Abstract
Background
Oxidized low‐density lipoprotein (oxLDL) and hs‐CRP (high‐sensitivity C‐reactive protein) plays an important role in cardiovascular diseases though inflammation and oxidative stress, etc. However, evidence on their combined effects on stroke prognosis is still limited. We aimed to explore the joint association of oxLDL and hs‐CRP with outcomes of minor stroke or transient ischemic attack.
Methods and Results
A subgroup of 3019 patients from the CHANCE trial (Clopidogrel in High‐Risk Patients With Acute Nondisabling Cerebrovascular Events) were analyzed. Baseline oxLDL and hs‐CRP levels were measured. The primary outcome was any stroke within 90 days. The secondary outcomes included any stroke within 1 year, and ischemic stroke, combined vascular events, and poor functional outcomes (modified Rankin Scale 2–6 or 3–6) at 90 days and 1 year. Vascular events outcomes were analyzed with Cox proportional hazards and poor functional outcomes with logistic models. Elevated oxLDL (>28.81 μg/dL) and hs‐CRP (>4.20 mg/L) was observed in 624 (20.67%) of the 3019 patients. Patients with oxLDL >28.81 μg/dL and hs‐CRP >4.20 mg/L had a higher risk of recurrent stroke within 90 days (adjusted hazard ratio, 1.52; 95% CI, 1.17–1.97), compared with those with oxLDL ≤28.81 μg/dL and hs‐CRP ≤4.20 mg/L, after adjusting relevant confounding factors (P=0.002). Similar results were observed for secondary outcomes (P<0.05 for all).
Conclusions
In patients with minor stroke or transient ischemic attack, joint high levels of oxLDL and hs‐CRP was associated with increased risk of recurrent stroke, combined vascular events, and poor functional outcome.
Keywords: high‐sensitivity C‐reactive protein, oxidized low‐density lipoprotein, poor functional outcome, recurrence, stroke
Subject Categories: Ischemic Stroke
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00979589.
Nonstandard Abbreviations and Acronyms
- CHANCE
Clopidogrel in High‐Risk Patients With Acute Nondisabling Cerebrovascular Events
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- oxLDL
oxidized low‐density lipoprotein
Clinical Perspective.
What Is New?
Joint high levels of oxidized low‐density lipoprotein and hs‐CRP (high‐sensitivity C‐reactive protein) were associated with increased risk of recurrent stroke and combined vascular events in patients with minor stroke or transient ischemic attack.
Joint high levels of oxidized low‐density lipoprotein and hs‐CRP were associated with increased risk of poor functional outcome in patients with minor stroke or transient ischemic attack.
What Are the Clinical Implications?
The application of this combined test in the clinical practice of stroke diseases may help clinical decision making and further improve the prognosis of patients with stroke.
Atherosclerosis is the main pathological basis of most ischemic stroke. 1 The role of inflammation and oxidative stress in the pathological progression of atherosclerosis and cardiovascular disease have been widely addressed before. 2 , 3 , 4 Specific mediators such as hs‐CRP (high‐sensitivity C‐reactive protein) and low‐density lipoprotein link these pathophysiological pathways. 5 Hs‐CRP is a well‐recognized inflammatory biomarker, its high level reflects the instability of atherosclerotic plaque 6 and is associated with a worse outcome after ischemic stroke including recurrent vascular events. 7 , 8 , 9 The oxidative modification of low‐density lipoprotein under the oxidative stress resulting in oxidized low‐density lipoprotein (oxLDL), 10 which is another factor induces proinflammatory and proatherogenic effects and is involved in the initiation and acceleration of atherosclerosis lesions, 2 , 11 Our previous study found that elevated concentrations of oxLDL could be a strong predictor of recurrent stroke in patients with minor stroke or transient ischemic attack (TIA). 12 Studies showed the levels of oxLDL positively correlated with hs‐CRP in men from the general population 13 and in patients with coronary heart disease, 14 , 15 implying they may be involved in some of the same pathophysiological pathways in the process of atherogenesis. One observational study showed the combined use of oxLDL and hs‐CRP have better predictive value for prognosis after acute coronary syndrome. 16 However, no studies have ever investigated the relationship between combined oxLDL and hs‐CRP levels and recurrent stroke and poor functional outcomes in patients with minor stroke or TIA.
Acute minor ischemic stroke and TIA are the most common cerebrovascular diseases, with a high risk of recurrent stroke or other vascular events in the early stage. Identifying more comprehensive and reliable predictors is crucial to treat patients precisely to reduce stroke burden. Thus, we aimed to investigate whether oxLDL and hs‐CRP have combined effects on outcomes of acute minor ischemic stroke or TIA, using the data derived from the CHANCE trial (Clopidogrel in High‐Risk Patients With Acute Nondisabling Cerebrovascular Events).
METHODS
The data and methods that support the findings of this study are available from the corresponding authors upon reasonable request.
Study Population
Details on the rationale, design, and results of the CHANCE trial have been published previously. 17 , 18 , 19 In brief, CHANCE was a randomized, double‐blind, placebo‐controlled clinical trial conducted at 114 centers in China between October 1, 2009, and July 30, 2012, with the aim to assess the efficacy of combined treatment with clopidogrel (loading dose of 300 mg followed by 75 mg daily for 90 days) plus aspirin (loading dose of 75–300 mg followed by 75 mg daily for 21 days) versus aspirin alone (loading dose of 75–300 mg followed by 75 mg daily for 90 days) in reducing the risk of recurrent stroke after 90 days of follow‐up. This trial enrolled 5170 patients with minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] ≤3) or high‐risk TIA (age, blood pressure, clinical features, duration of symptoms, and presence of diabetes 2 ≥4) within 24 hours after symptom onset. A total of 73 (64%) centers voluntarily participated in the blood substudy, and 3044 consecutive blood samples were collected. The CHANCE trial was registered with ClinicalTrials.gov (NCT00979589). Written informed consent was obtained from all participants or their legal proxies. The CHANCE protocol was approved by the ethics committee at each study center.
Data Collection
Baseline data on demographics, smoking status, and medical history of ischemic stroke, TIA, diabetes, hypertension, hyperlipidemia, myocardial infarction, angina, congestive heart failure, known atrial fibrillation, and valvular heart disease, were collected through face‐to‐face interviews by trained interviewers. Blood pressure and body mass index were measured by the trained nurses on admission according to the unified standard report via CHANCE protocol. 18 The NIHSS and age, blood pressure, clinical features, duration of symptoms, and presence of diabetes2 scores were assessed by the trained neurologists on admission. The medication use of antihypertensive, hypoglycemic, and statin agents was recorded during the 90‐day follow‐up period.
Measurement of Circulating oxLDL and hs‐CRP
Blood samples were collected from fasting patients within 24±12 hours after randomization. At each center, plasma samples were isolated and immediately frozen at −80 °C. Then all samples were transported through cold chain to the central laboratory in Beijing Tiantan Hospital. No freezing and thawing circle occurred before test. The oxLDL levels were measured in EDTA‐plasma samples by using ELISA (mAb‐4E6based ELISA Kit, RapidBio Lab, CALAS, CA). 20 The intra‐assay and interassay coefficients of variation of oxLDL level tests were 1.8% and 1.4%, respectively. The hs‐CRP levels were measured on a Roche Modular P800 analyzer (Roche, Basel, Switzerland) using a turbidimetric immunoassay (Ji'en Technique Co Ltd, Shanghai, China). The intra‐assay and interassay coefficients of variation of hs‐CRP level were 2.5% and 2.0%, respectively. All measurements were conducted in the clinical laboratory of Beijing Tiantan Hospital according to the manufacturer's guidelines by laboratory personnel who were masked to the study samples, study group assignments, and outcomes.
Follow‐Up and Efficacy Outcomes
Patients were followed up at 90 days and 1 year by trained site coordinators. 18 , 19 The primary outcome was a new stroke (ischemic or hemorrhagic) within 90 days. The secondary outcomes included any stroke within 1 year, and new ischemic stroke, combined vascular events (ischemic stroke, hemorrhagic stroke, myocardial infarction, or vascular death), and poor functional outcomes (defined as modified Rankin Scale [mRS] 2–6 or 3–6) at 90 days and 1 year. The definition of the above‐mentioned outcomes was consistent with those previously described in the CHANCE trial. 18 , 19 All reported events were reviewed and confirmed by a central adjudication committee that was masked to the study‐group assignments.
