
| Endometriosis assessment | |||||
| Self-report | 4 | 0.859 (0.642,1.149) | 0.989 (0.868,1.128) | 0.018 | 70.3 |
| surgery | 3 | 1.030 (0.925,1.148) | 0.996 (0.957,1.037) | 0.022 | 73.7 |
| ICD | 7 | 1.206 (1.000,1.455) | 1.127 (1.079,1.178) | 0.000 | 88.1 |
| Other | 2 | 1.040 (0.991,1.092) | 1.040 (0.991,1.092) | 0.341 | 0.0 |
| Ascertainment of breast cancer | |||||
| Histopathology reports | 2 | 0.547 (0.365,0.821) | 0.547 (0.365,0.821) | 0.632 | 0.0 |
| ICD | 11 | 1.134 (1.037,1.241) | 1.058 (1.030,1.086) | 0.000 | 86.1 |
| Other | 3 | 0.992 (0.915,1.076) | 0.990 (0.916,1.069) | 0.349 | 5.0 |
| NOS score | |||||
| <7 | 9 | 1.051 (0.991,1.114) | 1.026 (0.999,1.055) | 0.006 | 63.0 |
| ≥ 7 | 7 | 1.052 (0.836,1.327) | 1.159 (1.091,1.230) | 0.000 | 88.4 |
| Adjustment for age | |||||
| Yes | 11 | 1.060 (0.924,1.217) | 1.083 (1.042,1.125) | 0.000 | 85.4 |
| No | 5 | 1.055 (0.977,1.138) | 1.024 (0.991,1.058) | 0.010 | 69.7 |
| Adjustment for BMI | |||||
| Yes | 4 | 0.860 (0.696,1.164) | 0.942 (0.867,1.024) | 0.058 | 59.9 |
| No | 12 | 1.133 (1.041,1.233) | 1.059 (1.032,1.087) | 0.000 | 84.8 |
| Adjustment for oral contraceptive use history | |||||
| Yes | 3 | 0.968 (0.890,1.053) | 1.968 (0.890,1.053) | 0.834 | 0.000 |
| No | 13 | 1.101 (1.008,1.203) | 1.056 (1.029,1.084) | 0.000 | 85.5 |
| Adjustment for pregnancies | |||||
| Yes | 3 | 1.004 (0.959,1.051) | 1.004 (0.959,1.052) | 0.539 | 0.000 |
| No | 13 | 1.108 (0.998,1.231) | 1.067 (1.036,1.099) | 0.000 | 85.1 |
| Adjustment for smoking | |||||
| Yes | 3 | 0.711 (0.450,1.124) | 0.933 (0.852,1.022) | 0.027 | 72.3 |
| No | 13 | 1.122 (1.035,1.216) | 1.058 (1.031,1.085) | 0.000 | 83.5 |
| Adjustment for family history of breast cancer | |||||
| Yes | 4 | 0.860 (0.696,1.064) | 0.942 (0.867,1.024) | 0.058 | 59.9 |
| No | 12 | 1.133 (1.041,1.233) | 1.059 (1.032,1.087) | 0.000 | 84.8 |
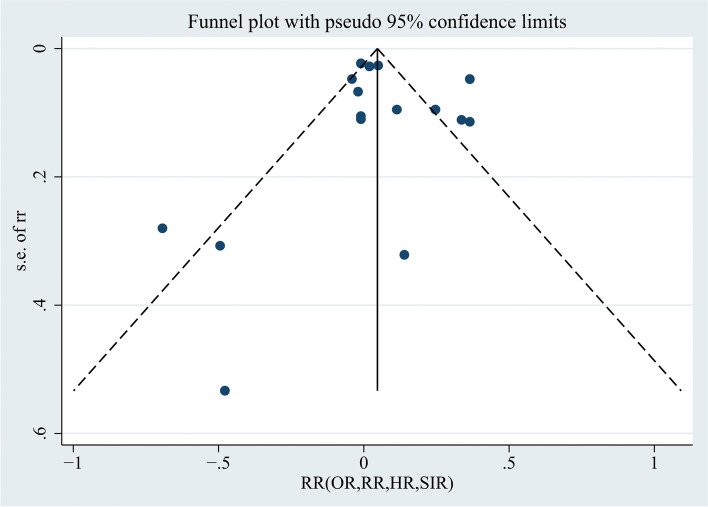
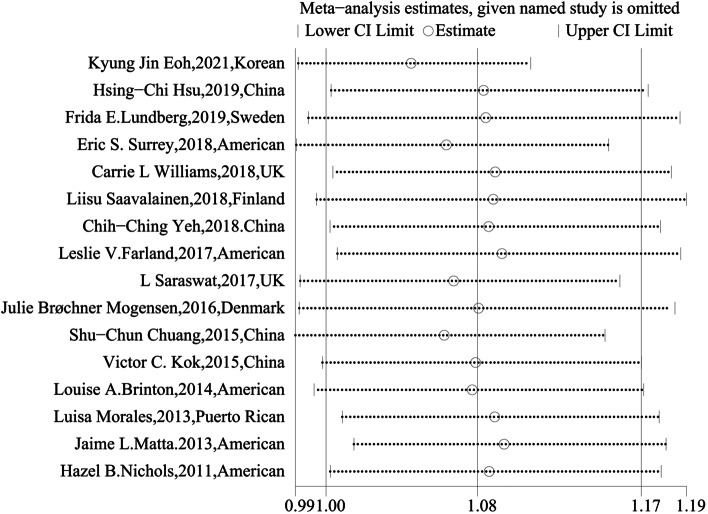
In Fig. 6, the funnel plot was visually symmetric, and the p values for Begg’s and Egger’s tests were p = 0.499 (> 0.05) and p = 0.698 (> 0.05), respectively, suggesting that there was no publication bias. In the leave-one-out sensitivity analysis, no significant changes were observed (Fig. 7), suggesting that this meta-analysis is stable.
Fig. 6.
Funnel plot using data from the 16 studies evaluating the association between endometriosis and breast cancer
Fig. 7.
Sensitivity analysis of the 16 studies evaluating the association between endometriosis and breast cancer
