Hypertension is an extremely common noncommunicable condition affecting an estimated 1.3 billion adults (aged 30–79 years) worldwide and with a prevalence that has doubled over the past 20 years. 1 Hypertension substantially increases the risk of cardiovascular disease and is the leading cause of premature mortality globally. In addition, the economic burden of hypertension is high, with estimates from 2012 to 2013 suggesting an annual cost of $51.2 billion in the United States alone. 2 Furthermore, the impact of this economic burden is likely to be higher in developing countries, where >80% of those with hypertension reside. 1 Therefore, strategies to reduce the health and economic burdens associated with hypertension have become a global priority. The benefits of optimal blood pressure (BP) control are well known. For instance, every 10‐mm Hg reduction in systolic BP leads to a 13% reduction in all‐cause mortality, while significantly reducing the risk of major cardiovascular events (20%), coronary heart disease (17%), stroke (27%), and heart failure (28%). 3
It is estimated that only about a fifth of the treated population achieves adequate BP control, 1 with a common barrier being poor adherence to medications for reasons including complex medication regimens and adverse effects of treatment. Also, existing antihypertensives do not target all the regulatory pathways affecting BP or completely suppress a single pathway, allowing counter‐regulatory escape mechanisms to reduce their long‐term efficacy. This unmet need among patients with hypertension can be overcome in part by the development of novel therapies that (1) target key regulatory mechanisms with minimal counter‐regulatory escape, (2) are better tolerated, and (3) have simplified therapeutic regimens. However, translation of novel approaches in hypertension to clinical treatments has been stagnant for decades, with the last such newly licensed medication being the first direct renin inhibitor, aliskiren, 15 years ago. 4 In recent years, small interfering RNAs (siRNAs) targeting angiotensinogen have generated significant interest as a novel therapeutic approach in hypertension. They are effective and long lasting, but concerns remain, particularly about reversing their effects. Strategies to overcome this concern would support clinical translation. The focus of this article was to briefly highlight current developments, potential advantages, and ongoing challenges for angiotensinogen‐targeting siRNA therapy in hypertension.
Angiotensinogen‐Targeting siRNA in Hypertension
RNA interference, using siRNAs, is a novel therapeutic strategy that has recently been translated from bench to bedside, and gradually evolved from use in rare genetic disorders to common chronic conditions with the licensing of inclisiran for the treatment of hypercholesterolemia by the US Food and Drug Administration in 2021. Zilebesiran is a novel first‐in‐class siRNA therapeutic that is currently in phase II clinical evaluation for the treatment of hypertension. 5 This subcutaneously administered N‐acetylgalactosamine (GalNAc)‐conjugated chemically modified siRNA specifically targets angiotensinogen synthesis in the liver (Figure A), thereby suppressing generation of angiotensin I and II (Ang II), with resultant lowering of BP. Standard renin‐angiotensin system (RAS) medications, such as angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers, cause a compensatory rise in renin with long‐term use because of reduction of BP and loss of negative feedback mediated by Ang II. This increase in renin, together with the high physiological concentrations of angiotensinogen, results over time in a restoration of Ang II levels (angiotensin‐converting enzyme inhibitors) and Ang II competition at the AT1 receptor (angiotensin II receptor blockers), known as RAS escape (Figure B). It is postulated that the near‐complete depletion of angiotensinogen by siRNA therapy could prevent RAS escape, even with the compensatory rise of renin, resulting in a major advantage of more effective RAS inhibition and long‐term BP control. 6
Figure . Mechanism of RNAi by GalNAc‐AGT‐siRNA (A) and RAS, mediators, clinical effects, and medications (B).
