Abstract
Background
Transcarotid artery revascularization (TCAR) was approved by the Food and Drug Administration in 2015 for patients with carotid artery stenosis. However, no randomized trial to evaluate TCAR has been performed to date, and previous reports have important limitations. Accordingly, we measured stroke or death after TCAR compared with carotid endarterectomy (CEA) and transfemoral carotid artery stenting (TF‐CAS).
Methods and Results
We used the Vascular Quality Initiative registry to study patients who underwent TCAR, CEA, or TF‐CAS from September 2016 to June 2021. Our primary outcomes were perioperative and 1‐year stroke or death. We used logistic regression for risk adjustment for perioperative outcomes and Cox regression for risk adjustment for 1‐year outcomes. We used a 2‐stage residual inclusion instrumental variable (IV) method to adjust for selection bias and other unmeasured confounding. Our instrument was a center's preference to perform TCAR versus CEA or TF‐CAS. We performed a subgroup analysis stratified by presenting neurologic symptoms. We studied 21 234 patients who underwent TCAR, 82 737 who underwent CEA, and 14 595 who underwent TF‐CAS across 662 centers. The perioperative rate of stroke or death was 2.0% for TCAR, 1.7% for CEA, and 3.7% for TF‐CAS (P<0.001). Compared with TCAR, the IV‐adjusted odds ratio of perioperative stroke or death for CEA was 0.74 (95% CI, 0.55–0.99) and for TF‐CAS was 1.66 (95% CI, 0.99–2.79). Results were similar among both symptomatic and asymptomatic patients. The 1‐year rate of stroke or death was 6.4% for TCAR, 5.2% for CEA, and 9.7% for TF‐CAS (P<0.001). Compared with TCAR, the IV‐adjusted hazard ratio of 1 year stroke or death for CEA was 0.97 (95% CI, 0.80–1.17), and for TF‐CAS was 1.45 (95% CI, 1.04–2.02). IV analysis further demonstrated that symptomatic patients with carotid stenosis had the lowest 1‐year likelihood of stroke or death with TCAR (compared with TCAR, symptomatic IV‐adjusted hazard ratio for CEA: 1.30 [95% CI, 1.04–1.64], and TF‐CAS: 1.86 [95% CI, 1.27–2.71]).
Conclusions
Perioperative stroke or death was greater following TCAR when compared with CEA. However, at 1 year there was no statistically significant difference in stroke or death between the 2 procedures. TCAR performed favorably compared with TF‐CAS at both time points. Although CEA remains the gold standard procedure for patients with carotid stenosis, TCAR appears to be a safe alternative to CEA and TF‐CAS when used selectively and may be useful when treating symptomatic patients.
Keywords: carotid endarterectomy, carotid revascularization, carotid stenting, CEA, instrumental variable, TCAR, TF‐CAS
Subject Categories: Cardiovascular Surgery, Cerebrovascular Disease/Stroke, Cerebrovascular Procedures, Ischemic Stroke, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- BMT
best medical therapy
- CEA
carotid endarterectomy
- TCAR
transcarotid artery revascularization
- TF‐CAS
transfemoral carotid artery stenting
- VQI
Vascular Quality Initiative
Clinical Perspective.
What Is New?
This is the first large‐scale study comparing transcarotid artery revascularization (TCAR), carotid endarterectomy, and transfemoral carotid artery stenting using instrumental variable methodology.
Perioperative stroke or death was greater following TCAR when compared with carotid endarterectomy. At 1 year, there was no statistically significant difference in stroke or death between the 2 procedures. TCAR performed favorably when compared with transfemoral carotid artery stenting at both time points.
Symptomatic patients with carotid stenosis demonstrated the most favorable results at 1 year with TCAR.
What Are the Clinical Implications?
Although carotid endarterectomy remains the gold standard procedure for treating carotid stenosis, TCAR appears to be a safe alternative to carotid endarterectomy and transfemoral carotid artery stenting.
