Abstract
Introduction:
Transgender/gender-diverse (TGD) youth are treated with gonadotropin-releasing hormone agonists (GnRHa) to halt endogenous puberty and prevent the development of secondary sex characteristics discordant with their gender identity. This treatment may have significant impact on growth and height velocity (HV).
Methods:
Participants were recruited prior to GnRHa initiation from four gender specialty clinics in the United States. Anthropometric, laboratory, and Tanner-stage data were abstracted from medical records.
Results:
Fifty-five TGD youth (47% designated male at birth) with a mean ± standard deviation age of 11.5 ± 1.2 years were included in the analysis. HV in the first year of GnRHa use was median (interquartile range) 5.1 (3.7 – 5.6) cm/year. Later Tanner stage at GnRHa initiation was associated with lower HV: 5.3 (4.4 – 5.6) cm/year for Tanner stage II, 4.4 (3.3 – 6.0) cm/year for Tanner stage III, and 1.6 (1.5 – 2.9) cm/year for Tanner stage IV (p = 0.001). When controlled for age, there was not a significant difference in mean HV between TGD youth and pre-pubertal youth; however, when stratified by Tanner stage individuals starting GnRHa at Tanner stage IV had a HV below that of prepubertal youth (1.6 (1.5 – 2.9) vs. 6.1 (4.3 – 6.5) cm/year, p =0.006).
Conclusions:
Overall, TGD youth treated with GnRHa have HV similar to that of prepubertal children, but TGD youth who start GnRHa later in puberty have a HV below the pre-pubertal range. Ongoing follow-up of this cohort will determine the impact of GnRHa treatment on adult height.
Implications and Contributions:
This study describes the HV in the first year of GnRHa treatment in pubertal participants in a large multi-site cohort of TGD youth in the United States. Overall, TGD youth treated with GnRHa have HV similar to that of prepubertal children, but TGD youth who start GnRHa later in puberty have a HV below the pre-pubertal range.
Keywords: Transgender, gender-diverse, GnRH analog, growth, height velocity, puberty
INTRODUCTION
Increasing numbers of transgender/gender-diverse (TGD) youth, individuals whose gender identity is not congruent with their sex designated at birth, are seeking gender-affirming medical care (1). Gender-affirming medical treatment may include gonadotropin-releasing hormone agonists (GnRHa) in youth entering puberty to halt further development of secondary sex characteristics that are incongruent with the individual’s gender identity (2). The rise in sex hormones during puberty stimulates increases in growth hormone (GH) and insulin-like growth factor 1 (IGF1) that drive skeletal growth in adolescents (3)(4). Thus altering endogenous puberty with GnRHa treatment has the potential to change height velocity (HV) and ultimately alter adult height. The typical height difference between men and women makes height an important part of how an individual’s gender is perceived. If a transmasculine individual desires an adult height in the typical range for cisgender men or if a transfeminine individual desires an adult height in the typical range for cisgender women, the height gained during puberty may be especially important.
Despite the importance of height to patients and families, there are limited data regarding growth in this population. In the absence of these data, providers must turn to HV data derived from children treated with GnRHa for central precocious puberty (CPP), but it is difficult to extrapolate data in children with CPP to TGD youth for several reasons. First, patients with CPP are experiencing an abnormally early puberty whereas TGD youth presenting for gender-affirming medical care typically experience a normally timed puberty. Second, patients with CPP are treated with GnRHa at younger ages than TGD youth. Third, because CPP is more common in children designated female at birth, studies of patients with CPP provide limited data for individuals designated male at birth (5)(6). Thus, children with CPP or other concerns about puberty and/or growth may have different HV responses to GnRHa compared to TGD youth with normal growth and pubertal development.
Data from the Netherlands have shown a drop in height standard deviation in TGD youth treated with GnRHa for various lengths of time (7)(8)(9), but have not evaluated HV based on Tanner stage at GnRHa initiation or compared HV to pre-pubertal cisgender controls. In addition, it is not clear whether findings from their population can be extrapolated to more diverse populations.
Our aim in this study was to quantify the growth of TGD youth starting GnRHa therapy. Here we present the HV in the first year of GnRHa treatment in pubertal participants in the Trans Youth Care - United States (TYCUS) study, the largest prospective study of TGD youth in the United States.
