Abstract
Background
This is the third update of the review first published in 2017.
Hypertension is a prominent preventable cause of premature morbidity and mortality. People with hypertension and established cardiovascular disease are at particularly high risk, so reducing blood pressure to below standard targets may be beneficial. This strategy could reduce cardiovascular mortality and morbidity but could also increase adverse events. The optimal blood pressure target in people with hypertension and established cardiovascular disease remains unknown.
Objectives
To determine if lower blood pressure targets (systolic/diastolic 135/85 mmHg or less) are associated with reduction in mortality and morbidity compared with standard blood pressure targets (140 mmHg to 160mmHg/90 mmHg to 100 mmHg or less) in the treatment of people with hypertension and a history of cardiovascular disease (myocardial infarction, angina, stroke, peripheral vascular occlusive disease).
Search methods
For this updated review, we used standard, extensive Cochrane search methods. The latest search date was January 2022. We applied no language restrictions.
Selection criteria
We included randomized controlled trials (RCTs) with more than 50 participants per group that provided at least six months' follow‐up. Trial reports had to present data for at least one primary outcome (total mortality, serious adverse events, total cardiovascular events, cardiovascular mortality). Eligible interventions involved lower targets for systolic/diastolic blood pressure (135/85 mmHg or less) compared with standard targets for blood pressure (140 mmHg to 160 mmHg/90 mmHg to 100 mmHg or less).
Participants were adults with documented hypertension and adults receiving treatment for hypertension with a cardiovascular history for myocardial infarction, stroke, chronic peripheral vascular occlusive disease, or angina pectoris.
Data collection and analysis
We used standard Cochrane methods. We used GRADE to assess the certainty of the evidence.
Main results
We included seven RCTs that involved 9595 participants. Mean follow‐up was 3.7 years (range 1.0 to 4.7 years). Six of seven RCTs provided individual participant data. None of the included studies was blinded to participants or clinicians because of the need to titrate antihypertensive drugs to reach a specific blood pressure goal. However, an independent committee blinded to group allocation assessed clinical events in all trials. Hence, we assessed all trials at high risk of performance bias and low risk of detection bias. We also considered other issues, such as early termination of studies and subgroups of participants not predefined, to downgrade the certainty of the evidence.
We found there is probably little to no difference in total mortality (risk ratio (RR) 1.05, 95% confidence interval (CI) 0.91 to 1.23; 7 studies, 9595 participants; moderate‐certainty evidence) or cardiovascular mortality (RR 1.03, 95% CI 0.82 to 1.29; 6 studies, 9484 participants; moderate‐certainty evidence). Similarly, we found there may be little to no differences in serious adverse events (RR 1.01, 95% CI 0.94 to 1.08; 7 studies, 9595 participants; low‐certainty evidence) or total cardiovascular events (including myocardial infarction, stroke, sudden death, hospitalization, or death from congestive heart failure (CHF)) (RR 0.89, 95% CI 0.80 to 1.00; 7 studies, 9595 participants; low‐certainty evidence). The evidence was very uncertain about withdrawals due to adverse effects. However, studies suggest more participants may withdraw due to adverse effects in the lower target group (RR 8.16, 95% CI 2.06 to 32.28; 3 studies, 801 participants; very low‐certainty evidence). Systolic and diastolic blood pressure readings were lower in the lower target group (systolic: mean difference (MD) –8.77 mmHg, 95% CI –12.82 to –4.73; 7 studies, 8657 participants; diastolic: MD –4.50 mmHg, 95% CI –6.35 to –2.65; 6 studies, 8546 participants). More drugs were needed in the lower target group (MD 0.56, 95% CI 0.16 to 0.96; 5 studies, 7910 participants), but blood pressure targets at one year were achieved more frequently in the standard target group (RR 1.20, 95% CI 1.17 to 1.23; 7 studies, 8699 participants).
Authors' conclusions
We found there is probably little to no difference in total mortality and cardiovascular mortality between people with hypertension and cardiovascular disease treated to a lower compared to a standard blood pressure target. There may also be little to no difference in serious adverse events or total cardiovascular events. This suggests that no net health benefit is derived from a lower systolic blood pressure target. We found very limited evidence on withdrawals due to adverse effects, which led to high uncertainty. At present, evidence is insufficient to justify lower blood pressure targets (135/85 mmHg or less) in people with hypertension and established cardiovascular disease. Several trials are still ongoing, which may provide an important input to this topic in the near future.
Keywords: Adult, Humans, Blood Pressure, Cardiovascular Diseases, Hypertension, Hypertension/complications, Hypotension, Myocardial Infarction, Stroke, Stroke/complications
Plain language summary
Blood pressure targets in people with cardiovascular disease
Key messages
The evidence identified in this review does not support lower blood pressure goals over standard goals in people with high blood pressure (also known as hypertension) and heart or vascular (blood vessels and circulatory system) problems
More new trials are needed to examine this question
What is high blood pressure?
Hypertension (high blood pressure) is a long‐term condition that increases the risk of health problems such as heart attack, stroke, or kidney disease.
How is high blood pressure treated?
Many people with heart or vascular problems also have high blood pressure. Some clinical guidelines recommend a lower blood pressure goal (135/85 mmHg or lower) for people with high blood pressure and previous heart or vascular problems than for with those without (140 mmHg to 160 mmHg or less systolic (pressure when heart pumps blood around the body) and 90 mmHg to 100 mmHg diastolic or less (pressure when heart rests between beats) are standard blood pressure goals). It is unclear whether lower goals lead to overall health benefits.
What did we want to find out?
We wanted to find out if lower blood pressure goals are better than standard blood pressure goals for people with high blood pressure who also have heart or vascular problems.
What did we do?
We searched for studies that compared lower blood pressure targets to standard blood pressure targets in people with high blood pressure and a history of cardiovascular disease (heart disease, angina, stroke, vascular disease). Studies had to talk about results such as deaths or other events caused by diseases of the heart or the blood vessels, such as heart attack, stroke, or heart failure. Studies could also talk about other types of health‐related side effects. We only chose randomized studies (where people were randomly put into one of two or more treatment groups) with 50 or more people in each group and that lasted at least six months.
What did we find?
In this update, we found one new study giving a total of seven studies with 9595 people included in the review. We found little to no difference in total numbers of deaths, or heart or vascular deaths between lower and standard blood pressure goals. There was also little to no difference for the total number of heart or vascular problems and total serious harms, but the evidence was less certain.
What are the limitations of the evidence?
Based on uncertainty and limited information, we found more people dropped out of the trials because of medicine‐related harms in the lower blood pressure target group and no overall health benefit among people in the lower target group.
How up to date is this evidence?
This is the third update of a review first published in 2017. The evidence is up to date to January 2022.
Summary of findings
Summary of findings 1. Lower blood pressure targets compared with standard blood pressure targets for mortality and morbidity.
| Lower blood pressure targets compared with standard blood pressure targets for mortality and morbidity | ||||||
| Patient or population: cardiovascular disease with high blood pressure Setting: outpatients (mean duration of trials 4 years) Intervention: lower blood pressure targets (≤ 135/85 mmHg) Comparison: standard blood pressure targets (≤ 140–160/90–100 mmHg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | certainty of the evidence (GRADE) | Comments | |
| Risk with standard blood pressure target | Risk with lower blood pressure target | |||||
|
Total mortality Follow‐up: mean 1–4.7 years |
Study population | RR 1.05 (0.91 to 1.23) | 9595 (7 RCTs) | ⊕⊕⊕⊝ Moderatea | — | |
| 68 per 1000 | 71 per 1000 (62 to 84) | |||||
|
Total serious adverse events Follow‐up: mean 1 to 4.7 years |
Study population | RR 1.01 (0.94 to 1.08) | 9595 (7 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | |
| 252 per 1000 | 255 per 1000 (237 to 272) | |||||
|
Total cardiovascular events Follow‐up: mean 1–4.7 years |
Study population | RR 0.89 (0.80 to 1.00) | 9595 (7 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | |
| 127 per 1000 | 113 per 1000 (102 to 127) | |||||
|
Cardiovascular mortality Follow‐up: mean 1–4.7 years |
Study population |
RR 1.03 (0.82 to 1.29) |
9484 (6 RCTs) |
⊕⊕⊕⊝ Moderatea | — | |
| 31 per 1000 | 32 per 1000 (25 to 40) |
|||||
|
Participant withdrawals due to adverse effects Follow‐up: mean 1–3.8 years |
Study population | RR 8.16 (2.06 to 32.28) | 801 (3 RCT) | ⊕⊝⊝⊝ Very lowb,c | — | |
| 7 per 1000 | 60 per 1000 (15 to 239) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level owing to serious imprecision (95% CI is wider than the minimal important difference). bDowngraded one level owing to high risk of bias. cDowngraded two levels owing to very serious imprecision (only two of the smaller studies reported this outcome).
Background
Description of the condition
Hypertension (high blood pressure) is one of the most preventable causes of premature morbidity and mortality worldwide. Hypertension was the leading risk factor for the global burden of disease in 2017 (GBD 2017 Risk factors). Hypertension is a major risk factor for stroke, myocardial infarction, heart failure, chronic kidney disease, cognitive decline, and premature death (NICE 2022).
Historically more emphasis was placed on diastolic blood pressure (DBP) than on systolic blood pressure (SBP) as a predictor of cardiovascular morbidity and fatal events. However, numerous observational studies have revealed that both SBP and DBP show a graded independent relationship with mortality and morbidity (ESH‐ESC 2007). Untreated hypertension may be associated with a progressive rise in blood pressure, possibly culminating in a treatment‐resistant state caused by associated vascular and kidney damage (NICE 2022).
Epidemiological studies suggest that the risk associated with high blood pressure is a continuous relationship, and for blood pressures above 115/70 mmHg, the risk of cardiovascular events doubles for every 20/10 mmHg (SBP/DBP) rise in blood pressure. This suggests that for every 20 mmHg lower SBP or 10 mmHg lower DBP, the risk of a cardiovascular event is reduced by about 50% (Lewington 2002).
Blood pressure is normally distributed within a population, and there is no natural cut‐off point above which hypertension definitively exists and below which it does not. In any individual person, SBP or DBP (or both) may be elevated. DBP is more commonly elevated among people younger than 50 years. With ageing, systolic hypertension becomes a more significant problem as a result of progressive stiffening and loss of compliance of larger arteries (NICE 2022).
Cardiovascular disease (CVD) remains the leading cause of death worldwide (Townsend 2016). CVD accounts for more deaths than all communicable, neonatal, maternal, and nutritional disorders combined, and almost double the number of deaths caused by cancers. Globally, CVD accounts for nearly 17 million deaths annually – more than one‐third of the total number of deaths. Despite this, between 2007 and 2017, age‐standardized death rates fell by 10% for cardiovascular and circulatory diseases (GBD 2017 Mortality). Ischaemic heart disease (IHD) was the leading global cause of years of life lost (YLLs), having increased by 17.3% from 2007 to 2017. Similarly, stroke ranked third and increased its mean percentage change number of YLLs by 12.1% from 2007 to 2017 (GBD 2017 Risk factors).
Thus, cardiovascular secondary prevention is considered a key issue. People who have had atherosclerotic stroke should be included among those deemed at high risk of further atherosclerotic coronary events (20% over 10 years). A significant percentage of those who have a first myocardial infarction are expected to experience recurrent myocardial infarction, heart failure, stroke, or fatal coronary heart disease (CHD). In fact, within five years of a first myocardial infarction, around 20% to 30% of the population aged over 65 years will experience recurrent myocardial infarction or fatal CHD (Mozaffarian 2015).
Description of the intervention
Clinicians use target blood pressures in clinical practice to make treatment decisions related to the intensity of antihypertensive therapy for each patient.
The standard blood pressure target has generally been an arbitrary threshold blood pressure above which treatment is recommended. Over time, this threshold has become lower. The standard SBP target declined from a target of 160 mmHg or less to 140 mmHg or less, and the DBP target decreased from 100 mmHg or less to 90 mmHg or less in people aged up to 80 years (ESH‐ESC 2007). Even lower blood pressure targets have been proposed for people with a history of cardiovascular events (AHA 2007; ESH‐ESC 2007; JNC‐7 2003).
Years later, a review of available evidence led to a reappraisal of some recommendations made by international guidelines, particularly among older people and people with diabetes or previous CVD (ESH‐ESC 2013; JNC‐8 2014; ESC 2016). However, the last updates of some US and European guidelines have turned again to recommend more intensive goals (ACC‐AHA 2017; ESH‐ESC 2018).
How the intervention might work
Some evidence suggests that for people at high risk, thresholds for antihypertensive treatment should be lower than for those at lower risk. It has also been suggested that to maximize the cost‐effectiveness of hypertension management, the intensity of the therapeutic approach should be graded as a function of total cardiovascular risk (ESH‐ESC 2007). However, we noted a trend towards homogenizing blood pressure goals (ACC‐AHA 2017; NICE 2022).
People with a history of CVD are considered to represent a high‐risk population. The effect of lowering blood pressure values in these people could include greater absolute reduction in morbidity and mortality but could also be associated with an absolute increase in adverse events.
Reducing blood pressure to below standard targets through drug therapy has been recommended in guidelines as a strategy for people with a history of CVD. Nevertheless, lower may not always be better. Researchers have described a J‐curve for blood pressure in coronary artery disease (Bangalore 2010; Messerli 2006). Bangalore 2010 reported that for people with coronary artery disease, low blood pressure (less than 110 mmHg to 120 mmHg/60 mmHg to 70 mmHg) was associated with increased risk of future cardiovascular events.
One cohort study explored the association between achieved blood pressure and cardiovascular events in people with hypertension and a history of coronary disease. These investigators concluded that when a goal less than 120/70 mmHg was reached, an association with more cardiovascular adverse events was detected, supporting the J‐curve hypothesis (Vidal‐Petiot 2016).
Uncertainty remains regarding many aspects of this controversial topic, leading to differing opinions (Carey 2020; Kaul 2020; Mancia 2014; Verdecchia 2014).
Why it is important to do this review
The arterial pressure threshold above which benefits of treatment outweigh harms in people with hypertension and CVD is unclear.
Some, but not all, clinical guidelines have recommended blood pressure targets lower than standard targets. Following are recommendations for blood pressure targets in people with hypertension and CVD as stated in recently published guidelines.
The Joint National Committee‐7 Report recommended blood pressure targets less than 140/90 mmHg for people with uncomplicated hypertension, and blood pressure targets less than 130/80 mmHg for people with hypertension and either diabetes or kidney disease (JNC‐7 2003). However, an updated statement in 2014 reflects some changes in the goals policy (JNC‐8 2014). JNC‐8 2014 suggests treating to goals of SBP less than 150 mmHg and DBP less than 90 mmHg in the general population aged 60 years and older. In the general population aged up to 60 years, the guideline maintains the recommendation of treating to goals of SBP less than 140 mmHg and DBP less than 90 mmHg. In people with diabetes or kidney disease, new targets are similar to those for the general population. JNC‐8 2014 provides no direct recommendation for people with previous CVD, although this is acknowledged as a relevant question to be assessed and answered. The latest US guideline recommends a blood pressure target less than 130/80 mmHg for adults with confirmed hypertension and known CVD (ACC‐AHA 2017). WHO 2021 also recommends a target SBP treatment goal of less than 130 mmHg in people with hypertension and known CVD.
The 2007 European Society of Hypertension and European Society of Cardiovascular Guidelines for Management of Arterial Hypertension recommended that blood pressure should be reduced to less than 140/90 mmHg and to lower values, if tolerated, in all people with hypertension (ESH‐ESC 2007). The blood pressure goal was less than 130/80 mmHg for people with diabetes and others at high risk, such as people with associated clinical conditions (stroke, myocardial infarction, kidney dysfunction, proteinuria). Reappraisal of European guidelines on hypertension management remarks that the recommendation to lower blood pressure to 130/80 mmHg or less for people with diabetes or a history of CVD is not supported by incontrovertible trial evidence (ESH 2009). The most recent update proposed an SBP goal of 120 mmHg to 129 mmHg for people younger than 65 years at low to moderate cardiovascular risk or with diabetes, and a goal of 130 mmHg to 139 mmHg, if tolerated, for older people at any level of cardiovascular risk and in people with and without established CVD. A DBP target of less than 80 mmHg is always recommended (ESH‐ESC 2018).
The 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice indicated that evidence was sufficient to recommend a blood pressure target less than 140/90 mmHg for all people who were hypertensive (except older people, for whom the benefit has not been tested in randomized trials) (ESC 2016). Nonetheless, its last update has become less conservative, recommending an SBP of 120 mmHg to 130 mmHg for secondary cardiovascular prevention in people aged less than 70 years and DBP less than 80 mmHg for all people receiving treatment (ESC 2022).
In its Recommendations for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension, the 2015 Canadian Hypertension Education Program made a proposal to reach blood pressure targets less than 140/90 mmHg in most situations, including for people with previous CVD (CHEP 2015). Nevertheless, the latest update of this guideline is prone to an intensive intervention in some people with high cardiovascular risk, including those with CVD (Hypertension CANADA 2020). Specifically, the guideline calls for consideration of a less than 120 mmHg target, taking into account the SPRINT (Systolic Blood Pressure Intervention Trial) results (SPRINT 2015).
One Cochrane Review found that treating hypertension to lower than the standard blood pressure target of 140 mmHg to 160 mmHg/90 mmHg to 100 mmHg or less was not proven to reduce mortality or total serious adverse events in the overall population, showing that benefits of trying to achieve a lower blood pressure target did not outweigh the harms associated with that intervention (Arguedas 2020). Another Cochrane Review analyzing the same question in people with diabetes found a reduction in the incidence of stroke with the lower goal but a significant increase in the number of serious adverse events (Arguedas 2013).
Two non‐Cochrane Reviews on this issue have also been published (Ettehad 2016; Xie 2016). Ettehad 2016 combined data from all relevant clinical trials published on blood pressure reduction. The authors estimated effects of a blood pressure decrease in terms of mortality or cardiovascular morbidity, and according to different basal characteristics, such as established CVD. There was a decrease in mortality and other cardiovascular events as blood pressure was reduced. The review found inconsistent results on safety issues. One meta‐analysis has also claimed beneficial effects with intensive targets on major cardiovascular events (BPLTTC 2021). Xie 2016 focused on the efficacy and safety of a blood pressure decrease for intensive strategies, including clinical trials with at least six months' follow‐up that randomized participants to more‐intensive versus less‐intensive blood pressure targets, different blood pressure targets, or different blood pressure changes from baseline. Participants in the more‐intensive group showed decreased risk in terms of less ictus and fewer relevant cardiovascular events.
Several guidelines that directly focus on the main objective of this Cochrane Review – cardiovascular secondary prevention – have been published. The 2007 guidelines for Treatment of Hypertension in the Prevention and Management of Ischemic Heart Disease from the American Heart Association recommended blood pressure targets less than 130/80 mmHg for people with demonstrated coronary artery disease or risk equivalents (carotid artery disease, peripheral arterial disease, abdominal aortic aneurysm) and for high‐risk people (AHA 2007). Subsequently, when performance measures based on these recommendations were proposed, limitations were admitted because of lack of clinical trials that directly compared clinical outcomes of large populations of people with coronary disease randomized to different blood pressure targets (Drozda 2011). The 2015 update of this guideline concluded that blood pressure less than 140/90 mmHg would seem a reasonable target for the secondary prevention of cardiovascular events in people with hypertension and coronary artery disease. Conversely, with less‐supportive evidence, a lower blood pressure target (less than 130/80 mmHg) could be appropriate for some people with coronary artery disease, previous myocardial infarction, stroke, or coronary artery disease equivalents (carotid artery disease, peripheral artery disease, abdominal aortic aneurysm) (Rosendorff 2015).
Limited data specifically assess the optimal blood pressure target in relation to secondary stroke prevention. American guidelines note that goals for target blood pressure level or reduction from pretreatment baseline are uncertain and should be individualized (Kernan 2014). For people who have had a recent lacunar stroke, an SBP less than 130 mmHg is accepted as reasonable; for people who have had other types of stroke, less than 140/90 mmHg is recommended.
Lowering blood pressure too much may cause adverse cardiovascular events (Filippone 2011). Some observations have suggested that excessive lowering of blood pressure through drug treatment is associated with an increased number of deaths due to CHD (Farnett 1991), particularly among people with coronary artery disease (Bangalore 2010; Messerli 2006). Given that controversy over a potential J‐curve phenomenon continues (Auer 2018; Mancia 2014; Verdecchia 2014), additional studies are expected to clarify the dilemma.
Therefore, at present, the optimal blood pressure target for reducing morbidity and mortality in people with hypertension and history of CVD is unknown. This review aimed to establish if a stricter blood pressure target should be recommended for these people.
Objectives
To determine if lower blood pressure targets (systolic/diastolic 135/85 mmHg or less) are associated with reduction in mortality and morbidity as compared with standard blood pressure targets (140 mmHg to 160 mmHg/90 mmHg to 100 mmHg or less) in the treatment of people with hypertension and a history of cardiovascular disease (myocardial infarction, angina, stroke, peripheral vascular occlusive disease).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) with more than 50 participants per group and at least six months' follow‐up. In addition, 70% or more of participants had to meet all cited criteria in the Types of participants section. Studies could also be included if individual participant data were available, or if data from relevant participants were provided separately, enabling specific inclusion of this population as defined. Blinding was not possible. To be eligible for inclusion, trial reports had to present data for at least one primary outcome.
We excluded trials that used anything other than accepted randomized allocation methods such as alternate allocation, week of presentation, or retrospective controls. We placed no restrictions on publication language.
Types of participants
Participants had to be at least 18 years of age with hypertension documented in a standard way, or had to be receiving treatment for hypertension, with a positive cardiovascular history of myocardial infarction, stroke (not including transient ischaemic attack (TIA)), chronic peripheral vascular occlusive disease, or angina pectoris.
Trials were not limited by any other factor or by baseline risk.
Types of interventions
Intervention: lower blood pressure treatment target: systolic/diastolic 135/85 mmHg or less; mean blood pressure (MBP) 102 mmHg or less.
Control: standard blood pressure treatment target: systolic/diastolic 140 mmHg to 160 mmHg/90 mmHg to 100 mmHg or less; MBP 107 mmHg to 120 mmHg or less.
MBP was accepted as a valid way of measuring interventions, while prespecified targets are taken into account and according to the following equation: MBP = [(2 × DBP) + SBP]/3.
Types of outcome measures
All primary and secondary outcomes were measured at longest reported follow‐up (except when other period was indicated) in clinical trials with a minimum follow‐up of six months.
Primary outcomes
Total mortality.
Total serious adverse events.
Total cardiovascular events including myocardial infarction, stroke, sudden death, hospitalization or death from CHF, and other significant vascular events such as ruptured aneurysms (excluding angina, TIA, surgical or other procedures, or accelerated hypertension). In practice, this was measured as total number of participants with at least one cardiovascular event, including fatal and non‐fatal cardiovascular events.
Cardiovascular mortality.
We defined serious adverse events according to the International Conference on Harmonisation Guidelines as any event that led to death, was life‐threatening, required hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability, or was a congenital anomaly or birth defect (ICH 1995).
