First Movement
Both in the United States and globally, cardiovascular disease (CVD) remains the number one cause of death. According to the American Heart Association (AHA) 2022 Heart Disease and Stroke Statistics Update, ≈121.5 million people in the United States have hypertension, 100 million have obesity, and more than 28 million people have type 2 diabetes. 1 Furthermore, only 1 in 4 adults report reaching the physical activity (PA) and exercise goals recommended by the US Department of Health and Human Services' Physical Activity Guidelines for Americans, 2nd edition. 2 Over the past 2 decades, several research studies reported that >80% of all cardiovascular events could be prevented by healthy lifestyle and management of their known risk factors. In 2010, after decades of decline in cardiovascular death rates, AHA expanded its focus on additional strategies, which could directly promote population‐ and individual‐level health. The central tenet of this new approach was the creation of a novel, operational definition of a construct called cardiovascular health (CVH). 3 In defining CVH, the AHA's 2010 writing group acknowledged that health is much broader and a more “positive” construct than merely the absence of disease. It turns out that the overall heritability of CVH is generally low, indicating that behavioral and environmental exposures are of paramount importance in determining CVH. 4 As such, following and sustaining healthier lifestyles at young ages seem to be the key ingredients of a successful recipe for maintaining better CVH into middle ages and perhaps healthy aging. 5 , 6 , 7 , 8 , 9 , 10 , 11 However, one's ability to choose healthy lifestyles across the life span is highly variable and strongly influenced by other factors, such as psychological health elements 12 , 13 and social or socioeconomic and structural determinants. 14
Created in 2010, Life's Simple 7 (LS7) was the AHA's effort to define ideal CVH based on risk factors that can be mitigated via lifestyle changes or interventions. Over time, LS7 has become a proven, powerful tool for understanding how to achieve healthy aging and ways to improve CVH, while decreasing the risks of developing cardiac or cerebrovascular disease, as well as cancer, dementia, and potentially other chronic disorders. The initial definition of CVH was based on 7 health behaviors and health factors that, when optimized, were associated with better CVD‐free survival, total longevity, and improved quality of life. The 7 components of CVH (LS7) included health behavior indicators of dietary quality, participation in PA, and exposure to cigarette smoking, and health factors such as body mass index (BMI), fasting blood glucose, total cholesterol, and blood pressure levels. Each metric was classified as poor (0), intermediate (1), or ideal (2) on the basis of generally accepted clinical thresholds. Based on this schema, ideal CVH was defined as having all 7 metrics at ideal levels. Interestingly, ideal CVH also formed the basis of a new definition of optimal brain health, which was published in 2017. 15
Perhaps unsurprisingly, in the United States the prevalence of ideal CVH is exceedingly low (<1%) for all age groups, including in those as young as 12 years, 16 and it declines over time. The prevalence of ≥5 metrics at ideal levels is only 45% among US adolescents, 32% in adults 20 to 39 years, 11% among adults 40 to 59 years, and only 4% in adults ≥60 years of age. 16 Thus, although some individuals can preserve higher levels of CVH, most will require some attention or sustained intervention to achieve it or maintain it into later years. The prevalence of ideal diet, as defined in 2010, is consistently negligible (<1%) across all age groups, in fact driving the overall low prevalence of ideal CVH. Population‐level CVH scores have been low and fairly stagnant over the past 2 decades in the United States, 1 but this general observation conceals several salient facts or “details.” First, although some segments of the population experience modest improvements in CVH, others (generally from lower socioeconomic strata) see worsening CVH, 17 reflecting bimodal distributions that are not well characterized by the usual measures of central tendency. Second, wider and more persistent differences seem to exist by race or ethnicity, urban versus rural environment, or by geography, with respect to the prevalence of CVH levels, and these disparities may begin at younger ages. 16 , 18 , 19 Furthermore, recent data show that the prevalence of high CVH is <10% during pregnancy, 20 and poor CVH in pregnancy is associated with poor CVH in offspring, suggesting that ideal CVH is not universal, even at birth. 21