METHODS
The TYCUS study is a multi-site, prospective observational study of gender-affirming medical care at four academic medical centers with multidisciplinary clinics dedicated to serving TGD youth: Children’s Hospital Los Angeles/University of Southern California, Boston Children’s Hospital/Harvard Medical School, the Ann & Robert H. Lurie Children’s Hospital of Chicago/Northwestern University, and the Benioff Children’s Hospital/University of California San Francisco. A full description of the study protocol is published (10). TGD youth initiating GnRHa treatment for puberty suppression were recruited between July 2016 and September 2018. Participants were excluded from the study if they had previously undergone GnRHa treatment, had a diagnosis of precocious puberty, had serious psychiatric symptoms, or could not read or understand English. Anthropometric and laboratory data collected in the course of clinical care were abstracted from the medical record and recorded prior to the participant beginning GnRHa (baseline) and at 6- and 12-month follow-up visits. Tanner stages were assigned by the participant’s clinician in the course of clinical care using the standards of Marshall and Tanner (11).
Annualized HV was calculated as the difference between the baseline and the 12-month visit heights (expressed in centimeters), divided by the time between the two visits (expressed as a fraction of one year). Because the interval between clinic visits was not always exactly 12 months, participants were included in the analysis if they had been treated with a GnRHa for at least 10 months and no more than 14 months. While the majority of participants started GnRHa in early puberty (Tanner stage II or III), some individuals were Tanner IV at baseline. Participants were excluded from analysis if they initiated treatment with gender-affirming hormones (GAH; estradiol or testosterone) prior to their 12-month follow-up visit. No participants were taking anti-androgen medications. Individuals taking stimulant medication for treatment of ADHD were included in the analysis. Luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol, and testosterone were measured as part of standard clinical care while individuals were treated with GnRHa. Laboratory measurements were completed at study sites and at outside facilities. Most of the study sites used LC-MS/MS and immunoassays for gonadotropin and sex-steroid measurements. Samples were not obtained at a particular time of day.
A comparison group of prepubertal, presumed cisgender youth not receiving hormonal intervention was drawn from the Bone Mineral Density in Childhood Study (BMDCS) (12). The HV of BMDCS and TGD youth were compared controlling for mid-age, the midpoint between the ages of the two visits used to calculate the HV. Growth data from the Centers for Disease Control and Prevention were used to determine participant height z-scores based on sex designated at birth (13).
Comparisons for normally distributed variables were made using Student’s t-test and multivariate linear regression. Non-normally distributed variables were compared using the Wilcoxon signed-rank test or the Kruskal-Wallis test. Proportions were compared using Fisher’s exact test. A p-value of < 0.05 was considered significant. Age, Tanner stage at GnRHa initiation, sex designated at birth, body mass index (BMI), race/ethnicity, baseline gonadotropins, and sex-hormone measurements were used as covariates. Stata Statistical Software: Release 16 (College Station, TX) was used for calculations.
RESULTS
Participant Characteristics at GnRHa Initiation
Of 92 youth who were enrolled prior to GnRHa initiation, 9 participants were excluded because they did not receive GnRHa treatment for at least 10 months, 12 were excluded from analysis because they did not have a documented height after 10 to 14 months of GnRHa treatment, and 16 participants were excluded because they started GAH prior to 12 months of GnRHa treatment (Figure 1). A total of 55 individuals were included in analysis, of whom 26 (47%) were designated male at birth (DMAB) and 29 (53%) were designated female at birth (DFAB; Table 1). Of the participants DMAB, 10 (38%) identified as female, 14 (54%) identified as transgender female, and two 2 (8%) identified as nonbinary. Of the participants DFAB, 15 (52%) identified as male, 13 (45%) identified as transgender male, and 1 (3%) identified as nonbinary.
Figure 1.