If a study used a different definition for serious adverse events, review authors resolved this inclusion of data by consensus.
We included all four primary outcomes in the summary of findings table.
Secondary outcomes
Participant withdrawals due to adverse effects.
SBP and the difference from baseline at one year, or both.
DBP and the difference from baseline at one year, or both.
Proportion of participants reaching the target blood pressure level.
Number of antihypertensive drugs that each participant needed at the end of the study.
We considered participant withdrawals due to adverse effects to be an important outcome and included these data in the summary of findings table.
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist conducted systematic searches of the following databases for RCTs without language, publication year, or publication status restrictions.
Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (to 2 February 2022);
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 12) via the Cochrane Register of Studies (to 26 January 2022);
MEDLINE Ovid (1946 to 25 January 2022);
Embase Ovid (1974 to 25 January 2022);
Latin American and Caribbean Health Sciences Literature (LILACS) Bireme (1982 to 27 January 2022);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov, to 27 January 2022);
World Health Organization International Clinical Trials Registry Platform (trialsearch.who.int, to 27 January 2022).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. When appropriate, subject strategies were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs (as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). We have provided search strategies for databases in Appendix 1. We did not apply a language restriction to the database searches.
Searching other resources
The Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE, Embase, and Epistemonikos for systematic reviews) to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
When necessary, we contacted the authors of key papers and abstracts to request additional information about their trials.
We attempted to identify additional trials by searching the reference lists of included trials and (systematic) reviews, meta‐analyses, and health technology assessment reports (Appendix 2). We contacted authors of trials reporting incomplete information to request the missing information.
Duplicate publications
When we identified more than one publication of an original trial, we assessed these articles together to maximize data collection. We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. In the case of substantial disagreements between articles, we contacted study authors.
References from published studies
We examined the references of included and excluded studies to identify further references linked to potentially eligible RCTs.
Language
We applied no language restrictions. We translated any study not published in English, French, or Spanish.
Correspondence
We contacted trial investigators to request data from subgroups of participants with CVD or missing data, or to clarify study details.
Data collection and analysis
Pairs of review authors independently assessed search results. One review author (LCS) reviewed all results. We used Early Review Organizing Software version 2.0 (www.eros-systematic-review.org) and Covidence (www.covidence.org) when screening and classifying references.
Selection of studies
Two review authors independently carried out the selection of papers, excluding records when title, keywords, and abstract showed that they were not RCTs, groups had fewer than 50 participants, follow‐up was less than six months, no review primary outcomes were addressed, participants did not match prespecified criteria, blood pressure targets were not the only intervention, or specific targets were different from those prespecified. We obtained the full text of all remaining articles considered for inclusion and excluded these if inclusion criteria were not met. We obtained the full text of papers that could not be assessed by information presented in the abstract. We provisionally included studies that were likely to include subgroups of participants who met our criteria, and we contacted study authors to request data for those subgroups.
We resolved discrepancies by discussion or by consultation with a third review author, if necessary. When we considered an issue to be a highly significant point, we scheduled a plenary discussion.
We constructed a PRISMA flow diagram depicting the study selection process (Figure 1).
1.
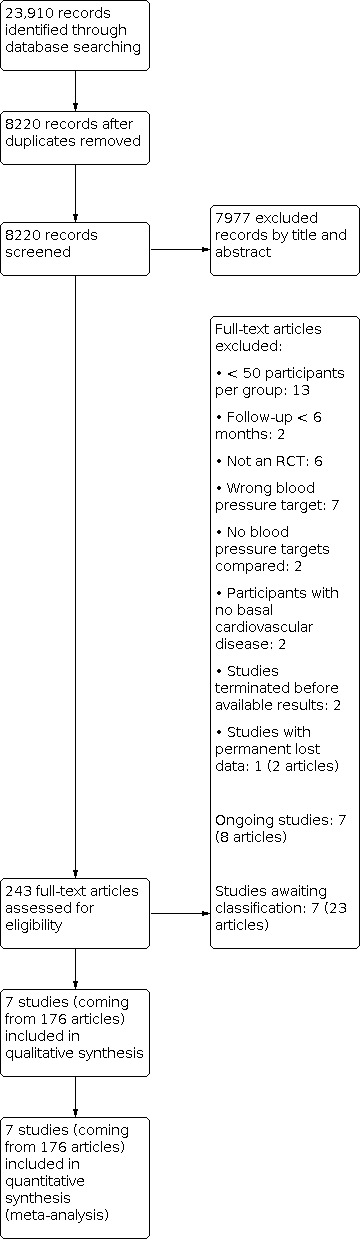
PRISMA flow diagram. RCT: randomized controlled trial.
Data extraction and management
Two review authors independently extracted data from included trials using a previously prepared data extraction form, including basic information, verification of study eligibility, assessment of risk of bias, baseline study characteristics, results in outcomes, and subgroup analyses. Another review author cross‐checked extracted data.
We resolved differences between review authors by discussion and by involvement of a third review author, when necessary. We used Review Manager 2014 for data analyses. We based quantitative analyses of outcomes on the intention‐to‐treat principle.
We used Microsoft Access and Microsoft Excel when organizing and analyzing individual participant data.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each study using the six domains of the Cochrane RoB 1 tool, according to the method described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). We resolved any differences in opinion by discussion among all review authors.
We tried to find study protocols for comparison with published study reports.
Review authors reported the overall risk of bias for all included studies according to the following.
Low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria were met.
Unclear risk of bias (plausible bias that raised some doubt about the results) if we assessed one or more criteria as unclear.
High risk of bias (plausible bias that seriously weakened confidence in the results) if one or more criteria were not met.
We performed sensitivity analyses excluding trials with high or unclear risk of bias.
Measures of treatment effect
We used Review Manager 2014 for analyses. We based quantitative analyses of outcomes on intention‐to‐treat results. We used risk ratios (RRs) and a fixed‐effect model, if appropriate, to combine dichotomous outcomes across trials. We calculated absolute risk reduction (ARR) or absolute risk increase (ARI) and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) for total mortality, total serious adverse events, and total cardiovascular events. We estimated 95% confidence intervals (CIs). We recorded combined outcomes and analyzed participants with at least one event in the outcome.
We combined data for blood pressure reached and the difference from baseline using mean difference (MD). This combines weight based on the number of participants in the trial and within‐study variance. If the trial did not report within‐study variance for decrease in blood pressure, we imputed the standard deviation (SD) from the mean SD provided by other trials. This imputation is a limitation, and to overcome it, we reported the 99% CI instead of the standard 95% CI as reported for all other data. We carried out sensitivity analyses to assess the impact of changing the assumptions made.
Unit of analysis issues
We based the analysis of outcomes on randomized participants, but if cluster‐randomized trials were included, we planned to conduct appropriate analyses. We have taken special care to identify if data presented signified the total number of events or the total number of participants with a first event. We contacted study authors for clarification when necessary.
We selected data for the longest follow‐up of the trial.
Dealing with missing data
We contacted study authors to obtain additional information not provided in published articles.
Assessment of heterogeneity
We used Chi² and I² statistics to test for heterogeneity of treatment effect among trials. We consider a Chi² P < 0.05 or I² statistic > 50% as indicative of heterogeneity. We used a random‐effects model to test for statistical significance when there was significant heterogeneity and 'random' distribution of intervention effects could be justified.
We planned to investigate reasons for data showing more than moderate heterogeneity (I² > 60%). If we could not identify sources of heterogeneity, we excluded studies from meta‐analysis.
Assessment of reporting biases
We planned to construct a funnel plot to test for asymmetry if we included 10 or more studies in the meta‐analysis.
Data synthesis
Two review authors analyzed data using Review Manager 2014 and reported data in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). If meta‐analysis was not appropriate, we planned to provide a narrative description of the results.
Subgroup analysis and investigation of heterogeneity
If possible, we planned subgroup analysis for:
participants with diabetes;
men and women; and
people aged 75 years or older.
We aimed to investigate clinical heterogeneity by examining differences in achieved blood pressure among trials, trial duration, different interventions used for hypertension, and history of stroke or CHD as inclusion criteria.
Sensitivity analysis
We tested the robustness of results using several sensitivity analyses including:
risk of bias of trials; and
industry‐sponsored versus non‐industry‐sponsored trials.
We also tested the robustness of results by repeating the analysis using different measures of effect size (e.g. odds ratio) and different statistical models.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table using the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions, along with GRADEpro GDT software (gradepro.org). The outcomes included:
total mortality;
total serious adverse events;
total cardiovascular events;
cardiovascular mortality;
participant withdrawals due to adverse effects.
We used all six GRADE domains (risk of bias, consistency of effect, imprecision, indirectness, publication bias, and other aspects) to assess certainty of evidence as it related to the studies contributing data for the prespecified outcomes. We justified decisions to downgrade the certainty of the evidence using footnotes, and made explanatory comments when necessary.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies tables.
Results of the search
The search identified 23,910 records. After removal of duplicates and partial screening, 8220 records remained; we assessed them on the basis of title and abstract and excluded 7977 records. We obtained the full text of 243 study reports; after exclusions, 21 reports remained. When needed, we contacted the authors of these studies for further information and subsequently labelled seven studies as ongoing projects and seven reports as awaiting classification studies.
Seven studies in this update met the review inclusion criteria (Figure 1). While all references are noted in the PRISMA diagram (Figure 1), we listed a subset of key references within the review. A full list is available on request.
Included studies
We included seven trials (AASK 2002; ACCORD BP 2010; HOT 1998; PAST BP 2016; PRESERVE 2021; SPRINT 2015; SPS3 2013).
Five trials compared two different SBP targets that met our inclusion criteria (PRESERVE 2021; SPS3 2013; and subgroups of participants with basal CVD in ACCORD BP 2010; PAST BP 2016; and SPRINT 2015). One trial compared two different DBP targets within our criteria for lower and standard targets in a subgroup of participants with secondary cardiovascular prevention (HOT 1998). One trial compared two MBP targets in a subgroup of participants who met our predefined inclusion criteria (AASK 2002). We described comparative basal characteristics of these seven trials in Table 2.
1. Baseline characteristics of included study participants.
| Mean unless otherwise stated | AASK 2002 | ACCORD BP 2010 | HOT 1998 | PAST BP 2016 | PRESERVE 2021a | SPRINT 2015 | SPS3 2013 |
| Number of participants | 155 | 1531 | 3232 | 295 | 111 | 1562 | 2709 |
| Sex (% male) | 68% | 63% | 53% | 64% | 59% | 76% | 62% |
| Age (years) | 57 (SD 9) | 62 (SD 8) | 62 (–) | 71 (SD 9) | 69 (SD 9) | 70 (SD 9) | 63 (SD 11) |
| Ethnic group (% Caucasian) | 0% | 62% | 92% | 98% | — | 71% | 53% |
| Diabetes | 0% | 100% | 12% | 10% | 2% | 0% | 36% |
| Current smoker | 31% | 13% | 16% | 13% | 14% | 14% | 20% |
| Systolic blood pressure (mmHg) | 149 (SD 28) | 138 (SD 16) | 174 (SD 15) | 143 (SD 14) | 149 (SD 13) | 138 (SD 16) | 146 (SD 18) |
| Diastolic blood pressure (mmHg) | 93 (SD 16) | 74 (SD 11) | 106 (SD 3) | 80 (SD 10) | — | 74 (SD 12) | 79 (SD 11) |
| Ischaemic heart disease | 25% | 86% | 95% | 22% | 5% | — | 11% |
| Stroke | 69% | 20% | 7% | 85% | 100% | 0% | 99% |
| Peripheral vascular disease | 23% | — | — | 7% | 2% | — | — |
| Thiazides | — | 51% | — | 35% | — | — | 35% |
| ACEI/ARB | — | 84% | — | 65% | — | — | 71% |
| Calcium channel blocker | — | 26% | — | 43% | — | — | 28% |
| Beta blocker | — | 57% | — | 20% | — | — | 27% |
| Other antihypertensive drugs | — | 28% | — | 11% | — | — | 8% |
| Number of antihypertensive drugs | — | 3.0 (SD 1.4) | 1.0 (–) | 1.1 (SD 0.8) | — | 2.1 (SD 1.0) | 1.7 (SD 1.1) |
(–) no information is available. Ischaemic heart disease, stroke, and peripheral vascular disease percentages are independent of each other because participants can have more than one cardiovascular event at the same time. A similar explanation can be offered with respect to percentages in the different classes of antihypertensive drugs.
ACEI: angiotensin‐converting enzyme inhibitor; ARB: angiotensin receptor blocker; SD: standard deviation.
aPRESERVE 2021: baseline demographics only provided for the 81 participants with complete diffusion tensor imaging data.
Methods
All included trials were randomized and open with blinded endpoint design. In AASK 2002, participants were also randomly assigned (in a 3 × 2 factorial design) to metoprolol, ramipril, or amlodipine treatment. In ACCORD BP 2010, participants were randomized to intensive or standard glycaemic control according to a 2 × 2 factorial design. HOT 1998 used a 3 × 2 factorial design and randomized participants to receive aspirin (acetylsalicylic acid) or placebo. SPS3 2013 had a 2 × 2 factorial design with additional randomization to aspirin plus placebo or aspirin plus clopidogrel.
Mean follow‐up duration was 3.7 years (range 1.0 years to 4.7 years).
Participants
The total number of participants included in the review was 9595 (lower target, 5356; standard target, 4239). AASK 2002 included 155 participants (14% of total AASK (African American Study of Kidney Disease and Hypertension) study); ACCORD BP 2010 included 1531 participants (32% of total ACCORD (Action to Control Cardiovascular Risk in Diabetes) study); HOT 1998 included 3232 participants (17% of total HOT (Hypertension Optimal Treatment) study); PAST BP 2016 295 participants (56% of total PAST BP (Prevention After Stroke – Blood Pressure) trial); PRESERVE 2021 111 participants (100% of total PRESERVE study); SPRINT 2015 included 1562 participants (17% of total SPRINT study); and SPS3 2013 included 2709 participants (90% of total SPS3 (Secondary Prevention of Small Subcortical Strokes) study).
AASK 2002 and SPRINT 2015 were conducted in the USA: ACCORD BP 2010 in the USA and Canada; PAST BP 2016 and PRESERVE 2021 in the UK; SPS3 2013 in eight countries in the Americas and Europe; and HOT 1998 in over 20 countries in Asia, the Americas, and Europe.
Basal participant characteristics differed among trials (Table 2).
For participants' basal cardiovascular condition, we accepted the following participant profiles as valid secondary prevention.
AASK 2002: participants with IHD, stroke, or peripheral vascular disease (PVD).
ACCORD BP 2010: participants with myocardial infarction, stroke, or angina.
HOT 1998: participants with myocardial infarction, stroke, or angina.
PAST BP 2016: participants had stroke or, less frequently, IHD.
PRESERVE 2021: participants had clinical lacunar stroke.
SPRINT 2015: participants all had IHD or PVD.
SPS3 2013: some participants had IHD, but all had recent lacunar stroke.
We considered myocardial infarction and angina identified by electrocardiogram (ECG) or coronary revascularization, and silent events, as meeting the inclusion criteria. In general, stroke was the prevalent condition in AASK 2002, PAST BP 2016, PRESERVE 2021, and SPS3 2013, whereas ischaemic myocardial infarction was the most prevalent condition in ACCORD BP 2010, HOT 1998, and SPRINT 2015.
AASK 2002 and SPRINT 2015 excluded people with history of diabetes, but HOT 1998, PAST BP 2016, PRESERVE 2021, and SPS3 2013 included some people with diabetes; all ACCORD BP 2010 participants had diabetes.
All trials included more men than women with mean age from 57 years to 71 years.
Ethnicity varied from all or mostly Caucasian (assumed to be white people) (HOT 1998; PAST BP 2016), to mixed populations (ACCORD BP 2010; SPRINT 2015; SPS3 2013), to African American people (AASK 2002). PRESERVE 2021 did not provide ethnicity data.
Trials included people with reduced kidney function (AASK 2002), additional cardiovascular risk factors (ACCORD BP 2010; SPRINT 2015), previous stroke (PAST BP 2016; PRESERVE 2021; SPS3 2013), or general hypertension (HOT 1998).
The baseline blood pressure required for inclusion varied. AASK 2002 required DBP 95 mmHg or greater and HOT 1998 required DBP 100 mmHg to 115 mmHg. ACCORD BP 2010 and SPRINT 2015 required SBP 130 mmHg to 180 mmHg, PAST BP 2016 sought SBP 125 mmHg, PRESERVE 2021 included people with SBP 140 mmHg or greater, or 125 mmHg to 140 mmHg while on antihypertensive medication, and SPS3 2013 had SBP 130 mmHg or greater or DBP 85 mmHg or greater (or both) or a history of hypertension with blood pressure‐lowering medication at randomization.
HOT 1998 was fully industry funded, AASK 2002 was partially industry funded, and PRESERVE 2021 was mainly funded by health charities. ACCORD BP 2010, PAST BP 2016, SPRINT 2015, and SPS3 2013 were fully publicly funded. ACCORD BP 2010, SPRINT 2015, and SPS3 2013 were supported by the National Institutes of Health in the USA. PAST BP 2016 was funded by the National Institute for Health Research (NIHR) in the UK.
Interventions
Participants in AASK 2002 were randomized to MBP 102 mmHg to 107 mmHg (standard target) or MBP less than 92 mmHg (lower target). ACCORD BP 2010 and SPRINT 2015 randomized participants to SBP less than 140 mmHg (standard target) or SBP less than 120 mmHg (lower target). Participants in PAST BP 2016 were randomized to SBP less than 140 mmHg (standard target) or less than 130 mmHg (lower target). PRESERVE 2021 randomized participants to SBP 130 mmHg to 140 mmHg (standard target) or less than 125 mmHg (lower target). Participants in SPS3 2013 randomized participants to SBP 130 mmHg to 149 mmHg (standard target) or SBP less than 130 mmHg (lower target). Finally, participants in HOT 1998 randomized participants to DBP 90 mmHg or less (standard target) or DBP 85 mmHg or less or 80 mmHg or less (lower target).
In AASK 2002, if the blood pressure goal could not be achieved by the drug used when initially randomized (metoprolol, ramipril, or amlodipine), researchers added open‐label antihypertensives sequentially (furosemide, doxazosin, clonidine, hydralazine, or minoxidil). Felodipine was proposed as basal therapy in HOT 1998, with other drugs added according to a five‐step regimen. In SPRINT 2015, the protocol encouraged the use of drug classes with strongest evidence for reduction in cardiovascular outcomes, including thiazide‐type diuretics (chlorthalidone encouraged as the first‐line agent), loop diuretics (for participants with advanced chronic kidney disease), and beta‐adrenergic blockers (for people with coronary artery disease). ACCORD BP 2010, PAST BP 2016, PRESERVE 2021, and SPS3 2013 provided no specific drug instructions.
Outcomes
The primary analysis in AASK 2002 focused on change in glomerular filtration rate, with relevant cardiovascular events measured as secondary outcomes. In ACCORD BP 2010, HOT 1998, and SPRINT 2015, the main outcome was occurrence of several types of cardiovascular events. The primary outcome in PAST BP 2016 was change in SBP between baseline and one year. The main endpoint in PRESERVE 2021 was a global cognitive score. Time to recurrent stroke was the main analysis in SPS3 2013.
Additional notes
AASK 2002 was conducted between February 1995 and September 2001; ACCORD BP 2010 between January 2001 and June 2009; HOT 1998 between October 1992 and August 1997; PAST BP 2016 between July 2008 and July 2012; PRESERVE 2021 between February 2012 and November 2017; SPRINT 2015 between November 2010 and March 2013; and SPS3 2013 between February 2003 and April 2012.
Excluded studies
We excluded 36 records following assessment of full‐text reports (Figure 1). Among them, we considered it useful to provide more detailed information about eight excluded studies (BBB 1994; HOSP 2006; INFINITY 2019; MDRD 1994; NCT01230216; PODCAST 2013; REIN‐2 2005; RESTART‐AP 2013).
BBB 1994 was a multicentre, prospective, randomized, and open trial conducted in Sweden with blinded endpoint (PROBE; prospective, randomized, open‐label, blinded endpoint) design. Adults aged 47 to 67 years were included if their treated DBP was in the range 90 mmHg to 100 mmHg on at least three consecutive visits. Specific exclusion criteria were: history of IHD or pathological ECG or both, somatic disorders expected to cause a significant deterioration in health within the next few years, or inability to participate. The study compared two interventions: standard (unchanged) target: DBP 90 mmHg to 100 mmHg versus intensive target: DBP 80 mmHg or less. Study data were lost. The principal author (Professor Lennart Hansson) is deceased; Dr Bjorn Dahlöf confirmed that data were not retained. We also contacted Bayer but they confirmed the company does not have any data available for the BBB (Behandla Blodtryck Bättre) study. The journal Blood Pressure, in which BBB results were published, confirmed the manuscript received was essentially as published, and the documentation was destroyed about 10 years before (following Professor Hansson's death). The Swedish Council on Health Technology Assessment assessed the study in a report (No. 170/2) but did not have access to the original data. We also approached the Östra Hospital, where Professor Hansson was working at the time the study was conducted. They found no records, and we were told that the legal requirement to keep records safe expired after 15 years.
HOSP 2006 randomized participants up to five years and intended to assess two home blood pressure target strategies. The number of recruited participants was much smaller than intended and was not sufficient for analysis of the effects of different levels of target home blood pressure.
INFINITY 2019 was a prospective, randomized, open‐label trial with blinded endpoints. It was designed to compare a standard target (24‐hour SBP less than 145 mmHg) versus an intensive target (24‐hour SBP less than 130 mmHg) in people older than 74 years with SBP greater than 150 mmHg and at risk for cerebrovascular disease. Participants had visible white matter hyperintensity lesions on cerebral magnetic resonance imaging (MRI) screening. It was confirmed that the trial included fewer than 50 participants per group (39 participants per group).
MDRD 1994 focused mainly on effects of dietary protein restriction and blood pressure control on progression of chronic kidney disease. The National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK, USA) provided individual participant data. However, after a first analysis, we excluded this study because researchers included fewer than 50 participants per group (an inclusion criterion) (lower target: 56 participants, eight total deaths; standard target: 47 participants, three total deaths).
NCT01230216 was designed to assess whether an intensive blood pressure target could reduce the per cent of atheroma volume measured by intravascular ultrasound in people with hypertension and coronary artery disease. This study was terminated early owing to slow patient enrolment.
The primary outcome for PODCAST 2013 was Addenbrooke's Cognitive Examination. Secondary outcomes included vascular events, quality of life, functional outcome, depression, and death. The trial recruited 83 participants during the pilot phase. Low recruitment meant that the trial did not proceed and did not meet the 50 participants per group inclusion criterion of this review.
REIN‐2 2005 was designed to establish whether further blood pressure‐lowering therapy in addition to angiotensin‐converting enzyme inhibitors (ACEIs) could benefit people with chronic kidney disease. Accordingly, the primary objective assessed the effect of intensified versus conventional blood pressure control on progression to end‐stage kidney disease. The Istituto di Ricerche Farmacologiche Mario Negri (Bergamo, Italy) provided individual participant data. It was confirmed that the trial included fewer than 50 participants per group, so this study did not meet this review inclusion criterion (lower target: 34 participants, two deaths; standard target: 39 participants, two deaths).
