Abstract
A 28‐year‐old man with ankylosing spondylitis (AS) who was treated with a tumour necrosis factor‐alpha (TNF‐α) inhibitor, adalimumab, presented with newly detected multiple bilateral pulmonary nodules on chest computed tomography (CT). We suspected bacterial infection, including those caused by acid‐fast bacilli, or adalimumab‐related condition, such as sarcoidosis. After adalimumab cessation, no resolution of the pulmonary shadows was observed. Moreover, pulmonary cavitation appeared on chest CT at 7 weeks, prompting surgical lung biopsy. Acid‐fast bacteria culture of the lung tissue showed negative results. Pathological examination suggested that confluent granulomas associated with sarcoidosis might have obstructed the blood vessels, causing necrosis and lung cavitation. Consequently, prednisolone was initiated, and these shadows were reduced. After administering anti‐interleukin (IL)‐17A antibody for treatment of AS and prednisolone withdrawal, these shadows were not exacerbated. TNF‐α inhibitor‐induced sarcoidosis could cause cavitary lesions due to vascular invasion of granulomas.
Keywords: adalimumab, ankylosing spondylitis, interleukin‐17, sarcoidosis, tumour necrosis factor‐alpha
We report a case of sarcoidosis, a cavitary lesion in the lung induced by the TNF‐α inhibitor, adalimumab.
INTRODUCTION
Tumour necrosis factor alpha (TNF‐α) inhibitors are used for treatment of sarcoidosis. However, TNF‐α inhibitor‐induced sarcoidosis has also been reported. 1 Treatment of TNF‐α inhibitor‐induced sarcoidosis includes withdrawal of offending drugs and steroids and changing the initial TNF‐α inhibitor of choice. 2 Herein, we report a case of sarcoidosis, a cavitary lesion in the lung induced by the TNF‐α inhibitor, adalimumab.
CASE REPORT
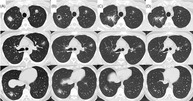
A 28‐year‐old man was referred to our department in June 2020 after presenting with bilateral pulmonary nodules on chest computed tomography (CT) 1 month prior to this referral. At the initial visit, he exhibited no chest symptoms. He had been administered adalimumab for ankylosing spondylitis (AS) since April 2019 at a dosage of 40 mg (subcutaneous self‐injection, alternate weekly). CT before starting adalimumab displayed no obvious abnormalities in the lung fields. However, 7 weeks after adalimumab initiation, multiple bilateral pulmonary nodules were seen on chest CT (Figure 1A). Our tentative differential diagnosis was bacterial infection, including an acid‐fast bacterial infection, drug‐induced pneumonia, or sarcoidosis. Although adalimumab was discontinued, the shadows did not resolve, prompting admission to our department for bronchoscopy.
FIGURE 1.
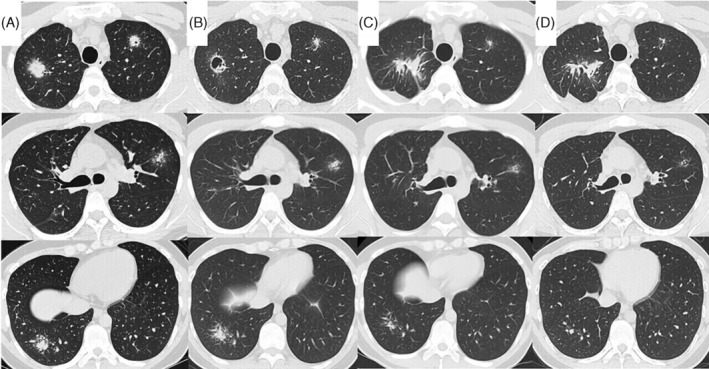
Diagram depicting the timeline of disease progression, CT findings, and management. CT taken at admission shows large nodules with small peripheral nodules, which resemble globular star clusters, in the bilateral upper lobes and right lower lobe (a), formation of cavity in the nodule in the right upper lobe follows (B). One month after starting prednisolone, CT showing the nodules in the left upper and right lower lobes decreased (C). Anti‐human IL‐17A monoclonal antibody is started in January 2021, and prednisolone is terminated in march due to resolution of AS symptoms o. Since then, the nodules in both the left upper and right lower lobes have been gradually decreased (D)
The patient had unremarkable medical history. In addition, the patient had no family history of serious diseases or allergies.