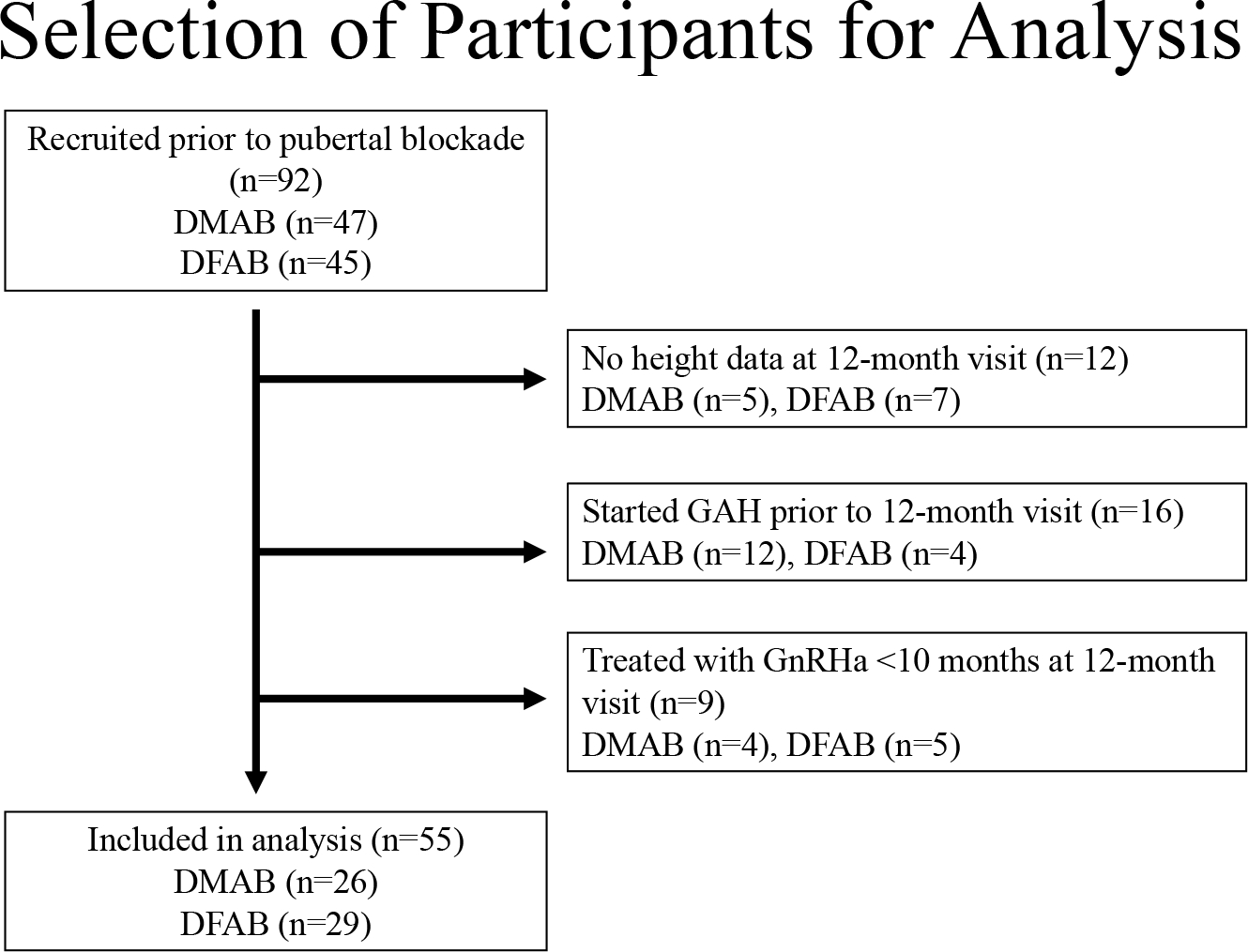
Flowsheet of participant selection.
DMAB = designated male at birth, DFAB = designated female at birth, GAH=gender-affirming hormones, GnRHa=gonadotropin-releasing hormone agonist
Table 1.
