Abstract
Background
Although endocrine therapy is an effective treatment for breast cancer, its antiestrogen effects are associated with increased risks of cardiovascular diseases and type 2 diabetes. This study aimed to investigate the association between endocrine therapy and the risk of cardiovascular diseases and type 2 diabetes among breast cancer survivors in Korea, in consideration of various age groups.
Methods and Results
In the National Health Insurance Service database of Korea, a total of 133 171 patients with breast cancer aged ≥20 years were included in the current study. Endocrine therapy was treated as time‐varying exposure, and patients were categorized as nonusers, selective estrogen receptor modulator users, aromatase inhibitor users, and both users. Time‐dependent Cox regression models were used to estimate hazard ratios (HRs) and 95% CIs. Age at diagnosis, socioeconomic status, histological type, other treatments, and comorbidities were adjusted in the model. Compared with nonusers, selective estrogen receptor modulator users were associated with higher risks of stroke (HR, 1.20 [95% CI, 1.04–1.40]) and venous thromboembolism (HR, 1.47 [95% CI, 1.13–1.90]), whereas aromatase inhibitor users were associated with a higher risk of coronary heart disease (HR, 1.22 [95% CI, 1.06–1.41]). The risk of type 2 diabetes was associated with selective estrogen receptor modulator users (HR, 1.13 [95% CI, 1.05–1.21]), aromatase inhibitor users (HR, 1.14 [95% CI, 1.05–1.23]), and both users (HR, 1.24 [95% CI, 1.10–1.39]). In particular, the risk of a composite of cardiovascular diseases was higher in younger or premenopausal patients.
Conclusions
In breast cancer survivors in Korea, endocrine therapy is associated with a higher risk of cardiovascular diseases and type 2 diabetes. Monitoring of cancer comorbidities after endocrine therapy is needed in younger and older patients.
Keywords: breast cancer, cardiovascular diseases, comorbidities, endocrine therapy, type 2 diabetes
Subject Categories: Cardiovascular Disease; Epidemiology; Diabetes, Type 2; Cardiotoxicity
Nonstandard Abbreviations and Acronyms
- AI
aromatase inhibitor
- NHIS
National Health Insurance Service
- PSM
propensity score matching
- SERM
selective estrogen receptor modulator
- T2D
type 2 diabetes
Clinical Perspective.
What Is New?
As a large, longitudinal, population‐based study of Korea (N=133 171), endocrine therapy was associated with elevated risks of a composite of cardiovascular diseases and type 2 diabetes in patients with breast cancer diagnosed from 2006 to 2016.
The increased risk of cardiovascular diseases could be affected by menopausal status and the age of diagnosis among patients with breast cancer, whereas the risk of type 2 diabetes was higher regardless of age.
What Are the Clinical Implications?
It is necessary to manage cancer comorbidities in younger endocrine therapy users, as well as in older breast cancer survivors.
Monitoring of cancer comorbidities after endocrine therapy in various age groups may contribute to the healthy prognosis of patients with breast cancer.
Breast cancer is the most commonly diagnosed cancer in women, and its prevalence is steadily increasing worldwide. 1 , 2 The incidence of breast cancer in Korea continues to increase across all age groups. Some of the key features of breast cancer in Korea are its higher incidence in younger women aged 40 to 49 years, an increasing proportion of premenopausal women, and a higher survival rate than in other countries. Improved cancer screening and advanced cancer treatment will further increase the number of breast cancer survivors. 2
It has been reported that many breast cancer survivors die from noncancer diseases, especially cardiovascular disease (CVD) and diabetes, rather than their diagnosed cancer. 3 , 4 This is likely to be affected by common risk factors (such as age, alcohol intake, tobacco use, obesity, physical activity, diet, and adverse events caused by cancer treatment) between breast cancer and other non‐communicable diseases. 5
Endocrine therapies used for hormone receptor‐positive patients with breast cancer are classified as selective estrogen receptor modulators (SERMs) such as tamoxifen, which are involved in binding to estrogen receptors as an antagonist in breast cancer tissues, and aromatase inhibitors (AIs), which are involved in the inhibition of estrogen production. 5 , 6 , 7 Although endocrine therapy is effective for breast cancer recurrence and mortality, its antiestrogen effects can increase the risk of cancer comorbidities. Previous studies investigating their antiestrogen roles in CVD and type 2 diabetes (T2D) have reported inconsistent results, and the findings on AI uses are relatively insufficient compared with SERM use. 8 , 9 , 10 , 11 , 12 , 13 Furthermore, most of the results were for elderly patients with breast cancer, and studies that reflect the characteristics of patients with breast cancer in Korea are necessary.
The National Health Insurance Service (NHIS) claims database provides information on the entire Korean population; thus, it is possible to identify nationwide patients with breast cancer diagnosed between 2006 and 2016 in Korea. The current study aimed to investigate the association between endocrine therapy and the risk of CVD and T2D among breast cancer survivors in Korea, using the NHIS database.
METHODS
The analytical methods and study material will be available to other researchers on request for purposes of reproducing the results or replicating the procedure. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of the NHIS committee.
Data Source and Study Population
The NHIS provides a population‐based database of the health information of the entire population in Korea, including insurance eligibility, medical treatment and history, and health screening records. We used the NHIS database between 2002 and 2017, which comprises the qualification and contribution health use database including diagnostic records and prescription records, health check‐up, and cancer screening databases. The detailed information of the NHIS database were described in previous studies. 14 , 15 The institutional review board of Seoul National Hospital, Seoul, Korea approved the current study protocol (C‐1905‐067‐1033). The informed consent was waived because the NHIS provides the database anonymized.
Our study population included patients with newly diagnosed invasive or in situ breast cancer between 2006 and 2016 (N=310 919). The International Classification of Diseases, Tenth Revision (ICD‐10) was used to identify the diagnostic diseases. Patients with invasive breast cancer were defined as those with a diagnostic code of C50 and a V193 code. Women who had a diagnostic code of D05 with the V193 code and without a diagnosis of C50 within 90 days were considered patients with in situ breast cancer. The V193 code has been used to identify patients with cancer more definitively. 7 The first diagnosed date of breast cancer between 2006 and 2016 was considered the entry date for the study population. Exclusion criteria were applied from 2002 until before the entry date for the following reasons: (1) subjects who had a history of diseases (any type of cancer, CVD, or T2D), (2) previous prescription records of endocrine therapy, (3) patients who survived ≤1 year after the first diagnosis of breast cancer, (4) the first diagnosis at age ≤19 years, and (5) patients without a V193 code. After applying the exclusion criteria, a total of 133 171 patients were included in the current study. Patients with breast cancer were categorized as nonusers (N=36 699, 27.6%), SERM users (N=61 195, 46.0%), AI users (N=24 633, 18.5%), and both users (N=10 644, 8.0%; Figure 1).
