Abstract
Background
Therapeutic strategies for preventing paradoxical reperfusion injury after myocardial ischemia are limited. We tested whether central nervous system actions of leptin induce important protective effects on cardiac function and metabolism after myocardial ischemia/reperfusion (I/R) injury, the role of cardiac sympathetic innervation in mediating these effects, and whether there are major sex differences in the cardioprotective effects of chronic central nervous system leptin infusion.
Methods and Results
Myocardial I/R was induced by temporary ligation of the left descending coronary artery in male and female Wistar rats instrumented with intracerebroventricular cannula in the lateral ventricle. Vehicle or leptin (0.62 μg/h) infusion was started immediately after reperfusion and continued for 28 days using osmotic minipumps connected to the intracerebroventricular cannula. Cardiac function was assessed by echocardiography, ventricular pressures, and exercise performance. Intracerebroventricular leptin treatment markedly attenuated cardiac dysfunction post‐I/R as evidenced by improved ejection fraction (56.7±1.9 versus 22.6%±1.1%), maximal rate of left ventricle rise (11 680±2122 versus 5022±441 mm Hg) and exercise performance (−4.2±7.9 versus −68.2±3.8 Δ%) compared with vehicle‐treated rats. Intracerebroventricular leptin infusion reduced infarct size in females, but not males, when compared with ad‐lib fed or pair‐fed saline‐treated rats. Intracerebroventricular leptin treatment also increased cardiac NAD+/NADH content (≈10‐fold) and improved mitochondrial function when compared with vehicle treatment. Cervical ganglia denervation did not attenuate the cardiac protective effects of leptin after I/R injury.
Conclusions
These data indicate that leptin, via its central nervous system actions, markedly improves overall heart function and mitochondrial metabolism after I/R injury regardless of sex, effects that are largely independent of cardiac sympathetic innervation.
Keywords: heart, mitochondria, myocardial ischemia/reperfusion
Subject Categories: Basic Science Research, Ischemia, Metabolism, Physiology
Nonstandard Abbreviations and Acronyms
- β‐OHB
beta‐hydroxybutyrate
- AMPK
adenosine monophosphate‐activated protein kinase
- CGx
cervical ganglia denervation
- H2O2
hydrogen peroxide
- I/R
ischemia/reperfusion
- OXPHOS
oxidative phosphorylation
- ROS
reactive oxygen species
Clinical Perspective.
What Is New?
Our study reveals a potent cardioprotective effects of leptin, mediated via the central nervous system, that protect the heart after ischemia/reperfusion injury.
Central nervous system leptin infusion improves cardiac function in male and female rats after ischemia/reperfusion injury.
The cardioprotective effect of central nervous system leptin administration is not mediated by cardiac sympathetic nerves.
What Are the Clinical Implications?
Understanding the mechanisms by which leptin acts on the central nervous system to regulate cardiac metabolism and function may lead to new, more effective therapeutic strategies for myocardial ischemia and heart failure.
Cardiovascular disease remains the leading cause of mortality and is a major health care burden in the United States and worldwide. 1 Approximately 18.2 million Americans suffer from coronary artery disease and ≈20% of patients who experience an acute myocardial infarction (MI) develop heart failure (HF) within 5 years. 2 Reperfusion strategies are currently the primary therapies for acute MI; however, reperfusion of ischemic areas may paradoxically exacerbate MI‐associated myocardial injury, accounting for up to 50% of the final infarct size and increasing the probability of HF development. 3 Although improvements have been made with timely reperfusion strategies and advances in percutaneous coronary intervention technology, effective therapies to protect the heart against ischemia/reperfusion (I/R) injury are still limited.
Our recent studies suggest that activation of brain leptin receptors may offer a new approach for preventing cardiac injury after ischemic insults. Leptin, an adipocyte derived peptide, plays an important role in glucose and lipid metabolism as well as in overall energy homeostasis via its central nervous system (CNS) actions. We previously reported that CNS leptin administration in insulin‐deficient diabetic rats not only restored euglycemia and enhanced cardiac glucose uptake, but also restored intrinsic heart rate, autonomic regulation of heart rate, and baroreflex sensitivity, which were all impaired in diabetes. 4
These observations led us to hypothesize that the CNS actions of leptin may improve cardiac metabolism and overall function in conditions of metabolic stress such as I/R injury. In this study we investigated whether leptin, through its CNS effects, protects the heart from I/R injury and if there are major sex differences in the cardioprotective effects of leptin. Our preliminary studies indicated that chronic infusion of leptin, directly into the CNS at rates that do not lead to leptin spillover into the systemic circulation, exerted remarkable protection against cardiac I/R injury, improving cardiac function and exercise performance. Therefore, we also tested whether chronic CNS leptin infusion improves cardiac mitochondrial function and metabolism and whether these effects may be mediated via sympathetic nervous system activation.
Previous studies from our laboratory and from others showed that leptin increases sympathetic activity to peripheral tissues including the heart. 5 , 6 , 7 , 8 Although some studies suggest that increased sympathetic activation to the heart may be detrimental in HF, 9 cardiac sympathetic innervation is an important compensatory mechanism for increasing cardiac function in situations of stress, such as during MI. 10 However, the role of the cardiac nerves in mediating the chronic effects of leptin on cardiac function after I/R injury have not, to our knowledge, been previously reported.
Therefore, the present study was designed to test the hypothesis that leptin's CNS actions protect against progressive HF in a model of myocardial I/R injury in male and female rats and whether cardiac sympathetic nerves mediate these cardioprotective effects. We also investigated potential mechanisms contributing to leptin's cardioprotective actions by assessing metabolic markers in the heart and plasma, as well as cardiac mitochondrial function.
METHODS
The data that support the findings of this study are available from the corresponding author upon request.
Animal Studies
All experimental protocols and procedures of this study were conducted and conformed with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (protocol #1406B) of the University of Mississippi Medical Center, Jackson, MS, USA.
Experiments were performed in 180 male and female Wistar rats (12‐ to 14‐weeks old) purchased from Charles River Laboratories (Houston, USA). The rats were placed in individual cages in a 12 hour/12 hour dark and light cycle room and given free access to food (#8640, Harlan/Envigo, USA) and water throughout the study.
Animal Surgeries
Intracerebroventriclar Cannulation
Rats were anesthetized with isoflurane and a stainless‐steel cannula (26 gauge, 10 mm long) was implanted into the brain right lateral ventricle for leptin or vehicle infusions. Animals were housed individually and allowed to recover from intracerebroventricular surgery for 7 days before baseline measurements were taken. The accuracy of implantation of the intracerebroventricular cannula was tested by determining the dipsogenic response to an acute injection of 100 ng of angiotensin II and cannula placement was examined post‐mortem.
Myocardial I/R
The rats were anesthetized with ketamine/xylazine cocktail (100 and 10 mg/kg, intraperitoneally), an endotracheal intubation was performed using polyethylene size 90 tubing, and then a mechanical ventilator (Harvard Apparatus, USA) was connected to the endotracheal tube to ventilate the animals at 80 breaths per minute and a tidal volume of 1.2 mL/kg. After steady breathing was established, the chest was opened at the fourth left intercostal space using a chest retractor inserted between the ribs. The pericardium was removed, and the left anterior descending coronary artery was ligated using 4‐0 prolene suture (Ethicon, USA) for 60 minutes followed by reperfusion. The chest retractor was removed, the ribs were drawn together, thoracic pressure was reestablished, and the skin was closed. A thin layer of antibiotics (penicillin G benzathine, 6 000 000U/mL, Pfizer) was applied in the chest before suturing the skin to prevent infections. Buprenorphine (0.1 mg/kg, sc) was administrated immediately after surgery and after 24 hours of postoperative recovery. Sham animals were subjected to the same surgical procedures with the exception of left anterior descending coronary artery ligation and reperfusion. The effectiveness of I/R surgery to induce MI was determined by the presence of left ventricle (LV) anterior wall akinesia at week 1 post‐I/R using echocardiography. Animals that do not develop LV anterior wall akinesia were excluded from the protocol.
Cervical Ganglia Denervation
Two weeks before I/R surgery, the rats were anesthetized with isoflurane and a midline incision in the ventral neck region was performed to remove the cervical ganglia as previously described. 11 , 12 Briefly, the salivary glands were exposed and retracted to access and dissect the underlying muscles until the cervical ganglia were identified. Then, the superior and medial cervical ganglia were gently pulled and removed bilaterally using an ophthalmic scissor. After cervical ganglia denervation (CGx), a thin layer of antibiotics (penicillin G benzathine, 6 000 000U/mL, Pfizer) was applied, the skin was sutured, and buprenorphine (0.1 mg/kg) was injected subcutaneously.
The success of the denervation was confirmed by bilateral ptosis and significant reduction in tyrosine hydroxylase immunofluorescence in cardiac fibers after euthanasia. The heart's ability to increase heart rate after a reduction in blood pressure induced by acute intravenous infusion of sodium nitroprusside in CGx and control animals was also used to test the efficacy of CGx surgery; under isoflurane (2%) anesthesia, a polyethylene catheter was implanted in the femoral artery and connected to a pressure transducer attached to a bridge amplifier (Bridge Amp, ADInstruments, NZ) and an analogical to digital interface (PowerLab 16/30, ADInstruments, NZ) for arterial pressure and heart rate recording. After 2 minutes of stable arterial pressure recording, sodium nitroprusside (32 μg/kg) was administered through the penile vein using a 27G needle coupled with a 1‐mL syringe.