Characteristics of Participants
| Total (n = 55) | Designated male at birth (n = 26) | Designated female at birth (n = 29) | |
|---|---|---|---|
|
| |||
| Age at GnRHa start, mean (range), y | 11.5 (9.0–14.5) | 11.9 (10.2–14.5) | 11.1 (9.0–13.9) |
|
| |||
| Gender, n (%) | |||
| Female | 10 (18%) | 10 (38%) | 0 (0%) |
| Male | 15 (27%) | 0 (0%) | 15 (52%) |
| Transgender female | 14 (25%) | 14 (54%) | 0 (0%) |
| Transgender male | 13 (24%) | 0 (0%) | 13 (45%) |
| Nonbinary | 3 (5%) | 2 (8%) | 1 (3%) |
|
| |||
| Race/ethnicity, n (%) | |||
| White | 34 (62%) | 13 (50%) | 21 (72%) |
| Black or African American | 2 (4%) | 1 (4%) | 1 (3%) |
| Multi-race | 5 (9%) | 2 (8%) | 3 (10%) |
| Hispanic or Latino | 12 (22%) | 9 (35%) | 3 (10%) |
| Unknown | 2 (4%) | 1 (4%) | 1 (3%) |
|
| |||
| Tanner stage at GnRHa start, n (%) | |||
| II | 34 (62%) | 21 (81%) | 13 (45%) |
| III | 16 (29%) | 3 (12%) | 13 (45%) |
| IV | 5 (9%) | 2 (8%) | 3 (10%) |
|
| |||
| BMI Z-score, mean (SD) | |||
| Baseline visit | 0.46 (0.89) | 0.56 (0.84) | 0.38 (0.94) |
| 12-month visit | 0.66 (0.97) | 0.68 (1.00) | 0.63 (0.95) |
| FSH, median (IQR), IU/L | |||
| Baseline visit | 2.3 (1.4–4.3) | 1.6 (1.1–2.3) | 4.1 (2.3–5.4) |
| 12-month visit | 0.8 (0.4–1.6) | 0.6 (0.15–1.2) | 1.1 (0.7–2.0) |
| LH, median (IQR), IU/L | |||
| Baseline visit | 0.8 (0.2–2.2) | 0.7 (0.3–1.6) | 0.8 (0.1–2.8) |
| 12-month visit | 0.3 (0.2–0.7) | 0.4 (0.2–1.0) | 0.3 (0.2–0.4) |
| Estradiol, median (IQR), pg/mL | |||
| Baseline visit | 7.0 (2.5–20.0) | 2.0 (2.0–6.0) | 12.0 (6.0–23.0) |
| 12-month visit | 2.0 (2.0–6.0) | 2.0 (2.0–2.0) | 2.0 (2.0–6.0) |
| Testosterone, median (IQR), ng/dL | |||
| Baseline visit | 12.0 (9.0–30.0) | 13.0 (9.5–71.0) | 12.0 (4.0–18.0) |
| 12-month visit | 8.5 (7.0–12.0) | 10.0 (7.5–11.5) | 6.0 (3.0–12.0) |
Most individuals in the cohort started GnRHa in early puberty (Tanner stage II or III). Of those participants DMAB, most were Tanner stage II at the initiation of GnRHa (21 individuals, 81%), 3 (12%) were Tanner III, and 2 (8%) were Tanner IV. Of those participants DFAB, 13 (45%) were Tanner II at initiation of GnRHa, 13 (45%) were Tanner III, and 3 (10%) were Tanner IV. The mean age at GnRHa initiation was 11.5 years (range 9.0 to 14.5 years). As expected given that children DMAB start puberty later than children DFAB, participants DMAB were older at initiation of GnRHa treatment compared to participants DFAB (11.9 ± 1.1 vs. 11.1 ± 1.2 years, p = 0.01).
The comparison group of BMDCS participants consisted of 226 participants. This group was 52% (n = 118) DMAB and 48% (n = 108) DFAB. The BMDCS cohort was younger than the TGD cohort (11.0 ± 2.8 years vs. 11.9 ± 1.2 years, p = 0.01), and all participants in BMDCS were prepubertal (Tanner I) at baseline.
Height Velocity During GnRHa Treatment
The median (IQR) HV for the entire TGD cohort after starting GnRHa was 5.1 (3.7–5.6) cm/year. There was no significant difference in the HV between participants DMAB and those DFAB (5.4 (4.2–5.7) cm/year vs. 4.8 (3.5–5.5) cm/year, p = 0.2). Compared to prepubertal, presumed cisgender youth in the BMDCS, TGD youth treated with GnRHa had similar HV when controlled for mid-age (p = 0.8).