Statistical Analysis
Continuous variables were presented as medians with interquartile ranges and categorical variables as frequencies with percentages. The nonparametric Wilcoxon or Kruskal–Wallis test was used to compare group differences for continuous variables, and χ2 tests for categorical variables. There is no consensus on the best cutoff value of oxLDL and hs‐CRP; our previous study showed patients in the highest oxLDL quartile had a higher risk of recurrent stroke within 90 days compared with those in the lowest oxLDL quartile. 12 Thus, we used the upper quartile to indicate high risk in the current study. Patients were classified into 4 groups: group 1: oxLDL ≤28.81 μg/dL and hs‐CRP ≤4.20 mg/L, group 2: oxLDL ≤28.81 μg/dL and hs‐CRP >4.20 mg/L, group 3: oxLDL >28.81 μg/dL and hs‐CRP ≤4.20 mg/L, group 4: oxLDL >28.81 μg/dL and hs‐CRP >4.20 mg/L. Cox proportional hazards model was used to calculate hazards ratios (HRs) and 95% CIs for the associations of oxLDL and hs‐CRP with recurrent stroke, ischemic stroke, and combined vascular events. For poor functional outcomes, logistic regression was used to estimate odds ratios (ORs) and 95% CIs. Patients with oxLDL level ≤28.81 μg/dL and hs‐CRP level ≤4.20 mg/L were set as the reference group. Variables including age, sex, body mass index, high‐density lipoprotein, history of ischemic stroke, myocardial infarction, hypertension, baseline NIHSS score, randomized treatment of aspirin alone or clopidogrel with aspirin, and the qualifying events of minor stroke or TIA, were adjusted in the multivariable regression analyses. The Kaplan–Meier analyses were used to generate survival plots of recurrent stroke, ischemic stroke, and combined vascular events, and groups were compared by the log‐rank test. We further performed sensitivity analysis by changing the cutoff points of oxLDL and hs‐CRP. Because previous study suggested patients with hs‐CRP >3 mg/L was associated with increased risk of recurrent stroke, 8 we used hs‐CRP cutoff of 3 mg/L to define high risk. For oxLDL, median value (13.96 μg/dL) was used. Totally, 3 sensitivity analyses were conducted with different combinations of oxLDL and hs‐CRP (patients were classified into 4 groups based on: (1) the upper quartile of oxLDL and hs‐CRP levels of 3.0 mg/L; (2) the median of oxLDL and the upper quartile of hs‐CRP; (3) the median of oxLDL and hs‐CRP levels of 3.0 mg/L). Also, subgroup analysis was performed according to statin agents and qualifying events of minor stroke or TIA with an interaction test.
Overall, a 2‐sided P<0.05 was considered statistically significant. All analyses were performed with SAS software version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Baseline Characteristics
Of the 5170 patients who participated in the CHANCE trial, 2151 cases without oxLDL or hs‐CRP measurements were excluded. Thus, a total of 3019 patients were included in the final analysis. The baseline characteristics of patients included and excluded were well balanced, except that the patients enrolled had slightly higher blood pressure levels, NIHSS scores, lower proportion of history of angina, diabetes, qualifying TIA, and were more likely to receive antihypertensive agents during follow‐up (Table 1). Of the 3019 patients included in this study, the median age was 62.31 (interquartile ranges, 54.74–71.18), and 1007 (33.36%) patients were women. Elevated oxLDL (>28.81 μg/dL) and hs‐CRP (>4.20 mg/L) was observed in 624 (20.67%) of the 3019 patients. Table 2 shows the baseline characteristics of the patients by oxLDL and hs‐CRP levels. Compared with patients with both lower oxLDL and hs‐CRP levels, those with both higher oxLDL and hs‐CRP levels were more likely to be older, have higher NIHSS score, and higher proportion of history of ischemic stroke, myocardial infarction, and hypertension.
Table 1.
Baseline Characteristics of Patients Included Versus Not Included in this Substudy of the CHANCE Trial
| Characteristics | Overall | Excluded | Included | P value |
|---|---|---|---|---|
| Patients, n | 5170 | 2151 | 3019 | |
| Age, median (IQR), y | 62.29 (54.69–71.27) | 62.29 (54.61–71.38) | 62.31 (54.74–71.18) | 0.865 |
| Women, n (%) | 1750 (33.85) | 743 (34.54) | 1007 (33.36) | 0.374 |
| BMI, median (IQR), kg/m2 | 24.49 (22.72–26.45) | 24.49 (22.67–26.32) | 24.49 (22.76–26.56) | 0.285 |
| SBP, median (IQR), mm Hg | 150 (136–161) | 150 (135–160) | 150 (139–164) | 0.003 |
| DBP, median (IQR), mm Hg | 90 (80–100) | 90 (80–98) | 90 (80–100) | 0.021 |
| HDL, median (IQR), mmol/L | 1.2 (1.00–1.46) | 1.21 (1.06–1.60) | 1.2 (0.99–1.46) | 0.551 |
| LDL, median (IQR), mmol/L | 3.12 (2.49–3.82) | 3.12 (2.56–4.23) | 3.12 (2.49–3.82) | 0.586 |
| oxLDL, median (IQR), μg/dL | 13.96 (6.65–28.81) | ‐ | 13.96 (6.65–28.81) | 0.586 |
| hs‐CRP, median (IQR), mg/L | 1.7 (0.8–4.2) | 1.4 (0.6–4.3) | 1.7 (0.8–4.2) | 0.476 |
| Medical history, n (%) | ||||
| Ischemic stroke | 1033 (19.98) | 456 (21.20) | 577 (19.11) | 0.064 |
| TIA | 174 (3.37) | 80 (3.72) | 94 (3.11) | 0.234 |
| Myocardial infarction | 96 (1.86) | 42 (1.95) | 54 (1.79) | 0.667 |
| Angina | 184 (3.56) | 92 (4.28) | 92 (3.05) | 0.019 |
| Congestive heart failure | 80 (1.55) | 27 (1.26) | 53 (1.76) | 0.151 |
| Known atrial fibrillation | 96 (1.86) | 39 (1.81) | 57 (1.89) | 0.844 |
| Valvular heart disease | 14 (0.27) | 4 (0.19) | 10 (0.33) | 0.322 |
| Hypertension | 3399 (65.74) | 1431 (66.53) | 1968 (65.19) | 0.317 |
| Diabetes | 1093 (21.14) | 485 (22.55) | 608 (20.14) | 0.037 |
| Hypercholesterolemia | 573 (11.08) | 256 (11.90) | 317 (10.50) | 0.114 |
| Current or previous smoking, n (%) | 2221 (42.96) | 928 (43.14) | 1293 (42.83) | 0.822 |
| Time to randomization <12 h, n (%) | 2573 (49.77) | 1071 (49.79) | 1502 (49.75) | 0.978 |
| Qualifying events, n (%) | ||||
| TIA | 1445 (27.95) | 635 (29.52) | 810 (26.83) | 0.034 |
| Minor stroke | 3725 (72.05) | 1516 (70.48) | 2209 (73.17) | 0.034 |
| Baseline NIHSS score, median (IQR) | 2 (0–2) | 1 (0–2) | 2 (0–2) | 0.036 |
| Antiplatelet assignment, n (%) | 0.825 | |||
| Clopidogrel and aspirin | 2584 (49.98) | 1079 (50.16) | 1505 (49.85) | |
| Aspirin alone | 2586 (50.02) | 1072 (49.84) | 1514 (50.15) | |
| Medications within 90‐d follow‐up period, n (%) | ||||
| Antihypertensive agents | 1814 (35.09) | 698 (32.45) | 1116 (36.97) | <0.001 |
| Hypoglycemic agents | 656 (12.69) | 283 (13.16) | 373 (12.36) | 0.393 |
| Stain agents | 2171 (41.99) | 912 (42.40) | 1259 (41.70) | 0.617 |
BMI indicates body mass index; CHANCE, clopidogrel in high‐risk patients with acute nondisabling cerebrovascular events; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; LDL, low‐density lipoprotein; NIHSS, National Institutes of Health Stroke Scale; oxLDL, oxidized low‐density lipoprotein; SBP, systolic blood pressure; and TIA, transient ischemic attack.
Table 2.