Discussion
Many studies have attempted to find an association between endometriosis and endometrial cancer or breast cancer, but the results are still controversial among different studies [13, 16, 26]. After excluding a low-quality study in which the NOS score was < 4, the NOS scores of the remaining studies ranged from 4 to 8, indicating moderate quality. Our meta-analysis showed that endometriosis can increase the risk of endometrial cancer and that this increase is statistically significant [RR, 1.662; 95% CI, (1.148–2.407)]; a slightly increased risk can also be found in breast cancer [RR, 1.082; 95% CI, (1.001–1.169)]. For patients with endometriosis, besides medication and laparoscopic surgery used to relieve symptoms and remove the lesion, long-term management is crucial [43]. For people with high risks of breast cancer, breast ultrasound (< 40 years) and mammograms (> 40 years) are recommended to screen for breast cancer, while regarding endometrial cancer, screening asymptomatic women for this disease is only recommended for those with Lynch syndrome (LS) [44]. In low- and mid-income countries, because of late diagnosis along with unhealthy lifestyles and eating habits, the burden of breast cancer is increasing exponentially; moreover, “no symptoms” and “no awareness of where to be screened” are among the major reasons for poor screening [45, 46]. The results of our study suggest that women with endometriosis have an increased risk of endometrial cancer and breast cancer; therefore, regular screening may be recommended for these individuals through strengthening their long-term management, which may result in the prevent or early detection of endometrial and breast cancer, but the exact method and timing still need further investigation.
Inevitably, there are some limitations in our meta-analysis. First, the between-study heterogeneity was significant in our analysis; we could not eliminate it through subgroup analysis, and some subgroups were limited to too few of the included studies, which may decrease the credibility of our results. Second, the diagnostic procedures for endometriosis in the included studies were different, which should also be taken into consideration in the conclusion. Third, most of the included studies are retrospective studies, as the risk of recall bias is inevitable compared with that of randomized controlled trials, with the former having a lower level of clinical evidence.
