Abstract
Background
Spinal cord ischemia (SCI) remains a devastating complication after aortic dissection or repair. A primary hypoxic damage is followed by a secondary damage resulting in further cellular loss via apoptosis. Affected patients have a poor prognosis and limited therapeutic options. Shock wave therapy (SWT) improves functional outcome, neuronal degeneration and survival in murine spinal cord injury. In this first‐in‐human study we treated 5 patients with spinal cord ischemia with SWT aiming to prove safety and feasibility.
Methods and Results
Human neurons were subjected to ischemic injury with subsequent SWT. Reactive oxygen species and cellular apoptosis were quantified using flow cytometry. Signaling of the antioxidative transcription factor NRF2 (nuclear factor erythroid 2‐related factor 2) and immune receptor Toll‐like receptor 3 (TLR3) were analyzed. To assess whether SWT act via a conserved mechanism, transgenic tlr3 −/− zebrafish created via CRISPR/Cas9 were subjected to spinal cord injury. To translate our findings into a clinical setting, 5 patients with SCI underwent SWT. Baseline analysis and follow‐up (6 months) included assessment of American Spinal Cord Injury Association (ASIA) impairment scale, evaluation of Spinal Cord Independence Measure score and World Health Organization Quality of Life questionnaire. SWT reduced the number of reactive oxygen species positive cells and apoptosis upon ischemia via induction of the antioxidative factor nuclear factor erythroid 2‐related factor 2. Inhibition or deletion of tlr3 impaired axonal growth after spinal cord lesion in zebrafish, whereas tlr3 stimulation enhanced spinal regeneration. In a first‐in‐human study, we treated 5 patients with SCI using SWT (mean age, 65.3 years). Four patients presented with acute aortic dissection (80%), 2 of them exhibited preoperative neurological symptoms (40%). Impairment was ASIA A in 1 patient (20%), ASIA B in 3 patients (60%), and ASIA D in 1 patient (20%) at baseline. At follow‐up, 2 patients were graded as ASIA A (40%) and 3 patients as ASIA B (60%). Spinal cord independence measure score showed significant improvement. Examination of World Health Organization Quality of Life questionnaires revealed increased scores at follow‐up.
Conclusions
SWT reduces oxidative damage upon SCI via immune receptor TLR3. The first‐in‐human application proved safety and feasibility in patients with SCI. SWT could therefore become a powerful regenerative treatment option for this devastating injury.
Keywords: shock wave therapy, spinal cord ischemia, spinal cord regeneration, Toll‐Like receptor 3
Subject Categories: Vascular Disease, Translational Studies, Cardiovascular Surgery
Nonstandard Abbreviations and Acronyms
- ASIA
American Spinal Cord Injury Association
- IRI
ischemia reperfusion injury
- NRF2
nuclear factor erythroid 2‐related factor 2
- SCI
spinal cord ischemia
- SCIM
Spinal Cord Independence Measure
- SWT
shock wave therapy
- TLR3
Toll‐like receptor 3
- WHOQOL
World Health Organization Quality of Life
Clinical Perspective.
What Is New?
The mechanical stimulus of shock wave therapy is neuroprotective after ischemic injury of the spinal cord.
It is at least partly mediated via mitigation of oxidative damage via the crucial transcription factor nuclear factor erythroid 2‐related factor 2 and depends on innate immune receptor Toll‐like receptor 3.
A first‐in‐human application showed safety and feasibility of shock wave therapy in patients with spinal cord ischemia.
What Are the Clinical Implications?
This new treatment option might constitute a potent novel therapeutic approach to alleviate oxidative damage; reduce secondary neuronal damage; and thus, preserve spinal cord tissue and function in patients with spinal cord ischemia.
Approximately 50 000 patients per year die from aortic disease in the United States. 1 Dilatation of the aorta with subsequent thinning of the aortic wall due to genetic predisposition and/or predisposing risk factors including hypertension or smoking can result in aortic dissection or rupture. 2 Currently, surgical replacement of the dilated aorta or endovascular repair is performed to avoid dissection or rupture. Paraplegia attributable to spinal cord ischemia (SCI) remains a devastating complication after spinal malperfusion because of dissection, surgery, or intervention without any effective treatment option. 3 Spinal blood supply is mainly provided by (1) the anterior spinal artery and a pair of posterior spinal arteries all of which are arising from the vertebral artery, and (2) paired intercostal and lumbar segmental arteries originating from the aorta itself, and (3) paraspinal collateral network. Depending on the type and location of the aneurysm or dissection, SCI was reported in 15% of Type I, 31% of Type II, 7% of Type III, and 4% of Type IV aneurysms according to the Crawford classification in early reports. 4 Over the last decades, improved surgical techniques and implication of preoperative preventive strategies to reduce the occurrence of SCI and to improve results have been established. 5 Left heart bypass with moderate or deep hypothermia is preferred over circulatory arrest, and reimplantation of segmental arteries (with special regard to the Adamkiewicz artery) is performed. 6 , 7 However, the success of reimplantation of segmental arteries is not well proven. Near‐infrared spectroscopy is used to detect potential ischemia at early stage. 8 , 9 Cerebrospinal fluid drainage to prevent spinal compartment syndrome upon ischemia has proven useful. 10 Moreover, the concept of ischemic preconditioning of the spinal cord via minimally invasive artery embolization has shown promising results. 11 Altogether, the implication of all the described perioperative efforts has markedly decreased the incidence of SCI to 3.3% for Type I, 6.3% for Type II, 2.6% for Type III and 1.4% for Type IV aneurysms. 12
The complexity of aortic repair, existence of acute aortic dissection, and the degree of urgency represent independent risk factors for the occurrence of SCI. 12 In the largest available case series of 116 patients with SCI, the outcome of affected patients was still devastating: 23% of the patients had died after 3 years and 42% required a wheelchair. Neurological impairment at nadir was the most significant predictor for prognosis. 13
Malperfusion of the spinal cord attributable to sudden disruption of blood supply during dissection, surgery or intervention results in necrotic and apoptotic cell death of neurons and glial cells. Release of cytoplasmic content further initiates inflammatory response and the release of proinflammatory cytokines. 14 Thus, adjacent resident astrocytes and pericytes are activated further promoting infiltration of immune cells and fibroblasts resulting in glial scar formation at the site of damage. 14 , 15 This secondary damage caused by the excitotoxic effects of inflammatory mediators results in further cellular loss via apoptosis hours to days after the primary ischemic event being responsible for the occurrence of ‘delayed‐paraplegia’. 11 Mitigation of apoptosis and thus, secondary spinal cord damage results in decreased lesion size and improved neuronal outcome in animal models preventing from the onset of delayed‐paraplegia. 16
Numerous strategies to regenerate spinal cord tissue have been investigated extensively including stem cell treatment, 17 enhancing myelination, limiting secondary damage, functional reorganization of spinal circuits, 18 inhibition of growth inhibitors (eg, neurite outgrowth inhibition protein 19 or chondritin sulfate proteoglycans 20 ), influencing astrocytic scar 21 and delivery of axonal growth factors. 22 Despite encouraging first results, none of the methods is yet available in routine clinical application. Thus, there is an urgent clinical need to alleviate the burden of SCI for affected patients to improve symptoms, quality of life, and outcomes.
