Malnutrition is one of the hallmarks of frailty in elderly patients and a predictor of worse outcomes in elderly patients with severe aortic valve stenosis. 1 In this context, Ishizu et al 2 present in this issue of the Journal of the American Heart Association (JAHA) their analyses on prevalence and prognostic value of the Controlling Nutritional Status (CONUT) score, Geriatric Nutritional Risk Index (GNRI), and Prognostic Nutritional Index for malnutrition assessment of Japanese elderly patients at high surgical risk undergoing transcatheter aortic valve implantation (TAVI). They found that malnourishment in their population was common and associated with increased mortality after TAVI regardless of the nutrition index used and irrespective of age, sex, body mass index, frailty, kidney function, and left ventricular ejection fraction. This phenomenon had already been observed by the OCEAN‐TAVI (Optimized Transcatheter Valvular Intervention–TAVI) investigators, 3 who found nutritional status as a surrogate marker for predicting worse clinical outcomes after TAVI. While one may argue that this finding might apply only to this select population (only Japanese and very old patients with a mean age >80 years) with a well‐defined risk profile (only at high risk), the current evidence shows that patients with different risk profiles (not only those at high risk) and other Asian and Western populations are under the negative impact of malnutrition as well.
Still, in Japan, after stratifying groups according to the Society of Thoracic Surgeons (STS) Predicted Risk of Mortality score (STS <4, STS 4–8, and STS >8), the OCEAN‐TAVI investigators 3 observed that the statistically significant differences in mortality between patients with good and poor nutritional status in the overall cohort remained statistically significant in the groups stratified according to STS Predicted Risk of Mortality score, which reveals that the negative impact of malnutrition on the outcomes after TAVI go beyond risk profile and do not affect only patients at high risk for surgical aortic valve replacement but also those at intermediate and low risk.
As for other Asian populations, Lee et al 4 studied in South Korea the clinical usefulness of nutritional assessment tools for predicting the risk of mortality following TAVI and found that low GNRI was independently associated with a higher risk of 1‐year mortality. Furthermore, Sihag et al 5 demonstrated that patients with worse nutritional status in China had a worse prognosis regardless of the nutritional score used (GNRI, Prognostic Nutritional Index, or CONUT). Both studies 4 , 5 showed that the nutritional aspect seems to be a problem not only in Japan but also in other Asian populations as well.
In North America, Koseki et al 6 found that the GNRI is an important surrogate marker for predicting prognosis and mortality, and Emami et al 7 found malnutrition independently associated with increased mortality, complications, readmission, and resource use in patients undergoing TAVI in the United States.
In Europe, Ferreiro et al 8 found the Nutritional Risk Index independently associated with increased risk of death during long‐term follow‐up after TAVI in Spain. Eichler et al 9 and Seoudy et al 10 observed that nutrition status of patients scheduled for TAVI offer prognostic information for 1‐ and 4‐year all‐cause mortality in Germany.
If we consider all the aforementioned Asian and Western studies, we could draw the conclusion that malnutrition affects TAVI outcomes on a global scale (although we are yet to see studies from Latin America, Africa, India, and other regions of the world).
We should also consider the impact of other possible factors at play in the context of the study by Ishizu et al. 2 For instance, the authors described statistically significant differences in the baseline characteristics regarding the presence of left ventricular outflow tract calcification, with higher prevalence in the group with moderate/severe malnutrition regardless of nutritional index applied. It has been demonstrated that the presence of significant left ventricular outflow tract calcification increases the risk of periprocedural and 1‐year mortality, stroke, myocardial infarction, paravalvular leak, and aortic annulus/root rupture after TAVI, 11 and this may have affected their results. Moreover, there is no mention of how many patients had bicuspid valves, which have shown different outcomes when compared with their tricuspid counterparts 12 or when treated with balloon or self‐expandable transcatheter heart valves. 13 Confounding factors should always be kept in mind when analyzing TAVI outcomes.