Baseline Characteristics of Patients According to oxLDL and hs‐CRP Levels
| Characteristics | Overall | Group 1* | Group 2* | Group 3* | Group 4* | P value |
|---|---|---|---|---|---|---|
| Patients, n | 3019 | 2138 | 125 | 132 | 624 | |
| Age, median (IQR), y | 62.31 (54.74–71.18) | 61.36 (54.10–70.19) | 62.51 (54.62–72.33) | 62.58 (55.24–70.58) | 65.34 (56.36–73.43) | <0.001 |
| Women, n (%) | 1007 (33.36) | 700 (32.74) | 48 (38.40) | 37 (28.03) | 222 (35.58) | 0.182 |
| BMI, median (IQR), kg/m2 | 24.49 (22.76–26.56) | 24.49 (22.72–26.37) | 24.46 (22.65–26.99) | 24.28 (22.72–26.74) | 24.54 (22.86–27.14) | 0.226 |
| SBP, median (IQR), mm Hg | 150 (139–164) | 150 (138–162) | 150 (140–160) | 150 (140–160) | 150 (140–168) | 0.297 |
| DBP, median (IQR), mm Hg | 90 (80–100) | 90 (80–100) | 90 (80–97) | 90 (80–99.5) | 90 (80–100) | 0.731 |
| HDL, median (IQR), mmol/L | 1.2 (0.99–1.46) | 1.21 (1.01–1.48) | 1.21 (1.06–1.41) | 1.14 (0.91–1.35) | 1.17 (0.98–1.44) | 0.009 |
| LDL, median (IQR), mmol/L | 3.12 (2.49–3.82) | 3.11 (2.50–3.78) | 3.28 (2.61–3.95) | 3.19 (2.53–3.79) | 3.13 (2.45–3.92) | 0.331 |
| oxLDL, median (IQR), μg/dL | 13.96 (6.65–28.81) | 9.46 (5.01–15.62) | 19.88 (12.44–25.23) | 35.19 (30.88–45.6) | 53.15 (38.01–81.19) | <0.001 |
| hs‐CRP, median (IQR), mg/L | 1.7 (0.8–4.2) | 1.1 (0.6–2.0) | 5.8 (4.7–8.4) | 2.9 (1.5–3.7) | 9.45 (6.0–13.9) | <0.001 |
| Medical history, n (%) | ||||||
| Ischemic stroke | 577 (19.11) | 379 (17.73) | 18 (14.40) | 26 (19.70) | 154 (24.68) | 0.001 |
| TIA | 94 (3.11) | 67 (3.13) | 1 (0.80) | 5 (3.79) | 21 (3.37) | 0.466 |
| Myocardial infarction | 54 (1.79) | 29 (1.36) | 2 (1.60) | 4 (3.03) | 19 (3.04) | 0.029 |
| Angina | 92 (3.05) | 62 (2.90) | 3 (2.40) | 3 (2.27) | 24 (3.85) | 0.583 |
| Congestive heart failure | 53 (1.76) | 34 (1.59) | 3 (2.40) | 2 (1.52) | 14 (2.24) | 0.672 |
| Known atrial fibrillation | 57 (1.89) | 35 (1.64) | 2 (1.60) | 4 (3.03) | 16 (2.56) | 0.354 |
| Valvular heart disease | 10 (0.33) | 5 (0.23) | 0 (0.00) | 1 (0.76) | 4 (0.64) | 0.312 |
| Hypertension | 1968 (65.19) | 1351 (63.19) | 87 (69.60) | 93 (70.45) | 437 (70.03) | 0.005 |
| Diabetes | 608 (20.14) | 421 (19.69) | 26 (20.80) | 26 (19.70) | 135 (21.63) | 0.757 |
| Hyperlipidemia | 317 (10.50) | 234 (10.94) | 9 (7.20) | 11 (8.33) | 63 (10.10) | 0.446 |
| Current or previous smoking, n (%) | 1293 (42.83) | 910 (42.56) | 53 (42.40) | 66 (50.00) | 264 (42.31) | 0.405 |
| Time to randomization <12 h, n (%) | 1502 (49.75) | 1069 (50.00) | 58 (46.40) | 71 (53.79) | 304 (48.72) | 0.628 |
| Qualifying events, n (%) | 0.116 | |||||
| TIA | 810 (26.83) | 597 (27.92) | 25 (20.00) | 31 (23.48) | 157 (25.16) | |
| Minor stroke | 2209 (73.17) | 1541 (72.08) | 100 (80.00) | 101 (76.52) | 467 (74.84) | |
| Baseline NIHSS score, median (IQR) | 2 (0–2) | 1 (0–2) | 2 (1–3) | 2 (1–2) | 2 (0.5–3) | 0.005 |
| Antiplatelet assignment, n (%) | 0.069 | |||||
| Clopidogrel and aspirin | 1505 (49.85) | 1079 (50.47) | 73 (58.40) | 60 (45.45) | 293 (46.96) | |
| Aspirin alone | 1514 (50.15) | 1059 (49.53) | 52 (41.60) | 72 (54.55) | 331 (53.04) | |
| Medications within 90‐d follow‐up period, n (%) | ||||||
| Antihypertensive agents | 1116 (36.97) | 777 (36.34) | 51 (40.80) | 50 (37.88) | 238 (38.14) | 0.668 |
| Hypoglycemic agents | 373 (12.36) | 258 (12.07) | 20 (16.00) | 15 (11.36) | 80 (12.82) | 0.585 |
| Statin agents | 1259 (41.70) | 887 (41.49) | 51 (40.80) | 58 (43.94) | 263 (42.15) | 0.939 |
BMI indicates body mass index; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; LDL, low‐density lipoprotein; NIHSS, National Institutes of Health Stroke Scale; oxLDL, oxidized low‐density lipoprotein; SBP, systolic blood pressure; and TIA, transient ischemic attack.
Patients were classified into 4 groups based on the upper quartile of oxLDL and hs‐CRP levels: group 1: oxLDL ≤28.81 μg/dL and hs‐CRP ≤4.20 mg/L; group 2: oxLDL ≤28.81 μg/dL and hs‐CRP >4.20 mg/L; group 3: oxLDL >28.81 μg/dL, hs‐CRP ≤4.20 mg/L; group 4: oxLDL >28.81 μg/dL and hs‐CRP >4.20 mg/L.
Associations of oxLDL and hs‐CRP With Recurrent Vascular Events
Overall, the cumulative incidence of recurrent stroke, ischemic stroke, combined vascular events was 9.74%, 9.54%, 9.80%, within 90 days of follow‐up and 12.06%, 11.63%, 12.42% within 1 year of follow‐up. All Kaplan–Meier curves by oxLDL and hs‐CRP levels appeared to separate early and to continue to diverge throughout the follow‐up period (Figure 1). Patients with oxLDL >28.81 μg/dL and hs‐CRP >4.20 mg/L showed a higher incidence of recurrent stroke, ischemic stroke, and combined vascular events within 90 days and at 1 year (all log‐rank test P<0.01). Compared with patients with oxLDL ≤28.81 μg/dL and hs‐CRP ≤4.20 mg/L, patients who had levels of oxLDL >28.81 μg/dL and hs‐CRP >4.20 mg/L had increased risk of recurrent stroke within 90 days (13.78% versus 8.51%; HR, 1.65 [95% CI, 1.28–2.13]) and 1 year (16.35% versus 10.76%; HR, 1.56 [95% CI, 1.24–1.97]). After adjusting for confounding factors, the association persisted (HR, 1.52 [95% CI, 1.17–1.97]; HR, 1.44 [95% CI, 1.14–1.83]). Similar results were observed for ischemic stroke and combined vascular events within 90 days and 1 year (Table 3). Figure 2 shows the sensitivity analysis results by using oxLDL cutoff of 13.96 μg/dL and hs‐CRP cutoff of 3 mg/L. Consistent with the main results, patients with both elevated levels of oxLDL and hs‐CRP had higher risk of recurrent stroke, ischemic stroke, and combined vascular events when applying different combinations of oxLDL and hs‐CRP in the sensitivity analysis.
Figure 1. Kaplan–Meier curves for incidence of recurrent stroke, ischemic stroke, and combined vascular events grouped by oxidized low‐density lipoprotein (oxLDL) and hs‐CRP (high‐sensitivity C‐reactive protein) levels within 90 days and 1 year.
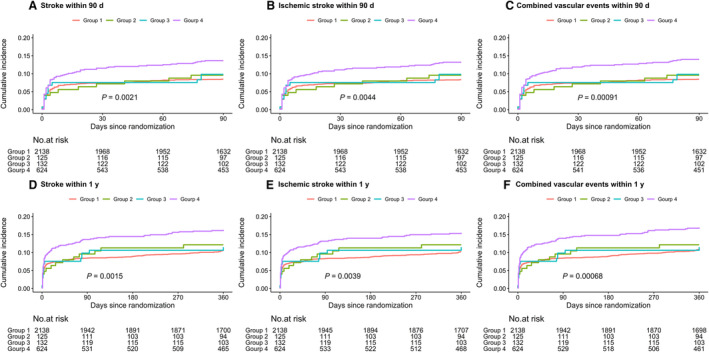
A, recurrent stoke within 90 days; (B) ischemic stroke within 90 days; (C) combined vascular events within 90 days; (D) recurrent stoke within 1 year; (E) ischemic stroke within 1 year; (F) combined vascular events within 1 year. Patients were classified into 4 groups based on the upper quartile of oxLDL and hs‐CRP levels: group 1: oxLDL ≤28.81 μg/dL and hs‐CRP ≤4.20 mg/L; group 2: oxLDL ≤28.81 μg/dL and hs‐CRP >4.20 mg/L; group 3: oxLDL >28.81 μg/dL, hs‐CRP ≤4.20 mg/L; group 4: oxLDL >28.81 μg/dL and hs‐CRP >4.20 mg/L.
