Abstract
Background
Although sufficient and healthy sleep is inversely associated with cardiovascular disease (CVD) and its risk factors, the American Heart Association's Life's Simple 7 (LS7), as a measure of cardiovascular health (CVH), did not include sleep. We evaluated an expanded measure of CVH that includes sleep as an eighth metric in relation to CVD risk.
Methods and Results
The analytic sample consisted of MESA (Multi‐Ethnic Study of Atherosclerosis) Sleep Study participants who had complete data on sleep characteristics from overnight polysomnography, 7‐day wrist actigraphy, validated questionnaires, and the outcome. We computed the LS7 score and 4 iterations of a new CVH score: score 1 included sleep duration, score 2 included sleep characteristics linked to CVD in the literature (sleep duration, insomnia, daytime sleepiness, and obstructive sleep apnea), scores 3 and 4 included sleep characteristics associated with CVD in MESA (score 3: sleep duration and efficiency, daytime sleepiness, and obstructive sleep apnea; score 4: score 3+sleep regularity). Multivariable‐adjusted logistic and Cox proportional hazards models evaluated associations of the LS7 and CVH scores 1 to 4 with CVD prevalence and incidence. Among 1920 participants (mean age: 69±9 years; 54% female), there were 95 prevalent CVD events and 93 incident cases (mean follow‐up, 4.4 years). Those in the highest versus lowest tertile of the LS7 score and CVH scores 1 to 4 had up to 80% lower odds of prevalent CVD. The LS7 score was not significantly associated with CVD incidence (hazard ratio, 0.62 [95% CI, 0.37–1.04]). Those in the highest versus lowest tertile of CVH score 1, which included sleep duration, and CVH score 4, which included multidimensional sleep health, had 43% and 47% lower incident CVD risk (hazard ratio, 0.57 [95% CI, 0.33–0.97]; and hazard ratio, 0.53 [95% CI, 0.32–0.89]), respectively.
Conclusions
CVH scores that include sleep health predicted CVD risk in older US adults. The incorporation of sleep as a CVH metric, akin to other health behaviors, may enhance CVD primordial and primary prevention efforts. Findings warrant confirmation in larger cohorts over longer follow‐up.
Keywords: cardiovascular diseases, cardiovascular health, health behaviors, Life's Essential 8, Life's Simple 7, primordial prevention, sleep
Subject Categories: Cardiovascular Disease, Epidemiology, Lifestyle, Primary Prevention, Risk Factors
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- CVH
cardiovascular health
- LS7
Life's Simple 7
- PA
physical activity
Clinical Perspective.
What Is New?
An expanded cardiovascular health score that includes the American Heart Association's Life's Simple 7 metrics plus a sleep health measure was evaluated in relation to cardiovascular disease prevalence and incidence.
The Life's Simple 7 score and all iterations of an “Essential Eight” score, that additionally incorporate sleep health measures, were related to cardiovascular disease prevalence.
Cardiovascular health scores that include sleep duration or a measure of multidimensional sleep health, in addition to the health factors and behaviors included in the Life's Simple 7, were additionally associated with cardiovascular disease incidence in older US adults.
What Are the Clinical Implications?
The incorporation of sleep as a cardiovascular health metric, akin to other health behaviors, may enhance primordial and primary cardiovascular disease prevention efforts at the population level.
The approach to promoting a healthy lifestyle, which traditionally focused heavily on diet and physical activity, should be expanded to encompass behaviors across the 24‐hour period, including sleep.
Additional research is needed to examine this expanded definition of cardiovascular health in relation to lifetime risk of cardiovascular disease and to evaluate the impact of screening for sleep problems and improving sleep hygiene on cardiovascular outcomes.
Sleep, alongside diet and physical activity (PA), is one of the 3 pillars of health, but unlike other health behaviors, healthy sleep is not included in the American College of Cardiology/American Heart Association (AHA) cardiovascular disease (CVD) prevention guidelines. 1 Similar to the high prevalence of unhealthy diet and physical inactivity, 2 poor sleep is ubiquitous. 3 Approximately 35% of US adults are short sleepers (<7 hours), ≈20% report excessive daytime sleepiness, and <50% report having a good night of sleep every night. 4 , 5 , 6 , 7 Importantly, the presence of sleep disorders, such as insomnia and obstructive sleep apnea (OSA), has been linked to other adverse sleep exposures such as short sleep, suggesting that multiple unhealthy sleep phenotypes may occur concurrently and potentially interact, further augmenting risk for chronic disease. 6 , 8 , 9 , 10
Sleep disorders and unhealthy sleep behaviors have been extensively linked to elevated cardiometabolic risk and CVD, 11 , 12 , 13 with effect sizes similar to those observed for other health behaviors such as diet and physical activity. A 2016 AHA statement on sleep concluded that short and poor‐quality sleep and sleep disorders are associated with higher obesity, hypertension, and diabetes risk. 11 Irregularity in sleep timing and duration have also been linked to metabolic abnormalities and higher CVD risk. 12 , 14 Further, poor sleep is related to poor diet quality and lower PA and may influence CVD risk by interacting with these lifestyle factors. 15 , 16
While sleep phenotypes have been examined in relation to individual health behaviors and cardiometabolic risk factors, the role of sleep in achieving ideal cardiovascular health (CVH) has not been well characterized. We previously demonstrated that self‐reported short sleep duration, poorer sleep quality, and higher insomnia severity and OSA risk are related to poorer CVH, 17 but the association of objectively assessed sleep with CVH has not been investigated. Furthermore, although sleep is increasingly acknowledged as a CVD risk factor, 2 , 11 healthy sleep is not included as a CVH metric in the AHA's Life's Simple 7 (LS7). 18 A higher LS7 composite score, indicative of more favorable CVH, has been linked to lower risk of cardiovascular outcomes 19 , 20 , 21 , 22 , 23 ; whether an expanded definition of CVH that includes sleep, that is, updating the LS7 to “Essential Eight,” is associated with CVD risk has not been previously evaluated. In addition, given that sleep is a multidimensional health construct, there is a need to evaluate which sleep parameters should be prioritized for CVD prevention.
We investigated the association of sleep characteristics with CVH and whether CVH scores that describe healthy sleep, as an additional eighth metric, would be associated with lower CVD prevalence and incidence among older US adults, a population at high risk for CVD. 2 We hypothesized that a CVH score that includes healthy sleep (ie, adding sleep metrics to the LS7) would be associated with CVD prevalence and incidence. We further hypothesized that a CVH score that includes several sleep characteristics, providing a global measure of multidimensional sleep health (akin to diet quality, which is assessed from intakes of multiple food groups), would represent the best measure for predicting future CVD risk.
Methods
Study Population
The MESA (Multi‐Ethnic Study of Atherosclerosis) mechanism for public access to clinical exam data are via the National Institutes of Health BioLINCC repository: https://biolincc.nhlbi.nih.gov/studies/mesa/. Data and materials from the MESA Sleep Ancillary Study are publicly available on the National Sleep Research Resource and can be accessed at: https://sleepdata.org/. MESA is an ongoing US prospective cohort study of subclinical CVD and CVD risk factors. 24 At baseline, 6814 adults (age, 45–84 years; 38% White, 12% Chinese‐American, 28% Black, and 22% Hispanic, with race and ethnicity based on self‐report), free of clinical CVD, were recruited. To date, 6 clinical examinations have been completed. Of 4655 participants at Exam 5 (2010–2012), 2261 (48.5%) enrolled in the MESA Sleep Ancillary Study (2010–2013), which included sleep assessments: single overnight polysomnography, 7‐day wrist actigraphy, and validated questionnaires. A total of 2060 participants had successful polysomnography data, 2156 had actigraphy data, and 2240 participants completed sleep questionnaires. The final analytical sample consisted of n=1920 participants with complete data on the sleep characteristics from actigraphy, polysomnography, and sleep questionnaires as well as the outcomes of interest. All participants gave written informed consent. Study protocols were approved by the institutional review boards at participating institutions.