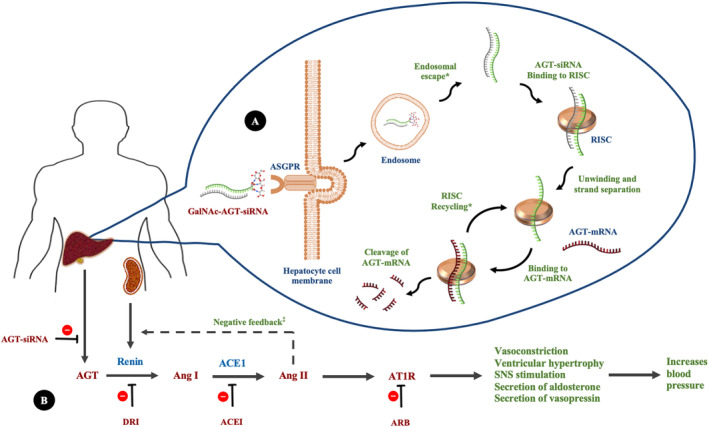
*Accumulation and stability of GalNAc‐siRNA in hepatic endosomes and RISC recycling results in prolonged biochemical and clinical effect of GalNAc‐AGT‐siRNA. ‡Standard RAS medications cause a compensatory rise in renin with long‐term use because of loss of negative feedback mediated by Ang II (RAS escape). Near complete depletion of AGT by siRNA therapy could potentially prevent RAS escape. ACE indicates angiotensin‐converting enzyme; ACEI, angiotensin‐converting enzyme inhibitor; AGT, angiotensinogen; Ang I, angiotensin I; Ang II, angiotensin II; ARB, angiotensin II receptor blocker; ASGPR, asialoglycoprotein receptor; AT1R, angiotensin II type 1 receptor; DRI, direct renin inhibitor; GalNAc, N‐acetylgalactosamine; mRNA, messenger RNA; RAS, renin‐angiotensin system; RISC, RNA‐induced silencing complex; RNAi, RNA interference; siRNA, small interfering RNA; and SNS, sympathetic nervous system.
GalNAc‐siRNA therapies also offer several other distinct advantages, including an ability to improve adherence because of their long duration of action. This partially stems from their inherent nature, because once bound to the RISC (RNA‐induced silencing complex), they are protected from nuclease degradation, allowing recycling and repeated degradation of target messenger RNAs (eg, angiotensinogen messenger RNA), resulting in a prolonged biochemical and clinical effect 7 (Figure A). In addition, recent evidence suggests that the accumulation and stability of GalNAc‐siRNA in acidic intracellular compartments, such as hepatic endosomes, is a major contributor to their prolonged action, acting as a reservoir for months after a single SC injection. 8 This long duration of action of siRNA therapeutics can translate to better 24‐hour BP control, which should result in improved cardiovascular outcomes. 9
Recently concluded phase I studies with zilebesiran (formerly ALN‐AGT01) confirm a sustained long‐term effect, with circulating angiotensinogen reduced by >90% for 6 months after a single SC dose (800 mg), 5 with the peak reduction of circulating angiotensinogen occurring after ≈3 weeks. 5 Sustained reductions of BP were also observed, with 24‐hour ambulatory systolic BP reduced by >15 mm Hg at 8 weeks after a single dose of zilebesiran (800 mg). 5 In addition, zilebesiran was well tolerated, with only mild‐to‐moderate injection site reactions (n=5/56) and no treatment‐related serious adverse events, hypotension, or significant alterations of renal or liver function. Zilebesiran is currently being evaluated in phase II trials in patients with mild‐to‐moderate hypertension without antihypertensive medications (KARDIA‐1: 375 patients; double‐blind, placebo‐controlled 5‐way study; zilebesiran at 150, 300, and 600 mg biannually and 300 mg quarterly) and among patients with inadequate BP control with standard medications (KARDIA‐2: 800 patients; initial open‐label 3‐way run‐in period of 4 weeks with indapamide/amlodipine/olmesartan, followed by double‐blind, placebo‐controlled 3‐way study for 6 months, followed by open‐label extension for up to a further 12 months; zilebesiran 600 mg on day 1 in the initial double‐blind period and every 6 months thereafter in the open‐label extension period). It is evident that zilebesiran has the potential to offer a distinct advantage over existing treatments, addressing an unmet need for good treatment adherence through providing a simplified therapeutic regimen that potentially involves dosing at 6‐month intervals.