To date, more than 2500 scientific articles have cited the original 2010 document describing the AHA's construct of CVH and explored its prevalence, determinants, outcomes, and mechanisms of CVH in diverse populations and across the life span. 3 Because of the data accumulated over the past dozen years of investigation, the definition and quantification of each of the original metrics in the LS7 needed calibration for inter‐ and intraindividual variances, as well as refinement and further validation against hard outcomes. As such, variability was studied, new metrics were considered, and the age spectrum was expanded to include the entire life span. The foundational contexts of social determinants of health and psychological health were also addressed as crucial factors in optimizing and preserving CVH. As the result of these efforts, an AHA presidential advisory group recently published 22 a modified concept and improved tool for assessing CVH, Life's Essential 8 (LE8), which includes the following:
Diet (updated): there is a new guide to assess diet quality for adults and children at individual level (ie, for individual health care and dietary counseling) and at population level (ie, for research and public health purposes). At the population level, dietary assessment is based on daily intake of various elements included in the Dietary Approaches to Stop Hypertension eating pattern, which has 8 subcomponents: high intake of fruits, vegetables, nuts and legumes, whole grains, and low‐fat dairy and low intake of sodium, red and processed meats, and sweetened drinks. At the individual level, the Mediterranean Eating Pattern for Americans is used to assess and monitor CVH. The Mediterranean Eating Pattern for Americans is a Dietary Approaches to Stop Hypertension‐style eating pattern that can be measured with 16 yes‐or‐no‐questions about the weekly frequency of consuming olive oil, vegetables, berries, meat, fish, dairy, grains, etc. Of note, the Mediterranean Eating Pattern for Americans screener does not include consumption of sugar‐sweetened beverages; as such, clinicians are encouraged to ask separately about the intake of this type of beverages at the time of their assessments.
PA (no changes): Activity is measured by the total number of minutes of moderate or vigorous physical activity per week, as defined by the Physical Activity Guidelines for Americans, 2nd edition. 2 The optimal level is at least 150 minutes per week of moderate PA, or 75 minutes per week of vigorous‐intensity PA for adults; 420 minutes or more per week for children ages 6 and older; and age‐specific modifications for younger children.
Nicotine exposure (updated): the use of inhaled nicotine‐delivery systems, that is, e‐cigarettes or vaping devices, was added, because the previous variable incorporated only traditional, combustible cigarettes. This reflects use by both adults and youth and their implications for long‐term health. LE8 also includes second‐hand smoke exposure for children and adults.
BMI (no changes): although BMI is an imperfect predictor, it is easily computed and widely available; therefore, BMI was maintained as a reasonable gauge to assess weight categories in relationship to various health problems. The BMI band of 18.5 to 24.9 is associated with the highest levels of CVH, although specific health risks associated with the BMI strata may differ in people from diverse racial or ethnic backgrounds or ancestries. This aligns with the World Health Organization's recommendations to adjust BMI ranges for people of Asian or Pacific Islander ancestry because recent evidence indicates their risk of conditions such as CVD or type 2 diabetes is higher at lower BMIs.
Blood lipids (updated): the metric for blood lipids (cholesterol and triglycerides) was updated to include non‐high‐density lipoprotein cholesterol as the preferred variable to monitor, rather than total cholesterol. This shift is made because non‐high‐density lipoprotein cholesterol can be measured in nonfasting status and can be reliably calculated in all people.
Blood glucose (updated): this metric was expanded to include the use of hemoglobin A1c (which can better reflect long‐term glycemic control) or blood glucose levels for people with or without type 1 or type 2 diabetes or prediabetes.
Blood pressure (no changes): these criteria remained unchanged from the AHA 2017 guidelines that established levels <120/80 mm Hg as optimal, and hypertension defined as 130 to 139 mm Hg systolic pressure (the top number in a reading) or 80 to 89 mm Hg diastolic pressure (bottom number).
Sleep duration (new): Sleep duration has been found repeatedly to be associated with CVH (in a U‐shaped pattern). Sleep duration, measured by average hours of sleep per night, is considered at an ideal level when it reaches 7 to 9 hours per day in adults.
Each metric has a new scoring system, ranging from 0 to 100 points, allowing generation of a new composite CVH score (as the unweighted average of all components), which also varies from 0 to 100 points.