TCAR may be useful in treating symptomatic patients with carotid stenosis.
Carotid artery stenosis is a major risk factor for stroke, the fifth leading cause of death in the United States. 1 The mainstay for stroke‐risk reduction for patients with carotid stenosis is noninvasive best medical therapy (BMT), including risk factor identification and amelioration using lifestyle interventions and appropriate medications. 2 , 3 , 4 In addition to BMT, carotid endarterectomy (CEA) has been demonstrated repeatedly to incrementally reduce the risk of stroke among appropriately selected patients (Table 1). 5 , 6 , 7 , 8 , 9
Table 1.
Patient Subgroups That Have a Stroke‐Risk Reduction Benefit With CEA Over BMT Alone Based on Historical Randomized Clinical Trials. 5 , 6 , 7 , 8 , 9
| 1 | Symptomatic women with 70%–99% carotid stenosis undergoing surgery within 2–3 weeks of their same‐sided neurologic event with a 3–5‐year life expectancy |
| 2 | Symptomatic men with 70%–99% carotid stenosis undergoing surgery within 3 months of an ipsilateral neurologic event with a 3–5‐year life expectancy |
| 3 | Symptomatic men with 50%–69% carotid stenosis undergoing surgery within 2–3 wks of their same sided neurologic event with a 3–5‐year life expectancy |
| 4 | Asymptomatic men with 60%–99% stenosis aged 75–80 years with a 5‐year life expectancy who were free of any major life‐threatening condition |
BMT indicates best medical therapy; and CEA, carotid endarterectomy.
Two additional procedures have since been widely adopted in the treatment of carotid occlusive disease, under the assumption that they too will provide a stroke‐reduction benefit over BMT. In the 2000s the Food and Drug Administration approved transfemoral carotid artery stenting (TF‐CAS) to treat patients with carotid stenosis, despite mixed evidence surrounding its periprocedural risks compared with CEA. 10 , 11 , 12 , 13 , 14 More recently, in 2015 the Food and Drug Administration approved a third procedure to treat high‐risk patients, called transcarotid artery revascularization (TCAR). 15 , 16 This approval was granted in the absence of a dedicated randomized trial comparing it to BMT or other procedures. TCAR has since been rapidly adopted into practice and now accounts for ≈1 in 5 carotid procedures across the 247 US centers that offer it as of June 2020. 17 Moreover, in May 2022 the approved indications were broadened to include standard‐risk patients. 18
However, despite its rapid uptake, TCAR's rightful place in the treatment armamentarium of carotid stenosis remains unknown. 3 , 4 With no completed or enrolling randomized trial underway, the evaluation of TCAR currently rests exclusively on observational studies comparing TCAR with CEA and TF‐CAS. It should be noted that prior reports comparing TCAR to CEA and TF‐CAS have important methodologic limitations, and have not accounted for selection bias and other forms of unmeasured confounding. 17 , 19 , 20 , 21 As such, optimal procedure selection remains a focus of controversy, and the quality of evidence to guide the use of TCAR in clinical practice remains low. 3 , 4
Therefore, it was our objective to compare results after TCAR, CEA, and TF‐CAS accounting for selection bias and other forms of unmeasured confounding. To do this, we used an instrumental variable (IV) method for risk‐adjustment. IV techniques are the optimal methods available to account for unmeasured factors in situations where randomization is not available, such as with TCAR. 22 Our hypothesis was that TCAR is a viable procedural alternative to CEA or TF‐CAS in the treatment of carotid stenosis. Our results add important information to guide clinical decision making for patients being considered for TCAR, CEA, or TF‐CAS.
Methods
Human Subjects Protection
This study was approved by the Institutional Review Board at Dartmouth‐Hitchcock Medical Center. All data were deidentified before analysis, and therefore the need for consent was waived. Data are available upon application and peer‐review approval from the VQI (Vascular Quality Initiative; www.vqi.org).