An interesting observation in the study by Ishizu 2 was that malnutrition was highly prevalent in patients with a body mass index >22 kg/m2 using the CONUT and GNRI criteria (65.5% and 61.2%, respectively). We tend to associate malnutrition only with low weight, however, high prevalence of malnutrition in individuals with normal weight or patients who are overweight/obese was also described, which underscores the importance of not excluding the diagnosis of malnutrition simply because an individual is not underweight.
An aspect not to be forgotten in the elderly population is the interface between frailty and malnutrition. These are different geriatric syndromes but inextricably intertwined. It did not go unnoticed that Ishizu et al 2 reported a higher prevalence of moderate/severe malnutrition regardless of index used in patients who are frail in comparison with patients who are not frail (statistically significant differences) as measured by the Clinical Frailty Scale. Looking closely at their Table 2 and the intersection between GNRI and Clinical Frailty Scale reported by Ishizu et al, 2 the overlap between frailty and moderate/severe malnutrition is evident. According to GNRI, 58% of all patients who were moderately/severely malnourished were found to be frail, and 70.6% of all patients who were frail were found to be malnourished. On the other hand, when we look at the other malnutrition assessments (CONUT score and Prognostic Nutritional Index), the same degree of overlap between frailty and moderate/severe malnutrition is not so obvious. According to the CONUT score, 64.5% of all malnourished patients were found to be frail but only 21.4% of all patients who were frail were found to be malnourished. According to the Prognostic Nutritional Index, 70.8% of all patients who were malnourished were found to be frail, but only 19.7% of all patients who were frail were found to be malnourished. The fact that we do not see a 100% overlap between frailty and moderate/severe malnutrition in this study may point to a multifaceted phenomenon whereby elderly individuals who are malnourished become frail and elderly individuals who are frail become malnourished. 14
It is highly likely that sarcopenia is the key element that ties frailty and malnutrition (Figure 1) in both epidemiological and pathophysiological realms. 15 Elderly patients with malnutrition are more prone to frailty at the beginning and exhibit progressive frailty in subsequent years, while patients who follow a high‐protein diet are less likely to exhibit muscle mass depletion and strength loss over time. 16 Unfortunately, this was an issue not well explored in the study by Ishizu et al 2 and has been the subject of investigation 17 including sarcopenia and presarcopenia.
Figure 1. The continuum of malnutrition, frailty, and sarcopenia and associated factors in elderly patients.
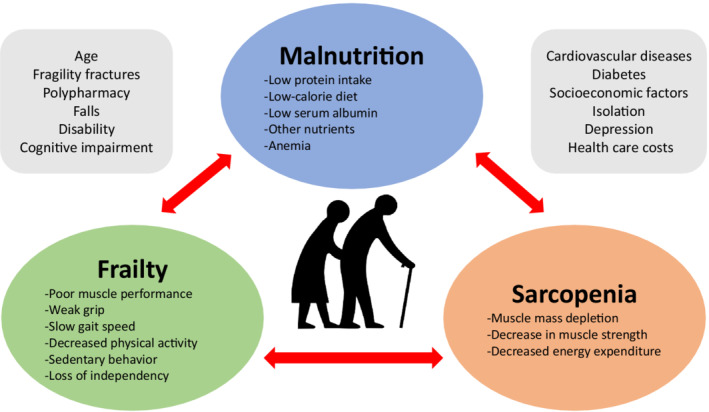
The FRAILTY‐AVR (Frailty in Aortic Valve Replacement) studies highlighted the interplay between frailty, malnutrition, sarcopenia, and TAVI outcomes. 1 , 17 , 18 These prospective multicenter cohort investigations were conducted in 14 centers including patients from both sides of the pond (Canada, the United States, and France), showing that malnutrition is associated with higher 1‐year mortality and 30‐day adverse events after TAVI (and after surgical aortic valve replacement as well) with the combination of malnutrition and severe frailty being associated with the highest mortality rate. 1
The FRAILTY‐AVR investigators 17 also assessed the prognostic value of sarcopenia in elderly patients undergoing TAVI, defining it as the concomitant presence of (A) low muscle mass and strength (defined as low psoas muscle area by computed tomographic imaging) and (B) low chair‐rise performance (defined as 5 sit‐to‐stands without using the arms in ≥15 seconds or an inability to complete the test). After adjustment for individual covariates, sarcopenia (found in 21% of the participants) was associated with 1‐year mortality, worsening disability, and discharge to a skilled‐care facility. Interestingly, 63% of the individuals were found to have presarcopenia (defined as the presence of one but not the other—either A or B, but not A and B), and this was also independently associated with 1‐year mortality and discharge to a skilled‐care facility.
