Abstract
Staphylococcus schleiferi subsp. schleiferi is associated with a range of nosocomial infections, but the pathogenic mechanisms by which these occur are poorly understood. This study provides phenotypic and genotypic evidence for the expression of a cell wall-anchored fibronectin-binding protein by this species.
Following the description of Staphylococcus schleiferi subsp. schleiferi in 1988 (7), this coagulase-negative staphylococcus has been implicated as the causative pathogen in a range of nosocomial infections. These include bacteremia (14, 19), brain abscess (9), pacemaker- and other intravenous-device-related infections (2, 5, 9), and infections of the urinary tract (21), orthopedic implants (14), and surgical wounds (9, 17). The pathogenic mechanisms by which S. schleiferi causes such diseases are unknown, but there is a degree of similarity between the spectrum of infections caused by this microorganism and those associated with Staphylococcus aureus (32). It is plausible, therefore, that the two species share one or more virulence determinants. S. aureus expresses a range of cell wall-associated and secreted proteins that are involved in pathogenesis, including the fibronectin- and fibrinogen-binding proteins, which promote adherence to host cells, and the extracellular matrix and plasma proteins (6, 16, 20, 22, 25). These adhesins play a central role in colonization of medical devices by interacting with the fibrinogen and fibronectin that coat prosthetic material following insertion in vivo (28–30). Isolation of S. schleiferi from cultures of prosthetic material (2, 9, 14, 27) suggests the presence of one or more bacterial cell surface-expressed adhesins with a host protein specificity similar to that of S. aureus. S. schleiferi has been reported to bind fibrinogen, as assessed by commercial agglutination kits (12, 23, 27), but adherence to fibronectin and the identification of cell wall-associated adhesins have not previously been described for this organism. We have investigated the possibility that S. schleiferi expresses a fibronectin-binding protein (FnBP).
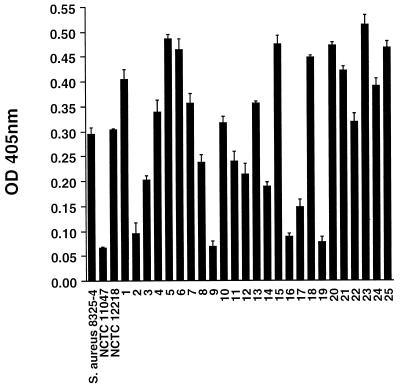
Adherence of bacterial isolates to purified human fibronectin (10 μg/ml) was assessed with a microtiter plate assay, as previously described (11). Bound bacteria were detected by staining with crystal violet (0.5%, [vol/vol]), and the absorbance was measured with an enzyme-linked immunosorbent assay plate reader (Labsystems Multiscan Plus). Each isolate was tested in quadruplicate in an individual assay, and each experiment was performed three times. All assay plates included S. aureus 8325-4 as a positive control and phosphate-buffered saline without bacteria as a negative control. The optical density at 405 nm (OD405) used in the analysis was the mean value for a given strain minus the OD405 for the negative control on the same plate. Adherence of S. schleiferi NCTC 12218 to purified fibronectin was compared with that of S. aureus 8325-4, which is known to adhere to fibronectin (10), and with that of Staphylococcus epidermidis NCTC 11047, which adheres poorly. The ability of S. schleiferi to adhere to purified fibronectin in vitro was demonstrated (Fig. 1), with no significant difference in OD405 between S. schleiferi NCTC 12218 and S. aureus 8325-4 (P = 0.45, unpaired t test). The absorbance values for S. schleiferi and S. aureus were significantly greater than that for S. epidermidis (P < 0.0001 for both, unpaired t test).
FIG. 1.