Figure 1. Flowchart for the included study population.
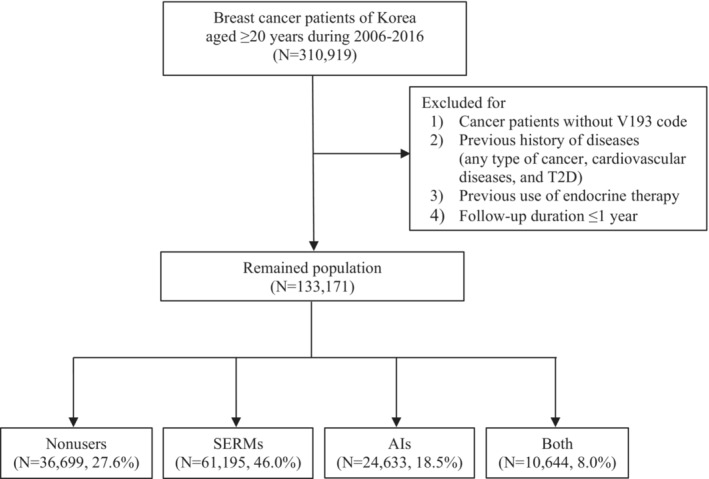
AIs indicates aromatase inhibitors; SERMs, selective estrogen receptor modulators; and T2D, type 2 diabetes.
Definition of Exposure and Outcomes
Exposure was categorized as: (1) nonusers, (2) SERM users, (3) AI users, and (4) both users. According to the Anatomical Therapeutic Chemical classification system, those who had at least 1 prescription record of antiestrogen (L02BA: tamoxifen, toremifene) and aromatase inhibitors (L02BG: anastrozole, letrozole, and exemestane) after the first diagnosis of breast cancer were defined as SERM and AI users, respectively. If SERM or AI users switched their regimen types after the first prescription, they were classified as both users to reflect the time‐updated definition. Those who had no prescription records of endocrine therapy during the follow‐up period were defined as nonusers.
Diagnostic CVDs and T2D were considered as the study outcomes. The CVD event was defined as those with at least 1 inpatient medical record or at least 2 outpatient medical records. The composite of CVDs was divided into stroke (ICD‐10: I60‐64), coronary heart disease (CHD; ICD‐10: I20‐25), venous thromboembolism (VTE; ICD‐10: I26, I80, I82), heart failure (HF; ICD‐10: I50), and arrhythmia (ICD‐10: I47‐49). 16 , 17 The T2D event was defined as having diagnostic codes of E11 to E14 with prescriptions of antidiabetic drugs or at least 2 claims of diagnostic codes of E11 to E14 within 365 days. 18 The follow‐up duration was calculated from the entry date until the first diagnosis of interest of outcomes (CVD or T2D), other cancers, all‐cause death, or last date of this study (December 31, 2017), whichever came first.
Covariates
Demographic characteristics at baseline were identified using qualification and contribution databases. Age at diagnosis was classified as <40, 40 to 44, 45 to 49, 50 to 54, 55 to 59, 60 to 64, 65 to 69, and ≥70 years. Insurance‐based income level for socioeconomic status was divided into quartile 1 (lowest), including medical aid beneficiaries, and quartile 2, quartile 3, and quartile 4 (highest). Residential region was classified as metropolitan, urban, or rural. Invasive and in situ breast cancers were considered histological subtypes. Information about patients who underwent surgery and other cancer treatments (chemotherapy, radiotherapy, and trastuzumab) within 1 year of the first diagnosis of breast cancer was included. A detailed definition of breast cancer–related treatments has been provided in a previous study. 7 Comorbidities were identified from medical records 1 year before the entry date. Hypertension, dyslipidemia, and osteoporosis were considered as the comorbidities.
Data on body mass index, lifestyle factors (smoking status, alcohol consumption, and physical activity), and menopausal status were available for some patients who underwent health screening before the entry date. Body mass index was categorized as <23 and ≥23.0 kg/m2. Smoking status, alcohol consumption, and physical activity were classified as never/ever or yes/no. Menopausal status was divided into premenopausal and postmenopausal status.
Statistical Analysis
The χ2 test for categorical variables and the ANOVA for continuous variables were conducted. Multinomial logistic regression models were used to estimate a mutually adjusted odds ratio and 95% CI for the comparison of the characteristics of the study population according to endocrine therapy. Time‐dependent Cox regression models were used to estimate the hazard ratio (HR) and 95% CI for associations between endocrine therapy and the risk of CVDs and T2D compared with nonusers. In a comparative analysis among endocrine therapy users, SERM users were considered as a reference group. Endocrine therapy users may have (the) immortal time between entry date and first prescription date. Thus, it was treated as a nonexposed period to consider time‐related bias. 19 , 20 The multivariable‐adjusted model for CVD and T2D included age at diagnosis (continuous), insurance‐based income level, region of residence, histological subtype, surgery, chemotherapy, radiotherapy, trastuzumab, hypertension, dyslipidemia, osteoporosis, and T2D (or CVD).
Subgroup analyses by various age groups (<55 versus ≥55 years, <60 versus ≥60 years; <55 years [40–54 and 45–54 years]; and <60 years [40–59 and 45–59 years]) were performed to investigate further associations in younger and older patients. Subgroup analyses by histological subtype (invasive and in situ), surgery, chemotherapy, radiotherapy, and trastuzumab were conducted to assess the differential effects on CVD or T2D. We also assessed the associations by regimen types of SERMs (tamoxifen and toremifene) and AIs (anastrozole, letrozole, and exemestane).
Various sensitivity analyses were performed to assess the robustness of our results. First, we excluded short‐term endocrine therapy users of <6 months or <12 months to consider the depletion of susceptible bias associated with an early increase in acute events. 21 Second, we excluded the patients who were followed‐up for <5 years to consider a sufficient time period to identify the incident cases of CVD. Third, the endocrine therapy users were defined as those who had at least 2 prescriptions of regimen to identify the possible misclassification of exposure definition. Fourth, body mass index, lifestyle factors (smoking, alcohol consumption, and physical activity), and menopausal status were further adjusted in patients who underwent health screening because of data availability. In addition, the subgroup analysis by menopausal status was performed in the health screening subjects. Fifth, we compared the results of CVDs in patients with a previous history of CVD at baseline. Lastly, the associations after the propensity score matching (PSM) between 2 groups (SERM users versus nonusers, AI users versus nonusers, and AI users versus SERM users) were also conducted. Each group was matched as the 1:1 ratio according to age at diagnosis (continuous), insurance‐based income level, region of residence, histological type, surgery, chemotherapy, radiotherapy, trastuzumab, hypertension, dyslipidemia, and osteoporosis. For the comparison of the results before PSM, T2D, or CVD was additionally adjusted for each CVD and T2D outcome. The balance of covariate was checked by standardized mean difference for each comparative group (standardized mean difference <0.1). In addition, a false discovery rate for multiple testing was performed, considering the number of P values from the analyses (P for false discovery rate <0.5). A heterogeneity test was conducted on the subgroup results. If the P value was <0.1 or I 2>50%, we hypothesized that the associations by the stratified factors were significantly heterogeneous. SAS statistical software version 9.4 (SAS Institute, Cary, NC) and the meta package of R version 4.1.0 software (R Foundation for Statistical Computing, Vienna, Austria) were used for all the analyses.