Ventricular Catheterization
At the end of the protocol (week 4 post‐I/R), the rats were anesthetized with urethane (1 g/kg) and placed on a temperature‐controlled heating pad to maintain body temperature. A pressure‐volume catheter (Millar 1.4F, SPR 838, ADInstruments, NZ) connected to an Mikro‐Tip® Pressure Volume System (MPVS) Ultra unit (ADInstruments, NZ) and a PowerLab digital data acquisition (ADInstruments, NZ), was inserted into the LV through the right carotid artery and ventricular pressure was recorded for at least 10 minutes. The following parameters were calculated: maximal rate of left ventricle pressure rise and drop and isovolumetric relaxation time constant.
Experimental Protocol
The rats were allowed to recover for 7 days after intracerebroventricular cannula implantation before baseline measurements were recorded. Daily food intake and weekly body weight were measured during the entire protocol. Echocardiographic images and videos were acquired at baseline (before) I/R surgery and at weeks 1, 2, 3, and 4 post‐I/R. A graded maximum velocity exercise tolerance test was also performed at baseline and at the end of week 4 post‐I/R.
Leptin (0.62 μg/h) or vehicle (saline 0.5 μL/h) infusion was started immediately after myocardial reperfusion using an osmotic minipump (model 2002, Durect Corp., CA) that was implanted subcutaneously in the scapular region and connected to the intracerebroventricular cannula using tygon tubing (Cole Parmer, 0.38 mm ID, USA). Leptin or vehicle was administered for 28 consecutive days with minipumps replaced on day 14 of infusion under brief isoflurane anesthesia. The rate of leptin infusion was based on our previous studies showing this dose is effective in decreasing food intake and improving glucose homeostasis. 6 , 13
On day 28, the LV was catheterized with a pressure‐volume Millar catheter (SPR‐383, ADInstruments, NZ) and after measurements the rats were euthanized. The heart was collected, weighed and divided into 3 pieces: the base was used to collect myocardial fibers for mitochondrial function analysis using Oxygraph2‐k electrodes (Oroboros Instruments, AT); a section of the mid‐LV was stored in 10% buffered formalin for histology; and noninfarcted areas of the midapical region were frozen for molecular analysis (Figure 1A).
Figure 1. Central leptin effects on food intake and body weight.
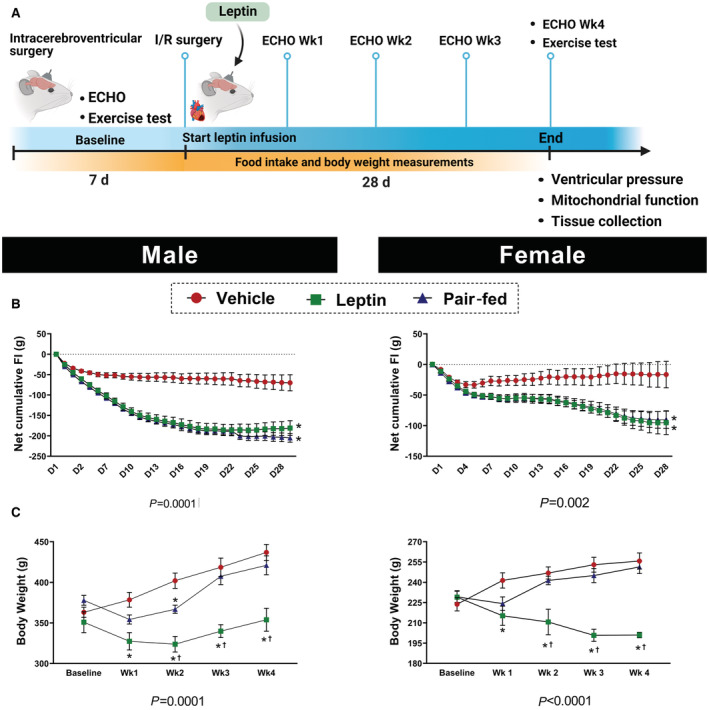
A, Experimental protocol; (B) Net cumulative food intake, which is the sum of daily food intake during the experimental period compared with the average baseline food intake; (C) body weight measured in male and female Wistar rats treated with intracerebroventricular vehicle, leptin, or vehicle+pair‐feeding (n=8 per sex/group). * Vs vehicle, P< 0.05; † vs pair‐fed, P<0.05. Two‐way repeated measures ANOVA (B and C).
Euthanasia
Euthanasia was performed by thoracotomy and removal of the heart after induction of deep anesthesia by intravenous injection of urethane (1g/kg) or by inhalation of isoflurane (5%).
Echocardiography
Transthoracic echocardiography was performed at baseline (before I/R) and on weeks 1, 2, 3, and 4 after I/R surgery using the VEVO 3100 system (VisualSonics, CA) equipped with a 21‐MHz transducer (MX250) at 100 frames/sec. Rats were anesthetized with 2.0% isoflurane and placed on a heating table and their extremities were fixed to 4 electrocardiography leads on the table. A parasternal long axis B‐mode was acquired at the maximum LV length. Three long axis B‐mode movies of the LV were also acquired at midventricular level for longitudinal strain analysis. Echocardiographic parameters of systolic function including LV end‐systolic and end‐diastolic volumes were calculated as previously described. 14 LV ejection fraction (EF) and stroke volume (SV) were calculated as EF=[(LV end‐diastolic volume−LV end‐systolic volume)/LV end‐diastolic volume]×100 and SV=LV end‐diastolic volume−LV end‐systolic volume, respectively. Global longitudinal strain was analyzed by 2‐dimensional echocardiography speckle‐tracking using the VevoLab software (VisualSonics, CA) which uses the Lagrangian method 15 as follows: global longitudinal strain =[L(t)‐L(t0)]/L(t0), where L represents the apex‐to‐base fiber length at time t after cardiac fibers deformation and t0, the length of the cardiac fiber before deformation.
Four‐Dimensional Echocardiography
Four‐dimensional images were collected using the VEVO 3100 System (VisualSonics, CA), a 21‐MHz transducer (MX250) and a translating linear step motor. Rats were anesthetized with isoflurane 2.0% and placed in supine position in a heating table with electrocardiography electrodes leads. Serial short‐axis ECG‐gated cine loops were acquired with ≈11 μm stepwise movement across the full length of the LV. Extracted ultrasound images were then analyzed using a MATLAB graphical user interface (Weldon School of Biomedical Engineering, Purdue University—Indiana, USA) 16 to measure regional surface area strain in the 17‐segment model of the LV. Regional surface area strain was calculated as regional surface area strain (θ,t)=A(θ,t)−A D(θ)/A D(θ) where A represents the surface area of the boundary voxel as calculated using the vector cross product at rotation θ and time t in the cardiac cycle, and A D is the respective surface area at end‐diastole (ie, t=0).
Maximum Graded Exercise Test
All animals were adapted to running on a motorized rodent treadmill (Columbus Instruments, USA) for 2 days before the test was performed. Each session of adaptation lasted ≈5 minutes and the animals ran at 9 m/min without inclination. Two maximum graded exercise performance tests were performed, the first during the baseline period before I/R surgery and the second at week 4 post‐I/R. Tests followed the protocol described by Petrosino and collaborators. 17 Blood lactate was measured before and after exercise testing using lactate strips (Lactate Plus, Nova Biomedical, USA) right after the exercise test. The work performed by each animal was calculated as the product of body weight (kg) and total vertical distance (meters), where vertical distance = (distance run) × (sin θ) × gravity, where θ is equal to the angle of the treadmill from 5° to 15° and assuming gravity is equal to 10 m/s2.
High‐Resolution Respirometry in Isolated Cardiac Fibers
Cardiac fibers were isolated, weighed, and permeabilized with saponin as previously described. 18 Respirometric oxidative phosphorylation (OXPHOS) analysis was performed using a 2‐chamber titration‐injection oxygraph (Oroboros Oxygraph2‐k, Oroboros Instruments, AT). ATP‐linked respiration was measured using the difference between succinate (10 mmol/L, substrate for succinate dehydrogenase, complex II) and oligomycin (4 ng, inhibitor of ATP synthase), and the difference between oligomycin and antimycin (2.5 μmol/L, complex III inhibitor) was used to calculate ATP‐leak. ATP reserve was calculated from the difference between carbonyl cyanide p‐(trifluoromethoxy) phenylhydrozone (0.1 mM) and oligomycin. High‐resolution respirometry was combined with the Fluorescent‐Sensor Green of the O2K‐Fluo LED2‐Module for hydrogen peroxide (H2O2) production. The respiratory control ratio was used as a quality assurance marker and cytochrome C (10 μmol/L) as a validation of proper tissue preparation.
Plasma β‐Hydroxybutyrate and Cardiac NAD +/NADH Measurements
Plasma β‐hydroxybutyrate (ab83390, Abcam, USA) and cardiac tissue NAD+/NADH (ab65348, Abcam, USA) concentrations were measured with ELISA kits.