RESTART‐AP 2013 was designed to determine whether restarting antithrombotic agents had an impact on the number of new‐onset cerebral microbleeds, and if intensive blood pressure lowering reduced their numbers. Study authors confirmed that insufficient funding was available, and the study was terminated early.
Studies awaiting classification
Seven studies await classification (ABCD‐H 1998; Cardio‐Sis 2014; ESH‐CHL‐SHOT 2014; RESPECT 2019; STABLE‐ICAS 2018; STEP 2021; Zeng 2016). Five studies did not report data for participants with CVD at baseline (ABCD‐H 1998; Cardio‐Sis 2014; RESPECT 2019; STEP 2021; Zeng 2016). We have requested these data from study authors but have not received them before publication of this review. STABLE‐ICAS 2018 showed severe inconsistencies when presenting outcomes. Despite our efforts, to date, we have been unable to reach an adequate clarification from the authors.
Ongoing studies
We identified seven ongoing studies (BPROAD 2019; EPICS‐Pilot 2020; ESPRIT 2019; IBIS 2019; OPTIMAL‐DIABETES 2019; OPTIMAL Stroke 2019; NCT03666351). We will evaluate these studies for possible inclusion in updates of this review when complete.
Risk of bias in included studies
The summary of the risk of bias assessment of each trial is shown in Figure 2. Assessment of risk of bias was based on both published and unpublished data.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
SPRINT 2015 did not report any specific random sequence generation in its protocol. However, the study authors confirmed via e‐mail that they used a permuted block randomization scheme with random block lengths, stratified by clinic (Reboussin 2020 [pers comm]). All other included trials specified a computerized system for randomization (random permuted blocks, minimization or Pocock‐Simon method). Thus, we judged methods used for allocation at low risk of bias for all seven studies (AASK 2002; ACCORD BP 2010; HOT 1998; PAST BP 2016; PRESERVE 2021; SPRINT 2015; SPS3 2013). The allocation concealment domain was at low risk of bias for all included trials.
Blinding
None of the included studies was blinded to participants or clinicians because of the need to titrate antihypertensive drugs to reach a specific blood pressure goal. However, an independent committee blinded to group allocation assessed clinical events in all trials. Hence, we assessed all trials at high risk of performance bias and low risk of detection bias.
Incomplete outcome data
Available information (both published and unpublished) for six trials did not suggest a significant imbalance between groups for withdrawals or dropouts (AASK 2002; ACCORD BP 2010; PAST BP 2016; PRESERVE 2021; SPRINT 2015; SPS3 2013); we assessed these trials at low risk of attrition bias.
In HOT 1998, 14% of total ECGs could not be obtained, leading to some uncertainty on silent myocardial infarctions. We decided to assume a conservative perspective and consider this trial to have unclear risk of bias.
Selective reporting
We assessed protocols and published articles for AASK 2002, ACCORD BP 2010, HOT 1998, PAST BP 2016, PRESERVE 2021, and SPRINT 2015 and confirmed no sign of reporting bias. We assessed these trials at low risk of reporting bias.
Serious adverse effects reported in SPS3 2013 were related to hypotension and blood pressure management only. We contacted study authors for clarification but received no response. Finally, the National Institute of Neurological Disorders and Stroke (NINDS) provided individual participant data. After reviewing all data, we assessed this study at low risk of selective reporting bias.
Other potential sources of bias
All data used in this Cochrane Review but PRESERVE 2021 came from subgroups of participants not predefined in the original study protocols, and this constitutes a potential source of bias.
Some studies were partially (e.g. AASK 2002) or fully (e.g. HOT 1998) funded by pharmaceutical industry sources, which constitutes another potential source of bias.
We also considered early termination of PRESERVE 2021 and SPRINT 2015 as a potential source of bias.
Effects of interventions
See: Table 1
Lower versus standard blood pressure targets
Seven RCTs met the inclusion criteria (AASK 2002; ACCORD BP 2010; HOT 1998; PAST BP 2016; PRESERVE 2021; SPRINT 2015; SPS3 2013). We obtained data from published and unpublished sources. We assumed that silent myocardial infarction complied with the definition of cardiovascular event when provided.
Primary outcomes
Total mortality
There was no evidence of a difference in total mortality between lower and standard blood pressure target groups (RR 1.05, 95% CI 0.91 to 1.23; P = 0.50; 7 studies, 9595 participants; Analysis 1.1). When the absolute effect was measured, results showed three additional total deaths per 1000 participants identified in the lower target (95% CI 6 fewer to 16 more total deaths per 1000 participants). Researchers reported a total of 367/5356 deaths in the lower target group and 287/4239 deaths in the standard target group (Figure 3).
1.1. Analysis.

Comparison 1: Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 1: Total mortality
3.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.1 Total mortality.
Total serious adverse events
All seven studies provided data for analysis of serious adverse events. We adopted a broad definition of serious adverse event, according to ICH 1995. We included participants with any cause of death, any cardiovascular event (as predefined in our protocol), or any other serious adverse event as defined by trial authors, while avoiding double‐counting of participants. When all data were pooled, there was no evidence of a difference in serious adverse events between lower and standard blood pressure target groups (RR 1.01, 95% CI 0.94 to 1.08; P = 0.75; 7 studies, 9595 participants; Analysis 1.2). When measuring the absolute effect, researchers identified three additional serious adverse events per 1000 participants in the lower target group (95% CI 15 fewer to 20 more serious adverse events per 1000 participants). Results showed 1210 (of 5356 participants) with at least one serious adverse event in the lower target group and 1061 (of 4239 participants) in the standard target group (Figure 4). We considered PRESERVE 2021 and SPRINT 2015 to report the full range of serious adverse events (Analysis 1.2.1), and five studies reported subsets of events (AASK 2002; ACCORD BP 2010; HOT 1998; PAST BP 2016; SPS3 2013; Analysis 1.2.2).
1.2. Analysis.

Comparison 1: Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 2: Total serious adverse events
4.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.2 Serious adverse events.
Total cardiovascular events
AASK 2002 analyzed data from 27 participants in relation to individual cardiovascular events for myocardial infarction, stroke, and heart failure hospitalization; as well as data from seven further participants from a direct cardiovascular mortality diagnosis.
Five studies provided data as a direct composite outcome (ACCORD BP 2010; HOT 1998; PAST BP 2016; SPRINT 2015; SPS3 2013), and the other two contributed on the basis of pooled individual cardiovascular events (AASK 2002; PRESERVE 2021). There was no evidence of a difference in total number of cardiovascular events between the lower blood pressure target group compared with the standard group (RR 0.89, 95% CI 0.80 to 1.00; P = 0.05; 7 trials, 9595 participants; Analysis 1.3). When measuring the absolute effect, researchers in these studies identified 14 fewer cardiovascular events per 1000 participants in the lower blood pressure target group (95% CI 0 to 25 fewer cardiovascular events per 1000 participants). Results showed 565/5356 participants had cardiovascular events in the lower target group and 535/4239 participants had cardiovascular events in the standard target group (Figure 5).
1.3. Analysis.

Comparison 1: Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 3: Total cardiovascular events
5.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.3 Cardiovascular events.
Cardiovascular mortality
We need to make some comments related to AASK 2002 before we report analysis results. AASK 2002 researchers used two different documents to register causes of death (CARDIO_REVW Form #38 and CC_DEATH Form #48). We noted no complete overlap between forms. After discussion, we considered there to be valid cardiovascular mortality when the researcher answered 'yes' to question 4 on Form #38: "Was there a cardiovascular death?" This indicated 11 deaths. Two clinicians (a cardiologist and a general practitioner) analyzed data from Form #48 case‐by‐case and identified two additional deaths after completing a careful validation process.
Five other trials provided data using well‐defined categories. Results showed no evidence of a difference in cardiovascular mortality between lower and standard blood pressure target groups (RR 1.03, 95% CI 0.82 to 1.29; P = 0.83; 6 trials, 9484 participants; Analysis 1.4). Researchers reported 172/5301 cardiovascular deaths in the lower target group and 131/4183 cardiovascular deaths in the standard target group (Figure 6).
1.4. Analysis.

Comparison 1: Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 4: Cardiovascular mortality
6.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.4 Cardiovascular mortality.
Secondary outcomes
Participant withdrawals due to adverse effects
Four trials provided no information about withdrawals due to adverse effects among participants with basal CVD (AASK 2002; ACCORD BP 2010; SPRINT 2015; SPS3 2013).
PRESERVE 2021 reported reasons for withdrawals. Additionally, review authors extracted data from free‐text notes only for HOT 1998; PAST BP 2016 provided data of better quality. Despite limited information, results showed a difference in withdrawals due to adverse effects between groups favouring standard blood pressure target (RR 8.16, 95% CI 2.06 to 32.28; P = 0.003; 3 trials, 801 participants; Analysis 1.5). Researchers reported 22/475 withdrawals due to adverse effects in the lower target group and 2/326 participants in the standard target group.
1.5. Analysis.

Comparison 1: Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 5: Participant withdrawals due to adverse effects
Systolic blood pressure change from baseline at one year
After the first year of therapy, the mean SBP achieved was lower in the lower blood pressure target group (MD –8.77 mmHg, 95% CI –12.82 to –4.73; P < 0.0001; 7 trials, 8657 participants; Analysis 1.6). Heterogeneity among trials was high, so we preferred a random‐effects model for this analysis. We considered the different targets and specific basal characteristics for each trial as the most likely causes of this heterogeneity.
1.6. Analysis.

Comparison 1: Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 6: Systolic blood pressure change from baseline at 1 year (mmHg)
Diastolic blood pressure change from baseline at one year
After the first year of therapy, the mean DBP achieved was lower in the lower blood pressure target group (MD –4.50 mmHg, 95% CI –6.35 to –2.65; P < 0.00001; 6 trials, 8546 participants; Analysis 1.7). Heterogeneity between trials for this outcome was high, so we chose a random‐effects model for this analysis. We considered the different targets and specific basal characteristics for each trial as the most likely causes of this heterogeneity.
1.7. Analysis.

Comparison 1: Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 7: Diastolic blood pressure change from baseline at 1 year (mmHg)
Proportion of participants reaching the target blood pressure level at one year
Results showed that 3120/4875 (64%) participants reached the target in the lower target group and 2849/3824 (75%) participants in the standard target group (7 trials, 8699 participants; Analysis 1.8). Therefore, more people in the standard group achieved particular blood pressure targets.
1.8. Analysis.

Comparison 1: Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 8: Blood pressure target achieved at 1 year
Number of antihypertensive drugs that each participant needed at the end of the study
At the end of the study, the number of antihypertensive drugs needed was lower in the standard blood pressure target group (mean 1.9 drugs) than in the lower blood pressure target group (mean 2.4 drugs) (MD 0.56, 95% CI 0.16 to 0.96; P = 0.0066; 5 trials, 7910 participants; Analysis 1.9). Heterogeneity between trials for this outcome was high, so we chose a random‐effects model for this analysis. We considered the different targets and specific basal characteristics for each trial as the most likely causes of this heterogeneity.
1.9. Analysis.

Comparison 1: Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 9: Number of antihypertensive drugs that each participant needed at the end of study
Discussion
Pharmacological treatment of high blood pressure aims to reduce morbidity and mortality. Specific blood pressure targets have been proposed in guidelines for people with hypertension who have established CVD, but optimal thresholds remain uncertain because the benefit‐to‐harm ratio of more intensive treatment has not been established.
This Cochrane Review explored current evidence from RCTs and assessed relevant outcomes linked to two alternative strategies: standard blood pressure target (140 mmHg to 160 mmHg/90 mmHg to 100 mmHg or less) and lower blood pressure target (135/85 mmHg or less).
We included seven RCTs with 9595 participants and a mean follow‐up of 3.7 years (range 1.0 to 4.7 years) (AASK 2002; ACCORD BP 2010; HOT 1998; PAST BP 2016; PRESERVE 2021; SPRINT 2015; SPS3 2013). Five studies compared SBP targets, one compared DBP targets, and one compared MBP targets. Six trials had individual participant data.
Regarding the analysis strategy, two other Cochrane Reviews considered each target (SBP or DBP) separately (Arguedas 2013; Arguedas 2020). Our Cochrane protocol did not specify any particular strategy (Gorricho 2013). For this Cochrane Review, we decided to use pooled data as the main analysis, but we also tested whether results were consistent when blood pressure targets were considered separately. To avoid misclassification problems, we added a third category (MBP) to SBP/DBP.
Summary of main results
Evidence from the seven included trials indicates that blood pressure targets were more frequently achieved in the standard blood pressure target group (2849/3724 (75%) participants) than in the lower target group (3120/4875 (64%) participants).
Researchers used more antihypertensive drugs in the lower blood pressure target group (mean 2.4 drugs) than in the standard group (mean 1.9 drugs).
Results show broad differences for SBP (−8.8 mmHg) and DBP (−4.5 mmHg) changes from baseline in the lower target group.
We detected no benefits for total mortality (RR 1.05, 95% CI 0.91 to 1.23) or cardiovascular mortality (RR 1.03, 95% CI 0.82 to 1.29). Subsequent analyses separating trials by SBP, DBP, or MBP targets did not change these results. We also found no difference with regard to total cardiovascular events (including myocardial infarction, stroke, sudden death, hospitalization, or death from CHF) (RR 0.89, 95% CI 0.80 to 1.00) and total serious adverse events (RR 1.01, 95% CI 0.94 to 1.08). When we considered SBP target trials separately, we identified no significant changes in the main results.
Most withdrawals due to adverse effects occurred in the lower target group (RR 8.16, 95% CI 2.06 to 32.28). However, little evidence was available, making establishment of a trustworthy global assessment of benefits and harms very challenging.
It is important to note that we detected no significant heterogeneity for any primary outcome. Therefore, at present, there does not seem to be sufficient sound evidence to justify stricter blood pressure targets (135/85 mmHg or less) than the standard range (140 mmHg to 160 mmHg/90 mmHg to 100 mmHg or less) for people with hypertension and established CVD.
We detected significant heterogeneity for two outcomes – blood pressure difference from baseline at one year and number of antihypertensive drugs that each participant needed at the end of study. We considered the different targets and the specific basal characteristics for each trial as the most likely causes for this heterogeneity. Subgroup analysis indicated significant heterogeneity in the male subgroup for cardiovascular mortality. The source of heterogeneity could be linked to a decrease in the numbers of participants and events, and differences in trial design between HOT 1998 and ACCORD BP 2010/SPRINT 2015.
The minimum 5‐mmHg difference in SBP or DBP targets predefined as clinically significant in our protocol is consistent with previous guideline decisions (NICE 2022). Nonetheless, as Arguedas 2013 reported, it could be argued that this difference is not large enough to show significant changes in relevant outcomes. To test this hypothesis, we conducted an additional sensitivity analysis of participants with diabetes, while excluding the intermediate less than 85 mmHg target in HOT 1998; results were very similar between the main analysis in participants with CVD and the subgroup analysis in participants with diabetes and showed large differences in targets (Table 3).
2. Lower versus standard blood pressure target; people with diabetes, difference in targets 10 mmHg or greater.
| Outcome | Studies | Participants | Statistical method | Effect estimate |
| Total mortality | ACCORD BP 2010; HOT 1998; SPS3 2013 | 2773 | Risk ratio (M‐H, fixed, 95% CI) | 1.15 (0.91 to 1.45) |
| Cardiovascular mortality | ACCORD BP 2010; HOT 1998; SPS3 2013 | 2773 | Risk ratio (M‐H, fixed, 95% CI) | 0.98 (0.69 to 1.39) |
| Cardiovascular events | ACCORD BP 2010; HOT 1998; SPS3 2013 | 2773 | Risk ratio (M‐H, fixed, 95% CI) | 0.88 (0.74 to 1.03) |
| Serious adverse events | ACCORD BP 2010; HOT 1998; SPS3 2013 | 2773 | Risk ratio (M‐H, fixed, 95% CI) | 1.01 (0.88 to 1.15) |
CI: confidence interval.
As for the risk of bias assessment, three domains (selection, detection, and reporting bias) showed a low risk in all included trials. Two other domains (attrition and other bias) presented mixed rates, while performance bias was at high risk for all included trials (Figure 7).
7.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We specified in our Cochrane protocol three subgroup analyses (people with diabetes, participants by sex, and people aged 75 years or older) designed to explore potential differences in specific populations (Gorricho 2013). Despite the large amount of information retrieved from individual participant data for this review, data available for people aged 75 years or older were too few to permit any definitive conclusions. When participant data were split according to sex, and when only participants with diabetes were considered, we found magnitudes of effect similar to those described in the main analysis. People with diabetes and established CVD could be seen at first as being in a higher risk category than people who do not have diabetes (Mancia 2011). However, estimates for people with diabetes were similar to estimates for the general population with basal CVD: there were no differences in total mortality, cardiovascular mortality, or total cardiovascular events associated with lower target; and no differences in both target strategies for serious adverse events. Evidence was insufficient to reveal greater effect from a lower blood pressure target in these subgroups, although sample sizes were not large enough to exclude a significant effect.
We planned two sensitivity analyses to test the robustness of results: risk of bias of the included trials and industry‐sponsored versus non‐industry‐sponsored trials.
Because we rated overall risk of bias as high, we could not perform sensitivity analyses. We found no difference in any main outcome favouring the lower blood pressure target in industry‐sponsored or non‐industry‐sponsored trials (ACCORD BP 2010; PAST BP 2016; PRESERVE 2021; SPRINT 2015; SPS3 2013).
Overall completeness and applicability of evidence
CVDs are prevalent, and hypertension is an added risk factor commonly treated in this population. Evidence‐based guidelines focused on this issue are needed. However, data derived from RCTs designed to clarify this uncertainty remain insufficient.
Six of seven studies contributed individual participant data for subgroups of participants (AASK 2002, 155 participants; ACCORD BP 2010, 1531 participants; HOT 1998, 3232 participants; PAST BP 2016, 295 participants; SPRINT 2015, 1562 participants; SPS3 2013, 2709 participants).
Although this review analyzed a significant body of evidence and results are considered to be robust, we cannot state these results as conclusive. Three ongoing trials have been designed to explicitly answer relevant questions for people with established CVD (EPICS‐Pilot 2020; IBIS 2019; OPTIMAL Stroke 2019); it is anticipated that these studies will yield additional evidence.
Over 6000 participants provided data on SBP targets, and over 3000 on DBP targets. Neither subanalysis substantially changed overall results in primary outcomes when all target strategies were considered together. From this perspective, results of this review can be generalized for physicians prescribing antihypertensive drugs, no matter the specific target strategy (SBP, DBP, or both) chosen.
As identified by Arguedas 2020, and probably fuelled by the intention‐to‐treat approach, this review found no real differences as wide as expected between groups in achieved SBPs and DBPs, according to the predefined targets for each study. All seven included trials achieved the standard target, but only ACCORD BP 2010, PAST BP 2016, and SPS3 2013 achieved the required blood pressure in the lower target group (in HOT 1998, participants did not achieve the 80 mmHg or less target). This underlines the difficulty of putting the intervention into practice, as often happens in real life. Accordingly, this aspect could be seen as both a limitation and a strength.
Quality of the evidence
We downgraded the certainty of the evidence for total mortality and cardiovascular mortality to moderate owing to imprecision and lack of data. In our opinion, other potential limitations (e.g. several studies did not predefine CVD subgroups) are unlikely to lower confidence in the estimate, given the large sample sizes, the design of SPS3 2013 (29% of total participants), the sensitivity analysis performed about potential risk of bias, and the strength of the individual participant data analysis.
We also downgraded the certainty of the evidence for other outcomes: we assessed total cardiovascular events and total serious adverse events as providing low‐certainty evidence, and withdrawals due to adverse effects as providing very low‐certainty evidence. Total cardiovascular events, total serious adverse events, and withdrawals due to adverse events data were affected by high risk of bias. As for withdrawals, imprecision was especially marked, leading to further downgrading of evidence certainty. (See Table 1.)
Potential biases in the review process
Because of study requirements, none of the included studies were blinded to participants or clinical researchers. However, all studies implemented mechanisms for assessment of outcomes by independent blinded committees. Consequently, we considered potential performance bias as high and detection bias as low.
Another potential source of bias came from the fact that all included participants but those from PRESERVE 2021 were also included in subgroup studies. In addition, to adapt study interventions to those defined in our review, we pooled participants in HOT 1998 in two different target groups (less than 85 mmHg and less than 80 mmHg) only for the lower blood pressure target.
Additionally, primary outcomes in AASK 2002 and PRESERVE 2021 were not aligned with the interests of our review. It must be stressed that most subgroups included a large number of participants, and the vast majority of findings were analyzed as individual participant data.
Differences between trials in types and definitions of outcomes could also be a source of bias (see 'Outcomes' in the Characteristics of included studies table). For example, not all studies provided adequate information about the ways silent myocardial infarctions were dealt with, revealing differences among studies that included heart failure hospitalization as an outcome.
We observed no homogeneous information among trials for serious adverse events – the most comprehensive outcome on safety. Only SPRINT 2015 and PRESERVE 2021 were deemed to report the total number of serious adverse events according to its international standardized definition (ICH 1995). Other included trials provided an unreliably low number of serious adverse events (HOT 1998); reported only events judged by researchers as probably related to the interventions (ACCORD BP 2010); considered serious adverse events from an extremely narrow perspective (PAST BP 2016); or did not offer any specific information on this outcome (AASK 2002). Deaths, major cardiovascular events, and serious adverse effects reported by trialists were included as serious adverse events in analyses when only partial or disaggregated information was available, as in SPS3 2013. Because of these concerns, we strongly suspect reporting bias for certain outcomes such as serious adverse events and withdrawals due to adverse effects, for which few data were reported.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review with meta‐analysis that assessed blood pressure targets in people with established CVD from RCTs that directly compared different target strategies.
We found no evidence of additional benefit from a lower blood pressure target compared to a standard blood pressure target in terms of total mortality, cardiovascular mortality, total cardiovascular events, or total serious adverse events.
In contrast, some hypertension guidelines have not issued direct recommendations on blood pressure targets for people with previous CVD (JNC‐8 2014). Those reviews or guidelines that include explicit recommendations obtained them from observational data or post hoc analyses of achieved blood pressure in trials designed for various purposes (Bangalore 2013). This perspective could easily lead to selection bias, favouring lower risk of experiencing a cardiovascular event in participants with lower achieved blood pressure. Only one study directly compared clinical outcomes in people who had stroke and were treated to different blood pressure targets (SPS3 2013); no studies have been conducted in people with CVD.
Our results do not seem to support widespread implementation of an intensive target strategy (135/85 mmHg or less) for cardiovascular secondary prevention. A similar systematic review on chronic kidney disease did not show that a blood pressure target less than 125/75 mmHg to 130/80 mmHg is more beneficial than a target less than 140/90 mmHg (Upadhyay 2011). This is consistent with the last update of the NICE guideline (NICE 2022), which recommends reducing clinic blood pressure to below 140/90 mmHg for adults with hypertension aged under 80 years, with and without CVD. For adults with hypertension aged 80 years and over, even more relaxed blood pressure targets (150/90 mmHg) are determined. In the NICE committee's opinion, the evidence did not show a robust or consistent clinical benefit from using lower blood pressure targets for people with CVD compared with standard blood pressure targets.