Similar to neurons, cardiomyocytes are postmitotic cells with very limited regenerative capacity. Our group showed the efficacy of shock wave therapy (SWT) for the regeneration of ischemic myocardium via induction of angiogenesis 23 and inhibition of apoptosis via activation of mitogen‐activated p42/44 extracellular‐signal regulated kinase (ERK)1/2 and Akt/protein kinase B. 24 Treatment reduced dysfunctional scar tissue and even improved cardiac function via activation of innate immune receptor Toll‐like receptor 3 (TLR3). 25 , 26 A pilot trial extending the indication of SWT proved efficacy in a murine model of SCI, improving functional outcome, neuronal degeneration and even survival. 27 Following up on this study, we found striking effects of SWT on spinal cord regeneration even in traumatic spinal cord injury in a mouse model. 15
In this project, we aimed to translate our promising preclinical results to achieve a clinical perspective to patients suffering from SCI. In this first‐in‐human study we treated 5 patients suffering from SCI because of aortic dissection or surgery with SWT aiming to reduce secondary spinal damage upon ischemia and prove safety and feasibility of the new treatment option.
Methods
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Cell Culture and Experimental Protocols
SH‐SY5Y cells (ATCC) were cultivated and maintained in DMEM (ThermoFisher, Waltham, MA) with 4.5 g/L glucose with 10% FCS (Sigma‐Aldrich, St. Louis, MO) and 1% penicillin/streptomycin solution (Sigma‐Aldrich, St Louis, MO). SWT was performed in a specifically designed water bath as described previously. 28 For inhibition of TLR3, a commercially available TLR3/dsRNA complex inhibitor (Merck, Darmstadt, Germany) was used at a concentration of 10 μg/mL. Activation of TLR3 was induced using TLR3 agonist Polyinosinic:polycytidylic acid (Poly(I:C); Invivogen, San Diego, CA) at a concentration of 10 μg/mL. Oxygen/glucose deprivation was performed as described previously. 29 Briefly, SH‐SY5Y cells were pretreated with either Poly(I:C) or SWT. Subsequently, media was exchanged to glucose‐free DMEM, preconditioned in anoxia to minimize dissolved oxygen levels. Cells were further placed in an anaerobic chamber to produce oxygen deprivation. Upon 4 hours, media were again exchanged towards standard cell culture medium and cells were maintained under normoxic conditions for 24 hours.
RNA Extraction and RT‐PCR
Total RNA was extracted by using a Monarch Total RNA Miniprep Kit (New England Biolabs, Ipswich, MA) according to manufacturer's instructions. Integrity of RNA was assessed by using a NanoDrop 2000c spectrophotometer and RT‐PCR was performed with ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Foster City, CA). Specific gene expression was expressed as 2−ΔCT formula by normalizing to the housekeeping gene GAPDH. Following primer sequences were used:
| NRF2 | fwd | 5'‐TCCACAGCTCATCATGATGGAC |
| rev | 5'‐TACTCTTTCCGTCGCTGACTG | |
| HO‐1 | fwd | 5'‐TTTCAGAAGGGCCAGGTGAC |
| rev | 5'‐TTGTTGCGCTCAATCTCCTC |
Western Blot
Total protein from cell lysates were extracted by a subcellular protein fractionation kit (ThermoFisher, Waltham, MA) according to manufacturer's instructions. Protein was separated with 10% SDS‐polyacrylamide gels and transferred to nitrocellulose membranes. Upon blocking with 5% BSA in 0.1% Tween/TBS, membranes were incubated with primary antibodies.
Following antibodies were used:
| NRF2 | Cell Signaling, Danvers, MA; #12721 |
| ß‐actin | Sigma‐Aldrich, St. Louis, MO; Clone:AC‐15 |
Immunofluorescence Staining
Cells were fixed in ice‐cold 100% methanol for 5 minutes and washed with PBS. Cells were further blocked with 10% goat serum in PBS for 30 minutes and then incubated with anti‐NRF2 (nuclear factor erythroid 2‐related factor 2) antibody (Abcam, Cambridge, UK; ab31163) for 1 hour at a dilution of 1:100 in 2% BSA/PBS. Upon careful washing with PBS, cells were incubated in the dark with secondary antibody (AlexaFluor488, MolecularProbes, Eugene, OR) for 30 minutes. Counterstaining was performed with DAPI and cells were imaged with a LSM980 confocal microscope (Zeiss, Jena, Germany).
Annexin V/7‐Aminoactinomycin D
To assess necrotic and apoptotic cell death upon oxygen/glucose deprivation, we performed Annexin V/ 7‐aminoactinomycin D staining as described previously. 30 Briefly, collected cells were washed with Annexin V binding buffer and incubated with FITC‐conjugated Annexin V (MolecularProbes, Eugene, OR) for 15 minutes at a concentration of 2.5 μg/mL. Upon washing in Annexin V binding buffer, cells were incubated with 7‐aminoactinomycin D at a concentration of 2 μg/mL for another 15 minutes. Analysis was performed by flow cytometry with a FACSCalibur device (BD biosciences, Franklin Lakes, NJ).
ROS Detection
To assess oxidative stress, reactive oxygen species (ROS) were detected by 2′,7′ –dichlorofluorescin diacetate (H2DCFDA, Abcam, Cambridge, UK). Collected cells were incubated with H2DCFDA for 30 minutes and immediately thereafter analyzed by flow cytometry.
Zebrafish Model
To generate a tlr3 −/− zebrafish line, synthetic guide RNAs (sgRNA) were designed to target Exon 2 and Exon 4 of tlr3, using the tool chopchop. Melanocyte inducing transcription factor A wild‐type embryos were co‐injected with the 2 sgRNAs (5 ng/μL) and 40 ng/μL Cas9 mRNA. Adult G0‐fish were in‐crossed to identify carriers of the modification. tlr3 +/− F1‐fish were in‐crossed, to yield tlr3 −/− F2‐fish. Genotyping was performed on polymerase chain reaction products spanning Exon 2 and Exon 4.
Following sgRNA and primers were used:
| Genotyping (forward) | 5'‐GCACTACAAATGCACGCAAG |
| Genotyping (reverse 1) | 5'‐CACACCAAACGTAGCCCTTT |
| Genotyping (reverse 2) | 5'‐TGGGTTTTCAGGTAATGTCGG |
| sgRNA (exon 2) | 5'‐CACTGGATGTATCTCACACC |
| sgRNA (exon 4) | 5'‐ACTTGTTGATGCCCATGCCC |
For induction of spinal cord injury, 4‐day old (4 dpf) embryos of wild‐type and in‐crossed tlr3 −/− fish were used. Therefore, a traumatic lesion was induced to the spinal cord above the anal pore as described previously. 15 Embryos were left to regenerate in E3‐medium with respective additives (0.1% DMSO, 100 μg/mL Poly(I:C) or 5 μg/mL TLR3 complex inhibitor) for 24 hours. To assess spinal cord regeneration, whole‐mount immunolabeling of embryos with acetylated Tubulin (Sigma‐Aldrich, St.Louis, MO) and glial fibrillary acidic protein (ThermoFisher, Waltham, MA) at a concentration of 1:1000 was performed, and fish were imaged with confocal microscopy using a LSM 5 Exciter microscope (Zeiss, Jena, Germany).
