Abstract
Background
Cardiovascular complications from COVID‐19 contribute to its high morbidity and mortality. The effect of COVID‐19 infection on the coronary vasculature is not known. The objective of this study was to investigate the prevalence of coronary vasomotor dysfunction identified by coronary flow reserve from cardiac positron emission tomography in patients with previous COVID‐19 infection.
Methods and Results
All patients who had polymerase chain reaction–confirmed SARS‐CoV‐2 infection referred for myocardial stress perfusion positron emission tomography imaging at Brigham and Women's Hospital from April 2020 to July 2021 were compared with a matched control group without prior SARS‐CoV‐2 infection imaged in the same period. The main outcome was the prevalence of coronary vasomotor dysfunction. Myocardial perfusion and myocardial blood flow reserve were quantified using N13‐ammonia positron emission tomography imaging. Thirty‐four patients with prior COVID‐19 were identified and compared with 103 matched controls. The median time from polymerase chain reaction–confirmed SARS‐CoV‐2 to cardiac positron emission tomography was 4.6 months (interquartile range,1.2–5.6 months). There were 16 out of 34 (47%) patients previously hospitalized for COVID‐19 infection. Baseline cardiac risk factors were common, and 18 (53%) patients in the COVID‐19 group had abnormal myocardial perfusion. Myocardial blood flow reserve was abnormal (<2) in 44.0% of the patients with COVID‐19 compared with 11.7% of matched controls (P<0.001). The mean myocardial blood flow reserve was 19.4% lower in patients with COVID‐19 compared with control patients (2.00±0.45 versus 2.48±0.47, P<0.001).
Conclusions
Myocardial blood flow reserve was impaired in patients with prior COVID‐19 infection compared with cardiovascular risk factor–matched controls, suggesting a relationship between SARS‐CoV‐2 infection and coronary vascular health. These data highlight the need to assess long‐term consequences of COVID‐19 on vascular health in future prospective studies.
Keywords: Cardiac PET, coronary vasomotor dysfunction, COVID‐19, SARS‐CoV‐2
Subject Categories: Nuclear Cardiology and PET
Cardiovascular complications of COVID‐19 contribute to its high morbidity and mortality. Patients with cardiovascular risk factors, established cardiovascular disease, and de novo myocardial injury have the highest case fatality rates. 1 , 2 , 3 This suggests an interaction between the SARS‐CoV‐2 virus and the cardiovascular system that is incompletely understood; inflammation‐induced and/or direct injury of the vascular endothelium and myocardium likely play a central role. 4 , 5 Myocardial blood flow reserve (MBFR) derived from cardiac positron emission tomography (PET) is a powerful quantitative imaging marker of clinical cardiovascular risk. MBFR provides a robust and reproducible clinical measure of the integrated hemodynamic effects of focal and diffuse epicardial coronary artery disease (CAD) and microvascular dysfunction on myocardial tissue perfusion. 6 Inflammation and vascular dysfunction are key mediators of cardiovascular disease, yet the consequences of COVID‐19 infection on these manifestations are unknown. We designed this case–control study to investigate the prevalence and severity of coronary vasomotor dysfunction in patients previously infected with SARS‐CoV‐2.
METHODS
The authors declare that all supporting data are available within the article.
We identified consecutive subjects with prior polymerase chain reaction–confirmed SARS‐CoV‐2 infection who underwent clinically indicated N13‐ammonia myocardial stress perfusion PET imaging at our institution between April 1, 2020 and July 1, 2021. Control patients without prior SARS‐CoV‐2 infection and with PET in the same time period were matched ≈3:1 average of controls to each patient with COVID‐19 based on age, sex, diabetes, obesity, hyperlipidemia, hypertension, and history of CAD (defined as prior myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention). A summed stress score <3 was used to define a normal PET scan without evidence of obstructive CAD. High‐risk coronary vasomotor dysfunction was defined as MBFR <2 associated with a maximal myocardial blood flow (MBF) <1.8 mL/min per gram. To account for differences in heart rate and blood pressure, which are major determinants of rest MBF, MBFR was corrected using the formula MBFRcorrected=MBFRuncorrected×RPP/10 000, where RPP is the product of the resting blood pressure and heart rate. 6 Quantitative measures of MBF and MBFR were recorded by a single experienced operator blinded to patient data.