However, based on SPRINT 2015 data, Hypertension CANADA 2020 recommends consideration of lower targets in some people at high cardiovascular risk. Similarly, ACC‐AHA 2017, ESC 2022, ESH‐ESC 2018, and WHO 2021 suggest lower goals for people with established CVD, according to SPRINT 2015 data and the conclusions of several meta‐analyses. However, there was no specific analysis performed on this population. Other guidelines, such as Rosendorff 2015 and Kernan 2014, only partially agree with our view. Two US guidelines focusing on coronary and stroke patients are available. Rosendorff 2015 suggests less than 140/90 mmHg as a reasonable target for secondary prevention of cardiovascular events in coronary patients but considers a lower target (less than 130/80 mmHg) as useful for some people; researchers admit that this is not supported by evidence and offer no additional details of potential benefit profiles. Kernan 2014 recommends a less than 140/90 mmHg target strategy as a general rule for people with stroke but points out that 130/80 mmHg could be reasonable for people with a recent lacunar stroke, based mainly on SPS3 2013 results. However, the SPS3 2013 study did not achieve a statistically significant difference between lower and standard targets for any of the primary or secondary outcomes measured. In SPS3 2013, the difference detected in intracerebral haemorrhages (a subtype of intracranial haemorrhages not preplanned even as a secondary outcome) could well have been due to chance. It is surprising that despite no evidence of substantial benefit confirmed with the lower target, the SPS3 2013 authors concluded that, based on their results, use of a SBP target less than 130 mmHg was likely to be beneficial in people with recent lacunar stroke.
Ettehad 2016, a systematic review, identified large‐scale blood pressure‐lowering trials to quantify the effects of reducing SBP by 10 mmHg in terms of mortality and cardiovascular outcomes. This analysis was conducted for the main comparison and for several subgroups, one of them including people with established CVD. Results showed benefit for this subgroup in terms of mortality and cardiovascular events when blood pressure was reduced but inconsistent results for safety outcomes. The review authors concluded that lowering current normotensive levels is supported by their review, provided there is a relevant absolute risk. In this regard, relevant limitations must be taken into account. First, heterogeneity was extremely high in Ettehad 2016, including large differences among populations, basal comorbidities, and comparisons between treatment groups. In fact, some included studies compared the effects of different blood pressure targets, the effects of different drugs, or even the effects of drugs versus placebo. Second, the review did not consider individual participant data, leading to particularly low accuracy when conclusions are assumed about participants with or without basal CVD. Finally, among the included studies comparing different blood pressure targets, researchers mixed strategies that were too diverse, from less than 120 mmHg to less than 150 mmHg SBP targets. Certainly, this review gathered a large amount of information, but, at the same time, a careful approach should be demanded to avoid misleading conclusions.
Similarly, one large individual participant‐level data meta‐analysis led by the Blood Pressure Lowering Treatment Trialists' Collaboration (BPLTTC) has recently concluded that a 5 mmHg reduction of SBP can reduce the risk of major cardiovascular events by about 10%, in patients with or without previous diagnosis of CVD and even at normal blood pressure values (BPLTTC 2021). As in Ettehad 2016, a mixed strategy was conceived, including trials not only focused on more versus less intensive treatment regimens but also on effects of medications versus placebo or other medications. This study did not investigate potential treatment harms and only considered BPLTTC trials, missing the broader approach of a systematic review. In this sense, several relevant trials included in our review as HOT 1998, PAST BP 2016, or SPS3 2013 were not incorporated into BPLTTC 2021. Of note, there were no benefits related to lower targets identified in terms of cardiovascular and all‐cause mortality, which is in line with our results. Adjusted data from one observational, multicentre, prospective cohort has even suggested an increased mortality among people with symptomatic coronary, cerebrovascular or peripheral artery disease and SBP less than 130 mmHg (Sánchez 2021). The potential beneficial effect on major cardiovascular outcomes claimed by BPLTTC 2021 is not currently supported by our data, remaining disputed.
Another systematic review included clinical trials comparing only blood pressure targets (Xie 2016). Although this design seems to be more appropriate than that used in the previous case, review authors established inclusion criteria with high laxity. Limits were not well‐defined with regard to what was considered an intensive or standard target. Because of this, two studies could share the same target while simultaneously assigning treatment to different groups – standard and intensive (Brunström 2016). Participants with a wide range of blood pressure targets were mixed, leading to few informative results, even when data from numerous participants were collected. The review authors declared that, with high cardiovascular risk, benefits from intensive treatment clearly overcome potential harms, even in people with targets less than 140 mmHg, calling for changes to current guidelines.
In contrast, findings of our systematic review are not aligned with this view. We have not identified any advantages of lower blood pressure targets after taking into account more appropriate inclusion criteria, most individual participant data, and informative outcomes such as serious adverse events. Furthermore, even though SPRINT 2015 mortality results show a trend favouring the lower target strategy, this is not consistent with the results in other primary outcomes such as total serious adverse events or total cardiovascular events. More importantly, we detected no overall benefits in mortality and noted that adverse events were poorly informed by all concerned clinical trials. Also, ACCORD BP 2010, which was conceived with a twin design to SPRINT 2015 but in younger people with diabetes, found no beneficial effect from an intensive scheme. In our opinion, as long as the scientific community is dealing with this key lack of information, recommendations on blood pressure targets for people with hypertension with CVD should give priority to caution.
Authors' conclusions
Implications for practice.
The evidence identified in this review from randomized controlled trials does not support lower blood pressure targets (less than 135/85 mmHg) as compared to standard blood pressure targets (less than 140 mmHg to 160 mmHg/90 mmHg to 100 mmHg) in people with hypertension and established cardiovascular disease (myocardial infarction, stroke, chronic peripheral vascular occlusive disease, or angina pectoris).
We analyzed systolic, diastolic, or mean blood pressure goals as a whole and separately and obtained similar findings of little or no difference. There is a lack of benefit for the lower blood pressure target in total or cardiovascular mortality, total cardiovascular events, and serious adverse events. Some uncertainties remain as the evidence is very low certainty for withdrawals due to adverse effects.
Predefined subgroup analyses in older people, in people with diabetes, or based on participant sex does not suggest any differences in these conclusions.
According to the best available evidence, lower targets for people with hypertension and established cardiovascular disease provide minimal or no net health benefit.
Implications for research.
Well‐designed randomized controlled trials assessing lower blood pressure targets in people with hypertension and established cardiovascular disease are needed to ascertain the benefits and harms derived from intensive and more conservative strategies.
We have identified seven ongoing studies in people with stroke and coronary disease (BPROAD 2019; EPICS‐Pilot 2020; ESPRIT 2019; IBIS 2019; NCT03666351; OPTIMAL‐DIABETES 2019; OPTIMAL Stroke 2019), but additional studies exploring other types of basal cardiovascular disease (e.g. peripheral vascular disease, haemorrhagic stroke) are required. Future research should aim to report mortality rates and all serious adverse event outcomes.
Having access to individual participant data and other relevant documents (protocols, clinical study reports, raw data) becomes a major strength of systematic reviews with meta‐analysis. Thus, the authors of past or future trials are highly encouraged to share their databases.
What's new
| Date | Event | Description |
|---|---|---|
| 14 March 2022 | New search has been performed | This systematic review has been updated with a new bibliographic search on 25 January 2022. One study (PRESERVE), previously discarded because of low sample size, has now been included after increasing the total population assessed. Two promising studies (STEP and ESH‐CHL‐SHOT) have been labelled as 'awaiting classification' after failing in getting enough information to include or exclude them from the review. The main conclusions remain unchanged after this update. |
| 14 March 2022 | New citation required but conclusions have not changed | The main conclusions remain unchanged after this update. |
History
Protocol first published: Issue 1, 2013 Review first published: Issue 10, 2017
| Date | Event | Description |
|---|---|---|
| 24 March 2020 | New search has been performed | This systematic review has been updated with a new bibliographic search on 6 November 2019. Two promising studies have been labelled as 'awaiting classification' after failing in getting enough information to include or exclude them from the review. Random sequence generation in SPRINT 2015 has been considered as low risk of bias, after authors confirmation that they used a permuted block randomization scheme with random block lengths, stratified by clinic. |
| 24 March 2020 | New citation required but conclusions have not changed | The main conclusions remain unchanged after this update. |
| 24 August 2018 | Amended | The Risk of Bias figure and table have been amended to correct an error affecting the SPRINT study. As the main text correctly explained, this trial must be rated as 'unclear' in the random sequence generation domain. The 'allocation concealment' domain must be rated as 'low' risk of bias. |
| 27 April 2018 | New citation required and conclusions have changed | Four review authors included in the original version of the review (Muruzábal L, Malón MD, Montoya R, López A) have not participated in this update, and two new contributors (Erviti J, Leache L) have now been included as review authors |
Acknowledgements
We are grateful to:
James M Wright and the Cochrane Hypertension Group, for their encouragement, support, and assistance;
Lourdes Muruzábal, María del Mar Malón, Rodolfo Montoya, and Antonio López, for their relevant contributions as authors to a previous version of this systematic review;
the Biomedical Information Center of Navarre, which provided most of the published documents reviewed in this report (Stephen Adams, Vancouver, Canada, provided assistance with some references that were especially difficult to find);
Miguel Ángel Imízcoz, cardiologist, who provided specific advice related to ascertainment of cardiovascular events from individual participant data;
Agustín Ciapponi and Demian Glujovsky, of the Institute of Clinical Effectiveness and Health Policy (IECS), Buenos Aires, Argentina, who provided access to Early Review Organizing Software;
Annalisa Perna, Giuseppe Remuzzi, and Piero Ruggenenti, of Istituto di Ricerche Farmacologiche Mario Negri, Bergamo, Italy, who provided individual participant data for REIN‐2 2005;
Kate Fletcher, of the University of Birmingham, Birmingham, UK, and Jonathan Mant, of the University of Cambridge, Cambridge, UK, who provided individual participant data for PAST BP 2016; and
Larrye Loss and Julie Ye, from AstraZeneca, Wilmington (DE), USA, who provided access to protocol, forms, Clinical Study Report, and individual participant data for HOT 1998. This manuscript was not prepared in collaboration with AstraZeneca staff and does not necessarily reflect the opinions or views of the company. According to the Data Transfer Agreement, AstraZeneca was entitled to make comments to the final report, but approval was given with no remarks.
Data from SPS3 2013 were supplied by the National Institute of Neurological Disorders and Stroke (NINDS). This review does not necessarily reflect the opinions or views of the SPS3 study, the NINDS Central Repositories, or NINDS.
AASK 2002 and MDRD 1994 were conducted by the AASK/MDRD Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Data from the AASK 2002 and MDRD 1994 studies reported here were supplied by the NIDDK Central Repositories. This review was not prepared in collaboration with investigators in the AASK 2002 and MDRD 1994 studies, and does not necessarily reflect the opinions or views of the AASK/MDRD studies, the NIDDK Central Repositories, or the NIDDK.
This manuscript was prepared with ACCORD and SPRINT_POP Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordination Center and does not necessarily reflect the opinions or views of ACCORD, SPRINT_POP, or the National Heart, Lung and Blood Institute (NHLBI).
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) ALL <1946 to January 24, 2022>
Search Date: 25 January 2022
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp cardiovascular diseases/
2 ((heart or myocardial) adj5 (attack* or disease* or infarc*)).tw,kf.
3 (coronary adj5 (disease* or syndrome*)).tw,kf.
4 ((cardiovascular or peripheral or vascular) adj5 disease*).tw,kf.
5 atrial fibril*.tw,kf.
6 ((cardiac or heart) adj failure).tw,kf.
7 angina*.tw,kf.
8 exp ischemia/
9 (ischaemi* or ischemi*).tw,kf.
10 exp stroke/
11 (CVA or poststroke or post‐stroke or stroke or strokes).tw,kf.
12 apoplexy.tw,kf.
13 cerebrovascul*.tw,kf.
14 cerebral vascular.tw,kf.
15 ((brain* or cerebral* or lacunar) adj2 (accident* or infarct*)).tw,kf.
16 or/1‐15
17 ((goal? or intensive* or strict* or target* or tight*) adj6 (antihypertensive? or anti‐hypertensive? or bp or control or dbp or diastolic or pressure? or sbp or systolic or treat*)).tw,kf.
18 hypertension/
19 essential hypertension/
20 hypertens*.tw,kf.
21 exp blood pressure/
22 (blood pressure or bloodpressure).tw,kf.
23 or/18‐22
24 randomized controlled trial.pt.
25 controlled clinical trial.pt.
26 randomized.ab.
27 placebo.ab.
28 clinical trials as topic/
29 randomly.ab.
30 trial.ti.
31 or/24‐30
32 animals/ not (humans/ and animals/)
33 31 not 32
34 16 and 17 and 23 and 33 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Hypertension Group Specialised Register via Cochrane Register of Studies
Search Date: 2 February 2022
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 ((intensive* NEAR bp) OR (intensive* NEAR dbp) OR (intensive* NEAR pressure*) OR (intensive* NEAR sbp)) AND INREGISTER
#2 ((strict* NEAR bp) OR (strict* NEAR dbp) OR (strict* NEAR pressure*) OR (strict* NEAR sbp)) AND INREGISTER
#3 ((target* NEAR bp) OR (target* NEAR dbp) OR (target* NEAR pressure*) OR (target* NEAR sbp)) AND INREGISTER
#4 ((tight* NEAR bp) OR (tight* NEAR dbp) OR (tight* NEAR pressure*) OR (tight* NEAR sbp)) AND INREGISTER
#5 #1 OR #2 OR #3 OR #4
#6 ((cardiovascular NEAR disease*) OR (heart NEAR attack*) OR (heart NEAR disease*) OR (heart NEAR infarct*)) AND INREGISTER
#7 ((peripheral NEAR disease*) OR (myocardial NEAR attack*) OR (myocardial NEAR disease*) OR (myocardial NEAR infarct*)) AND INREGISTER
#8 ((coronary NEAR disease*) OR (coronary NEAR syndrome*) OR (vascular NEAR disease*) OR (atrial fibril*)) AND INREGISTER
#9 ((cardiac failure) OR (heart failure) OR (angina*) OR (ischemi*)) AND INREGISTER
#10 (stroke OR (strokes) OR (ischaemi*) OR (CVA)) AND INREGISTER
#11 (apoplexy OR (cerebrovascul*) OR (cerebral vascular) OR (brain accident*)) AND INREGISTER
#12 ((brain infarct*) OR (cerebral NEAR accident*) OR (lacunar NEAR accident*) OR (lacunar NEAR infarct*)) AND INREGISTER
#13 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12
#14 RCT:DE AND INREGISTER
#15 Review:ODE AND INREGISTER
#16 #14 OR #15 #17 #5 AND #13 AND #16 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Cochrane Central Register of Controlled Trials via Cochrane Register of Studies
Search Date: 26 January 2022
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 "A129 202201 FID_17362":FOLDER AND INSEGMENT
#2 "A129 2019 and earlier FID_17230":FOLDER AND INSEGMENT
#3 #1 NOT #2
#4 #3 NOT EXCL:EDT
#5 ((intensive* NEAR bp) OR (intensive* NEAR dbp) OR (intensive* NEAR pressure*) OR (intensive* NEAR sbp)) AND CENTRAL:TARGET
#6 ((strict* NEAR bp) OR (strict* NEAR dbp) OR (strict* NEAR pressure*) OR (strict* NEAR sbp)) AND CENTRAL:TARGET
#7 ((target* NEAR bp) OR (target* NEAR dbp) OR (target* NEAR pressure*) OR (target* NEAR sbp)) AND CENTRAL:TARGET
#8 ((tight* NEAR bp) OR (tight* NEAR dbp) OR (tight* NEAR pressure*) OR (tight* NEAR sbp)) AND CENTRAL:TARGET
#9 #5 OR #6 OR #7 OR #8 AND CENTRAL:TARGET
#10 ((cardiovascular NEAR disease*) OR (heart NEAR attack*) OR (heart NEAR disease*) OR (heart NEAR infarct*)) AND CENTRAL:TARGET
#11 ((peripheral NEAR disease*) OR (myocardial NEAR attack*) OR (myocardial NEAR disease*) OR (myocardial NEAR infarct*)) AND CENTRAL:TARGET
#12 ((coronary NEAR disease*) OR (coronary NEAR syndrome*) OR (vascular NEAR disease*) OR (atrial fibril*)) AND CENTRAL:TARGET
#13 ((cardiac failure) OR (heart failure) OR (angina*) OR (ischemi*)) AND CENTRAL:TARGET
#14 (stroke OR (strokes) OR (ischaemi*) OR (CVA)) AND CENTRAL:TARGET
#15 (apoplexy OR (cerebrovascul*) OR (cerebral vascular) OR (brain accident*)) AND CENTRAL:TARGET
#16 ((brain infarct*) OR (cerebral NEAR accident*) OR (lacunar NEAR accident*) OR (lacunar NEAR infarct*)) AND CENTRAL:TARGET
#17 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 AND CENTRAL:TARGET
#18 #9 AND #17 AND CENTRAL:TARGET ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Embase <1974 to 2022 January 24>
Search Date: 25 January 2022
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp cardiovascular disease/
2 ((heart or myocardial) adj5 (attack* or disease* or infarc*)).tw.
3 (coronary adj5 (disease* or syndrome*)).tw.
4 ((cardiovascular or peripheral or vascular) adj5 disease*).tw.
5 atrial fibril*.tw.
6 ((cardiac or heart) adj failure).tw.
7 angina*.tw.
8 exp ischemia/
9 (ischaemi* or ischemi*).tw.
10 exp stroke/
11 (CVA or poststroke or post‐stroke or stroke or strokes).tw.
12 apoplexy.tw.
13 cerebrovascul*.tw.
14 cerebral vascular.tw.
15 ((brain* or cerebral* or lacunar) adj2 (accident* or infarct*)).tw.
16 or/1‐15
17 ((goal? or intensive* or strict* or target* or tight*) adj6 (antihypertensive? or anti‐hypertensive? or bp or control or dbp or diastolic or pressure? or sbp or systolic or treat*)).tw.
18 exp hypertension/
19 hypertens*.tw.
20 exp blood pressure/
21 (blood pressure or bloodpressure).mp.
22 or/18‐21
23 randomized controlled trial/
24 crossover procedure/
25 double‐blind procedure/
26 (randomi?ed or randomly).tw.
27 (crossover* or cross‐over*).tw.
28 placebo.ab.
29 (doubl* adj blind*).tw.
30 assign*.ab.
31 allocat*.ab.
32 or/23‐31
33 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
34 32 not 33
35 16 and 17 and 22 and 34 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: LILACS (Latin American & Caribbean Health Sciences Literature) Search Date: 27 January 2022 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ ((ti:((angina OR apoplexy OR atrial fibrillation OR brain infarction OR cardiac failure OR cardiovascular disease OR cardiovascular disease OR cerebral infarction OR cerebrovascular OR coronary disease OR coronary diseases OR coronary syndrome OR coronary syndromes OR cva OR heart attack OR heart attacks OR heart disease OR heart diseases OR heart failure OR ischaemia OR ischemia OR lacunar OR myocardial infarction OR myocardial infarctions OR myocardial disease OR myocardial diseases OR peripheral disease OR peripheral diseases OR poststroke OR post‐stroke OR stroke OR vascular disease) )) OR (ab:((angina OR apoplexy OR atrial fibrillation OR brain infarction OR cardiac failure OR cardiovascular disease OR cardiovascular disease OR cerebral infarction OR cerebrovascular OR coronary disease OR coronary diseases OR coronary syndrome OR coronary syndromes OR cva OR heart attack OR heart attacks OR heart disease OR heart diseases OR heart failure OR ischaemia OR ischemia OR lacunar OR myocardial infarctio OR myocardial infarctions OR myocardial disease OR myocardial diseases OR peripheral disease OR peripheral diseases OR poststroke OR post‐stroke OR stroke OR vascular disease) ))) AND ((ti:((intensive* OR strict* OR target* OR tight*) )) OR (ab:((intensive* OR strict* OR target* OR tight*) ))) AND ((ti:((hypertens* OR blood pressure OR bloodpressure))) OR (mh:((hypertens* OR blood pressure OR bloodpressure)))) AND ( db:("LILACS") AND type_of_study:("clinical_trials")) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: ClinicalTrials.gov
Search Date: 27 January 2022
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Condition or disease: (hypertension) AND (angina OR cardiovascular OR myocardial infarction OR peripheral vascular OR stroke) Other terms: (intensive OR strict OR target OR tight) AND (randomized) Study type: Interventional Studies Outcome Measure: blood pressure or BP
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: WHO International Clinical Trials Registry Platform Search Date: 27 January 2022
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 intensive AND blood pressure AND randomized #2 strict AND blood pressure AND randomized #3 target* AND blood pressure AND randomized #4 tight AND blood pressure AND randomized #5 #1 OR #2 OR #3 OR #4
Appendix 2. Reviews and guidelines checked
ACC‐AHA 2017; Arguedas 2013; Arguedas 2020; Bangalore 2011; Bangalore 2013; Bangalore 2017; BPLTTC 2013; BPLTTC 2014; BPLTTC 2021; Drozda 2011; ESH‐ESC 2013; ESH‐ESC 2018; Ettehad 2016; Feldstein 2014; Hypertension CANADA 2020; Lim 2019; Lv 2012; Lv 2013; McBrien 2012; NICE 2022; Rosendorff 2009; Rosendorff 2015; Roy 2010; SBU 2007; Verdecchia 2016; Vidal‐Petiot 2018; WHO 2021; Xie 2016.