In the era of increasing interest in sex‐specific determinants of outcomes (with women accounting for a large proportion of patients undergoing TAVI, and two‐thirds of the population studied by Ishizu et al 2 were women), it is noteworthy that the FRAILTY‐AVR investigators carried out a study 18 that showed that women were more physically frail before TAVI and more deconditioned after the procedure in comparison with their male counterparts, but frailty was associated with adverse outcomes in both women and men undergoing TAVI. These outcomes underline the significance of evaluating and countering frailty in women and men before TAVI and preparing adequately to meet the higher demands for rehabilitation of older women who are frail.
The FRAILTY‐AVR studies 1 , 17 , 18 showed that frailty assessment adds incremental value to well‐established risk models to predict mortality and progressive disability after TAVI, raising the question of whether pre‐ and postoperative nutritional and physical interventions should be recommended in malnourished elderly patients who are frail to improve postoperative outcomes. Nutritional supplementation with proteins has been recommended for elderly patients with malnutrition, 19 with quantifiable effects on outcomes. 20
It is clear that there are some components that can be intervened upon before or after TAVI to possibly optimize outcomes in this population; however, whether the nutritional and physical condition will be an actionable aspect to improve outcomes after TAVI remains an open question that might be answered by the PERFORM‐TAVR (Protein and Exercise to Reverse Frailty in Older Men and Women Undergoing Transcatheter Aortic Valve Replacement [NCT03522454]) trial. The investigators will screen consecutive patients ≥70 years of age before TAVI and enroll those who have evidence of physical frailty. The investigators will randomly allocate patients to receive interventions consisting of a home‐based exercise program with a protein‐rich oral nutritional supplement versus usual lifestyle counseling. We hope this randomized controlled trial will shed some light on this issue in the near future and bring a possible new target of intervention to improve TAVI outcomes in elderly patients (Figure 2).
Figure 2. Summary of key aspects in the scenario of frailty and malnutrition in elderly patients undergoing transcatheter aortic valve implantation.

FRAILTY‐AVR indicates Frailty in Aortic Valve Replacement; PERFORM‐TAVR, Protein and Exercise to Reverse Frailty in Older Men and Women Undergoing Transcatheter Aortic Valve Replacement; and TAVI, transcatheter aortic valve implantation.
A last word of caution: Even if trials (such as the PERFORM‐TAVR) show that improving the nutritional status of patients who will undergo TAVI would be recommended to achieve better outcomes, this may not always be possible in a short period of time in symptomatic patients. Questions will remain about how to manage elderly patients who are frail and malnourished with severe aortic valve stenosis who present for TAVI in terms of reasonable time frames. These patients, as shown in large randomized controlled trials, have demonstrated improved survival following TAVI. In other words, malnutrition and frailty are known risk factors before TAVI that should be addressed, but we should bear in mind that this may be appropriate only if it does not cause undue delay in TAVI as therapy.
Disclosures
Basel Ramlawi has received financial support from Medtronic, Corcym, and AtriCure. All other authors have reported that they have no relationships relevant to the contents of this editorial to disclose.