Although endometriosis can increase the risk of endometrial cancer and breast cancer, the risk is relatively low. Undifferentiated screening of all patients with endometriosis may result in a waste of medical resources and increase the financial and psychological pressure on such individuals. Therefore, whether we can narrow down the increased risk in populations with endometriosis to limit the necessary screening to specific subgroups. The American Fertility Society (AFS) classification system for EMS, proposed in 1979 and revised in 1985, is commonly used in the clinic. According to its total scores, EMS is classified into 4 stages: 1–5 denotes stage I (minimal lesions); 6–15 denotes stage II (mild lesions); 16–40 denotes stage III (moderate lesions); and 41–150 denotes stage IV (severe endometriosis) [47, 48]. A study showed that CA125 levels were higher in stage IV than in other stages [49]. Although CA125 is commonly regarded as a specific tumor biomarker of ovarian cancer [50], it is also increased in other cancers, including breast cancer and endometrial cancer [51–53]. Therefore, we wondered whether endometriosis with a higher stage also confers a higher risk of endometrial cancer and breast cancer. Unfortunately, none of the studies we included in this meta-analysis analyzed the risk stratified by the AFS stage of endometriosis. Whether screening can improve outcomes for patients, including decreasing morbidity and mortality, still needs further exploration. Knowing this connection may be beneficial to the management of endometriosis in the future.
Like endometrial cancer and breast cancer, endometriosis is also an estrogen-dependent disease. In endometriotic tissue, aromatase stimulated by PGE2 (Prostaglandin E2), which is essential in the compounding of estrogen, is present at high levels, as well as a lack of 17β-HSD (17β-hydroxysteroid-dehydrogenase) type 2, which can convert estradiol (E2) to the less potent estrone (E1), leading to the accumulation of excess estrogen [54]. In addition, progesterone, which can antagonize estrogen-driven growth in the endometrium, can increase the level of progesterone in ectopic endometrium; however, we found a hyporesponsiveness to progesterone and a low expression of progesterone receptor (PR) in ectopic endometrium, which can also be found in the eutopic endometrium [55]. The excess accumulation of estrogen and the accompanying progesterone resistance causes the excess proliferation of ectopic endometrium. Endometrial cancer can be divided into type 1 and type 2. Type 1 endometrial cancer is endometrioid and estrogen-sensitive, which constitutes 80–85% of all endometrial cancers. Type 2 tumors are estrogen-independent and have a poor prognosis. A study that we included in our analysis showed that endometriosis can increase the risk of type 1 endometrial cancer [SIR, 1.54; 95% CI (1.20–1.96)], while the association cannot be found in type 2 tumors [SIR, 1.06; 95% CI (0.28–2.71) ][20]. Breast cancer is the most frequently diagnosed cancer among women, and one of its risk factors is increasing exposure of breast tissue to estrogen [56]. Based on the hormone receptor status, we can divide breast cancer into three categories: estrogen and progesterone receptor-positive (ER+/PR+) breast cancer, ER+/PR- breast cancer, and ER−/BR- breast cancer (ER−/PR+ has been indicated to not be a reproducible subtype). A study we included showed that endometriosis can increase the risk of ER+/PR- breast cancer [aHR, 1.90; 95% CI (1.44–2.50)] while having no association with the other two cancer types [35]. Five estrogen-responsive genes, CYP19A1, EGFR, ESR2, FOS, and IGF1, were found to be modified in human endometriosis, uterine tumor and breast tumor tissues. We can speculate that estrogen may play an important role between endometriosis and the increasing risk of endometrial cancer and breast cancer, but the study that researched the association between endometriosis and specific types of cancer is not sufficient, and the corresponding result may be contingent. Although we have not found an increasing level of E2 in the serum of populations with endometriosis [57], we have detected a local increase in estrogen in breast cancer and endometrial cancer tissue [58, 59]; as an inflammatory condition, endometriosis may promote the accumulation of E2 in local tissue through the action of a series of inflammatory factors such as IL-1, IL-6, IL-8, PGE, etc., and the exact mechanism is worthy of further exploration.