Data and analyses
Comparison 1. Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Total mortality | 7 | 9595 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.91, 1.23] |
| 1.2 Total serious adverse events | 7 | 9595 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.08] |
| 1.2.1 Total serious adverse events | 2 | 1673 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| 1.2.2 Subset of total serious adverse events | 5 | 7922 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| 1.3 Total cardiovascular events | 7 | 9595 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 1.00] |
| 1.4 Cardiovascular mortality | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| 1.5 Participant withdrawals due to adverse effects | 3 | 801 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.16 [2.06, 32.28] |
| 1.6 Systolic blood pressure change from baseline at 1 year (mmHg) | 7 | 8657 | Mean Difference (IV, Random, 95% CI) | ‐8.77 [‐12.82, ‐4.73] |
| 1.7 Diastolic blood pressure change from baseline at 1 year (mmHg) | 6 | 8546 | Mean Difference (IV, Random, 95% CI) | ‐4.50 [‐6.35, ‐2.65] |
| 1.8 Blood pressure target achieved at 1 year | 7 | 8699 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.17, 1.23] |
| 1.9 Number of antihypertensive drugs that each participant needed at the end of study | 5 | 7910 | Mean Difference (IV, Random, 95% CI) | 0.56 [0.16, 0.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AASK 2002.
| Study characteristics | ||
| Methods | Multicentre, 3 × 2 factorial design, ITT strategy. Participants randomized equally to a usual mean arterial pressure goal of 102–107 mmHg or to a lower mean arterial pressure goal of ≤ 92 mmHg, and to treatment with metoprolol, ramipril, or amlodipine. When the blood pressure goal was not achieved using the randomized drug, other open‐labelled antihypertensive agents were added to participants' treatment. Participants and investigators were not masked to the blood pressure goal. Follow‐up: 3–6.4 years (mean 3.8 years) |
|
| Participants | African American men and women, aged 18–70 years, with hypertension defined as sitting DBP ≥ 95 mmHg and reduced kidney function, defined as GFR 20–65 mL/min/1.73 m². Exclusion criteria: DBP < 95 mmHg, history of diabetes mellitus, urinary protein‐to‐creatinine ratio > 2.5, accelerated or malignant hypertension within 6 months, secondary hypertension, evidence of non‐blood pressure‐related causes of CKD, serious systemic disease, or clinical CHF. Baseline characteristics of 155 participants (% or mean): men/women: 68%/32%; age: 57 (SD 9) years; SBP: 149 (SD 28) mmHg; DBP: 93 (SD 16) mmHg; MBP: 112 (SD 19) mmHg; current smoker: 31%; types of drugs at 1 year: no information available. Previous cardiovascular condition: IHD: 25%; stroke: 69%; PVD: 23%. Country: USA |
|
| Interventions | Standard (usual) target: MBP 102–107 mmHg Lower target: MBP < 92 mmHg |
|
| Outcomes | Primary outcome: change in GFR (GFR slope). Key secondary outcomes: all cardiovascular events including cardiovascular deaths and hospitalizations for MI, stroke, heart failure, revascularization procedures, and other hospitalized cardiovascular events were reviewed and classified by a blinded endpoints committee according to a prespecified protocol. |
|
| Funding sources | NIDDK Also partially funded by other NIH grants, Office of Research in Minority Health, Pfizer, AstraZeneca, and King Pharmaceuticals. |
|
| Declarations of interest | Quote: "Dr Wright has no stock ownership but has received research grants, honoraria, and consult fees from Astra, Bayer, Bristol‐Myers Squibb, Eli Lilly and Co, Merck & Co, Novartis Pharma AG, Pharmacia, Pfizer, Sankyo Inc, GlaxoSmithKline, and Solvay/Unimed. Dr Appel has received honoraria from Astra and Novartis Pharma AG. Dr Cheek is a speaker for Wyeth, Novartis, and Sanofi‐Synthélabo, and investigator for Abbott Laboratories. Dr Middleton is a speaker for Merck and a consultant for King Pharmaceuticals." | |
| Notes | Among the 3 drug arms planned in the factorial design, the amlodipine group was halted in September 2000. Blood pressure achieved at the end of the trial was: standard target: MBP 104 (SD 7) mmHg; lower target: MBP 95 (SD 8) mmHg. A public repository provided individual participant data from people with hypertension with established CVD for use in this systematic review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The computer screen displayed the blood pressure group to which the patient had been randomized (usual or low). Random permuted blocks with randomly varying block sizes were utilized" (p. S157). |
| Allocation concealment (selection bias) | Low risk | Quote: "The computer screen displayed the blood pressure group to which the patient had been randomized (usual or low)" (p. S157). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design was not compatible with blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All cardiovascular events including cardiovascular deaths and hospitalizations for MIs, strokes, heart failure, revascularization procedures, and other hospitalised cardiovascular events were reviewed and classified by a blinded endpoints committee according to a prespecified protocol" (p. S161). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There did not seem to be a significant imbalance in follow‐up flow diagram, according to Figure 1 (pp. 2421–31). |
| Selective reporting (reporting bias) | Low risk | Protocol checked against cardiovascular outcomes. |
| Other bias | Unclear risk | Subgroup of participants with basal CVD not predefined. |
ACCORD BP 2010.
| Study characteristics | ||
| Methods | Multicentre, 2 × 2 factorial design, ITT strategy. Participants and investigators were not blinded to blood pressure goals. All participants in the ACCORD BP trial were randomly assigned to intensive or standard glycaemic control, and were also randomly assigned to intensive or standard blood pressure control. Follow‐up: 4–8 years (mean 4.7 years) |
|
| Participants | Men and women with type 2 diabetes mellitus and a glycated haemoglobin level ≥ 7.5%, aged 40–79 years with CVD, or 55–79 years with anatomical evidence of a substantial amount of atherosclerosis, albuminuria, or LVH, or ≥ 2 additional risk factors for CVD (dyslipidaemia, hypertension, smoking, or obesity). Participants with SBP of 130–180 mmHg who were taking ≤ 3 antihypertensive medications and who had the equivalent of a 24‐hour protein excretion rate < 1.0 g were also eligible for the blood pressure trial. Exclusion criteria: BMI > 45, sCR level > 1.5 mg/dL, and other serious illness. Baseline characteristics of 1531 participants (% or mean): men/women: 63%/37%; age: 62 (SD 8) years; age ≥ 75 years: 7%; SBP: 138 (SD 16) mmHg; DBP: 74 (SD 11) mmHg; current smoker: 13%; ethnic group: white: 62%, non‐white: 38%. Types of drugs at 1 year: thiazides: 51%; ACEIs/ARBs: 84%; CCB: 26%; BB: 57%; other: 28%. Previous cardiovascular condition: IHD: 86%; stroke: 20%. Countries: USA, Canada |
|
| Interventions | Standard target: SBP < 140 mmHg Lower (intensive) target: SBP < 120 mmHg |
|
| Outcomes | Primary outcome: first occurrence of a major cardiovascular event, defined as the composite of non‐fatal MI, non‐fatal stroke, or cardiovascular death. Secondary outcomes included: combination of the primary outcome plus revascularization or hospitalization for CHF; combination of a fatal coronary event, non‐fatal MI, or unstable angina; non‐fatal MI; fatal or non‐fatal stroke; non‐fatal stroke; death from any cause; death from cardiovascular causes; and hospitalization or death due to heart failure. |
|
| Funding sources | Supported by contracts from the NHLBI. The NIDDK, the National Institute on Aging, the National Eye Institute, and the Centers for Disease Control and Prevention also contributed funding. General Clinical Research Centers provided support at many sites. Several companies provided study medications. | |
| Declarations of interest | Drs Bigger, Buse, Byington, Corson, Cushman, Cutler, Evans, Friedewald, Gerstein, Goff, Grimm, Ismail‐Beigi, Katz, Peterson, and Probstfield declared different types of relationships with NIH institutions and pharmaceutical companies (consultancy, grants, honoraria). | |
| Notes | The glycaemia ACCORD trial was stopped on 6 February 2008. Blood pressures achieved at the end of the trial were as follows: standard target: SBP 133.5 (SD 0.4) mmHg; lower target: SBP 119.3 (SD) 0.4 mmHg. A public repository provided individual participant data from people with hypertension with established CVD for use in this systematic review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed centrally on the study's Web site with the use of permuted blocks to maintain concealment of future study‐group assignments" (pp. 1575–85). |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed centrally on the study's Web site with the use of permuted blocks to maintain concealment of future study‐group assignments" (pp. 1575–85). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design was not compatible with blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "ACCORD utilized a centralized adjudication process for all deaths, and hospitalizations for myocardial infarction and strokes. Upon identification of a potential outcome, clinical site staff obtained medical records or details regarding the case. Personal identifiers and information that may have alerted adjudicators to treatment assignment (e.g. A1C values) were masked by the clinical site and the medical records sent to the Coordinating Center" (pp. 1575–85; Appendix 1). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Consort diagram (section 2) (pp. 1575–85; Appendix 1). |
| Selective reporting (reporting bias) | Low risk | No reporting bias (protocol was checked). |
| Other bias | Unclear risk | Subgroup of participants with basal CVD not predefined. |
HOT 1998.
| Study characteristics | ||
| Methods | Multicentre, 3 × 2 factorial design, ITT strategy. PROBE trial. All participants in the HOT trial were randomly assigned to achieve 3 therapeutic goals (DBP ≤ 90 mmHg, ≤ 85 mmHg, or ≤ 80 mmHg) and to receive aspirin (acetylsalicylic acid) 75 mg daily or placebo under double‐blind conditions. Participants were randomized on the basis of the following baseline variables: age, sex, previous antihypertensive therapy, smoking, previous MI, previous other CHD, previous stroke, and diabetes mellitus. Follow‐up: 3.3–4.9 years (mean 3.8 years) |
|
| Participants | 3232 men and women aged 50–80 years (mean 62 years) with essential hypertension. Required DBP ≥ 100 mmHg and ≤ 115 mmHg on 2 occasions, at least 1 week apart. Exclusion criteria included: malignant or secondary hypertension; stroke or MI within 12 months before randomization; decompensated CHF; serious disease affecting survival during the next 2–3 years; requirement for BB, ACEI, or diuretic treatment for reasons other than hypertension; requirement for antiplatelet or anticoagulant treatment; and diabetes requiring insulin. Baseline characteristics of 3232 participants (% or mean): men/women: 53%/47%; age: 62 years; SBP: 174 (SD 15) mmHg; DBP: 106 (SD 3) mmHg; diabetes: 12%; current smoker: 16%; and ethnic group: white: 92%, non‐white: 8%. Previous cardiovascular condition: IHD: 95%; stroke: 7%. Countries: Argentina, Austria, Belgium, Canada, Denmark, East Asia, Finland, France, Germany, Great Britain, Greece, Hungary, Israel, Italy, Mexico, Norway, South East Asia, Spain, Sweden, Switzerland, Netherlands, and the USA. |
|
| Interventions | Standard target: DBP ≤ 90 mmHg Lower target: DBP ≤ 85 mmHg or ≤ 80 mmHg |
|
| Outcomes | Primary outcomes: pooled major cardiovascular events (non‐fatal MI, non‐fatal stroke, or cardiovascular death) and target blood pressures or DBP achieved during treatment. Secondary outcomes: target DBP and specific outcomes, such as total or cardiovascular mortality, fatal and non‐fatal CHD, and stroke and hospitalization. |
|
| Funding sources | Astra AB (Sweden), Astra Merck Inc. (USA), Teva (Israel), Hoechst (Argentina) | |
| Declarations of interest | Not reported | |
| Notes | Silent MIs were documented by ECG at randomization and final visit. Blood pressures achieved at end of the trial: standard target: DBP 85 (SD 5) mmHg; lower target: DBP 82 (SD 5) mmHg. A private repository provided individual participant data from people with hypertension with established CVD that were used in this Cochrane Review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was computer‐generated based on communications by fax between investigators and the Study Coordinating Centre" (pp. 1755–62). Comment: the randomization procedure was a version of the Pocock‐Simon randomization (Pocock SJ, 1975) Protocol, section 7.3. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was computer‐generated based on communications by fax between investigators and the Study Coordinating Centre" (pp. 1755–62; and protocol, section 7.3). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design was not compatible with blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An Independent Clinical Event Committee evaluated all events (masked)" (pp. 1755–62; and protocol, section 7.2). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "14% of the ECG could not be obtained leading to uncertainty on silent myocardial infarctions. On the other hand, no significant differences among targets have been detected" (Clinical Study Report, p. 23, pp. 1755–62, Figure 1). |
| Selective reporting (reporting bias) | Low risk | The database showed all required results (study protocol, sections 3.1 and 3.2). |
| Other bias | Unclear risk | Subgroup of participants with basal CVD not predefined. |
PAST BP 2016.
| Study characteristics | ||
| Methods | Multicentre, primary care‐based, pragmatic RCT Randomization method used minimization to balance groups on the basis of age (< 80 years, ≥ 80 years), sex, diabetes mellitus, atrial fibrillation, baseline SBP, and practice. Follow‐up: 1 year |
|
| Participants | Men and women with stroke/TIA diagnosis obtained through review of medical records and participant interview. Exclusion criteria: SBP < 125 mmHg at baseline, already taking ≥ 3 antihypertensive agents, orthostatic hypotension, treatment target of 130 mmHg SBP specified, or insufficient corroborative evidence of stroke/TIA from medical record and participant interview. Baseline characteristics of 295 participants (% or mean): men/women: 64%/36%; age: 71 (SD 9) years; SBP: 143 (SD 14) mmHg; DBP: 80 (SD 10) mmHg; current smoker: 13%; ethnic group: Caucasian: 98%. Types of drugs at 1 year: thiazides: 35%; ACEIs/ARBs: 65%; CCB: 43%; BB: 20%; other: 11%. Previous cardiovascular condition: IHD: 22%; stroke: 85%; PVD: 7%. |
|
| Interventions | Standard target: SBP ≤ 140 mmHg Intensive target: SBP ≤ 130 mmHg, or 10 mmHg reduction if baseline SBP 125–140 mmHg |
|
| Outcomes | Primary outcome: change in SBP between baseline and 12 months. Key secondary outcomes: adverse effects, tolerability, and adverse events; clinical outcomes (including major cardiovascular events (composite of fatal and non‐fatal stroke, MI or fatal CHD, and other cardiovascular death), all‐cause mortality and hospital admissions). Key secondary events (stroke, MI, fatal CHD, and other cardiovascular death) were reviewed by independent clinicians blinded to treatment to ensure unbiased coding of these events. |
|
| Funding sources | Financial support from the NIHR Programme Grants for Applied Research funding scheme. | |
| Declarations of interest | Quote: "JM has received grants from Ferrer and the NIHR; RJMcM has received grants from Ferrer during the conduct of the study and grants and personal fees from Omron, grants from Lloyds Pharmacy, personal fees from the Japanese Society of Hypertension, and personal fees from the American Society of Nephrology outside the submitted work; AR has received grants from the University of Birmingham during the conduct of the study; FDRH has received grants from the NIHR and non‐financial support from Omron and Microlife during the conduct of the study; no other relationships or activities that could appear to have influenced the submitted work." | |
| Notes | This study has been concluded and published. Agreement was made with study authors to include data from people with hypertension with established CVD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: "The central study team at the University of Birmingham randomized patients, with minimisation based on age, sex, diabetes mellitus, atrial fibrillation, baseline systolic blood pressure, and general practice. The research nurse ascertained treatment allocation either by telephone or online" (p. i708). "If the patient is eligible and willing to take part, the nurse will also gain written informed consent prior to randomization, and will telephone the randomization service to obtain treatment group allocation" (p. 37). |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization will use minimisation to balance the randomized groups on the basis of age (< 80, ≥ 80), sex, diabetes mellitus, atrial fibrillation (because of the difficulties of obtaining accurate BP measurements in this group), baseline systolic BP and practice" (p. 37). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design was not compatible with blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The outcome measure was not blinded, but a nurse not directly involved in the participant's care obtained it by using an automated sphygmomanometer, so systematic recording bias is unlikely" (p. i708). Quote: "Key secondary events (stroke; myocardial infarction; fatal coronary heart disease and other cardiovascular death) will be reviewed by independent clinicians blinded to treatment to ensure unbiased coding of these events" (p. 37). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Primary outcome data were available for 379 participants at one year follow‐up (182 (68%) in the intensive target arm and 197 (75%) in the standard target arm). All patients were followed up for clinical events and deaths" (p. i708, Figure 1). |
| Selective reporting (reporting bias) | Low risk | No reporting bias (the protocol publication was checked). |
| Other bias | Unclear risk | Only half of the total number of study participants met the review inclusion criteria (participants with previous stroke). |
PRESERVE 2021.
| Study characteristics | ||
| Methods | A 2‐year, randomized, parallel, multicentre controlled, blinded‐outcomes clinical trial, which tested intensive versus standard blood pressure treatment regimens in SVD. Follow‐up: 2 years |
|
| Participants | Participants had a clinical lacunar stroke with an anatomically corresponding lacunar infarct on MRI, in addition to confluent white matter hyperintensities graded ≥ 2 on the Fazekas scale, and were recruited ≥ 3 months after stroke. Participants were aged ≥ 40 years with hypertension defined as SBP > 140 mmHg, or 125–140 mmHg while on antihypertensive medication. Exclusion criteria: known single gene disorder causing SVD, cause of stroke other than SVD (e.g. carotid or vertebral stenosis > 50%, cortical infarction), dementia, life expectancy < 2 years, symptomatic postural hypotension, women of child‐bearing potential, and inability to fulfil study data collection. Countries: UK |
|
| Interventions | Standard target: SBP 130–140 mmHg Lower (intensive) target: SBP < 125 mmHg |
|
| Outcomes | The initial endpoint was a global cognitive score, with DTI‐MRI as a secondary endpoint. After the SPS3 cognition study was published, DTI‐MRI became the primary endpoint. Information on total mortality, cardiovascular events, serious adverse events, withdrawals, and changes in blood pressure were also reported. | |
| Funding sources | Funded by a joint British Heart Foundation and the Stroke Association programme grant (TSA BHF 2010/01). Additional infrastructural support was provided by the NIHR‐funded Newcastle Biomedical Research Centre, the Cambridge University Hospitals NIHR Comprehensive Biomedical Research Centre, and the Sheffield Hospitals NIHR funded Clinical Research Facility. HS Markus, GA Ford, and JT O'Brien are supported by NIHR Senior Investigator awards. The funding organization and sponsor had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication. | |
| Declarations of interest | GA Ford reports personal fees from Amgen, Bayer, Daiichi Sankyo, Medtronic, Pfizer, and Stryker outside the submitted work. JT O'Brien reports personal fees from TauRx, Axon, GE Healthcare, Avid/Lilly, Eisai, Roche, ND Merck outside the submitted work. K Harkness reports financial activities outside the submitted work from Medtronic. The other authors reported no conflicts. | |
| Notes | In SPS3, cognitive change could not be detected over 2 years in 2916 participants with lacunar stroke. Following this, the PRESERVE steering committee met, and with funders agreement, halted recruitment to the cognitive only arm which had a planned sample size of 422, and only recruited to the DTI‐MRI arm (which had a sample size of 180). Target blood pressure difference was achieved by 3 months (intensive, 127 mmHg; standard, 140 mmHg) and maintained for 2 years. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients will be randomised to Usual or Intensive blood pressure lowering. Randomisation will be in the ratio 1:1 and performed via an online randomisation system, available 24 hours, based at the Mental Health & Neuroscience Clinical Trials Unit (MH&N CTU) at the Institute of Psychiatry. Randomisation will be stratified by centre" (Protocol, section 9.3 Randomisation procedure). |
| Allocation concealment (selection bias) | Low risk | Quote: "After recruitment, participants were randomized (stratified by centre) with random allocation concealed until the intervention was assigned" (Stroke 2021, p. 2485). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Treatment allocation was known to the participants and clinical staff, but analysis of MRI and cognitive outcomes was performed blind to treatment allocation" (Stroke 2021, p. 2485). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Treatment allocation was known to the participants and clinical staff, but analysis of MRI and cognitive outcomes was performed blind to treatment allocation" (Stroke 2021, p. 2485). "Stroke and death outcome events were recorded on a proforma and reviewed by 2 adjudicators blinded to treatment" (Stroke 2021, p. 2486). "To avoid bias in outcome assessment there will be blinded assessment of clinical endpoints" (Protocol, section 6.1). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant imbalance between groups |
| Selective reporting (reporting bias) | Low risk | Protocol and main article checked |
| Other bias | High risk | All participants met the base CVD criteria. Trial was assessed as biased because it was stopped early for lack of funding. |
SPRINT 2015.
| Study characteristics | ||
| Methods | Multicentre, randomized, parallel, controlled trial. Blinded to outcomes assessor. Intervention was stopped early after a median follow‐up of 3.26 years. |
|
| Participants | Men and women aged ≥ 50 years with SBP 130–180 mmHg (on 0 or 1 medication), 130–170 mmHg (on up to 2 medications), 130–160 mmHg (on up to 3 medications), or 130–150 mmHg (on up to 4 medications). Participants also had ≥ 1 of the following risk factors:
2 major exclusion criteria: diabetes mellitus and stroke. Other exclusion criteria were secondary hypertension, proteinuria, recent cardiovascular event or procedure, and symptomatic heart failure within the past 6 months. Baseline characteristics of 1562 participants (% or mean): men/women: 76%/24%; age: 70 (SD 9) years; SBP: 138 (SD 16) mmHg; DBP: 74 (SD 12) mmHg; current smoker: 14%; ethnic group: white: 71%. |
|
| Interventions | Standard target: SBP < 140 mmHg Intensive target: SBP < 120 mmHg |
|
| Outcomes | Primary outcome: composite of non‐fatal MI, acute coronary syndrome not resulting in MI, non‐fatal stroke, non‐fatal acute decompensated heart failure, and death from CVD. 3 subgroups were of particular interest: participants with and without CKD, black or non‐black participants, and participants aged < 75 years or ≥ 75 years. SPRINT prespecified secondary outcomes included components of the primary outcome, total mortality, and a composite of the primary outcome (i.e. CVD‐free survival). Additional secondary CVD outcomes included peripheral arterial disease, coronary revascularization, TIA, LVH on ECG, and atrial fibrillation or flutter. Peripheral arterial disease included carotid and peripheral revascularization, abdominal aortic aneurysm repair, and other objectively defined peripheral arterial disease events. |
|
| Funding sources | Federal funds from the NHLBI, NIDDK, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke. | |
| Declarations of interest | Study authors declared no conflicts of interest (in Clinical Trials 2014;11(5):532–46). In New England Journal of Medicine 2015;26;373(22):2103–16, Dr Ambrosius, Dr Johnson, Dr Rahman, Dr Reboussin, Dr Rocco, Dr Sink, Dr Williamson, and Dr Wright Jr, reported grant support from NIH/NHLBI and non‐financial support from Takeda Pharmaceuticals International, Inc, and Arbor Pharmaceuticals, LLC, during the conduct of the study. Dr Cheung and Dr Goff reported grant support from the NIH during the conduct of the study. Dr Cushman reported grant support from the NIH and non‐financial support from Takeda Pharmaceuticals International, Inc, and Arbor Pharmaceuticals, LLC, during the conduct of the study; and personal fees from Takeda and Novartis outside the submitted work. Dr Cutler reported non‐financial support from Takeda International Pharmaceuticals Inc, and Arbor Pharmaceuticals, Inc, during the conduct of the study, and personal fees from the NHLBI outside the submitted work. Dr Fine, Ms Snyder, and Dr Whelton reported non‐financial support from Takeda Pharmaceuticals International, Inc, and Arbor Pharmaceuticals, LLC, during the conduct of the study. Dr Kimmel reported personal fees from Academic Press outside the submitted work. Dr Lewis reported grant support from the NIH and non‐financial support from Takeda Pharmaceuticals International and Arbor Pharmaceuticals during the conduct of the study; and grant support from Novo Nordisk outside the submitted work. Dr Oparil reported grant support from the NIH/NHLBI during the conduct of the study; grant support from Merck and Co, the NIH/NHLBI, Novartis, and Arbor Pharmaceuticals, LLC; grant support and personal fees from AstraZeneca and Bayer; grant support, personal fees, and non‐financial support from Medtronic; and personal fees from Forest Laboratories, Inc, Amgen (Onyx – Subsidiary), Boehringer Ingelheim, and GlaxoSmithKline outside the submitted work. In addition, Dr Oparil was co‐chair (JNC 8): quote: "Evidence‐Based Guideline for the Management of High Blood Pressure in Adults: Report from the Panel Members Appointed to the Eighth Joint National Committee (JNC 8), and Co‐Chair, 2007–2013" (JAMA 2014;311(5):507–20). |
|
| Notes | 4 institutes of the NIH cosponsored SPRINT. Study authors declared no conflicts of interest. A public repository provided individual participant data on people with hypertension with established CVD for use in this Cochrane Review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No detailed information provided on the randomization system used in the trial. Contacted study authors who confirmed via e‐mail that they used a permuted block randomization scheme with random block lengths, stratified by clinic. |
| Allocation concealment (selection bias) | Low risk | Quote: "Participant randomization: SPRINT will use an internet‐based, web browser randomization procedure. Clinical Sites access the randomization application through the study web site. Access to this application is password protected and its communications are encrypted. Once security requirements are satisfied, a series of questions identify and verify the eligibility of the participant. When the session is complete, an e‐mail is sent to the Clinic Coordinator, the appropriate CCN, and the CC indicating that the participant has been properly randomized and appended to the database" (pp. 2103–16). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design was not compatible with blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Participants and study personnel were aware of the study‐group assignments, but outcome adjudicators were not" (pp. 2103–16). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias detected (pp. 2103–16, Figure 1). |
| Selective reporting (reporting bias) | Low risk | No reporting bias detected (protocol checked). |
| Other bias | High risk | Only about 17% of total participants met review inclusion criteria (participants with established CVD). Trial was assessed as biased because it was stopped early for benefit. |
SPS3 2013.