Assessment of Sleep Characteristics
Actigraphy was performed for 7 consecutive days using the Actiwatch Spectrum wrist actigraph (Philips Respironics, Murrysville, PA) worn on participants' nondominant wrists. In‐home, overnight polysomnography was conducted using a 15‐channel monitor (Compumedics Somte System; Compumedics Ltd., Abbotsville, Australia). Actigraphy and polysomnography data were scored centrally at the Brigham and Women's Hospital Sleep Reading Center by trained technicians blinded to other data, with high levels of inter‐and intrascorer reliability. 25 Sleep duration, efficiency, and regularity were ascertained from actigraphy. Sleep duration ≥7 and <9 hours was considered sufficient. 11 , 25 , 26 Adequate sleep efficiency was defined as percentage of time in bed after lights off spent sleeping ≥85%. 27 Low night‐to‐night variability in sleep duration and timing were defined as having a sleep duration and sleep onset timing SD <90 minutes. An apnea‐hypopnea index was calculated on the basis of the average number of all apneas plus hypopneas associated with a 4% desaturation per hour of sleep. 25 Participants were considered to have no or mild OSA if they had an apnea‐hypopnea index <15 events/h. Insomnia was ascertained using the 5‐item Women's Health Initiative Insomnia Rating Scale (score range, 0–20), 28 and those with scores ≥9 were considered to have insomnia. The Epworth Sleepiness Scale 29 was used to measure daytime sleepiness (score range, 0–24); scores >10 indicated excessive daytime sleepiness.
Assessment of Cardiovascular Health Metrics
Information on CVH metrics, collected during Exam 5, was used to operationalize the CVH scores. Body mass index (kg/m2) was calculated from weight and height. Three readings of seated systolic and diastolic blood pressure were obtained; the average of the last 2 readings was used. Total cholesterol and blood glucose levels were measured from venous blood samples collected after a 12‐hour fast and shipped to the MESA central laboratory for analysis. Minutes per week of moderate and vigorous PA were estimated from the MESA Typical Week Physical Activity Survey. 30 A validated 128‐item food frequency questionnaire was administered to measure habitual diet. 31 Cigarette smoking was self‐reported; participants were categorized as current, former, or never smokers.
Operationalization of the CVH Scores
Detailed description of the operationalization of the AHA's Life's Simple 7 (LS7) and the new potential Essential Eight CVH scores including sleep is shown in Table 1. The LS7 score was computed on the basis of meeting recommendations for body mass index, cholesterol, blood pressure, blood glucose, diet, PA, and smoking, 18 consistent with previous studies. 17 , 19 , 20 , 21 Briefly, a score of 2 was assigned to each metric for meeting the ideal guideline, whereas a score of 1 or 0 was assigned for intermediate or poor achievement of the guideline. The component scores were summed to create the LS7 score, with higher scores being indicative of more favorable CVH. The LS7 score was categorized as follows: 0 to 7 (poor), 8 to 11 (moderate), and 12 to 14 (high).
Table 1.
Operationalization of the AHA LS7 and New CVH Scores*
| Cardiovascular health metric | Ideal (score=2) | Intermediate (score=1) | Poor (score=0) |
|---|---|---|---|
| Smoking | Never smoker | Former smoker | Current smoker |
| Diet† | Meets 4–5 recommendations | Meets 2–3 recommendations | Meets 0–1 recommendations |
| Physical activity‡ | ≥150 min/wk moderate intensity or ≥75 min/wk vigorous intensity or ≥150 min/wk moderate+vigorous intensity | 1–149 min/wk moderate intensity or 1–74 min/wk vigorous intensity or 1 to 149 min/wk moderate+vigorous intensity | No moderate or vigorous activity |
| BMI | <25 kg/m2 | 25–29.9 kg/m2 | ≥30 kg/m2 |
| Blood pressure | SBP <120 and DBP <80 mm Hg untreated | SBP 120–129 or DBP: <80 mm Hg or treated to ideal level | SBP ≥130 or DBP ≥80 mm Hg |
| Total cholesterol | <200 mg/dL | 200–239 mg/dL or treated to ideal level | ≥240 mg/dL |
| Fasting glucose | <100 mg/dL | 100–125 mg/dL or treated to ideal level | ≥126 mg/dL |
| Sleep in CVH score 1§ | Sleep duration ≥7 h and<9 h | Sleep duration ≥6 h and <7 h | Sleep duration <6 h or >9 h |
| Sleep in CVH score 2 | Meets all the following sleep metrics:
|
Meets 2–3 of sleep metrics for ideal sleep score | Meets 0–1 of sleep metrics for ideal sleep score |
| Sleep in CVH score 3 | Meets all the following sleep metrics:
|
Meets 2–3 of sleep metrics for ideal sleep score | Meets 0–1 of sleep metrics for ideal sleep score |
| Sleep in CVH score 4 | Meets 4–5 of metrics below:
|
Meets 2–3 of sleep metrics for ideal sleep score | Meets 0–1 of sleep metrics for ideal sleep score |
AHA LS7 indicates American Heart Association Life Simple 7; AHI, apnea‐hypopnea index; BMI, body mass index; CVH, cardiovascular health; DBP, diastolic blood pressure; ESS, Epworth Sleepiness Scale; OSA, obstructive sleep apnea; SBP, systolic blood pressure; and WHIIRS, Women's Health Initiative Insomnia Rating Scale.
The AHA LS7 score was computed on the basis of level of meeting recommendations for BMI, cholesterol, blood pressure, blood glucose, diet, physical activity, and smoking. Participants received a score of 0–2 based on their level of meeting each metric. Component scores were summed to create the AHA LS7 score, which ranges from 0 to 14. CVH scores of 1–4 consist of the AHA LS7 metrics plus a sleep score (sleep score range, 0–2) and therefore range from 0 to 16, such that higher scores are indicative of more favorable CVH.
The diet score was based on habitual dietary intake from a validated 120‐item food frequency questionnaire, modified from the Insulin Resistance Atherosclerosis Study instrument. A healthy diet score was computed on the basis of meeting the AHA recommendations for intakes of fruits and vegetables, fish, whole grains, sodium, and sugar‐sweetened beverages. The AHA diet recommendations are as follows: (1) fruits and vegetables: ≥4.5 cups per day; (2) fish: ≥two 3.5‐oz servings per week (preferably oily fish); (3) fiber‐rich whole grains (≥1.1 g of fiber per 10 g of carbohydrate): ≥three 1‐oz‐equivalent servings per day; (4) sodium: <1500 mg per day; (5) sugar‐sweetened beverages: ≤450 kcal (36 oz) per week.
The times spent in vigorous and moderate physical activity were self‐reported on the MESA Typical Week Physical Activity Survey.
Sleep duration of ≥7 h and <9 h was considered “ideal” consistent with the definition put forth by the 2016 AHA statement on sleep (reference 11). Sleep duration ≥6 h and <7 h was considered “intermediate” given that many cohort studies in the literature, including in MESA, define short sleep as sleeping <6 h/night.
