Abstract
Background
We elucidated the safety of treatment with alteplase at 0.6 mg/kg within 24 hours for patients on direct oral anticoagulants (DOACs) before ischemic stroke onset.
Methods and Results
Consecutive patients with acute ischemic stroke who underwent intravenous thrombolysis using alteplase at 0.6 mg/kg from 2011 to 2021 were enrolled from our single‐center prospective stroke registry. We compared outcomes between patients taking DOACs and those not taking oral anticoagulants within 48 hours of stroke onset. The primary safety outcome was the rate of symptomatic intracranial hemorrhage with a ≥4‐point increase on the National Institutes of Health Stroke Scale score from baseline. The efficacy outcome was defined as 3‐month modified Rankin Scale score of 0 to 2 after stroke onset. Of 915 patients with acute ischemic stroke who received intravenous thrombolysis (358 women; median age, 76 years; median National Institutes of Health Stroke Scale score, 10), 40 patients took DOACs (6 took dabigatran, 8 took rivaroxaban, 16 took apixaban, and 10 took edoxaban) within 24 hours of onset and 753 patients did not take any oral anticoagulants. The rate of symptomatic intracranial hemorrhage was comparable between patients on DOACs and those not on oral anticoagulants (2.5% versus 2.4%, P=0.95). The rate of favorable outcomes was comparable between the 2 groups (59.4% versus 58.2%, P=0.46), although the admission National Institutes of Health Stroke Scale score was higher in patients on DOACs. No significant differences showed in any intracranial hemorrhage within 36 hours or mortality at 3 months.
Conclusions
Intravenous thrombolysis would be safely performed for patients on DOACs following the recommendations of the Japanese guidelines.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02251665.
Keywords: direct oral anticoagulants, ischemic stroke, low‐dose alteplase, thrombolysis
Subject Categories: Ischemic Stroke, Cerebrovascular Disease/Stroke, Anticoagulants
Nonstandard Abbreviations and Acronyms
- AIS
acute ischemic stroke
- APTT
activated partial thromboplastin time
- DOAC
direct oral anticoagulant
- HI
hemorrhagic infarction
- ICH
intracranial hemorrhage
- IVT
intravenous thrombolysis
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- OAC
oral anticoagulant
- PH
parenchymal hematoma
- PT‐INR
prothrombin time–international normalized ratio
Clinical Perspective
What Is New?
We investigated the outcomes of patients with acute ischemic stroke treated with intravenous low‐dose (0.6 mg/kg) alteplase within 24 hours after taking direct oral anticoagulants (DOACs).
The rate of symptomatic intracranial hemorrhage was comparable between patients on DOACs and those not on oral anticoagulants.
The rate of 3‐month modified Rankin Scale score of 0 to 2 was also comparable between patients on DOACs and those not on oral anticoagulants.
What Are the Clinical Implications?
Ischemic stroke patients taking DOACs might be safely treated with intravenous low dose alteplase for patients on DOACs within 24 hours from the final dose of DOACs.
Further investigations are needed to examine the safety of intravenous thrombolysis based on DOAC plasma levels.
Several guidelines recommend against the use of intravenous thrombolysis (IVT) with standard‐dose (0.9 mg/kg) alteplase in patients with acute ischemic stroke (AIS) who are taking direct oral anticoagulants (DOACs) (direct thrombin inhibitors and factor Xa inhibitors) because of the potential risk of hemorrhagic complications, 1 , 2 including symptomatic intracerebral hemorrhage. 3 However, a recent meta‐analysis of observational cohort studies suggested no increased risk of symptomatic intracranial hemorrhage (ICH) following administration of alteplase for patients within 48 hours from the last intake of DOACs compared with patients taking a vitamin K antagonist or not taking oral anticoagulants (OACs) at stroke onset. 4 Moreover, the hospital‐based stroke registry in the United States (Get With The Guidelines‐Stroke) reported that the rate of symptomatic ICH in patients within 7 days from last DOAC intake was comparable with that in patients who did not take OACs by administering alteplase 0.9 mg/kg. 5 Therefore, the safety and efficacy of IVT for patients on DOACs remain controversial.
The approved dose of alteplase in Japan is 0.6 mg/kg based on the Japan Alteplase Clinical Trial. 6 A randomized controlled trial showed that IVT with alteplase at 0.6 mg/kg did not increase the rate of death or disability compared with IVT with alteplase at 0.9 mg/kg. 7 In addition to this dose issue, the Japanese guidelines have a unique recommendation on IVT for patients taking DOACs: patients whose most recent DOAC intake was >4 hours previously can undergo IVT if their prothrombin time–international normalized ratio (PT‐INR) and activated partial thromboplastin time (APTT) are within the acceptable range for IVT in patients taking vitamin K antagonists. 8 , 9 This recommendation is mainly based on the pharmacokinetic theory that DOACs reach their highest concentration ≈ 4 hours after dosing 9 , 10 ; it has not been fully investigated in the clinical practice. Therefore, we performed the present study to elucidate the safety of IVT with alteplase at 0.6 mg/kg for patients on DOACs before stroke onset as recommended by Japanese guidelines.
Methods
Anonymized data that support the findings of this study are available from the corresponding author upon reasonable request and after permission has been granted by the ethics committee. The present study conforms to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines. 11 A completed STROBE checklist is included Data S1.