Tanner stage at the start of GnRHa had a significant effect on HV, with participants who started GnRHa at later Tanner stages having lower HV: Tanner stage II 5.3 (4.4 – 5.6) cm/year, Tanner stage III 4.4 (3.3 – 6.0) cm/year, Tanner stage IV 1.6 (1.5 – 2.9) cm/year, p = 0.001 (Table 2, Figure 2). When stratified by Tanner stage and controlled for midage, TGD youth who started on a GnRHa at Tanner stage II or stage III had HV comparable to prepubertal youth in BMDCS (5.3 (4.1 – 5.6) cm/year vs. 6.1 (4.3 – 6.5) cm/year, p = 0.7 and 4.4 (3.3 – 6.0) cm/year vs. 6.1 (4.3 – 6.5) cm/year, p = 0.9). However, individuals starting on GnRHa at Tanner stage IV had lower HV compared to pre-pubertal youth in the BMDCS (1.6 (1.5 – 2.9) cm/year vs. 6.1 (4.3 – 6.5) cm/year, p =0.006). There was no difference in HV between participants DMAB and those DFAB when controlled for Tanner stage.
Table 2.
Height Velocity by Tanner Stage at Baseline
| Total | Designated male at birth | Designated female at birth | |
|---|---|---|---|
|
| |||
| Height velocity, median (IQR), cm/year | |||
| Tanner stage II | 5.3 (4.4 – 5.6) | 5.6 (4.7 – 5.7) | 5. (4.2 – 5.4) |
| Tanner stage III | 4.4 (3.3 – 6.0) | 4.2 (2.3 – 6.4) | 4.4 (4.0 – 5.5) |
| Tanner stage IV | 1.6 (1.5 – 2.9) | 1.5 (1.4 – 1.6) | 2.9 (1.5 – 3.5) |
Figure 2.
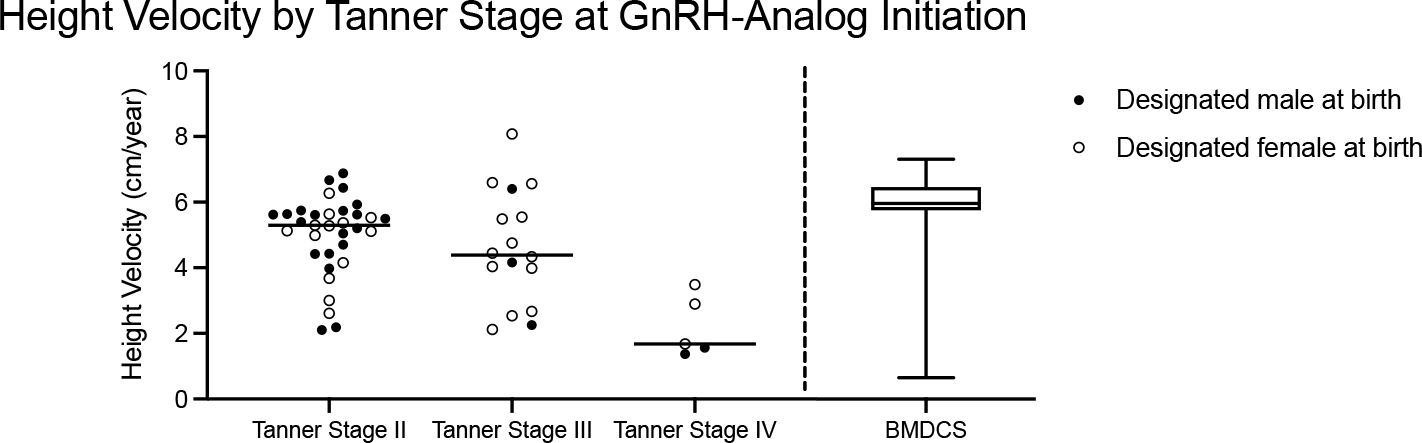
Height velocity by Tanner stage at GnRH-analog initiation. Closed circles indicate youth designated male at birth (DMAB), and open circles indicate youth designated female at birth (DFAB). Horizontal line indicates the median for DMAB and DFAB combined. Comparison is made to height velocities of pre-pubertal youth in the Bone Mineral Density in Childhood Study (BMDCS), represented by the box and whisker plot in which the box and line indicate the median and interquartile range, respectively, and whiskers indicate minimum and maximum values. There was a significant difference in height velocity between participants who were Tanner Stage IV at GnRH analog initiation and those at Tanner Stage II and III (p = 0.002) and pre-pubertal participants in the BMDCS (p = 0.004).