Confocal Image Analyses
Analysis of fish spinal cord injuries was performed as described previously. 15 Briefly, immunostaining was imaged with a Zeiss LSM Exciter 5 using a 40×/0.80 W ACHROPLAN objective (Carl Zeiss, Oberkochen, Germany). Lesion sites were documented as confocal image stacks using standardized settings. To evaluate neuronal volume, acetylated tubulin signals at the lesion site were quantified using the ImageJ Plugin “color pixel counter”. In a first step signal intensities were normalized to motoaxon signals (spinal cord exit points) to compensate for experiment to experiment variation. Next a 313×215px region of interest containing the lesion site and spanning the entire spinal cord was selected to determine number of voxels with signal intensities above 8% of maximum intensity.
Statistical Analysis
All results are expressed as mean±SEM. Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA). Statistical significance between multiple groups was calculated using Kruskal–Wallis test or 1‐way ANOVA. Statistical comparisons between 2 groups were performed by Student t‐ or Mann–Whitney U test as appropriate. P<0.05 was considered as statistically significant. All analyses were exploratory in nature.
Patient Treatment and Data
Off‐label use of SWT in ischemic spinal cord injury was approved by the institutional ethical board. Patients were included upon the clinical diagnosis of spinal cord ischemia confirmed via neurologic assessment and spinal magnetic resonance imaging (MRI). Patients were informed about the option of an off‐label use of SWT and informed consent was obtained. The clinical application was approved by an institutional review committee.
Baseline analysis before treatment included assessment of the patient's American Spinal Cord Injury Association (ASIA) impairment scale (performed by a neurologist), evaluation of Spinal Cord Independence Measure (SCIM) score, and the World Health Organization Quality of Life (WHOQOL) questionnaire. Patients were treated thereafter with spinal SWT once a week for 6 consecutive weeks. Therapy was applied at the height of lesion including 5 cranial and 5 distal segments. End point analysis was performed 6 months after the first therapy including MRI scan, ASIA scale, SCIM score, and WHOQOL questionnaire.
SWT was performed with a commercially available extracorporeal shock wave device (Flashwave, Konstanz, Germany) once a week for 6 weeks. Treatments included each time 1000 impulses on both paravertebral sites, covering 5 segments above and below the determined level of injury. Neurological level of injury was assessed via MRI as well as by physical examination by a neurologist according to the ASIA‐scoring system. Subjective percepts of the participants on their independence and quality of life were assessed by obtaining the SCIM‐Score as well as the WHOQOL‐questionnaire.
Results
SWT Reduces Oxidative Damage and Neuronal Apoptosis via NRF2
In a first series of experiments, we aimed to determine whether SWT protects from neuronal cell death upon ischemia/reperfusion injury (IRI). ROS are generated during IRI and are able to initiate apoptosis upon IRI. 31 We subjected a human neuroblastoma cell line (SH‐SY5Y) to ischemic injury and treated the cells with SWT. Treatment reduced the number of ROS positive cells (oxygen/glucose deprivation 2.308±0.93 versus SWT 1.681±0.5376; P=0.0035; n=4) (Figure 1A) and resulted in a significant reduction of apoptotic cells upon IRI (Control 2.274±2.498 versus oxygen/glucose deprivation 8.884±4.686 versus SWT 5.022±3.026; P=0.0003; n=12–16) (Figure 1B). SWT activates innate immune receptor TLR3, 15 , 25 and TLR3 induces the antioxidative transcription factor NRF2, protecting against oxidative damage. 32 , 33 Thus, we presumed that a TLR3‐dependent induction of NRF2 might be responsible for the neuroprotective effect upon IRI. Both the TLR3 agonist Poly(I:C) and SWT induced expression of NRF2 protein (Control 9.650±5.478 versus Poly(I:C) 39.45±18.48 versus SWT 49.03±19.61 versus SWT + Inhibitor 17.94±6.821; F (3, 16)=8.364; P=0.0014; n=5) (Figure 1C and 1D) and mRNA levels (Control 1±0.1385 versus Poly(I:C) 1.841±0.1973 versus SWT 1.671±0.7202 versus SWT + Inhibitor 1.386±0.1312; F (3, 19)=5.208; P=0.0086; n=5–6) (Figure 1E), whereas inhibition of TLR3 before SWT abolished the upregulation of the transcription factor. The cytoplasmatic transcription factor NRF2 translocates into the nucleus inducing the oxidative stress mitigating factor HO‐1 (heme oxygenase‐1). 34 Again, SWT induced HO‐1 in a TLR3‐dependent manner (Control 1±0.3180 versus Poly(I:C) 1.801±0.1970 versus SWT 1.752±0.5315 versus SWT + Inhibitor 1.245±0.05788; F (3, 17)=6.784; P=0.0033; n=4–6) (Figure 1F). Moreover, both TLR3 agonists Poly(I:C) and SWT induced NRF2 upon IRI in vitro, indicating its crucial role in the reduction of oxidative damage upon SWT (Figure 1G).
Figure 1. Shock wave therapy (SWT) reduces oxidative damage and neuronal apoptosis via NRF2.
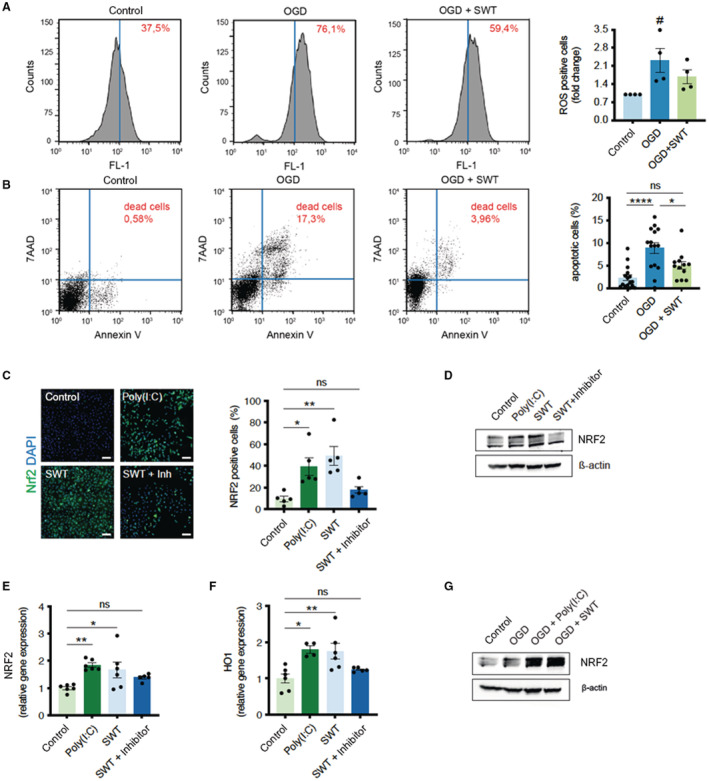
A, SH‐SY5Y cells underwent oxygen/glucose deprivation for 4 hours. After 24 hours of reoxygenation, the amount of reactive oxygen species was detected by H2DCFDA via FACS analysis. Shock wave–treated cells showed reduced oxidative stress. Data are means±SEM. # P<0.05. n=4. B, FACS analysis of Annexin V/PI staining to determine apoptotic and necrotic cell death upon oxygen/glucose deprivation. Shock wave–treated cells showed greater survival in this setting. Data are means±SEM. *P<0.05; ****P<0.0001. n=16 (Control), n=15 (OGD), n=12 (OGD + SWT). C, Immunofluorescence staining revealed an increased number of NRF2 positive SH‐SY5Y cells after treatment with shock waves or Toll‐like receptor 3 agonist Poly(I:C). Addition of a Toll‐like receptor 3 inhibitor abolished shock wave‐mediated NRF2 expression. Scale bar: 100 μm. Data are means±SEM. *P<0.05; **P<0.01. n=5. D, Immunoblot analysis of NRF2 protein expression upon treatment with shock waves and Poly(I:C). Inhibition of TLR3 prevented NRF2‐expression upon SWT. E and F, Quantitative polymerase chain reaction analysis revealed increased gene expression levels of NRF2 and its downstream target HO‐1 (heme oxygenase‐1) upon SWT and treatment with TLR3 agonist Poly(I:C). Data are means±SEM. *P<0.05; **P<0.01. n=6. G, SH‐SY5Y cells undergoing oxygen/glucose deprivation showed increased protein expression of antioxidative NRF2 when pretreated with shock waves or Poly(I:C). Statistical comparison by ranks: Kruskal–Wallis test (A). Statistical comparisons between multiple groups: 1‐way ANOVA with Tukey post hoc analysis (B, C, E, F). OGD indicates oxygen/glucose deprivation; and SWT, shock wave therapy.