For sleep health, the recommended measurement in LE8 is either self‐reported average hours of sleep per night or objective sleep/actigraphy data from wearable technology, if available. The scoring system in adults assigns 100 points for 7 to 9, 90 points for 9 to 10, 70 points for 6 to 7, 40 points for 5 to 6 or ≥10, 20 points for 4 to 5 and 0 for <4 hours of sleep. In children, it uses age‐appropriate ranges and scoring based on deviations from these intervals, as follows: 100 points for age‐appropriate optimal range; 90 points for <1 hour above optimal range; 70 points for <1 hour below optimal range; 40 points for 1to 2 hours below or ≥1 hour above optimal; 20 points for 2 to 3 hours below optimal range; and 0 points for ≥3 hours below optimal range. For children, age‐appropriate optimal sleep durations used were as follows: 4 to 12 months: 12 to 16 hours per 24 hours (includes naps); 1 to 2 years: 11 to 14 hours per 24 hours; 3 to 5 years: 10 to 13 hours per 24 hours; 6 to 12 years: 9 to 12 hours; and age 13 to 18 years, 8 to 10 hours. 23
Second Movement
Together with diet and PA, sleep is one of the most important pillars of health. Similar to the high rates of unhealthy diet and PA, poor sleep is also very prevalent in the general population, across all ages. Approximately 1 in 3 US adults are short sleepers (getting <7 hours of habitual sleep per day), less than half report having a good night of sleep every night, and about 1 in 5 report excessive daytime sleepiness. 24 , 25 , 26 Furthermore, prevalent sleep disorders such as insomnia and obstructive sleep apnea (OSA) have been linked to short or impaired sleep, suggesting that multiple sleep disturbances may occur concurrently and potentially interact, further augmenting the risk for development of chronic diseases. 25 , 26 , 27 , 28 However, disentangling these collinearities is neither easy nor practical at the point of care.
In this issue of the Journal of the American Heart Association (JAHA), Makarem and collaborators 29 present in greater detail work that started several years earlier, which was presented at the AHA Epidemiology and Prevention | Lifestyle and Cardiometabolic Health Lifestyle Scientific Sessions in Phoenix, Arizona, March 3 to 6, 2020, and published in abstract form. 30 The authors started from the observation that, although there is already extensive work published showing that sufficient and healthy sleep is inversely associated with CVD and its risk factors, the AHA's LS7 tool did not include sleep as a separate domain. The authors studied the inclusion of sleep as a separate CVH metric, akin to other health behaviors, and hypothesized that its inclusion may enhance primordial and primary CVD prevention efforts at the population level. They studied a sample from the MESA (Multi‐Ethnic Study of Atherosclerosis) Sleep Study, of participants who had complete data on sleep characteristics from overnight polysomnography, 7‐day wrist actigraphy, validated questionnaires, and adjudicated cardiovascular outcomes, both at baseline and during the follow‐up period. The authors computed the LS7 scores based on the 0 to 2 grading system and derived 4 iterations of a new CVH score or model: score 1 included LS7+mean sleep duration, score 2 included LS7+sleep characteristics linked to CVD in the general literature (mean sleep duration, insomnia, daytime sleepiness, and OSA), and scores 3 and 4 included LS7 plus sleep characteristics associated with CVD in the MESA trial (score 3: mean sleep duration and efficiency, daytime sleepiness, and OSA; score 4: score 3 plus sleep regularity). Among the 1920 participants analyzed (mean age: 69±9 years, 54% female), there were 95 prevalent CVD events and 93 incident cases observed during a mean follow‐up of 4.4 years. In the sample, mean LS7 score was 7.3, whereas averages of the alternate CVH scores 1 to 4 ranged from 7.4 to 7.8. Actigraphy monitoring showed that almost two thirds of the participants slept <7 hours, almost one third slept <6 hours habitually, and 39% and 25% had high night‐to‐night variability in sleep duration and sleep onset timing, respectively. Overall, 10% had poor sleep efficiency, 1 in 7 had excessive daytime sleepiness, more than a third were classified as having significant insomnia symptoms, and 47% had moderate or severe OSA. Short sleepers were significantly more likely to have low sleep efficiency, excessive daytime sleepiness, high sleep duration and timing variability, and OSA; they also had higher prevalence of overweight or obesity, type 2 diabetes or high blood pressure, and lower mean LS7 scores (P<0.001).