On admission, physical examination revealed vital signs: temperature, 37.0°C; blood pressure, 115/75 mmHg; pulse rate, 80 beats/min with regular cardiac rhythm; respiratory rate, 16 breaths/min; percutaneous O2 saturation, 99% (on room air). Heart and lung auscultation revealed no unusual findings.
Chest radiography at the initial visit showed bilateral pulmonary nodules. Approximately 1 month after adalimumab withdrawal, a chest radiograph showed no improvement. Therefore, the patient was admitted for further examination. CT showed multiple bilateral pulmonary nodules (Figure 1A) without significant enlargement of the mediastinal hilar lymph nodes.
Blood test results showed no obvious inflammatory findings. Furthermore, tests for collagen‐related autoantibodies and anti‐neutrophil cytoplasmic antibody were negative. Tests for soluble IL‐2 receptor, angiotensin‐converting enzyme, and Krebs von den Lungen 6 were also within normal limits. Aspergillus antigen test and interferon gamma releasing assay for tuberculosis showed negative results. Tests for anti‐Mycobacterium avium complex antibody, and beta‐D glucan were negative. Sputum examination showed no acid‐fast bacteria. Bronchoalveolar lavage fluid (BALF) cytology revealed macrophages (87.5%), lymphocytes (10%), and neutrophils (2.5%). Acid‐fast bacterial culture in BALF was negative. In addition, CD4/CD8 ratio in BALF was 2.74. The tissues obtained by transbronchial lung biopsies showed epithelioid granulomas without necrosis, suggesting sarcoidosis. Two months later, CT showed cavitation of the nodules in the right upper lobe (Figure 1B). We performed surgical lung biopsy to exclude acid‐fast bacterial infection. Both culture and polymerase chain reaction for acid‐fast bacteria of the lung tissue showed negative results. Pathological findings showed granulomas along the peribronchial and granulomas invasion into the airway and vascular walls, with particularly prominent vascular invasion and obstruction (Figure 2). These results indicated no acid‐fast bacterial infection. Therefore, we diagnosed the patient with sarcoidosis.
FIGURE 2.
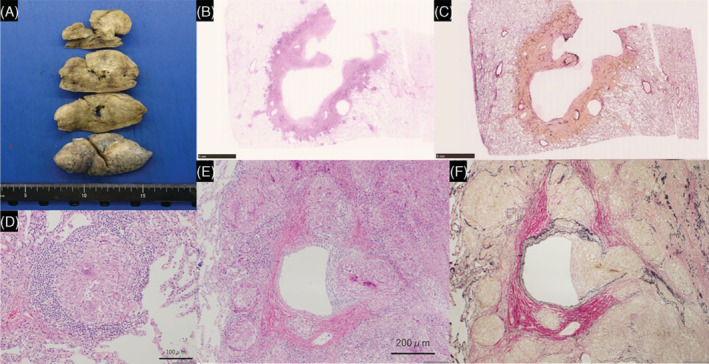
Gross and microscopic histopathology from lung biopsy. Gross view shows a cavity in the resected right upper lobe (A). The image shows granuloma invasion of the vascular walls (B–F), and a large cavity with no airway involvement, suggesting that the cavity is formed as a result of necrosis due to vascular obstruction (E, F). (D: Haematoxylin–eosin, ×200, E: Haematoxylin–eosin, ×100, F: Elastic van Gieson, ×100)
Four months following his first admission, a flare‐up of his AS was noted upon presentation with joint pains. Consequently, prednisolone was initiated. One month later, a repeat follow‐up chest CT showed shrinkage of the bilateral nodules (Figure 1C). At that time, secukinumab, which is an anti‐IL‐17 monoclonal antibody, was initiated for AS. In addition, prednisolone was discontinued due to resolution of joint pain from AS. Repeat chest CT showed no recurrence (Figure 1D).
DISCUSSION
In this case, multiple pulmonary nodules suggestive of sarcoidosis appeared while using adalimumab, a TNF‐α inhibitor, for AS.