To determine whether CVH scores that include a measure of sleep health are associated with CVD risk, we computed 4 separate novel CVH scores that include sleep as an eighth metric (LS7 metrics plus a sleep score). Similar to other metrics, participants received a score of 0 to 2 for the sleep metric such that sleep scores of 0, 1, and 2 represented poor, intermediate, and ideal sleep. Therefore, the new CVH scores ranged from 0 to 16, with higher scores being indicative of more favorable CVH. Four iterations of the new CVH score that incorporate different sleep metrics were tested. In CVH score 1, the sleep score was based on objectively assessed sleep duration, the most widely measured aspect of sleep health. In CVH score 2, the sleep score was based on meeting the guidelines for 4 a priori sleep characteristics strongly related to cardiovascular risk in the literature: sleep duration, insomnia, daytime sleepiness, and OSA. 11 , 32 In CVH scores 3 and 4, the sleep score was based on meeting recommendations for sleep characteristics previously linked to cardiovascular risk in this cohort 12 , 14 , 33 , 34 , 35 and to CVH maintenance in this study.
CVD Ascertainment
MESA cardiologists, physician epidemiologists, and neurologists adjudicated CVD end points. 36 In this study, the composite cardiovascular event outcome encompassed incident myocardial infarction, definite angina, probable angina (if followed by coronary artery bypass grafting or percutaneous coronary intervention), resuscitated cardiac arrest, stroke, stroke death, coronary heart disease, or other CVD death as defined by the MESA protocol. Participants who developed CVD at or before the Sleep Exam were considered prevalent cases, and those who developed CVD after the Sleep Exam were considered incident cases. There were 95 prevalent CVD events at the Sleep Exam and 93 incident cases that occurred during a mean follow‐up of 4.4 years after the Sleep Exam.
Statistical Analysis
Participant demographic, lifestyle, and medical characteristics were described using mean±SD for continuous variables and frequencies for categorical variables in the overall sample and by sleep duration (<6 versus ≥6 hours). T‐tests and chi‐square tests were used to examine differences in descriptive characteristics by categories of sleep duration. Linear and logistic regression models were used to examine sleep characteristics in relation to the LS7 score and the odds of having poor CVH, respectively. Sleep duration (hours), efficiency (percentage), and variability (SD of sleep duration and sleep timing); insomnia (Women's Health Initiative Insomnia Rating Scale); and daytime sleepiness (Epworth Sleepiness Scale) were evaluated as continuous and categorical variables. OSA was evaluated as a categorical variable (moderate to severe versus mild to none). All models were adjusted for age (years), sex (male, female), race and ethnicity (White, Black, Hispanic, and Chinese‐American), education (college or greater, less than college), health insurance (has health insurance, does not have health insurance) and alcohol use (current drinker, does not currently consume alcohol).
For the main analysis, to investigate several novel CVH scores that include sleep, compared with the traditional LS7 score, in relation to CVD, multivariable‐adjusted logistic regression models were used to examine the LS7 score and 4 potential "Essential Eight" scores in relation to odds of prevalent CVD at the MESA Sleep Exam. Next, Cox proportional hazards models were used to evaluate the LS7 and the new potential "Essential Eight" scores in relation to risk of developing incident CVD after the Sleep Exam. Given the modest number of CVD cases, we compared CVD risk among tertiles of the LS7 and "Essential Eight" scores. A P value <0.05 was considered significant, and SAS version 9.4 (SAS Institute, Cary, NC) was used to conduct analyses.
Results
Descriptive Characteristics of the Study Population and Differences by Sleep Duration
The mean age at Exam 5 was 69±9 years and 54% of participants were female (Table 2). The racial and ethnic distribution was 40% White, 27% Black, 23% Hispanic, and 10% Chinese. Prevalence of overweight/obesity and diabetes was 73% and 18%, respectively. The mean LS7 score was 7.3, and the means of the alternate CVH scores that included sleep ranged from 7.4 to 7.8. Actigraphy showed that 63% of participants slept <7 hours and 30% slept <6 hours, while 39% and 25% had high night‐to‐night variability in sleep duration and sleep onset timing, respectively (Table 2). Overall, 10% had sleep efficiency <85%; 14% and 36% were classified as having excessive daytime sleepiness and high insomnia symptoms, respectively; and 47% had moderate‐to‐severe OSA. Notably, short sleepers were significantly more likely to have low sleep efficiency, excessive daytime sleepiness, high sleep duration and timing variability, and OSA (P<0.001). Short sleepers also had higher prevalence of overweight/obesity, diabetes, and hypertension, and had lower mean LS7 scores (P<0.001).
Table 2.
| Overall sample (n=1920) | Sleep duration <6 h/night (n=582) | Sleep duration ≥6 h/night (n=1338) | P value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, y | 68.5 (9.1) | 68.5 (9.5) | 68.5 (9.0) | 0.990 |
| Female | 1038 (54) | 261 (45) | 777 (58) | <0.001 |
| White | 759 (40) | 147 (25) | 612 (46) | <0.001 |
| Black | 510 (27) | 221 (38) | 290 (21) | |
| Hispanic | 449 (23) | 146 (25) | 305 (23) | |
| Chinese‐American | 202 (10) | 70 (12) | 131 (10) | |
| Married | 1157 (61) | 313 (54) | 844 (64) | <0.001 |
| Have health insurance | 1824 (95) | 548 (94) | 1276 (95) | 0.300 |
| Education≥college | 1346 (70) | 402 (69) | 944 (71) | 0.500 |
| Born in the United States | 1298 (68) | 368 (63) | 930 (70) | 0.007 |
| Cardiometabolic risk factors | ||||
| BMI, kg/m2 | 28.8 (5.6) | 29.7 (5.8) | 28.5 (5.5) | <0.001 |
| BMI in overweight or obese categories | 1407 (73) | 463 (80) | 944 (71) | <0.001 |
| SBP, mm Hg | 122.5 (19.9) | 124.5 (21.5) | 121.6 (19.1) | 0.003 |
| DBP, mm Hg | 68.2 (9.9) | 69.9 (10.5) | 67.5 (9.6) | <0.001 |
| Hypertension | 1091 (57) | 359 (62) | 732 (55) | 0.005 |
| Fasting glucose, mg/dL | 101.5 (27.1) | 103.4 (27.8) | 100.7 (26.8) | 0.04 |
| Type 2 diabetes (treated or untreated) | 353 (18) | 120 (21) | 233 (17) | 0.020 |
| Total AHA LS7 score | 7.3 (2.5) | 7.0 (2.5) | 7.4 (2.5) | <0.001 |
| Sleep habits | ||||
| Sleep duration (h)‡ | 6.5 (1.3) | 4.9 (0.9) | 7.2 (0.8) | <0.001 |
| Poor sleep efficiency‡ , § | 186 (10) | 93 (16) | 93 (7) | <0.001 |
| Sleep efficiency, %‡ , § | 89.8 (3.7) | 88.5 (4.3) | 90.4 (3.3) | <0.001 |
| SD of sleep duration, min‡ | 84.0 (48) | 102.2 (43) | 76.1 (48) | <0.001 |
| High night‐to‐night variability in sleep duration (SD of sleep duration ≥90 vs <90 min)‡ | 38.6 (741) | 57.7 (336) | 30.3 (405) | <0.001 |
| SD of sleep onset timing, min‡ | 81.7 (97) | 112.3 (109) | 68.4 (64) | <0.001 |
| High night‐to‐night variability in sleep onset timing (SD of sleep onset timing ≥90 vs <90 min)‡ | 24.9 (479) | 45.0 (262) | 16.2 (217) | <0.001 |
| Insomnia‖ | 679 (36) | 215 (38) | 464 (35) | 0.360 |
| Excessive daytime sleepiness¶ | 270 (14) | 112 (20) | 158 (12) | <0.001 |
| Moderate to severe obstructive sleep apnea# | 812 (47) | 277 (54) | 535 (45) | 0.001 |
| Cardiovascular health metrics | ||||
| Total AHA LS7 score categories | ||||
| Poor | 984 (51) | 332 (57) | 652 (49) | 0.003 |
| Intermediate | 842 (44) | 228 (39) | 614 (46) | |
| Ideal | 94 (5) | 22 (4) | 72 (5) | |
| Diet score | ||||
| Poor | 809 (42) | 266 (46) | 543 (41) | 0.040 |
| Intermediate | 1027 (54) | 286 (49) | 741 (55) | |
| Ideal | 84 (4) | 30 (5) | 54 (4) | |
| Physical activity score | ||||
| Poor | 427 (22) | 140 (24) | 287 (22) | 0.440 |
| Intermediate | 281 (15) | 82 (14) | 199 (15) | |
| Ideal | 1212 (63) | 360 (62) | 852 (64) | |
| Smoking score | ||||
| Poor | 138 (7) | 58 (10) | 80 (6) | 0.007 |
| Intermediate | 899 (47) | 270 (46) | 629 (47) | |
| Ideal | 883 (46) | 254 (44) | 629 (47) | |
| Cholesterol score | ||||
| Poor | 828 (43) | 601 (39) | 601 (45) | 0.008 |
| Intermediate | 395 (21) | 281 (20) | 281 (21) | |
| Ideal | 697 (36) | 456 (41) | 456 (34) | |
| BMI score | ||||
| Poor | 695 (36) | 245 (42) | 450 (34) | <0.001 |
| Intermediate | 712 (37) | 218 (37) | 494 (37) | |
| Ideal | 513 (27) | 119 (21) | 394 (29) | |
| Blood pressure score | ||||
| Poor | 1288 (67) | 424 (73) | 864 (65) | 0.002 |
| Intermediate | 118 (6) | 87 (5) | 87 (7) | |
| Ideal | 514 (27) | 387 (22) | 387 (29) | |
| Glucose score | ||||
| Poor | 332 (17) | 115 (20) | 217 (16) | 0.012 |
| Intermediate | 416 (22) | 141 (24) | 275 (21) | |
| Ideal | 1172 (61) | 326 (56) | 846 (63) | |
AHA LS7 indicates American Heart Association Life's Simple 7; BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; OSA, obstructive sleep apnea; PA, physical activity; and SBP, systolic blood pressure.