The authors found that those in the highest versus the lowest tertile of the LS7 score and CVH scores 1 to 4 had up to 80% lower odds of prevalent CVD. Interestingly, the LS7 score was not significantly associated with incident CVD (hazard ratio [HR], 0.62 [95% CI, 0.37–1.04]). Those in the highest versus lowest tertile of CVH score 1 (which included sleep duration) and CVH score 4 (which included multidimensional sleep health) had 43% and 47% lower incident CVD risk (HR, 0.57 [95% CI, 0.33–0.97] and 0.53 [95% CI, 0.32–0.89]), respectively. The authors were able to demonstrate in this cohort of older adults that, although the LS7 score and new CVH scores that included sleep were all related to prevalent CVD, only CVH scores that include sleep predicted incident CVD. Contrary to their initial hypothesis, even a CVH score that included only objectively assessed sleep duration (a common measure of sleep health, now incorporated in LE8), predicted CVD incidence. Notably, the CVH score that incorporated objective measures of sleep duration, quality, and regularity as well as sleep disorders, was also significantly associated with both CVD prevalence and incidence.
Scherzo or Coda, But Not Finale
Where Do We Go From Here?
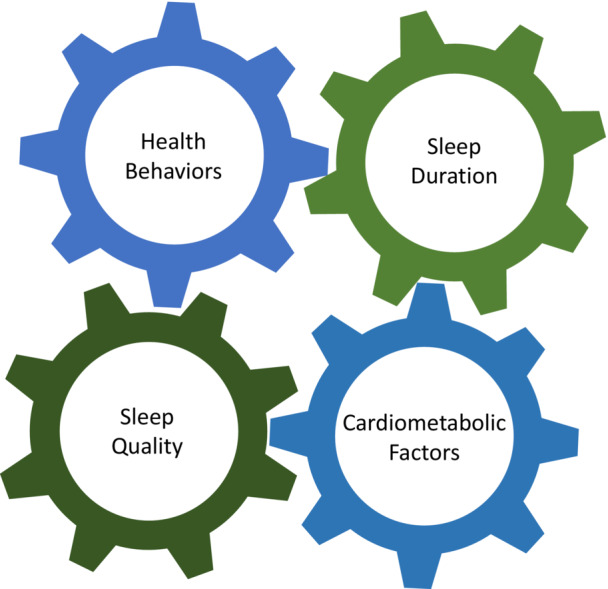
Sleep found its way as a new “wedge” in the pictorial or the “side” in the octagon determined by the individual determinants of CVH in LE8 (Figure 1), almost concurrently with explorations such as the one presented here by Makarem et al. 29 These new data show that, in a manner similar to our evidence journey from LS7 to LE8, more investigative and validation efforts are needed to determine how to best measure and incorporate sleep as a relatively independent predictive factor of CVH, including with the use of the more refined scores from the 0 to 100 scale. It is very possible, likely, and desirable that measures of sleep continuity or other measures of sleep quality (Figure 2) may make their way into our next iteration of LE8, and, of course, awaiting the arrival of some ever‐elusive biomarkers of known sleep disorders, in the LE8.x. Until then, let us all get back to the work bench …
Figure 1. Life's Essential 8 elements, with healthy sleep as the new element for cardiovascular health.22 .
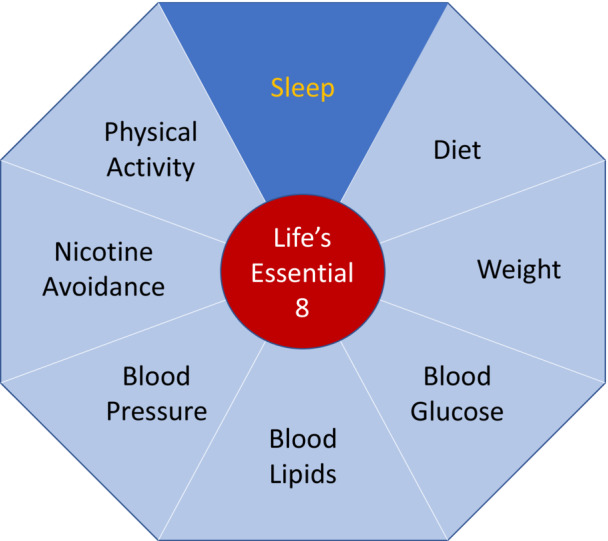
Figure 2. The interrelationship between health or cardiometabolic factors, health behaviors, sleep duration, and sleep quality (different type of impairments, various disorders, circadian misalignments, etc.).