Immunofluorescence
Paraffin embedded heart tissue sections (5 μm) were dewaxed and rehydrated, the sections were treated with 0.1 mol/L sodium citrate buffer for antigen retrieval and Opal 4‐color IHC kit (PerkinElmer, USA) was used for simultaneous detection of tyrosine hydroxylase (1:300; AB152, Milipore, USA) stained with Opal570 fluorophore (550 to 570 nm, green color), α‐actinin sarcomeric protein (1:300; A781, Sigma, USA) stained with Opal620 fluorophore (588 to 616 nm, red color) and nuclear counterstain (spectral DAPI, 358 to 461 nm, blue color). Sections were observed under fluorescence microscope (LionHeart FX, Agilent, USA) using 100× magnification.
Histological Analysis and Infarct Size
Heart samples were sectioned (5 μm) and stained using picrosirius red for quantification of interstitial collagen. Stained cross‐sections were captured using light microscopy (NIKON, Eclipse 50i, JP) at 40× magnification. To estimate the fraction area (%) of collagen using picrosirius red‐stained sections, 15 to 20 images of the septum were randomly captured for posterior analysis using NIH ImageJ software. LV infarct size was measured using picrosirius red‐stained sections and calculated by dividing the length of the infarcted area by the total circumference of the LV (expressed as percentage). Cardiomyocyte diameter was estimated in ≈30 cells/animal with well‐defined round cell membranes and visible cell nuclei at midwall depth on sections stained with hematoxylin and eosin.
Statistical Analysis
The results are expressed as mean ± SEM and scatter plots with bars were used to represent the data. The data points show individual values, indicating the upper and lower extremes, and the bars represent the mean of all individual values. The data were analyzed for Gaussian distribution (normality) using 3 different tests (Kolmogorov–Smirnov, Shapiro–Wilk, and Anderson–Darling). Sample distributions were considered normal when all groups passed in at least 1 normality test. Nonparametric test (Kruskal–Wallis or Mann–Whitney) was used for data with non‐Gaussian distribution and parametric test (1‐way ANOVA or t‐test) was used for samples with Gaussian distribution. Comparisons between baseline and experimental values within each group and comparisons between different groups were made by 2‐way ANOVA with repeated measures followed by Tukey post hoc test when appropriate. Single‐time point differences between 2 groups were compared using t‐test. Statistical significance was accepted at a level of P<0.05. Data were plotted and analyzed using GraphPad Prism 9 (GraphPad Software, La Jolla, CA). The number of animals calculated for each experiment was based on our previous studies and strong preliminary data for similar experimental designs (paired, unpaired), expected variance of experimental measurements, and anticipated differences between means. There were no missing data, and all treatment groups were equivalent at baseline, thus no baseline adjustment was necessary.
RESULTS
Chronic Intracerebroventricular Leptin Administration Reduces Food Intake and Body Weight
Figure 1A is a schematic representation of the experimental design followed in the present study. Compared with vehicle treatment, chronic intracerebroventricular leptin infusion reduced net cumulative food intake (Figure 1B, P<0.0001) as well as body weight (Figure 1C, P=0.0001) in male and female rats. Pair feeding was performed in additional groups of vehicle‐treated male and female rats to reduce food intake to the same level observed during chronic intracerebroventricular leptin infusion to control for potential effects of reduced food intake on cardiac function. Despite the same reduction in cumulative food intake, pair‐fed animals did not exhibit the same weight loss experienced by leptin‐treated rats. This is likely caused by the fact that leptin not only promotes satiety but also increases energy expenditure. 19
Chronic Intracerebroventricular Leptin Administration Improves Cardiac Function After I/R Injury
Time‐course evaluation of cardiac function by echocardiography showed that vehicle‐treated and pair‐fed rats, male and female, presented a reduction in EF, SV, and global longitudinal strain at weeks 1, 2, 3, and 4 post‐I/R compared with baseline values (Figure 2A through 2G, P<0.0001). However, chronic intracerebroventricular leptin infusion markedly improved EF in male and female rats submitted to I/R injury (Figure 2A and 2E, P<0.0001), SV (Figure 2B and 2F, P<0.0001) and global longitudinal strain (Figure 2C and 2G, P=0.0004) at weeks 3 and 4 post‐I/R compared with vehicle‐treated and pair‐fed rats.
Figure 2. Cardiac function assessed by echocardiography at baseline and at weeks 1 to 4 of central leptin infusion post‐I/R in male and female rats.
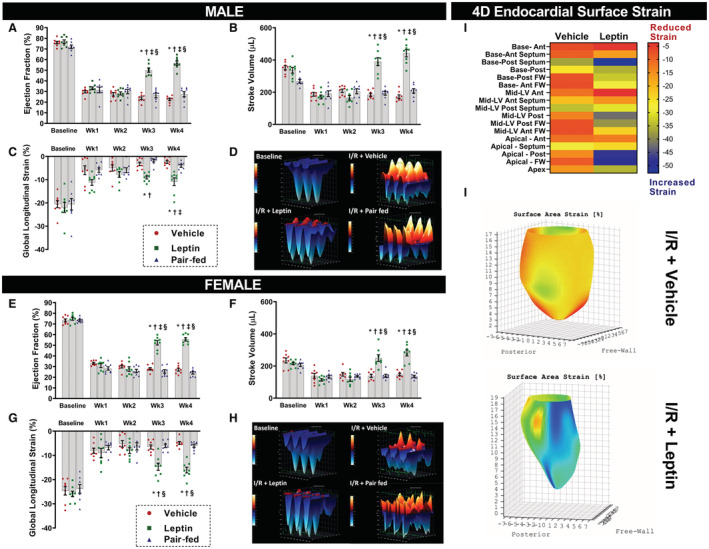
A, and E, Ejection fraction; (B and F) stroke volume; (C and G) global longitudinal strain. D and H, are 3‐dimensional representations of longitudinal strain in male and female rats at baseline (top left) and 4 weeks post‐I/R in vehicle‐treated (top right), leptin‐treated (left bottom) and pair‐fed (right bottom) rats. I and J, are a heat map representation of regional endocardial surface strain and a 4‐dimensional reconstruction of the endocardial surface strain, respectively, in vehicle and leptin‐treated animals analyzed by 4‐dimensional echocardiography technology (Videos [Link], [Link]) (n=8 per sex/group). * Vs vehicle, P<0.05; † vs pair‐fed, P<0.05. ‡ Vs leptin Wk2, P<0.05; § vs leptin Wk1, P<0.05. Two‐way repeated measures ANOVA (A through D and E through H). FW indicates free wall; I/R, ischemia/reperfusion; and LV, left ventricle.
Figure 2D and 2H show a 3‐dimensional representation of longitudinal strain in 3 consecutive cardiac cycles in vehicle and leptin‐treated rats at baseline and at week 4 post‐I/R. Note that after I/R injury, shortening of the cardiac fibers was reduced (red color positive waves) in vehicle and pair‐fed treated animals when compared with baseline (Figure 2D and 2H). However, the normal pattern of shortening (blue color negative waves) was restored in leptin‐treated male and female rats (Figure 2D and 2H).
Four‐dimensional endocardial strain analysis was also performed in vehicle and leptin‐treated rats for better visualization of endocardial contractility patterns in the entire LV (Figure 2J). The LV was divided into 17 regions and the endocardial surface peak strain was calculated in each region and represented in a heat map (Figure 2I). Intracerebroventricular leptin‐treated rats presented better surface strain pattern in several LV regions, especially in the base‐posterior septum, apical‐posterior and apical‐free wall (blue squares), compared with vehicle‐treated rats post‐I/R (Figure 2I and 2J). Video S1 and S2 show leptin‐treated animals and vehicle‐treated animals, respectively.
Chronic Intracerebroventricular Leptin Administration Improves Heart Contractility, Relaxation and Exercise Capacity After I/R Injury
We observed a significant improvement in maximal rate of left ventricle pressure rise (Figure 3A and 3D, P≤0.005) and isovolumetric relaxation time constant (Figure 3B and 3E, P≤0.02), assessed by ventricular catheterization at the end of the experimental protocol, in male and female rats infused with intracerebroventricular leptin when compared with vehicle‐treated and pair‐fed animals. These observations corroborate the echocardiographic data and suggest that chronic intracerebroventricular leptin infusion improved systolic and diastolic functions post‐I/R injury.
Figure 3. Effects of central leptin infusion on ventricular pressures and exercise performance 4 weeks after I/R injury in male and female rats.
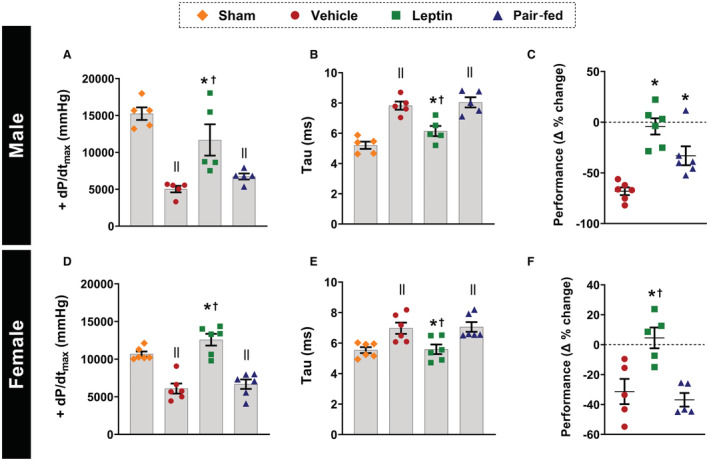
A and D, maximal rate of left ventricle pressure rise; (B and E), isovolumetric relaxation time constant; (C and F) exercise performance percentage change (n=5–6 per sex/group). * Vs vehicle, P<0.05; † vs pair‐fed, P<0.05; II vs sham, P<0.05. Kruskal–Wallis test (C–E) and 1‐way ANOVA (A, B, and F). +dP/dtmax indicates maximal rate of left ventricle pressure rise.