For endometriosis populations, the combined oral contraceptive pill (COCP) is the first-line drug to relieve symptoms and is widely used in adolescents < 16 years of age. COCP is a compound preparation of a certain amount of estrogen and progesterone that can directly act on the endometrium and can simultaneously act on the hypothalamus through negative feedback and inhibit the secretion of gonadotropin-releasing hormone (GnRH). Studies show that COCP can decrease the risk of endometrial cancer, which means that for people with endometriosis, it may protect the endometrium from malignant transformation [60, 61], but for breast cancer, the result is still controversial. Some meta-analyses have shown that COCP has no association with an increased risk of breast cancer [62], but many studies have shown that endometriosis can increase the risk of breast cancer. The differing results may be related to the age and the duration of oral contraceptives, as well as the years elapsed after stopping such treatment [63–65]. Louise ‘s study showed that for patients < 50 years of age, any OC use before age 20 can increase breast cancer events by approximately threefold, but in patients ≥50 years of age with estrogen receptor-positive tumors, previous OC use at any age can significantly decrease the risk of breast cancer events among patients [66], which may account for the increasing risk between endometriosis and breast cancer for some people. However, restricted to the original research, we cannot perform a further stratification study based on these factors. For individuals who have not accepted any treatment or undergone other medical or surgical therapies, no study shows whether there are any differences in the risks of breast cancer or endometrial cancer. Therefore, knowing the association may be important for the choice of time and methods of endometriosis treatment.
Conclusion
Knowing the association between endometriosis and cancer has important public and prevalent clinical implications. Our meta-analysis clearly showed that endometriosis can increase the risk of endometrial cancer and breast cancer, which may be significant for long-term management, but we cannot ignore the between-study heterogeneity, which may influence the credibility of the results of our study. For future research, we should perform further stratification research based on the AFS stage or macrophenotype, restricting the increased risk of cancer to specific populations, which may be more valuable for regular screening.
Supplementary Information
Additional file 1. Supplementary files NO.1 Basic characteristics and quality evaluation.
Additional file 2. Supplementary files NO.2 Search strategy.
Additional file 3. Supplementary files NO.3 Stata analysis.
Acknowledgments
The authors thank all researchers who contributed to this article.
Abbreviations
- EMS
Endometriosis
- SUP
Superficial endometriosis
- OMA
Ovarian endometrioma
- DIE
Deep infiltrating endometriosis
- PGE2
Prostaglandin E2
- 17β-HSD
17β-hydroxysteroid-dehydrogenase
- PR
Progesterone receptor
- COCP
Combined oral contraceptive pill
Authors’ contributions
JTY: Data collection and management, Data analysis, Manuscript writing. HLP: Project development, Data collection and management, Manuscript editing. XH: Data collection and management, Manuscript editing. XRQ: Data collection and management, Manuscript editing. The author(s) read and approved the final manuscript.
Funding
This meta-analysis was performed without any funding support.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiatian Ye and Hongling Peng contributed equally to this work.
References
- 1.Borghese B, Santulli P, Marcellin L, Chapron C. Definition, description, clinicopathological features, pathogenesis and natural history of endometriosis: CNGOF-HAS Endometriosis Guidelines. Gynecol Obstet Fertil Senol. 2018;46:156–167. doi: 10.1016/j.gofs.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Gruber TM, Mechsner S. Pathogenesis of Endometriosis: The Origin of Pain and Subfertility. Cells. 2021;10. 10.3390/cells10061381. [DOI] [PMC free article] [PubMed]