Table 3.
Associations of oxLDL and hs‐CRP Levels With Stroke, Ischemic Stroke, and Combined Vascular Events Within 90 Days and 1 Year
| Outcomes | Outcomes within 90 d | Outcomes within 1 y | ||||
|---|---|---|---|---|---|---|
| Events, n (%) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Events, n (%) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Stroke | ||||||
| Group 1 | 182 (8.51) | Reference | Reference | 230 (10.76) | Reference | Reference |
| Group 2 | 12 (9.60) | 1.21 (0.63–2.01) | 1.06 (0.59–1.90) | 15 (12.00) | 1.13 (0.67–1.90) | 1.09 (0.64–1.83) |
| Group 3 | 14 (10.61) | 1.25 (0.73–2.15) | 1.21 (0.70–2.08) | 17 (12.88) | 1.20 (0.73–1.97) | 1.16 (0.71–1.91) |
| Group 4 | 86 (13.78) | 1.65 (1.28–2.13) | 1.52 (1.17–1.97) | 102 (16.35) | 1.56 (1.24–1.97) | 1.44 (1.14–1.83) |
| Ischemic stroke | ||||||
| Group 1 | 179 (8.37) | Reference | Reference | 222 (10.38) | Reference | Reference |
| Group 2 | 12 (9.60) | 1.14 (0.64–2.05) | 1.07 (0.60–1.93) | 15 (12.00) | 1.17 (0.69–1.97) | 1.12 (0.66–1.89) |
| Group 3 | 14 (10.61) | 1.27 (0.74–2.19) | 1.23 (0.71–2.11) | 17 (12.88) | 1.25 (0.76–2.04) | 1.20 (0.73–1.97) |
| Group 4 | 83 (13.30) | 1.62 (1.25–2.10) | 1.49 (1.15–1.94) | 97 (15.54) | 1.54 (1.21–1.95) | 1.41 (1.11–1.79) |
| Combined vascular events | ||||||
| Group 1 | 182 (8.51) | Reference | Reference | 237 (11.09) | Reference | Reference |
| Group 2 | 12 (9.60) | 1.21 (0.63–2.01) | 1.06 (0.59–1.90) | 15 (12.00) | 1.10 (0.65–1.85) | 1.05 (0.62–1.78) |
| Group 3 | 14 (10.61) | 1.25 (0.73–2.15) | 1.21 (0.70–2.08) | 17 (12.88) | 1.17 (0.71–1.91) | 1.12 (0.69–1.84) |
| Group 4 | 88 (14.10) | 1.69 (1.31–2.18) | 1.56 (1.21–2.02) | 106 (16.99) | 1.58 (1.26–1.99) | 1.46 (1.16–1.84) |
Adjusted hazard ratio (95% CI) was calculated after adjusting for age, sex, body mass index, high‐density lipoprotein, history of ischemic stroke, myocardial infarction, hypertension, baseline National Institutes of Health Stroke Scale score, randomized treatment of aspirin alone or clopidogrel with aspirin, and the qualifying events of minor stroke or transient ischemic attack. Patients were classified into 4 groups based on the upper quartile of oxidized low‐density lipoprotein (oxLDL) and hs‐CRP (high‐sensitivity C‐reactive protein) levels: group 1: oxLDL ≤28.81 μg/dL and hs‐CRP ≤4.20 mg/L; group 2: oxLDL ≤28.81 μg/dL and hs‐CRP >4.20 mg/L; group 3: oxLDL >28.81 μg/dL, hs‐CRP ≤4.20 mg/L; group 4: oxLDL >28.81 μg/dL and hs‐CRP >4.20 mg/L. HR indicates hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein; and oxLDL, oxidized low‐density lipoprotein.
Figure 2. Sensitivity analysis of oxidized low‐density lipoprotein (oxLDL) and hs‐CRP (high‐sensitivity C‐reactive protein) levels with clinical outcomes at 90 days and 1 year.
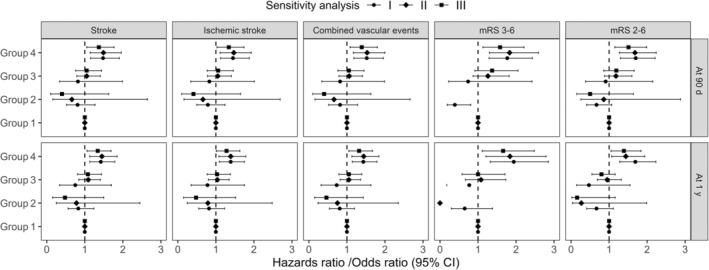
Adjusted hazard ratio/odds ratio (95% CI) was calculated after adjusting for age, sex, body mass index, high‐density lipoprotein, history of ischemic stroke, myocardial infarction, hypertension, baseline National Institutes of Health Stroke Scale score, randomized treatment of aspirin alone or clopidogrel with aspirin, and the qualifying events of minor stroke or transient ischemic attack. Sensitivity analysis I: Patients were classified into 4 groups based on the upper quartile of oxLDL and hs‐CRP levels of 3.0 mg/L: group 1: oxLDL ≤28.81 μg/dL and hs‐CRP ≤3.0 mg/L; group 2: oxLDL ≤28.81 μg/dL and hs‐CRP >3.0 mg/L; group 3: oxLDL >28.81 μg/dL, hs‐CRP ≤3.0 mg/L; group 4: oxLDL >28.81 μg/dL and hs‐CRP >3.0 mg/L. Sensitivity analysis II: Patients were classified into 4 groups based on the median of oxLDL and the upper quartile of hs‐CRP: group 1: oxLDL ≤13.96 μg/dL and hs‐CRP ≤4.2 mg/L; group 2: oxLDL ≤13.96 μg/dL and hs‐CRP >4.2 mg/L; group 3: oxLDL >13.96 μg/dL, hs‐CRP ≤4.2 mg/L; group 4: oxLDL >13.96 μg/dL and hs‐CRP >4.2 mg/L. Sensitivity analysis III: Patients were classified into 4 groups based on the median of oxLDL and hs‐CRP levels of 3.0 mg/L: group 1: oxLDL ≤13.96 μg/dL and hs‐CRP ≤3.0 mg/L; group 2: oxLDL ≤13.96 μg/dL and hs‐CRP >3.0 mg/L; group 3: oxLDL >13.96 μg/dL, hs‐CRP ≤3.0 mg/L; group 4: oxLDL >13.96 μg/dL and hs‐CRP >3.0 mg/L. mRS indicates modified Rankin Scale.
Associations of oxLDL and hs‐CRP With Poor Functional Outcome
Totally, there were 200 (6.67%) and 130 (4.45%) patients who had poor functional outcome (defined as mRS of 3–6) at 90 days and 1 year, respectively. The corresponding values of patients had mRS of 2–6 were 316 (10.53%) and 281 (9.61%) at 90 days and 1 year, respectively. The risk of poor functional outcome (defined as mRS 3–6) significantly increased in patients with oxLDL >28.81 μg/dL and hs‐CRP >4.20 mg/L compared with those with oxLDL ≤28.81 μg/dL and hs‐CRP ≤4.20 mg/L, the adjusted OR (95% CI) was 1.90 (1.38–2.61) at 90 days and 1.98 (1.35–2.93) at 1 year, respectively (Table 4). When poor functional outcome was defined as mRS 2–6, significantly increased risks were also found in patients with oxLDL >28.81 μg/dL and hs‐CRP >4.20 mg/L, the adjusted OR (95% CI) was 1.76 (1.34–2.30) at 90 days and 1.62 (1.22–2.16) at 1 year, respectively. Robust results were observed in sensitivity analysis (Figure 2).
Table 4.