Intracerebroventricular leptin infusion also prevented deterioration of exercise capacity following I/R injury in male and female rats, as evidenced by maintenance of similar exercise performances at baseline and week 4 post‐I/R surgery, expressed as delta % change (difference between baseline and week 4 post‐I/R values, Figure 3C and 3F, P≤0.023). In contrast, vehicle‐treated and pair‐fed animals showed marked reductions in exercise capacity as expected following I/R injury.
Chronic Intracerebroventricular Leptin Administration Reduces Septal Fibrosis and Infarct Size After I/R Injury
Infusion of leptin intracerebroventricular reduced infarct size in females, but not in males, when compared with vehicle and pair‐fed treated rats (Figure 4A and 4F, P≤0.0056). However, male and female rats receiving intracerebroventricular leptin exhibited reduced heart weight/tibia length (Figure 4B and 4G, P≤0.04) and septal collagen deposition (Figure 4C and 4H, P≤0.011) compared with vehicle and pair‐fed rats. No differences in myocyte diameter were observed between groups for either sex (Figure 4D and 4I, P≥0.33). Figure 4E and 4J show representative images of the histological cross sectional mid‐LV, septal collagen deposition (40x), and septal myocytes (40×).
Figure 4. Effects of central leptin infusion on cardiac remodeling 4 weeks after I/R injury in male and female rats.
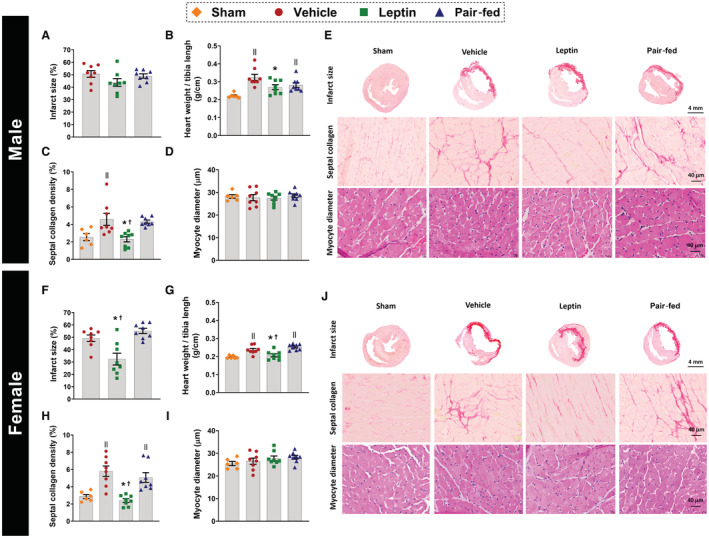
A and F, infarction size; (B and G) heart weight/tibia length; (C and H) septal collagen deposition; (D and I) myocyte diameter. E and J, are representative pictures of mid‐ventricular histological sections of the heart showing infarction size (4×), septal collagen deposition (40×) and myocyte diameter (40×) (n=8 per sex/group). * Vs vehicle, P<0.05; † vs pair‐fed, P<0.05; II vs sham, P<0.05. One‐way ANOVA (A through D and F through I).
Chronic Intracerebroventricular Leptin Administration Improves Cardiac Mitochondrial Function and Cardiac Metabolism After I/R Injury
Mitochondrial function in isolated cardiac fibers from the LV remote region was significantly improved in intracerebroventricular leptin‐treated male and female rats compared with vehicle and pair‐fed controls. For instance, leptin treatment increased ATP‐linked respiration (Figure 5A and 5G, P≤0.01) and reduced proton leak in male and female rats (Figure 5B and 5H, P≤0.008). Males, but not females, treated with leptin also showed increased mitochondrial reserve (Figure 5C and 5I, P=0.03) compared with vehicle and pair‐fed rats, and a reduction in H2O2 production (Figure 5D and 5J, P≤0.01) compared with the pair‐fed group. Intracerebroventricular leptin treatment also increased cardiac NAD+/NADH content in males and females (Figure 5E and 5K, P≤0.027) compared with all other groups and reduced plasma β‐hydroxybutyrate levels in both sexes (Figure 5F and 5L, P≤0.031) back to levels observed in sham‐operated rats, suggesting an improvement in cardiac metabolism. Figure 5M is a representative trace of the oxygen consumption measured in permeabilized cardiac fibers at 37 °C using Oroboros high‐resolution respirometry.
Figure 5. Effects of central leptin infusion on cardiac mitochondrial function and metabolism 4 weeks after I/R injury in male and female rats.
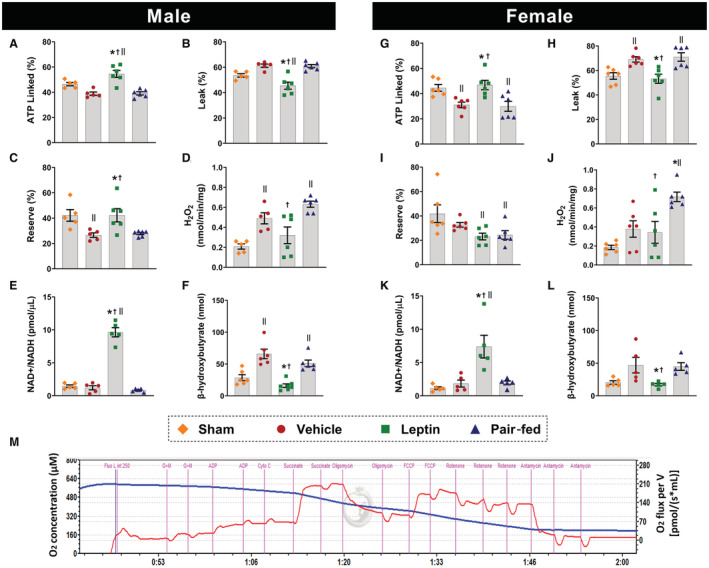
A and G, ATP‐linked respiration; (B and H) proton leak; (C and I) mitochondrial reserve; (D and J) H2O2 production; (E and K) cardiac NAD+/NADH content; (F and L) plasma levels of β‐hydroxybutyrate. M, is a representative tracing of permeabilized cardiac fiber's oxygen consumption rate measured by Oroboros high‐resolution respirometry at 37 °C (n=5–6 per sex/group). * Vs vehicle, P<0.05; † vs pair fed, P<0.05; II vs sham, P<0.05. One‐way ANOVA (A through L).
Cardiac Sympathetic Denervation Does Not Alter the Cardioprotective Effects of Chronic Intracerebroventricular Leptin Infusion After I/R Injury
To test if the CNS‐mediated cardioprotective effects of leptin after I/R injury are mediated by changes in cardiac sympathetic activity, we performed bilateral surgical removal of the cervical ganglia (CGx) before induction of myocardial I/R injury and intracerebroventricular leptin infusion (Figure 6A). Successful cardiac sympathetic denervation was confirmed by ≈70% reduction in tyrosine hydroxylase staining in cardiac fibers (Figure 6B, P<0.001) and a 50% attenuation of the compensatory rise in heart rate after an acute reduction in blood pressure induced by intravenous injection of sodium nitroprusside (Figure 6C). Bilateral ptosis, a marker of successful denervation, was also observed after CGx. Despite this significant reduction in cardiac sympathetic innervation, intracerebroventricular leptin infusion still improved cardiac function as evidenced by an increase in LV contraction and relaxation (Figure 6D and 6E, P≤0.03), maximum exercise capacity (Figure 6F, P=0.04), EF (Figure 6G, P<0.0001), SV (Figure 6H, P=0.012), and global longitudinal strain (Figure 6I, P=0.019) when compared with CGx animals treated with vehicle. Data from male and female CGx rats were combined since we did not observe sex differences within each group.
Figure 6. Effects of cardiac sympathetic denervation on cardioprotective actions of central leptin infusion 4 weeks after I/R injury in male and female rats.