Continuous variables are shown as mean±SD, and categorical variables are shown as n (%).
T tests and chi‐square tests were used to examine differences in descriptive characteristics by sleep duration.
Sleep duration, sleep efficiency, and sleep variability were assessed from actigraphy measures performed using the Actiwatch Spectrum wrist actigraph (Philips Respironics, Murrysville, PA) worn on participants' nondominant wrists for 7 consecutive days.
Sleep efficiency was defined as the percentage of time in bed after lights off spent sleeping and was considered poor if <85%.
Insomnia was evaluated using the validated 5‐item Women's Health Initiative Insomnia Rating Scale (WHIIRS), which assesses insomnia symptoms including long sleep latency, sleep maintenance insomnia, early morning awakening, and poor sleep quality over the past 4 weeks. The score ranges from 0 to 4 for each item on the WHIIRS (0–20 total score range). Participants with WHIIRS scores ≥9 were considered to have insomnia.
The Epworth Sleepiness Scale (ESS) was used to measure daytime sleepiness. The total score of the ESS ranges from 0 to 24, and those with an ESS score >10 were considered to have excessive daytime sleepiness.
OSA was assessed using overnight polysomnography, which was conducted using a 15‐channel monitor (Compumedics Somte System; Compumedics Ltd., Abbotsville, Australia). An apnea‐hypopnea index (AHI) was calculated on the basis of the average number of all apneas plus hypopneas associated with a 4% desaturation per hour of sleep. Participants were considered to have moderate to severe OSA if they had an AHI ≥15 events/h.
Associations of Sleep Phenotypes With Cardiovascular Health
In linear models (Table 3), longer sleep duration was related to higher LS7 scores (P=0.023). Greater daytime sleepiness (P=0.010), high night‐to‐night variability in sleep duration and sleep timing, and moderate‐to‐severe OSA were associated with lower LS7 scores (P<0.001). Higher sleep efficiency—a marker of less fragmented sleep—was associated with a higher LS7 score (P=0.002). In logistic models (Table 3), short sleep (odds ratio [OR], 1.25 [95% CI, 1.01–1.55]), high night‐to‐night variability in sleep duration (OR, 1.24 [95% CI, 1.02–1.51]) and sleep timing (OR, 1.31 [95% CI, 1.04–1.64]), and moderate‐to‐severe OSA (OR, 2.21 [95% CI, 1.78–2.73]) were associated with greater odds of having poor CVH.
Table 3.
Multivariable‐Adjusted Linear and Logistic Models for Associations of Sleep Characteristics with Cardiovascular Health Defined by the American Heart Association Life's Simple 7 Score (n=1920)*
| Linear regression models | Logistic regression models† | |||
|---|---|---|---|---|
| Sleep characteristics | B(SE) | P value | Sleep characteristics | OR (95% CI) |
| Sleep duration, h‡ | 0.01 (0.04) | 0.023 | Sleep duration (<6 vs ≥6 h) | 1.25 (1.01–1.55) |
| Sleep duration (<6 h vs ≥6 h) | −0.27 (0.12) | 0.025 | ||
| Sleep efficiency (%)c | 0.05 (0.01) | 0.002 | Sleep efficiency (<85% vs ≥ 85%) | 1.19 (0.86–1.65) |
| Sleep efficiency (<85% vs ≥85%) | −0.26 (0.18) | 0.144 | ||
| SD of sleep duration (h)‡ | −0.25 (0.07) | <0.001 | Increased night‐to‐night variability in sleep duration (SD of sleep duration ≥90 vs <90 min) | 1.24 (1.02–1.51) |
| Increased night‐to‐night variability in sleep duration (SD of sleep duration ≥90 vs <90 min) | −0.45 (0.11) | <0.001 | ||
| SD of sleep onset timing (h)‡ | −0.03 (0.03) | 0.382 | Increased night‐to‐night variability in sleep onset timing (SD of sleep onset timing ≥90 vs <90 min) | 1.31 (1.04–1.64) |
| Increased night‐to‐night variability in sleep onset timing (SD of sleep onset timing ≥90 vs <90 min) | −0.50 (0.12) | <0.001 | ||
| WHIIRS‡ | −0.04 (0.11) | 0.746 | Insomnia (WHIIRS score ≥9 vs <9) | 1.03 (0.84–1.25) |
| ESS‡ | −0.40 (0.16) | 0.010 | Excessive daytime sleepiness (ESS >10 vs ≤10) | 1.17 (0.89–1.54) |
| OSA (moderate to severe vs mild or none)§ | −0.95 (0.11) | <0.001 | OSA (moderate to severe vs mild or none)§ | 2.21 (1.78–2.73) |
AHA LS7 indicates American Heart Association Life's Simple 7; ESS, Epworth Sleepiness Scale; OR, odds ratio; OSA, obstructive sleep apnea; and WHIIRS, Women's Health Initiative Insomnia Rating Scale.
Models are adjusted for age, sex, race/ethnicity, education, health insurance, and alcohol use.
In linear models, the sleep characteristics were evaluated in relation to the AHA LS7 score. In logistic regression models, the sleep characteristics were evaluated in relation to odds of having poor cardiovascular health, defined as an AHA LS7 score of 0–7.
Variables were used on the continuous scale. A higher standard deviation of sleep duration and sleep onset timing indicates greater night‐to‐night variability in sleep patterns. A higher ESS score indicates greater daytime sleepiness. A higher Women's Health Initiative Insomnia Rating Scale score indicates greater insomnia severity.
Additional adjustment of models on OSA for BMI attenuated associations, but the results remained significant. In linear models: B(SE)=−0.30(0.10), P‐value=0.005, and in logistic regression models: OR (95%CI), 1.35 (1.07–1.72).