- 3.Saunders PTK, Horne AW. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807–2824. doi: 10.1016/j.cell.2021.04.041. [DOI] [PubMed] [Google Scholar]
- 4.Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, Missmer SA. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51. 10.1016/j.bpobgyn.2018.06.001. [DOI] [PubMed]
- 5.Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers. 2018;4:9. doi: 10.1038/s41572-018-0008-5. [DOI] [PubMed] [Google Scholar]
- 6.Mousa M, Al-Jefout M, Alsafar H, Kirtley S, Lindgren CM, Missmer SA, Becker CM, Zondervan KT, Rahmioglu N. Prevalence of Common Gynecological Conditions in the Middle East: Systematic Review and Meta-Analysis. Front Reprod Health. 2021;3. 10.3389/frph.2021.661360. [DOI] [PMC free article] [PubMed]
- 7.Artemova D, Vishnyakova P, Khashchenko E, Elchaninov A, Sukhikh G, Fatkhudinov T. Endometriosis and Cancer: Exploring the Role of Macrophages. Int J Mol Sci. 2021;22. 10.3390/ijms22105196. [DOI] [PMC free article] [PubMed]
- 8.Mikhaleva LM, Davydov AI, Patsap OI, Mikhaylenko EV, Nikolenko VN, Neganova ME, Klochkov SG, Somasundaram SG, Kirkland CE, Aliev G. Malignant Transformation and Associated Biomarkers of Ovarian Endometriosis: A Narrative Review. Adv Ther. 2020;37:2580–2603. doi: 10.1007/s12325-020-01363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barca D, Liguori Bjosvik L, Edman G, Eliasson UH, Gervino G, Philemark C, Svendson BD. Indoor Radon Concentration and Risk Estimation: the EURA PROJECT. J Human Earth Future. 2021;2:323–333. doi: 10.28991/HEF-2021-02-04-01. [DOI] [Google Scholar]
- 10.Shchegolikhina LV, Minkina AN. An innovative approach to the combination treatment of endometriosis. Vopr Kurortol Fizioter Lech Fiz Kult. 2019;96:32–36. doi: 10.17116/kurort20199606132. [DOI] [PubMed] [Google Scholar]
- 11.Trabert B, Sherman ME, Kannan N, Stanczyk FZ. Progesterone and Breast Cancer. Endocr Rev. 2020;41. 10.1210/endrev/bnz001. [DOI] [PMC free article] [PubMed]
- 12.Bhyan SB, Zhao L, Wee Y, Liu Y, Zhao M. Genetic links between endometriosis and cancers in women. PeerJ. 2019;7:e8135. doi: 10.7717/peerj.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Liu R, Tang S, Feng F, Liu C, Wang L, Zhao W, Zhang T, Yao Y, Wang X, Sun C. Impact of endometriosis on risk of ovarian, endometrial and cervical cancers: a meta-analysis. Arch Gynecol Obstet. 2019;299:35–46. doi: 10.1007/s00404-018-4968-1. [DOI] [PubMed] [Google Scholar]
- 14.Kajiyama H, Suzuki S, Yoshihara M, Tamauchi S, Yoshikawa N, Niimi K, Shibata K, Kikkawa F. Endometriosis and cancer. Free Radic Biol Med. 2019;133:186–192. doi: 10.1016/j.freeradbiomed.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Guidozzi F. Endometriosis-associated cancer. Climacteric. 2021;24:587–592. doi: 10.1080/13697137.2021.1948994. [DOI] [PubMed] [Google Scholar]
- 16.Kvaskoff M, Mahamat-Saleh Y, Farland LV, Shigesi N, Terry KL, Harris HR, Roman H, Becker CM, As-Sanie S, Zondervan KT, et al. Endometriosis and cancer: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:393–420. doi: 10.1093/humupd/dmaa045. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Kim TH, Chung HH, Song YS. Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis. Br J Cancer. 2014;110:1878–1890. doi: 10.1038/bjc.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, Liang Z, Liu X, Zhang Q, Li S. The Association between Endometriosis, Tubal Ligation, Hysterectomy and Epithelial Ovarian Cancer: Meta-Analyses. Int J Environ Res Public Health. 2016;13. 10.3390/ijerph13111138. [DOI] [PMC free article] [PubMed]
- 19.Eoh KJ, Han M, Kim EH, Jung I, Kim YT. Markedly increased risk of malignancies in women with endometriosis. Gynecol Oncol. 2021. 10.1016/j.ygyno.2021.01.019. [DOI] [PubMed]