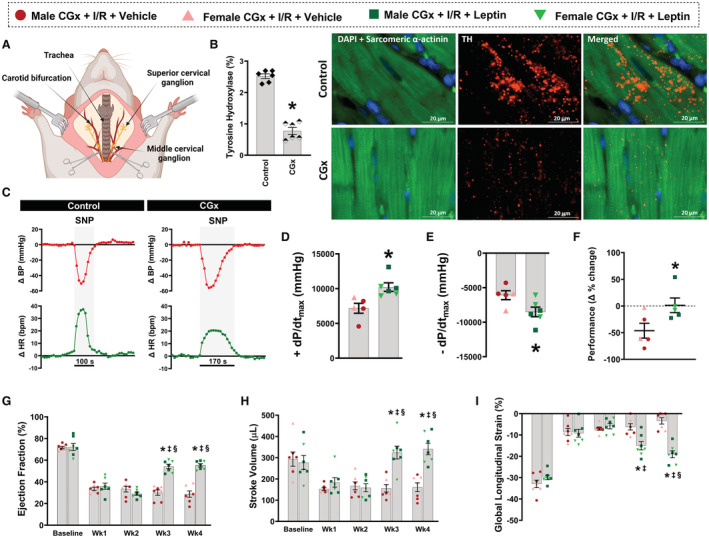
A, Schematic showing anatomical location of the cervical ganglia and its surgical removal in Wistar rats; (B) Immunofluorescence for tyrosine hydroxylase in the heart of denervated (cervical ganglia denervation) and control rats; (C) Representative trace recording of blood pressure and heart rate responses during intravenous infusion of sodium nitroprusside; (D) Maximal rate of left ventricle pressure rise; (E) maximal rate of left ventricle pressure fall; (F) percentage change of exercise performance; (G) ejection fraction; (H) stroke volume; (I) global longitudinal strain. (n=5–7 per sex/group). * Vs cervical ganglia denervation+ischemia/reperfusion+vehicle, P<0.05; ‡ vs leptin Wk2, P<0.05; § vs leptin Wk1, P<0.05. One‐way ANOVA (A through E), Mann–Whitney test (F), and 2‐way repeated measures ANOVA (G through I). BP indicates blood pressure; CGx, cervical ganglia denervation; HR, heart rate; I/R, ischemia/reperfusion; DAPI, 4',6‐diamidino‐2‐phenylindole; SNP, sodium nitroprusside; and −dP/dtmax, maximal rate of left ventricle pressure drop.
Cardiac Sympathetic Denervation Does Not Alter the Beneficial Effects of Intracerebroventricular Leptin Infusion on Cardiac Remodeling, Mitochondrial Function, and Cardiac Metabolism after I/R Injury
We also examined if CGx would impair leptin's effects to attenuate adverse myocardial remodeling after I/R. However, CGx did not impair the CNS actions of leptin to reduce heart weight/tibia length and septal collagen deposition when compared with vehicle‐treated CGx rats (Figure 7A and 7B, P≤0.006), despite similar infarct sizes between the groups (Figure 7C, P=0.89). No differences were observed in cardiomyocyte diameter between groups (Figure 7D, P=0.92). Figure 7E show representative images of the histological cross‐sectional mid‐LV, septal collagen deposition (40×), and septal myocytes (40×).
Figure 7. Effects of central leptin infusion on cardiac remodeling, mitochondrial function, and metabolism in cardiac sympathetic denervated male and female rats 4 weeks post‐ischemia/reperfusion (I/R) injury.
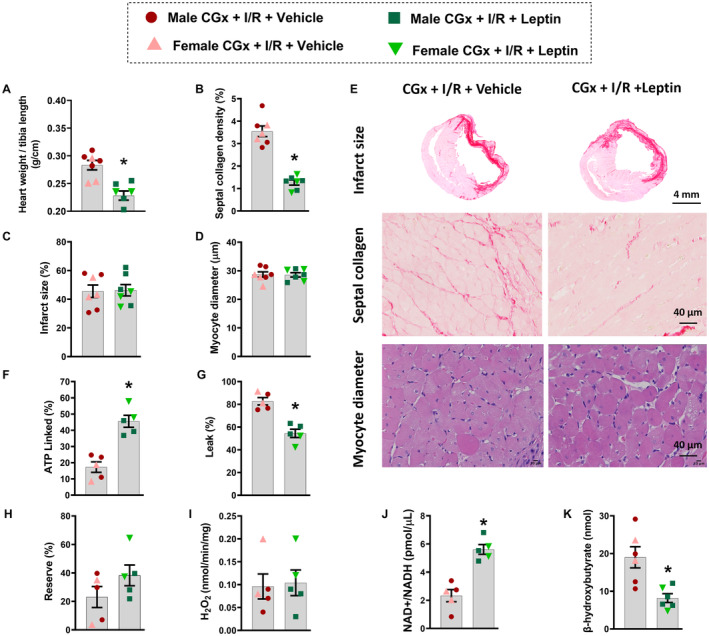
A, Heart weight/tibia length; (B) septal collagen deposition; (C) infarct size; (D) myocyte diameter; (F) ATP‐linked respiration; (G) proton leak; (H) reserve; (I) H2O2 production in isolated cardiac fibers from cervical ganglia denervation rats 4 weeks after I/R injury. J, Cardiac NAD+/NADH content and (K) plasma levels of β‐hydroxybutyrate from cervical ganglia denervation rats 4 weeks after I/R injury. E, Representative pictures of mid‐ventricular histological sections of the heart showing infarction size (4×), septal collagen deposition (40×) and myocyte diameter (40×). (n=5–7 per sex/group). * Vs cervical ganglia denervation+I/R+vehicle, P<0.05. Unpaired t test. CGx indicates cervical ganglia denervation; and I/R, ischemia/reperfusion.
CGx also did not impair the beneficial effects of intracerebroventricular leptin infusion to improve cardiac mitochondrial function at week 4 post‐I/R, as demonstrated by an increase in ATP‐linked respiration and reduction in proton leak compared with vehicle‐treated CGx rats (Figure 7F and 7G, P=0.0004). No differences were observed in mitochondrial reserve or H2O2 production between groups (Figure 7H and 7I, P≥0.18). In addition, cardiac metabolic improvement, as evidenced by increased NAD+/NADH ratio (Figure 7J, P=0.0003) and reduction in β‐hydroxybutyrate (β‐OHB) circulating levels (Figure 7K, P=0.0052), was not affected by CGx.
DISCUSSION
A major new finding of the current study is that intracerebroventricular leptin infusion for 28 days greatly improved cardiac function after I/R injury in male and female rats as evidenced by marked improvements in echocardiographic parameters at weeks 3 and 4 post‐I/R, and in LV intraventricular pressures (maximal rate of left ventricle pressure rise and drop) and exercise capacity at week 4 post‐I/R. Additionally, central leptin administration attenuated adverse cardiac remodeling, including reductions in septal collagen deposition and heart weight to tibia length ratio. Surprisingly, female, but not male, rats treated with leptin also showed a significant reduction in infarction size.
Another important new finding is that leptin, administered directly into the CNS after I/R injury, markedly increased cardiac mitochondrial oxidative capacity and NAD+/NADH redox state. Furthermore, we showed that leptin's CNS‐mediated effects on cardiac metabolism and mitochondrial function as well as its overall cardiac protective effects after I/R injury are not dependent on intact cardiac sympathetic innervation since bilateral CGx did not diminish these improvements in cardiac function elicited by chronic intracerebroventricular leptin infusion. We also provided strong evidence that these cardiac protective actions of leptin are equally effective in males and females, and occur independently of leptin's anorexic effects since pair‐feeding did not elicit similar protection against myocardial I/R injury.
Leptin plays an important role in controlling adipose tissue homeostasis by regulating appetite and energy expenditure according to the body's nutritional status. 19 , 20 We previously showed that central leptin administration reversed the deleterious effects of uncontrolled diabetes on the cardiovascular system 4 and improved heart function in male rats with MI induced by permanent coronary artery ligation. 21 We also showed that these effects were mediated by the CNS since this dose of leptin infused intracerebroventricular does not increase circulating levels of leptin. 13 In addition, chronic intraperitoneal leptin infusion at the same dose infused intracerebroventricular in the present study (0.62 μg/h) did not alter food intake, body weight, blood pressure, heart rate, blood glucose, or insulin levels in male and female rats. 13
Although intracerebroventricular leptin infusion reduces insulin plasma levels in male and female rats, 13 we have previously shown that insulin‐deficient diabetic rats treated with central leptin also presented cardiovascular improvements, 4 indicating that the cardiometabolic effects of intracerebroventricular leptin administration are independent of insulin. Therefore, we are confident that the beneficial cardiac protective effects of intracerebroventricular leptin infusion after I/R injury are mediated by CNS actions and not by direct peripheral effects of leptin or reductions in plasma insulin levels.
The impact of leptin on cardiac function has been a topic of debate with some studies showing a deleterious effect of high circulating leptin levels on cardiac structure and function, 22 , 23 while other studies show beneficial effects of leptin on cardiac physiology in animals with low baseline leptin levels 24 or when leptin is infused directly into the CNS. 21 It is important to note that most previous studies supporting a deleterious effect of leptin on cardiac function were correlative studies in individuals with obesity who could have additional confounding factors associated with increased adiposity. 25 , 26 Since obesity is usually accompanied by other comorbidities, such as hypertension and diabetes, it is difficult to separate the effects of hyperleptinemia on cardiac function from other factors that occur concurrently in obesity. Conversely, preclinical research suggests a role for intramyocardial leptin signaling in the maintenance of proper cardiac structure, function, and glycolytic metabolism. 27 , 28 Our present study provides new evidence that leptin, via its actions of the CNS, exerts remarkable protective effects that improve cardiac function after myocardial I/R injury and that this effect is not mediated by leptin spillover into the systemic circulation.
Cardioprotective Effects of Intracerebroventricular Leptin Infusion After I/R Injury Are Not Attributable to Reduced Food Intake
Long‐term intracerebroventricular leptin infusion significantly reduced food intake and body weight during the first 2 weeks of treatment leading to weight loss compared with vehicle‐treated rats. We included a vehicle‐infused pair‐fed group to serve as an additional control for leptin's anorexic effects. We found that vehicle‐treated pair‐fed rats exhibited similar impairments in cardiac function and other parameters related to cardiac dysfunction post‐I/R injury when compared with ad libitum‐fed vehicle‐treated rats. Thus, we found no evidence that the anorexic effect of leptin contributes importantly to leptin's ability to improve cardiac function and attenuate progression to HF after myocardial I/R injury.