LS7 and Potential "Essential Eight" CVH Scores in Relation to Prevalent and Incident CVD
Higher LS7 and CVH scores 1 to 4 were all related to lower odds of having CVD at Exam 5 (Table 4 and Figure). Those in the highest versus lowest tertile of the LS7 score had 75% lower CVD odds (OR, 0.25 [95% CI, 0.13–0.49]). Similarly, those in the highest versus lowest tertile of CVH score 1, which included sleep duration, and CVH score 2, which included sleep characteristics linked to CVD in the literature (sleep duration, insomnia, daytime sleepiness, and OSA), had 71% and 80% lower odds of prevalent CVD, respectively (OR, 0.29 [95% CI, 0.16–0.54]; and OR, 0.20 [95% CI, 0.10–0.41]). Consistent with these findings, those in the highest versus lowest tertile of CVH score 3, which included commonly studied sleep characteristics linked to cardiovascular risk in MESA (sleep duration, sleep efficiency, daytime sleepiness, and OSA), and CVH score 4, which included the same sleep characteristics as CVH score 3 plus measures of sleep regularity as novel sleep‐related CVD risk factors, had 68% and 67% lower odds of prevalent CVD, respectively (OR, 0.32 [95% CI, 0.17–0.60]; and OR, 0.33 [95% CI, 0.18–0.59]).
Table 4.
Association of LS7 Score and Alternate CVH Scores That Include Sleep With CVD (n=1920)
| Cardiovascular health scores | Operationalization of sleep metric | Tertiles | CVD prevalence OR (95% CI)* , † , ‡ | CVD incidence HR (95% CI)§ , † , ‡ |
|---|---|---|---|---|
| AHA LS7 score‖ | No sleep metric |
Tertile 1 Tertile 2 Tertile 3 |
1.00 0.74 (0.46–1.19) 0.25 (0.13–0.49) |
1.00 0.65 (0.39–1.09) 0.62 (0.37–1.04) |
|
AHA LS7+ sleep score based on sleep duration |
Ideal sleep score=2:
Intermediate sleep score=1:
Poor sleep score=0:
|
Tertile 1 Tertile 2 Tertile 3 |
1.00 0.58 (0.35–0.96) 0.29 (0.16–0.54) |
1.00 0.82 (0.50–1.35) 0.57 (0.33–0.97) |
|
CVH score 2 ‖ AHA LS7+sleep score based on sleep characteristics linked to CVD in the literature |
Ideal sleep score=2:
Intermediate sleep score=1: Meets 2–3 of sleep metrics for ideal sleep score Poor sleep score=0: Meets 0–1 of metrics for ideal sleep score |
Tertile 1 Tertile 2 Tertile 3 |
1.00 0.53 (0.33–0.84) 0.20 (0.10–0.41) |
1.00 0.91 (0.56–1.49) 0.66 (0.37–1.18) |
|
CVH score 3 ‖ AHA LS7+sleep score based on sleep characteristics previously linked to cardiovascular risk in MESA |
Ideal sleep score=2:
Intermediate sleep score=1: Meets 2–3 of sleep metrics for ideal sleep score Poor sleep score=0: Meets 0–1 of metrics for ideal sleep score |
Tertile 1 Tertile 2 Tertile 3 |
1.00 0.78 (0.48–1.27) 0.32 (0.17–0.60) |
1.00 0.58 (0.34–1.00) 0.63 (0.38–1.05) |
|
CVH score 4 § AHA LS7+sleep score based on sleep characteristics recently linked to cardiovascular risk in MESA |
Ideal sleep score=2: Meets 4–5 of metrics below:
Intermediate sleep score=1: Meets 2–3 of sleep metrics for ideal sleep score Poor sleep score=0: Meets 0–1 of metrics for ideal sleep score |
Tertile 1 Tertile 2 Tertile 3 |
1.00 0.74 (0.45–1.20) 0.33 (0.18–0.59) |
1.00 0.64 (0.38–1.09) 0.53 (0.32–0.89) |
AHA LS7 indicates American Heart Association's Life's Simple 7; AHI, Apnea‐Hypopnea Index; CVD, cardiovascular disease; CVH, cardiovascular health; ESS, Epworth Sleepiness Scale; HR, hazard ratio; MESA, Multi‐Ethnic Study of Atherosclerosis; OR, odds ratio; OSA, obstructive sleep apnea; and WHIIRS, Women's Health Initiative Insomnia Rating Scale.
Logistic Regression Models were used to examine associations of the AHA LS7 score and alternate CVH scores with odds of having a CVD event.
Models were adjusted for age, sex, race/ethnicity, education, health insurance, and alcohol use.
CVD events were defined as all CVD (CVDa) per MESA protocol. CVDa includes myocardial infarction, resuscitated cardiac arrest, definite angina, probable angina (if followed by revascularization), stroke, stroke death, coronary heart disease death, other atherosclerotic death, other CVD death.
Cox proportional hazards models were used to examine associations of the AHA LS7 score and alternate CVH scores with risk of developing a CVD event.
The AHA LS7 score was computed on the basis of level of meeting recommendations for body mass index, cholesterol, blood pressure, blood glucose, diet, physical activity, and smoking. Participants received a score of 0–2 based on their level of meeting each metric. Component scores were summed to create the AHA LS7 score, which ranges from 0 to 14. CVH scores of 1–4 consist of the AHA LS7 metrics plus a sleep score (sleep score range, 0–2) and therefore range from 0 to 16, such that higher scores are indicative of more favorable CVH.
Sleep duration of ≥7 h and <9 h was considered “ideal,” consistent with the definition put forth by the 2016 AHA statement on sleep. Sleep duration ≥6 h and <7 h was considered “intermediate” given that many cohort studies in the literature, including in MESA, define short sleep as sleeping <6 h/night.
Figure 1. The Life's Simple 7 and iterations of potential “Essential Eight” CVH scores that include sleep in relation to CVD.
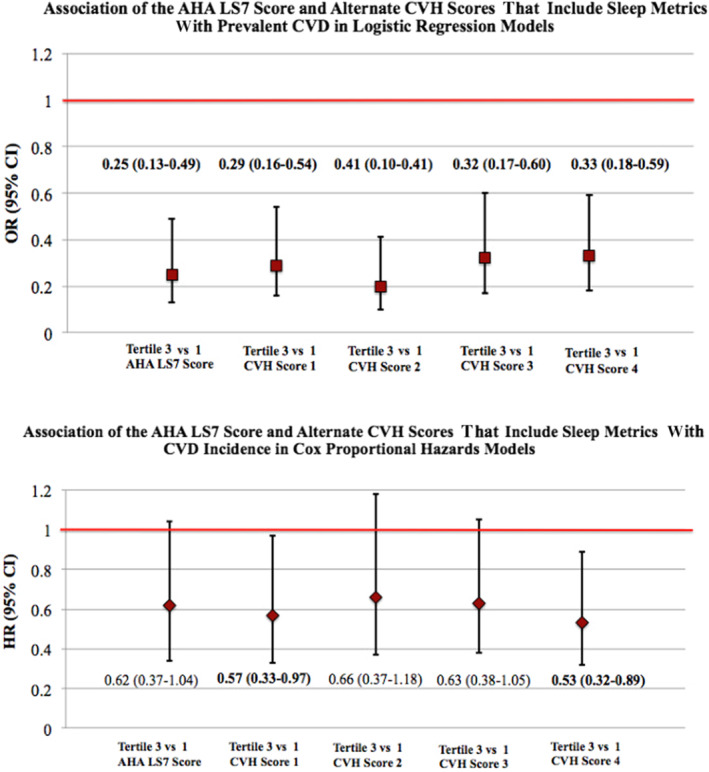
The AHA's LS7 score and 4 iterations of potential “Essential Eight” scores, that include the same LS7 metrics but additionally incorporate sleep, were evaluated in relation to CVD risk. The upper panel shows the LS7 and alternate CVH scores in relation to prevalent CVD using multivariable logistic models in 1920 adults in the MESA Sleep Study. The lower panel shows associations of the LS7 score and alternate CVH scores with risk of developing new CVD using multivariable Cox proportional hazards models. Models were adjusted for age, sex, race and ethnicity, education, health insurance, and alcohol use. AHA indicates American Heart Association; CVD, cardiovascular disease; CVH, cardiovascular health; HR, hazard ratio; LS7, Life's Simple 7; MESA, Multi‐Ethnic Study of Atherosclerosis; and OR, odds ratio.