The majority of participants in the study received an implantable histrelin (84%), and the remainder received injectable leuprolide (16%). Of the 12 participants (9 DMAB and 3 DFAB) with ineffective blockade based on laboratory measurements (LH > 1 IU/L, FSH > 4.3 IU/L, estradiol > 40 pg/ml, and/or testosterone >30 ng/dl), 8 (67%) received injectable leuprolide. When the HV analysis was repeated excluding individuals with findings suggestive of ineffective blockade, the findings remained statistically significant. In addition, there was no difference in the HV between these individuals and those with suppressed gonadotropins (5.2 (3.2 – 5.6) cm/year vs. 5.1 (3.7 – 5.6) cm/year, p = 0.8).
HV was also negatively associated with age at GnRHa start even when Tanner stage at start was included as a covariate, demonstrating that some but not all of the effect of age was mediated by Tanner stage (R2= 0.3 p = 0.02). Baseline sex-hormone and gonadotropin concentrations, laboratory indicators of pubertal status, were examined to evaluate their effect on HV. While baseline sex hormone concentrations did not have a significant association with HV in either participants DMAB or DFAB (estradiol: β=0.001, 95% CI [−0.03, 0.04], R2 = 0.0002, p = 0.9; testosterone: β= −0.003, 95% CI [−0.006, 0.001] R2 = 0.05, p = 0.2), baseline LH was found to have an inverse association with HV (R2 = 0.08, p = 0.04); however, this was not independent of its association with Tanner stage. Thus, decreased HV with higher LH concentrations simply represented the previous finding that HV was lower in participants with later Tanner stage at baseline. Decreased weight gain can have a negative effect on height velocity, and thus we also evaluated BMI as a covariate. Baseline BMI did not have a significant association with HV in either participants DMAB or DFAB (β=0.30, 95% CI [−0.17, 0.78], R2 = 0.03, p = 0.2). Also, there was no statistically significant difference in either baseline or 12-month BMI z-scores in DMAB compared to DFAB participants (Table 1).
Seven participants were treated for ADHD (4 DMAB, 3 DFAB), and these participants had lower BMI Z-scores than those not being treated for ADHD (−0.29 ± 0.76 vs. 0.59 ± 0.85, p=0.009). The HV in participants with ADHD was not significantly different from HV of participants without ADHD (5.2 (2.1–5.5) vs. 5.0 (4.0–5.6) cm/year, p =0.5); however, the degree of difference that could be detected was limited by the relatively small sample size.
DISCUSSION
TGD youth treated with GnRHa for 12 months have growth rates similar to those of prepubertal youth. However, individuals with later Tanner stage and chronological age at initiation of GnRHa had significantly lower HV during the first year of GnRHa treatment.
Although GnRHa are routinely used in the treatment of TGD youth, studies assessing the impact of GnRHa treatment on growth are largely derived from studies of youth with CPP. Previous studies of GnRHa treatment in youth with CPP have shown that pubertal blockade can lead to a HV that is lower than expected for a prepubertal individual (14). In contrast, our data demonstrate similar HV between TGD youth treated with GnRHa and prepubertal youth. In CPP, HV has been found to decrease inversely with bone age; this decreased HV has been suggested to be due to premature growth-plate senescence induced by prior estrogen exposure (14). A similar mechanism could also explain our observation of lower HV in those who initiated GnRHa at later Tanner stages with limited remaining growth potential. Alternatively, the lower HV in individuals Tanner stage IV at treatment initiation could represent catch-down growth, in which individuals have a pre-determined growth trajectory and thus will have a decreased height velocity upon starting GnRHa treatment to compensate for the prior pubertal growth acceleration they had experienced (15). Under this theory, an individual would be expected to resume a prepubertal HV even in the absence of sex steroids once they return to their pre-determined growth curve. A third possibility is that longer exposure to sex hormones could permanently alter an individual’s ability to grow without the presence of sex steroids, and thus HV will remain depressed until sex steroids are reintroduced. Ongoing follow-up of this cohort will elucidate if individuals have changes in their HV over time while continuing to receive GnRHa treatment prior to introduction of GAH.
Later age at GnRHa initiation was associated with lower HV even when controlled for Tanner stage. This suggests that chronological age has an effect on HV independent of pubertal status. While it is possible that Tanner stage is not a precise enough measurement of pubertal status and thus we were not able to fully capture the association between age and pubertal status, it is also possible that this reflects a true association between age and growth, as it is known that, in the general population, individuals with later pubertal onset have lower prepubertal HV as well as lower peak HV compared to individuals with average or earlier pubertal onset (12).