Data Source
We used the VQI registry to study patients treated with TCAR, CEA, or TF‐CAS. The VQI is an international quality improvement registry for the Society for Vascular Surgery and includes more than 900 centers in the United States, Europe, and Canada (www.vqi.org). As part of TCAR's Food and Drug Administration approval process, the TCAR Surveillance Project was started, which requires that all patients who undergo the procedure be captured by the VQI registry on a prospective basis. Audits of device sales records indicate that more than 95% of TCAR procedures are included in the Surveillance Project, which began in September 2016. 23 Therefore, we analyzed data surrounding the 3 procedures from September 2016 (start of the TCAR Surveillance Project) until June 2021 (end of data availability).
Inclusion and Exclusion Criteria
All patients in the VQI registry who underwent TCAR, CEA, or TF‐CAS during the study interval were considered for inclusion. We excluded patients who underwent TCAR, CEA, or TF‐CAS for reasons other than atherosclerotic disease or neointimal hyperplasia (eg, for traumatic injury or arterial dissection). We excluded patients who underwent TCAR, CEA, or TF‐CAS combined with another procedure (eg, as an adjunct to an intracranial procedure or combined with coronary artery bypass grafting).
Primary Exposure and Assumptions
Our primary exposure was procedure type: TCAR, CEA, or TF‐CAS. We assumed that the associated risks and benefits of TCAR, CEA, or TF‐CAS, respectively, in addition to BMT, were discussed by the treating clinician with each patient, and the decision was then made to undertake a procedure. It was not our objective to imply anything about the indications for a specific procedure or the respective efficacy compared with BMT in reducing the risk of stroke. These questions will optimally be addressed by currently enrolling trials. 24 However, because TCAR is not included in these studies, observational research remains an important component of assessing TCARs safety profile.
Primary Outcomes and Definitions
Our primary outcome was a composite of any stroke or death. We determined outcomes in the perioperative (in‐hospital) period, and at 1 year. Secondary outcomes included any stroke alone (defined by the VQI registry as new clinical neurologic symptoms lasting more than 24 hours after the index procedure), any ipsilateral stroke (ipsilateral to the index carotid procedure), death alone (as assessed from the Social Security Death Index), cranial nerve injury (any clinically detected neurologic changes that were deemed related to the technical conduct of the procedure rather than an ischemic or hemorrhagic cerebral event), transient ischemic attack (any transient neurologic event resolving within 24 hours without evidence of stroke on imaging), myocardial infarction (any rise in cardiac biomarkers, clinical ischemic symptoms, new electrocardiographic changes, or new wall motion abnormalities), reperfusion syndrome (symptoms clinically attributed to increased cerebral flow at the discretion of the treating clinician), dysrhythmia (any postoperative change in cardiac rhythm requiring treatment with medications or cardioversion), acute heart failure (pulmonary edema requiring treatment or monitoring in an intensive care unit or step‐down unit), operative time (time from skin incision to procedure completion), reoperation or additional procedures to control bleeding (percutaneous or surgical procedures to control bleeding or hematoma evacuation that were caused by the index procedure), hospital length of stay more than 1 day, and technical failure of the intended procedure (the intended procedure was aborted and a different procedure may or may not have been performed).
Statistical Analysis
Patient characteristics and outcomes were calculated out of the known (nonmissing) values for each variable. We summarized continuous measures with means and SDs or medians with interquartile ranges as appropriate and compared them with Student's t test or the Wilcoxon rank‐sum test as appropriate. We report proportions as percentages and compared them with chi‐square analysis. We used Kaplan–Meier estimation for 1‐year outcomes.