Statistical Analysis
The presence and extent of coronary artery calcium was assessed using semiquantitative visual analysis of the low‐dose, noncontrast computed tomography scan obtained for attenuation correction of the PET images. 7 A normal‐response linear mixed model was used for continuous variables, and conditional binary logistic regression was used for dichotomous variables, which accounted for the matching between COVID‐19 and control patients and to compare hemodynamics, MBF, and prevalence of coronary vasomotor dysfunction. A Fisher exact test was used for CAC assessment and clinical risk categories between groups. P<0.05 was considered statistically significant, and all testing was 2‐tailed. All analyses were performed by using SAS University Edition 9.4 (SAS Institute). The study was approved by the Mass General Brigham Institutional Review Board and conducted in accordance with institutional guidelines, and informed consent was waived.
RESULTS
We studied 34 patients with COVID‐19 and 103 matched controls. The median time from polymerase chain reaction–confirmed SARS‐CoV‐2 to cardiac PET was 4.6 months (interquartile range [IQR], 1.2–5.6 months). There were 15 out of 34 (43%) patients who required hospitalization for COVID‐19 infection. Among those hospitalized, the median length of stay was 8 days (IQR, 5–32 days). Treatments included: 9 out of 15 (60%) remdesivir, 6 out of 15 (40%) steroids, and 2 out of 15 (13%) tocilizumab. Three (20%) patients required the intensive care unit and 11 out of 15 (73%) required supplemental oxygen ≥2 L. Cardiac risk factors were common among the COVID‐19 cohort including obesity (67.7%), hypertension (91.2%), hyperlipidemia (91.2%), and diabetes (55.8%) (Table 1). The most common symptoms reported at the time of cardiac PET testing in the COVID‐19 group were chest pain and dyspnea. Among the 34 COVID‐19 cases, 35.3% (12/34) reported chest pain, 35.3% (12/34) reported dyspnea, 5.8% (2/34) reported palpitations, and 32% (11/34) were denoted as other.
Table 1.
Baseline Demographic of the Study Cohort
| Clinical characteristics | COVID‐19, n=34 | Control, n=103 | P value* |
|---|---|---|---|
| Age, y, mean (SD) | 65.7 (9.1) | 66.7 (8.9) | NS |
| Women, n (%) | 13 (38.2) | 42 (41) | NS |
| Time from SARS‐CoV‐2 infection to cardiac PET, mo, median (IQR) | 4.6 (2.6–7.7) | NA | |
| Previously hospitalized for COVID‐19, n (%) | 15 (43) | NA | |
| Cardiovascular risk factors, n (%) | |||
| Obesity | 23 (67.7) | 71 (69) | NS |
| Diabetes | 19 (55.8) | 53 (51.5) | NS |
| Hypertension | 31 (91.2) | 92 (89.3) | NS |
| Dyslipidemia | 31 (91.2) | 89 (86.4) | NS |
| Known CAD | 10 (29.4) | 40 (38.8) | NS |
| Prior revascularization | 9 (26.4) | 33 (32) | 0.5 |
| Statin use | 32 (94) | 84/98 (86) | 0.2 |
Control subjects matched on age, sex, and cardiovascular risk factors (diabetes, hypertension, known CAD, obesity, dyslipidemia). CAD indicates coronary artery disease, and IQR, interquartile range.
P values represents conditional regression for dichotomous variables. P<0.05 is considered statistically significant.
At rest, MBF was globally homogeneous and similar across the 2 groups (Table 2). However, despite a similar coronary risk factor profile and calcified atherosclerotic burden (Table 2), the maximal hyperemic MBF was 19.4% lower in patients with COVID‐19 compared with controls (2.0±0.45 versus 2.48±0.47, P<0.001). Consequently, MBFR was abnormal (<2) in 44% of the patients with COVID‐19 compared with 11.7% of matched controls (P<0.001). Similar results were obtained when the analysis was restricted to patients with visually normal PET scans (MBFR: 2.02±0.4 versus 2.54±0.4, P<0.001, in COVID‐19 and controls, respectively) (Table 2), suggesting that these findings are not simply related to epicardial CAD but reflective of an abnormal microcirculatory response to stress. In a subanalysis, there was no significant differences in MBFR among patients with COVID‐19 who were hospitalized versus those who were not (COVID‐19 hospitalized [n=16] MBFR=2.01 [0.5] versus COVID‐19 not hospitalized [n=18] MBFR=1.99 [0.42], P=0.5), although this is limited by the small sample size. Apart from a slightly higher resting heart rate at rest among patients with COVID‐19, hemodynamic parameters, including blood pressure, at rest and following vasodilator stress, were not statistically significantly different between patients with COVID‐19 and controls (Table 2). Similarly, MBFR was reduced when corrected to resting myocardial blood flow normalized to the resting heart rate and blood pressure (MBFR_co), although results were less dramatic and not statistically significant when a clinical cutoff of 2 for coronary microvacular dysfunction was applied to the corrected flows (Table 2). Among the patients with COVID‐19 with abnormal stress myocardial perfusion, 55.6% (10/18) had evidence of obstructive CAD, 22.2% (4/18) had nonobstructive CAD, and 22.2% (4/18) were unknown.