| Study characteristics | ||
| Methods | Multicentre open‐label, clinical trial, ITT strategy, 2 × 2 factorial design with randomization to both an antiplatelet intervention and a target level of SBP control. Follow‐up: 0–8.6 years (mean 3.7 years) |
|
| Participants | Participants aged ≥ 30 years; normotensive or hypertensive; had a recent (within 180 days), symptomatic, MRI‐confirmed lacunar stroke; and were without surgically amenable ipsilateral carotid artery stenosis or high‐risk cardioembolic sources. Main exclusion criteria: disabling stroke (modified Rankin score ≥ 4), previous intracranial haemorrhage from non‐traumatic causes, or cortical ischaemic stroke. Baseline characteristics of 2709 participants (% or mean): men/women: 62%/38%; age: 63 (SD 11) years; SBP: 146 (SD 18) mmHg; DBP: 79 (SD 11) mmHg; diabetes: 36%; current smoker: 20%; ethnic groups: white: 53%, non‐white: 49%. Types of drugs at 1 year: thiazides: 35%; ACEIs/ARBs: 71%; CCBs: 28%; BBs: 27%; other: 8%. Previous cardiovascular condition: IHD: 11%; stroke: 99%. Countries: USA, Canada, Mexico, Ecuador, Peru, Chile, Argentina, and Spain. |
|
| Interventions | Standard (higher) target: SBP 130–149 mmHg Lower target: SBP < 130 mmHg |
|
| Outcomes | Primary outcome: time to recurrent stroke (first of fatal or non‐fatal ischaemic stroke or central nervous system haemorrhage). All possible clinical stroke events were assessed at the clinical site by both the local neurology investigator and a neurologist blinded to assigned treatment groups. Secondary outcomes included acute MI and death, classified as vascular or non‐vascular. Safety events were major cognitive decline, major extracranial (systemic) haemorrhage, serious complication of hypotension, and other SPS3‐related serious adverse events. Serious adverse events were major vascular events and severe adverse events related to hypotension. No information about non‐vascular deaths or severe adverse events other than hypotension‐related events was provided. |
|
| Funding sources | National Institute of Neurological Disorders and Stroke (USA) | |
| Declarations of interest | Study authors declared no conflicts of interest. | |
| Notes | The antiplatelet component of the trial was terminated at the recommendation of the data and safety monitoring committee because of lack of efficacy combined with evidence of harm. Blood pressures achieved at the end of the trial were: standard target SBP 138 (SD 1) mmHg; lower target SBP 127 (SD 1) mmHg. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization assignments were generated using a permuted‐block design (variable block size), stored in each clinical centre's electronic data entry system, and protected from preview" (pp. 164–75). |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization assignments were generated using a permuted‐block design (variable block size), stored in each clinical centre's electronic data entry system, and protected from preview" (pp. 164–75). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design was not compatible with blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The Prospective, Randomized, Open‐label, Blinded Endpoint (PROBE) study design, a standard for international blood pressure trials, was utilised" (pp. 164–75). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After review of individual participant data, no imbalance was found between interventions in relation to reasons for end of SPS3 participation |
| Selective reporting (reporting bias) | Low risk | Only serious adverse events related to hypotension and blood pressure management were reported. Despite repeated attempts to obtain clarification from the study authors, no response was received. At a later stage, all data were reviewed thanks to the National Institute of Neurologic Diseases and Stroke, which provided full access to individual participant data. |
| Other bias | Low risk | All participants met the base CVD criteria. |
ACEI: angiotensin‐converting enzyme inhibitor; ACCORD: Action to Control Cardiovascular Risk in Diabetes; ARB: angiotensin receptor blocker; BB: beta‐blocker; BMI: body mass index; CCB: calcium channel blocker; CHF: congestive heart failure; CKD: chronic kidney disease; CVD: cardiovascular disease; DBP: diastolic blood pressure; DTI‐MRI: diffusion tensor imaging‐magnetic resonance imaging; ECG: electrocardiography/electrocardiogram; GFR: glomerular filtration rate; HOT: Hypertension Optimal Treatment; IHD: ischaemic heart disease; ITT: intention‐to‐treat; LVH: left ventricular hypertrophy; MBP: mean blood pressure; MI: myocardial infarction; min: minute; MRI: magnetic resonance imaging; NHLBI: National Heart, Lung and Blood Institute; NIDDK: National Institute of Diabetes and Digestive and Kidney Diseases; NIH: National Institutes of Health; NIHR: National Institute for Health Research; PROBE: prospective, randomized, open‐label, blinded endpoint; PVD: peripheral vascular disease; RCT: randomized controlled trial; SBP: systolic blood pressure; sCR: serum creatinine; SD: standard deviation; SPRINT: Systolic Blood Pressure Intervention Trial; SPS3: Secondary Prevention of Small Subcortical Strokes; TIA: transient ischaemic attack; SVD: small vessel disease.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| BBB 1994 | Study data have been lost. The principal author (Professor Lennart Hansson) is deceased; Dr Bjorn Dahlöf confirmed that data have not been retained. Bayer was also contacted but confirmed the company does not have any data available for the BBB (Behandla Blodtryck Bättre) study. The journal Blood Pressure, in which BBB results were published, confirmed the manuscript received was essentially as the published version, and the documentation was destroyed about 10 years before (following Professor Hansson's death). The Swedish Council on Health Technology Assessment assessed the study in a report (No. 170/2) but did not have access to the original data. We also approached the Östra Hospital, where Professor Hansson was working at the time the study was conducted. No records were found, and we were told that the legal requirement to keep records safe expired after 15 years. |
| HOSP 2006 | Number of recruited participants much smaller than intended and not enough for analysis of the effects of different levels of target home blood pressure. |
| INFINITY 2019 | < 50 participants in each group with cardiovascular disease at baseline. |
| MDRD 1994 | < 50 participants in each group with cardiovascular disease at baseline. |
| NCT01230216 | Study not completed owing to slow participant enrolment. |
| PODCAST 2013 | < 50 participants in each group with cardiovascular disease at baseline. Study is in progress, but the recruitment phase has closed. |
| REIN‐2 2005 | < 50 participants in each group with cardiovascular disease at baseline. |
| RESTART‐AP 2013 | Study not completed owing to lack of funding, according to information provided by study authors. |
Characteristics of studies awaiting classification [ordered by study ID]
ABCD‐H 1998.
| Methods | Multicentre, controlled, randomized, 2 × 2 factorial design. The ABCD‐H trial included hypertensive (DBP ≥ 90.0 mmHg) NIDDM. Participants were randomized to 1 of 4 groups: intensive treatment with nisoldipine, intensive treatment with enalapril, moderate treatment with nisoldipine, or moderate treatment with enalapril. Participants and investigators were not blinded to blood pressure goals. Follow‐up: 5 years |
| Participants | Adults with NIDDM aged 40–74 years with minimum DBP ≥ 90.0 mmHg. Exclusion criteria: MI, unstable angina or CVA within the previous 6 months, CABG surgery within the previous 3 months, Class III or IV NYHA CHF, absolute need for therapy with ACEI or CCB, haemodialysis or peritoneal dialysis, or sCR concentration > 3 mg/dL (265 mmol/L). Country: USA |
| Interventions | Standard (moderate) target: DBP 80–89 mmHg Intensive target: DBP ≤ 75 mmHg |
| Outcomes | Primary endpoint: effect of intensive or moderate blood pressure control on the change in 24‐hour creatinine clearance assessed every 6 months. Secondary endpoints: effects of intensive as compared with moderate blood pressure control on the incidence of cardiovascular events, retinopathy, clinical neuropathy, urinary albumin excretion, and LVH. All cardiovascular events were reviewed by an independent endpoints committee blinded to participants' assigned treatment groups. Cardiovascular outcomes were defined as death due to cardiovascular events (sudden death, progressive heart failure, fatal MI, fatal arrhythmias, CVAs, or ruptured aortic aneurysm); non‐fatal MI; non‐fatal CVA; heart failure requiring hospital admission; or pulmonary infarction. |
| Notes | Trial included a number of unspecified participants with basal angina as reported in the published article. Study authors were contacted to clarify this issue, but no definitive answer was received before publication of this review. After 67 months of study, the committee recommended discontinuation of nisoldipine therapy among participants with hypertension. |
Cardio‐Sis 2014.
| Methods | Prospective, multicentre, randomized study with 2 parallel groups, ITT strategy, open‐label design. Follow‐up: 2 years |
| Participants | Adults aged > 55 years with uncontrolled SBP (≥ 150 mmHg) and ≥ 1 additional cardiovascular risk factor (cigarette smoking, total cholesterol ≥ 5.2 mmol/L, HDL‐C < 1.0 mmol/L, LDL‐C ≥ 3.4 mmol/L, family history of premature CVD in a first‐degree relative (aged < 65 years in women and < 55 years in men), previous TIA or stroke, or established coronary or peripheral arterial disease). Exclusion criteria: diabetes, kidney failure, chronic atrial fibrillation or flutter, clinically significant hepatic or haematological disorders, alcoholism, or drug addiction, with causes precluding ECG interpretation for LVH, significant valvular heart disease, or any disease causing reduced life expectancy. Baseline characteristics (% or mean): men/women: 52%/48%; age: 71 (SD 7) years; SBP: 159 (SD 9) mmHg; DBP: 85 (SD 9) mmHg; current smoker: 7%; ethnic group: white: 100%. Country: Italy |
| Interventions | Standard (conventional) target: SBP < 140 mmHg Lower (aggressive) target: SBP < 130 mmHg |
| Outcomes | Primary outcome: prevalence of ECG LV hypertrophy at the final 2‐year visit. Main secondary outcome: composite of all‐cause mortality, non‐fatal MI, non‐fatal stroke, TIA, CHF NYHA stage III or IV requiring hospitalization, angina pectoris with objective evidence of myocardial ischaemia, new‐onset atrial fibrillation, coronary revascularization, aortic dissection, occlusive peripheral arterial disease, and kidney failure requiring dialysis. For participants with > 1 event, survival time up to the first event was used in the analysis. The comparison between groups in serial changes in SBP and DBP was another secondary endpoint of the study. |
| Notes | 216 participants (115 in standard group, 101 in lower group) met the inclusion criteria for the review, but additional information on outcomes is needed to obtain useful data. Study authors were contacted, and they forwarded our questions to the Steering Committee. An answer had not been received from the committee before review publication. Blood pressures achieved at the end of the trial were: standard target: SBP 139 (SD 14) mmHg; lower target: SBP 134 (SD 14) mmHg. |
ESH‐CHL‐SHOT 2014.
| Methods | Prospective, multinational, randomized trial, with a 3 × 2 factorial design: 3 different SBP targets; 2 different LDL‐C targets. The trial is designed as a PROBE trial. Expected mean follow‐up: 4 years |
| Participants | Men and women aged ≥ 65 years. Qualifying event is stroke or TIA 1–6 months before randomization. Untreated people should have SBP ≥ 140 mmHg, and those on antihypertensive treatment could be included irrespective of their blood pressure. People not receiving statin treatment with LDL‐C > 2.8 mmol/L, and those on statin treatment with any LDL‐C value could be included. All participants should receive antiplatelet therapy (or anticoagulant whenever indicated) unless contraindicated. Exclusion criteria: people with unstable clinical conditions; clinical disturbances caused by non‐stroke pathology; haemodynamically significant carotid stenosis or requiring carotid revascularization; secondary hypertension; SBP > 140 mmHg with 3 antihypertensive drugs at full doses and orthostatic hypotension; people with LDL‐C > 2.8 mmol/L under full dose of a statin, LDL‐C > 4.5 mmol/L with low dose of a statin or untreated, history of MI if baseline LDL‐C < 1.8 mmol/L; dementia; severe disability (modified Rankin scale > 4); severe CKD defined as sCR > 250 mmol/L. |
| Interventions | Standard target: SBP < 135–145 mmHg Intensive target: SBP < 125–135 mmHg or < 125 mmHg |
| Outcomes | Primary endpoint: occurrence of (recurrent) stroke (fatal and non‐fatal). Secondary cardiovascular endpoints are time to occurrence of: first major cardiovascular event: a composite of cardiovascular death, non‐fatal stroke, non‐fatal MI, vascular interventions, and hospitalized heart failure; CHD events: a composite of sudden death, fatal and non‐fatal MI, unstable angina, and coronary interventions; all‐cause death; cardiovascular death: a composite of fatal stroke, fatal MI, sudden death, any other death attributed to CVD; hospitalized heart failure; new‐onset atrial fibrillation; ischaemic stroke; haemorrhagic stroke; composite of stroke and TIA. |
| Notes | The published byline includes 53 co‐authors; no reported conflicts of interest. The activity of the General Coordinating Centre in Milan is supported by institutional research funds of Fondazione Istituto Auxologico Italiano. It also collaborates the European Society of Hypertension and the Chinese Hypertension League. The trial was terminated in 2020 with less than half the 7500 planned participants, due to slow and insufficient recruitment. We are waiting for the analysis of the data collected in order to be included in this review. |
RESPECT 2019.
| Methods | Prospective, multicentre, open, blinded endpoint, randomized clinical trial that included 140 hospitals in Japan between 20 October 2010 and 7 December 2016. Follow‐up: 3.9 years |
| Participants | Aged 50–85 years, independent ambulation, SBP 130–180 mmHg or DBP 80–110 mmHg on a regimen of 0–3 antihypertensive medications, and a history of stroke within the previous 3 years (evidence of an acute disturbance of focal neurological functions, with symptoms lasting > 24 hours, and symptomatic ischaemic stroke or intracerebral haemorrhage confirmed by MRI or computed tomography). Participation required written informed consent, and approval was provided by all local ethics committees for human research. Exclusion criteria: people in whom stroke onset occurred within previous month. |
| Interventions | Standard target: SBP < 140/90 mmHg or < 130/80 mmHg for people with diabetes, CKD, or history of MI Lower target: SBP < 120/80 mmHg |
| Outcomes | Primary endpoint: recurrent stroke, including ischaemic stroke and intracerebral haemorrhage. Recurrent stroke was clinically defined as a focal neurological deficit persisting for > 24 hours, as confirmed by MRI or computed tomography. Stroke was deemed fatal if death occurred within 30 days. Secondary endpoints: reductions in ischaemic stroke, subtype of ischaemic stroke (including atherothrombotic infarction, cardioembolic infarction, lacunar infarction, or infarction due to other and unknown aetiology), intracerebral haemorrhage, subarachnoid haemorrhage, TIA, acute MI defined by standard criteria (compatible clinical history with changes on ECG or in cardiac enzyme concentration), composite cardiovascular events (cardiovascular death, non‐fatal stroke, and non‐fatal MI), all‐cause death, and the composite of all‐cause death, non‐fatal stroke, and non‐fatal MI. Cardiovascular death was defined as sudden death, fatal stroke, fatal MI, fatal CHF, or death attributed to other CVD. All reported efficacy outcomes were confirmed by a central adjudication committee that was blinded to treatment assignment. Serious adverse events were defined as those that were fatal or life‐threatening, that resulted in clinically significant or persistent disability, that required hospitalization, or that were judged as a significant hazard or harm that required medical or surgical interventions. |
| Notes | Participants assigned to a standard target < 130/80 mmHg should be excluded from our database because they overlap with our lower target criteria (≤ 135/85 mmHg). To date, we have been unable to obtain individual participant data from the study authors to resolve this issue. |
STABLE‐ICAS 2018.
| Methods | Prospective, randomized, open, blinded endpoint trial conducted at 10 centres in South Korea Follow‐up: 6 months |
| Participants | People aged ≥ 40 years with symptomatic ICAS 7–42 days after index ischaemic stroke. Symptomatic ICAS was defined as from the previous trials. Index ischaemic stroke lesions were elucidated on DWIs with a significant stenosis (> 50%) or occlusion at the corresponding middle cerebral artery (M1 portion) or distal ICA documented by MRI or computed tomography angiogram. Among them, those with a mean SBP ≥ 140 mmHg or who were taking antihypertensive medication were enrolled. After 5 minutes of stabilization, blood pressure was measured 3 times at 1‐minute intervals from a sitting position. The participants whose mean SBP of the second and third measurements exceeded 140 mmHg were eligible. Key exclusion criteria: intractable hypertension on screening SBP 150 mmHg with > 3 antihypertensive medications), thrombolytic therapy for index ischaemic stroke without residual steno‐occlusion, orthostatic hypotension, presumed cardioembolic stroke, planned cerebrovascular surgery or intervention within 7 months, absent baseline FLAIR image, severe stroke (National Institute of Health Stroke Scale 16), severe hypertension (mean SBP > 200 mmHg) during screening period and severe heart failure (NYHA class III or IV). The ethics committees of all participating centres approved the protocol. |
| Interventions | Standard (modest) target: SBP 130–140 mmHg Lower (intensive) target: SBP 110–120 mmHg |
| Outcomes | Primary endpoint: WML volume change in the whole forebrain on FLAIR image between baseline and 24 weeks. Secondary radiological endpoints: ischaemic lesion volume change in the ipsilateral hemisphere to the symptomatic ICAS between baseline and 24 weeks and NIL on 24‐weeks FLAIR image. Secondary clinical endpoints were recurrent stroke, MI, and vascular death at 24 weeks. |
| Notes | We were interested in clarifying what type of cardiovascular events were included in the study because information in the article and ClinicalTrials.gov was not coherent. Other controversial issues were the real number of total serious adverse events and ischaemic strokes reported. We made several attempts to contact the study authors but received no response. ClinicalTrials.gov: NCT01104311 |
STEP 2021.
| Methods | Multicentre, prospective, randomized, open‐label, blind endpoint trial Follow‐up: 4 years |
| Participants | Men and women aged 60–80 years, with SBP 140–190 mmHg in the 3 screening visits or currently under antihypertensive treatment and having signed the written informed content. Exclusion criteria: SBP ≥ 190 mmHg or DBP < 60 mmHg; known secondary cause of hypertension; history of large atherosclerotic cerebral infarction or haemorrhagic stroke (not lacunar infarction and TIA); hospitalization for MI or unstable angina within the previous 6 months; coronary revascularization (PCI or CABG) within the previous 12 months; planned to perform coronary revascularization (PCI or CABG) in the next 12 months; history of sustained atrial fibrillation or ventricular arrhythmias at entry influencing the measurement of electronic blood pressure; NYHA class III or IV heart failure at entry or hospitalization for exacerbation of chronic heart failure within the previous 6 months; severe valvular disease or valvular disease likely to require surgery or percutaneous valve replacement during the trial; dilated or hypertrophic cardiomyopathy, rheumatic heart disease, or congenital heart disease; uncontrolled diabetes (serum fasting glucose ≥ 200 mg/dL (11.1 mmol/L, glycated haemoglobin > 8%); laboratory tests indicating abnormal liver or kidney function (ALT > 3 times the upper limit of normal value, or ESKD on dialysis, or eGFR < 30 mL/minute, or sCR > 2.5 mg/dL (> 221 µmol/L)); severe somatic disease such as cancer; severe cognitive impairment or mental disorders; participating in other clinical trials. |
| Interventions | Standard target: 130–149 mmHg Intensive target: 110–129 mmHg |
| Outcomes | Primary outcomes: a composite endpoint comprising MI, first occurrence of symptomatic stroke (ischaemic or haemorrhagic, fatal or non‐fatal), hospitalization for unstable angina or acute decompensated heart failure, coronary revascularization (PCI, CABG), and death from cardiovascular causes. Secondary outcomes: major coronary events comprising MI, hospitalization for unstable angina or acute decompensated heart failure, coronary revascularization (PCI, CABG), and death from cardiovascular causes; first occurrence of symptomatic stroke (ischaemic or haemorrhagic, fatal or non‐fatal); all‐cause death; cardiovascular death; MI; hospitalization for unstable angina; hospitalization for acute decompensated heart failure; coronary revascularization (PCI, CABG); first occurrence of diabetes mellitus; decline in cognitive function; decline in renal function or development of ESKD; major artery function changes. |
| Notes | A formal request has been made to the trial authors, applying for the data of those participants with basal CVD (540 participants, 6.4% of the total sample). |
Zeng 2016.
| Methods | Prospective, controlled, open‐label study Follow‐up: 10 years |
| Participants | People with hypertension aged > 65 years with chronic renal disease III to IV stage and macroproteinuria. Before randomization, all participants had been treated for 1 year with ACEI or ARBs and other antihypertensive drugs, but their SBP > 140 mmHg and < 150 mmHg. |
| Interventions | Standard target: SBP ≤ 150 mmHg Lower target: SBP ≤ 120 mmHg |
| Outcomes | Progress of renal disease and risk of development of CVD. |
| Notes | Complete information for this study is lacking. To date, only an abstract has been published in spite of the fact that the current follow‐up is 10 years. For this reason, the study has been considered as a finished project awaiting classification. |
ABCD‐H: Appropriate Blood Pressure Control in Diabetes – Hypertension; ACEI: angiotensin‐converting enzyme inhibitor; ALT: alanine aminotransferase; ARB: angiotensin receptor blocker; CABG: coronary artery bypass graft; CCB: calcium channel blocker; CHF: congestive heart failure; CKD; chronic kidney disease; CVA: cerebrovascular accident; CVD: cardiovascular disease; DBP: diastolic blood pressure; DWI: diffusion weighted imaging; ECG: electrocardiogram; eGFR: estimated glomerular filtration rate; ESKD: end‐stage kidney disease; FLAIR: fluid‐attenuated inversion recovery; HDL‐C: high‐density lipoprotein cholesterol; ICA: internal carotid artery; ICAS: intracranial atherosclerosis; IHD: ischaemic heart disease; ITT: intention‐to‐treat; LDL‐C: low‐density lipoprotein cholesterol; LV: left ventricular; LVH: left ventricular hypertrophy; MI: myocardial infarction; MRI: magnetic resonance imaging; NIDDM: non‐insulin‐dependent diabetes mellitus; NIL; new ischaemic lesion; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; PROBE: prospective, randomized, open‐label, blinded endpoint; SBP: systolic blood pressure; sCR: serum creatinine; SD: standard deviation; TIA: transient ischaemic attack; WML: white matter lesion.