Zebrafish Regenerate Spinal Cord Injury via TLR3
Zebrafish show remarkable regenerative capacities of spinal cord injuries being able to induce axonal growth and full restoration of spinal function and are therefore studied frequently to elucidate conserved spinal regeneration mechanisms. 15 , 35 , 36 To analyze whether SWT operates via a conserved mechanism of spinal regeneration, zebrafish were subjected to spinal cord injury followed by treatment with a TLR3 inhibitor or a TLR3 agonist. Stimulation of TLR3 resulted in improved spinal cord regeneration, whereas treatment with a TLR3 inhibitor impaired axonal growth (DMSO 54401±7879 (n=11) versus Poly(I:C) 58 967±3494 (n=8) versus TLR3‐inhibitor 47 234±5760 (n=10); F (2, 26)=8.253; P=0.0017) (Figure 2A). To further confirm these results, we created transgenic tlr3 knockout fish using CRISPR/Cas9 and repeated the experiment (Figure 2B). In line with our previous results, tlr3 −/− fish showed decreased neuron volume within the lesion compared with wild‐type fish (wild‐type 49583±6512 (n=13) versus tlr3 −/− 36 829±7184 (n=20); P<0.0001) (Figure 2C). These findings support the hypothesis that TLR3‐mediated spinal cord regeneration represents an evolutionary conserved mechanism.
Figure 2. Zebrafish regenerate spinal cord injury via Toll‐like receptor 3 (TLR3).
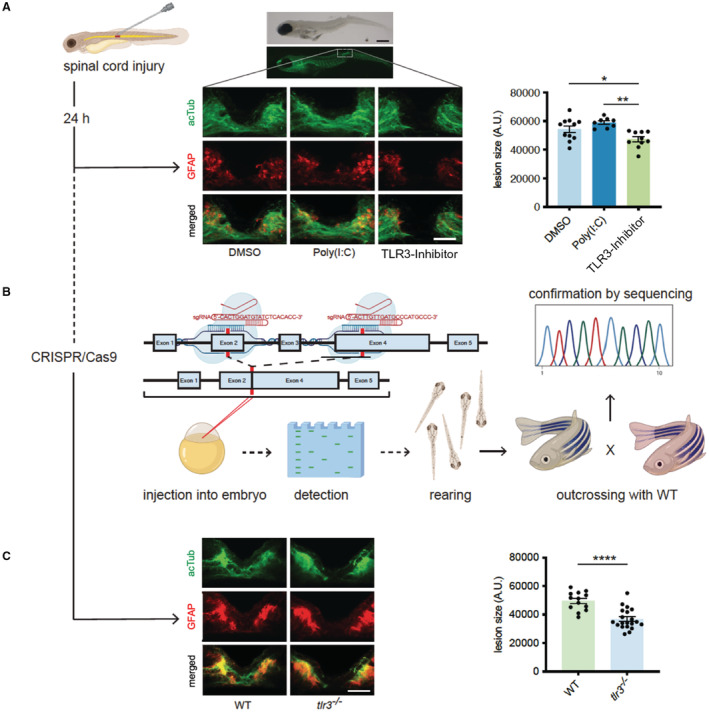
A, Zebrafish larvae (4dpf) underwent traumatic spinal cord injury. After 24 hours, regeneration of spinal cord was assessed via immunofluorescence staining of acetylated Tubulin and glial fibrillary acidic protein. Inhibition of tlr3 prevents neuronal regeneration in zebrafish upon spinal cord injury. Scale bar: 500 μm (top); 50 μm (bottom). Data are means±SEM. *P<0.05; **P<0.01. n=11 (DMSO), n=8 (Poly(I:C)), n=10 (TLR3‐Inhibitor). B, TLR3‐deficient zebrafish were generated as illustrated using CRISPR/Cas9. C, Zebrafish lacking tlr3 failed neuronal regeneration upon spinal cord injury. Data are means±SEM. ****P<0.0001 n=13 (wild‐type), n=20(tlr3 −/−). Statistical comparisons between multiple groups: 1‐way ANOVA with Tukey post hoc analysis (A). Statistical comparisons between 2 groups: Student t‐test (B). acTUB, acetylated Tubulin; DMSO, dimethyl sulfoxide; GFAP, glial fibrillary acidic protein; TLR3, Toll‐like receptor 3; and WT, wild‐type.
First‐in‐Human Application: SWT in Patients With SCI
To translate our previous findings into a clinical setting, we obtained ethical approval from the institutional ethics board and treated 5 patients suffering from spinal cord ischemia after aortic surgery with SWT. Mean age was 65.3 years, and there were 4 male (80%) and 1 female patients (20%). All patients had a history of hypertension, 3 patients had a history of smoking (60%), and 3 patients were suffering from diabetes (60%). Two patients had a diagnosed underlying aortic pathology (40%). Four patients presented with acute aortic dissection (80%), 2 of them exhibited preoperative neurological symptoms (40%). Mean cardiopulmonary bypass time was 68.8 minutes (68.8±70.9), mean X‐clamp time was 146.8 minutes (146.8±91.2) with mean brain perfusion of 19.4 minutes (19.4±19.0) (Table 1). The initial ischemic spinal cord lesion was at the level of C8 (1 patient), T6 (1 patient), or T12 (3 patients) (Figure 3A). Patients were included upon the clinical diagnosis of spinal cord ischemia confirmed via neurological examination and spinal MRI. Baseline analysis before treatment included assessment of the patient's ASIA impairment scale (performed by a neurologist), evaluation of SCIM and the WHOQOL questionnaire. Patients were treated thereafter with spinal SWT once a week for 6 consecutive weeks (Figure 3B). Therapy was applied at the height of lesion including 5 cranial and 5 distal segments (Figure 3C). End point analysis was performed 6 months after the first therapy including MRI scan, ASIA scale, SCIM score, and WHOQOL questionnaire.
Table 1.