Table 2.
PET MBF, Coronary Flow Reserve, and Hemodynamics in COVID‐19 and Matched Controls
| PET imaging characteristics | COVID‐19, n=34 | Controls, n=103 | P value |
|---|---|---|---|
| Rest myocardial blood flow, mL/min per gram | 0.79 (0.17) | 0.73 (0.16) | 0.06 |
| Corrected rest myocardial blood flow, mL/min per gram | 0.82 (0.2) | 0.83 (0.2) | 0.65 |
| Rest coronary vascular resistance, mm Hg/mL per g per minute | 120 (35.7) | 126.7 (33.2) | 0.35 |
| Stress myocardial blood flow, mL/min per gram | 1.56 (0.44) | 1.79 (0.39) | <0.001 |
| Stress coronary vascular resistance, mm Hg/mL per gram per minute | 58.2 (23.7) | 50.2 (12.4) | 0.008 |
| Abnormal stress MBF, MBF <1.8, n (%) | 25 (73.5) | 58 (56.3) | 0.1 |
| MBFR | 2.0 (0.45) | 2.48 (0.47) | <0.001 |
| Corrected MBFR (MBFRco)# | 1.95 (0.5) | 2.24 (0.66) | 0.01 |
| MBFR in patients with normal scans, SSS <3 | 2.02 (0.43), n=15 | 2.54 (0.43), n=63 | <0.001 |
| Coronary vasomotor dysfunction, MBFR <2, n (%) | 15 (44.1) | 12 (11.7) | <0.001 |
| Coronary vasomotor dysfunction corrected, corrected MBFR <2, n (%) | 20 (59) | 47 (45) | 0.16 |
| Abnormal perfusion, SSS >3, n (%) | 18 (53) | 40 (39) | 0.2 |
| SSS, median (IQR) | 4 (0–9) | 0 (0–6) | 0.1 |
| Coronary artery calcium, n (%) | 0.92 | ||
| None | 5 (14.7) | 14 (13.6) | |
| Mild–moderate | 8 (23.5) | 31 (30.1) | |
| Severe | 21 (61.8) | 58 (56.3) | |
| Hemodynamics | |||
| Resting heart rate, bpm | 73 (10.4) | 68 (12) | 0.02 |
| Peak heart rate, bpm | 90 (15.6) | 90 (16) | 0.95 |
| Resting systolic blood pressure, mm Hg | 134 (23) | 136 (24) | 0.79 |
| Peak systolic blood pressure, mm Hg | 129 (20) | 132 (29) | 0.7 |
All values represent mean and standard deviation unless otherwise noted. P values represent linear mixed model for continuous variables and conditional logistic regression for dichotomous variables. P<0.05 is considered statistically significant. IQR indicates interquartile range; MBF, myocardial blood flow; MBFR, myocardial blood flow reserve; PET, positron emission tomography; and SSS, summed stress score.
MBFRcorrected=MBFRuncorrected×RPP/10 000. RPP=Resting HR×resting SBP.
The Figure illustrates the differences in cardiovascular risk based on the patient‐level measures of maximal MBF and MBFR, both markers of clinical risk that reflect the global coronary vasodilator capacity. 6 Compared with controls, the COVID‐19 group had a greater proportion of patients with concordant abnormal maximal MBF and MBFR, reflecting high clinical risk (44% versus 9.7%, P<0.001). Conversely, the proportion of patients with COVID‐19 with concordant normal maximal MBF and MBFR, reflecting low risk, was lower than in the control group though not statistically significant (20.6% versus 40.8%, P=0.06).