Adherence of S. schleiferi to purified human fibronectin in vitro. By using a microtiter plate assay, absorbance was compared among S. aureus 8325-4, S. epidermidis NCTC 11047, S. schleiferi NCTC 12218, and 25 clinical isolates of S. schleiferi cultured from the following sources: blood cultures (bars 1 to 11); pacemaker leads, boxes, or wounds (bars 12 to 19); an intravenous cannula (bar 20); a brain abscess (bar 21); cerebrospinal fluid (bar 22); and wound infections (bars 23 to 25). Each isolate was tested in quadruplicate in an individual assay, and each experiment was performed in triplicate. Values are means ± SEM.
To evaluate whether the ability to adhere to fibronectin is also present in recent isolates associated with disease, adherence assays were performed with 25 clinical isolates of S. schleiferi obtained from the Centre Nationale de Reference des Toxemies à Staphylocoques, Lyon, France. These were isolated from blood cultures (11 isolates), pacemaker leads, devices, or wounds (8 isolates), an intravenous cannula (1 isolate), a brain abscess (1 isolate), cerebrospinal fluid (1 isolate), and wound infections (3 isolates). There was a marked variation in OD405 values within the group, with a 7.4-fold difference in absorbance between the lowest and highest binders (Fig. 1). This variation was highly reproducible, as reflected by the standard errors of the means (SEM) (Fig. 1). Colony count experiments excluded variations in bacterial inocula as the cause (data not shown). The reason for this variability is unclear, but similar observations about the adherence of a collection of clinical isolates of S. aureus to purified collagen in vitro have recently been made (26). It is possible that there is variation between isolates in the number of cell surface-expressed binding sites or the efficiency with which FnBPs bind fibronectin, which could, in turn, be related to the relative genetic heterogeneity that is reported to exist for S. schleiferi (9). The small number of isolates in each disease group does not permit comment on the level of adherence versus clinical diagnosis.
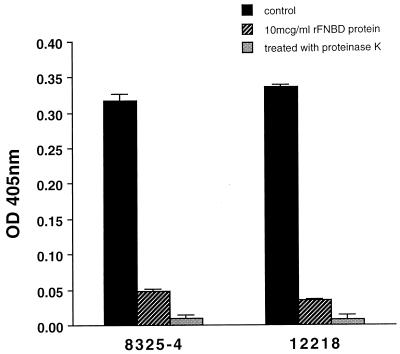
Surface proteins were removed from the bacterial pellet of 10-ml overnight cultures of S. schleiferi NCTC 12218 and S. aureus 8325-4 by using proteinase K, as previously described (4). Adherence to purified human fibronectin by organisms pre-treated with proteinase K and untreated controls was compared (Fig. 2). Proteinase K treatment resulted in a highly significant reduction in adherence for both S. schleiferi NCTC 12218 (P < 0.0001) and S. aureus 8325-4 (P = 0.0002, paired t test). These results are consistent with the proteolytic degradation of cell surface-associated FnBP.
FIG. 2.
Effect of proteinase K and the recombinant form of the ligand binding domain of FnBPB from Streptococcus dysgalactiae on adherence to purified human fibronectin in vitro. Adherence of S. schleiferi NCTC 12218 and S. aureus 8325-4 was evaluated in the presence of 10 μg of the recombinant form of the ligand-binding domain of FnBP encoded by fnbB of Streptococcus dysgalactiae per ml and following treatment with proteinase K. Each isolate was tested in quadruplicate in an individual assay, and each experiment was performed in triplicate. Values are means ± SEM.
The microtiter plate assay was repeated for S. aureus 8325-4 and S. schleiferi NCTC 12218 in the presence of the recombinant form of the ligand-binding domain of FnBPB of Streptococcus dysgalactiae (15) (rFNBD-B, a gift from Magnus Höök, Houston, Texas, referred to below as rFNBD protein), which was added to the wells immediately prior to bacterial inoculation at a final concentration of 10 μg/ml. Control wells were incubated with bacteria in the absence of rFNBD protein. The total volume was maintained at 100 μl for all wells. Absorbance was significantly reduced to a level of 15.2% compared with the control for S. aureus (P = 0.0002, paired t test) (Fig. 2), a finding consistent with published data (15). A statistically significant reduction was also seen for S. schleiferi, the absorbance value in the presence of rFNBD protein being 10.1% of that for the control (P < 0.0001, paired t test). This provides further evidence for the presence of a cell surface-associated FnBP that either has homology to rFNBD protein or has its function sterically hindered by its presence.