We created a logistic regression model to estimate the adjusted odds ratio (OR) of stroke or death for TCAR versus CEA and TF‐CAS in the perioperative period. We then created a Cox‐proportional hazards model to estimate the adjusted hazard ratio (HR) of stroke or death for TCAR versus CEA and TF‐CAS over time. In both models, we included all variables in Table 2 in the regression, and we additionally adjusted for the association of the hospital center and of calendar time. TCAR served as the referent value for all point estimates. We performed a subanalysis of the primary outcomes stratified by the presence or absence of focal neurologic symptoms at the time of the procedure (ie, asymptomatic or symptomatic patients with carotid stenosis). Symptomatic status was defined as the presence of temporary or permeant focal neurologic symptoms upon evaluation by the attending proceduralist. We conducted sensitivity analyses including percent carotid stenosis as a covariate and adjusting for the association of the proceduralist as a random effect.
Table 2.
Patient Characteristics
| TCAR | CEA | TF‐CAS | TCAR versus CEA | TCAR versus TF‐CAS | |
|---|---|---|---|---|---|
| Variable | n=21 234 | n=82 737 | n=14 595 | P value | P value |
| Characteristic, % (unless otherwise noted) | |||||
| Age, y (SD) | 73.2 (9.0) | 70.7 (9.5) | 70.2 (9.6) | <0.001 | <0.001 |
| Female sex | 36.4 | 39.2 | 35.5 | <0.001 | 0.089 |
| Obesity (BMI, kg/m2 >30) | 33.4 | 34.6 | 35.7 | 0.001 | <0.001 |
| Race | |||||
| White | 90.4 | 89.4 | <0.001 | <0.001 | <0.001 |
| Black | 0.4 | 4.7 | <0.001 | <0.001 | 0.296 |
| Other Race* | 9.1 | 5.8 | <0.001 | <0.001 | <0.001 |
| Neurologic symptoms | 49.6 | 50.1 | 65.7 | 0.204 | <0.001 |
| CAD | 51.4 | 26.4 | 46.0 | <0.001 | <0.001 |
| CHF | 17.0 | 11.5 | 17.3 | <0.001 | 0.554 |
| Coronary revascularization | 39.9 | 34.1 | 37.1 | <0.001 | <0.001 |
| Hypertension | 90.9 | 89.6 | 89.1 | <0.001 | <0.001 |
| COPD | 25.6 | 23.1 | 26.9 | <0.001 | 0.011 |
| Home oxygen | 3.4 | 2.2 | 2.9 | 0.016 | |
| Diabetes | 38.4 | 36.7 | 39.5 | <0.001 | 0.050 |
| Chronic kidney disease (creatinine >1.7 mg/dL) | 6.2 | 5.4 | 6.2 | <0.001 | 0.997 |
| Smoking | |||||
| Never | 26.9 | 26.2 | 26.0 | 0.030 | 0.054 |
| Active | 22.1 | 25.0 | 27.4 | <0.001 | <0.001 |
| Prior | 50.9 | 48.8 | 46.4 | <0.001 | <0.001 |
| Prior ipsilateral carotid procedure | 14.4 | 1.8 | 20.0 | 20.0 | <0.001 |
| Prior contralateral carotid procedure | 14.2 | 13.5 | 13.2 | 0.005 | 0.010 |
| Preoperative medications | |||||
| Aspirin | 89.8 | 84.3 | 86.1 | <0.001 | <0.001 |
| P2y12 inhibitor | 87.6 | 37.4 | 77.4 | <0.001 | <0.001 |
| Dual antiplatelet | 80.5 | 31.6 | 70.8 | <0.001 | <0.001 |
| Statin | 89.7 | 85.4 | 83.5 | <0.001 | <0.001 |
| Beta blocker | 56.5 | 54.1 | 53.1 | <0.001 | <0.001 |
| Anticoagulation | 14.4 | 6.6 | 13.2 | <0.001 | 0.001 |
| Angiotensin‐converting enzyme inhibitor | 53.1 | 53.3 | 49.8 | 0.621 | <0.001 |
| Functional status | <0.001 | ||||
| Ambulatory | 94.7 | 98.6 | 94.9 | <0.001 | 0.579 |
| Wheelchair | 3.7 | 1.2 | 4.0 | <0.001 | 0.059 |
| Confined to bed | 0.1 | 0.1 | 0.2 | <0.028 | 0.098 |
| Insurance | |||||
| Medicare | 69.21 | 53.02 | 59.79 | <0.001 | <0.001 |
| Medicaid | 3.11 | 3.88 | 4.92 | <0.001 | <0.001 |
| Private | 26.54 | 35.86 | 33.45 | <0.001 | <0.001 |
| Non US, or none | 26.54 | 35.86 | 33.45 | <0.001 | <0.001 |