Associations of oxLDL and hs‐CRP Levels With Poor Functional Outcomes at 90 Days and 1 Year
| Outcomes | Outcomes at 90 d | Outcomes at 1 y | ||||
|---|---|---|---|---|---|---|
| Events, n (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Events, n (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| mRS 3–6 | ||||||
| Group 1 | 118 (5.55) | Reference | Reference | 71 (3.43) | Reference | Reference |
| Group 2 | 3 (2.40) | 0.42 (0.13–1.34) | 0.35 (0.11–1.12) | 3 (2.54) | 0.73 (0.23–2.37) | 0.60 (0.18–1.95) |
| Group 3 | 9 (6.87) | 1.26 (0.62–2.53) | 1.19 (0.59–2.42) | 7 (5.43) | 1.62 (0.73–3.59) | 1.46 (0.65–3.30) |
| Group 4 | 70 (11.33) | 2.17 (1.59–2.97) | 1.90 (1.38–2.61) | 49 (8.09) | 2.48 (1.70–3.61) | 1.98 (1.35–2.93) |
| mRS 2–6 | ||||||
| Group 1 | 189 (8.89) | Reference | Reference | 168 (8.12) | Reference | Reference |
| Group 2 | 10 (8.00) | 0.89 (0.46–1.73) | 0.75 (0.38–1.47) | 8 (6.78) | 0.82 (0.40–1.72) | 0.68 (0.32–1.44) |
| Group 3 | 16 (12.21) | 1.43 (0.83–2.46) | 1.33 (0.77–2.31) | 17 (13.18) | 1.72 (1.01–2.93) | 1.63 (0.94–2.81) |
| Group 4 | 101 (16.34) | 2.00 (1.54–2.60) | 1.76 (1.34–2.30) | 88 (14.52) | 1.92 (1.46–2.53) | 1.62 (1.22–2.16) |
Adjusted odds ratio (95% CI) was calculated after adjusting for age, sex, body mass index, high‐density lipoprotein, history of ischemic stroke, myocardial infarction, hypertension, baseline National Institutes of Health Stroke Scale score, randomized treatment of aspirin alone or clopidogrel with aspirin, and the qualifying events of minor stroke or transient ischemic attack. Patients were classified into 4 groups based on the upper quartile of oxidized low‐density lipoprotein and hs‐CRP (high‐sensitivity C‐reactive protein) levels: group 1: oxidized low‐density lipoprotein ≤28.81 μg/dL and hs‐CRP ≤4.20 mg/L; group 2: oxidized low‐density lipoprotein ≤28.81 μg/dL and hs‐CRP >4.20 mg/L; group 3: oxidized low‐density lipoprotein >28.81 μg/dL, hs‐CRP ≤4.20 mg/L; group 4: oxidized low‐density lipoprotein >28.81 μg/dL and hs‐CRP >4.20 mg/L. hs‐CRP indicates high‐sensitivity C‐reactive protein; mRS, modified Rankin Scale; OR, odds ratio; and oxLDL, oxidized low‐density lipoprotein.
Subgroup Analysis
The associations of oxLDL and hs‐CRP with recurrent stroke within 90 days were consistent with respect to the use of statins agents (yes or no) (P for interaction=0.542; Table 5) and the qualifying events of minor stroke or TIA (P for interaction=0.794; Table 6). We found similar results for secondary outcomes of interest (all P for interactions >0.05).
Table 5.
Subgroup Analysis According to Stain Agents Within 90‐Day Follow‐up Period for Associations of oxLDL and hs‐CRP Levels With Clinical Outcomes At 90 Days and 1 Year
| Outcomes | Outcomes within 90 d | Outcomes within 1 y | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | P int | Group 1 | Group 2 | Group 3 | Group 4 | P int | |
| Stroke | ||||||||||
| No | Reference | 0.95 (0.44–2.05) | 1.32 (0.67–2.61) | 1.74 (1.27–2.39) | 0.542 | Reference | 1.03 (0.52–2.04) | 1.32 (0.71–2.45) | 1.61 (1.20–2.17) | 0.645 |
| Yes | Reference | 1.17 (0.47–2.91) | 1.06 (0.43–2.63) | 1.16 (0.73–1.84) | Reference | 1.16 (0.51–2.66) | 0.99 (0.43–2.27) | 1.18 (0.80–1.76) | ||
| Ischemic stroke | ||||||||||
| No | Reference | 0.98 (0.45–2.10) | 1.33 (0.67–2.63) | 1.71 (1.24–2.36) | 0.538 | Reference | 1.06 (0.54–2.09) | 1.35 (0.73–2.50) | 1.58 (1.16–2.13) | 0.653 |
| Yes | Reference | 1.18 (0.48–2.95) | 1.07 (0.43–2.66) | 1.13 (0.71–1.80) | Reference | 1.19 (0.52–2.74) | 1.02 (0.45–2.35) | 1.14 (0.76–1.72) | ||
| Combined vascular events | ||||||||||
| No | Reference | 0.96 (0.44–2.06) | 1.32 (0.67–2.61) | 1.78 (1.29–2.44) | 0.582 | Reference | 1.00 (0.51–1.97) | 1.28 (0.69–2.38) | 1.65 (1.23–2.20) | 0.599 |
| Yes | Reference | 1.17 (0.47–2.91) | 1.06 (0.43–2.63) | 1.21 (0.77–1.91) | Reference | 1.13 (0.49–2.58) | 0.96 (0.42–2.20) | 1.18 (0.80–1.75) | ||
| mRS 3–6 | ||||||||||
| No | Reference | 0.19 (0.03–1.43) | 0.73 (0.22–2.42) | 1.97 (1.29–3.01) | 0.518 | Reference | 1.08 (0.32–3.71) | 0.88 (0.20–3.79) | 2.06 (1.20–3.52) | 0.807 |
| Yes | Reference | 0.57 (0.13–2.44) | 1.65 (0.66–4.11) | 1.80 (1.10–2.94) | Reference | … | 1.95 (0.71–5.34) | 1.89 (1.07–3.34) | ||
| mRS 2–6 | ||||||||||
| No | Reference | 0.48 (0.17–1.37) | 0.87 (0.36–2.08) | 1.84 (1.29–2.62) | 0.261 | Reference | 1.22 (0.55–2.69) | 1.24 (0.54–2.83) | 1.82 (1.25–2.65) | 0.612 |
| Yes | Reference | 1.15 (0.47–2.84) | 1.89 (0.90–3.97) | 1.64 (1.08–2.49) | Reference | … | 1.99 (0.93–4.27) | 1.39 (0.89–2.18) | ||
Adjusted hazards ratio or odds ratio (95% CI) was calculated after adjusting for age, sex, body mass index, high‐density lipoprotein, history of ischemic stroke, myocardial infarction, hypertension, baseline National Institutes of Health Stroke Scale score, randomized treatment of aspirin alone or clopidogrel with aspirin, and the qualifying events of minor stroke or transient ischemic attack. Patients were classified into 4 groups based on the upper quartile of oxidized low‐density lipoprotein (oxLDL) and hs‐CRP (high‐sensitivity C‐reactive protein) levels: group 1: oxLDL ≤28.81 μg/dL and hs‐CRP ≤4.20 mg/L; group 2: oxLDL ≤28.81 μg/dL and hs‐CRP >4.20 mg/L; group 3: oxLDL >28.81 μg/dL, hs‐CRP ≤4.20 mg/L; group 4: oxLDL >28.81 μg/dL and hs‐CRP >4.20 mg/L. Hs‐CRP indicates high‐sensitivity C‐reactive protein; mRS, modified Rankin Scale; oxLDL, oxidized low‐density lipoprotein; P int , P for interaction; and TIA, transient ischemic attack.
Table 6.