In Cox proportional hazards models that evaluated the LS7 and new CVH scores that incorporate sleep in relation to risk of developing CVD (Table 4 and Figure), only CVH scores 1 and 4 were significantly associated with CVD incidence (although hazard ratios <1 were observed when comparing the highest versus lowest tertile of all scores). Those in the highest versus lowest tertile of CVH score 1, which included only sleep duration, had 43% lower CVD risk (hazard ratio, 0.57 [95% CI, 0.33–0.97]). Similarly, those in the highest versus lowest tertile of CVH score 4, which included a measure of multidimensional sleep health, had 47% lower risk of developing CVD (hazard ratio, 0.53 [95% CI, 0.32–0.89]).
Discussion
To our knowledge, this represents the first investigation of the addition of objectively assessed sleep to the AHA's LS7 framework, as a novel eighth metric of CVH, for CVD outcome prediction. We uniquely demonstrate that while the LS7 score and new CVH scores that include sleep were all related to prevalent CVD, only CVH scores that include sleep predicted CVD incidence in this cohort of older adults. Contrary to our initial hypothesis, even a CVH score that includes only objectively assessed sleep duration, a common measure of sleep health, predicted CVD incidence. Notably, the CVH score that incorporated objective measures of sleep duration, quality, and regularity as well as sleep disorders was also significantly associated with both CVD prevalence and incidence. This finding underscores the importance of embracing a holistic vision of sleep health that includes sleep behaviors and highly prevalent, mild sleep problems rather than strictly focusing on sleep disorders when assessing cardiovascular risk.
The criteria used to define CVH include face validity; consistency with clinic and public health guidelines; simplicity and accessibility to practitioners and individuals providing guidance regarding lifestyle change for CVH promotion; providing actionable targets for individuals, practitioners, and policy makers; allowing all subsets of the population to make progress achieving or maintaining CVH; and being readily measured to allow for current assessment and monitoring over time. 18 Our findings herein are consistent with our prior work and that of others 11 , 17 , 32 in demonstrating that sleep is important for achieving CVH. Our results also support the utility of a multidimensional sleep health approach, consistent with the framework proposed by Buysse et al 37 , 38 and supported by emerging evidence from MESA and other cohorts, 13 , 26 , 38 , 39 to more comprehensively capture, monitor, and address actionable targets predictive of CVD risk, when comprehensive sleep health assessment is possible. However, given that criteria used to define the original CVH metrics include simplicity, accessibility, and ease of monitoring of lifestyle CVH metrics for providers and individuals alike, sleep duration may represent the easiest, most actionable new metric to recommend for inclusion. Indeed, short sleep duration was interrelated to multiple other poor sleep dimensions, likely contributing to its utility as a single measure of sleep health in this sample.
Although there are no previous studies on the role of sleep as an eighth metric of CVH, sleep has been extensively linked to CVD. 11 Meta‐analyses of cohort studies demonstrate that short sleep duration is associated with up to 48% higher risk of developing or dying from CHD and 15% higher incident stroke risk. 40 In Italian men, the presence of severe sleep disturbances (difficulty falling and remaining asleep, daytime sleepiness) was associated with 80% higher risk of CVD, particularly from age 48 years onward. 41 Further, compared with those sleeping 7 to 8 hours, long sleepers had 56% higher CVD risk. In MESA, irregular sleep duration and timing have been linked to >2‐fold higher CVD risk. 12 OSA was associated with ≈2‐fold higher incident CVD risk 42 ; severe OSA and short and long sleep have also been associated with ≈2‐fold higher risk of peripheral artery disease in this cohort. 43
Combinations of sleep characteristics have also been linked to CVD outcomes. Short sleepers with poor sleep quality had 63% higher CVD risk compared with normal sleepers with good sleep quality among Dutch adults. 44 In Italian men, those who slept ≤6 hours and had sleep disturbances had 69% greater risk of developing CVD. 41 Finally, in elderly US adults, multidimensional sleep health, encompassing sleep duration, timing, and quality; napping habits; and sleep disorder symptoms was related to ≈2‐fold higher risk of cardiovascular mortality. 13 Our finding that CVH scores that include both sleep duration only and multidimensional sleep health are associated with CVD risk may be explained, at least in part, by the clustering of poor sleep characteristics. In our cohort, short sleepers also had a higher prevalence of poor sleep efficiency, high sleep variability, excessive daytime sleepiness, and OSA. Therefore, screening for sleep duration may represent a realistic and practical approach for assessing sleep health in clinic or public health settings when comprehensive multidimensional sleep health assessment is not feasible.
Potential mechanisms underlying the association of sleep with cardiovascular risk include the influence of sleep on health factors and behaviors included in the LS7. 11 , 45 , 46 There is convincing evidence that short sleep and to a lesser extent long sleep, poor‐quality sleep, insomnia, and OSA are associated with greater risk for obesity, diabetes, and hypertension. 11 A growing body of evidence has also linked irregular sleep patterns to cardiometabolic diseases. 45 , 46 Indeed, in MESA, short sleep, low sleep efficiency, and high sleep variability were related to higher body mass index and overweight/obesity, 47 while OSA was associated with obesity and higher blood pressure. 34 Sleep variability predicted adverse cardiometabolic profiles in this cohort, as every 1‐hour increase in sleep duration and timing SD was associated with up to 36% greater odds of metabolic syndrome. 14
Short sleep, poor‐quality sleep, and insomnia have been associated with higher caloric intake and unhealthy food choices, including lower intake of plant‐based foods and higher intakes of added sugar and sodium. 48 , 49 Daytime sleepiness and short sleep have been linked to lower PA. 50 , 51 In MESA, OSA was associated with poorer‐quality diet and less PA. 52 , 53 To our knowledge, only 1 previous study has evaluated sleep characteristics in relation to overall CVH, as measured by the LS7. 17 In that study, women with sleep duration ≥7 versus <7 hours, good versus poor sleep quality, no insomnia, and low versus high OSA risk were more likely to meet >4 LS7 metrics. This is consistent with our results that short sleep, lower sleep efficiency, higher daytime sleepiness, and OSA are associated with poor CVH.