RESULTS
Table 1 shows the baseline characteristics of the included study population for CVD and T2D. The mean (SD) age for nonusers, SERM users, AI users, and both users were 50.1 (10.8), 45.5 (8.3), 58.2 (7.8), and 51.2 (8.7) years, respectively. Compared with nonusers, SERM users were younger, and patients were more likely to have in situ breast cancer. However, the AI users were older and were patients with invasive breast cancer. All of the SERM, AI, and both users underwent surgery, were treated with radiotherapy, and were not treated with trastuzumab compared with nonusers. Although SERM and AI users were treated less with chemotherapy, both users were treated more with chemotherapy than nonusers. In the health screening subjects (N=72 063; 54% of the total population), the SERM users were patients who were premenopausal, and AI users were patients who were postmenopausal and overweight or obese (Table S1).
Table 1.
Baseline Characteristics of Study Population for Cardiovascular Diseases and Type 2 Diabetes
| Characteristic | Nonusers, N=36 699, 27.6% | SERM users, N=61 195, 46.0% | SERM users vs nonusers, OR (95% CI)* | AI users, N=24 633, 18.5% | AI users vs nonusers, OR (95% CI)* | Both, N=10 644, 8.0% | Both vs nonusers, OR (95% CI)* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at diagnosis, y | ||||||||||||||
| Mean (SD) | 50.1 | (10.8) | 45.5 | (8.3) | 58.2 | (7.8) | 51.2 | (8.7) | ||||||
| <40 | 6058 | (16.5) | 11 702 | (19.1) | 1.89 | (1.81–1.98) | 90 | (0.4) | 0.02 | (0.01–0.02) | 607 | (5.7) | 0.25 | (0.23–0.28) |
| 40–44 | 5153 | (14.0) | 16 817 | (27.5) | 3.08 | (2.94–3.23) | 257 | (1.0) | 0.05 | (0.04–0.06) | 1266 | (11.9) | 0.63 | (0.58–0.68) |
| 45–49 | 6752 | (18.4) | 18 856 | (30.8) | 2.58 | (2.47–2.70) | 1789 | (7.3) | 0.27 | (0.25–0.28) | 3226 | (30.3) | 1.23 | (1.15–1.30) |
| 50–54 | 7270 | (19.8) | 8082 | (13.2) | 1.00 | (Reference) | 6882 | (27.9) | 1.00 | (Reference) | 2651 | (24.9) | 1.00 | (Reference) |
| 55–59 | 5115 | (13.9) | 2161 | (3.5) | 0.38 | (0.35–0.40) | 6368 | (25.9) | 1.39 | (1.32–1.47) | 1259 | (11.8) | 0.72 | (0.66–0.77) |
| 60–64 | 2941 | (8.0) | 1410 | (2.3) | 0.41 | (0.38–0.45) | 4432 | (18.0) | 1.64 | (1.54–1.74) | 752 | (7.1) | 0.72 | (0.66–0.79) |
| 65–69 | 1623 | (4.4) | 939 | (1.5) | 0.50 | (0.46–0.55) | 2445 | (9.9) | 1.68 | (1.56–1.81) | 448 | (4.2) | 0.78 | (0.69–0.88) |
| ≥70 | 1787 | (4.9) | 1228 | (2.0) | 0.56 | (0.52–0.61) | 2370 | (9.6) | 1.47 | (1.36–1.59) | 435 | (4.1) | 0.69 | (0.61–0.77) |
| Insurance‐based income | ||||||||||||||
| Q1 (lowest) | 9214 | (25.1) | 13 924 | (22.8) | 1.00 | (Reference) | 6241 | (25.3) | 1.00 | (Reference) | 2610 | (24.5) | 1.00 | (Reference) |
| Q2 | 7029 | (19.2) | 11 407 | (18.6) | 1.02 | (0.98–1.07) | 4494 | (18.2) | 1.04 | (0.98–1.09) | 2034 | (19.1) | 1.03 | (0.97–1.11) |
| Q3 | 8686 | (23.7) | 13 994 | (22.9) | 1.02 | (0.97–1.06) | 5719 | (23.2) | 1.05 | (1.00–1.11) | 2339 | (22.0) | 1.01 | (0.95–1.08) |
| Q4 (highest) | 11 770 | (32.1) | 21 870 | (35.7) | 1.08 | (1.04–1.12) | 8179 | (33.2) | 1.01 | (0.97–1.06) | 3661 | (34.4) | 1.08 | (1.02–1.15) |
| Region of residence | ||||||||||||||
| Metropolitan | 18 619 | (50.7) | 30 269 | (49.5) | 1.00 | (Reference) | 12 662 | (51.4) | 1.00 | (Reference) | 5432 | (51.0) | 1.00 | (Reference) |
| Urban | 7736 | (21.1) | 14 277 | (23.3) | 1.05 | (1.01–1.09) | 4747 | (19.3) | 0.98 | (0.93–1.02) | 2299 | (21.6) | 1.01 | (0.95–1.07) |
| Rural | 10 244 | (27.9) | 16 526 | (27.0) | 1.05 | (1.02–1.09) | 7189 | (29.2) | 1.01 | (0.97–1.05) | 2899 | (27.2) | 0.96 | (0.91–1.01) |
| Histological type | ||||||||||||||
| Invasive | 33 252 | (90.6) | 52 004 | (85.0) | 1.00 | (Reference) | 24 530 | (99.6) | 1.00 | (Reference) | 10 459 | (98.3) | 1.00 | (Reference) |
| In situ | 3447 | (9.4) | 9191 | (15.0) | 1.29 | (1.23–1.36) | 103 | (0.4) | 0.03 | (0.02–0.03) | 185 | (1.7) | 0.16 | (0.14–0.19) |
| Surgery | ||||||||||||||
| No | 7004 | (19.1) | 7087 | (11.6) | 1.00 | (Reference) | 2587 | (10.5) | 1.00 | (Reference) | 1613 | (15.2) | 1.00 | (Reference) |
| Yes | 29 695 | (80.9) | 54 108 | (88.4) | 1.85 | (1.78–1.93) | 22 046 | (89.5) | 1.96 | (1.86–2.07) | 9031 | (84.9) | 1.15 | (1.08–1.23) |
| Chemotherapy | ||||||||||||||
| No | 11 404 | (31.1) | 27 663 | (45.2) | 1.00 | (Reference) | 8575 | (34.8) | 1.00 | (Reference) | 2748 | (25.8) | 1.00 | (Reference) |
| Yes | 25 295 | (68.9) | 33 532 | (54.8) | 0.45 | (0.44–0.47) | 16 058 | (65.2) | 0.76 | (0.73–0.79) | 7896 | (74.2) | 1.09 | (1.03–1.15) |