Patient Characteristics
| Parameter | Value |
|---|---|
| Demographics | |
| Mean (SD) age, y | 63.3 (14.9) |
| Men, n (%) | 4 (80) |
| Previous aortic surgery, n (%) | 2 (40) |
| Risk factors | |
| Cerebrovascular family history, n (%) | 0 |
| Previous neurologic dysfunction, n (%) | 0 |
| Hypertension, n (%) | 5 (100) |
| Peripheral vascular disease, n (%) | 0 |
| Chronic heart disease, n (%) | 0 |
| Atrial fibrillation, n (%) | 0 |
| Hypercholesterolemia, n (%) | 0 |
| Smoking, n (%) | 2 (40) |
| Diabetes, n (%) | 2 (40) |
| COPD, n (%) | 0 |
| Degenerative arotic pathology, n (%) | 1 (20) |
| Connective tissue disease, n (%) | 0 |
| Atherosclerosis, n (%) | 0 |
| Surgical characteristics | |
| Urgent, n (%) | 4 (80) |
| Acute dissection, n (%) | 4 (80) |
| Peroperative neurolgical symptoms, n (%) | 2 (40) |
| Mean (SD) cardiopulmonary bypass time, min | 68.8 (70.9) |
| Mean (SD) crossclamp time, min | 146.8 (91.2) |
| Mean (SD) deep hypothermic arrest, min | 22.2 (21.7) |
| Mean (SD) brain perfusion, min | 19.4 (19.0) |
| CSF drainage postoperatively, n (%) | 0 |
COPD indicates chronic obstructive pulmonary disease; CSF, cerebrospinal fluid.
Figure 3. First‐in‐human application: shock wave therapy in patients with spinal cord ischemia.
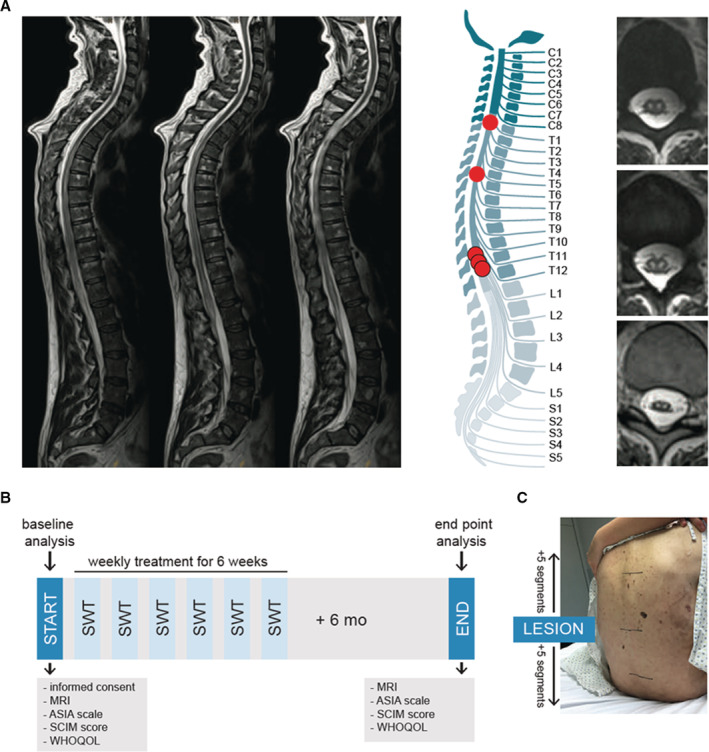
A, Magnetic resonance imaging was used to confirm diagnosis of ischemic spinal cord injury. On the left, sagittal T2‐weighted turbo‐spin echo sequences show a long‐range cord hyperintensity involving the midaspect of the thoracic cord reaching from thoracic vertebra 3 to the expanded conus medullaris. The illustration shows the level of injury of each patient. Abnormal T2 hyperintense spots within the central gray matter (“owl's eye” pattern) are shown on the right side. n=5. B, The illustration shows the applied study protocol. Upon consent of participation, neurological level of injury was assessed via magnetic resonance imaging and physical examination by a neurologist using the Classification of the American Spinal Cord Injury Association scale. Subjective condition of the patients was assessed via the Spinal Cord Independence Measure and the World Health Organization Quality of Life questionnaire. Patients were treated once a week for 6 weeks and end point analysis was done 6 months upon the last treatment. C, Shock wave therapy included 5 segments above and below the defined level of injury. ASIA indicates American Spinal Injury Association; MRI, magnetic resonance imaging; SCIM, Spinal Cord Independence Measure; SWT, shock wave therapy; and WHOQOL, World Health Organization Quality of Life.
SWT Is Safe and Feasible in Patients With SCI
Severity of impairment was evaluated as ASIA A in 1 patient (20%), ASIA B in 3 patients (60%), and ASIA D in 1 patient at baseline (20%) (Figure 4A). At follow‐up, 2 patients were graded as ASIA A (40%) and 3 patients as ASIA C (60%) (Figure 4A). The neurological level of injury changed to level Th1 (1 patient), Th11 (1 patient), Th12 (1 patient), and L1 (2 patients) at follow‐up (Figure 4B). SCIM score showed significant improvement in all aspects including self‐care, respiration, and sphincter management, mobility indoors and outdoors, and mobility in room and toilet manifesting in a higher total SCIM score (baseline 16.8±9.783 versus follow‐up 45.2±12.74; P=0.0042; n=5) (Figure 4B and C). Examination of the WHOQOL questionnaires revealed increased scores in the environment (baseline 28.25±5.737 versus follow‐up 30.40±4.827) and physical health at follow‐up (baseline 15.25±1.893 versus follow‐up 20.60±6.148) (Figure 4D). No side effects of the new treatment option have been observed.
Figure 4. Shock wave therapy is safe and feasible in patients with spinal cord ischemia.
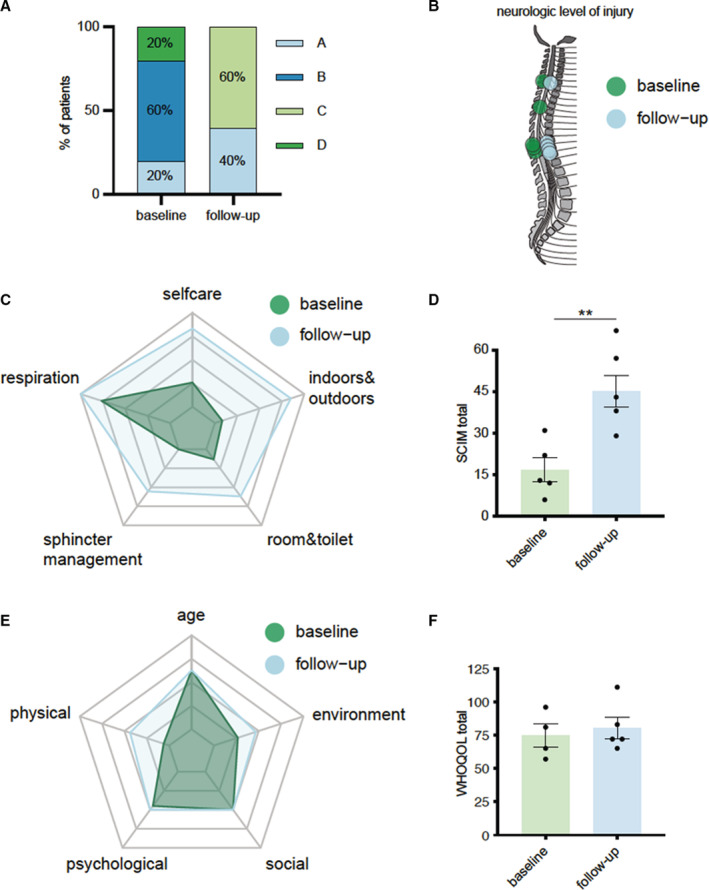
A, Classification of neurological impairment according to the American Spinal Injury Association at baseline and 6 months after therapy. B, Neurological levels of injury at baseline and 6 months after therapy. C, Radar chart comparing baseline levels to follow‐up levels in each category of the Spinal Cord Independence Measure. D, Total scores of Spinal Cord Independence Measure at baseline and 6 months after therapy. Data are means±SEM. **P<0.01. E, Radar chart showing the measurements of each category of the World Health Organization Quality of Life questionnaire. F, Total scores of the World Health Organization Quality of Life questionnaire at baseline and 6 months after therapy. Data are means±SEM. Statistical comparisons between 2 groups: Student t‐test (D). ASIA indicates American Spinal Injury Association; SCIM, Spinal Cord Independence Measure; and WHOQOL, World Health Organization Quality of Life.