Figure 1. MBFR and maximal myocardial blood flow.
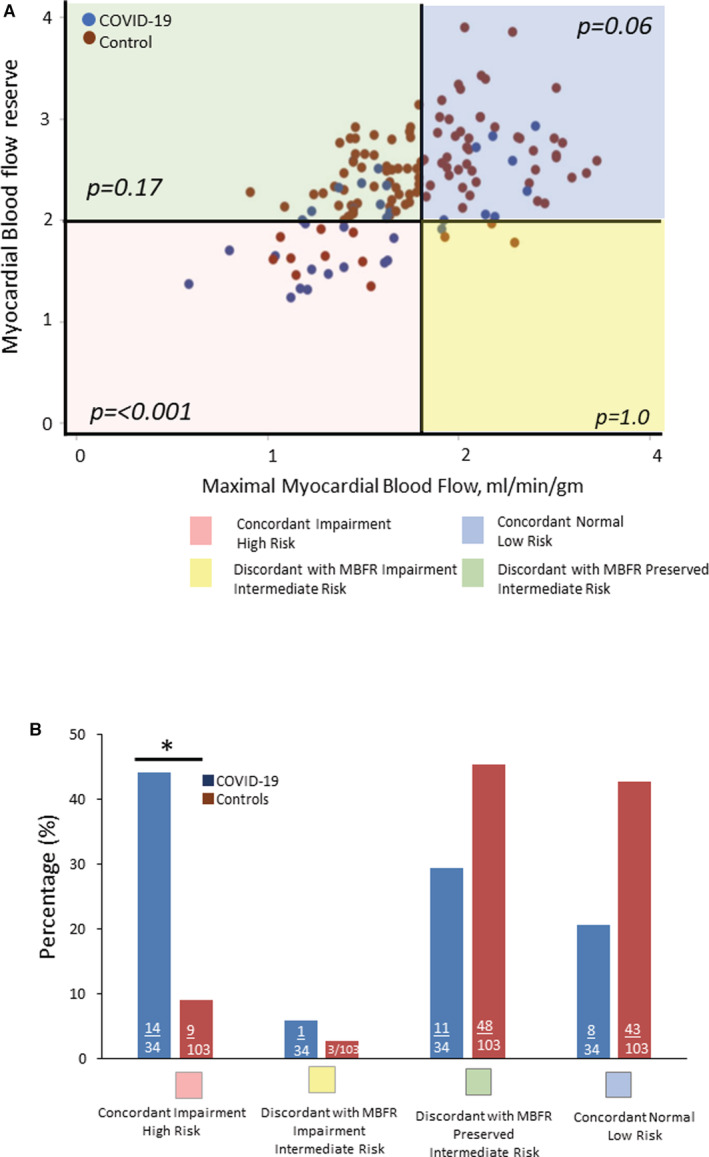
Shown is a scatter plot (A) and bar graph (B) demonstrating the concordant and discordant impairment of coronary flow reserve and maximal myocardial blood flow in patients with COVID‐19 compared with matched controls. MBFR <2 and maximal myocardial blood flow <1.8 mL/g−1 per minute−1 were defined as impaired. MBFR indicates myocardial blood flow reserve. *P<0.001.
DISCUSSION
We identified a cohort of patients with prior SARS‐CoV‐2 infection who underwent clinically indicated stress myocardial perfusion PET imaging. When compared with a matched control group without prior COVID‐19 within a similar time frame, patients with prior COVID‐19 had greater frequency and severity of coronary vasomotor dysfunction with lower maximal MBF and MBFR, a concordance that reflects the highest clinical risk. Conversely, the lowest clinical risk, patients with normal MBFR and maximal MBF, were proportionally lower in patients with COVID‐19 compared with controls. That the burden of calcified atherosclerosis was similar in both groups and the lower MBFR in patients with COVID‐19 who had normal perfusion suggest that the reduced MBFR was not simply a manifestation of more epicardial CAD or diffuse atherosclerosis, but may represent an abnormal microcirculatory response to stress. SARS‐CoV‐2 infection has been shown to be associated with endothelial dysfunction, and these results may suggest an acceleration of endothelial dysfunction or even a progression of atherosclerosis that occurs in the setting of SARS‐CoV‐2 infection. 8
In survivors, the longer‐term cardiovascular consequences of COVID‐19 are unknown and important to delineate to inform therapeutic strategies for long‐term sequelae beyond acute illness, including the risk of myocardial infarction and stroke from endothelial damage. Our findings are novel and extend the observations of prior studies in 2 important ways: (1) by showing a high prevalence of coronary vasomotor abnormalities in high‐risk patients with prior SARS‐CoV‐2 infection and (2) by demonstrating that the severity of these abnormalities may represent excess microvascular dysfunction not accounted for by common associated coronary risk factors or the burden of atherosclerosis. The excess microvascular risk in this study supports a potential role for vascular endothelial damage and/or inflammation in driving coronary vasomotor abnormalities that might contribute to excess cardiovascular risk in this population.