Disclosures
No relevant disclosures for this work.
Acknowledgments
Seven Sweethearts was a 1942 musical film directed by Frank Borzage, starring Kathryn Grayson, Marsha Hunt, and Van Heflin. Life Begins at Eight Thirty was a 1942 romance film starring Monty Woolley, Ida Lupino, and Cornel Wilde; it was based on the West End play The Light of Heart by Emlyn Williams.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
See article by Makarem et al.
For Disclosures, see page 4.
References
- 1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke Statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. [DOI] [PubMed] [Google Scholar]
- 2. U.S. Department of Health and Human Services . Physical Activity Guidelines for Americans. 2nd ed. Washington, DC: Health and Human Services; 2022. [Google Scholar]
- 3. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 4. Allen NB, Hwang SJ, Cupples LA, Levy D, Fox C, O'Donnell C, Lloyd‐Jones DM. The heritability of ideal cardiovascular health: the Framingham heart study (abstract 17245). Circulation. 2010;122:A17245. [Google Scholar]
- 5. Pahkala K, Laitinen TT, Niinikoski H, Kartiosuo N, Rovio SP, Lagstrom H, Loo BM, Salo P, Jokinen E, Magnussen CG, et al. Effects of 20‐year infancy‐onset dietary counselling on cardiometabolic risk factors in the special Turku coronary risk factor intervention project (STRIP): 6‐year post‐intervention follow‐up. Lancet Child Adolesc Health. 2020;4:359–369. doi: 10.1016/S2352-4642(20)30059-6 [DOI] [PubMed] [Google Scholar]
- 6. Matthews LA, Rovio SP, Jaakkola JM, Niinikoski H, Lagstrom H, Jula A, Viikari JSA, Ronnemaa T, Simell O, Raitakari OT, et al. Longitudinal effect of 20‐year infancy‐onset dietary intervention on food consumption and nutrient intake: the randomized controlled STRIP study. Eur J Clin Nutr. 2019;73:937–949. doi: 10.1038/s41430-018-0350-4 [DOI] [PubMed] [Google Scholar]
- 7. Allen NB, Lloyd‐Jones D, Hwang SJ, Rasmussen‐Torvik L, Fornage M, Morrison AC, Baldridge AS, Boerwinkle E, Levy D, Cupples LA, et al. Genetic loci associated with ideal cardiovascular health: a meta‐analysis of genome‐wide association studies. Am Heart J. 2016;175:112–120. doi: 10.1016/j.ahj.2015.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu K, Daviglus ML, Loria CM, Colangelo LA, Spring B, Moller AC, Lloyd‐Jones DM. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the coronary artery risk development in (Young) adults (CARDIA) study. Circulation. 2012;125:996–1004. doi: 10.1161/CIRCULATIONAHA.111.060681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S, Liu K. Healthy lifestyle change and subclinical atherosclerosis in young adults: coronary artery risk development in Young adults (CARDIA) study. Circulation. 2014;130:10–17. doi: 10.1161/CIRCULATIONAHA.113.005445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gooding HC, Shay CM, Ning H, Gillman MW, Chiuve SE, Reis JP, Allen NB, Lloyd‐Jones DM. Optimal lifestyle components in Young adulthood are associated with maintaining the ideal cardiovascular health profile into middle age. J Am Heart Assoc. 2015;4:e002048. doi: 10.1161/JAHA.115.002048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pahkala K, Hietalampi H, Laitinen TT, Viikari JS, Ronnemaa T, Niinikoski H, Lagstrom H, Talvia S, Jula A, Heinonen OJ, et al. Ideal cardiovascular health in adolescence: effect of lifestyle intervention and association with vascular intima‐media thickness and elasticity (the special Turku coronary risk factor intervention project for children [STRIP] study). Circulation. 2013;127:2088–2096. doi: 10.1161/CIRCULATIONAHA.112.000761 [DOI] [PubMed] [Google Scholar]