Study Population
All patients with AIS admitted to our institute within 7 days from symptom onset or the last known well time were prospectively registered in the NCVC (National Cerebral and Cardiovascular Center) Stroke Registry (ClinicalTrials.gov identifier: NCT02251665). 12 , 13 , 14 Data for the period from March 2011 to January 2021 were retrospectively reviewed. The inclusion criteria were administration of IVT (alteplase at 0.6 mg/kg) and the acquisition of clinical data at 3 months after symptom onset. Patients taking DOACs who developed ischemic stroke within 48 hours after the last DOAC intake were included. This is because DOAC plasma levels in patients with ischemic stroke are heterogeneous, and high plasma levels of DOAC have been reported beyond 24 hours after the last intake of DOAC. 15 We excluded patients who were treated with any anticoagulants other than DOACs (vitamin K antagonist or unfractionated heparin). Patients taking DOACs who developed ischemic stroke >48 hours after the last DOAC intake were also excluded.
Among the patients who did not receive IVT, the data of those patients with a duration of ≤24 hours from the last DOAC intake to stroke onset were extracted, and the reasons for not administering IVT were also investigated.
Data were prospectively collected in a database and retrieved for retrospective analysis. The study was conducted in accordance with the standards of the Declaration of Helsinki and approved by the local ethics committee of NCVC. Written informed consent for IVT with alteplase was obtained from each patient (or a relative if the patient had communication difficulties).
IVT and Endovascular Therapy
IVT was performed by using alteplase at 0.6 mg/kg for patients with AIS within 4.5 hours from stroke onset according to the Japanese guidelines. 8 , 9 , 10 Before November 2017, IVT for patients on DOACs could be considered if the PT‐INR was <1.7 and the APTT was <1.5 times the baseline value (>40 seconds as a guide) regardless of the time since the last DOAC dose. 9 After November 2017, IVT was not recommended if the time since the last dose was <4 hours, even if the coagulant markers met the above criteria. 8 , 9 All endovascular therapy procedures were performed according to the American Heart Association/American Stroke Association Guideline 1 and the Japanese Guidelines for Neuroendovascular Mechanical Thrombectomy. 16
Clinical Data Collection
The following clinical data were collected: age, sex, prestroke modified Rankin Scale (mRS) score, baseline systolic blood pressure, baseline National Institutes of Health Stroke Scale (NIHSS) score, and antiplatelet drugs before index stroke. The ischemic core was graded with the Alberta Stroke Program Early Computed Tomography Score on diffusion‐weighted magnetic resonance imaging or noncontrast computed tomography. 17 , 18 Time metrics included the duration from the last known well time to IVT and the duration from the last DOAC intake to IVT. Medical history included current smoking, atrial fibrillation, congestive heart failure, hypertension, diabetes, dyslipidemia, chronic kidney disease, ischemic stroke, or transient ischemic attack before index stroke, and ischemic heart disease (history of myocardial infarction, angina, or coronary revascularization treatment). Large vessel occlusion was defined as occlusion of the internal carotid artery (either intracranial or extracranial), M1 or M2 segment of the middle cerebral artery, or basilar artery. Occluded sites were determined using digital subtraction angiography, magnetic resonance angiography, or computed tomography angiography on admission. Laboratory data at admission included the platelet count, serum glucose concentration, serum creatinine concentration, PT‐INR, and APTT. Noncontrast computed tomography was performed around 24 hours after IVT in all patients, and an additional computed tomography examination was performed if neurological deterioration was observed. The stroke subtype was determined by board‐certified stroke neurologists according to the Trial of ORG 10172 in Acute Stroke Treatment criteria. 19
DOACs before the index stroke included dabigatran, rivaroxaban, apixaban, and edoxaban. The approved doses of rivaroxaban in Japan (15 mg once daily in patients with a creatinine clearance rate of ≥50 mL/min and 10 mg once daily in those with a creatinine clearance rate pf 30–49 mL/min) are lower than the global dose based on the unique pharmacokinetics of rivaroxaban in Japanese patients 20 and the results of a Japanese phase III trial. 21 Approved doses of apixaban, edoxaban, and dabigatran in Japan are the same as global doses.
Outcomes
The primary safety outcome was symptomatic ICH within 36 hours after symptom onset. The definition of symptomatic ICH was based on the European Cooperative Acute Stroke Study III criteria. 22 The secondary safety outcomes were any ICH within 36 hours after symptom onset, major hemorrhagic events that fulfilled the International Society on Thrombosis and Hemostasis criteria 23 within 36 hours, and mortality at 3 months. Hemorrhagic transformation was classified into the following categories as described by the Heidelberg Bleeding Classification: hemorrhagic infarction (HI) 1, HI 2, parenchymal hematoma (PH) 1, PH 2, PH remote from infarcted brain tissue (remote PH), intraventricular hemorrhage, subarachnoid hemorrhage, and subdural hemorrhage. 24 The efficacy outcomes were the mRS score and a favorable outcome (mRS score of 0–2).
Statistical Analysis
The data are summarized as median (interquartile range) for continuous variables and as frequency and percentage for categorical variables. The enrolled patients were divided based on OACs before the index stroke: patients who were taking DOACs within 24 hours before the index stroke and those not on any OACs. Differences between the 2 cohorts were assessed for significance using the Mann–Whitney U test or the 2‐sided Fisher exact test, as appropriate. Baseline characteristics and outcomes were compared by univariate analysis. Logistic regression models were constructed for each binary outcome, and odds ratios (ORs) with 95% CIs for patients not on OACs were calculated. Since it is well known that the maximum likelihood estimation of the logistic regression model may be biased by small numbers of events, the Firth penalized likelihood estimation model was used to reduce the bias. 25 The following prespecified variables were included: age, sex, baseline NIHSS score, and Alberta Stroke Program Early Computed Tomography Score, each of which is reportedly associated with safety outcomes. 26 , 27 A comparison of the overall distribution of the mRS score at 3 months and a comparison of the baseline PT‐INR or APTT according to the duration from last DOAC intake to administration of alteplase were performed, and the PT‐INR or APTT before administration of alteplase was compared between the 2 cohorts.