Characteristics of ongoing studies [ordered by study ID]
BPROAD 2019.
| Study name | BPROAD study |
| Methods | Multicentre, open‐label, parallel‐group, randomized controlled trial that will be conducted across mainland China. Expected follow‐up: 5 years |
| Participants | Inclusion criteria: men and women aged ≥ 50 years; diabetes defined as: a self‐reported previous diagnosis by healthcare professionals and taking antidiabetic medications; fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); SBP ≥ 140 mmHg on 0 medication; 130–180 mmHg on 1 medication; 130–170 mmHg on up to 2 medications; 130–160 mmHg on up to 3 medications; or 130–150 mmHg on up to 4 medications; increased risk of CVD. Exclusion criteria: history consistent with type 1 diabetes; known secondary cause of hypertension; 1‐minute standing SBP < 110 mmHg; arm circumference too large to allow accurate blood pressure measurement with available devices; cardiovascular event or procedure or hospitalization for unstable angina within past 3 months; symptomatic heart failure within past 6 months or left ventricular ejection fraction (by any method) < 35% within the past 6 months; ALT or AST levels more than twice the upper limit of the normal range or active liver diseases; dialysis, kidney transplantation, eGFR < 30 mL/minute/1.73 m², or sCR > 2.0 mg/dL; proteinuria; previous diagnosis of polycystic kidney disease or glomerulonephritis; a medical condition likely to limit survival to < 5 years; any factors judged by the clinic team to be likely to limit adherence to interventions; failure to obtain informed consent from participant; currently participating in another intervention study; currently living with another BPROAD participant; pregnancy, currently trying to become pregnant, or of child‐bearing potential and not using contraception. |
| Interventions | Standard target: SBP < 140 mmHg Lower target: SBP < 120 mmHg |
| Outcomes | Primary outcome: major cardiovascular events. Secondary outcomes: composite of the primary outcome and all‐cause mortality; macrovascular outcomes; major coronary artery diseases; total stroke; heart failure; cardiovascular death; total mortality; cognitive function; health‐related quality of life; kidney outcomes. |
| Starting date | February 2019 |
| Contact information | Guang Ning, MD, PhD; XUYANRR@yahoo.com.cn |
| Notes | Sponsor: Shanghai Jiao Tong University School of Medicine |
EPICS‐Pilot 2020.
| Study name | EPICS‐Pilot 2020 |
| Methods | PROBE assessed randomised, parallel‐group pilot trial |
| Participants | Inclusion criteria: age ≥ 40 years; ischaemic stroke, confirmed by imaging (including TIA with imaging evidence of acute brain ischaemia; living at home and independent (walking without the aid of another person, but may have some help for daily activities – equivalent to Rankin score ≤ 3); SBP ≥ 130 mmHg at entry (mean of 2 measures, seated, after resting alone in office for 5 minutes); qualifying stroke/TIA between 7 days and 1 year of randomization. eGFR ≥ 50 mL/minute/m2; medically stable and capable of participating in a randomised trial, including home blood pressure measures, in the opinion of the study physician; willing to provide informed consent (no surrogate consent will apply). Exclusion criteria: stroke/TIA due to cardioembolism or other defined causes (e.g. dissection, endocarditis, other specified); severe stenosis of large cranio‐cervical artery (> 70% stenosis of cervical carotid, vertebral, or Circle of Willis artery); medical history of primary intracerebral haemorrhage (asymptomatic cerebral microbleeds detected on brain MRI are not excluded); SBP < 110 mmHg after 3 minutes of standing or other contraindication to intensive SBP lowering in opinion of treating clinician (e.g. syncope or presyncope, recurrent falls); unlikely to comply with study procedures (home blood pressure measures, follow‐up visits) due to severe or fatal comorbid illness (e.g. dementia, active malignancy, severe frailty) or other factor (e.g. inability to travel); women of child‐bearing potential. |
| Interventions | Standard target: SBP 130–139 mmHg Lower target: SBP 115–125 mmHg |
| Outcomes | Primary outcome: difference in achieved SBP. Secondary outcomes: time to first composite major vascular event; proportions of participants assigned to target goals successfully reaching target; number of dose‐titrations required; time in target range; loss to follow‐up; time taken to reach target range; change in cognition; change in Montreal cognitive assessment score (range 0–30) at last follow‐up compared with baseline score; quality of life score; change in EQ‐5D‐5L score; difference in mean achieved DBP between groups; change in SBP and DBP from baseline to end‐of‐trial; time required per follow‐up visit; feasibility of remote blood pressure titration; disability in intensive SBP and guideline‐based SBP target participants assessed by modified Rankin score (shift analysis and proportion with no, mild, or moderate disability, Rankin score 0–3); number of adverse events, serious adverse events, and suspected unexpected serious adverse reactions; number of prespecified adverse events; qualitative patient feedback obtained via workshops and questionnaires; total, direct and indirect (e.g. via lost income to study participants or family members) costs associated with face‐to‐face visits for study participants will be quantified. |
| Starting date | June 2021 |
| Contact information | Katrina Tobin; +353 1 716 4576; katrina.tobin@ucd.ie |
| Notes | Responsible party: University College Dublin |
ESPRIT 2019.
| Study name | ESPRIT study |
| Methods | Multicentre, open‐label, randomized controlled trial Expected follow‐up: 4 years |
| Participants | Participants aged ≥ 50 years with a mean baseline SBP ≥ 130 mmHg and history of CVDs or at high vascular risk. |
| Interventions | Standard target: SBP < 140 mmHg Intensive target: SBP < 120 mmHg |
| Outcomes | Primary outcome: number of participants with composite of major CVD events Secondary outcomes: number of participants with: MI; coronary revascularization; non‐coronary revascularization; chronic or acute decompensated heart failure hospitalization or emergency department visit; stroke; cardiovascular death; all‐cause death; composite outcome of the primary composite with all‐cause death; ESKD, a sustained decline in eGFR to < 10 mL/minute/1.73 m², renal death, or a sustained decline of ≥ 40% in eGFR from randomization; all‐cause dementia or mild cognitive impairment. |
| Starting date | August 2019 |
| Contact information | Jing Li, MD, PhD; +86 (10) 6086 6077; jing.li@fwoxford.org Xinghe Huang, PhD; +86 18800120831; xinghe.huang@fwoxford.org |
| Notes | Sponsor: China National Center for Cardiovascular Diseases |
IBIS 2019.
| Study name | IBIS study |
| Methods | Multicentre, randomized, controlled trial |
| Participants | Inclusion criteria: men and women aged ≥ 40 years; history of symptomatic, MRI/CT‐confirmed ischaemic stroke (3–12 months since last acute onset); SBP ≥ 140 mmHg on 0 medication; 135–180 mmHg on 1 medication; 135–170 mmHg on up to 2 medications; 135–160 mmHg on up to 3 medications; or 135–150 mmHg on up to 4 medications. Exclusion criteria: documented symptomatic intracranial or extracranial stenosis (≥ 50%) (or both), or asymptomatic intracranial or extracranial stenosis (≥ 70%) (or both); disabling stroke (modified Rankin score ≥ 4); previous intracranial haemorrhage from a non‐traumatic cause; any symptoms of orthostatic hypotension during the standing blood pressure measurement, or standing SBP < 110 mmHg; severe heart failure (NYHA class III and IV) within the past 6 months or left ventricular ejection fraction (by any method) < 35%; any history of atrial fibrillation, ventricular aneurysm, or suspicion of cardioembolic pathology for stroke; other specific cause of stroke identified by routine clinical care (e.g. arteritis, dissection, migraine/vasospasm, drug abuse); dialysis, eGFR < 20 mL/minute/1.73 m², urine protein‐to‐creatinine ratio ≥ 1 g/g, or albumin‐to‐creatinine ratio ≥ 600 mg/g; planned or probable revascularization (any angioplasty or vascular surgery) within 3 months after screening; a medical condition likely to limit survival to < 3 years; a cancer diagnosed and treated within the past 2 years that, in the judgement of clinical study staff, would compromise a person's ability to comply with the protocol and complete the trial (except non‐melanoma skin cancer, early‐stage prostate cancer, or localized breast cancer); any factors judged by the clinic team to be likely to limit adherence to the intervention; failure to obtain informed consent from a participant; currently participating in another intervention study; pregnant, currently trying to become pregnant, or of child‐bearing potential and not using contraception. |
| Interventions | Standard target: SBP < 140 mmHg Intensive target: SBP < 120 mmHg |
| Outcomes | Primary outcome: stroke event Secondary outcomes: composite major CVD events; MI; non‐MI acute coronary syndrome; heart failure; dementia; all‐cause mortality |
| Starting date | July 2020 |
| Contact information | Jiang He, MD, PhD; 504‐988‐5165; jhe@tulane.edu Yilong Wang, MD, PhD; 011‐86‐13911666571; yilong528@aliyun.com |
| Notes | Sponsors: Tulane University and Beijing Tiantan Hospital |
NCT03666351.
| Study name | NCT03666351 |
| Methods | A prospective, multicentre, randomized, open label, evaluator blind study |
| Participants | Inclusion criteria: men or women aged ≥ 19 years and < 80 years; diagnosis of mild–moderate AS or mild‐moderate AR; applicable to 2.0–3.9 m/second of aortic jet velocity for mild–moderate AS or to 0.2–0.6 cm of vena contracta for mild–moderate AR; diagnosis of hypertension (SBP > 130 mmHg if being treated or SBP > 140 mmHg if being untreated); for women of child‐bearing potential; negative pregnancy test results during the screening period and prior to administration of the investigational product, and agreement on use of medically allowable contraceptive measures (condom, oral contraceptive pills, injectable or implantable contraceptives, intrauterine devices, contraceptive patches, etc.) during the study period; voluntary written consent to taking part in the clinical study and willingness to comply with requirements of the study. Main exclusion criteria: history of a cardiac valve replacement surgery (replacement surgery of mitral valve, aortic valve, or tricuspid valve); accompanied by severe mitral regurgitation; admitted to needing a surgery by the current treatment guidelines; accompanied by symptoms such as angina pectoris, exertional dyspnoea, syncope, etc.; < 50% of left ventricular ejection fraction. |
| Interventions | Standard target: SBP ≤ 140 mmHg Lower target: SBP ≤ 130 mmHg |
| Outcomes | Primary outcome: changes from baseline in left ventricular mass at 24 months Secondary outcomes: changes from baseline in left ventricular global longitudinal strain at 24 months; changes from baseline in E/E' (E: early diastolic LV inflow velocity, E': early diastolic mitral annulus velocity) at 24 months; changes from baseline in LV volumes at 24 months; rate of disease progression; changes from baseline in SBP at 6, 12, 18, and 24 months; cumulative incidence rate for each visit time point; changes from baseline in stroke volume index at 24 months; changes from baseline in left ventricular ejection fraction at 24 months |
| Starting date | August 2018 |
| Contact information | Jong‐Hwan Jeon; +821040984928; jonghwan06@hanmi.co.kr |
| Notes | Sponsor: Hanmi Pharmaceutical Company Limited |
OPTIMAL‐DIABETES 2019.
| Study name | OPTIMAL‐DIABETES study |
| Methods | 2‐group, multicentre, randomized clinical trial Expected follow‐up: 3.5 years |
| Participants | Inclusion criteria: SBP 130–180 mmHg, 130–150 mmHg (if on 0–4 medications), 130–160 mmHg (if on 0–3 medications), 130–170 mmHg (if on 0–2 medications), 130–180 mmHg (if on 0–1 medications); type 2 diabetes; to be considered as having a high cardiovascular risk, including ≥ 1 of the following factors: established CVD, subclinical CVD, CKD, additional cardiovascular risk factors. Main exclusion criteria: refusal to provide written informed consent; body mass index > 45 kg/m², known secondary cause of hypertension, severe renal dysfunction with GFR < 20 mL/minute/1.73 m² calculated by the CKD‐EPI equation; angina at rest Class IV Canadian Cardiovascular Society; acute coronary syndrome in the last 6 months; symptomatic heart failure Class IV NYHA or ejection fraction < 35% on Doppler echocardiography in the last 6 months. |
| Interventions | Standard target: SBP < 140 mmHg Intensive target: SBP < 120 mmHg |
| Outcomes | Primary outcome: time to cardiovascular death, non‐fatal MI, non‐fatal stroke, hospitalization for unstable angina, or hospitalization for heart failure. Secondary outcomes: time to cardiovascular death, non‐fatal MI, or non‐fatal stroke; time to total death, non‐fatal MI, non‐fatal stroke, hospitalization for unstable angina, or hospitalization for heart failure; time to death, cardiovascular death, renal death; time to MI; stroke, ischaemic stroke, haemorrhagic stroke, undetermined type of stroke, TIA; time to hospitalization for unstable angina, heart failure; time to renal outcome; time to mild cognitive impairment, mild cognitive impairment or all‐cause probable dementia, all‐cause probable dementia; total brain volume; white matter lesions volume. |
| Starting date | August 2019 |
| Contact information | Karla Santo, MD, PhD; +55 11 2151‐5915; karla.santo@einstein.br Diogo Moia; +55 11 2151‐5915; diogo.moia@einstein.br |
| Notes | Sponsors: Hospital Israelita Albert Einstein and Ministry of Health, Brazil |
OPTIMAL Stroke 2019.
| Study name | OPTIMAL Stroke study |
| Methods | 2‐group, multicentre, randomized clinical trial Expected follow‐up: 3.5 years |
| Participants | Inclusion criteria: history of ischaemic stroke or TIA, considered clinically stable in the 48 hours prior to inclusion in the study (they will be classified into a recent stroke < 120 days or chronic when > 120 days), AND SBP 130–180 mmHg, 130–180 mmHg and use of up to 1 antihypertensive drug, 130–170 mmHg and use of up to 2 drugs, 130–160 mmHg and use of up to 3 drugs, 130–150 mmHg and use of up to 4 drugs, AND to be considered as having a high cardiovascular risk. Main exclusion criteria: severe disability after the event that qualified for the study, defined as a modified Rankin scale ≥ 4; being part of another clinical trial involving interventions for cardiovascular prevention; body mass index > 45 kg/m²; pregnancy or breastfeeding; secondary hypertension; Class IV Canadian Cardiovascular Society resting angina; refusal to consent; symptomatic heart failure – Class IV NYHA or ejection fraction < 35% on Doppler echocardiography; conditions that, at the investigators' discretion, limit the patient's participation in the study. |
| Interventions | Standard target: SBP < 140 mmHg Intensive target: SBP < 120 mmHg |
| Outcomes | Primary outcome: time to cardiovascular death, non‐fatal MI, non‐fatal stroke, hospitalization for unstable angina or hospitalization for heart failure. Secondary outcomes: time to cardiovascular death, non‐fatal MI or non‐fatal stroke; time to total death, non‐fatal MI, non‐fatal stroke, hospitalization for unstable angina or hospitalization for heart failure; time to non‐fatal MI, non‐fatal stroke, or total death; time to death, renal death; time to renal outcome; time to cardiovascular death; time to stroke, haemorrhagic stroke, ischaemic stroke; unclassified stroke; time to TIA; time to MI; time to hospitalization due to heart failure; unstable angina; time to a composite outcome of mild cognitive impairment or probable all‐cause dementia, mild cognitive impairment, all‐cause probable dementia; total brain volume; white matter lesions volume. |
| Starting date | August 2019 |
| Contact information | Maria Julia Machline Carrion, MD, PhD; 11 2151‐5915 ext 75915; mjuliacarrion@gmail.com Gisele Sampaio Silva, MD, MPH, PhD; 11 2151‐5915 ext 75915; giselesampaio@hotmail.com |
| Notes | Sponsors: Hospital Israelita Albert Einstein and Ministry of Health, Brazil |
ALT: alanine aminotransferase; AR: aortic regurgitation; AS: aortic stenosis; AST: aspartate aminotransferase; BPROAD: Blood Pressure Control Target in Diabetes; CKD: chronic kidney disease; CKD‐EPI: Chronic Kidney Disease Epidemiology Collaboration; CT: computer tomography; CVD: cardiovascular disease; DBP: diastolic blood pressure; DWI: diffusion weighted imaging; eGFR: estimated glomerular filtration rate; ESKD: end‐stage kidney disease; GFR: glomerular filtration rate; HbA1c: glycosylated haemoglobin; LDL‐C: low‐density lipoprotein cholesterol; LV: left ventricular; MI: myocardial infarction; MRI: magnetic resonance imaging; NYHA: New York Heart Association; PROBE: Prospective, Open‐label, Blinded Endpoint; SBP: systolic blood pressure; sCR: serum creatinine; TIA: transient ischaemic attack.
Differences between protocol and review
No amendments to the original protocol have been implemented.
Contributions of authors
LCS is the lead author. He co‐ordinated the review, entered the text of the review into Review Manager 5, conducted external correspondence, appraised inclusion criteria and certainty of evidence, and extracted and analyzed study data.
JGo led the protocol, appraised inclusion criteria and certainty of evidence, extracted study data, and drafted the final review.
JGa appraised inclusion criteria and certainty of evidence, extracted study data, and drafted the final review.
MCC appraised inclusion criteria and certainty of evidence and drafted the final review.
JE appraised inclusion criteria and certainty of evidence, and drafted the final review.
LL appraised inclusion criteria and certainty of evidence, extracted study data, and drafted the final review.
All review authors participated in writing of the Discussion and Conclusions.
Sources of support
Internal sources
-
Navarre Health Service and Health Department of the Government of Navarre, Spain
Working time of authors (employees of the Government of Navarre).
Facilities.
External sources
-
European Social Fund Operational Programme 2007‐2013, Other
50% of the full research project, as salary from September 2012 to December 2015 for the Pharmacotherapy Research Coordinator in the Navarre Health Service (LCS).
-
University of British Columbia, Vancouver, Canada
Bibliographic searches. Methodological support.
Declarations of interest
LCS: none.
JGo: none.
JGa: none.
MCC: has received funding from Servier for a training course on innovation in hospital settings, unrelated to the topic of this systematic review.
JE: none.
LL: none.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
AASK 2002 {published and unpublished data}
- AASK. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial – correction. JAMA 2006;295(23):2726. [DOI: 10.1001/jama.295.23.2726-c] [DOI] [PubMed] [Google Scholar]
- Appel LJ, Middleton J, Miller ER 3rd, Lipkowitz M, Norris K, Agodoa LY, et al. The rationale and design of the AASK cohort study. Journal of the American Society of Nephrology 2003;14(7 Suppl 2):S166-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gassman JJ, Greene T, Wright JT Jr, Agodoa L, Bakris G, Beck GJ, et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). Journal of the American Society of Nephrology 2003;14(7 Suppl 2):S154-65. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K, Bourgoigne J, Gassman J, Hebert L, Middleton J, Phillips RA, et al. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) trial. American Journal of Kidney Diseases 2006;48(5):739-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288(19):2421-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
ACCORD BP 2010 {published and unpublished data}
- ACCORD Study Group, Cushman C, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. New England Journal of Medicine 2010;362(17):1575-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. American Journal of Cardiology 2007;99(12A):21i-33i. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Cushman WC, Grimm RH Jr, Cutler JA, Evans GW, Capes S, Corson MA, et al, ACCORD Study Group. Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. American Journal of Cardiology 2007;99(12A):44i-55i. [PMID: ] [DOI] [PubMed] [Google Scholar]
HOT 1998 {published and unpublished data}
- Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351(9118):1755-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A. The Hypertension Optimal Treatment (HOT) Study: 24-month data on blood pressure and tolerability. Blood Pressure 1997;6(5):313-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A. The Hypertension Optimal Treatment (HOT) Study – patient characteristics: randomization, risk profiles, and early blood pressure results. Blood Pressure 1994;3(5):322-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hansson L. The Hypertension Optimal Treatment Study (the HOT Study). Blood Pressure 1993;2(1):62-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Hansson L, Clement D, Elmfeldt D, Julius S, Rosenthal T, et al. Benefits and risks of more intensive blood pressure lowering in hypertensive patients of the HOT study with different risk profiles: does a J-shaped curve exist in smokers? Journal of Hypertension 2003;21(4):797-804. [DOI: 10.1097/01.hjh.0000052482.18130.b2] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Hansson L, Dahlöf B, Elmfeldt D, Kjeldsen S, Kolloch R, et al. Effects of individual risk factors on the incidence of cardiovascular events in the treated hypertensive patients of the Hypertension Optimal Treatment Study. HOT Study Group. Journal of Hypertension 2001;19(6):1149-59. [PMID: ] [DOI] [PubMed] [Google Scholar]
PAST BP 2016 {published data only}29062286
- Fletcher K, Mant J, McManus R, Campbell S, Betts J, Taylor C, et al. Protocol for PAST BP: a randomised controlled trial of different blood pressure targets for people with a history of stroke of transient ischaemic attack (TIA) in primary care. BMC Cardiovascular Disorders 2010;10:37. [DOI: 10.1186/1471-2261-10-37] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mant J, McManus R, Roalfe A, Fletcher K, Taylor C, Martin U, et al. RCT of different systolic blood pressure targets for people with a history or stroke or transient ischaemic attack: the PAST-BP (Prevention After Stroke – Blood Pressure) study. Journal of Human Hypertension 2014;28:627-8. [Google Scholar]
- Mant J, McManus RJ, Roalfe A, Fletcher K, Taylor CJ, Martin U, et al. Different systolic blood pressure targets for people with history of stroke or transient ischaemic attack: PAST-BP (Prevention After Stroke – Blood Pressure) randomised controlled trial. BMJ 2016;352:i708. [DOI: 10.1136/bmj.i708] [DOI] [PMC free article] [PubMed] [Google Scholar]
PRESERVE 2021 {published data only}ISRCTN37694103
- Croall ID, Tozer DJ, Moynihan B, Khan U, O'Brien JT, Morris RG, et al. Effect of standard vs intensive blood pressure control on cerebral blood flow in small vessel disease. The PRESERVE randomized clinical trial. JAMA Neurology 2018;75(6):720-7. [DOI: 10.1001/jamaneurol.2017.5153] [ISRCTN37694103] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN37694103. How intensively should we treat blood PRESsure in established cERebral small VEssel disease? www.isrctn.com/ISRCTN37694103 (first received 25 January 2012).