Subgroup Analysis According to Qualifying Events for Associations of oxLDL and hs‐CRP Levels With Clinical Outcomes At 90 Days and 1 Year
| Outcomes | Outcomes within 90 d | Outcomes within 1 y | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | P int | Group 1 | Group 2 | Group 3 | Group 4 | P int | |
| Stroke | ||||||||||
| Minor stroke | Reference | 0.98 (0.50–1.94) | 1.28 (0.69–2.37) | 1.58 (1.18–2.13) | 0.794 | Reference | 0.96 (0.52–1.77) | 1.16 (0.66–2.03) | 1.44 (1.10–1.89) | 0.853 |
| TIA | Reference | 1.33 (0.41–4.27) | 0.94 (0.29–3.07) | 1.22 (0.71–2.10) | Reference | 1.61 (0.58–4.46) | 1.16 (0.42–3.22) | 1.33 (0.81–2.17) | ||
| Ischemic stroke | ||||||||||
| Minor stroke | Reference | 1.00 (0.51–1.98) | 1.31 (0.71–2.43) | 1.57 (1.16–2.13) | 0.729 | Reference | 0.99 (0.54–1.83) | 1.20 (0.68–2.12) | 1.42 (1.07–1.87) | 0.857 |
| TIA | Reference | 1.32 (0.41–4.25) | 0.95 (0.29–3.09) | 1.15 (0.66–2.01) | Reference | 1.62 (0.58–4.49) | 1.17 (0.42–3.25) | 1.27 (0.77–2.10) | ||
| Combined vascular events | ||||||||||
| Minor stroke | Reference | 0.98 (0.50–1.94) | 1.28 (0.69–2.37) | 1.61 (1.20–2.17) | 0.840 | Reference | 0.92 (0.50–1.70) | 1.10 (0.63–1.94) | 1.42 (1.09–1.86) | 0.880 |
| TIA | Reference | 1.32 (0.41–4.26) | 0.95 (0.29–3.10) | 1.30 (0.76–2.21) | Reference | 1.61 (0.58–4.45) | 1.17 (0.42–3.26) | 1.47 (0.91–2.37) | ||
| mRS 3–6 | ||||||||||
| Minor stroke | Reference | 0.39 (0.12–1.26) | 1.30 (0.61–2.78) | 1.92 (1.35–2.72) | 0.962 | Reference | 0.64 (0.20–2.11) | 1.38 (0.57–3.31) | 1.82 (1.20–2.77) | 0.742 |
| TIA | Reference | … | 0.71 (0.09–5.57) | 1.67 (0.75–3.72) | Reference | … | 2.06 (0.23–18.39) | 2.96 (0.98–8.95) | ||
| mRS 2–6 | ||||||||||
| Minor stroke | Reference | 0.67 (0.32–1.42) | 1.54 (0.86–2.75) | 1.89 (1.41–2.53) | 0.318 | Reference | 0.56 (0.24–1.33) | 1.85 (1.04–3.29) | 1.60 (1.17–2.19) | 0.463 |
| TIA | Reference | 1.38 (0.30–6.26) | 0.45 (0.06–3.49) | 1.12 (0.54–2.33) | Reference | 1.59 (0.33–7.58) | 0.62 (0.08–4.93) | 1.59 (0.77–3.27) | ||
Adjusted hazards ratio or odds ratio (95% CI) was calculated after adjusting for age, sex, body mass index, high‐density lipoprotein, history of ischemic stroke, myocardial infarction, hypertension, baseline National Institutes of Health Stroke Scale score, and randomized treatment of aspirin alone or clopidogrel with aspirin. Patients were classified into 4 groups based on the upper quartile of oxidized low‐density lipoprotein (oxLDL) and hs‐CRP (high‐sensitivity C‐reactive protein) levels: group 1: oxLDL ≤28.81 μg/dL and hs‐CRP ≤4.20 mg/L; group 2: oxLDL ≤28.81 μg/dL and hs‐CRP >4.20 mg/L; group 3: oxLDL >28.81 μg/dL, hs‐CRP ≤4.20 mg/L; group 4: oxLDL >28.81 μg/dL and hs‐CRP >4.20 mg/L. Hs‐CRP indicates high‐sensitivity C‐reactive protein; mRS, modified Rankin Scale; oxLDL, oxidized low‐density lipoprotein; P int , P for interaction; and TIA, transient ischemic attack.
DISCUSSION
In this subgroup analysis of the CHANCE trial, we found that patients with joint high levels of oxLDL and hs‐CRP had higher risk of stroke recurrence, ischemic stroke, combined vascular events, and poor functional outcome at 90 days and 1 year than both low levels of oxLDL and hs‐CRP. Further analysis by changing the cut‐off value of oxLDL to 13.96 μg/dL and hs‐CRP to 3 mg/L in sensitivity analysis showed consistent results.
The role of oxLDL and hs‐CRP in the pathophysiological pathway of stroke has been widely explored in previous studies. Both oxLDL and hs‐CRP are independent predictors of recurrent stroke, ischemic stroke, and combined vascular events after minor stroke or TIA, and may reflect levels of inflammatory response and oxidative stress in the systemic vasculature. 12 , 21 Inflammatory responses and oxidative stress have been associated with atherosclerotic plaque instability in patients with ischemic stroke. Previous studies have found that elevated oxLDL has been associated with increased plaque instability in patients undergoing carotid endarterectomy 22 and high levels of hs‐CRP have also been proved to be significantly associated with stroke recurrence in patients with symptomatic intracranial atherosclerotic stenosis after intracranial stent implantation. 23 In addition, endothelial dysfunction is the first step in the development of an atherosclerotic plaque and local inflammation. 24 OxLDL and hs‐CRP can also affect the clinical outcome of stroke by damaging endothelial cells. 25 , 26 , 27 Zhang et al have shown a positive relationship between elevated level of oxLDL and hs‐CRP in patients with acute coronary syndrome. 14 Subsequently, in their other study, combining oxLDL with hs‐CRP could better predict the prognosis of patients with acute coronary syndrome. 16 A recent animal study found that oxLDL treatment contributes to the inflammatory response and oxidative stress in human aortic endothelial cells, linking oxLDL and C‐reactive protein to atherosclerosis, endothelial dysfunction, oxidative stress, and inflammatory response from a molecular perspective. 28 However, the evidence on the association of combined oxLDL and hs‐CRP with poor prognosis after stroke is limited. In the current study, we used the upper quartile of oxLDL and hs‐CRP to differentiate between high and low risk, and found the association of oxLDL and hs‐CRP and adverse vascular outcomes appears only at both high levels, when both low levels treated as the reference group, which is in accord with other studies on the association of oxLDL and hs‐CRP with coronary events. 14 , 16 Consistent with prior studies which indicated oxLDL and hs‐CRP can independently predict a poorer functional prognosis in patients with minor stroke or TIA, 8 , 29 , 30 , 31 we also found patients with both elevated levels of oxLDL and hs‐CRP had highest risk of poor functional outcome, no matter defined as mRS 3–6 or mRS 2–6. Increased hs‐CRP levels may affect neurological function by activating the complement system and exacerbating cytotoxic damage in brain tissue. 8 , 9 Elevated oxLDL may directly accelerate foam cell formation and promote the development of atherosclerosis. 32 Endothelial cells, an important link in the recovery of the ischemic semidark zone, are also affected by hs‐CRP and oxLDL. 33 All of these factors increase the risk of poor functional prognosis in patients with ischemic stroke. These findings suggested the combination of serum oxLDL and hs‐CRP were powerful biological markers of poor prognosis after stroke or TIA, which would be beneficial for the stroke risk stratification approaches and further identifying individuals in high‐risk. However, although we observed a higher incidence of recurrent stroke, ischemic stroke, and combined vascular events in patients with high oxLDL and low hs‐CRP levels, or high hs‐CRP with low oxLDL levels, than those with both low oxLDL and hs‐CRP levels, the HRs were not significant in this study. This may be partly because of the different definition of the study population, in which we considered the role of both factors at the same time, and the low sample size in the 2 groups. In connection with the existing studies on combined oxLDL and hs‐CRP levels, we speculate on the plausible path‐physiological mechanisms linking them to minor stroke or TIA include the following. High concentrations of oxLDL can induce macrophage differentiation 34 and stimulate endothelial cell production of CRP. 35 CRP forms a positive feedback loop with oxLDL by activating the complement system, continuously enhancing phagocytosis of oxLDL by macrophages and promoting foam cell formation, 32 and causing cellular autophagy under oxidative stress conditions. 36 On the other hand, activation of the inflammatory response also triggers atherosclerotic protective fibrous membrane rupture. 37 Both of these aspects cause atherosclerotic plaque instability. In addition, imbalances in the oxidative‐antioxidant system play an important role in increasing the risk of recurrence, vascular events, and poor functional prognosis in patients with stroke. OxLDL and hs‐CRP together induce endothelial cell damage, 38 , 39 , 40 expose blood to procoagulant tissues, 26 and promote lipoprotein oxidation in a vicious cycle. 36 , 41 , 42 Furthermore, it has been reported that CRP can bind phosphatidylcholine on oxLDL in a calcium‐dependent manner, forming a CRP/oxLDL complex 43 , 44 with positive 43 or negative 44 effects on the progression of atherosclerosis. However, there is no conclusive evidence about the effect of such complexes on atherosclerosis.