Variable definitions: age, age in years at the time of the index procedure; female, sex at birth; obesity, BMI >30 at the time of the index procedure; race, self‐reported where available, otherwise identified from the medical record; neurologic symptoms, see article text; CAD, history of coronary disease on medical record review; CHF, history of heart failure on medical record review; COPD, history of COPD on medical record review; coronary revascularization, any prior coronary bypass or percutaneous revascularization procedure; hypertension, based on medical record review or any blood pressure documented >130/80 mm Hg; smoking, patient reported where available, otherwise based on medical record review; prior carotid procedure, any history of a carotid procedure on medical record review; preoperative medications, medications being taken within 36 hours of the procedure; functional status, based on medical record review; insurance, based on medical record review.
BMI indicates body mass index; CAD, coronary artery disease; CEA, carotid endarterectomy; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; TCAR, transcarotid artery revascularization; and TF‐CAS, transfemoral carotid artery stenting.
This category includes: Asian, American Indian, Alaskan Native, Native Hawaiian or Pacific Islander, and More than One Race.
Instrumental Variable Analysis
To account for selection bias and other unmeasured confounding when modeling whether or not stroke or death occurs, we employed an IV procedure designed for nonlinear models known as 2‐stage residual inclusion. 25 The proposed IV analysis identifies patients who would have undergone TCAR at one institution, but CEA or TF‐CAS at another, in relation to the value of the instrument. 22 Under the assumptions of the model, the IV analysis accounts for unmeasured and unmeasurable confounding between the type of procedure and the outcome of stroke or death in patients who are eligible for both procedures. 22 , 26 , 27
We conducted 1 set of models for the perioperative results, and 1 for the 1‐year results. For the perioperative results, in the first stage of the method a linear regression model regresses procedure type on the instrument and all measured potential confounding variables. In the second stage of the method, a logistic regression model regresses the binary dependent variable indicator for stroke or death on procedure type and all potential measured confounders and the residuals from the first stage model. Under the IV assumptions, controlling for the residuals serves the purpose of approximately controlling for the net effect of any unmeasured confounders.
We used a similar technique for the 1‐year results. To account for the greater proportion of censored observations at 1 year of follow‐up, we used a recently developed 2‐stage residual inclusion method adapted for time‐to‐event outcomes analyzed using the Cox model. 28 , 29 , 30 , 31 The first stage is the same as for the perioperative outcome. In the second stage the independent variables are the procedure type, the observed covariates, and the residuals from the first stage, and the dependent variable is time to stroke or death, which could be observed or censored. In addition, the predictor side of the second stage equation includes a frailty term which accounts for the part of the residual from the first stage that is independent of the unmeasured confounders and whose inclusion helps the residual to control for unmeasured confounders. Therefore, the second stage of the procedure involves a Cox proportional hazards frailty model, not the standard Cox model. 28 , 29
Our proposed instrument was a center's preference to perform TCAR versus other procedures for carotid revascularization. 32 We calculated this preference as the proportion of TCAR out of the total procedures performed at a given center in the 6 months before the index procedure for each patient, similar to prior work by us and others. 28 , 29 , 30 , 31 , 32 The F‐statistic was strong for both of these instruments individually and overall (Figure S1). More details on the IV procedures are available in Data S1.