| Radiotherapy | ||||||||||||||
| No | 15 348 | (41.8) | 18 670 | (30.5) | 1.00 | (Reference) | 7292 | (29.6) | 1.00 | (Reference) | 3579 | (33.6) | 1.00 | (Reference) |
| Yes | 21 351 | (58.2) | 42 525 | (69.5) | 1.60 | (1.55–1.65) | 17 341 | (70.4) | 1.84 | (1.77–1.91) | 7065 | (66.4) | 1.34 | (1.28–1.41) |
| Trastuzumab | ||||||||||||||
| No | 30 794 | (83.9) | 56 672 | (92.6) | 1.00 | (Reference) | 22 283 | (90.5) | 1.00 | (Reference) | 9867 | (92.7) | 1.00 | (Reference) |
| Yes | 5905 | (16.1) | 4523 | (7.4) | 0.57 | (0.54–0.59) | 2350 | (9.5) | 0.45 | (0.43–0.48) | 777 | (7.3) | 0.35 | (0.33–0.38) |
| Hypertension | ||||||||||||||
| No | 32 012 | (87.2) | 56 704 | (92.7) | 1.00 | (Reference) | 18 591 | (75.5) | 1.00 | (Reference) | 9223 | (86.7) | 1.00 | (Reference) |
| Yes | 4687 | (12.8) | 4491 | (7.3) | 1.03 | (0.98–1.09) | 6042 | (24.5) | 1.15 | (1.10–1.21) | 1421 | (13.4) | 1.07 | (1.00–1.15) |
| Dyslipidemia | ||||||||||||||
| No | 33 331 | (90.8) | 57 561 | (94.1) | 1.00 | (Reference) | 20 738 | (84.2) | 1.00 | (Reference) | 9787 | (92.0) | 1.00 | (Reference) |
| Yes | 3368 | (9.2) | 3634 | (5.9) | 0.94 | (0.89–0.99) | 3895 | (15.8) | 1.06 | (1.00–1.12) | 857 | (8.1) | 0.85 | (0.78–0.92) |
| Osteoporosis | ||||||||||||||
| No | 33 696 | (91.8) | 57 711 | (94.3) | 1.00 | (Reference) | 21 617 | (87.8) | 1.00 | (Reference) | 9638 | (90.6) | 1.00 | (Reference) |
| Yes | 3003 | (8.2) | 3484 | (5.7) | 0.97 | (0.92–1.02) | 3016 | (12.2) | 0.94 | (0.89–0.99) | 1006 | (9.5) | 1.19 | (1.10–1.29) |
Data are presented as n (%) unless otherwise indicated. AI indicates aromatase inhibitor; OR, odds ratio; Q, quartile; and SERM, selective estrogen receptor modulator.
The OR for each covariate was adjusted for other covariates in the multinomial logistic regression model.
Overall Association of CVD and T2D
Table 2 shows the overall association of endocrine therapy on CVD and T2D outcomes. The median follow‐up duration was 4.73 years, and the mean follow‐up duration was 5.27 (SD, 3.05) years. Compared with nonusers, the higher risks of a composite of CVDs were observed in SERM users (HR, 1.13 [95% CI, 1.05–1.21]), AI users (HR, 1.14 [95% CI, 1.05–1.23]), and both users (HR, 1.24 [95% CI, 1.10–1.39]). For specific CVD outcomes, the risk of stroke was higher in SERM users (HR, 1.20 [95% CI, 1.04–1.40]), the risk of CHD was higher in AI users (HR, 1.22 [95% CI, 1.06–1.41]), and the risk of VTE was higher in SERM (HR, 1.47 [95% CI, 1.13–1.90]) and both users (HR, 1.72 [95% CI, 1.15–2.55]) than in nonusers.
Table 2.
Associations of Endocrine Therapy With CVD and T2D Comparison With Nonusers
| Total | No. of events | (%) | 1000 PY | Age‐adjusted HR (95% CI) | Multivariable‐adjusted HR (95% CI)* | P value for FDR† | |||
|---|---|---|---|---|---|---|---|---|---|
| Composite of CVD | |||||||||
| Nonusers | 36 699 | 1339 | (3.65) | 190.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| SERM users | 61 195 | 1526 | (2.49) | 318.0 | 1.05 | (0.98–1.13) | 1.13 | (1.05–1.21) | 0.003 |
| AI users | 24 633 | 1159 | (4.71) | 120.2 | 1.08 | (1.00–1.16) | 1.14 | (1.05–1.23) | 0.003 |
| Both | 10 644 | 377 | (3.54) | 73.4 | 1.20 | (1.07–1.34) | 1.24 | (1.10–1.39) | 0.002 |
| Stroke | |||||||||
| Nonusers | 36 699 | 335 | (0.91) | 190.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| SERM users | 61 195 | 356 | (0.58) | 318.0 | 1.15 | (0.99–1.33) | 1.20 | (1.04–1.40) | 0.048 |
| AI users | 24 633 | 337 | (1.37) | 120.2 | 1.09 | (0.94–1.26) | 1.16 | (0.99–1.35) | 0.124 |
| Both | 10 644 | 88 | (0.83) | 73.4 | 1.11 | (0.88–1.41) | 1.13 | (0.89–1.44) | 0.462 |
| CHD | |||||||||
| Nonusers | 36 699 | 414 | (1.13) | 190.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| SERM users | 61 195 | 465 | (0.76) | 318.0 | 1.02 | (0.89–1.16) | 1.03 | (0.90–1.18) | 0.679 |
| AI users | 24 633 | 389 | (1.58) | 120.2 | 1.18 | (1.03–1.35) | 1.22 | (1.06–1.41) | 0.033 |
| Both | 10 644 | 110 | (1.03) | 73.4 | 1.12 | (0.90–1.39) | 1.14 | (0.92–1.42) | 0.442 |
| VTE | |||||||||
| Nonusers | 36 699 | 96 | (0.26) | 190.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| SERM users | 61 195 | 154 | (0.25) | 318.0 | 1.35 | (1.04–1.74) | 1.47 | (1.13–1.90) | 0.012 |
| AI users | 24 633 | 74 | (0.30) | 120.2 | 1.10 | (0.82–1.49) | 1.17 | (0.86–1.58) | 0.388 |
| Both | 10 644 | 35 | (0.33) | 73.4 | 1.65 | (1.11–2.45) | 1.72 | (1.15–2.55) | 0.015 |
| HF | |||||||||
| Nonusers | 36 699 | 231 | (0.63) | 190.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| SERM users | 61 195 | 206 | (0.34) | 318.0 | 0.75 | (0.63–0.90) | 0.98 | (0.81–1.18) | 0.794 |