Although pair‐feeding experiments did not completely mimic the effects of intracerebroventricular leptin on body weight, probably because of leptin's thermogenic effect, it is unlikely that the increase in total body energy expenditure could explain the improvement observed in cardiac metabolism and function in rats with myocardial I/R injury that were treated with intracerebroventricular leptin. Leptin also stimulates lipolysis and increases free fatty acids in the circulation, 29 which could provide an extra source of fuel to the heart. This lipolytic effect of leptin, however, occurs acutely whereas we began to observe significant cardiac functional improvement only 2 weeks after initiating leptin treatment. Moreover, diabetic animals with elevated circulating free fatty acids are not protected from myocardial dysfunction after ischemic injury. 30 Whether increased lipolysis and circulating free fatty acids contribute to the beneficial CNS‐mediated effects of leptin on the heart post‐I/R injury is unclear and beyond the scope of this study.
Leptin's CNS‐Mediated Effects to Improve Cardiac Function After I/R Develop Slowly
An interesting observation that remains unexplained is the slowly developing but remarkable improvement of cardiac function after I/R injury that occurs during chronic intracerebroventricular leptin infusion. Although the mechanisms involved are unclear, we previously demonstrated that intracerebroventricular leptin infusion significantly increases phosphorilated adenosine monophosphate‐activated protein kinase (p‐AMPK)/adenosine monophosphate‐activated protein kinase (AMPK) ratio, an important metabolic energy sensor, at the end of the second week of leptin treatment post‐MI. 21 However, the increase in p‐AMPK/AMPK expression at week 2 was followed by a decrease at week 4 post‐MI. This transient increase in p‐AMPK expression may be attributable to restoration of an adequate energy supply to the heart that coincides with improvement of cardiac function. Thus, the restoration of myocardial bioenergetics observed at week 2 of leptin infusion may be necessary for subsequent improvements in cardiac function at weeks 3 and 4 post‐MI. At this later time, expression of this metabolic energy sensor may be reduced back to normal levels as energy production is normalized. However, the factors that link leptin's CNS effects to the slowly developing improvements in cardiac metabolism and function are still unclear and will require further investigation.
Cardioprotective Effects of Intracerebroventricular Leptin Infusion After I/R Occur in Male and Female Rats
In the current study, we also demonstrate that leptin, via its CNS actions, protects the myocardium of female rats submitted to myocardial I/R. The only significant sex difference noted was that intracerebroventricular leptin infusion reduced the infarcted area in female, but not male, rats when compared with vehicle treatment, indicating an even more pronounced cardioprotective effect of leptin in females. Previous studies suggest that estrogens may confer important protection against I/R injury in females and a decline in estrogens after menopause may contribute to age‐related increased vulnerability to I/R injury. 31 Lagranha and collaborators showed that cardiac functional recovery in premenopausal female rats after myocardial I/R injury was accompanied by smaller infarct size associated with increased post‐translational modifications of mitochondrial enzymes involved in regulating reactive oxygen species (ROS) generation and oxidative metabolism. 32 Despite previous evidence demonstrating that premenopausal females may be protected against I/R injury, our vehicle‐treated young females exhibited similar infarct size and cardiac functional impairment compared with male rats.
Although some studies suggest that there may be sex differences in leptin's effects on sympathetic activity, our results in the present study and in previous studies suggest that chronic intracerebroventricular leptin infusion appears to alter cardiac function to the same extent in conscious male and female rats. 4 , 13 Additionally, other studies indicate that increases in sympathetic activity and heart rate evoked by intracerebroventricular leptin infusion are similar in male and pro‐estrus female rats. 33 Although levels of estrogen were not measured in the present study, we speculate that intracerebroventricular leptin treatment may potentiate estrogen‐related cardiac protection, contributing to smaller infarcts in leptin‐treated females. Future studies are needed to test this possibility and to investigate other potential mechanisms responsible for increased resistance of the myocardium to ischemia and/or reperfusion insults in females during intracerebroventricular leptin treatment.
CNS‐Mediated Effects of Leptin Improve Cardiac Diastolic Function After I/R
Another important and novel observation of the present study is the improvement in isovolumetric relaxation time constant, a gold‐standard index of diastolic function, and improved exercise capacity 4 weeks post‐I/R in male and female rats treated with leptin. After MI, the active relaxation phase of the myocardium is delayed by a decrease in the elastic recoil energy stored in titin molecules following cardiomyocyte contraction. 34 Therefore, impaired contractility after MI also impairs relaxation since the stored energy in the titin molecule is a function of the maximal cardiomyocyte shortening. 34 Depending on the extent of the infarct, LV passive relaxation may also be compromised by increased fibrosis leading to reduced LV compliance. In the present study, we showed that chronic intracerebroventricular leptin infusion not only improved active relaxation, as evidenced by a reduction in isovolumetric relaxation time constant (probably because of an increase in cardiac fiber shortening, ie, increased global longitudinal strain), but also reduced septal fibrosis which may have improved LV compliance leading to better passive relaxation. Altogether, restoration of cardiac systolic and diastolic function by CNS leptin administration improved the ability of the myocardium to increase cardiac output during states of high metabolic demand (ie, maximum exercise test), resulting in normal exercise performance after I/R.
CNS‐Mediated Effects of Leptin Improve Mitochondrial Function and Cardiac Metabolism After I/R
We also investigated whether the increased cardiac contractility observed in viable areas of the heart during intracerebroventricular leptin infusion after I/R was related to improved mitochondrial function and cardiac metabolism. The heart has high energy demands and must continuously produce large amounts of ATP to sustain its contractile function. A large part of ATP produced in the heart is generated by mitochondrial OXPHOS of substrates (approaching 95% under normal circumstances). 35 , 36 However, during states of metabolic stress, such as following I/R, dramatic alterations in cardiac metabolism occur and energy production may become compromised because of reduction in mitochondrial OXPHOS capacity. 35
OXPHOS is an uncoupled process where protons translocated to the intermembrane space by specific respiratory complexes can return to the mitochondrial matrix independent of F0F1‐ATP synthase in a process known as proton leak, which is tightly linked with ROS production. 37 ROS are generally considered to be toxic byproducts of aerobic metabolism and increased ROS production in periods of stress, such as during I/R injury, can damage organelles and whole cells, exacerbating the injury. We found that chronic intracerebroventricular leptin treatment not only improved ATP production and reduced proton leak measured in isolated cardiac fibers of male and female rats after myocardial I/R, but also reduced H2O2 levels, an important cellular ROS, in these fibers. These findings suggest an additional mechanism for the CNS‐mediated cardiac protective effect of leptin after I/R injury.
Mitochondria damage is an important mediator of I/R injury and opening of mitochondrial permeability transition pore, a non‐selective channel of the inner mitochondrial membrane, during the reperfusion process results in membrane depolarization leading to enhanced OXPHOS uncoupling, ROS formation, and ATP depletion. 3 Previous studies showed that mitochondrial permeability transition pore inhibitors administered at the onset of reperfusion reduced infarct size and improved cardiac function in small and large animal models. 3 , 38 Thus, we speculate that increased cardiac ATP production and reduced ROS formation elicited by intracerebroventricular leptin infusion may prevent/attenuate the opening of mitochondrial permeability transition pore and protect the heart after I/R.
Nicotinamide adenine dinucleotide (NAD+) is the major electron carrier coenzyme in fuel oxidation and mitochondrial ATP generation and has recently been proposed as a potential target for future metabolic therapies in HF. 39 Although the mechanisms leading to impaired NAD+ metabolism in HF are not fully understood, NAD+ levels or NAD+/NADH redox state are reduced in HF, and supplementation with NAD+ precursors has beneficial cardiac effects in preclinical models of HF and in patients with HF. 39 , 40 , 41 Since we observed increased ATP production in the hearts of intracerebroventricular leptin treated rats, we also measured cardiac NAD+/NADH content in and found an impressive 10‐fold increase in NAD+/NADH content compared with sham, vehicle and pair‐fed groups. One potential mechanism for the protection provided by increasing NAD+ levels in the heart is reduced acetylation of proteins that regulate cardiac metabolism since NAD+ is an important co‐substrate for sirtuin deacetylases. 42 Sirtuin 3 is the major deacetylase localized in the mitochondria that has been linked to the cardioprotective effects of increased NAD+ levels and prevents mitochondrial permeability transition pore opening during reperfusion of the ischemic myocardium. 43 Additional studies are needed to determine Sirtuin 3 activity within the mitochondria in the myocardium of intracerebroventricular leptin‐treated animals after I/R.
CNS‐Mediated Effects of Leptin Normalize β‐Hydroxybutyrate After I/R
We also found a reduction in circulating β‐OHB, the predominant ketone body oxidized by the heart, 4 weeks after I/R in rats treated with leptin. Ketone bodies provide an important source of energy during fasting, starvation, prolonged exercise, or uncontrolled diabetes. 44 , 45 Circulating ketone bodies are increased in patients with HF and have been proposed as an important source of energy for failing hearts. 46 Our findings indicate that cardiac I/R injury is associated with increased circulating β‐OHB levels, and that chronic intracerebroventricular leptin administration completely normalized plasma β‐OHB concentrations. Although the precise mechanisms responsible for normalization of β‐OHB levels in intracerebroventricular leptin‐treated rats after I/R injury are still unclear, increased glucose and fatty acid OXPHOS in the heart, and potentially in several other organs, by leptin's CNS effects may have contributed to normalization of β‐OHB plasma levels. Unfortunately, we did not quantify cardiac β‐OHB levels in the present study and additional studies are needed to determine whether intracerebroventricular leptin treatment alters β‐OHB oxidation by the heart in situations of metabolic stress.