- 12. Whitaker KM, Jacobs DR Jr, Kershaw KN, Demmer RT, Booth JN III, Carson AP, Lewis CE, Goff DC Jr, Lloyd‐Jones DM, Gordon‐Larsen P, et al. Racial disparities in cardiovascular health behaviors: the coronary artery risk development in Young adults study. Am J Prev Med. 2018;55:63–71. doi: 10.1016/j.amepre.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine GN, Cohen BE, Commodore‐Mensah Y, Fleury J, Huffman JC, Khalid U, Labarthe DR, Lavretsky H, Michos ED, Spatz ES, et al. Psychological health, well‐being, and the mind‐heart‐body connection: a scientific statement from the American Heart Association. Circulation. 2021;143:e763–e783. [DOI] [PubMed] [Google Scholar]
- 14. Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. [DOI] [PubMed] [Google Scholar]
- 15. Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd‐Jones DM, Bae HJ, Bauman MA, Dichgans M, Duncan PW, et al. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48:e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 17. Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd‐Jones DM. Cardiovascular health behavior and health factor changes (1988‐2008) and projections to 2020: results from the national health and nutrition examination surveys. Circulation. 2012;125:2595–2602. doi: 10.1161/CIRCULATIONAHA.111.070722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pilkerton CS, Singh SS, Bias TK, Frisbee SJ. Changes in cardiovascular health in the United States, 2003‐2011. J Am Heart Assoc. 2015;4:e001650. doi: 10.1161/JAHA.114.001650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lawrence E, Hummer RA, Harris KM. The cardiovascular health of young adults: disparities along the urban‐rural continuum. Ann Am Acad Pol Soc Sci. 2017;672:257–281. doi: 10.1177/0002716217711426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perak AM, Ning H, Khan SS, Van Horn LV, Grobman WA, Lloyd‐Jones DM. Cardiovascular health among pregnant women, aged 20 to 44 years, in the United States. J Am Heart Assoc. 2020;9:e015123. doi: 10.1161/JAHA.119.015123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perak AM, Lancki N, Kuang A, Labarthe DR, Allen NB, Shah SH, Lowe LP, Grobman WA, Lawrence JM, Lloyd‐Jones DM, et al. Associations of maternal cardiovascular health in pregnancy with offspring cardiovascular health in early adolescence. JAMA. 2021;325:658–668. doi: 10.1001/jama.2021.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lloyd‐Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, et al. Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paruthi S, Brooks LJ, D'Ambrosio C, Hall WA, Kotagal S, Lloyd RM, Malow BA, Maski K, Nichols C, Quan SF, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of sleep medicine. J Clin Sleep Med. 2016;12:785–786. doi: 10.5664/jcsm.5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:137–141. doi: 10.15585/mmwr.mm6506a1 [DOI] [PubMed] [Google Scholar]
- 25. Ford ES, Cunningham TJ, Giles WH, Croft JB. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372–378. doi: 10.1016/j.sleep.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohayon MM. Determining the level of sleepiness in the American population and its correlates. J Psychiatr Res. 2012;46:422–427. doi: 10.1016/j.jpsychires.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 27. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep‐disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36:417–440. doi: 10.1146/annurev-publhealth-031914-122838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Makarem N, Castro‐Diehl C, St‐Onge M‐P, Redline S, Shea S, Lloyd‐Jones DM, Ning H, Aggarwal B. Abstract 36: the role of sleep as a cardiovascular health metric: does it improve cardiovascular disease risk prediction? Results from the multi‐ethnic study of atherosclerosis. Circulation. 2020;141:A36. doi: 10.1161/circ.141.suppl_1.36 [DOI] [Google Scholar]
- 30. Makarem N, Castro‐Diehl C, St‐Onge M‐P, Redline S, Shea S, Lloyd‐Jones DM, Ning H, Aggarwal B. The role of sleep as a cardiovascular health metric: does it improve cardiovascular disease risk prediction? Results from the multi‐ethnic study of atherosclerosis (abstract 36). Ciculation. 2020;141. doi: 10.1161/circ.141.suppl_1.36 [DOI] [Google Scholar]