This study has multiple limitations. Given the lack of data on the patients' coronary vascular health before COVID‐19 infection, we are unable to determine causality. However, when compared with a matched control group with a comparably high burden of cardiovascular risk factors and atherosclerosis, we found a greater degree of impairment in MBFR in the patients with COVID‐19. Second, the small sample size and single‐center study limited the power to understand the impact of severity or timing of initial COVID‐19 infection relative to vascular assessment. Finally, this study does not ascertain the impact of coronary vasomotor dysfunction in long COVID‐19 (or post‐COVID), which is an important question to address in the future. 9 , 10 , 11 , 12
In conclusion, these results suggest an association between SARS‐CoV‐2 infection and coronary microvascular dysfunction. Importantly, these data highlight the need to assess long‐term consequences of COVID‐19 in future prospective studies with inclusion of coronary vascular assessment before COVID‐19 infection.
Sources of Funding
D.M.H.: T32HL094301. B.W.: American Heart Association 21CDA851511, NHLBI K23 HL159276‐01, 2021 ASNC/Institute for the Advancement of Nuclear Cardiology (IANC) Research Fellowship Award. S.D.: 2020 ASNC IANC Research Award, Joint KL2/Catalyst Medical Research Investigator Training (CMeRIT) Award from Harvard Catalyst and the Boston Claude D. Pepper Older Americans Independence Center (5P30AG031679). J.M.B.: American Heart Association 21CDA852429, National Heart, Lung, and Blood Institute K23, HL159279, KL2/Catalyst Medical Research Investigator Training Award from Harvard Catalyst UL1 TR002541.
Disclosures
The authors have reported that they have no conflict‐of‐interest relationships relevant to the contents of this article to disclose. The authors' financial disclosures include: Dr Di Carli reports grants from Gilead Sciences and Spectrum Dynamics, and personal consulting fees from Janssen and Bayer, outside the submitted work. Dr Dorbala reports grants from Pfizer and GE Healthcare and personal consulting fees from GE Healthcare and Pfizer, outside the submitted work. Dr Blankstein reports grants from Amgen and Astellas, and personal consulting fees from Amgen outside of the submitted work.
Presented in part at ASNC2021: 26th Annual Scientific Session and Exhibition of the American Society of Nuclear Cardiology held virtually from September 30 to October 2, 2021 and published in abstract form (J Nucl Cardiol. 2021;28:2425–2455 or doi: 10.1007/s12350‐021‐02760‐1).
For Sources of Funding and Disclosures, see page 5.
References
- 1. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington state. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, Osborne MT, Seidelmann SB, Vita T, Bibbo CF, et al. Integrated non‐invasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation. 2017;136:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiles C, Duan F, Gladish GW, Ravenel JG, Baginski SG, Snyder BS, DeMello S, Desjardins SS, Munden RF, NLST Study Team . Association of coronary artery calcification and mortality in the National Lung Screening Trial: a comparison of three scoring methods. Radiology. 2015;276:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al‐Aly Z, Xie Y, Bowe B. High‐dimensional characterization of post‐acute sequelae of COVID‐19. Nature. 2021;594:259–264. [DOI] [PubMed] [Google Scholar]
- 10. Al‐Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS‐CoV‐2 infection. Nat Med. 2022;28:1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, Khunti K, Alwan NA, Walker AS. Trajectory of long covid symptoms after covid‐19 vaccination: community based cohort study. BMJ. 2022;377:e069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whitaker M, Elliott J, Chadeau‐Hyam M, Riley S, Darzi A, Cooke G, Ward H, Elliott P. Persistent COVID‐19 symptoms in a community study of 606,434 people in England. Nat Commun. 2022;13:1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