Sleep may contribute to CVD risk via psychological and physiological pathways. 11 , 45 , 46 , 54 , 55 Sleep deprivation and sleep disorders have been linked to increased sympathetic activity, reduced parasympathetic activation, increased inflammation, and oxidative stress, which collectively lead to dysfunction of the vascular endothelium. In addition, short‐duration sleep and irregular sleep patterns could disrupt circadian rhythmicity and result in circadian misalignment, which can lead to metabolic dysfunction predisposing to CVD. 11 , 45 , 46 Finally, poor sleep has also been linked to depression and stress. 55
Our study has several important strengths including the community‐based multiethnic nature of our cohort, rigorous CVD adjudication procedures, comprehensive assessment of cardiovascular risk factors included in our models, and the use of standardized polysomnography and actigraphy to measure sleep, which resulted in less measurement error. A limitation worth noting is the modest number of CVD cases attributable to the follow‐up period of 4.4 years. We also had limited power to test for subgroup differences by sex and race and ethnicity and did not adjust for type 1 error. Finally, although our sleep health scores included important sleep‐related CVD risk factors, they may not capture the full picture of an individual's sleep health. For instance, in patients with OSA, elevated OSA‐specific hypoxic burden has been linked to major adverse cardiovascular events. 56
Additional studies in external cohorts with larger sample sizes and longer follow‐up are warranted to confirm these findings and examine this expanded definition of CVH in relation to subclinical CVD and lifetime risk of CVD outcomes. Clinical trials are also needed to evaluate the influence of screening for sleep problems and improving sleep hygiene on cardiovascular outcomes. We show here that those who are short sleepers are more likely to have sleep disorders and engage in other poor sleep behaviors, suggesting that sleep problems may cluster. Therefore, the value of screening for self‐reported sleep duration during a clinic visit, as a feasible and time efficient approach for assessing sleep, in improving CVD risk prediction also warrants further investigation.
Conclusions
Our findings demonstrate that the incorporation of sleep as a CVH metric, akin to other health behaviors, may enhance primordial and primary CVD prevention efforts at the population level. The approach to promoting a healthy lifestyle, which traditionally focused heavily on diet and PA, should be expanded to encompass behaviors across the 24‐hour period, including sleep. 10 , 11 , 32 Health care providers should assess their patients' sleep patterns, discuss sleep‐related problems, and educate patients about the importance of prioritizing sleep to promote CVH. Furthermore, the formal integration of sleep health into CVH promotion guidance could provide benchmarks for surveillance and ensure that sleep becomes an equal counterpart in public health policy to the attention and resources given to other lifestyle behaviors.
Sources of Funding
Dr Makarem is supported by National Heart, Lung, and Blood Institute Grant R00‐HL148511; and American Heart Association Grant #AHA855050. Dr St‐Onge was funded by National Institutes of Health Grants R01HL128226 and R01HL142648. Dr Redline was funded by National Heart, Lung, and Blood Institute R35 HL135818. Dr Aggarwal is supported by an American Heart Association Research Goes Red Award (Grant #AHA811531).
MESA is conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, N01‐HC‐95169, UL1‐TR‐000040, UL1‐TR‐001079, UL1‐TR‐001881, and DK06349. Funding for the MESA Sleep Exam was by grant HL098433.
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
None.
Acknowledgments
We thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa‐nhlbi.org.
Presented in part at the American Heart Association EPI|Lifestyle Scientific Sessions in Phoenix, Arizona, March 3‐6, 2020, and published in abstract form [Circulation. 2020;141:A36 or https://doi.org/10.1161/circ.141.suppl_1.36].
For Sources of Funding and Disclosures, see page 11.
References
- 1. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–e595. doi: 10.1161/CIR.0000000000000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Muntner P, Bittencourt MS. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 3. Institute of Medicine Committee on Sleep Medicine and Research . In: Colten HR, Altevogt BM, eds Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 4. National Sleep Foundation . 2013 Executive Summary, National Bedroom Poll. 2013. http://sleepfoundation.org/sites/default/files/RPT495a.pdf [Google Scholar]
- 5. Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults—United States, 2014. Morbid Mortal Wkly Rep. 2016;65:137–141. doi: 10.15585/mmwr.mm6506a1 [DOI] [PubMed] [Google Scholar]
- 6. Ford ES, Cunningham TJ, Giles WH, Croft JB. Trends in insomnia and excessive daytime sleepiness among US adults from 2002 to 2012. Sleep Med. 2015;16:372–378. doi: 10.1016/j.sleep.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ohayon MM. Determining the level of sleepiness in the American population and its correlates. J Psychiatr Res. 2012;46:422–427. doi: 10.1016/j.jpsychires.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 8. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186 [DOI] [PubMed] [Google Scholar]
- 9. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep‐disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36:417–440. doi: 10.1146/annurev-publhealth-031914-122838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. St‐Onge M, Grandner MA, Brown D, Conroy MB, Jean‐Louis G, Coons M, Bhatt DL. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–e386. doi: 10.1161/CIR.0000000000000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang T, Mariani S, Redline S. Sleep irregularity and risk of cardiovascular events: the Multi‐Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2020;75:991–999. doi: 10.1016/j.jacc.2019.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wallace ML, Buysse DJ, Redline S, Stone KL, Ensrud K, Leng Y, Ancoli‐Israel S, Hall MH. Multidimensional sleep and mortality in older adults: a machine‐learning comparison with other risk factors. J Gerontol. 2019;74:1903–1909. doi: 10.1093/gerona/glz044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang T, Redline S. Cross‐sectional and prospective associations of actigraphy‐assessed sleep regularity with metabolic abnormalities: the multi‐ethnic study of atherosclerosis. Diabetes Care. 2019;42:1422–1429. doi: 10.2337/dc19-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frank S, Gonzalez K, Lee‐Ang L, Young MC, Tamez M, Mattei J. Diet and sleep physiology: public health and clinical implications. Front Neurol. 2017;8:393. doi: 10.3389/fneur.2017.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kline CE. The bidirectional relationship between exercise and sleep: implications for exercise adherence and sleep improvement. Am J Lifestyle Med. 2014;8:375–379. doi: 10.1177/1559827614544437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Makarem N, St‐Onge M, Liao M, Lloyd‐Jones DM, Aggarwal B. Association of sleep characteristics with cardiovascular health among women and differences by race/ethnicity and menopausal status: findings from the American Heart Association Go Red for Women Strategically Focused Research Network. Sleep Health. 2019;5:501–508. doi: 10.1016/j.sleh.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 19. Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, Miedema MD, Konety S, Chang PP, Solomon SD. American Heart Association's Life's Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128:970–976. doi: 10.1016/j.amjmed.2015.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ogunmoroti O, Oni E, Michos ED, Spatz ES, Allen NB, Rana JS, Virani SS, Blankstein R, Aronis KN, Blumenthal RS, et al. Life's Simple 7 and incident heart failure: the multi‐ethnic study of atherosclerosis. J Am Heart Assoc. 2017;6:e005180. doi: 10.1161/JAHA.116.005180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life's Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44:1909–1914. doi: 10.1161/STROKEAHA.111.000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lloyd‐Jones DM. Cardiovascular health and protection against CVD: more than the sum of the parts? Circulation. 2014;130:1671–1673. doi: 10.1161/CIRCULATIONAHA.114.012869 [DOI] [PubMed] [Google Scholar]