Discussion
Spinal cord blood supply is at risk in patients with aortic dissection or in patients undergoing aortic repair. Disruption of blood flow has devastating consequences for affected patients with obscure prognosis. 13 The incidence of SCI after aortic surgery has decreased over the last years, mainly because of improvement of preoperative interventions designed to prevent spinal cord damage. 12 However, if SCI occurs postoperatively, therapeutic options to alleviate the burden of SCI are still limited in spite of remarkable scientific efforts. 3
During spinal cord ischemia, a primary hypoxic damage with neuronal necrosis is followed by a secondary neuronal damage, caused by excitotoxic proinflammatory mediators released upon neuronal cell loss. 37 During this phase, reactive oxygen metabolites are produced, causing apoptosis and further aggravation of the primary spinal cord damage. 38 This secondary injury causes spreading of the lesion to uninjured tissue further exacerbating functional impairment. 39
One strategy to improve outcome in SCI is to prevent the secondary damage and thus, spare functional spinal cord tissue from further injury. 40 However, the therapeutic window for this strategy is narrow, as secondary damage occurs within the first hours to days after spinal cord ischemia. 11 The postoperative diagnosis of SCI remains challenging, as some patients remain intubated for a longer postoperative period because of a complex postoperative course and thus, neurological deficits sometimes only become apparent after the therapeutic window has closed. Therefore, strategies for the early diagnosis of spinal malperfusion like near‐infrared spectroscopy or serum levels of neuron specific enolase are crucial to further improve outcome of patients with SCI. 9 , 41 In this study, patients were treated as soon as SCI was confirmed. Earlier treatment (immediately after the ischemic event) or maybe even prophylactic treatment might even improve SWT effects.
In this study, we provide evidence for a neuroprotective effect of SWT from preclinical data and for the first time translate this to a clinical safety and feasibility examination. The mechanical stimulus of SWT promotes angiogenesis in tissues containing postmitotic cell types including the heart and spinal cord. 42 SWT enhances proliferation and angiogenesis via the release of angiogenic factors including vascular endothelial growth factor, fibroblast growth factor, and placental growth factor and inhibits apoptosis via phosphorylation of ERK and AKT. 24 Moreover, specific extracellular vesicles containing angiogenic cargo are released upon the stimulus promoting tissue regeneration and functional improvement. 23 These effects are mainly mediated via the innate immune receptor TLR3 which is stimulated via extracellular RNA. 25 We show in a recent article that wild‐type animals with traumatic spinal cord injury improved locomotor function upon SWT, whereas Tlr3 −/− animals showed no improvement upon therapy. Treatment caused reduction of scar size and reduced numbers of degenerating neurons via regulation of interleukin‐6. 15
In this study, our purpose was to analyze potential beneficial effects of SWT in ischemic spinal cord injury and to prove safety and feasibility of the clinical application. SWT reduced the formation of ROS upon ischemia resulting in decreased apoptosis via regulation of the antioxidative transcription factor NRF2. Its TLR3‐dependent upregulation induced transcription of HO‐1 which mediates protection of ischemic damage via oxidative cleavage of heme groups. 43 Oxidative stress represents a major hallmark in the pathophysiology of SCI; thus, its mitigation appears as an effective target to reduce secondary damage and preserve spinal cord function. 38
To substantiate the significance of the uncovered mechanism, we created tlr3‐deficient zebrafish via CRISPR/Cas9. Fish were subjected to spinal cord injury and axonal growth was assessed. Unlike mammals, zebrafish are able to regenerate their spinal cords entirely upon injury. In contrast to humans, zebrafish are able to form bridges across injured spinal cord tissue via expression of connective tissue growth factor a. 44 This results in complete restoration of morphology and function making them an exciting organism to elucidate conserved mechanisms of spinal cord regeneration.
In a previous study we found promising effects of SWT in a murine model of spinal cord ischemia. Locomotor function was significantly preserved in treated animals and even resulted in improved survival. 27 These findings drove us to aspire rapid and efficient translation of our promising findings. To prove efficacy of SWT on human spinal cord tissue, we treated spinal slice cultures and found decreased neuronal degeneration upon SWT. 15 To translate our preclinical results even further, we treated in this first‐in‐human study 5 patients with SCI attributable to aortic dissection or surgery with SWT. SCI was diagnosed by a neurologist, and we evaluated the extent of the spinal cord injury via MRI and well‐established functional scores including ASIA scale and SCIM evaluation. Neurologic scores were to some extent improved 6 months after start of treatment.
Two studies have been published investigating long‐term outcome after spinal cord ischemia. Robertson et al. have analyzed 115 patients with SCI with a mean follow‐up of 3 years. They found that 23% of the patients had died at the last follow‐up, 42% required a wheelchair, and 54% catheterization. 13 Older age, severity of impairment at nadir and the presence of peripheral artery disease were independent risk factors for mortality. Nedeltchev et al. followed up on 57 patients with spinal cord ischemia with a mean‐follow‐up of 4.5 years. While initially 30% of the patients showed severe impairment (ASIA A and B), 28% moderate impairment (ASIA C), and 42% mild impairment (ASIA D), 41% showed full walking ability, 30% could walk with aids, 20% needed a wheelchair and 9 had died. 45 In the present cohort, there was no death in the follow‐up period. Initial impairment was severe in 80% (ASIA A and B), and moderate in 20% (ASIA D). At follow‐up, 2 patients were graded as ASIA A (40%) and 3 patients as ASIA C (60%). It remains difficult to compare the present patient cohort with the previously published cohorts. The described studies were performed by neurologists and included patients with neurological etiology underlying spinal cord ischemia (eg, degenerative spine disease, cryptogenic, epidural anesthesia). Our cohort included only patients with aortic pathologies. Initial impairment was markedly higher in our group. Moreover, follow‐up was limited to 6 months in the present study, whereas the described studies included longer follow‐ups.
SWT might also be a valuable tool to improve outcomes in patients with traumatic spinal cord injuries. SWT promotes spinal cord repair in a murine model of spinal cord injury resulting in decreased lesion size and improved motor function. 15 The NEUROwave (extracorporeal shock wave therapy [ESWT] in acute traumatic complete [AIS A] and incomplete [AIS B‐D] cross‐sectional lesions on motor and sensory function within six months after injury) trial is a prospective, multicenter randomized controlled trial investigating the effect of SWT on patients with acute traumatic spinal cord injury. 46 It is currently in its recruitment phase and will validate a possible beneficial effect of SWT in traumatic spinal cord injury. First experimental trials have applied SWT to the brain after infarction and have reported a reduction of infarction volume with improvement of neurological function in rats. 47 First off‐label applications were even reported in patients with Parkinson Disease. 48 Future trials are needed to elucidate possible beneficial SWT effects on the brain.