| AI users | 24 633 | 156 | (0.63) | 120.2 | 0.94 | (0.77–1.15) | 1.05 | (0.85–1.29) | 0.794 |
| Both | 10 644 | 60 | (0.56) | 73.4 | 1.24 | (0.93–1.66) | 1.41 | (1.05–1.89) | 0.065 |
| Arrhythmia | |||||||||
| Nonusers | 36 699 | 263 | (0.72) | 190.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| SERM users | 61 195 | 346 | (0.57) | 318.0 | 1.12 | (0.96–1.31) | 1.14 | (0.97–1.34) | 0.244 |
| AI users | 24 633 | 205 | (0.83) | 120.2 | 1.07 | (0.89–1.28) | 1.08 | (0.90–1.30) | 0.493 |
| Both | 10 644 | 84 | (0.79) | 73.4 | 1.28 | (1.00–1.64) | 1.28 | (0.99–1.64) | 0.244 |
| T2D | |||||||||
| Nonusers | 36 699 | 1581 | (4.31) | 190.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| SERM users | 61 195 | 2136 | (3.49) | 318.0 | 1.16 | (1.09–1.24) | 1.22 | (1.14–1.30) | <0.001 |
| AI users | 24 633 | 1490 | (6.05) | 120.2 | 1.22 | (1.14–1.31) | 1.22 | (1.14–1.31) | <0.001 |
| Both | 10 644 | 477 | (4.48) | 73.4 | 1.25 | (1.13–1.38) | 1.24 | (1.12–1.38) | <0.001 |
AI indicates aromatase inhibitor; CHD, coronary heart disease; CVD, cardiovascular disease; FDR, false discovery rate; HF, heart failure; HR, hazard ratio; PY, person‐years; SERM, selective estrogen receptor modulator; T2D, type 2 diabetes; and VTE, venous thromboembolism.
Multivariable‐adjusted model included age at diagnosis (continuous), insurance‐based income, region of residence, histological subtype, surgery, chemotherapy, radiotherapy, trastuzumab, hypertension, dyslipidemia, osteoporosis, and T2D (or CVD).
Significance remained after FDR for multiple test (P value for FDR<0.05).
Table 3 shows the associations of CVD and T2D among endocrine therapy users compared with SERM users. Although the risk of CHD was higher in AI than SERM users, there were no significant associations after multiple testing.
Table 3.
Associations of Endocrine Therapy With CVD and T2D Comparison With SERMs
| Total | No. of events | (%) | 1000 PY | Age‐adjusted HR (95% CI) | Multivariable‐adjusted HR (95% CI)* | P value for FDR† | |||
|---|---|---|---|---|---|---|---|---|---|
| Composite of CVD | |||||||||
| SERM users | 61 195 | 1526 | (2.49) | 318.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| AI users | 24 633 | 1159 | (4.71) | 120.2 | 1.02 | (0.95–1.11) | 1.01 | (0.93–1.09) | 0.841 |
| Both | 10 644 | 377 | (3.54) | 73.4 | 1.14 | (1.01–1.27) | 1.10 | (0.98–1.24) | 0.116 |
| Stroke | |||||||||
| SERM users | 61 195 | 356 | (0.58) | 318.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| AI users | 24 633 | 337 | (1.37) | 120.2 | 0.95 | (0.82–1.10) | 0.96 | (0.83–1.12) | 0.624 |
| Both | 10 644 | 88 | (0.83) | 73.4 | 0.97 | (0.76–1.22) | 0.94 | (0.74–1.19) | 0.624 |
| CHD | |||||||||
| SERM users | 61 195 | 465 | (0.76) | 318.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| AI users | 24 633 | 389 | (1.58) | 120.2 | 1.16 | (1.01–1.33) | 1.19 | (1.03–1.37) | 0.051 |
| Both | 10 644 | 110 | (1.03) | 73.4 | 1.10 | (0.89–1.36) | 1.11 | (0.90–1.37) | 0.495 |
| VTE | |||||||||
| SERM users | 61 195 | 154 | (0.25) | 318.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| AI users | 24 633 | 74 | (0.30) | 120.2 | 0.82 | (0.62–1.09) | 0.80 | (0.60–1.06) | 0.176 |
| Both | 10 644 | 35 | (0.33) | 73.4 | 1.23 | (0.85–1.79) | 1.17 | (0.80–1.71) | 0.416 |
| HF | |||||||||
| SERM users | 61 195 | 206 | (0.34) | 318.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| AI users | 24 633 | 156 | (0.63) | 120.2 | 1.25 | (1.01–1.54) | 1.07 | (0.87–1.33) | 0.794 |
| Both | 10 644 | 60 | (0.56) | 73.4 | 1.66 | (1.24–2.22) | 1.44 | (1.08–1.94) | 0.065 |
| Arrhythmia | |||||||||
| SERM users | 61 195 | 346 | (0.57) | 318.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| AI users | 24 633 | 205 | (0.83) | 120.2 | 1.14 | (0.90–1.45) | 0.95 | (0.79–1.14) | 0.584 |
| Both | 10 644 | 84 | (0.79) | 73.4 | 1.05 | (1.04–1.05) | 1.12 | (0.88–1.43) | 0.493 |
| T2D | |||||||||
| SERM users | 61 195 | 2136 | (3.49) | 318.0 | 1.00 | (Reference) | 1.00 | (Reference) | |
| AI users | 24 633 | 1490 | (6.05) | 120.2 | 1.08 | (0.97–1.19) | 1.00 | (0.94–1.08) | 0.901 |
| Both | 10 644 | 477 | (4.48) | 73.4 | 1.05 | (1.05–1.05) | 1.02 | (0.92–1.13) | 0.830 |
AI indicates aromatase inhibitor; CHD, coronary heart disease; CVD, cardiovascular disease; FDR, false discovery rate; HF, heart failure; HR, hazard ratio; PY, person‐years; SERM, selective estrogen receptor modulator; T2D, type 2 diabetes; and VTE, venous thromboembolism.