Cardiac Sympathetic Nerves Do Not Mediate the Chronic CNS Cardioprotective Actions of Leptin After Myocardial I/R
Since leptin stimulates sympathetic activity to several organs including the heart, 4 we hypothesized that leptin‐induced increases in cardiac sympathetic activity could contribute, at least in part, to the beneficial effects of leptin on cardiac contractility and overall function in rats with I/R injury. To test this hypothesis, we performed bilateral CGx in male and female rats before myocardial I/R surgery and intracerebroventricular leptin infusion. Most sympathetic fibers innervating the heart come from postganglionic adrenergic neurons with cell bodies in the cervical sympathetic chain ganglia that run bilaterally to the spinal cord. 47 , 48 Although 3 major ganglia send projections to the heart, the superior and middle cervical ganglia are located in the cervical level, whereas the stellate ganglion is located in the thoracic level which makes its surgical removal challenging for in vivo protocols. Furthermore, complete sympathetic denervation of the heart could increase I/R mortality since cardiac sympathetic fibers play an important role in the acute compensatory hemodynamic changes that occur after myocardial ischemia. 10 In fact, we observed a 10% increase in mortality rate during I/R surgery in CGx rats when compared with animals with intact ganglia, reinforcing the importance of cardiac sympathetic innervation for the acute compensatory response after cardiac ischemia. Thus, only the cervical ganglia were removed leading to a 70% decrease in myocardial tyrosine hydroxylase expression, a precursor of noradrenaline formation in sympathetic fibers. CGx efficacy was also confirmed by a 50% reduction in compensatory tachycardia in response to acute hypotension induced by sodium nitroprusside. The 50% elevation in heart rate may be explained by increased catecholamines from the adrenal medulla following the large reduction in blood pressure and by the 30% remaining cardiac sympathetic fibers.
A previous report showed that superior cervical ganglia denervation improved cardiac function and inflammation after MI. 11 In our study, however, CGx did not prevent cardiac I/R injury in vehicle‐treated rats, and CGx failed to attenuate the cardiac protection provided by intracerebroventricular leptin administration. These observations suggest that any potential changes in sympathetic activity to the heart during intracerebroventricular leptin administration did not contribute significantly to leptin's CNS‐mediated cardiac protective effects during I/R.
Leptin, acting via the CNS, has important effects on peripheral glucose and lipid usage. 8 , 49 However, activation of the autonomic nervous system cannot fully explain leptin's effects on metabolism in peripheral tissues, including the heart. 5 , 6 Our results corroborate these previous studies examining the chronic effects of leptin on glucose regulation, and suggest that leptin's CNS cardioprotective effects that improve mitochondrial function and myocardial contractility in hearts after I/R injury are not mediated primarily by the sympathetic nervous system. Using parabiosis, we recently provided evidence that the CNS‐mediated antidiabetic effects of leptin may involve release of a brain‐derived factor that circulates in the blood and produces a powerful glucose‐lowering effect. 50 Thus, it is possible that a similar mechanism may be responsible for leptin's CNS‐mediated effect to improve cardiac metabolism and overall function in situations of extreme stress such as after myocardial I/R. However, the identity of this factor(s) remains elusive and additional studies beyond the scope of the present study are needed to test this possibility and to identify this factor(s) and its (their) downstream pathways in the heart.
In summary, chronic intracerebroventricular leptin treatment elicited profound cardioprotective effects after myocardial I/R injury in male and female rats (Figure 8). These beneficial cardiac effects of leptin included improvements in mitochondrial function, increased ATP production, reduced proton leak and H2O2 production, and increased cardiac NAD+ levels, leading to improved cardiac contraction (systolic function), relaxation (diastolic function), and increased exercise capacity. We also provide strong evidence that leptin's CNS cardiac protective effects are largely independent of reductions in food intake or increases in cardiac sympathetic activity and occur in males and females. These important effects of leptin suggest novel therapeutic targets for ischemic heart disease and reperfusion injuries.
Figure 8. Central leptin infusion improves cardiac function after ischemia/reperfusion injury regardless of cardiac sympathetic denervation.
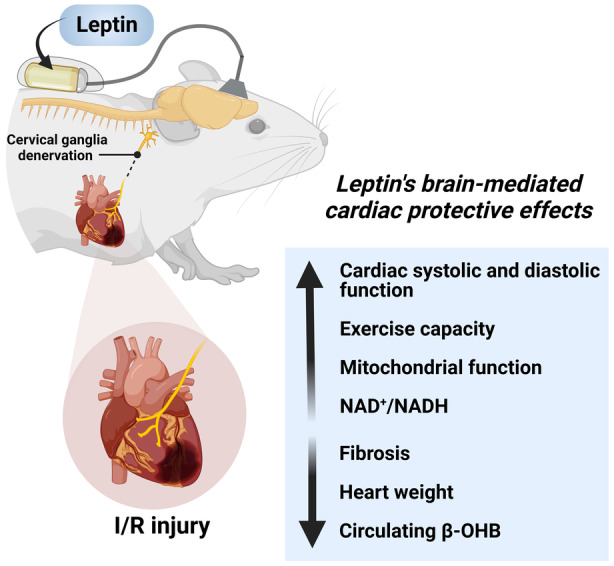
Chronic infusion of leptin directly into the brain improves cardiac function and exercise capacity while it attenuates cardiac remodeling and adverse collagen accumulation after ischemia/reperfusion injury. This improvement in cardiac function during central leptin administration was accompanied by increased cardiac mitochondrial function and NAD+/NADH redox state, and reduced circulating β‐hydroxybutyrate levels. Ablation of cardiac sympathetic nerves did not abolish the cardioprotective effects of chronic central leptin infusion after ischemia/reperfusion injury. β‐OHB, β‐hydroxybutyrate; and I/R indicates ischemia/reperfusion.
Translational Perspective
Current therapies for myocardial I/R injury offer limited benefits, and alternative approaches are urgently needed. Our findings highlight the CNS as an important, albeit unconventional, target for new approaches to treat myocardial I/R and heart failure. Our study reveals potent cardioprotective effects of leptin, mediated via the CNS, that improve mitochondrial function and cardiac metabolism and protect the heart after ischemic insults. Although intracerebroventricular infusions are impractical in a clinical setting, understanding the mechanisms by which leptin acts on the CNS to regulate cardiac metabolism and function may lead to new, more effective therapeutic strategies for myocardial ischemia and heart failure. For example, activating a major downstream mediator of leptin – the brain melanocortin system, particularly melanocortin‐4 receptors – may be a viable therapeutic approach since new melanocortin‐4 receptors agonists given systemically cross the blood–brain barrier and are currently approved to treat some forms of genetic obesity. In addition, we recently demonstrated that the intracerebroventricular administration of melanotan II, an melanocortin‐4 receptors agonist, also has important cardioprotective effects after permanent ligation of the left anterior descending coronary artery. 21 Thus, the CNS leptin‐melanocortin system may offer a novel therapeutic target for MI and I/R injury of the heart.
Sources of Funding
This work was supported by the American Heart Association (grant 835218); the National Institute of General Medicine Sciences (grants P20 GM104357); and the National Institute of Diabetes and Digestive and Kidney Diseases (grant R01 DK121411, R00DK113280).
Disclosures
None.
Supporting information
Video S1‐S2
Video S2
Data S1
Acknowledgments
The authors thank Mr Brent Shows for informatics assistance.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027081
For Sources of Funding and Disclosures, see page 16.