- 23. Younus A, Aneni EC, Spatz ES, Osondu CU, Roberson L, Ogunmoroti O, Malik R, Ali SS, Aziz M, Feldman T, et al. A systematic review of the prevalence and outcomes of ideal cardiovascular health in US and non‐US populations. Mayo Clin Proceed. 2016;91:649–670. doi: 10.1016/j.mayocp.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 24. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, JacobsJr DR, Kronmal R, Liu K, et al. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 25. Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, Jackson CL, Williams MA, Redline S. Racial/ethnic differences in sleep disturbances: the Multi‐Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38:877–888. doi: 10.5665/sleep.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chung J, Goodman M, Huang T, Bertisch S, Redline S. Multidimensional sleep health in a diverse, aging adult cohort: concepts, advances, and implications for research and intervention. Sleep Health. 2021;7:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fung MM, Peters K, Ancoli‐Israel S, Redline S, Stone KL, Barrett‐Connor E. Total sleep time and other sleep characteristics measured by actigraphy do not predict incident hypertension in a cohort of community‐dwelling older men. J Clin Sleep Med. 2013;9:585–591. doi: 10.1093/sleep/27.7.1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levine DW, Lewis MA, Bowen DJ, Kripke DF, Kaplan RM, Naughton MJ, Shumaker SA. Reliability and validity of women's health initiative insomnia rating scale. Psychol Assess. 2003;15:137–148. doi: 10.1037/1040-3590.15.2.137 [DOI] [PubMed] [Google Scholar]
- 29. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. [DOI] [PubMed] [Google Scholar]
- 30. Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross‐Cultural Activity Participation Study. J Womens Health Gend Based. 1999;8:805–813. doi: 10.1089/152460999319129 [DOI] [PubMed] [Google Scholar]
- 31. Mayer‐Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a multi‐cultural epidemiologic study. Ann Epidemiol. 1999;9:314–324. doi: 10.1016/s1047-2797(98)00070-2 [DOI] [PubMed] [Google Scholar]
- 32. Redline S, Foody J. Sleep disturbances: time to join the top 10 potentially modifiable cardiovascular risk factors? Circulation. 2011;124:2049–2051. doi: 10.1161/CIRCULATIONAHA.111.062190 [DOI] [PubMed] [Google Scholar]
- 33. Bakker JP, Weng J, Wang R, Redline S, Punjabi NM, Patel SR. Associations between obstructive sleep apnea, sleep duration, and abnormal fasting glucose. The Multi‐Ethnic Study of Atherosclerosis. Am J Resp Crit Care Med. 2015;192:745–753. doi: 10.1164/rccm.201502-0366OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dean DA, Wang R, Jacobs DR Jr, Duprez D, Punjabi NM, Zee PC, Shea S, Watson K, Redline S. A systematic assessment of the association of polysomnographic indices with blood pressure: the Multi‐Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38:587–596. doi: 10.5665/sleep.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwon Y, Gharib SA, Biggs ML, Jacobs DR, Alonso A, Duprez D, Lima J, Lin GM, Soliman EZ, Mehra R, et al. Association of sleep characteristics with atrial fibrillation: the Multi‐Ethnic Study of Atherosclerosis. Thorax. 2015;70:873–879. doi: 10.1136/thoraxjnl-2014-206655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow‐mediated dilation for incident cardiovascular events in a population‐based study: the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buysse D, Wallace M. Sleep health: a new paradigm for sleep and aging research. Innov Aging. 2018;2:595. [Google Scholar]
- 38. Wallace ML, Yu L, Buysse DJ, Stone KL, Redline S, Smagula SF, Stefanick ML, Kritz‐Silverstein D, Hall MH. Multidimensional sleep health domains in older men and women: an actigraphy factor analysis. Sleep. 2021;44:zsaa181. doi: 10.1093/sleep/zsaa181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ensrud KE, Kats AM, Schousboe JT, Langsetmo L, Vo TN, Blackwell TL, Buysse DJ, Ancoli‐Israel S, Stone KL. Multidimensional sleep health and subsequent health‐care costs and utilization in older women. Sleep. 2020;43:zsz230. doi: 10.1093/sleep/zsz230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta‐analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- 41. Gianfagna F, Veronesi G, Bertù L, Cesana G, Grassi G, Stranges S, Callegari C, Ferrario MM. Influence of sleep disturbances on age at onset and long‐term incidence of major cardiovascular events: the MONICA‐Brianza and PAMELA cohort studies. Sleep Med. 2016;21:126–132. doi: 10.1016/j.sleep.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 42. Yeboah J, Redline S, Johnson C, Tracy R, Ouyang P, Blumenthal RS, Burke GL, Herrington DM. Association between sleep apnea, snoring, incident cardiovascular events and all‐cause mortality in an adult population: MESA. Atherosclerosis. 2011;219:963–968. doi: 10.1016/j.atherosclerosis.2011.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nagayoshi M, Lutsey PL, Benkeser D, Wassel CL, Folsom AR, Shahar E, Iso H, Allison MA, Criqui MH, Redline S. Association of sleep apnea and sleep duration with peripheral artery disease: the Multi‐Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2016;251:467–475. doi: 10.1016/j.atherosclerosis.2016.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoevenaar‐Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren W. Sleep duration and sleep quality in relation to 12‐year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487–1492. doi: 10.5665/sleep.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zuraikat FM, Makarem N, Redline S, Aggarwal B, Jelic S, St‐Onge M. Sleep regularity and cardiometabolic heath: is variability in sleep patterns a risk factor for excess adiposity and glycemic dysregulation? Current Diab Rep. 2020;20:38. doi: 10.1007/s11892-020-01324-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Makarem N, Zuraikat FM, Aggarwal B, Jelic S, St‐Onge M. Variability in sleep patterns: an emerging risk factor for hypertension. Curr Hypertens Rep. 2020;22:19. doi: 10.1007/s11906-020-1025-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ogilvie RP, Redline S, Bertoni AG, Chen X, Ouyang P, Szklo M, Lutsey P. Actigraphy measured sleep indices and adiposity: the Multi‐Ethnic Study of Atherosclerosis (MESA). Sleep. 2016;39:1701–1708. doi: 10.5665/sleep.6096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zuraikat FM, Makarem N, Liao M, St‐Onge M, Aggarwal B. Measures of poor sleep quality are associated with higher energy intake and poor diet quality in a diverse sample of women from the go red for women strategically focused research network. J Am Heart Assoc. 2020;9:e014587. doi: 10.1161/JAHA.119.014587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mossavar‐Rahmani Y, Weng J, Wang R, Shaw PA, Jung M, Sotres‐Alvarez D, Castañeda SF, Gallo LC, Gellman MD, Qi Q, et al. Actigraphic sleep measures and diet quality in the Hispanic Community Health Study/Study of Latinos Sueño ancillary study. J Sleep Res. 2017;26:739–746. doi: 10.1111/jsr.12513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Loprinzi PD, Cardinal BJ. Association between objectively‐measured physical activity and sleep, NHANES 2005–2006. Ment Health Phys Act. 2011;4:65–69. [Google Scholar]
- 51. McClain JJ, Lewin DS, Laposky AD, Kahle L, Berrigan D. Associations between physical activity, sedentary time, sleep duration and daytime sleepiness in US adults. Prev Med. 2014;66:68–73. doi: 10.1016/j.ypmed.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 52. Reid M, Maras JE, Shea S, Wood AC, Castro‐Diehl C, Johnson DA, Huang T, Jacobs DR Jr, Crawford A, St‐Onge MP, et al. Association between diet quality and sleep apnea in the Multi‐Ethnic Study of Atherosclerosis. Sleep. 2019;42:zsy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Billings ME, Johnson DA, Simonelli G, Moore K, Patel SR, Roux AV, Redline S. Neighborhood walking environment and activity level are associated with OSA: the Multi‐Ethnic Study of Atherosclerosis. Chest. 2016;150:1042–1049. doi: 10.1016/j.chest.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Makarem N, Alcántara C, Williams N, Bello NA, Abdalla M. Effect of sleep disturbances on blood pressure. Hypertension. 2021;77:1036–1046. doi: 10.1161/HYPERTENSIONAHA.120.14479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hall MH, Brindle RC, Buysse DJ. Sleep and cardiovascular disease: Emerging opportunities for psychology. Am Psychol. 2018;73:994–1006. doi: 10.1037/amp0000362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Trzepizur W, Blanchard M, Ganem T, Balusson F, Feuilloy M, Girault JM, Meslier N, Oger E, Paris A, Pigeanne T, et al. Sleep apnea–specific hypoxic burden, symptom subtypes, and risk of cardiovascular events and all‐cause mortality. Am J Respirat Crit Care Med. 2022;205:108–117. doi: 10.1164/rccm.202105-1274OC [DOI] [PubMed] [Google Scholar]