Multivariable‐adjusted model included age at diagnosis (continuous), income level, region of residence, histological subtype, surgery, chemotherapy, radiotherapy, trastuzumab, hypertension, dyslipidemia, osteoporosis, and T2D (or CVD).
Significance remained after FDR for multiple test (P for FDR<0.05).
Subgroup and Sensitivity Analyses
Summarized results of various subgroup and sensitivity analyses of each outcome were presented as forest plots (Figures 2 and 3, and Figures S1 through S5). More detailed results were presented in Tables S2 through S23.
Figure 2. Summary of results for composite of cardiovascular diseases (comparison with nonusers).
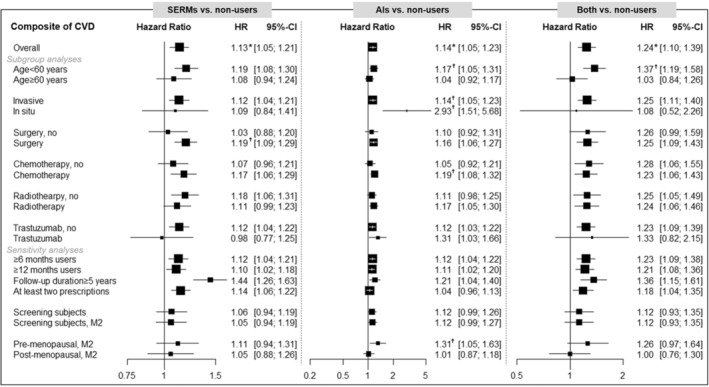
The multivariable‐adjusted model included age at diagnosis (continuous), insurance‐based income level, region of residence, histological subtype, surgery, chemotherapy, radiotherapy, trastuzumab, hypertension, dyslipidemia, osteoporosis, and type 2 diabetes. However, Model 2 (M2) additionally includes body mass index, lifestyle factors, and menopausal status. The asterisk (*) indicates the significance after multiple testing by a false discovery rate (FDR) (P for FDR<0.05) in Table 2. The dagger (†) symbol indicates the results of heterogeneity (P <0.1 or I 2>50%) by the stratified factor in the supplementary material. AIs indicates aromatase inhibitors; CVD, cardiovascular disease; HR, hazard ratio; and SERMs, selective estrogen receptor modulators.
Figure 3. Summary of results for type 2 diabetes (comparison with nonusers).
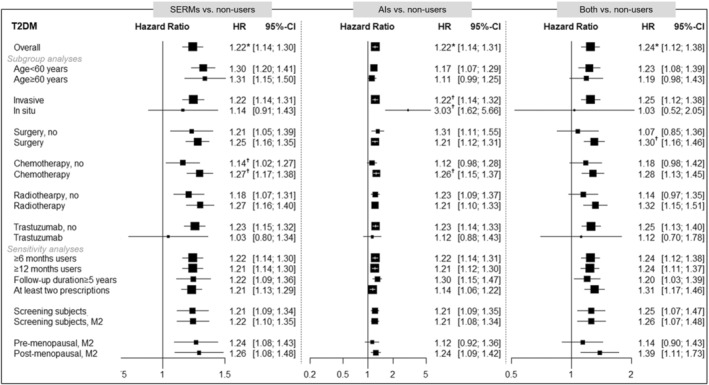
The multivariable‐adjusted model included age at diagnosis (continuous), insurance‐based income level, region of residence, histological subtype, surgery, chemotherapy, radiotherapy, trastuzumab, hypertension, dyslipidemia, osteoporosis, and cardiovascular disease. However, Model 2 (M2) additionally includes body mass index, lifestyle factors, and menopausal status. The asterisk (*) indicates the significance after multiple testing by a false discovery rate (FDR) (P for FDR<0.05) in Table 2. The dagger (†) symbol indicates the results of heterogeneity (P<0.1 or I 2>50%) by the stratified factor in the supplementary material. AIs indicates aromatase inhibitors; HR, hazard ratio; SERMs, selective estrogen receptor modulators; and T2D, type 2 diabetes.
The risk of composite CVDs was significantly heterogeneous with age at diagnosis in SERM, AI, and both users (Figure 2). Moreover, SERM users have a significant difference by surgery, whereas AI users have significant heterogeneity by histological type, chemotherapy, trastuzumab, and menopausal status. In particular, the risk of composite CVD with AI users was significantly elevated in younger or patients who were premenopausal. The risk of T2D was significantly heterogeneous with age at diagnosis, histological type, and chemotherapy (Figure 3). Unlike CVD, the risk of T2D was higher in older, in situ, and patients who were treated with chemotherapy. Figures S1 through S5 show the summarized forest plots for stroke, CHD, VTE, HF, and arrhythmia. In regimen types, the risk of VTE was higher in tamoxifen, and the risk of T2D was higher in tamoxifen and letrozole, compared with nonusers. Tables S2 through S12 show all of the results of subgroup analyses by age at diagnosis, histological type, surgery, chemotherapy, radiotherapy, trastuzumab, regimen type, and menopausal status.
To assess the robustness of our results, various sensitivity analyses were performed. First, similar associations were observed after excluding users <6 or <12 months; however, the significant association of stroke disappeared in SERM users who were prescribed for >12 months (Table S13). Second, patients who were followed up for >5 years showed stronger associations of a composite of CVDs, stroke, and CHD in both SERM and AI users; whereas the risk of VTE with SERM users was insignificant (Table S14). Third, the overall associations were not different in the users having at least 2 prescriptions for endocrine therapy (Table S15). Fourth, there was no difference in the results of further adjustments for body mass index, lifestyle factors, and menopausal status, although statistical significance was maintained only in the risk of VTE by SERM users (Table S16). Fifth, in the patients with a previous history of CVD, higher risks of stroke and HF by SERM users were observed (Table S17). Lastly, the results after PSM were compared with the overall findings. The matched covariates achieved the balance in each comparative group (Tables S18 through S20). After PSM, the risks of VTE and T2D remained in SERM users compared with nonusers (Table S21). However, the significance of results by AI users compared with nonusers disappeared after PSM (Table S22). In the PSM of SERM users with AI users, a lower risk of T2D in AI users was observed than in SERM users (Table S23).
DISCUSSION
Among Koreans diagnosed with breast cancer from 2006 to 2016 using the NHIS of Korea, SERM users were associated with higher risks of stroke and VTE, whereas AI users were associated with higher risk of CHD, compared with nonusers. Compared with nonusers, the risk of T2D was higher in both SERM and AI users, regardless of age. The higher risks of VTE and T2D in SERM users compared with nonusers still remained after PSM. In addition, from the results considering various age subgroups, our findings suggest that younger patients or patients who are premenopausal should also be monitored for cancer comorbidities.