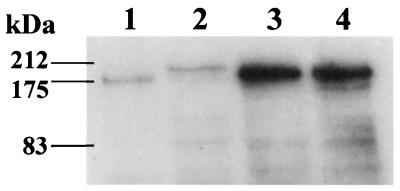
Cell wall-associated protein extracts were prepared from S. aureus 8325-4 and S. schleiferi, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and evaluated by Western ligand affinity blotting by established methods (11, 18, 31). S. aureus 8325-4 was used as a positive control and was shown to express a protein with an apparent molecular mass of approximately 180 kDa (Fig. 3), which was consistent with previous reports (1, 10, 31). The extracts from S. schleiferi NCTC 12218 and two randomly selected clinical S. schleiferi isolates (Fig. 1, bars 3 and 18) also contained a reactive protein band (Fig. 3). The band for NCTC 12218 had a molecular mass of approximately 200 kDa. The bands for the two clinical S. schleiferi isolates had greater density on visual inspection than those for S. schleiferi NCTC 12218 and S. aureus 8325-4. Western ligand affinity blotting was repeated with a 1:4 dilution of the initial cell wall extract from the clinical isolates to facilitate assessment of the molecular mass of the positive bands. The reactive bands had a molecular mass of 180 kDa for both clinical isolates (data not shown). The greater band density for the two clinical isolates obtained with the original extract occurred despite standardization of the bacterial inocula used during cell wall-associated protein extraction and was reproducible on repeat testing with an independently prepared protein extract (data not shown). However, greater band density was not predictive of an enhanced adherence to fibronectin in vitro; the S. schleiferi isolates represented by bars 18 and 3 were more and less adherent, respectively, than NCTC 12218 (Fig. 1). The visualization of a band from lysostaphin cell wall preparations suggests that the protein is cell wall anchored. The reason for the variations in apparent molecular mass of the FnBPs from S. schleiferi NCTC 12218 and the two clinical isolates is not apparent. One possibility is that the proteins have one or more domains that are characterized by a variable number of tandem repeats. This has been well documented for S. aureus in, e.g., the B-repeat region of the collagen-binding protein (8) and the D-repeat domain of the FnBP in clinical isolates (24). Protein A variants that result in variations in apparent molecular mass have also been described (3). An alternative explanation is that the protein from the clinical strains underwent degradation before processing, in which case the small N-terminal fragment would be lost.
FIG. 3.
Visualization of FnBPs by Western ligand affinity blotting of cell wall-associated protein extracts. Equal amounts of cell wall-associated protein extract were loaded into each lane. Lane 1, S. aureus 8325-4; lane 2, S. schleiferi NCTC 12218; lanes 3 and 4, clinical S. schleiferi isolates (numbered 18 and 3 in Fig. 1, respectively).
PCR analysis was performed to determine the presence of homology between D1 to D3 of the binding domain of the fnbA of S. aureus 8325-4 and genomic DNA from S. schleiferi NCTC 12218. The rationale for this choice was that the D-repeat region is highly conserved between the FnBPA and FnBPB of S. aureus 8325-4, probably as a result of functional requirements of the binding domains, and represents the area of greatest homology between FnBPs of distantly related species (15). Oligonucleotide primers were synthesized by Genosys, as follows: 5′-GGCCAAAATAGCGGTAACC-3′ (forward) and 5′-GCTTACTTTTGGAAGTGTATC-3′ (reverse), corresponding to bases 2349 to 2367 and 2674 to 2694 of fnbA, respectively (as assigned by GenBank). S. schleiferi NCTC 12218 and S. aureus 8325-4 chromosomal DNA was prepared as previously described (13). PCR amplifications were performed in a DNA thermal cycler (Perkin-Elmer Cetus) with Taq polymerase (Boehringer Mannheim). The PCR mixtures contained 100 pmol of forward and reverse primers, 100 ng of genomic DNA, 200 μM deoxynucleoside triphosphate, reaction buffer (1×), 1.5 mM MgSO4, and 2.5 U of Taq polymerase in a 100-μl volume. This was overlaid with 100 μl of mineral oil and amplified for 30 temperature cycles consisting of a 1-min denaturation step at 94°C, a 1-min annealing step at 54°C, and elongation for 3 min. This was followed by incubation at 72°C for 10 min.