References
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke Statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/cir.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 3. Hausenloy DJ, Yellon DM. Myocardial ischemia‐reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/jci62874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. do Carmo JM, Hall JE, da Silva AA. Chronic central leptin infusion restores cardiac sympathetic‐vagal balance and baroreflex sensitivity in diabetic rats. Am J Physiol Heart Circ Physiol. 2008;295:H1974–H1981. doi: 10.1152/ajpheart.00265.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. da Silva AA, Hall JE, Moak SP, Browning J, Houghton HJ, Micheloni GC, do Carmo JM. Role of autonomic nervous system in chronic CNS‐mediated antidiabetic action of leptin. Am J Physiol Endocrinol Metab. 2017;312:E420–e428. doi: 10.1152/ajpendo.00301.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. da Silva AA, Tallam LS, Liu J, Hall JE. Chronic antidiabetic and cardiovascular actions of leptin: role of CNS and increased adrenergic activity. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1275–R1282. doi: 10.1152/ajpregu.00187.2006 [DOI] [PubMed] [Google Scholar]
- 7. Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–2043. doi: 10.2337/diab.46.12.2040 [DOI] [PubMed] [Google Scholar]
- 8. Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189–12197. doi: 10.1523/jneurosci.2336-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14:30–38. doi: 10.1038/nrcardio.2016.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Young DB. Colloquium series on integrated systems physiology: from molecule to function to disease. Control of Cardiac Output. Vol 2. Morgan & Claypool Life Sciences; 2010:1–97. doi: 10.4199/C00008ED1V01Y201002ISP006 [DOI] [PubMed] [Google Scholar]
- 11. Ziegler KA, Ahles A, Wille T, Kerler J, Ramanujam D, Engelhardt S. Local sympathetic denervation attenuates myocardial inflammation and improves cardiac function after myocardial infarction in mice. Cardiovasc Res. 2018;114:291–299. doi: 10.1093/cvr/cvx227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Savastano LE, Castro AE, Fitt MR, Rath MF, Romeo HE, Muñoz EM. A standardized surgical technique for rat superior cervical ganglionectomy. J Neurosci Methods. 2010;192:22–33. doi: 10.1016/j.jneumeth.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 13. da Silva AA, Pinkerton MA, Spradley FT, Palei AC, Hall JE, do Carmo JM. Chronic CNS‐mediated cardiometabolic actions of leptin: potential role of sex differences. Am J Physiol Regul Integr Comp Physiol. 2021;320:R173–r181. doi: 10.1152/ajpregu.00027.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wahr DW, Wang YS, Schiller NB. Left ventricular volumes determined by two‐dimensional echocardiography in a normal adult population. J Am Coll Cardiol. 1983;1:863–868. doi: 10.1016/s0735-1097(83)80200-9 [DOI] [PubMed] [Google Scholar]
- 15. Hoit BD. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging. 2011;4:179–190. doi: 10.1161/circimaging.110.959817 [DOI] [PubMed] [Google Scholar]
- 16. Damen FW, Salvas JP, Pereyra AS, Ellis JM, Goergen CJ. Improving characterization of hypertrophy‐induced murine cardiac dysfunction using four‐dimensional ultrasound‐derived strain mapping. Am J Physiol Heart Circ Physiol. 2021;321:H197–h207. doi: 10.1152/ajpheart.00133.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petrosino JM, Heiss VJ, Maurya SK, Kalyanasundaram A, Periasamy M, LaFountain RA, Wilson JM, Simonetti OP, Ziouzenkova O. Graded maximal exercise testing to assess mouse cardio‐metabolic phenotypes. PloS One. 2016;11:e0148010. doi: 10.1371/journal.pone.0148010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krajcova A, Urban T, Megvinet D, Waldauf P, Balik M, Hlavicka J, Budera P, Janousek L, Pokorna E, Duska F. High resolution respirometry to assess function of mitochondria in native homogenates of human heart muscle. PloS One. 2020;15:e0226142. doi: 10.1371/journal.pone.0226142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534 [DOI] [PubMed] [Google Scholar]
- 20. Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab. 2019;1:754–764. doi: 10.1038/s42255-019-0095-y [DOI] [PubMed] [Google Scholar]
- 21. Gava FN, da Silva AA, Dai X, Harmancey R, Ashraf S, Omoto ACM, Salgado MC, Moak SP, Li X, Hall JE, et al. Restoration of cardiac function after myocardial infarction by long‐term activation of the CNS leptin‐Melanocortin system. JACC Basic Transl Sci. 2021;6:55–70. doi: 10.1016/j.jacbts.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khafaji HA, Bener AB, Rizk NM, Al SJ. Elevated serum leptin levels in patients with acute myocardial infarction; correlation with coronary angiographic and echocardiographic findings. BMC Res Notes. 2012;5:262. doi: 10.1186/1756-0500-5-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leifheit‐Nestler M, Wagner NM, Gogiraju R, Didié M, Konstantinides S, Hasenfuss G, Schäfer K. Importance of leptin signaling and signal transducer and activator of transcription‐3 activation in mediating the cardiac hypertrophy associated with obesity. J Transl Med. 2013;11:170. doi: 10.1186/1479-5876-11-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barouch LA, Berkowitz DE, Harrison RW, O'Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.Cir.0000083716.82622.Fd [DOI] [PubMed] [Google Scholar]
- 25. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376 [DOI] [PubMed] [Google Scholar]
- 26. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight‐reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777 [DOI] [PubMed] [Google Scholar]
- 27. McGaffin KR, Sun CK, Rager JJ, Romano LC, Zou B, Mathier MA, O'Doherty RM, McTiernan CF, O'Donnell CP. Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury. Cardiovasc Res. 2008;77:54–63. doi: 10.1093/cvr/cvm023 [DOI] [PubMed] [Google Scholar]
- 28. McGaffin KR, Witham WG, Yester KA, Romano LC, O'Doherty RM, McTiernan CF, O'Donnell CP. Cardiac‐specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc Res. 2011;89:60–71. doi: 10.1093/cvr/cvq288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, Lu YH, Kozlova A, Voss H, Martins GG, et al. Sympathetic neuro‐adipose connections mediate leptin‐driven lipolysis. Cell. 2015;163:84–94. doi: 10.1016/j.cell.2015.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ouwens DM, Diamant M, Fodor M, Habets DDJ, Pelsers M, El Hasnaoui M, Dang ZC, van den Brom CE, Vlasblom R, Rietdijk A, et al. Cardiac contractile dysfunction in insulin‐resistant rats fed a high‐fat diet is associated with elevated CD36‐mediated fatty acid uptake and esterification. Diabetologia. 2007;50:1938–1948. doi: 10.1007/s00125-007-0735-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fels JA, Manfredi G. Sex differences in ischemia/reperfusion injury: the role of mitochondrial permeability transition. Neurochem Res. 2019;44:2336–2345. doi: 10.1007/s11064-019-02769-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res. 2010;106:1681–1691. doi: 10.1161/circresaha.109.213645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi Z, Brooks VL. Leptin differentially increases sympathetic nerve activity and its baroreflex regulation in female rats: role of oestrogen. J Physiol. 2015;593:1633–1647. doi: 10.1113/jphysiol.2014.284638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thune JJ, Solomon SD. Left ventricular diastolic function following myocardial infarction. Curr Heart Fail Rep. 2006;3:170–174. doi: 10.1007/s11897-006-0018-6 [DOI] [PubMed] [Google Scholar]
- 35. Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res. 2021;128:1487–1513. doi: 10.1161/circresaha.121.318241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009 [DOI] [PubMed] [Google Scholar]
- 37. Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg. 2018;1859:940–950. doi: 10.1016/j.bbabio.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 38. Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia‐reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025 [DOI] [PubMed] [Google Scholar]
- 39. Diguet N, Trammell SAJ, Tannous C, Deloux R, Piquereau J, Mougenot N, Gouge A, Gressette M, Manoury B, Blanc J, et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation. 2018;137:2256–2273. doi: 10.1161/circulationaha.116.026099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee CF, Chavez JD, Garcia‐Menendez L, Choi Y, Roe ND, Chiao YA, Edgar JS, Goo YA, Goodlett DR, Bruce JE, et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation. 2016;134:883–894. doi: 10.1161/circulationaha.116.022495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou B, Wang DD, Qiu Y, Airhart S, Liu Y, Stempien‐Otero A, O'Brien KD, Tian R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J Clin Invest. 2020;130:6054–6063. doi: 10.1172/jci138538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin AS, Abraham DM, Hershberger KA, Bhatt DP, Mao L, Cui H, Liu J, Liu X, Muehlbauer MJ, Grimsrud PA, et al. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich's ataxia cardiomyopathy model. JCI Insight. 2017;2. doi: 10.1172/jci.insight.93885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parodi‐Rullán RM, Chapa‐Dubocq X, Rullán PJ, Jang S, Javadov S. High sensitivity of SIRT3 deficient hearts to ischemia‐reperfusion is associated with mitochondrial abnormalities. Front Pharmacol. 2017;8:275. doi: 10.3389/fphar.2017.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koeslag JH, Noakes TD, Sloan AW. Post‐exercise ketosis. J Physiol. 1980;301:79–90. doi: 10.1113/jphysiol.1980.sp013190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15:412–426. doi: 10.1002/(sici)1520‐7560(199911/12)15:6<412::aid‐dmrr72>3.0.co;2‐8 [DOI] [PubMed] [Google Scholar]
- 46. Lommi J, Koskinen P, Näveri H, Härkönen M, Kupari M. Heart failure ketosis. J Intern Med. 1997;242:231–238. doi: 10.1046/j.1365-2796.1997.00187.x [DOI] [PubMed] [Google Scholar]
- 47. Pardini BJ, Lund DD, Schmid PG. Organization of the sympathetic postganglionic innervation of the rat heart. J Auton Nerv Syst. 1989;28:193–201. doi: 10.1016/0165-1838(89)90146-x [DOI] [PubMed] [Google Scholar]
- 48. Franzoso M, Zaglia T, Mongillo M. Putting together the clues of the everlasting neuro‐cardiac liaison. Biochim Biophys Acta. 2016;1863:1904–1915. doi: 10.1016/j.bbamcr.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 49. Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiuchi M, Shimazu T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes. 1999;48:1706–1712. doi: 10.2337/diabetes.48.9.1706 [DOI] [PubMed] [Google Scholar]
- 50. da Silva AA, Hall JE, Dai X, Wang Z, Salgado MC, do Carmo MC. Chronic antidiabetic actions of leptin: evidence from Parabiosis studies for a CNS‐derived circulating antidiabetic factor. Diabetes. 2021;70:2264–2274. doi: 10.2337/db21-0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1‐S2
Video S2
Data S1