In the additional analysis, we divided patients taking DOACs within 24 hours before the index stroke into 3 groups according to the duration from the last DOAC intake to IVT (<4 hours, 4–12 hours, and 12–24 hours). This is because the second edition of the guideline published in 2012 indicated special care to perform IVT within 12 hours from the last intake of DOACs because of the half‐life of DOACs, 10 and the third edition in 2019 contraindicated IVT within 4 hours from the last intake. 9
All reported P values were 2‐tailed, with the level of statistical significance set at P<0.05. All analyses were performed using STATA 17 (StataCorp, College Station, TX).
Results
Patients' Characteristics
A flowchart of patient selection is shown in Figure S1. Of all 6033 patients with AIS registered during the observational period, 915 patients received IVT. We further excluded 104 patients who were taking vitamin K antagonists; 15 patients treated with intravenous anticoagulant agents, including those for dialysis, before stroke onset; and 3 patients who received IVT >48 hours from the last intake of DOACs. Of the remaining 793 patients (women, 290 [36.6%]; median age, 76 years [interquartile range, 68–84 years]), 40 patients took DOACs (6 took dabigatran, 8 took rivaroxaban, 16 took apixaban, and 10 took edoxaban) within 24 hours of onset (Table S1), and 753 patients took no OACs. The time from last DOAC intake to administration of alteplase is shown in Figure 1. There were no patients with last intake 24 to 48 hours in this study. Idarucizumab was given to reverse dabigatran in 2 of 6 patients on dabigatran; 1 of these patients was previously reported. 28
Figure 1.
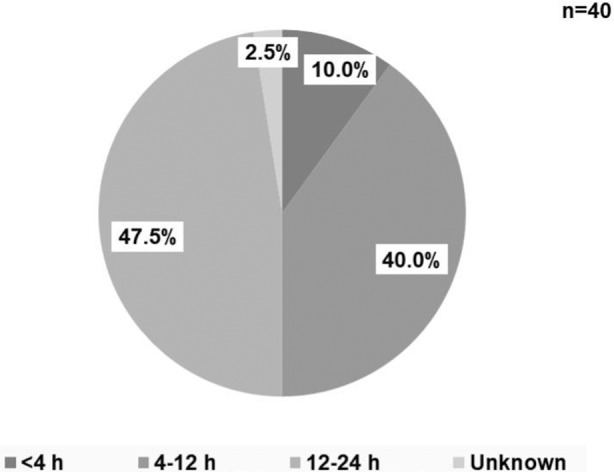
Time from last direct oral anticoagulant intake to administration of alteplase.
DOAC indicates direct oral anticoagulant.
The baseline characteristics of patients receiving IVT are shown in Table 1. Patients on DOACs were older (80 years versus 76 years, P=0.03); had a higher median baseline NIHSS score (15 versus 9, P=0.03), and more frequently had atrial fibrillation (90.0% versus 31.3%, P<0.01), congestive heart failure (22.5% vs. 9.7%, P=0.02), and ischemic stroke or transient ischemic attack before the index stroke (47.8% versus 16.1%, P<0.01) than patients not on OACs.
Table 1.
Baseline Characteristics of Patients Who Received IVT According to Prior OACs
| Patients on DOACs (n=40) | Patients not on OACs (n=753) | Pvalue | |
|---|---|---|---|
| Women | 11 (27.5) | 279 (37.1) | 0.24 |
| Age, y | 80 [74–87] | 76 [68–84] | 0.03 |
| Premorbid mRS score | 0 [0–2] | 0 [0–1] | 0.03 |
| Baseline systolic BP, mm Hg | 157 [143–180] | 160 [141–180] | 0.70 |
| Baseline NIHSS score | 15 [5–24] | 9 [4–17] | 0.03 |
| Antiplatelet drugs prior to index stroke | 7 (17.5) | 206 (27.3) | 0.20 |
| ASPECTS* | 8 [7–10] | 9 [8–10] | 0.24 |
| Time from LKW to administration of alteplase, min | 148 [103–190] | 122 [90–173] | 0.12 |
| Endovascular therapy | 16 (40.0) | 194 (25.8) | 0.06 |
| Medical history | |||
| Current smoker | 11 (27.5) | 197 (26.2) | 0.85 |
| Atrial fibrillation | 36 (90.0) | 236 (31.3) | <0.01 |
| Congestive heart failure | 9 (22.5) | 73 (9.7) | 0.02 |
| Hypertension | 33 (82.5) | 537 (71.3) | 0.15 |
| Diabetes | 8 (20.0) | 154 (20.5) | 1.00 |
| Dyslipidemia | 21 (52.5) | 398 (52.9) | 1.00 |
| Chronic kidney disease | 11 (27.5) | 156 (20.8) | 0.32 |
| Ischemic stroke or TIA before index stroke | 11 (47.8) | 103 (16.1) | <0.01 |
| Ischemic heart disease | 8 (20.0) | 96 (12.8) | 0.22 |
| Occluded vessel site | |||
| Internal carotid artery | 1 (2.5) | 80 (10.6) | 0.11 |
| M1 segment of middle cerebral artery | 12 (30.0) | 136 (18.1) | 0.09 |
| M2 segment of middle cerebral artery | 11 (27.5) | 133 (17.7) | 0.14 |
| Basilar artery | 3 (7.5) | 21 (2.8) | 0.12 |
| Laboratory data | |||
| Platelet count, ×103/μL | 185 [148–224] | 194 [162–234] | 0.28 |
| Serum glucose, mg/dL | 128 [111–153] | 123 [105–149] | 0.59 |
| Serum creatinine, mg/dL | 0.99 [0.89–1.12] | 0.84 [0.70–1.03] | <0.01 |
| PT‐INR | 1.15 [1.04–1.21] | 1.01 [0.96–1.06] | <0.01 |
| APTT, seconds | 29 [28–32] | 28 [26–30] | 0.02 |
| Stroke subtype | |||
| Large‐artery atherosclerosis | 3 (7.5) | 128 (17.0) | <0.01 |
| Cardioembolism | 34 (85.0) | 314 (41.7) | |
| Small‐vessel occlusion | 0 | 65 (8.6) | |
| Stroke of other determined pathogenesis | 1 (2.5) | 141 (18.7) | |
| Stroke of undetermined pathogenesis | 2 (5.0) | 102 (13.6) | |
Data are presented as median [interquartile range] or n (%).