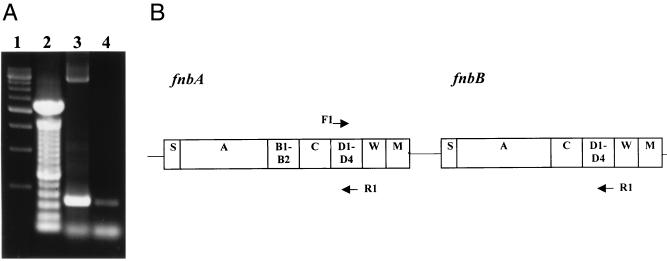
Agarose gel electrophoresis of an aliquot of the PCR mixtures is shown in Fig. 4A. A predicted band of approximately 350 bp was seen for S. aureus 8325-4, together with a second band of approximately 3.7 kb. The primer recognition sites used for fnbA had only 1- and 2-bp differences for forward and reverse primers, respectively, between fnbA and fnbB. Figure 4B shows a map of the possible positions at which the fnbA primers could anneal in the fnb locus. It is likely that the 3.7-kb band represents amplification of the region from D1 of fnbA to D3 of fnbB. The results for S. schleiferi, shown in lane 4 of Fig. 4A, demonstrate a single band of approximately 350 bp. Sequence analysis of this fragment was performed with Big-Dye terminator chemistry (ABI Prism) and visualized on an ABI 377 sequencer. This demonstrated homology between the fragment from S. schleiferi and the ligand-binding domain of FnBPs expressed by other bacterial species, including S. aureus 8325-4 (data not shown). Studies are in progress to clone and sequence the gene as a forerunner to the construction of an isogenic mutant that will facilitate the evaluation of FnBPs in the pathogenesis of S. schleiferi infection.
FIG. 4.
PCR analysis of FnBP gene fragments. (A) Genomic DNA from S. aureus 8325-4 (lane 3) and S. schleiferi NCTC 12218 (lane 4) was amplified with primers complementary to the 345-bp D1 to D3 binding domain of S. aureus 8325-4 fnbA. Lane 1, 500-bp molecular size marker; lane 2, 100-bp molecular size marker. (B) Map showing the possible positions at which the fnbA primers could anneal in the S. aureus 8325-4 fnb locus (F, forward primer; R, reverse primer). Regions marked S, A, B, C, D, W, and M are domains in the FnBPA and FnBPB proteins.
Acknowledgments
This work was supported by Wellcome Trust Microbiology Training Fellowship grant 044331, Wellcome Trust grant 52320, and BioResearch Ireland.
We are grateful to Magnus Höök for providing rFNBD protein. We are grateful to Mark Enright for his assistance in DNA sequencing.