Although zilebesiran overcomes many key pharmacokinetic and pharmacodynamic challenges to the use of siRNA therapy (ie, poor tissue penetration, nuclease inactivation, rapid renal elimination, immune activation, and off‐target effects) by means of innovative chemical modifications and GalNAc‐conjugated delivery, several challenges remain. Only larger population studies will enable the proper establishment of its safety, especially in high‐risk subpopulations with hypertension, such as those with heart failure, diabetes, and chronic kidney disease. However, the recent licensing of inclisiran, a similar GalNAc‐conjugated siRNA for hypercholesterolemia, and its excellent safety profile emerging from larger clinical trials (≈4000 participants) is reassuring. 10 Also, RNA interference leads to a slow onset of action (days/weeks), so zilebesiran would be unsuitable for the initial treatment of accelerated hypertension or as a sole therapy in those with hypertensive urgency. In addition, whether zilebesiran prevents/reduces target organ damage, incidence of cardiovascular events, and mortality remains to be answered. Furthermore, interaction with other long‐term medications, cost‐effectiveness (pharmacoeconomics), and the impact of biomarkers and genetic variation on clinical response/safety (pharmacogenomics) are areas that will merit future exploration. However, an important potential safety issue in broader therapeutic use of zilebesiran is its long‐acting/ongoing, RAS‐inhibiting, and BP‐lowering effect in circumstances of hypovolemia and hypotension.
Reversibility of Angiotensinogen‐Targeting siRNA Mediated BP Lowering
There is a need to rapidly restore BP to ensure adequate tissue perfusion in settings of hypovolemia, hypotension, and acute kidney injury to prevent serious morbidity and mortality. Based on the potential clinical need to reverse the effects of angiotensinogen‐targeting siRNA, Uijl et al have examined strategies to overcome the BP‐lowering effects of these agents in vivo in spontaneously hypertensive rats, 11 with results that address an important knowledge gap, lead to potential solutions for clinical use, and support the clinical translation of angiotensinogen‐targeting siRNA therapy.
The Uijl study 11 explored the acute and chronic reversibility of BP lowering in male spontaneously hypertensive rats (11 weeks old, body weight 325 ± 13 g) treated with angiotensinogen‐targeting siRNA by using vasopressors (IV Ang II or norepinephrine) and vasopressor‐sparing agents/strategies (SC fludrocortisone, SC midodrine, or high‐salt diet). In brief, the methodology involved the administration of a low‐salt diet (0.4% NaCl) to spontaneously hypertensive rats (n=19) from day 1, followed by 2 injections of SC angiotensinogen‐targeting siRNA on days 7 and 21 (10 mg/kg). Subsequently, on day 21, animals were randomized to receive either fludrocortisone (n=7, 6 mg/kg daily), midodrine (n=6, 4 mg/kg daily), or a high‐salt diet (n=6, 4% NaCl) for a period of 2 weeks, with the evaluation of BP using an implanted radiotelemetry transmitter, together with assessment of other clinical and biochemical parameters. To circumvent the possibility of poor delivery via the SC route, midodrine was administered for a further 4 days at a dose of 8 mg/kg dissolved in drinking water. Ascending bolus doses of Ang II (n=11, 0.05–5 μg/kg) and norepinephrine (n=8, 0.1–10 μg/kg) were used to assess acute BP response to vasopressors just before each dose of siRNA on days 7 and 21.
Angiotensinogen‐targeting siRNA caused a near complete depletion of angiotensinogen (by 99.2 ± 0.1%) and a reduction of mean arterial pressure (MAP) by 19 mm Hg, similar to that observed with zilebesiran (800 mg) in the phase I trial. 5 Also, the responses to both vasopressors were retained, with Ang II and norepinephrine rapidly and substantially increasing MAP during siRNA administration. Both fludrocortisone and a high‐salt diet gradually increased MAP, which returned to baseline on days 5 and 7, respectively. However, midodrine failed to normalize MAP, either by the subcutaneous or oral route. Fludrocortisone also partially restored hepatic angiotensinogen levels, possibly because of diminished renin‐mediated cleavage. These preclinical data provide a valuable proof of concept for reversing angiotensinogen‐targeting siRNA‐mediated BP lowering, although they will require relevant clinical testing. Preliminary data from the zilebesiran phase I trial show that a high‐salt diet reduces its BP lowering effect, 12 suggesting that oral sodium (or intravenous, alongside vasopressors, if severe) could be used clinically where hypotension and hypovolemia need to be reversed.
Clinically, oral fludrocortisone is used in the treatment of postural hypotension at a dose range of 100 to 400 μg daily, whereas midodrine is initiated at a dose of 2.5 mg 3 times a day, and then titrated to a maximum of 10 mg at the same frequency. 13 By comparison, a much higher dose of fludrocortisone than midodrine was used in the study by Uijl et al, 11 with a human equivalent dose 14 that would be considered substantial (0.96 mg/kg). Also, 5 to 7 days were required for the complete restoration of MAP with fludrocortisone or a high‐salt diet in spontaneously hypertensive rats, indicating a rate of response that would be insufficient in a situation of clinical urgency. A rapid short‐lasting increase in MAP was noted with bolus doses of vasopressors, but clinically, vasopressor infusions would be needed for a sustained effect, requiring hospital admission, intensive monitoring, and the use of additional human and health care resources.