APTT indicates activated partial thromboplastin time; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; BP, blood pressure; DOAC, direct oral anticoagulant; IVT, intravenous thrombolysis; LKW, last known well; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; OAC, oral anticoagulant; PT‐INR, prothrombin time–international normalized ratio; and TIA, transient ischemic attack.
ASPECTS was measured on non‐contrast computed tomography (n=13) or diffusion‐weighted imaging (n=653).
Clinical Outcomes
Table 2 shows the primary and secondary outcomes in both groups. The primary safety outcome of symptomatic ICH was found in 1 patient on a DOAC and 18 patients not on OACs; the rate was comparable between the 2 groups (2.5% versus 2.4%; adjusted OR, 0.95 [95% CI, 0.17–5.28]). For the secondary safety outcomes, the rate of any ICH within 36 hours was comparable between patients on DOACs and those not on OACs (12.5% versus 16.2%; adjusted OR, 0.61 [95% CI, 0.24–1.59]). There was no significant difference in the rate of major hemorrhagic events fulfilling the International Society on Thrombosis and Hemostasis criteria (2.5% versus 1.1%, respectively) or mortality at 3 months (5.0% versus 5.7%, respectively) between the groups (Table 2).
Table 2.
Primary and Secondary Outcomes
| Patients on DOACs (n=40) | Patients not on OACs (n=753) | Adjusted OR* (95% CI) | Pvalue | |
|---|---|---|---|---|
| Primary outcome | ||||
| Symptomatic ICH† | 1 (2.5) | 18 (2.4) | 0.95 (0.17–5.28) | 0.95 |
| Secondary outcomes | ||||
| Safety outcomes | ||||
| Any ICH within 36 h‡ | 5 (12.5) | 122 (16.2) | 0.61 (0.24–1.59) | 0.32 |
| Major hemorrhagic event | 1 (2.5) | 8 (1.1) | 2.70 (0.45–16.20) | 0.28 |
| Mortality at 3 mo | 2 (5.0) | 43 (5.7) | 0.56 (0.10–3.14) | 0.51 |
| Efficacy outcomes§ | ||||
| mRS score at 3 mo** | 2 [0–4] | 2 [1–4] | 0.59 (0.26–1.39) | 0.23 |
| mRS score of 0–2 at 3 mo | 19 (59.4) | 375 (58.2) | 1.33 (0.62–2.82) | 0.46 |
Data are presented as n (%) or median [interquartile range].
Models for clinical outcomes adjusted by age, sex, baseline National Institutes of Health Stroke Scale score, and ASPECTS as reference for patients not on oral anticoagulants.
Symptomatic intracranial hemorrhage as the predominant cause of a ≥ 4‐point increase in the National Institutes of Health Stroke Scale score from baseline.
Any intracranial hemorrhage with a total National Institutes of Health Stroke Scale score of ≥4 points at the time of diagnosis compared with immediately before neurological worsening, or ≥2 points in one National Institutes of Health Stroke Scale category.
Patients with a premorbid modified Rankin Scale score of ≥3 (117 patients) were excluded.
Adjusted odds ratio per 1‐point increase in modified Rankin Scale score.
ASPECTS indicates Alberta Stroke Program Early Computed Tomography Score; DOAC, direct oral anticoagulant; ICH, intracranial hemorrhage; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; OAC, oral anticoagulant; and OR, odds ratio.
The details of patients on DOACs who developed hemorrhagic events after IVT are shown in Table S2. A patient receiving IVT about 8 hours from the last DOAC intake had symptomatic ICH with an extensive infarct due to occlusion of the internal carotid artery. His baseline NIHSS score was 32. He had a history of diabetes and chronic kidney disease at baseline and died of cerebral herniation with PH 2 on the second hospital day (Case 1 in Table S2). With respect to secondary safety outcomes, 5 patients on DOACs developed any ICH within 36 hours, including 1 symptomatic ICH (HI 1, n=3; HI 2, n=1; PH1, n=1; and subarachnoid hemorrhage, n=2), 4 ICHs occurring between 12 and 24 hours from the last DOAC intake, and the remaining ICH occurring between 4 and 12 hours (Figure 2). On admission, all 5 of these patients showed a normal PT‐INR and APTT under the upper 25th percentile of the levels for patients on DOACs (1.22 and 32 seconds, respectively). The remaining patient had a major endovascular therapy‐related hemorrhagic event fulfilling the International Society on Thrombosis and Hemostasis criteria at the site of vascular access; this patient's PT‐INR on admission was 1.45.
Figure 2.