There was no difference in the HV between participants with suppressed gonadotropins and those with laboratory measurements suggesting ineffective blockade. This was unexpected, as we would anticipate higher HV in those with pubertal gonadotropins. Of the 12 participants with ineffective blockade based on laboratory measurements, 8 (67%) received injectable leuprolide, compared to 16% in the cohort overall. Because depot formulations of leuprolide contain some free leuprolide, measurements taken shortly after leuprolide injection represent stimulated levels (16). It is therefore possible that at least some of the gonadotropin measurements in this study represented temporary stimulated levels. In addition, random gonadotropin levels collected during GnRHa treatment have been shown to be unreliable in assessing pubertal suppression (17)(18); thus, elevated levels may not indicate ineffective blockade. Alternatively, this comparison may have been underpowered due to the small number of participants with laboratory measurements suggesting ineffective blockade.
Limitations of this study include a relative lack of diversity of participants and lack of data on bone age and pretreatment HV. With respect to the first limitation, participants were primarily non-Hispanic white and recruited at urban academic institutions and thus may not represent more racially, ethnically, and geographically diverse populations. With respect to the second limitation, bone age was not routinely collected at all sites. With respect to the third limitation, participants were not enrolled in the study prior to initiating GnRHa, and thus we were unable to compare their HV during GnRHa treatment with their HV prior to treatment. Finally, although the HV in the first year of GnRHa treatment is informative, its ability to predict adult height is limited, especially in TGD youth who typically proceed with GAH treatment after varying durations of GnRHa therapy.
In summary, early pubertal transgender youth treated with GnRHa for pubertal suppression have growth rates comparable to those of prepubertal children. TGD youth started on GnRHa at later stages of puberty had lower HV, and ongoing follow-up of this cohort will determine the effects of initiation of GAH and will ultimately delineate the effects of gender affirming treatment on adult height. A better understanding of growth outcomes during gender-affirming treatment of TGD youth will inform clinical care and clinical guidelines and will better equip clinicians to counsel patients who may desire an adult height in the typical range for cisgender men or women.
Acknowledgements:
We thank Dr. Andrea Kelly for assistance with growth data from the Bone Mineral Density in Childhood Study.
Funding Sources:
This work was supported by the National Institutes of Health [grant numbers T32-DK007699, R01 HD082554] and Doris Duke Charitable Foundation Grant 2019–119 (KM).
Abbreviations:
- ADHD
Attention Deficit Hyperactivity Disorder
- BMDCS
Bone Mineral Density in Childhood Study
- BMI
Body mass index
- CPP
Central precocious puberty
- DFAB
Designated female at birth
- DMAB
Designated male at birth
- FSH
Follicle stimulating hormone
- GAH
Gender-Affirming Hormones
- GH
Growth Hormone
- GnRHa
Gonadotropin-releasing hormone agonist
- HV
Height velocity
- IGF1
Insulin-like growth factor 1
- LH
Luteinizing hormone
- TGD
Transgender/ Gender diverse
- TYCUS
Trans Youth Care – United States
Footnotes
Disclosure Statement: The authors have no conflicts of interest to declare.
Statement of Ethics: This study was approved by the Institutional Review Boards at the Ann & Robert H Lurie Children’s Hospital of Chicago (IRB-2016–389), Boston Children’s Hospital (IRB-P00021315), Children’s Hospital Los Angeles (CHLA-16–00108), and the University of California San Francisco (16–19371). All participants provided written informed assent and their parents/guardians provided written informed consent.