- 20.Mogensen JB, Kjær SK, Mellemkjær L, Jensen A. Endometriosis and risks for ovarian, endometrial and breast cancers: a nationwide cohort study. Gynecol Oncol. 10.1016/j.ygyno.2016.07.095. [DOI] [PubMed]
- 21.Surrey ES, Soliman AM, Johnson SJ, Davis M, Castelli-Haley J, Snabes MC. Risk of Developing Comorbidities Among Women with Endometriosis: A Retrospective Matched Cohort Study. J Womens Health (Larchmt) 2018;27:1114–1123. doi: 10.1089/jwh.2017.6432. [DOI] [PubMed] [Google Scholar]
- 22.Saraswat L, Ayansina D, Cooper KG, Bhattacharya S, Horne AW, Bhattacharya S. Impact of Endometriosis on Risk of Further Gynaecological Surgery and Cancer: A National Cohort Study. Obstet Gynecol Survey. 2018;73:92–94. doi: 10.1097/01.ogx.0000529864.64356.76. [DOI] [PubMed] [Google Scholar]
- 23.Matta JL, Flores I, Morales LM, Monteiro J, Alvarez-Garriga C, Bayona M. Women with endometriosis have a higher DNA repair capacity and diminished breast cancer risk. Mol Cancer Biol. 2013;1. 10.9777/mcb.2013.10005. [DOI] [PMC free article] [PubMed]
- 24.Morales L, Alvarez-Garriga C, Matta J, Ortiz C, Vergne Y, Vargas W, Acosta H, Ramírez J, Perez-Mayoral J, Bayona M. Factors associated with breast cancer in Puerto Rican women. J Epidemiol Global Health. 2013;3:205–215. doi: 10.1016/j.jegh.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams CL, Jones ME, Swerdlow AJ, Botting BJ, Davies MC, Jacobs I, Bunch KJ, Murphy MFG, Sutcliffe AG. Risks of ovarian, breast, and corpus uteri cancer in women treated with assisted reproductive technology in Great Britain, 1991–2010: data linkage study including 2.2 million person years of observation. BMJ. 2018;362:k2644. doi: 10.1136/bmj.k2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandini S, Lazzeroni M, Peccatori FA, Bendinelli B, Saieva C, Palli D, Masala G, Caini S. The risk of extra-ovarian malignancies among women with endometriosis: A systematic literature review and meta-analysis. Crit Rev Oncol Hematol. 2019;134:72–81. doi: 10.1016/j.critrevonc.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Wells GA, Shea B, O'Connell J. The Newcastle-Ottawa Scale (NOS) for Assessing The Quality of Nonrandomised Studies in Meta-analyses. 2014. [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kok VC, Tsai HJ, Su CF, Lee CK. The Risks for Ovarian, Endometrial, Breast, Colorectal, and Other Cancers in Women With Newly Diagnosed Endometriosis or Adenomyosis: A Population-Based Study. Int J Gynecol Cancer. 2015;25:968–976. doi: 10.1097/IGC.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 30.Brinton LA, Scoccia B, Moghissi KS, Westhoff CL, Niwa S, Ruggieri D, Trabert B, Lamb EJ. Long-term relationship of ovulation-stimulating drugs to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2014;23:584–593. doi: 10.1158/1055-9965.EPI-13-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saavalainen L, Lassus H, But A, Tiitinen A, Harkki P, Gissler M, Heikinheimo O, Pukkala E. A Nationwide Cohort Study on the risk of non-gynecological cancers in women with surgically verified endometriosis. Int J Cancer. 2018;143:2725–2731. doi: 10.1002/ijc.31721. [DOI] [PubMed] [Google Scholar]
- 32.Lundberg FE, Iliadou AN, Rodriguez-Wallberg K, Gemzell-Danielsson K, Johansson ALV. The risk of breast and gynecological cancer in women with a diagnosis of infertility: a nationwide population-based study. Eur J Epidemiol. 2019;34:499–507. doi: 10.1007/s10654-018-0474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu HC, Lin CY, Chang WC, Shen BJ, Chang WP, Chuang CM, Task Force on Carcinogenesis of Endometrial C Increased association between endometriosis and endometrial cancer: a nationwide population-based retrospective cohort study. Int J Gynecol Cancer. 2015;25:447–452. doi: 10.1097/IGC.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeh CC, Su FH, Tzeng CR, Muo CH, Wang WC. Women with adenomyosis are at higher risks of endometrial and thyroid cancers: A population-based historical cohort study. PLoS One. 2018;13:e0194011. doi: 10.1371/journal.pone.0194011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farland LV, Tamimi RM, Eliassen AH, Spiegelman D, Hankinson SE, Chen WY, et al. Laparoscopically confirmed endometriosis and breast cancer in the nurses’ health study II. Obstet Gynecol. 2016;128. 10.1097/AOG.0000000000001684. [DOI] [PMC free article] [PubMed]