Sarcoidosis caused by such paradoxical reactions is mostly seen in patients with rheumatoid arthritis. The incidence of paradoxical sarcoidosis is 1/2800 in patients with rheumatoid arthritis. The average time of onset is 22 months after induction, with lung involvement as the most common site. The most common drug inducing paradoxical sarcoidosis is etanercept followed by adalimumab. 2 Treatment of paradoxical sarcoidosis involves TNF‐α inhibitors withdrawal with or without administration of systemic steroids, to which treatment response is favorable. 2 However, in this case, cavitation appeared after adalimumab withdrawal, prompting a surgical lung biopsy. The pathologic findings showed that cavitation did not involve the airway, suggesting that the cavity was formed as a result of necrosis due to vascular invasion and obstruction by granulomas. The etiology of sarcoidosis is still unknown and has been reported to be associated with infection, specific HLA predisposition, and cellular immune dysregulation. 2 , 3 , 4 TNF‐α levels increased in both tissues and serum in patients with sarcoidosis, often correlating with disease activity. 1 Sarcoidosis is associated with many T‐cell abnormalities, including increased expression of Th1 chemokines and cytokines, elevated tissue CD4/CD8 ratio, and increased but dysfunctional Treg cells. 3 , 4
Development of TNF‐α inhibitor‐induced sarcoidosis may result from an unbalanced activation of TNF receptor 2, which is a major regulator of Treg and controls T‐cell differentiation. In this case, the patient developed sarcoidosis after adalimumab initiation. Therefore, we considered TNF‐α inhibitor‐induced sarcoidosis. However, it could be possible that adalimumab may have accelerated the onset of de novo sarcoidosis. We hypothesized that TNF‐α inhibitor withdrawal may have relatively enhanced the effect of TNF‐α in situ and facilitated the progression of granulomas invading or obstructing the vessels, thereby causing the cavitary lesions after adalimumab withdrawal. 1
Vascular invasion of granulomas in patients with sarcoidosis is a frequent finding. 5 Progression could lead to more advanced tissue or organ destruction. We recommend that when chest CT findings suggestive of granulomatous lesions are observed during TNF‐α inhibitors administration, an early lung biopsy should be performed, particularly in cases of cavitary lesions. Moreover, early treatment should be started.
AUTHOR CONTRIBUTIONS
Eriko Hamada, Yoshifumi Yamamoto, Yosuke Okuda, Kazuhiro Sakaguchi, and Noriyoshi Sawabata were involved in the collection of samples and data. Eriko Hamada, Yoshifumi Yamamoto, Kentaro Suzuki, Yoshiro Kai, Maiko Takeda, Chiho Ohbayashi, and Shigeo Muro were involved in the interpretation, writing, and editing of the manuscript. Eriko Hamada, Shigeto Hontsu, Motoo Yamauchi, Masanori Yoshikawa, Noriyoshi Sawabata, Chiho Ohbayashi, and Shigeo Muro pre‐pared the draft and final version of the manuscript.
CONFLICT OF INTEREST
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
ACKNOWLEDGMENTS
We would like to thank Editage (https://www.editage.jp) for English language editing.
Hamada E, Yamamoto Y, Okuda Y, Sakaguchi K, Suzuki K, Kai Y, et al. Pulmonary sarcoidosis with a cavitary lesion in the lung caused by a TNF‐α inhibitor: A case report. Respirology Case Reports. 2022;10:e01065. 10.1002/rcr2.1065
Associate Editor: Yet Hong Khor
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Amber KT, Bloom R, Mrowetz U, Hertl M. TNF‐α: treatment target or cause of sarcoidosis? J Eur Acad Dermatol Venereol. 2015;29:2104–11. 10.1111/jdv.13246 [DOI] [PubMed] [Google Scholar]
- 2. Sim JK, Lee SY, Shim JJ, Kang KH. Pulmonary sarcoidosis induced by adalimumab: a case report and literature review. Yonsei Med J. 2016;57:272–3. 10.3349/ymj.2016.57.1.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agostini C, Meneghin A, Semenzato G. T‐lymphocytes and cytokines in sarcoidosis. Curr Opin Pulm Med. 2002;8:435–40. 10.1097/00063198-200209000-00016 [DOI] [PubMed] [Google Scholar]
- 4. Grunewald J, Eklund A. Role of CD4+ T cells in sarcoidosis. Proc Am Thorac Soc. 2007;4:461–4. 10.1513/pats.200606-130MS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tamiko T, Yasuo M, Shigeki S, Riichiroh M. Pulmonary vascular involvement in sarcoidosis: a report of 40 autopsy cases. Hum Pathol. 1992;23:1216–23. 10.1016/0046-8177(92)90288-E [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