Many studies have demonstrated the cholesterol‐lowering, anti‐inflammatory and anti‐oxidant effects of statins, which have a positive prognostic effect on acute ischemic stroke. 45 , 46 Both oxLDL alone and hs‐CRP alone levels were reported to be lower in patients with stroke receiving statin therapy than in those not receiving statin therapy. 45 , 46 In JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin), a clinical trial among apparently healthy people without hyperlipidemia but with elevated hs‐CRP levels, rosuvastatin significantly reduced the incidence of major cardiovascular events. 47 Besides, it was reported that high statin doses could reduce adiponectin's capacity to suppress intracellular cholesteryl ester levels in oxLDL‐loaded macrophages. 48 However, there was no significant interaction between combined oxLDL and hs‐CRP and use of statins agents in our study, which was in line with the previously reported relationship between oxLDL/HDL and statins. 49
Our study had some limitations. First, although most trials use oxLDL‐4E6 antibody to determine oxLDL levels, in fact there may be antibody reaction variation because of potential cross‐reactivity of oxLDL‐4E6 antibodies with natural low‐density lipoprotein and lysine modification of apoB100. 20 Second, oxLDL and hs‐CRP levels were obtained by fasting venous blood collection after admission in all patients, so oxLDL and hs‐CRP levels at acute onset could not be recorded, and potential differences in acute and post‐acute oxLDL and hs‐CRP levels could not be investigated. Third, oxLDL and hs‐CRP levels during follow‐up were not recorded in this study, so we could not assess changes in plasma oxLDL and hs‐CRP levels over time and their effect on stroke outcomes.
CONCLUSIONS
In summary, this substudy of CHANCE trial suggested that joint higher levels of oxLDL and hs‐CRP was associated with increased risk of recurrent stroke, combined vascular events, and poor functional outcome in patients with minor stroke or TIA. The application of this combined test in the clinical practice of stroke diseases may help clinical decision making and further improve the prognosis of patients with stroke.
SOURCES OF FUNDING
This work was supported by National Natural Science Foundation of China (81870905, U20A20358), Beijing Municipal Science & Technology Commission (D171100003017002), Beijing Municipal Administration of Hospitals Incubating Program (PX2020021).
DISCLOSURES
None.
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Anxin Wang, Email: wanganxin@bjtth.org.
Xia Meng, Email: mengxia@ncrcnd.org.cn.
REFERENCES
- 1. Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013;12:1106–1114. doi: 10.1016/s1474-4422(13)70195-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ishigaki Y, Oka Y, Katagiri H. Circulating oxidized LDL: a biomarker and a pathogenic factor. Curr Opin Lipidol. 2009;20:363–369. doi: 10.1097/MOL.0b013e32832fa58d [DOI] [PubMed] [Google Scholar]
- 3. Marchio P, Guerra‐Ojeda S, Vila JM, Aldasoro M, Victor VM, Mauricio MD. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev. 2019;2019:8563845. doi: 10.1155/2019/8563845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, Vujacic‐Mirski K, Helmstädter J, Kröller‐Schön S, Münzel T, et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. 2019;2019:7092151. doi: 10.1155/2019/7092151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, Pan Y, Xu J, Li S, Wang M, Quan K, Meng X, Li H, Lin J, Wang Y, et al. Residual inflammatory risk predicts poor prognosis in acute ischemic stroke or transient ischemic attack patients. Stroke. 2021;52:2827–2836. doi: 10.1161/STROKEAHA.120.033152 [DOI] [PubMed] [Google Scholar]
- 6. Bouki KP, Katsafados MG, Chatzopoulos DN, Psychari SN, Toutouzas KP, Charalampopoulos AF, Sakkali EN, Koudouri AA, Liakos GK, Apostolou TS. Inflammatory markers and plaque morphology: an optical coherence tomography study. Int J Cardiol. 2012;154:287–292. doi: 10.1016/j.ijcard.2010.09.059 [DOI] [PubMed] [Google Scholar]
- 7. Guo D, Zhu Z, Zhong C, Wang A, Xie X, Xu T, Peng Y, Peng H, Li Q, Ju Z, et al. Prognostic metrics associated with inflammation and atherosclerosis signaling evaluate the burden of adverse clinical outcomes in ischemic stroke patients. Clin Chem. 2020;66:1434–1443. doi: 10.1093/clinchem/hvaa201 [DOI] [PubMed] [Google Scholar]
- 8. Li J, Zhao X, Meng X, Lin J, Liu L, Wang C, Wang A, Wang Y, Wang Y, CHANCE Investigators . High‐sensitive C‐reactive protein predicts recurrent stroke and poor functional outcome: subanalysis of the clopidogrel in high‐risk patients with acute nondisabling cerebrovascular events trial. Stroke. 2016;47:2025–2030. doi: 10.1161/STROKEAHA.116.012901 [DOI] [PubMed] [Google Scholar]
- 9. Cai Z, He W, Zhuang FJ, Chen Y. The role of high high‐sensitivity C‐reactive protein levels at admission on poor prognosis after acute ischemic stroke. Int J Neurosci. 2019;129:423–429. doi: 10.1080/00207454.2018.1538139 [DOI] [PubMed] [Google Scholar]
- 10. Surendran A, Zhang H, Winter T, Edel A, Aukema H, Ravandi A. Oxylipin profile of human low‐density lipoprotein is dependent on its extent of oxidation. Atherosclerosis. 2019;288:101–111. doi: 10.1016/j.atherosclerosis.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Xu Z, Wang X, Zheng J, Peng L, Zhou Y, Song Y, Lu Z. Extracellular‐vesicle containing miRNA‐503‐5p released by macrophages contributes to atherosclerosis. Aging. 2021;13:12239–12257. doi: 10.18632/aging.103855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang A, Xu J, Chen G, Wang D, Johnston SC, Meng X, Lin J, Li H, Cao Y, Zhang N, et al. Oxidized low‐density lipoprotein predicts recurrent stroke in patients with minor stroke or TIA. Neurology. 2018;91:e947–e955. doi: 10.1212/WNL.0000000000006118 [DOI] [PubMed] [Google Scholar]
- 13. Hulthe J, Fagerberg B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study). Arterioscler Thromb Vasc Biol. 2002;22:1162–1167. doi: 10.1161/01.atv.0000021150.63480.cd [DOI] [PubMed] [Google Scholar]
- 14. Zhang YC, Wei JJ, Wang F, Chen MT, Zhang MZ. Elevated levels of oxidized low‐density lipoprotein correlate positively with C‐reactive protein in patients with acute coronary syndrome. Cell Biochem Biophys. 2012;62:365–372. doi: 10.1007/s12013-011-9295-0 [DOI] [PubMed] [Google Scholar]
- 15. Holvoet P, Harris TB, Tracy RP, Verhamme P, Newman AB, Rubin SM, Simonsick EM, Colbert LH, Kritchevsky SB. Association of high coronary heart disease risk status with circulating oxidized LDL in the well‐functioning elderly: findings from the health, aging, and body composition study. Arterioscler Thromb Vasc Biol. 2003;23:1444–1448. doi: 10.1161/01.Atv.0000080379.05071.22 [DOI] [PubMed] [Google Scholar]
- 16. Zhang YC, Tang Y, Chen Y, Huang XH, Zhang M, Chen J, Sun YG, Li YG. Oxidized low‐density lipoprotein and C‐reactive protein have combined utility for better predicting prognosis after acute coronary syndrome. Cell Biochem Biophys. 2014;68:379–385. doi: 10.1007/s12013-013-9718-1 [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Johnston SC, Investigators C. Rationale and design of a randomized, double‐blind trial comparing the effects of a 3‐month clopidogrel‐aspirin regimen versus aspirin alone for the treatment of high‐risk patients with acute nondisabling cerebrovascular event. Am Heart J. 2010;160:380–386.e1. doi: 10.1016/j.ahj.2010.05.017 [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11–19. doi: 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Pan Y, Zhao X, Li H, Wang D, Johnston SC, Liu L, Meng X, Wang A, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (CHANCE) trial: one‐year outcomes. Circulation. 2015;132:40–46. doi: 10.1161/CIRCULATIONAHA.114.014791 [DOI] [PubMed] [Google Scholar]
- 20. Itabe H, Ueda M. Measurement of plasma oxidized low‐density lipoprotein and its clinical implications. J Atheroscler Thromb. 2007;14:1–11. doi: 10.5551/jat.14.1 [DOI] [PubMed] [Google Scholar]