Previous studies have focused on elderly patients (mean age >65 years) 22 , 23 ; thus, conducting further studies considering various age groups has been suggested. 24 In addition, the incidence of breast cancer in Korea is higher in patients who are young (especially aged 40–49 years) and premenopausal than in Western countries. 2 Thus, we conducted various subgroup analyses including young patients with breast cancer to reflect these characteristics, and also compared AI users with SERM users and compared users (SERM, AI, and both users) with nonusers to investigate the antiestrogen roles of endocrine therapy in CVD and T2D. Consistent with previous studies, 8 , 23 , 25 we identified that the VTE risk was higher in SERM users, especially tamoxifen (HR, 1.36 [95% CI, 1.03–1.79]; Table S11), compared with nonusers. In contrast, the risks of CHD and stroke were inconsistent, 23 , 26 , 27 and we found a higher risk of stroke in SERM users and a higher risk of CHD in AI users compared with nonusers. However, the higher risk of VTE in SERM users compared with nonusers only remained after PSM.
In our subgroup analyses, we identified that a higher risk of CVD outcomes remained in patients who were younger or premenopausal. A possible explanation for our subgroup results is that premenopausal women with a high risk of recurrence can optionally receive ovarian suppression treatments in addition to endocrine therapy. 6 In this case, premenopausal patients with breast cancer may have more severe estrogen deprivation, 6 , 28 which could be a risk factor for CVD. Further studies are necessary to determine the clinical characteristics of patients with breast cancer.
The evidence of myocardial diseases (HF and arrhythmia) is likely to be more inconclusive than other CVD outcomes. 8 , 22 , 23 , 26 , 29 Although the current study did not observe an overall association with endocrine therapy, we identified the higher risk of HF by SERM users in sensitivity analyses for patients followed up >5 years and patients with a history of CVD at baseline. Further studies are required to verify this association.
It has been reported that the risk of T2D was higher in tamoxifen users, but the evidence in AI users was insufficient. 12 , 13 , 30 Similar to SERMs, AIs can be involved in glucose metabolism by reducing endogenous estrogen levels. 25 Unlike previous studies with small sample sizes (range, 570–22 000), 31 our large population‐based study found an elevated risk of T2D among SERM, AI, and both users. In addition, a lower risk of T2D was observed in AI users than SERM users after PSM. Although the subgroup analysis by regimen type did not show difference in the present study, a recent study has suggested the potentially harmful effect of the toremifene regimen type. 32 Further studies based on a larger study population are required to compare with our findings.
This study has several limitations; therefore, our findings should be interpreted with caution. First, the clinical information of patients with breast cancer could not be considered, because the NHIS database was collected for administrative purposes. Although we considered other cancer treatments and histological subtypes in the analyses, insufficient clinical information, such as hormone receptors and cancer stage, may have interfered with the clear associations between endocrine therapy and CVD and T2D risk. CVD and T2D as the outcomes were defined by both ICD‐10 codes for diseases and Anatomical Therapeutic Chemical codes for regimen types based on the previous studies; however, the misclassification bias may still remain. Second, only some health‐screening subjects had information on lifestyle factors and menopause status. However, consistent results were identified from the sensitivity analysis after excluding subjects without health checkup information. Third, there was an age difference between the treatment groups, but our results could reflect the real‐world clinical practice of prescribing endocrine therapy depending on the menopausal status of the patient. However, these age differences were identified only in younger patients and not in elderly patients. In addition, through various subgroup analyses by age group, we confirmed that our results were comparable with those of previous studies. We also conducted the PSM to determine the robustness of our results. The significance of some results with AI users compared with nonusers disappeared; however, the statistical power was reduced compared with the overall findings. Thus, further studies to assess the AI users with CVD and T2D outcomes are required.
Nevertheless, our study had several strengths. This is a large population‐based study including all Korean patients with breast cancer from to 2006 to 2016 in the NHIS database covering the nationwide population of Korea. The definition of the study population was based on a previous study that proved similar to the number of patients with breast cancer in the Korea Central Cancer Registry database. 7 We also identified the differential effects of various age ranges and conducted comparisons of regimen types. By including a large number of AI users compared with previous studies, we could provide evidence of the individual effects of AIs against CVD and T2D. In addition, we attempted to consider the time‐related bias in the current study. A time‐dependent Cox model was used to consider the immortal time bias. Various sensitivity analyses were conducted to assess the robustness of our findings.
In conclusion, endocrine therapy is associated with a higher risk of CVD and T2D in breast cancer survivors. CHD risk was higher in AI users, whereas the risks of stroke and VTE were higher in SERM users than in nonusers. The increased risk of CVD could be affected by menopausal status and the age of patients with breast cancer. Although the risk of T2D did not differ between SERM and AI use, the risk was higher regardless of age. Therefore, it is necessary to manage cancer comorbidities in younger endocrine therapy users, as well as in older breast cancer survivors. Further studies are required to confirm these findings.
Sources of Funding
This research was supported by the grant of the Cancer Research Institute (0431–20190010), Seoul National University Hospital (2022), the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (HI19C1178), and the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF‐2018R1A2A3075397 and NRF‐2022R1A2B5B01002471).
Disclosures
None.
Supporting information
Tables S1–S23
Figures S1–S5
Acknowledgments
This work was supported by the institutional review board of Seoul National Hospital, Seoul, Korea (C‐1905‐067‐1033). This study used data collected by the NHIS (NHIS‐2019‐1‐660).