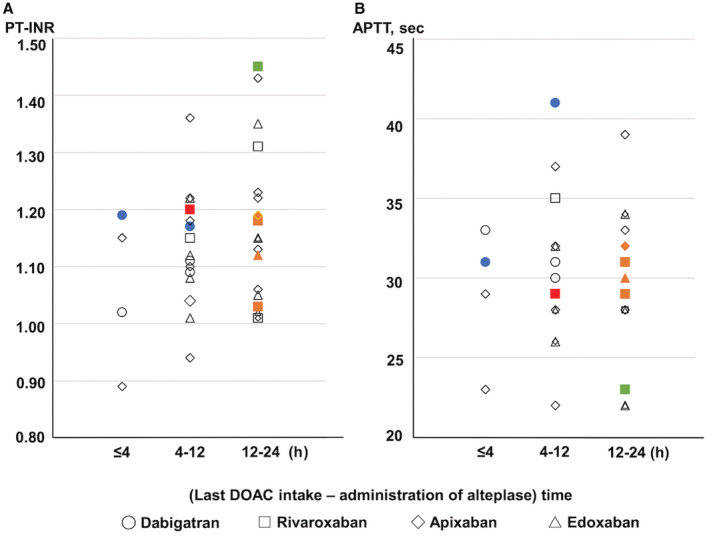
Baseline prothrombin time–international normalized ratio/ activated partial thromboplastin time according to time from last direct oral anticoagulant intake to administration of alteplase.
Distribution of (A) prothrombin time–international normalized ratio and (B) activated partial thromboplastin time according to time from last direct oral anticoagulant intake to administration of alteplase. Red symbols indicate symptomatic intracranial hemorrhage. Orange symbols indicate any intracranial hemorrhage within 36 hours from administration of alteplase. Green symbols indicate hemorrhagic event fulfilling International Society on Thrombosis and Hemostasis criteria. White symbols indicate no hemorrhagic events. Blue symbols indicate use of idarucizumab before thrombolysis. APTT indicates activated partial thromboplastin time; DOAC, direct oral anticoagulant; and PT‐INR, prothrombin time–international normalized ratio.
The distribution of the mRS score at 3 months is shown in Figure 3. The rate of favorable outcome was comparable between patients on DOACs and those not on OACs (59.4% versus 58.2%, P=0.46) (Table 2).
Figure 3.
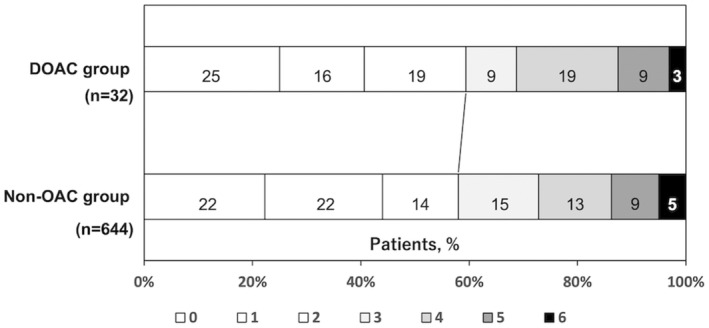
Distribution of modified Rankin Scale score at 3 months according to prior oral anticoagulants.
Patients with a prestroke modified Rankin Scale score of ≥3 (117 patients) were excluded. DOAC indicates direct oral anticoagulant; and OAC, oral anticoagulant.
Reasons for Not Administering Alteplase in Patients on DOACs
Of 5118 patients with AIS who did not receive IVT in the NCVC Stroke Registry, 302 patients took DOACs before stroke onset (Figure S1). Of these, 204 patients (67.5%) arrived at our hospital >4.5 hours from stroke onset or the last known well time, 58 (19.2%) patients had a mild neurological symptom or rapid symptom improvement, 17 (5.6%) had a large early ischemic change on admission imaging, 9 (3.0%) had a PT‐INR of >1.7 or APTT of >40 seconds, and the remaining 4 (1.3%) developed stroke immediately after taking DOACs (Table S3).
Discussion
The major finding of the present study was that rate of symptomatic ICH in patients on DOACs before AIS onset was comparable with that in patients not on OACs after IVT. This treatment also achieved a comparably favorable outcome between patients on DOACs and those not on OACs. These results suggested IVT with alteplase at 0.6 mg/kg might be safely performed for patients on DOACs, which supported the results with alteplase with 0.9 mg/kg. 4 , 5
Hemorrhagic complications are a major problem when administering alteplase. 26 In particular, patients who receive alteplase within 4 hours from the last dose of DOACs might have a high risk of hemorrhagic complications because the plasma levels of DOACs peak ≈ 2 to 4 hours after oral DOAC intake, and hemorrhagic events are reportedly associated with high‐peak levels of DOACs. 29 , 30 In a questionnaire survey in Japan before the revision of the recommendation in 2017, the frequency of asymptomatic ICH increased in patients with AIS within 4 hours after the last DOAC intake. 31 Another important timepoint after the last DOAC intake is 12 hours, which is the approximate half‐life of DOACs. In the present study, nearly half of patients with AIS on DOACs received alteplase within 12 hours from the last DOAC intake; however, the rate of symptomatic ICH in patients on DOACs was comparable with that in patients not on OACs. These results indicate that hemorrhagic events were not excessively caused by alteplase complying with the Japanese guidelines for patients within 12 hours from the last DOAC intake.
Among 6 patients on DOACs who developed all hemorrhagic events after IVT, 5 patients developed ICH. These 5 patients had large vessel occlusion on admission, which is known as a high‐risk factor for HI. 32 Twenty‐three patients (57.5%) in the DOAC group, including these 5 patients, received the lower dosages of DOACs and it may have affected outcomes in those cases. Moreover, 1 elderly patient with symptomatic ICH had diabetes and chronic kidney disease, which are risk factors for ICH after administering alteplase. 33 , 34 Clinicians should be mindful for the development of HI in patients with AIS on DOACs who have risk factors for ICH during treatment with alteplase. Although the patient with a hemorrhagic event fulfilling the International Society on Thrombosis and Hemostasis criteria 23 had a relatively prolonged PT‐INR on admission, his hemorrhagic event was endovascular therapy‐related bleeding at the vascular access site. Therefore, no major hemorrhagic event attributable to intrinsic disease was observed in this study.