REFERENCES
- 1.Skordis N, Butler G, De Vries MC, Main K, Hannema SE. ESPE and PES International Survey of Centers and Clinicians Delivering Specialist Care for Children and Adolescents with Gender Dysphoria HORMONE RESEARCH IN PAEDIATRIC S. 2019. [DOI] [PubMed]
- 2.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine Treatment of Gender-Dysphoric/ Gender-Incongruent Persons: An Endocrine Society* Clinical Practice Guideline. 2017. [DOI] [PubMed] [Google Scholar]
- 3.HO KY, EVANS WS, BLIZZARD RM, VELDHUIS JD, MERRIAM GR, SAMOJLIK E, et al. Effects of Sex and Age on the 24-Hour Profile of Growth Hormone Secretion in Man: Importance of Endogenous Estradiol Concentrations*. J Clin Endocrinol Metab [Internet]. 1987. Jan 1;64(1):51–8. [DOI] [PubMed] [Google Scholar]
- 4.Martha PM, Rogol AD, Veldhuis JD, Kerrigan JR, Goodman DW, Blizzard RM, et al. Alterations in the Pulsatile Properties of Circulating Growth Hormone Concentrations during Puberty in Boys* [Internet]. Vol. 69, Journal of Clinical Endocrinology and Metabolism. 1989. [DOI] [PubMed] [Google Scholar]
- 5.Colmenares A, González L, Gunczler P, Lanes R. Is the growth outcome of children with idiopathic short stature and isolated growth hormone deficiency following treatment with growth hormone and a luteinizing hormone-releasing hormone agonist superior to that obtained by GH alone? J Pediatr Endocrinol Metab. 2012. Aug;25(7–8):651–7. [DOI] [PubMed] [Google Scholar]
- 6.Benabbad I, Rosilio M, Tauber M, Paris E, Paulsen A, Berggren L, et al. Growth hormone in combination with leuprorelin in pubertal children with idiopathic short stature. Endocr Connect. 2018. May 1;7(5):708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delemarre-van de Waal HA, Cohen-Kettenis PT. Clinical management of gender identity disorder in adolescents: a protocol on psychological and paediatric endocrinology aspects. Eur J Endocrinol. 2006. Oct 30; (suppl_1):S131–7. [Google Scholar]
- 8.Schagen SEE, Cohen-Kettenis PT, Delemarre-van de Waal HA, Hannema SE. Efficacy and Safety of Gonadotropin-Releasing Hormone Agonist Treatment to Suppress Puberty in Gender Dysphoric Adolescents. J Sex Med. 2016. Jul 1;13(7):1125–32. [DOI] [PubMed] [Google Scholar]
- 9.Klink D, Caris M, Heijboer A, Van Trotsenburg M, Rotteveel J. Bone mass in young adulthood following gonadotropin-releasing hormone analog treatment and cross-sex hormone treatment in adolescents with gender dysphoria. J Clin Endocrinol Metab. 2015. Feb 1;100(2):E270–5. [DOI] [PubMed] [Google Scholar]
- 10.Olson-Kennedy J, Chan YM, Garofalo R, Spack N, Chen D, Clark L, et al. Impact of early medical treatment for transgender youth: Protocol for the longitudinal, observational trans youth care study. J Med Internet Res. 2019;21(7):e14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly A, Winer KK, Kalkwarf H, Oberfield SE, Lappe J, Gilsanz V, et al. Age-based reference ranges for annual height velocity in US children. J Clin Endocrinol Metab. 2014;99(6):2104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: Methods and development. Nationale Center for Health Statistics. Vol. 11, Vital and Health Statistics. 2002. [PubMed] [Google Scholar]
- 14.Weise M, Flor A, Barnes KM, Cutler GB, Baron J. Determinants of Growth during Gonadotropin-Releasing Hormone Analog Therapy for Precocious Puberty. J Clin Endocrinol Metab. 2004. Jan;89(1):103–7. [DOI] [PubMed] [Google Scholar]
- 15.Ong KKL, Ahmed ML, Dunger DB, Emmett PM, Preece MA. Association between postnatal catch-up growth and obesity in childhood: Prospective cohort study. Br Med J. 2000. Apr 8;320(7240):967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatia S, Neely EK, Wilson DM. Serum luteinizing hormone rises within minutes after depot leuprolide injection: implications for monitoring therapy. Pediatrics. 2002. Feb 1;109(2):e30–e30. [DOI] [PubMed] [Google Scholar]
- 17.Wiromrat P, Panamonta O. Elevated random luteinizing hormone is an unreliable indicator for pubertal suppression in girls treated with monthly leuprolide for idiopathic central precocious puberty. JCRPE J Clin Res Pediatr Endocrinol. 2019. Sep 1;11(3):227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis KA, Eugster EA. Random luteinizing hormone often remains pubertal in children treated with the histrelin implant for central precocious puberty. J Pediatr. 2013. Mar;162(3):562–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