- 36.Poole EM, Lin WT, Kvaskoff M, De Vivo I, Terry KL, Missmer SA. Endometriosis and risk of ovarian and endometrial cancers in a large prospective cohort of U.S. nurses. Cancer Causes Control. 2017;28:437–445. doi: 10.1007/s10552-017-0856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu HC, Tseng KY, Wang HC, Sung FC, Ma WF. Risk of endometriosis and subsequent ovary and breast cancers in nurses: a population-based cohort study in Taiwan. Int J Environ Res Public Health. 2019;16. 10.3390/ijerph16183469. [DOI] [PMC free article] [PubMed]
- 38.Nichols HB, Visvanathan K, Newcomb PA, Hampton JM, Egan KM, Titus-Ernstoff L, Trentham-Dietz A. Bilateral oophorectomy in relation to risk of postmenopausal breast cancer: confounding by nonmalignant indications for surgery? Am J Epidemiol. 2011;173:1111–1120. doi: 10.1093/aje/kwq510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen F, Liu Y, Lin L, Zhao M, Chen Q. Association of benign gynaecological diseases and risk of endometrial and ovarian cancers. J Cancer. 2020;11:3186–3191. doi: 10.7150/jca.39626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowlands IJ, Nagle CM, Spurdle AB, Webb PM. Gynecological conditions and the risk of endometrial cancer. Gynecol Oncol. 2011;123:537–541. doi: 10.1016/j.ygyno.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 41.Chuang SC, Wu GJ, Lu YS, Lin CH, Hsiung CA. Associations between medical conditions and breast cancer risk in Asians: a nationwide population-based study in Taiwan. PLOS ONE. 2015;10. 10.1371/journal.pone.0143410. [DOI] [PMC free article] [PubMed]
- 42.Burghaus S, Häberle L, Schrauder MG, Heusinger K, Thiel FC, Hein A, Wachter D, Strehl J, Hartmann A, Ekici AB, et al. Endometriosis as a risk factor for ovarian or endometrial cancer - results of a hospital-based case-control study. BMC Cancer. 2015;15. 10.1186/s12885-015-1821-9. [DOI] [PMC free article] [PubMed]
- 43.(2018) [Chinese consensus on the long term management of endometriosis]. Zhonghua fu chan ke za zhi,53:836–841 doi: 10.3760/cma.j.issn.0529-567x.2018.12.007 [DOI] [PubMed]
- 44.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16–41. doi: 10.1093/annonc/mdv484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvaro LR, Wilner M-L, Beatriz M, Juan MC. Epidemiologic Evidence for Association between a High Dietary Acid Load and the Breast Cancer Risk. SciMedicine J. 2021;3:166–176. doi: 10.28991/SciMedJ-2021-0302-8. [DOI] [Google Scholar]
- 46.Olabode Ebenezer O, Ghadier M, Elizabeth FO, Amira M, Ghada MA-R, Sucheta M, Nabanita C. Practice of Breast Self-Examination and Knowledge of Breast and Cervical Cancer Screening. SciMedicine J. 2021;3:219–229. doi: 10.28991/SciMedJ-2021-0303-3. [DOI] [Google Scholar]
- 47.Zhong Q, Yang F, Chen X, Li J, Zhong C, Chen S. Patterns of Immune Infiltration in Endometriosis and Their Relationship to r-AFS Stages. Front Genet. 2021;12:631715. doi: 10.3389/fgene.2021.631715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultes G. Classification of endometriosis. Wien Med Wochenschr. 1999;149:361–365. [PubMed] [Google Scholar]
- 49.Maiorana A, Cicerone C, Niceta M, Alio L. Evaluation of serum CA 125 levels in patients with pelvic pain related to endometriosis. Int J Biol Markers. 2007;22:200–202. doi: 10.5301/jbm.2008.5555. [DOI] [PubMed] [Google Scholar]
- 50.Dochez V, Caillon H, Vaucel E, Dimet J, Winer N, Ducarme G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12:28. doi: 10.1186/s13048-019-0503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nazmeen A, Maiti S, Mandal K, Roy SK, Ghosh TK, Sinha NK, Mandal K. Better Predictive Value of Cancer Antigen125 (CA125) as Biomarker in Ovary and Breast Tumors and its Correlation with the Histopathological Type/Grade of the Disease. Med Chem. 2017;13:796–804. doi: 10.2174/1573406413666170424155452. [DOI] [PubMed] [Google Scholar]