The functional outcome with the mRS score at 3 months has not been fully clarified in patients on DOACs with alteplase at 0.6 mg/kg. In our study, the rate of an mRS score of 0 to 2 at 3 months was comparable between patients on DOACs and those not on OACs, although the admission NIHSS score was much higher in patients on DOACs. The distribution of the mRS score in both groups was similar to that in a previous report including patients administered alteplase at 0.9 mg/kg (rate of mRS score of 0–2 at 3 months in the previous report: patients on DOACs, 31.2%; patients not on OACs, 39.1%). 35
One of the limitations of this study is its single‐center design with a relatively small sample size. Second, it might be difficult to extrapolate our results to patients using alteplase at 0.9 mg/kg because alteplase at 0.6 mg/kg is currently used only in Japan and some Asian countries. Third, we did not measure DOACs plasma levels in this study. In several studies and national guidelines, 1 , 2 , 36 , 37 , 38 , 39 IVT is considered based on the blood concentration of DOACs, but non‐specific coagulation assays (PT‐INR <1.7 and APTT <1.5 times the baseline value) are used for judgment in Japanese guidelines. Low correlations between PT‐INR/APTT and DOAC plasma levels were reported in several studies, 40 , 41 , 42 while we reported the positive correlation between them in the patients who treated with rivaroxaban was reported in a single‐center study. 43 Therefore, further investigations are needed to examine the safety of IVT based on DOAC plasma levels.
Conclusions
In this study, we examined the safety outcomes of IVT with alteplase at 0.6 mg/kg in patients taking DOACs in a real clinical practice setting. IVT would be safely performed for patients on DOACs since symptomatic ICH or major bleeding events appear to be comparable with those not taking DOACs. Further research involving multicenter studies with large sample sizes is warranted to confirm our results.
Sources of Funding
This study was supported by the Japan Agency for Medical Research and Development [grant number JP21ek0210139].
Disclosures
Yoshimoto.T reports lecture fees from Takeda Pharmaceutical and Nippon Boehringer Ingelheim. Ihara.M reports lecture fees from Daiichi Sankyo and Eisai and grant support from Panasonic, GE Precision Healthcare LLC, Bristol‐Myers Squibb, and Shimadzu Corporation. Toyoda.K reports lecture fees from Daiichi Sankyo, Bayer Yakuhin, Otsuka, Novartis, Abbott Medical, and Bristol‐Myers Squibb. Koga.M reports honoraria from Daiichi‐Sankyo; and reports research support from Takeda, Daiichi‐Sankyo, Nippon Boehringer Ingelheim, and Shionogi. The remaining authors have no disclosures to report.
Supporting information
Table S1–S3
Figure S1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025809
For Sources of Funding and Disclosures, see page 8.
See Editorial by Kan and Xian
REFERENCES
- 1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk DM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 2. Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C, de la Ossa NP, Strbian D, Tsivgoulis G, Turc G. European stroke organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6:I–LXII. doi: 10.1177/2396987321989865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rota E, Bruzzone G, Agosti S, Pastorino R, Morelli N. A case report of parenchymal hematoma after intravenous thrombolysis in a rivaroxaban‐treated patients: Is it a true rivaroxaban hemorrhagic complication? Medicine (Baltimore). 2017;96:e9435. doi: 10.1097/MD.0000000000009435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shahjouei S, Tsivgoulis G, Goyal N, Sadighi A, Mowla A, Wang M, Seiffge DJ, Zand R. Safety of intravenous thrombolysis among patients taking direct oral anticoagulants: A systematic review and meta‐analysis. Stroke. 2020;51:533–541. doi: 10.1161/STROKEAHA.119.026426 [DOI] [PubMed] [Google Scholar]
- 5. Kam W, Holmes DN, Hernandez AF, Saver JL, Fonarow GC, Smith EE, Bhatt DL, Schwamm LH, Reeves MJ, Matsouaka RA, et al. Association of Recent use of non‐vitamin K antagonist Oral anticoagulants with intracranial hemorrhage among patients with acute ischemic stroke treated with alteplase. JAMA. 2022;327:760–771. doi: 10.1001/jama.2022.0948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, Shinohara Y. Japan alteplase clinical trial group. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan alteplase clinical trial (J‐ACT). Stroke. 2006;37:1810–1815. doi: 10.1161/01.STR.0000227191.01792.e3 [DOI] [PubMed] [Google Scholar]
- 7. Anderson CS, Robinson T, Lindley RI, Arima H, Lavados PM, Lee TH, Broderick JP, Chen X, Chen G, Sharma VK, et al. Low‐dose versus standard‐dose intravenous alteplase in acute ischemic stroke. N Engl J Med. 2016;374:2313–2323. doi: 10.1056/NEJMoa1515510 [DOI] [PubMed] [Google Scholar]