Finally, only clinical studies will be able to demonstrate the duration of fludrocortisone treatment required until angiotensinogen‐targeting siRNA effects wane, in instances where sustained complete reversal is required. A different strategy proposed to reverse siRNA effects is the use of an oligonucleotide complementary to the siRNA anti‐sense strand, that can bind to the RISC‐bound siRNA, a method that has been shown to reverse GalNAc‐siRNA‐mediated gene silencing in vivo in mice and restore normal levels of the target protein in 4 days. However, this strategy remains an experimental approach. 15
Other Novel Therapeutics in Hypertension
At present there are 68 different medications from 15 different pharmacological classes licensed for the long‐term treatment of hypertension in the United States. 16 Recent developments include interventional strategies and novel drug classes that aim not only to control BP but also reduce target organ damage and improve cardiovascular outcomes. Examples of interventional/device‐based strategies include renal sympathetic denervation, baroreflex activation therapy, carotid body ablation, and central iliac arteriovenous anastomosis. 17 Nevertheless, except for renal denervation, others remain a long way from routine clinical use. Evidence from a recent meta‐analysis suggests that renal denervation significantly but modestly reduces ambulatory and office BP (by ~4/2 mm Hg). 18 However, the long‐term maintenance of this reduction, its effect on cardiovascular outcomes, and cost‐effectiveness all remain unclear.
Given its important role in regulation of BP, several novel classes of drugs targeting the renin–angiotensin–aldosterone system are currently in various stages of clinical development (Table 1). Examples include aldosterone synthase inhibitors (phase II), angiotensin (1‐7) analogues (preclinical), and Ang II‐directed vaccines (phase IIa). 17 In addition, neprilysin inhibitors (phase II), endothelin receptor antagonists (phase III), dopamine β‐hydroxylase inhibitors (phase I), and aminopeptidase A inhibitors (phase III) are examples of other novel therapeutics in development. Two noteworthy drug classes that have generated recent interest include dual angiotensin receptor–neprilysin inhibitors and SGLT2 (sodium glucose co‐transporter 2) inhibitors. Sacubitril/valsartan, the first‐in‐class angiotensin receptor–neprilysin inhibitor, is currently licensed for use in chronic heart failure with reduced ejection fraction. A recent meta‐analysis of 12 randomized clinical trials has shown that sacubitril/valsartan outperforms angiotensin II receptor blockers in reducing BP, suggesting it may address an unmet need in patients with hypertension and heart failure. 19 First licensed for use in type 2 diabetes, emerging evidence suggests that SGLT2 inhibitors have beneficial effects on BP, with multiple meta‐analyses suggesting a reduction on BP, although modest in magnitude. 17 However, their proven ability to reduce cardiovascular events and improve renal outcomes, independent of BP control, merit their further evaluation in patients with hypertension.
Table .
Other Novel Therapeutics in Hypertension
| Drug class | Drug(s)/compound name(s) | Target site(s); route | Stage* |
|---|---|---|---|
| RAAS | |||
|
AVE0991, HP‐β‐CD/Ang1‐7 | Mas receptor; oral | Preclinical |
|
CYT006‐AngQB, AGMG0201 | Circulation; SC, IM | Phase IIa |
|
LCI699, LY3045697, RO6836191 | Adrenal cortex; oral | Phase II |
| Other enzymes/receptors | |||
|
Etamicastat, zamicastat | Adrenal medulla; oral | Phase I |
|
LHW090 | Kidney; oral | Phase II |
|
Firibastat, NI956 | Brain; oral | Phase III |
|
Aprocitentan, atrasentan | Blood vessels; oral | Phase III |
|
Sacubitril/valsartan | AT2R, kidney; oral | Phase IV |
|
Gliflozins | Kidney; oral | Phase IV |
Ordered by stage of development. AT2R indicates angiotensin II type 2 receptor; RAAS, renin–angiotensin–aldosterone system; and SGLT2, sodium glucose co‐transporter 2.