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026743
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Kang SY, Kim YS, Kim Z, Kim HY, Kim HJ, Park S, Bae SY, Yoon KH, Lee SB, Lee SK, et al. Breast cancer statistics in Korea in 2017: data from a breast cancer registry. J Breast Cancer. 2020;23:115–128. doi: 10.4048/jbc.2020.23.e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shin DW, Ahn E, Kim H, Park S, Kim YA, Yun YH. Non‐cancer mortality among long‐term survivors of adult cancer in Korea: National Cancer Registry Study. Cancer Causes Control. 2010;21:919–929. doi: 10.1007/s10552-010-9521-x [DOI] [PubMed] [Google Scholar]
- 5. Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz‐Flores S, Dent S, Kondapalli L, Ky B, Okwuosa T, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137:e30–e66. doi: 10.1161/CIR.0000000000000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Awan A, Esfahani K. Endocrine therapy for breast cancer in the primary care setting. Curr Oncol. 2018;25:285–291. doi: 10.3747/co.25.4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung IY, Lee J, Park S, Lee JW, Youn HJ, Hong JH, Hur H, Study of multi‐disciplin ATobcsG . Nationwide analysis of treatment patterns for Korean breast cancer survivors using National Health Insurance Service data. J Korean Med Sci. 2018;33:e276. doi: 10.3346/jkms.2018.33.e276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matthews A, Stanway S, Farmer RE, Strongman H, Thomas S, Lyon AR, Smeeth L, Bhaskaran K. Long term adjuvant endocrine therapy and risk of cardiovascular disease in female breast cancer survivors: systematic review. BMJ. 2018;363:k3845. doi: 10.1136/bmj.k3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lipscombe LL, Fischer HD, Yun L, Gruneir A, Austin P, Paszat L, Anderson GM, Rochon PA. Association between tamoxifen treatment and diabetes: a population‐based study. Cancer. 2012;118:2615–2622. doi: 10.1002/cncr.26559 [DOI] [PubMed] [Google Scholar]
- 10. Hamood R, Hamood H, Merhasin I, Keinan‐Boker L. Risk of cardiovascular disease after radiotherapy in survivors of breast cancer: a case‐cohort study. J Cardiol. 2019;73:280–291. doi: 10.1016/j.jjcc.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 11. Santorelli ML, Hirshfield KM, Steinberg MB, Rhoads GG, Lin Y, Demissie K. Hormonal therapy for breast cancer and diabetes incidence among postmenopausal women. Ann Epidemiol. 2016;26:436–440. doi: 10.1016/j.annepidem.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 12. Wang CY, Shih SR, Huang KC. Increasing risk of diabetes mellitus in postmenopausal women with newly diagnosed primary breast cancer. J Diabetes Investig. 2020;11:490–498. doi: 10.1111/jdi.13112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun LM, Chen HJ, Liang JA, Li TC, Kao CH. Association of tamoxifen use and increased diabetes among Asian women diagnosed with breast cancer. Br J Cancer. 2014;111:1836–1842. doi: 10.1038/bjc.2014.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, Park JY, Lee KU, Ko KS, Lee BW. Background and data configuration process of a nationwide population‐based study using the Korean National Health Insurance System. Diabetes Metab J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee H, Cho SMJ, Park JH, Park S, Kim HC. ACC/AHA blood pressure classification and cardiovascular disease in 15 million adults of age 20‐94 years. J Clin Med. 2017;2019:8. doi: 10.3390/jcm8111832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park SJ, Lee MG, Jo M, Kim G, Park S. Joint effect of depression and health behaviors or conditions on incident cardiovascular diseases: a Korean population‐based cohort study. J Affect Disord. 2020;276:616–622. doi: 10.1016/j.jad.2020.07.009 [DOI] [PubMed] [Google Scholar]
- 18. Koo BK, Lee CH, Yang BR, Hwang SS, Choi NK. The incidence and prevalence of diabetes mellitus and related atherosclerotic complications in Korea: a National Health Insurance Database Study. PLoS One. 2014;9:e110650. doi: 10.1371/journal.pone.0110650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolkewitz M, Allignol A, Harbarth S, de Angelis G, Schumacher M, Beyersmann J. Time‐dependent study entries and exposures in cohort studies can easily be sources of different and avoidable types of bias. J Clin Epidemiol. 2012;65:1171–1180. doi: 10.1016/j.jclinepi.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 20. Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16:241–249. doi: 10.1002/pds.1357 [DOI] [PubMed] [Google Scholar]
- 21. Suissa S, Dell'Aniello S. Time‐related biases in pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2020;29:1101–1110. doi: 10.1002/pds.5083 [DOI] [PubMed] [Google Scholar]
- 22. Khosrow‐Khavar F, Filion KB, Bouganim N, Suissa S, Azoulay L. Aromatase inhibitors and the risk of cardiovascular outcomes in women with breast cancer: a population‐based cohort study. Circulation. 2020;141:549–559. doi: 10.1161/CIRCULATIONAHA.119.044750 [DOI] [PubMed] [Google Scholar]
- 23. Matthews AA, Peacock Hinton S, Stanway S, Lyon AR, Smeeth L, Lund JL, Bhaskaran K. Endocrine therapy use and cardiovascular risk in postmenopausal breast cancer survivors. Heart. 2020;107:1327–1335. doi: 10.1136/heartjnl-2020-317510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ren C, Li P, Chen HZ. Letter by Ren et al regarding article, "Aromatase inhibitors and the risk of cardiovascular outcomes in women with breast cancer: a population‐based cohort study". Circulation. 2020;142:e156–e157. doi: 10.1161/CIRCULATIONAHA.120.047304 [DOI] [PubMed] [Google Scholar]
- 25. Cheung YM, Ramchand SK, Yeo B, Grossmann M. Cardiometabolic effects of endocrine treatment of estrogen receptor‐positive early breast cancer. J Endocr Soc. 2019;3:1283–1301. doi: 10.1210/js.2019-00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haque R, Shi J, Schottinger JE, Chung J, Avila C, Amundsen B, Xu X, Barac A, Chlebowski RT. Cardiovascular disease after aromatase inhibitor use. JAMA Oncol. 2016;2:1590–1597. doi: 10.1001/jamaoncol.2016.0429 [DOI] [PubMed] [Google Scholar]
- 27. Ligibel JA, James O'Malley A, Fisher M, Daniel GW, Winer EP, Keating NL. Risk of myocardial infarction, stroke, and fracture in a cohort of community‐based breast cancer patients. Breast Cancer Res Treat. 2012;131:589–597. doi: 10.1007/s10549-011-1754-1 [DOI] [PubMed] [Google Scholar]
- 28. Krauss K, Stickeler E. Endocrine therapy in early breast cancer. Breast Care (Basel). 2020;15:337–346. doi: 10.1159/000509362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Colleoni M, Giobbie‐Hurder A, Regan MM, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Lang I, Smith I, et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1‐98 study. J Clin Oncol. 2011;29:1117–1124. doi: 10.1200/JCO.2010.31.6455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamood R, Hamood H, Merhasin I, Keinan‐Boker L. Diabetes after hormone therapy in breast cancer survivors: a case‐cohort study. J Clin Oncol. 2018;36:2061–2069. doi: 10.1200/JCO.2017.76.3524 [DOI] [PubMed] [Google Scholar]
- 31. Ye F, Wen J, Yang A, Wang Y, Li N, Yu P, Wei W, Tang J. The influence of hormone therapy on secondary diabetes mellitus in breast cancer: a meta‐analysis. Clin Breast Cancer. 2022;22:e48–e58. doi: 10.1016/j.clbc.2021.06.014 [DOI] [PubMed] [Google Scholar]
- 32. Choi YJ, Bak K, Yeo Y, Choi Y, Shin S. Incident type 2 diabetes risk of selective estrogen receptor modulators in female patients with breast cancer. Pharmaceuticals (Basel). 2021;14:925. doi: 10.3390/ph14090925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S23
Figures S1–S5