- 8. Toyoda K, Yamagami H, Koga M. Consensus guides on stroke thrombolysis for anticoagulated patients from Japan: Application to other populations. J Stroke. 2018;20:321–331. doi: 10.5853/jos.2018.01788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toyoda K, Koga M, Iguchi Y, Itabashi R, Inoue M, Okada Y, Ogasawara K, Tsujino A, Hasegawa Y, Hatano T, et al. Guidelines for intravenous thrombolysis (recombinant tissue‐type plasminogen activator), the third edition, march 2019: A guideline from the Japan stroke society. Neurol Med Chir (Tokyo). 2019;59:449–491. doi: 10.2176/nmc.st.2019-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minematsu K, Toyoda K, Hirano T, Kimura K, Kondo R, Mori E, Nakagawara J, Sakai N, Shiokawa Y, Tanahashi N, et al. Guidelines for the intravenous application of recombinant tissue‐type plasminogen activator (alteplase), the second edition, October 2012: A guideline from the Japan stroke society. J Stroke Cerebrovasc Dis. 2013;22:571–600. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 11. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative STROBE. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 12. Sato S, Uehara T, Ohara T, Suzuki R, Toyoda K, Minematsu K, Stroke Unit Multicenter Observational (SUMO) Study Investigators . Factors associated with unfavorable outcome in minor ischemic stroke. Neurology. 2014;83:174–181. doi: 10.1212/WNL.0000000000000572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujita K, Tanaka K, Yamagami H, Ide T, Ishiyama H, Sonoda K, Satow T, Takahashi JC, Ihara M, Koga M, et al. Detrimental effect of chronic hypertension on leptomeningeal collateral flow in acute ischemic stroke. Stroke. 2019;50:1751–1757. doi: 10.1161/STROKEAHA.119.025142 [DOI] [PubMed] [Google Scholar]
- 14. Yoshimoto T, Inoue M, Tanaka K, Kanemaru K, Koge J, Shiozawa M, Kamogawa N, Kimura S, Chiba T, Satow T, et al. Identifying large ischemic core volume ranges in acute stroke that can benefit from mechanical thrombectomy. J Neurointerv Surg. 2021;13:1081–1087. doi: 10.1136/neurintsurg-2020-016934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seiffge DJ, Kägi G, Michel P, Fischer U, Béjot Y, Wegener S, Zedde M, Turc G, Cordonnier C, Sandor PS, et al. Rivaroxaban plasma levels in acute ischemic stroke and intracerebral hemorrhage. Ann Neurol. 2018;83:451–459. doi: 10.1002/ana.25165 [DOI] [PubMed] [Google Scholar]
- 16. Yamagami H, Hayakawa M, Inoue M, Iihara K, Ogasawara K, Toyoda K, Hasegawa Y, Ohata K, Shiokawa Y, Nozaki K, et al. Guidelines for mechanical thrombectomy in Japan the Fourth Edition, March 2020: A Guideline from the Japan Stroke Society, the Japan Neurological Society, and the Japanese Society for Neuroendovascular Therapy. Neurol Med Chir (Tokyo). 2021;61:163–192. doi: 10.2176/nmc.nmc.st.2020-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pexman JH, Barber PA, Hill MD, Sevick RJ, Demchuk AM, Hudon ME, Hu WY, Buchan AM. Use of the Alberta stroke program early CT score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22:1534–1542. [PMC free article] [PubMed] [Google Scholar]
- 18. Hirai T, Sasaki M, Maeda M, Ida M, Katsuragawa S, Sakoh M, Takano K, Arai S, Hirano T, Kaiet Y, et al. Diffusion‐weighted imaging in ischemic stroke: Effect of display method on observers' diagnostic performance. Acad Radiol. 2009;16:305–312. doi: 10.1016/j.acra.2008.09.012 [DOI] [PubMed] [Google Scholar]
- 19. Adams H, Bendixen B, Kapelle L, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35 [DOI] [PubMed] [Google Scholar]
- 20. Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, Izumi T, Koretsune Y, Kajikawa M, Kato M, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J‐ROCKET AF study. Circ J. 2012;76:2104–2111. doi: 10.1253/circj.cj-12-0454 [DOI] [PubMed] [Google Scholar]
- 21. Tanigawa T, Kaneko M, Hashizume K, Kajikawa M, Ueda H, Tajiri M, Paolini JF, Mueck W. Model‐based dose selection for phase III rivaroxaban study in Japanese patients with non‐valvular atrial fibrillation. Drug Metab Pharmacokinet. 2013;28:59–70. doi: 10.2133/dmpk.dmpk-12-rg-034 [DOI] [PubMed] [Google Scholar]
- 22. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 23. Schulman S, Kearon C. Subcommittee on control of anticoagulation of the scientific and standardization Committee of the International Society on thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 24. von Kummer R, Broderick JP, Campbell BCV, Demchuk A, Goyal M, Hill MD, Treurniet KM, Majoie CBLM, Marquering HA, Mazya MV, et al. The Heidelberg bleeding classification: Classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 25. Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. doi: 10.1093/biomet/80.1.27 [DOI] [Google Scholar]
- 26. Das S, Mondal GP, Bhattacharya R, Ghosh KC, Das S, Pattem HK, Paul SA, Patra C. Predictors of postthrombolysis outcome and symptomatic postthrombolysis hemorrhage following intravenous thrombolysis with alteplase for acute ischemic stroke. J Neurosci Rural Pract. 2020;11:315–324. doi: 10.1055/s-0040-1709946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirano T, Sasaki M, Tomura N, Ito Y, Kobayashi S. Japan alteplase clinical trial group. Low Alberta stroke program early computed tomography score within 3 hours of onset predicts subsequent symptomatic intracranial hemorrhage in patients treated with 0.6 mg/kg alteplase. J Stroke Cerebrovasc Dis. 2012;21:898–902. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.018 [DOI] [PubMed] [Google Scholar]