Highest stage of development for compound(s) in the class.
Further Unmet Needs and Future Directions in Hypertension Therapy
siRNA therapeutics have the potential to meet a hitherto largely unmet need in relation to adherence in patients with hypertension. However, current data on safety and efficacy for zilebesiran, the first‐in‐class angiotensinogen‐targeting siRNA therapy, are restricted to well‐defined populations from small clinical trials, and will require further evaluation. It can be expected that the results from an on‐going component of the zilebesiran phase I studies (Part D: in patients who are obese) and recently started phase II studies (KARDIA‐1 and 2), will be informative. For licensing, we will also need to know how the drug performs in higher‐risk subpopulations with hypertension such as those with chronic kidney disease, diabetes, heart failure, and obesity, and in the elderly. In addition, its value as an add‐on therapy in those with uncontrolled hypertension or treatment‐resistant hypertension is yet to be established. Furthermore, with their ability to specifically target key proteins, siRNA therapeutics have the potential to knockdown other important nonhepatic mediators in hypertension, such as renin and endothelin. However, challenges in delivery currently limit the use of siRNA therapies for nonliver targets in hypertension.
Other important unmet needs in hypertension include effective treatments to minimize/prevent cardiovascular events and target organ damage associated with hypertension, thereby improving morbidity and mortality. Here, emerging therapies, such as SGLT2 inhibitors and angiotensin receptor–neprilysin inhibitors, could potentially offer solutions. Treatment‐resistant hypertension, affecting nearly 10% of patients with hypertension, 20 is another priority challenge because of its higher likelihood of adverse outcomes.
In summary, siRNA‐based therapeutic approaches have substantial potential to address treatment adherence, one of the major unmet needs in antihypertensive therapy. Emerging evidence from larger ongoing trials and long‐term follow‐up studies will help us understand whether siRNA therapy offers solutions to other unmet needs in hypertension therapy. Challenges remain, but the valuable study from Uijl et al suggests effective ways to mitigate against hypovolemia and hypotension with angiotensinogen‐targeting siRNA therapy, which will be important to the successful clinical translation of this valuable novel therapeutic approach to hypertension.
Disclosures
D.J.W. has received nonpersonal support for research and consultancy from AbbVie, Actelion, AstraZeneca, Idorsia, Johnson & Johnson, and Novartis. D.J.W. has received funding for research in hypertension from the British Heart Foundation. University/British Heart Foundation Centre for Cardiovascular Science received funding from Alnylam Pharmaceuticals for phase I trials with zilebesiran. The remaining authors have no disclosures to report.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Disclosures, see page 5.
References
- 1. Zhou B, Carrillo‐Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, Gregg EW, Bennett JE, Solomon B, Singleton RK, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population‐representative studies with 104 million participants. Lancet. 2021;398:957–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang G, Grosse SD, Schooley MW. Conducting research on the economics of hypertension to improve cardiovascular health. Am J Prev Med. 2017;53:S115–s117. doi: 10.1016/j.amepre.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta‐analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 4. Pool JL, Schmieder RE, Azizi M, Aldigier JC, Januszewicz A, Zidek W, Chiang Y, Satlin A. Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. Am J Hypertens 2007;20:11–20. doi: 10.1016/j.amjhyper.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 5. Huang S, Taubel J, Fiore G, Dewland P, Bakris G, Desai A, Cheng Y, Agarwal S, Harrop J, Nguyen H, et al. Abstract 14387: Dose‐related reductions in blood pressure with a rna interference (rnai) therapeutic targeting angiotensinogen in hypertensive patients: Interim results from a first‐in‐human phase 1 study of aln‐agt01. Circulation. 2020;142:A14387. doi: 10.1161/circ.142.suppl_3.14387. [Google Scholar]