RESULTS
Patients
We studied 21 234 patients who underwent TCAR, 82 737 who underwent CEA, and 14 595 who underwent TF‐CAS across 662 centers (Table 2; Figure S2). Patients were ≈70 years of age (TCAR: mean 73.2±9.0 years, CEA: mean 70.7±9.5 years, TF‐CAS: mean 70.2±9.6 years, P<0.001); and one third were female (TCAR: 36.4%, CEA: 39.2%, TF‐CAS: 35.5%, P<0.001). Approximately half of patients presented with neurologic symptoms, but this was most common for TF‐CAS (TF‐CAS 65.7%, versus TCAR: 49.6%, CEA 50.1%, P<0.001). Patients who underwent TCAR or TF‐CAS were more likely to have had a prior ipsilateral carotid procedure (TCAR: 14.4%, TF‐CAS 20.0%, versus CEA 1.8%, P<0.001) and to be on dual antiplatelet therapy (TCAR: 80.5%, TF‐CAS 70.8%, versus CEA 31.6%, P<0.001).
The indication for TCAR and TF‐CAS was an anatomic high‐risk lesion in 44.6% and 41.6% of cases respectively (Table 2, see legend for high‐risk definitions). 33 Most carotid procedures were performed for severe (≥70%) carotid stenosis (TCAR: 83.2%, CEA: 77.7%, TF‐CAS: 83.2%, P<0.001). Most TCAR and CEA procedures were performed under general anesthesia (TCAR: 82.4%, CEA 93.2%, versus TF‐CAS 20.0%, P<0.001).
Stroke or Death: Perioperative
The perioperative rate of stroke or death was 2.0% for TCAR, 1.7% for CEA, and 3.7% for TF‐CAS (P<0.001; Table 3). Compared with TCAR, the adjusted OR of perioperative stroke or death was 0.82 (95% CI, 0.72–0.95) for CEA, and 1.41 (95% CI, 1.18–1.69) for TF‐CAS (Figure 1). After IV adjustment for unmeasured confounding and selection bias, the OR of stroke or death was 0.74 (95% CI, 0.55–0.99) for CEA, and 1.66 (95% CI, 0.99–2.79) for TF‐CAS (ANOVA simultaneous test of the 3 procedures P=0.022).
Table 3.
Procedural Characteristics and Perioperative Outcomes
| TCAR | CEA | TF‐CAS | TCAR versus CEA | TCAR versus TF‐CAS | |
|---|---|---|---|---|---|
| Variable | n=21 234 | n=82 737 | n=14 595 | P value | P value |
| Procedural characteristics, % | |||||
| High risk | |||||
| Anatomic | 44.6 | 3.9 | 41.6 | <0.001 | <0.001 |
| Medical | 54.1 | NA | 38.0 | <0.001 | |
| Refused for surgery | 21.0 | NA | 22.2 | 0.009 | |
| Degree of stenosis | |||||
| <50% | 3.2 | 3.9 | 4.0 | <0.001 | <0.001 |
| 50–69% | 12.0 | 14.5 | 11.0 | <0.001 | 0.004 |
| 70–79% | 32.3 | 34.3 | 28.7 | <0.001 | <0.001 |
| 80–99% | 49.5 | 42.1 | 49.9 | <0.001 | 0.535 |
| Occluded | 1.5 | 1.3 | 4.6 | 0.011 | <0.001 |
| Urgency | |||||
| Elective | 88.8 | 86.7 | 74.5 | <0.001 | <0.001 |
| Urgent | 11.0 | 12.7 | 20.7 | <0.001 | <0.001 |
| Emergent | 0.2 | 0.6 | 4.8 | <0.001 | <0.001 |
| American Society of Anesthesiologists class | |||||
| 1 | 0.6 | 0.5 | 1.5 | 0.042 | <0.001 |
| 2 | 3.2 | 3.5 | 15.0 | 0.015 | <0.001 |
| 3 | 68.0 | 74.2 | 58.4 | <0.001 | <0.001 |
| 4 | 27.5 | 21.6 | 17.1 | <0.001 | <0.001 |