- 28. Hosoki S, Takagi M, Yamagami H, Ando D, Toyoda K, Koga M. Paradoxical elevation of plasma dabigatran after reversal with idarucizumab in stroke thrombolysis. J Neurol. 2018;265:2451–2453. doi: 10.1007/s00415-018-9011-8 [DOI] [PubMed] [Google Scholar]
- 29. Wada S, Toyoda K, Sato S, Matsuki T, Okata T, Kumamoto M, Tagawa N, Inoue M, Okamoto A, Ihara M, et al. Anti‐Xa activity and event risk in patients with direct factor Xa inhibitors initiated early after stroke. Circ J. 2018;82:2872–2879. doi: 10.1253/circj.CJ-18-0506 [DOI] [PubMed] [Google Scholar]
- 30. Shin H, Cho MC, Kim RB, Kim CH, Choi NC, Kim SK, Koh EH. Laboratory measurement of apixaban using anti‐factor Xa assays in acute ischemic stroke patients with non‐valvular atrial fibrillation. J Thromb Thrombolysis. 2018;45:250–256. doi: 10.1007/s11239-017-1590-1 [DOI] [PubMed] [Google Scholar]
- 31. Suzuki K, Aoki J, Sakamaoto Y, Abe A, Suda S, Okubo S, Nagao T, Kimura K. Low risk of ICH after reperfusion therapy in acute stroke patients treated with direct oral anti‐coagulant. J Neurol Sci. 2017;379:207–211. doi: 10.1016/j.jns.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 32. Hornig CR, Bauer T, Simon C, Trittmacher S, Dorndorf W. Hemorrhagic transformation in cardioembolic cerebral infarction. Stroke. 1993;24:465–568. doi: 10.1161/01.str.24.3.465 [DOI] [PubMed] [Google Scholar]
- 33. Demchuk AM, Morgenstern LB, Krieger DW, Chi TL, Hu W, Wein TH, Hardy RJ, Grotta JC, Buchan AM. Serum glucose level and diabetes predict tissue plasminogen activator‐related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30:34–39. doi: 10.1161/01.str.30.1.34 [DOI] [PubMed] [Google Scholar]
- 34. Tütüncü S, Ziegler AM, Scheitz JF, Slowinski T, Rocco A, Endres M, Nolte CH. Severe renal impairment is associated with symptomatic intracerebral hemorrhage after thrombolysis for ischemic stroke. Stroke. 2013;44:3217–3219. doi: 10.1161/STROKEAHA.113.002859 [DOI] [PubMed] [Google Scholar]
- 35. Xian Y, Federspiel JJ, Hernandez AF, Laskowitz DT, Schwamm LH, Bhatt DL, Smith EE, Fonarow GC, Peterson ED. Use of intravenous recombinant tissue plasminogen activator in patients with acute ischemic stroke who take non‐vitamin K antagonist oral anticoagulants before stroke. Circulation. 2017;135:1024–1035. doi: 10.1161/CIRCULATIONAHA.116.023940 [DOI] [PubMed] [Google Scholar]
- 36. Touzé E, Gruel Y, Gouin‐Thibault I, De Maistre E, Susen S, Sie P, Derex L. Intravenous thrombolysis for acute ischaemic stroke in patients on direct oral anticoagulants. Eur J Neurol. 2018;25:747–e52. doi: 10.1111/ene.13582 [DOI] [PubMed] [Google Scholar]
- 37. Seiffge DJ, Van Hooff RJ, Nolte CH, Béjot Y, Turc G, Ikenberg B, Berge E, Persike M, Dequatre‐Ponchelle N, Strbian D, et al. Recanalization therapies in acute ischemic stroke patients: Impact of prior treatment with novel oral anticoagulants on bleeding complications and outcome. Circulation. 2015;132:1261–1269. doi: 10.1161/CIRCULATIONAHA.115.015484 [DOI] [PubMed] [Google Scholar]
- 38. Seiffge DJ, Traenka C, Polymeris AA, Thilemann S, Wagner B, Hert L, Müller MD, Gensicke H, Peters N, Nickel CH, et al. Intravenous thrombolysis in patients with stroke taking rivaroxaban using drug specific plasma levels: Experience with a standard operation procedure in clinical practice. J Stroke. 2017;19:347–355. doi: 10.5853/jos.2017.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seiffge DJ, Meinel T, Purrucker JC, Kaesmacher J, Fischer U, Wilson D, Wu TY. Recanalisation therapies for acute ischaemic stroke in patients on direct oral anticoagulants. J Neurol Neurosurg Psychiatry. 2021;92:534–541. doi: 10.1136/jnnp-2020-325456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ebner M, Birschmann I, Peter A, Härtig F, Spencer C, Kuhn J, Rupp A, Blumenstock G, Zuern CS, Ziemann U, et al. Limitations of specific coagulation tests for direct Oral anticoagulants: a critical analysis. J Am Heart Assoc. 2018;7:e009807. doi: 10.1161/JAHA.118.009807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Härtig F, Birschmann I, Peter A, Hörber S, Ebner M, Sonnleitner M, Spencer C, Bombach P, Stefanou MI, Tünnerhoff J, et al. Point‐of‐care testing for emergency assessment of coagulation in patients treated with direct oral anticoagulants including edoxaban. Neurol Res Pract. 2021;3:9. doi: 10.1186/s42466-021-00105-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Härtig F, Poli S, Ebner M, Birschmann I, Kuhn J, Ziemann U, Häring HU, Lehmann R, Peter A, Hörber S. Monitoring of low dabigatran concentrations: Diagnostic performance at clinically relevant decision thresholds. J Thromb Thrombolysis. 2020;49:457–467. doi: 10.1007/s11239-019-01981-z [DOI] [PubMed] [Google Scholar]
- 43. Okata T, Toyoda K, Okamoto A, Miyata T, Nagatsuka K, Minematsu K. Anticoagulation intensity of rivaroxaban for stroke patients at a special low dosage in Japan. PLoS One. 2014;9:e113641. doi: 10.1371/journal.pone.0113641 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S3
Figure S1