- 6. Ren L, Colafella KMM, Bovee DM, Uijl E, Danser AHJ. Targeting angiotensinogen with rna‐based therapeutics. Curr Opin Nephrol Hypertens. 2020;29:180–189. doi: 10.1097/MNH.0000000000000586 [DOI] [PubMed] [Google Scholar]
- 7. Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, Manygoats K, Seifert S, Andree C, Stöter M, et al. Image‐based analysis of lipid nanoparticle‐mediated sirna delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31:638–646. doi: 10.1038/nbt.2612 [DOI] [PubMed] [Google Scholar]
- 8. Brown CR, Gupta S, Qin J, Racie T, He G, Lentini S, Malone R, Yu M, Matsuda S, Shulga‐Morskaya S, et al. Investigating the pharmacodynamic durability of galnac–sirna conjugates. Nucleic Acids Res. 2020;48:11827–11844. doi: 10.1093/nar/gkaa670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishikawa J, Hoshide S, Eguchi K, Ishikawa S, Shimada K, Kario K. Japan morning surge‐home blood pressure study investigators G. nighttime home blood pressure and the risk of hypertensive target organ damage. Hypertension. 2012;60:921–928. doi: 10.1161/HYPERTENSIONAHA.112.198101 [DOI] [PubMed] [Google Scholar]
- 10. Khan SA, Naz A, Qamar Masood M, Shah R. Meta‐analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69–73. doi: 10.1016/j.amjcard.2020.08.018 [DOI] [PubMed] [Google Scholar]
- 11. Uijl E, Ye D, Ren L, Mirabito Colafella KM, van Veghel R, Garrelds IM, Lu HS, Daugherty A, Hoorn EJ, Nioi P, et al. Conventional vasopressor and vasopressor‐sparing strategies to counteract the blood pressure‐lowering effect of small interfering rna targeting angiotensinogen. J Am Heart Assoc. 2022;11:e026426. doi: 10.1161/JAHA.122.026426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alnylam Pharmaceuticals . Alnylam presents new data for zilebesiran, an investigational rnai therapeutic for the treatment of hypertension, at the american heart association scientific sessions. 2021. BusinessWire:https://www.businesswire.com/news/home/20211113005228/en/Alnylam‐Presents‐New‐Data‐for‐Zilebesiran‐an‐Investigational‐RNAi‐Therapeutic‐for‐the‐Treatment‐of‐Hypertension‐at‐the‐American‐Heart‐Association‐Scientific‐Sessions‐2021. Published: 11/13/2021 Accessed: 09/09/2022
- 13. British National Formulary . Inclisiran. 2022. National Institute for Health Care Excellence:https://bnf.nice.org.uk/drug/inclisiran.html. Published: 27/07/2022 Accessed: 09/09/2022
- 14. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) . Guidance for industry estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers 2005. US FDA:https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/estimating‐maximum‐safe‐starting‐dose‐initial‐clinical‐trials‐therapeutics‐adult‐healthy‐volunteers. Published: 07/2005 Accessed: 09/09/2022
- 15. Zlatev I, Castoreno A, Brown CR, Qin J, Waldron S, Schlegel MK, Degaonkar R, Shulga‐Morskaya S, Xu H, Gupta S, et al. Reversal of sirna‐mediated gene silencing in vivo. Nat Biotechnol. 2018;36:509–511. doi: 10.1038/nbt.4136 [DOI] [PubMed] [Google Scholar]
- 16. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) . Guidance for industry hypertension indication: Drug labeling for cardiovascular outcome claims. 2011. US FDA:https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/hypertension‐indication‐drug‐labeling‐cardiovascular‐outcome‐claims. Published: 03/2011 Accessed: 09/09/2022
- 17. Hunter PG, Chapman FA, Dhaun N. Hypertension: Current trends and future perspectives. Br J Clin Pharmacol. 2021;87:3721–3736. doi: 10.1111/bcp.14825 [DOI] [PubMed] [Google Scholar]
- 18. Ahmad Y, Francis Darrel P, Bhatt Deepak L, Howard JP. Renal denervation for hypertension. J Am Coll Cardiol Intv. 2021;14:2614–2624. doi: 10.1016/j.jcin.2021.09.020 [DOI] [PubMed] [Google Scholar]
- 19. Geng Q, Yan R, Wang Z, Hou F. Effects of lcz696 (sacubitril/valsartan) on blood pressure in patients with hypertension: A meta‐analysis of randomized controlled trials. Cardiology. 2020;145:589–598. doi: 10.1159/000507327 [DOI] [PubMed] [Google Scholar]
- 20. Achelrod D, Wenzel U, Frey S. Systematic review and meta‐analysis of the prevalence of resistant hypertension in treated hypertensive populations. Am J Hypertens. 2015;28:355–361. doi: 10.1093/ajh/hpu151 [DOI] [PubMed] [Google Scholar]