Limitations
The findings of this study have to be handled with care, as our study represents a very small series of a first‐in‐human application proving safety and feasibility of a novel therapeutic treatment modality rather than showing efficacy. Patients with SCI represent a heterogeneous patient group, as the period of ischemia, the height of the lesion, the extent of the lesion and the extent of the repair differ markedly. In this first clinical application patients were treated at relatively late time‐points after onset of their ischemic injury meaning that the early window of opportunity to prevent secondary apoptotic injury had already been closed. The current clinical data therefore stand for the feasibility of the new treatment option and thereby raise the clear vision for further investigation in a prospective randomized‐controlled setting. Moreover, spinal cord injury in zebrafish was traumatic, not ischemic.
Altogether, we provide evidence for neuroprotection upon ischemic injury after SWT, which is at least partly mediated via mitigation of oxidative damage via the crucial transcription factor NRF2 and depends on innate immune receptor TLR3. Thereby, SWT stimulates an evolutionary conserved mechanism of spinal cord regeneration. In a first‐in‐human application we show safety and feasibility of SWT. This new treatment option might constitute a potent novel therapeutic approach to alleviate oxidative damage, reduce secondary neuronal damage and thus, preserve spinal cord tissue and function.
Sources of Funding
This work was supported by an Allgemeine Unfallversicherungsanstalt – the Austrian Workers' Compensation Board unrestricted research grant to J. Holfeld and C.G.T.
Disclosures
J. Holfeld and M. Grimm. are shareholders of Heart Regeneration Technologies GmbH, an Innsbruck Medical University spin‐off aiming to promote cardiac SWT (www.heart‐regeneration.com). All other authors have nothing to disclose.
For Sources of Funding and Disclosures, see page 11.
References
- 1. Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, Eggebrecht H, Elefteriades JA, Erbel R, Gleason TG, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent‐grafts. Ann Thorac Surg. 2008;85:S1–S41. doi: 10.1016/j.athoracsur.2007.10.099 [DOI] [PubMed] [Google Scholar]
- 2. Sakalihasan N, Michel J‐B, Katsargyris A, Kuivaniemi H, Defraigne J‐O, Nchimi A, Powell JT, Yoshimura K, Hultgren R. Abdominal aortic aneurysms. Nat Rev Dis Primers. 2018;4:34. doi: 10.1038/s41572-018-0030-7 [DOI] [PubMed] [Google Scholar]
- 3. Sofroniew MV. Dissecting spinal cord regeneration. Nature. 2018;557:343–350. doi: 10.1038/s41586-018-0068-4 [DOI] [PubMed] [Google Scholar]
- 4. Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357–368; discussion 368–370. [PubMed] [Google Scholar]
- 5. Coselli JS, Bavaria JE, Fehrenbacher J, Stowe CL, Macheers SK, Gundry SR. Prospective randomized study of a protein‐based tissue adhesive used as a hemostatic and structural adjunct in cardiac and vascular anastomotic repair procedures. J Am Coll Surg. 2003;197:243–252; discussion 252–243. doi: 10.1016/s1072-7515(03)00376-4 [DOI] [PubMed] [Google Scholar]
- 6. Griepp RB, Di Luozzo G. Hypothermia for aortic surgery. J Thorac Cardiovasc Surg. 2013;145:S56–S58. doi: 10.1016/j.jtcvs.2012.11.072 [DOI] [PubMed] [Google Scholar]
- 7. Conlon N, Grocott HP, Mackensen GB. Neuroprotection during cardiac surgery. Expert Rev Cardiovasc Ther. 2008;6:503–520. doi: 10.1586/14779072.6.4.503 [DOI] [PubMed] [Google Scholar]
- 8. ter Wolbeek C, Hartert M, Conzelmann LO, Peivandi AA, Czerny M, Gottardi R, Beyersdorf F, Weigang E. Value and pitfalls of neurophysiological monitoring in thoracic and thoracoabdominal aortic replacement and endovascular repair. Thorac Cardiovasc Surg. 2010;58:260–264. doi: 10.1055/s-0030-1249904 [DOI] [PubMed] [Google Scholar]
- 9. Etz CD, von Aspern K, Gudehus S, Luehr M, Girrbach FF, Ender J, Borger M, Mohr FW. Near‐infrared spectroscopy monitoring of the collateral network prior to, during, and after thoracoabdominal aortic repair: a pilot study. Eur J Vasc Endovasc Surg. 2013;46:651–656. doi: 10.1016/j.ejvs.2013.08.018 [DOI] [PubMed] [Google Scholar]
- 10. Weigang E, Sircar R, von Samson P, Hartert M, Siegenthaler MP, Luehr M, Richter H, Szabo G, Czerny M, Zentner J, et al. Efficacy and frequency of cerebrospinal fluid drainage in operative management of thoracoabdominal aortic aneurysms. Thorac Cardiovasc Surg. 2007;55:73–78. doi: 10.1055/s-2006-924708 [DOI] [PubMed] [Google Scholar]
- 11. Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73 [DOI] [PubMed] [Google Scholar]
- 12. Etz CD, Weigang E, Hartert M, Lonn L, Mestres CA, Di Bartolomeo R, Bachet JE, Carrel TP, Grabenwoger M, Schepens MA, et al. Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: a position paper of the vascular domain of the European Association for Cardio‐Thoracic Surgery†. Eur J Cardiothorac Surg. 2015;47:943–957. doi: 10.1093/ejcts/ezv142 [DOI] [PubMed] [Google Scholar]
- 13. Robertson CE, Brown RD Jr, Wijdicks EF, Rabinstein AA. Recovery after spinal cord infarcts: long‐term outcome in 115 patients. Neurology. 2012;78:114–121. doi: 10.1212/WNL.0b013e31823efc93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15:541–553. doi: 10.1007/s13311-018-0631-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gollmann‐Tepekoylu C, Nagele F, Graber M, Polzl L, Lobenwein D, Hirsch J, An A, Irschick R, Rohrs B, Kremser C, et al. Shock waves promote spinal cord repair via TLR3. JCI Insight. 2020;5. doi: 10.1172/jci.insight.134552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kakinohana M, Kida K, Minamishima S, Atochin DN, Huang PL, Kaneki M, Ichinose F. Delayed paraplegia after spinal cord ischemic injury requires caspase‐3 activation in mice. Stroke. 2011;42:2302–2307. doi: 10.1161/STROKEAHA.110.600429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, Van Gorp S, Leerink M, Tadokoro T, Marsala S, et al. A first‐in‐human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22:941–950.e946. doi: 10.1016/j.stem.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 18. McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin‐3 and brain‐derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Nogo‐A‐specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12:790–792. doi: 10.1038/nm1436 [DOI] [PubMed] [Google Scholar]
- 20. Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory‐ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu P, Tuszynski MH. Growth factors and combinatorial therapies for CNS regeneration. Exp Neurol. 2008;209:313–320. doi: 10.1016/j.expneurol.2007.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gollmann‐Tepekoylu C, Polzl L, Graber M, Hirsch J, Nagele F, Lobenwein D, Hess MW, Blumer MJ, Kirchmair E, Zipperle J, et al. miR‐19a‐3p containing exosomes improve function of ischemic myocardium upon shock wave therapy. Cardiovasc Res. 2019;116:1226–1236. doi: 10.1093/cvr/cvz209 [DOI] [PubMed] [Google Scholar]