- 52.Fang C, Cao Y, Liu X, Zeng XT, Li Y. Serum CA125 is a predictive marker for breast cancer outcomes and correlates with molecular subtypes. Oncotarget. 2017;8:63963–63970. doi: 10.18632/oncotarget.19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bian J, Sun X, Li B, Ming L. Clinical Significance of Serum HE4, CA125, CA724, and CA19–9 in Patients With Endometrial Cancer. Technol Cancer Res Treat. 2017;16:435–439. doi: 10.1177/1533034616666644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Worley MJ, Welch WR, Berkowitz RS, Ng S-W. Endometriosis-associated ovarian cancer: a review of pathogenesis. Int J Mol Sci. 2013;14:5367–5379. doi: 10.3390/ijms14035367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anifantaki F, Boutas I, Kalampokas T, Kalampokas E, Sofoudis C, Salakos N. Association of endometriosis and breast cancer: mini review of the literature. Arch Gynecol Obstet. 2016;293:5–10. doi: 10.1007/s00404-015-3809-8. [DOI] [PubMed] [Google Scholar]
- 57.Pedachenko N, Anagnostis P, Shemelko T, Tukhtarian R, Alabbas L. Serum anti-Mullerian hormone, prolactin and estradiol concentrations in infertile women with endometriosis. Gynecol Endocrinol. 2021;37:162–165. doi: 10.1080/09513590.2020.1855634. [DOI] [PubMed] [Google Scholar]
- 58.Zhao H, Zhou L, Shangguan AJ, Bulun SE. Aromatase expression and regulation in breast and endometrial cancer. J Mol Endocrinol. 2016;57:R19–R33. doi: 10.1530/JME-15-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rižner TL. Estrogen biosynthesis, phase I and phase II metabolism, and action in endometrial cancer. Mol Cellular Endocrinol. 2013;381:124–139. doi: 10.1016/j.mce.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 60.Cote ML, Alhajj T, Ruterbusch JJ, Bernstein L, Brinton LA, Blot WJ, Chen C, Gass M, Gaussoin S, Henderson B, et al. Risk factors for endometrial cancer in black and white women: a pooled analysis from the Epidemiology of Endometrial Cancer Consortium (E2C2) Cancer Causes Control. 2015;26:287–296. doi: 10.1007/s10552-014-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali AT. Reproductive factors and the risk of endometrial cancer. Int J Gynecol Cancer. 2014;24:384–393. doi: 10.1097/igc.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 62.Moorman PG, Havrilesky LJ, Gierisch JM, Coeytaux RR, Lowery WJ, Peragallo Urrutia R, Dinan M, McBroom AJ, Hasselblad V, Sanders GD, Myers ER. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188–4198. doi: 10.1200/jco.2013.48.9021. [DOI] [PubMed] [Google Scholar]
- 63.Marquardt RM, Kim TH, Shin JH, Jeong JW. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int J Mol Sci. 2019;20. 10.3390/ijms20153822. [DOI] [PMC free article] [PubMed]
- 64.Bardaweel SK, Akour AA, Al-Muhaissen S, AlSalamat HA, Ammar K. Oral contraceptive and breast cancer: do benefits outweigh the risks? A case - control study from Jordan. BMC Womens Health. 2019;19:72. doi: 10.1186/s12905-019-0770-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Del Pup L, Codacci-Pisanelli G, Peccatori F. Breast cancer risk of hormonal contraception: Counselling considering new evidence. Crit Rev Oncol Hematol. 2019;137:123–130. doi: 10.1016/j.critrevonc.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 66.Huzell L, Persson M, Simonsson M, Markkula A, Ingvar C, Rose C, Jernström H. History of oral contraceptive use in breast cancer patients: impact on prognosis and endocrine treatment response. Breast Cancer Res Treatment. 2015;149:505–515. doi: 10.1007/s10549-014-3252-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary files NO.1 Basic characteristics and quality evaluation.
Additional file 2. Supplementary files NO.2 Search strategy.
Additional file 3. Supplementary files NO.3 Stata analysis.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).