Acknowledgments
Michel Pompeu Sá receives support from The Thoracic Surgery Foundation (charitable arm of The Society of Thoracic Surgeons) through the TSF Every Heartbeat Matters Global Structural Heart Fellowship Award for the project “Structural Heart/Minimally Invasive Cardiac Surgery.”
See Article by Ishizu et al.
For Disclosures, see page 4.
REFERENCES
- 1. Goldfarb M, Lauck S, Webb JG, Asgar AW, Perrault LP, Piazza N, Martucci G, Lachapelle K, Noiseux N, Kim DH, et al. Malnutrition and mortality in frail and non‐frail older adults undergoing aortic valve replacement. Circulation. 2018;138:2202–2211. doi: 10.1161/CIRCULATIONAHA.118.033887 [DOI] [PubMed] [Google Scholar]
- 2. Ishizu K, Shirai S, Tashiro H, Kitano K, Tabata H, Nakamura M, Morofuji T, Murakami N, Morinaga T, Hayashi M, et al. Prevalence and prognostic significance of malnutrition in older, Japanese adults at high surgical risk undergoing transcatheter aortic valve implantation. J Am Heart Assoc. 2022. doi: 10.1161/JAHA.122.026294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shibata K, Yamamoto M, Kano S, Koyama Y, Shimura T, Kagase A, Yamada S, Kobayashi T, Tada N, Naganuma T, et al. Importance of geriatric nutritional risk index assessment in patients undergoing transcatheter aortic valve replacement. Am Heart J. 2018;202:68–75. doi: 10.1016/j.ahj.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 4. Lee K, Ahn JM, Kang DY, Ko E, Kwon O, Lee PH, Lee SW, Kim DH, Kim HJ, Kim JB, et al. Nutritional status and risk of all‐cause mortality in patients undergoing transcatheter aortic valve replacement assessment using the geriatric nutritional risk index and the controlling nutritional status score. Clin Res Cardiol. 2020;109:161–171. doi: 10.1007/s00392-019-01497-9 [DOI] [PubMed] [Google Scholar]
- 5. Sihag V, Li W, Hagar A, Peng Y, Feng Y, Bhushan S, Chen M. The impact of nutritional status on the outcome of transcatheter aortic valve implantation. Heart Surg Forum. 2022;25:E267–E272. doi: 10.1532/hsf.4547 [DOI] [PubMed] [Google Scholar]
- 6. Koseki K, Yoon SH, Kaewkes D, Koren O, Patel V, Kim I, Sharma R, Sekhon N, Chakravarty T, Nakamura M, et al. Impact of the geriatric nutritional risk index in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2021;157:71–78. doi: 10.1016/j.amjcard.2021.07.016 [DOI] [PubMed] [Google Scholar]
- 7. Emami S, Rudasill S, Bellamkonda N, Sanaiha Y, Cale M, Madrigal J, Christian‐Miller N, Benharash P. Impact of malnutrition on outcomes following transcatheter aortic valve implantation (from a National Cohort). Am J Cardiol. 2020;125:1096–1101. doi: 10.1016/j.amjcard.2019.12.038 [DOI] [PubMed] [Google Scholar]
- 8. González Ferreiro R, Muñoz‐García AJ, López Otero D, Avanzas P, Pascual I, Alonso‐Briales JH, González‐Juanatey JR, Pun F, Jiménez‐Navarro MF, Hernández‐García JM, et al. Nutritional risk index predicts survival in patients undergoing transcatheter aortic valve replacement. Int J Cardiol. 2019;276:66–71. doi: 10.1016/j.ijcard.2018.11.097 [DOI] [PubMed] [Google Scholar]