- 21. Coveney S, Murphy S, Belton O, Cassidy T, Crowe M, Dolan E, de Gaetano M, Harbison J, Horgan G, Marnane M, et al. Inflammatory cytokines, high‐sensitivity C‐reactive protein, and risk of one‐year vascular events, death, and poor functional outcome after stroke and transient ischemic attack. Int J Stroke. 2022;17:163–171. doi: 10.1177/1747493021995595 [DOI] [PubMed] [Google Scholar]
- 22. Uno M, Kitazato KT, Suzue A, Itabe H, Hao L, Nagahiro S. Contribution of an imbalance between oxidant‐antioxidant systems to plaque vulnerability in patients with carotid artery stenosis. J Neurosurg. 2005;103:518–525. doi: 10.3171/jns.2005.103.3.0518 [DOI] [PubMed] [Google Scholar]
- 23. Yu Y, Yan L, Lou Y, Cui R, Kang K, Jiang L, Mo D, Gao F, Wang Y, Lou X, et al. Multiple predictors of in‐stent restenosis after stent implantation in symptomatic intracranial atherosclerotic stenosis. J Neurosurg. 2021;1‐10:1716–1725. doi: 10.3171/2021.6.Jns211201 [DOI] [PubMed] [Google Scholar]
- 24. Paffen E, Demaat M. C‐reactive protein in atherosclerosis: a causal factor? Cardiovasc Res. 2006;71:30–39. doi: 10.1016/j.cardiores.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 25. Tsai NW, Hung SH, Huang CR, Chang HW, Chang WN, Lee LH, Wang HC, Lin YJ, Lin WC, Cheng BC, et al. The association between circulating endothelial progenitor cells and outcome in different subtypes of acute ischemic stroke. Clin Chim Acta. 2014;427:6–10. doi: 10.1016/j.cca.2013.09.029 [DOI] [PubMed] [Google Scholar]
- 26. Obermayer G, Afonyushkin T, Binder CJ. Oxidized low‐density lipoprotein in inflammation‐driven thrombosis. J Thromb Haemost. 2018;16:418–428. doi: 10.1111/jth.13925 [DOI] [PubMed] [Google Scholar]
- 27. Shantikumar S, Grant PJ, Catto AJ, Bamford JM, Carter AM. Elevated C‐reactive protein and long‐term mortality after ischaemic stroke: relationship with markers of endothelial cell and platelet activation. Stroke. 2009;40:977–979. doi: 10.1161/strokeaha.108.525105 [DOI] [PubMed] [Google Scholar]
- 28. Zhu B, Liu W, Xu Q, Liu HL. MicroRNA‐486‐5p functions as a diagnostic marker for carotid artery stenosis and prevents endothelial dysfunction through inhibiting inflammation and oxidative stress. Bioengineered. 2022;13:8667–8675. doi: 10.1080/21655979.2022.2054500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsai NW, Chang YT, Huang CR, Lin YJ, Lin WC, Cheng BC, Su CM, Chiang YF, Chen SF, Huang CC, et al. Association between oxidative stress and outcome in different subtypes of acute ischemic stroke. BioMed research international. 2014;2014:256879. doi: 10.1155/2014/256879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang A, Yang Y, Su Z, Yue W, Hao H, Ren L, Wang Y, Cao Y, Wang Y. Association of oxidized low‐density lipoprotein with prognosis of stroke and stroke subtypes. Stroke. 2017;48:91–97. doi: 10.1161/STROKEAHA.116.014816 [DOI] [PubMed] [Google Scholar]
- 31. Wang A, Zhang X, Li S, Zhao X, Liu L, Johnston SC, Meng X, Lin J, Zuo Y, Li H, et al. Oxidative lipoprotein markers predict poor functional outcome in patients with minor stroke or transient ischaemic attack. Eur J Neurol. 2019;26:1082–1090. doi: 10.1111/ene.13943 [DOI] [PubMed] [Google Scholar]
- 32. Marnell L, Mold C, Du Clos TW. C‐reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104–111. doi: 10.1016/j.clim.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 33. Nitzsche A, Poittevin M, Benarab A, Bonnin P, Faraco G, Uchida H, Favre J, Garcia‐Bonilla L, Garcia MCL, Léger PL, et al. Endothelial S1P(1) signaling counteracts infarct expansion in ischemic stroke. Circ Res. 2021;128:363–382. doi: 10.1161/circresaha.120.316711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin‐Ventura JL, Rodrigues‐Diez R, Martinez‐Lopez D, Salaices M, Blanco‐Colio LM, Briones AM. Oxidative stress in human atherothrombosis: sources, markers and therapeutic targets. Int J Mol Sci. 2017;18:2315. doi: 10.3390/ijms18112315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stancel N, Chen CC, Ke LY, Chu CS, Lu J, Sawamura T, Chen CH. Interplay between CRP, atherogenic LDL, and LOX‐1 and its potential role in the pathogenesis of atherosclerosis. Clin Chem. 2016;62:320–327. doi: 10.1373/clinchem.2015.243923 [DOI] [PubMed] [Google Scholar]
- 36. Carresi C, Mollace R, Macri R, Scicchitano M, Bosco F, Scarano F, Coppoletta AR, Guarnieri L, Ruga S, Zito MC, et al. Oxidative stress triggers defective autophagy in endothelial cells: role in atherothrombosis development. Antioxidants (Basel). 2021;10:387. doi: 10.3390/antiox10030387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Libby P. Collagenases and cracks in the plaque. J Clin Invest. 2013;123:3201–3203. doi: 10.1172/JCI67526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khatana C, Saini NK, Chakrabarti S, Saini V, Sharma A, Saini RV, Saini AK. Mechanistic insights into the oxidized low‐density lipoprotein‐induced atherosclerosis. Oxid Med Cell Longev. 2020;2020:5245308. doi: 10.1155/2020/5245308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang W, Speiser JL, Ye F, Tsai MY, Cainzos‐Achirica M, Nasir K, Herrington DM, Shapiro MD. High‐sensitivity C‐reactive protein modifies the cardiovascular risk of lipoprotein(a): multi‐ethnic study of atherosclerosis. J Am Coll Cardiol. 2021;78:1083–1094. doi: 10.1016/j.jacc.2021.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Daub K, Seizer P, Stellos K, Kramer BF, Bigalke B, Schaller M, Fateh‐Moghadam S, Gawaz M, Lindemann S. Oxidized LDL‐activated platelets induce vascular inflammation. Semin Thromb Hemost. 2010;36:146–156. doi: 10.1055/s-0030-1251498 [DOI] [PubMed] [Google Scholar]
- 41. Sakakura K, Nakano M, Otsuka F, Ladich E, Kolodgie FD, Virmani R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013;22:399–411. doi: 10.1016/j.hlc.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 42. Qiao J, Arthur JF, Gardiner EE, Andrews RK, Zeng L, Xu K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol. 2018;14:126–130. doi: 10.1016/j.redox.2017.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tabuchi M, Inoue K, Usui‐Kataoka H, Kobayashi K, Teramoto M, Takasugi K, Shikata K, Yamamura M, Ando K, Nishida K, et al. The association of C‐reactive protein with an oxidative metabolite of LDL and its implication in atherosclerosis. J Lipid Res. 2007;48:768–781. doi: 10.1194/jlr.M600414-JLR200 [DOI] [PubMed] [Google Scholar]
- 44. Singh SK, Agrawal A. Functionality of C‐Reactive protein for atheroprotection. Front Immunol. 2019;10:1655. doi: 10.3389/fimmu.2019.01655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tsai NW, Lee LH, Huang CR, Chang WN, Chang YT, Su YJ, Chiang YF, Wang HC, Cheng BC, Lin WC, et al. Statin therapy reduces oxidized low density lipoprotein level, a risk factor for stroke outcome. Critical care (London, England). 2014;18:R16. doi: 10.1186/cc13695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kitagawa K, Hosomi N, Nagai Y, Kagimura T, Ohtsuki T, Origasa H, Minematsu K, Uchiyama S, Nakamura M, Matsumoto M. Reduction in high‐sensitivity C‐reactive protein levels in patients with ischemic stroke by statin treatment: Hs‐CRP sub‐study in J‐STARS. J Atheroscler Thromb. 2017;24:1039–1047. doi: 10.5551/jat.39354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 48. Gasbarrino K, Hafiane A, Zheng H, Daskalopoulou SS. Intensive statin therapy compromises the adiponectin‐adipor pathway in the human monocyte‐macrophage lineage. Stroke. 2019;50:3609–3617. doi: 10.1161/strokeaha.119.026280 [DOI] [PubMed] [Google Scholar]
- 49. Wang A, Li S, Zhang N, Dai L, Zuo Y, Wang Y, Meng X, Wang Y. Oxidized low‐density lipoprotein to high‐density lipoprotein ratio predicts recurrent stroke in minor stroke or transient ischemic attack. Stroke. 2018;49:2637–2642. doi: 10.1161/STROKEAHA.118.022077 [DOI] [PubMed] [Google Scholar]