REFERENCES
- 1.Bisognano C, Vaudaux P E, Lew D P, Ng E Y W, Hooper D C. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 1997;41:906–913. doi: 10.1128/aac.41.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celard M, Vandenesch F, Darbas H, Grando J, Jean-Pierre H, Kirkorian G, Etienne J. Pacemaker infection caused by Staphylococcus schleiferi, a member of the human preaxillary flora: four case reports. Clin Infect Dis. 1997;24:1014–1015. doi: 10.1093/clinids/24.5.1014. [DOI] [PubMed] [Google Scholar]
- 3.Cheung A L, Bayer A S, Peters J, Ward J I. Analysis by gel electrophoresis, Western blot, and peptide mapping of protein A heterogeneity in Staphylococcus aureus strains. Infect Immun. 1987;55:843–847. doi: 10.1128/iai.55.4.843-847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung A L, Fischetti V A. Variation in the expression of cell wall proteins of Staphylococcus aureus grown on solid and liquid media. Infect Immun. 1988;56:1061–1065. doi: 10.1128/iai.56.5.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dacosta A, Lelièvre H, Kirkorian G, Célard M, Chevalier P, Vandenesch F, Etienne J, Touboul P. Role of preaxillary flora in pacemaker infections. A prospective study. Circulation. 1998;97:1791–1795. doi: 10.1161/01.cir.97.18.1791. [DOI] [PubMed] [Google Scholar]
- 6.Foster T J, McDevitt D. Surface-associated proteins of Staphylococcus aureus: their possible roles in virulence. FEMS Microbiol Lett. 1994;118:199–206. doi: 10.1111/j.1574-6968.1994.tb06828.x. [DOI] [PubMed] [Google Scholar]
- 7.Freney J, Brun Y, Bes M, Meugnier H, Grimont F, Grimont P A D, Nervi C, Fleurette J. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38:168–172. [Google Scholar]
- 8.Gillaspy A F, Lee C Y, Sau S, Cheung A L, Smeltzer M S. Factors affecting the collagen binding capacity of Staphylococcus aureus. Infect Immun. 1998;66:3170–3178. doi: 10.1128/iai.66.7.3170-3178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grattard F, Etienne J, Pozzetto B, Tardy F, Gaudin O G, Fleurette J. Characterization of unrelated strains of Staphylococcus schleiferi by using ribosomal DNA fingerprinting, DNA restriction patterns, and plasmid profiles. J Clin Microbiol. 1993;31:812–818. doi: 10.1128/jcm.31.4.812-818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene C, McDevitt D, Francois P, Vaudaux P E, Lew D P, Foster T J. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of the fnb genes. Mol Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- 11.Hartford O, Francois P, Vaudaux P, Foster T J. The dipeptide repeat region of the fibrinogen-binding protein (clumping factor) is required for functional expression of the fibrinogen-binding domain on the Staphylococcus aureus cell surface. Mol Microbiol. 1997;25:1065–1076. doi: 10.1046/j.1365-2958.1997.5291896.x. [DOI] [PubMed] [Google Scholar]
- 12.Hébert G A. Hemolysins and other characteristics that help differentiate and biotype Staphylococcus lugdunensis and Staphylococcus schleiferi. J Clin Microbiol. 1990;28:2425–2431. doi: 10.1128/jcm.28.11.2425-2431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hull R A, Gill R E, Hsu P, Minshew B H, Falkow S. Construction and expression of recombinant plasmids encoding type 1 or d-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981;33:933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jean-Pierre H, Darbas H, Jean-Roussenq A, Boyer G. Pathogenicity in two cases of Staphylococcus schleiferi, a recently described species. J Clin Microbiol. 1989;27:2110–2111. doi: 10.1128/jcm.27.9.2110-2111.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joh H J, House-Pompeo K, Patti J M, Gurusiddappa S, Hook M. Fibronectin receptors from Gram-positive bacteria: comparison of active sites. Biochemistry. 1994;33:6086–6092. doi: 10.1021/bi00186a007. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson K, Signas C, Muller H-P, Lindberg M. Two different genes encode fibronectin-binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991;202:1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- 17.Kluytmans J, Berg H, Steegh P, Vandenesch F, Etienne J, van Belkum A. Outbreak of Staphylococcus schleiferi wound infections: strain characterization by randomly amplified polymorphic DNA analysis, PCR ribotyping, conventional ribotyping, and pulsed-field gel electrophoresis. J Clin Microbiol. 1998;36:2214–2219. doi: 10.1128/jcm.36.8.2214-2219.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Latorre M, Rojo P M, Unzaga M J, Cisterna R. Staphylococcus schleiferi: a new opportunistic pathogen. Clin Infect Dis. 1993;16:589–590. doi: 10.1093/clind/16.4.589. [DOI] [PubMed] [Google Scholar]
- 20.McDevitt D, Francois P, Vaudaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 21.Ozturkeri H, Kocabeyoglu O, Yergok Y Z, Kosan E, Yenen O S, Keskin K. Distribution of coagulase-negative staphylococci, including the newly described species Staphylococcus schleiferi, in nosocomial and community acquired urinary tract infections. Eur J Clin Microbiol Infect Dis. 1994;13:1076–1079. doi: 10.1007/BF02111833. [DOI] [PubMed] [Google Scholar]
- 22.Patti J M, Allen B L, McGavin M J, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 23.Personne P, Bes M, Lina G, Vandenesch F, Brun Y, Etienne J. Comparative performances of six agglutination kits assessed by using typical and atypical strains of Staphylococcus aureus. J Clin Microbiol. 1997;35:1138–1140. doi: 10.1128/jcm.35.5.1138-1140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice K C, McGavin M J, Papakyriacou H. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Variance in fibronectin-binding and distribution of fnb genes in Staphylococcus aureus, abstr. D-19; pp. 215–216. [Google Scholar]
- 25.Signas C, Raucci G, Jonsson K, Lindgren P-E, Anantharamaiah G M, Hook M, Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci USA. 1989;86:699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas M G, Peacock S, Daenke S, Berendt A R. Adhesion of Staphylococcus aureus to collagen is not a major virulence determinant for septic arthritis, osteomyelitis or endocarditis. J Infect Dis. 1999;179:291–293. doi: 10.1086/314576. [DOI] [PubMed] [Google Scholar]
- 27.Vandenesch F, Lebeau C, Bes M, Lina G, Lina B, Greenland T, Benito Y, Brun Y, Fleurette J, Etienne J. Clotting activity in Staphylococcus schleiferi subspecies from human patients. J Clin Microbiol. 1994;32:388–392. doi: 10.1128/jcm.32.2.388-392.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaudaux P, Pittet D, Haeberli A, Huggler E, Nydegger U E, Lew D P, Waldvogel F A. Host factors selectively increase staphylococcal adherence on inserted catheters: a role for fibronectin and fibrinogen or fibrin. J Infect Dis. 1989;160:865–875. doi: 10.1093/infdis/160.5.865. [DOI] [PubMed] [Google Scholar]
- 29.Vaudaux P, Pittet D, Haeberli A, Lerch P G, Morgenthaler J J, Proctor R A, Waldvogel F A, Lew D P. Fibronectin is more active than fibrin or fibrinogen in promoting Staphylococcus aureus adherence to inserted intravascular devices. J Infect Dis. 1993;167:633–641. doi: 10.1093/infdis/167.3.633. [DOI] [PubMed] [Google Scholar]
- 30.Vaudaux P E, François P, Proctor R A, McDevitt D, Foster T J, Albrecht R M, Lew D P, Wabers H, Cooper S L. Use of adhesion-defective mutants of Staphylococcus aureus to define the role of specific plasma proteins in promoting bacterial adhesion to canine arteriovenous shunts. Infect Immun. 1995;63:585–590. doi: 10.1128/iai.63.2.585-590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaudaux P E, Monzillo V, Francois P, Lew D P, Foster T J, Berger-Bächi B. Introduction of the mec element (methicillin resistance) into Staphylococcus aureus alters in vitro functional activities of fibrinogen and fibronectin adhesins. Antimicrob Agents Chemother. 1998;42:564–570. doi: 10.1128/aac.42.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldvogel F A. Staphylococcus aureus. In: Mandell G L, Bennett J E, Dolins R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1995. pp. 1754–1777. [Google Scholar]