- 9. Eichler S, Salzwedel A, Harnath A, Butter C, Wegscheider K, Chiorean M, Völler H, Reibis R. Nutrition and mobility predict all‐cause mortality in patients 12 months after transcatheter aortic valve implantation. Clin Res Cardiol. 2018;107:304–311. doi: 10.1007/s00392-017-1183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seoudy H, Al‐Kassou B, Shamekhi J, Sugiura A, Frank J, Saad M, Bramlage P, Seoudy AK, Puehler T, Lutter G, et al. Frailty in patients undergoing transcatheter aortic valve replacement: prognostic value of the geriatric nutritional risk index. J Cachexia Sarcopenia Muscle. 2021;12:577–585. doi: 10.1002/jcsm.12689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sá MP, Van den Eynde J, Malin JH, Torregrossa G, Sicouri S, Ramlawi B. Impact of left ventricle outflow tract calcification on the outcomes of transcatheter aortic valve implantation: a study‐level meta‐analysis. J Card Surg. 2022;37:1379–1390. doi: 10.1111/jocs.16306 [DOI] [PubMed] [Google Scholar]
- 12. Sá MP, Van den Eynde J, Jacquemyn X, Tasoudis P, Erten O, Dokollari A, Torregrossa G, Sicouri S, Ramlawi B. Late outcomes of transcatheter aortic valve implantation in bicuspid versus tricuspid valves: meta‐analysis of reconstructed time‐to‐event data. Trends Cardiovasc Med. 2022. [epub ahead of print]. doi: 10.1016/j.tcm.2022.04.008 [DOI] [PubMed] [Google Scholar]
- 13. Sá MPBO, Simonato M, Van den Eynde J, Cavalcanti LRP, Alsagheir A, Tzani A, Fovino LN, Kampaktsis PN, Gallo M, Laforgia PL, et al. Balloon versus self‐expandable transcatheter aortic valve implantation for bicuspid aortic valve stenosis: a meta‐analysis of observational studies. Catheter Cardiovasc Interv. 2021;98:E746–E757. doi: 10.1002/ccd.29538 [DOI] [PubMed] [Google Scholar]
- 14. Verlaan S, Ligthart‐Melis GC, Wijers SLJ, Cederholm T, Maier AB, de van der Schueren MAE. High prevalence of physical frailty among community‐dwelling malnourished older adults‐a systematic review and meta‐analysis. J Am Med Dir Assoc. 2017;18:374–382. doi: 10.1016/j.jamda.2016.12.074 [DOI] [PubMed] [Google Scholar]
- 15. Afilalo J. Conceptual models of frailty: the sarcopenia phenotype. Can J Cardiol. 2016;32:1051–1055. doi: 10.1016/j.cjca.2016.05.017 [DOI] [PubMed] [Google Scholar]
- 16. Lorenzo‐López L, Maseda A, de Labra C, Regueiro‐Folgueira L, Rodríguez‐ Villamil JL, Millán‐Calenti JC. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. 2017;17:108. doi: 10.1186/s12877-017-0496-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mamane S, Mullie L, Lok Ok Choo W, Piazza N, Martucci G, Morais JA, Kim DH, Lauck S, Webb JG, Afilalo J, et al. Sarcopenia in older adults undergoing transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;74:3178–3180. doi: 10.1016/j.jacc.2019.10.030 [DOI] [PubMed] [Google Scholar]
- 18. Pighi M, Piazza N, Martucci G, Lachapelle K, Perrault LP, Asgar AW, Lauck S, Webb JG, Popma JJ, Kim DH, et al. Sex‐specific determinants of outcomes after transcatheter aortic valve replacement. Circ Cardiovasc Qual Outcomes. 2019;12:e005363. doi: 10.1161/CIRCOUTCOMES.118.005363 [DOI] [PubMed] [Google Scholar]
- 19. Mueller C, Compher C, Ellen DM; for the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors . A.S.P.E.N. clinical guidelines: nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35:16–24. doi: 10.1177/0148607110389335 [DOI] [PubMed] [Google Scholar]
- 20. Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009;CD003288. doi: 10.1002/14651858.CD003288.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]