- Markus HS, Egle M, Croall ID, Sari H, Khan U, Hassan A, et al. PRESERVE. Randomized trial of intensive versus standard blood pressure control in small vessel disease. Stroke 2021;52:2484-93. [DOI] [PubMed] [Google Scholar]
SPRINT 2015 {published data only}
- Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clinical Trials 2014;11(5):532-46. [DOI: 10.1177/1740774514537404] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard blood-pressure control. New England Journal of Medicine 2015;373(22):2103-16. [DOI: 10.1056/NEJMoa1511939] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow G, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA 2016;315(24):2673-82. [DOI: 10.1001/jama.2016.7050] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
SPS3 2013 {published data only}
- Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, et al, SPS3 Investigators. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. International Journal of Stroke 2011;6(2):164-75. [DOI: 10.1111/j.1747-4949.2010.00573.x] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure LA, Szychowski JM, Benavente O, Hart RG, Coffey CS. A post hoc evaluation of a sample size re-estimation in the Secondary Prevention of Small Subcortical Strokes study. Clinical Trials 2016;13(5):537-44. [DOI: 10.1177/1740774516643689] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPS3 Study Group, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382(9891):507-15. [DOI: 10.1016/S0140-6736(13)60852-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
BBB 1994 {published data only}
- BBB Study Group. The BBB study: a prospective randomized study of intensified antihypertensive treatment. Journal of Hypertension 1988;6(9):693-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hannson L. The BBB study: the effect of intensified antihypertensive treatment on the level of blood pressure, side-effects, morbidity and mortality in "well-treated" hypertensive patients. Behandla Blodtryck Battre. Blood Pressure 1994;3(4):248-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
HOSP 2006 {published data only}
- Kawano Y, Mannami T, Saitoh D, Kojima S, Etani H, Tsuchihashi T, et al. Hypertension control based on HOme Systolic Pressure (HOSP) study: design and interim report of the main study. Journal of Hypertension 2006;24(Suppl 6):329-30. [Google Scholar]
- Kawano Y. HOSP study. Nihon Rinsho [Japanese Journal of Clinical Medicine] 2006;64(Suppl 6):465-9. [PMID: ] [PubMed] [Google Scholar]
INFINITY 2019 {published data only}
- White WB, Marfatia R, Schmidt J, Wakefield DB, Kaplan RF, Bohannon RW, et al. INtensive versus standard ambulatory blood pressure lowering to prevent functional DeclINe in the ElderlY (INFINITY). American Heart Journal 2013;165(3):258-65.e1. [DOI: 10.1016/j.ahj.2012.11.008] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- White WB, Wakefield DB, Moscufo N, Guttmann CR, Kaplan RF, Bohannon RW, et al. Effects of intensive versus standard ambulatory blood pressure control on cerebrovascular outcomes in older people (INFINITY). Circulation 2019;140:1626-35. [DOI: 10.1161/CIRCULATIONAHA.119.041603] [DOI] [PMC free article] [PubMed] [Google Scholar]
MDRD 1994 {published and unpublished data}
- Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. New England Journal of Medicine 1994;330(13):877-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT01230216 {published data only}
- NCT01230216. Effect of intensive blood pressure control on progression of coronary atherosclerosis: randomized evaluation by intravascular ultrasound. clinicaltrials.gov/show/NCT01230216 (first received 28 October 2010).
PODCAST 2013 {published data only}85562386
- Blackburn DJ, Krishnan K, Fox L, Ballard C, Burns A, Ford GA, et al. Prevention of Decline in Cognition after Stroke Trial (PODCAST): a study protocol for a factorial randomised controlled trial of intensive versus guideline lowering of blood pressure and lipids. Trials 2013;14:401. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
REIN‐2 2005 {published data only}
- Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005;365(9463):939-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
RESTART‐AP 2013 {published data only}
- Arima H, Wang J, Bladin C, Heeley E, Billot L, Barzi F, et al. Rationale of the REstart or STop Antithrombotics Randomised Trial in Asia Pacific (RESTART-AP). Cerebrovascular Diseases 2013;36(Suppl 1):39-40. [Google Scholar]
References to studies awaiting assessment
ABCD‐H 1998 {published data only}
- Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. New England Journal of Medicine 1998;338(10):645-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Estacio RO, Savage S, Nagel NJ, Schrier RW. Baseline characteristics of participants in the Appropriate Blood Pressure Control in Diabetes trial. Controlled Clinical Trials 1996;17(3):242-57. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Savage S, Johnson Nagel N, Estacio RO, Feig PU, MacCarthy EP, Lukken NJ, et al. The ABCD (Appropriate Blood Pressure Control in Diabetes) trial. Rationale and design of a trial of hypertension control (moderate or intensive) in type II diabetes. Online Journal of Current Clinical Trials 1993;2(1):1-25. [PMID: ] [PubMed] [Google Scholar]
Cardio‐Sis 2014 {published data only}
- Cardio-Sis Study Group, Verdecchia P, Staessen JA, Achilli A, Simone G, Ganau A, Mureddu G, et al. Randomized study of traditional versus aggressive systolic blood pressure control (Cardio-Sis): rationale, design and characteristics of the study population. Journal of Human Hypertension 2008;22(4):243-51. [DOI: 10.1038/sj.jhh.1002313] [DOI] [PubMed] [Google Scholar]
- Reboldi G, Angeli F, Simone G, Staessen JA, Verdecchia P, Cardio-Sis Investigators. Tight versus standard blood pressure control in patients with hypertension with and without cardiovascular disease. Hypertension 2014;63(3):475-82. [DOI: 10.1161/HYPERTENSIONAHA.113.02089] [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Staessen JA, Angeli F, Simone G, Achilli A, Ganau G, et al, Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009;374(9689):525-33. [DOI: 10.1016/S0140-6736(09)61340-4] [DOI] [PubMed] [Google Scholar]
ESH‐CHL‐SHOT 2014 {published data only}
- Liu L, Mancia G. Termination of the ESH-CHL-SHOT trial. Journal of Hypertension 2020;38(12):2542-3. [DOI: 10.1097/HJH.0000000000002660] [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Liu L, Mancia G, Parati G, Grassi G, Stramba-Badiale M, et al. Blood pressure and LDL-cholesterol targets for prevention of recurrent strokes and cognitive decline in the hypertensive patient: design of the European Society of Hypertension-Chinese Hypertension League Stroke in Hypertension Optimal Treatment randomized trial. Journal of Hypertension 2014;32(9):1888-97. [DOI: 10.1097/HJH.0000000000000254] [PMID: ] [DOI] [PubMed] [Google Scholar]
RESPECT 2019 {published data only (unpublished sought but not used)}
- Kitagawa K, Yamamoto Y, Arima H, Maeda T, Sunami N, Kanzawa T, et al. Effect of standard vs intensive blood pressure control on the risk of recurrent stroke. A randomized clinical trial and meta-analysis. JAMA Neurology 2019;76(11):1309-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
STABLE‐ICAS 2018 {published data only (unpublished sought but not used)}
- Park JM, Kim BJ, Kwon SU, Hwang YH, Heo SH, Rha JH, et al. Intensive blood pressure control may not be safe in subacute ischemic stroke by intracranial atherosclerosis: a result of randomized trial. Journal of Hypertension 2018;36:1936-41. [DOI] [PubMed] [Google Scholar]
STEP 2021 {published data only}
- NCT03015311. Strategy of blood pressure intervention in the elderly hypertensive patients (STEP) [Strategy of systolic blood pressure intervention in the elderly hypertensive patients: a prospective randomized open-label blinded-endpoint trial]. clinicaltrials.gov/show/NCT03015311 (first received 2 January 2017).
- Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, et al. Trial of intensive blood-pressure control in older patients with hypertension. New England Journal of Medicine 2021;385(14):1268-79. [DOI: 10.1056/NEJMoa2111437] [DOI] [PubMed] [Google Scholar]
Zeng 2016 {published data only (unpublished sought but not used)}
- Zeng X, Zeng XH, Liu ZH, Li YY, Zeng XJ, Chen XY. Ten year target systolic blood pressure less than 120 mmHg for more than 65 aged hypertension patients with chronic renal disease. European Heart Journal Supplements 2016;37:264. [Abstract: P1456] [Google Scholar]
References to ongoing studies
BPROAD 2019 {published data only}
- NCT03808311. Blood Pressure Control Target in Diabetes (BPROAD). clinicaltrials.gov/ct2/show/NCT03808311 (first received 17 January 2019).
EPICS‐Pilot 2020 {published data only}
- NCT04647292. European blood pressure intensive control after stroke (EPICS-Pilot). clinicaltrials.gov/ct2/show/NCT04647292 (first received 30 November 2020).
ESPRIT 2019 {published data only}
- NCT04030234. Effects of Intensive Systolic Blood Pressure Lowering Treatment in Reducing RIsk of Vascular evenTs (ESPRIT). clinicaltrials.gov/ct2/show/NCT04030234 (first received 23 July 2019).
IBIS 2019 {published data only}
- NCT03585595. Intensive Blood Pressure Intervention in Stroke (IBIS) trial. clinicaltrials.gov/ct2/show/NCT03585595 (first received 13 July 2018).
NCT03666351 {published data only}
- NCT03666351. Study to evaluate the effect on improvement of LVH by the control of BP in hypertension patients with AV disease. clinicaltrials.gov/ct2/show/NCT03666351 (first received 11 September 2018).
OPTIMAL‐DIABETES 2019 {published data only}
- NCT04040634. OPtimal blood pressure for the prevenTIon of Major vAscuLar Events in patients with DIABETES Mellitus (OPTIMAL-DIABETES). clinicaltrials.gov/ct2/show/NCT04040634 (first received 1 August 2019).
OPTIMAL Stroke 2019 {published data only}
- NCT04036409. OPtimal blood pressure for the prevenTIon of Major vAscuLar Events in Stroke Patients (OPTIMAL Stroke). clinicaltrials.gov/ct2/show/NCT04036409 (first received 29 July 2019).
Additional references
ACC‐AHA 2017
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. Journal of the American College of Cardiology 2017;17:S0735-S1097. [DOI: 10.1016/j.jacc.2017.11.006] [DOI] [Google Scholar]
AHA 2007
- Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL, et al American Heart Association Council for High Blood Pressure Research, American Heart Association Council on Clinical Cardiology, American Heart Association Council on Epidemiology and Prevention. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Council on Clinical Cardiology and Epidemiology and Prevention. Circulation 2007;115(21):2761-88. [DOI: 10.1161/circulationaha.107.183885] [DOI] [PubMed] [Google Scholar]
Arguedas 2013
- Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD008277. [DOI: 10.1002/14651858.CD008277.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arguedas 2020
- Arguedas JA, Leiva V, Wright JM. Blood pressure targets in adults with hypertension. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD004349. [DOI: 10.1002/14651858.CD004349.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Auer 2018
- Auer J, Sharman JE, Weber T. J-curves in hypertension: what do they tell us about treatment of high blood pressure? European Heart Journal 2018;0:1-4. [DOI: 10.1093/eurheartj/ehy337] [DOI] [PubMed] [Google Scholar]
Bangalore 2010
- Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, et al. J-curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) trial. European Heart Journal 2010;31(23):2897-908. [DOI: 10.1093/eurheartj/ehq328] [DOI] [PubMed] [Google Scholar]
Bangalore 2011
- Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and Bayesian random-effects meta-analyses of randomized trials. Circulation 2011;123(24):2799-810. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bangalore 2013
- Bangalore S, Kumar S, Volodarskiy A, Messerli FH. Blood pressure targets in patients with coronary artery disease: observations from traditional and Bayesian random effects meta-analysis of randomised trials. Heart (British Cardiac Society) 2013;99(9):601-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bangalore 2017
- Bangalore S, Toklu B, Gianos E, Schwartzbard A, Weintraub H, Ogedegbe G, et al. Optimal systolic blood pressure target after SPRINT: insights from a network meta-analysis of randomized trials. American Journal of Medicine 2017;130(6):707-19. [DOI: 10.1016/j.amjmed.2017.01.004] [DOI] [PubMed] [Google Scholar]
BPLTTC 2013
- Blood Pressure Lowering Treatment Trialists' Collaboration, Ninomiya T, Perkovic V, Turnbull F, Neal B, Barzi F, Cass A, et al. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta-analysis of randomised controlled trials. BMJ 2013;347:f5680. [DOI: 10.1136/bmj.f5680] [DOI] [PMC free article] [PubMed] [Google Scholar]
BPLTTC 2014
- Blood Pressure Lowering Treatment Trialists' Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet 2014;384(9943):591-8. [DOI: 10.1016/S0140-6736(14)61212-5] [DOI] [PubMed] [Google Scholar]
BPLTTC 2021
- Blood Pressure Lowering Treatment Trialists' Collaboration. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet 2021;397:1625-36. [DOI: 10.1016/S0140-6736(21)00590-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brunström 2016
- Brunström M, Carlberg B. Lower blood pressure targets: to whom do they apply? Lancet 2016;387(10017):405-6. [DOI: 10.1016/S0140-6736(15)00816-8] [DOI] [PubMed] [Google Scholar]
Carey 2020
- Carey RM. Evidence for the universal blood pressure goal of <130/80 mm Hg is strong: controversies in hypertension – pro side of the argument. Hypertension 2020;76(5):1384-90. [DOI: 10.1161/HYPERTENSIONAHA.120.14647] [DOI] [PMC free article] [PubMed] [Google Scholar]
CHEP 2015
- Daskalopoulou SS, Rabi DM, Zarnke KB, Dasgupta K, Nerenberg K, Cloutier L, et al. The 2015 Canadian Hypertension Education Program. Recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Canadian Journal of Cardiology 2015;31(5):549-68. [DOI: 10.1016/j.cjca.2015.02.016] [DOI] [PubMed] [Google Scholar]
Drozda 2011
- Drozda J Jr, Messer JV, Spertus J, Abramowitz B, Alexander K, Beam CT, et al. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with coronary artery disease and hypertension: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. Circulation 2011;124(2):248-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
ESC 2016
- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. European Heart Journal 2016;37(29):2315-81. [DOI: 10.1093/eurheartj/ehw106] [DOI] [PMC free article] [PubMed] [Google Scholar]
ESC 2022
- Visseren FL, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. European Heart Journal 2022;29(1):5-115. [DOI: 10.1093/eurjpc/zwab154] [DOI] [PubMed] [Google Scholar]
ESH 2009
- Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. Journal of Hypertension 2009;18(6):308-47. [DOI: 10.1097/hjh.0b013e328333146d] [DOI] [PubMed] [Google Scholar]
ESH‐ESC 2007
- Mancia G, Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European Heart Journal 2007;28(12):1462-536. [DOI: 10.1093/eurheartj/ehm236] [PMID: ] [DOI] [PubMed] [Google Scholar]
ESH‐ESC 2013
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European Heart Journal 2013;34(28):2159-219. [DOI: 10.1093/eurheartj/eht151] [DOI] [PubMed] [Google Scholar]
ESH‐ESC 2018
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. European Heart Journal 2018;39(33):3021-104. [DOI: 10.1093/eurheartj/ehy339] [DOI] [PubMed] [Google Scholar]
Ettehad 2016
- Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387(10022):957-67. [DOI] [PubMed] [Google Scholar]
Farnett 1991
- Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA 1991;265(4):489-95. [PubMed] [Google Scholar]
Feldstein 2014
- Feldstein CA. Lowering blood pressure to prevent stroke recurrence: a systematic review of long-term randomized trials. Journal of the American Society of Hypertension 2014;8(7):503-13. [DOI: 10.1016/j.jash.2014.05.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Filippone 2011
- Filippone EJ, Foy A, Newman E. Goal-directed antihypertensive therapy: lower may not always be better. Cleveland Clinic Journal of Medicine 2011;78(2):123-33. [DOI: 10.3949/ccjm.78a.10101] [DOI] [PubMed] [Google Scholar]
GBD 2017 Mortality
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1736-88. [10.1016/S0140-6736(18)32203-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2017 Risk factors
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1923-94. [DOI: 10.1016/S0140-6736(18)32225-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane 2022. Available from www.training.cochrane.org/handbook.
Hypertension CANADA 2020
- Rabi DM, McBrien KA, Sapir-Pichhadze R, Nakhla M, Ahmed SB, Dumanski SM, et al. Hypertension Canada's 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Canadian Journal of Cardiology 2020;36:596-624. [DOI: 10.1016/j.cjca.2020.02.086] [DOI] [PubMed] [Google Scholar]
ICH 1995
- European Medicines Agency. Clinical safety data management: definitions and standards for expedited reporting (CPMP/ICH/377/95), 1995. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002749.pdf (accessed before 25 September 2017).
JNC‐7 2003
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The JNC 7 Report. JAMA 2003;289(19):2560-72. [DOI: 10.1001/jama.289.19.2560] [DOI] [PubMed] [Google Scholar]
JNC‐8 2014
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311(5):507-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaul 2020
- Kaul S. Evidence for the universal blood pressure goal of <130/80 mm Hg is strong: controversies in hypertension – con side of the argument. Hypertension 2020;76(5):1391-9. [DOI: 10.1161/HYPERTENSIONAHA.120.14648] [DOI] [PubMed] [Google Scholar]
Kernan 2014
- Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45(7):2160-236. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lewington 2002
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360(9362):1903-13. [DOI] [PubMed] [Google Scholar]
Lim 2019
- Lim MK, Ha SC, Luk KH, Yip WK, Tsang CS, Wong MC. Update on the Hong Kong Reference Framework for Hypertension Care for Adults in Primary Care Settings – review of evidence on the definition of high blood pressure and goal of therapy. Hong Kong Medical Journal 2019;25(1):64-7. [DOI: 10.12809/hkmj187701] [DOI] [PubMed] [Google Scholar]
Lv 2012
- Lv J, Neal B, Ehteshami P, Ninomiya T, Woodward M, Rodgers A, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: a systematic review and meta-analysis. PLOS Medicine 2012;9(8):e1001293. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lv 2013
- Lv J, Ehteshami P, Sarnak MJ, Tighiouart H, Jun M, Ninomiya T, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. Canadian Medical Association Journal 2013;185(11):949-57. [DOI: 10.1503/cmaj.121468] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mancia 2011
- Mancia G, Grassi G, Zanchetti A. Antihypertensive treatment and blood pressure in diabetic and nondiabetic patients: the lower, the better? Diabetes Care 2011;34(Suppl 2):S304-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mancia 2014
- Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: the J-curve: pro side of the argument. Hypertension 2014;63(1):29-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
McBrien 2012
- McBrien K, Rabi DM, Campbell N, Barnieh L, Clement F, Hemmelgarn BR, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Archives of Internal Medicine 2012;172(17):1296-303. [PMID: ] [DOI] [PubMed] [Google Scholar]
Messerli 2006
- Messerli FH, Mancia G, Conti R, Hewkin AC, Kupfer S, Champion A, et al. Dogma disputed: can aggressively lowering pressure in hypertensive patients with coronary artery disease be dangerous? Annals of Internal Medicine 2006;144(12):884-93. [DOI] [PubMed] [Google Scholar]
Mozaffarian 2015
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 2015;131(4):e29-322. [PMID: ] [DOI] [PubMed] [Google Scholar]
NICE 2022
- National Institute for Heath and Care Excellence. Hypertension in adults: diagnosis and management. www.nice.org.uk/guidance/ng136 (accessed 22 March 2022).
Reboussin 2020 [pers comm]
- Reboussin DM. Random sequence generation method in SPRINT [personal communication]. E-mail to: LC Saiz 2 July 2020.
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosendorff 2009
- Rosendorff C, Black HR. Evidence for a lower target blood pressure for people with heart disease. Current Opinion in Cardiology 2009;24(4):318-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosendorff 2015
- Rosendorff C, Lackland DT, Allison M, Aronow WS, Black HR, Blumenthal RS, et al, American Heart Association, American College of Cardiology, and American Society of Hypertension. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension 2015;65(6):1372-407. [PMID: ] [DOI] [PubMed] [Google Scholar]
Roy 2010
- Roy M, Mahmood N, Rosendorff C. Evidence for aggressive blood pressure-lowering goals in patients with coronary artery disease. Current Atherosclerosis Reports 2010;12(2):134-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sánchez 2021
- Sánchez JF, Escudero-Sánchez G, Calderón-García JF, Rico-Martín S, Robles NR, Bacaicoa MA, et al. Systolic blood pressure and outcomes in stable outpatients with recent symptomatic artery disease: a population-based longitudinal study. International Journal of Environmental Research and Public Health 2021;18(17):9348. [DOI: 10.3390/ijerph18179348] [DOI] [PMC free article] [PubMed] [Google Scholar]
SBU 2007
- SBU. Moderately elevated blood pressure. A systematic literature review. Swedish Council on Health Technology Assessment in Health Care (SBU); 2007. SBU report no 170. www.sbu.se/en/publications/sbu-assesses/moderately-elevated-blood-pressure/ (accessed prior to 25 September 2017).
Townsend 2016
- Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. European Heart Journal 2016;37(42):3232-45. [DOI: 10.1093/eurheartj/ehw334] [DOI] [PubMed] [Google Scholar]
Upadhyay 2011
- Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Annals of Internal Medicine 2011;154(8):541-8. [DOI: 10.7326/0003-4819-154-8-201104190-00335] [PMID: ] [DOI] [PubMed] [Google Scholar]
Verdecchia 2014
- Verdecchia P, Angeli F, Mazzotta G, Garofoli M, Reboldi G. Aggressive blood pressure lowering is dangerous: the J-curve: con side of the argument. Hypertension 2014;63(1):37-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Verdecchia 2016
- Verdecchia P, Angeli F, Gentile G, Reboldi G. More versus less intensive blood pressure-lowering strategy. Cumulative evidence and trial sequential analysis. Hypertension 2016;68:642-53. [DOI: 10.1161/HYPERTENSIONAHA.116.07608] [DOI] [PubMed] [Google Scholar]
Vidal‐Petiot 2016
- Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016;388(10056):2142-52. [DOI: 10.1016/S0140-6736(16)31326-5] [DOI] [PubMed] [Google Scholar]
Vidal‐Petiot 2018
- Vidal-Petiot E, Sorbets E, Bhatt DL, Ducrocq G, Elbez Y, Ferrari R, et al. Potential impact of the 2017 ACC/AHA guideline on high blood pressure in normotensive patients with stable coronary artery disease: insights from the CLARIFY registry. European Heart Journal 2018;0:1-9. [DOI: 10.1093/eurheartj/ehy488] [ISRCTN43070564] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2021
- World Health Organization. Guideline for the pharmacological treatment of hypertension in adults. apps.who.int/iris/bitstream/handle/10665/344424/9789240033986-eng.pdf (accessed 16 August 2022). [ISBN (ELECTRONIC VERSION): 978-92-4-003398-6]
Xie 2016
- Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 2016;387(10017):435-43. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gorricho 2013
- Gorricho J, Garjón J, Celaya MC, Muruzábal L, Montoya R, López Andrés A, et al. Blood pressure targets for the treatment of patients with hypertension and cardiovascular disease. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD010315. [DOI: 10.1002/14651858.CD010315] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saiz 2017
- Saiz LC, Gorricho J, Garjón J, Celaya MC, Muruzábal L, Malón MD, et al. Blood pressure targets for the treatment of people with hypertension and cardiovascular disease. Cochrane Database of Systematic Reviews 2017, Issue 10. Art. No: CD010315. [DOI: 10.1002/14651858.CD010315.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saiz 2020
- Saiz LC, Gorricho J, Garjón J, Celaya MC, Erviti J, Leache L. Blood pressure targets for the treatment of people with hypertension and cardiovascular disease. Cochrane Database of Systematic Reviews 2020, Issue 9. Art. No: CD010315. [DOI: 10.1002/14651858.CD010315.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