- 24. Gollmann‐Tepeköylü C, Lobenwein D, Theurl M, Primessnig U, Lener D, Kirchmair E, Mathes W, Graber M, Pölzl L, An A, et al. Shock wave therapy improves cardiac function in a model of chronic ischemic heart failure: evidence for a mechanism involving VEGF signaling and the extracellular matrix. J Am Heart Assoc. 2018;7:e010025. doi: 10.1161/jaha.118.010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holfeld J, Tepekoylu C, Reissig C, Lobenwein D, Scheller B, Kirchmair E, Kozaryn R, Albrecht‐Schgoer K, Krapf C, Zins K, et al. Toll‐Like receptor 3 signalling mediates angiogenic response upon shock wave treatment of ischaemic muscle. Cardiovasc Res. 2016;109:331–343. doi: 10.1093/cvr/cvv272 [DOI] [PubMed] [Google Scholar]
- 26. Holfeld J, Zimpfer D, Albrecht‐Schgoer K, Stojadinovic A, Paulus P, Dumfarth J, Thomas A, Lobenwein D, Tepekoylu C, Rosenhek R, et al. Epicardial shock‐wave therapy improves ventricular function in a porcine model of ischaemic heart disease. J Tissue Eng Regen Med. 2016;10:1057–1064. doi: 10.1002/term.1890 [DOI] [PubMed] [Google Scholar]
- 27. Lobenwein D, Tepekoylu C, Kozaryn R, Pechriggl EJ, Bitsche M, Graber M, Fritsch H, Semsroth S, Stefanova N, Paulus P, et al. Shock wave treatment protects from neuronal degeneration via a Toll‐Like receptor 3 dependent mechanism: implications of a first‐ever causal treatment for ischemic spinal cord injury. J Am Heart Assoc. 2015;4:e002440. doi: 10.1161/JAHA.115.002440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holfeld J, Tepekoylu C, Kozaryn R, Mathes W, Grimm M, Paulus P. Shock wave application to cell cultures. J Vis Exp. 2014. doi: 10.3791/51076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao LP, Ji C, Lu PH, Li C, Xu B, Gao H. Oxygen glucose deprivation (OGD)/re‐oxygenation‐induced in vitro neuronal cell death involves mitochondrial cyclophilin‐D/P53 signaling axis. Neurochem Res. 2013;38:705–713. doi: 10.1007/s11064-013-0968-5 [DOI] [PubMed] [Google Scholar]
- 30. Rieger AM, Nelson KL, Konowalchuk JD, Barreda DR. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp. 2011. doi: 10.3791/2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan TF, Wu TF, Bu LL, Ma SR, Li YC, Mao L, Sun ZJ, Zhang WF. Dihydromyricetin promotes autophagy and apoptosis through ROS‐STAT3 signaling in head and neck squamous cell carcinoma. Oncotarget. 2016;7:59691–59703. doi: 10.18632/oncotarget.10836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohan S, Gupta D. Crosstalk of Toll‐Like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomed Pharmacother. 2018;108:1866–1878. doi: 10.1016/j.biopha.2018.10.019 [DOI] [PubMed] [Google Scholar]
- 33. Yin S, Cao W. Toll‐Like receptor signaling induces Nrf2 pathway activation through p62‐triggered Keap1 degradation. Mol Cell Biol. 2015;35:2673–2683. doi: 10.1128/mcb.00105-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Biswas C, Shah N, Muthu M, La P, Fernando AP, Sengupta S, Yang G, Dennery PA. Nuclear heme oxygenase‐1 (HO‐1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti‐oxidant defenses. J Biol Chem. 2014;289:26882–26894. doi: 10.1074/jbc.M114.567685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wehner D, Tsarouchas TM, Michael A, Haase C, Weidinger G, Reimer MM, Becker T, Becker CG. Wnt signaling controls pro‐regenerative Collagen XII in functional spinal cord regeneration in zebrafish. Nat Comm. 2017;8:126. doi: 10.1038/s41467-017-00143-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oosterhof N, Holtman IR, Kuil LE, van der Linde HC, Boddeke EW, Eggen BJ, van Ham TJ. Identification of a conserved and acute neurodegeneration‐specific microglial transcriptome in the zebrafish. Glia. 2017;65:138–149. doi: 10.1002/glia.23083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. 2016;10:98. doi: 10.3389/fncel.2016.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. Oxidative stress in spinal cord injury and antioxidant‐based intervention. Spinal Cord. 2012;50:264–274. doi: 10.1038/sc.2011.111 [DOI] [PubMed] [Google Scholar]
- 39. Scott GS, Cuzzocrea S, Genovese T, Koprowski H, Hooper DC. Uric acid protects against secondary damage after spinal cord injury. Proc Natl Acad Sci USA. 2005;102:3483–3488. doi: 10.1073/pnas.0500307102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp. 2011;71:281–299. [DOI] [PubMed] [Google Scholar]
- 41. Pouw MH, Hosman AJF, van Middendorp JJ, Verbeek MM, Vos PE, van de Meent H. Biomarkers in spinal cord injury. Spinal Cord. 2009;47:519–525. doi: 10.1038/sc.2008.176 [DOI] [PubMed] [Google Scholar]
- 42. Holfeld J, Tepekoylu C, Blunder S, Lobenwein D, Kirchmair E, Dietl M, Kozaryn R, Lener D, Theurl M, Paulus P, et al. Low energy shock wave therapy induces angiogenesis in acute hind‐limb ischemia via VEGF receptor 2 phosphorylation. PloS One. 2014;9:e103982. doi: 10.1371/journal.pone.0103982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Araujo J, Zhang M, Yin F. Heme oxygenase‐1, oxidation, inflammation, and atherosclerosis. Front Pharmacol. 2012;3. doi: 10.3389/fphar.2012.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mokalled MH, Patra C, Dickson AL, Endo T, Stainier DYR, Poss KD. Injury‐induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science. 2016;354:630–634. doi: 10.1126/science.aaf2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nedeltchev K, Loher TJ, Stepper F, Arnold M, Schroth G, Mattle HP, Sturzenegger M. Long‐term outcome of acute spinal cord ischemia syndrome. Stroke. 2004;35:560–565. doi: 10.1161/01.STR.0000111598.78198.EC [DOI] [PubMed] [Google Scholar]
- 46. Leister I, Mittermayr R, Mattiassich G, Aigner L, Haider T, Machegger L, Kindermann H, Grazer‐Horacek A, Holfeld J, Schaden W. The effect of extracorporeal shock wave therapy in acute traumatic spinal cord injury on motor and sensory function within 6 months post‐injury: a study protocol for a two‐arm three‐stage adaptive, prospective, multi‐center, randomized, blinded, placebo‐controlled clinical trial. Trials. 2022;23:245. doi: 10.1186/s13063-022-06161-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yuen CM, Chung SY, Tsai TH, Sung PH, Huang TH, Chen YL, Chen YL, Chai HT, Zhen YY, Chang MW, et al. Extracorporeal shock wave effectively attenuates brain infarct volume and improves neurological function in rat after acute ischemic stroke. Am J Transl Res. 2015;7:976–994. [PMC free article] [PubMed] [Google Scholar]
- 48. Novak P, Lohse‐Busch H. Parkinson's disease treatment with shockwaves. Brain Stimul. 2021;14:1628. doi: 10.1016/j.brs.2021.10.130 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