| 5 | 0.0 | 0.0 | 0.3 | 0.638 | <0.001 |
| Anesthetic type | |||||
| General | 82.4 | 93.2 | 20.0 | <0.001 | <0.001 |
| Local/Regional | 17.5 | 6.8 | 79.8 | <0.001 | <0.001 |
| Procedural anticoagulation | 98.9 | 99.1 | 96.1 | <0.001 | <0.001 |
| Protamine | 83.5 | 73.0 | 13.4 | <0.001 | <0.001 |
| Balloon angioplasty after stenting | 41.0 | 0.0 | 63.7 | <0.001 | <0.001 |
| Perioperative outcomes, % (unless otherwise noted) | |||||
| Stroke/death | 2.0 | 1.7 | 3.7 | 0.007 | <0.001 |
| Stroke | 1.4 | 1.2 | 2.2 | 0.058 | <0.001 |
| Ipsilateral stroke | 1.2 | 0.9 | 1.8 | 0.001 | <0.001 |
| TIA | 0.6 | 0.5 | 0.8 | 0.100 | 0.003 |
| In hospital death | 0.4 | 0.3 | 1.1 | 0.005 | <0.001 |
| 30‐d death | 0.8 | 0.7 | 1.9 | 0.098 | <0.001 |
| MI | 0.5 | 0.7 | 0.5 | 0.086 | 0.543 |
| Reperfusion syndrome | 0.2 | 0.1 | 0.8 | 0.040 | <0.001 |
| Dysrhythmia | 1.4 | 1.3 | 1.6 | 0.083 | 0.178 |
| Acute heart failure | 0.3 | 0.3 | 0.4 | 0.791 | 0.177 |
| Cranial nerve injury | 0.2 | 2.5 | 0.0 | <0.001 | <0.001 |
| Operative time, median [interquartile range] | 66 [51–85] | 110 [86–139] | 61 [45–85] | <0.001 | <0.001 |
| Reoperation for bleeding | 0.9 | 1.6 | 0.4 | <0.001 | <0.001 |
| Length of stay >1 d | 29.2 | 31.1 | 35.5 | <0.001 | <0.001 |
| Technical failure | 0.5 | 0.0 | 0.6 | <0.001 | 0.017 |
High‐risk criteria include anatomic: a contralateral carotid artery occlusion, tandem stenoses >70%, “high” carotid lesion, restenosis after CEA, bilateral carotid stenosis requiring treatment, or a hostile neck; clinical: patient age>75 years, >2‐vessel coronary artery disease or unstable angina, New York Heart Association class III or IV heart failure, severe left ventricular dysfunction, recent MI, severe pulmonary disease, or creatinine >2.5 mg/dL6.
Urgency is defined as elective (planned or scheduled procedure), urgent (surgery within 24 hours of admission or the patient cannot be discharged until after surgery), and emergent (surgery within 6 hours of admission).
Stroke: Permanent focal neurologic symptoms detected clinically or evidence of stroke on imaging attributable to the procedure; TIA: transient focal neurologic symptoms detected clinically without imaging evidence of a stroke; MI: evidence of infarction on electrocardiogram or by enzyme assay; reperfusion syndrome: clinical changes attributable to increased cerebral blood flow; dysrhythmia: new rhythm disturbance requiring treatment with medications or cardioversion; acute heart failure: new pulmonary edema requiring treatment in intensive care unit or stepdown. CEA indicates carotid endarterectomy; MI, myocardial infarction; TCAR, transcarotid artery revascularization; and TF‐CAS, transfemoral carotid artery stenting.
Figure 1. Relative likelihood of stroke or death after TCAR, CEA, and TF‐CAS, perioperative, and at 1 year.
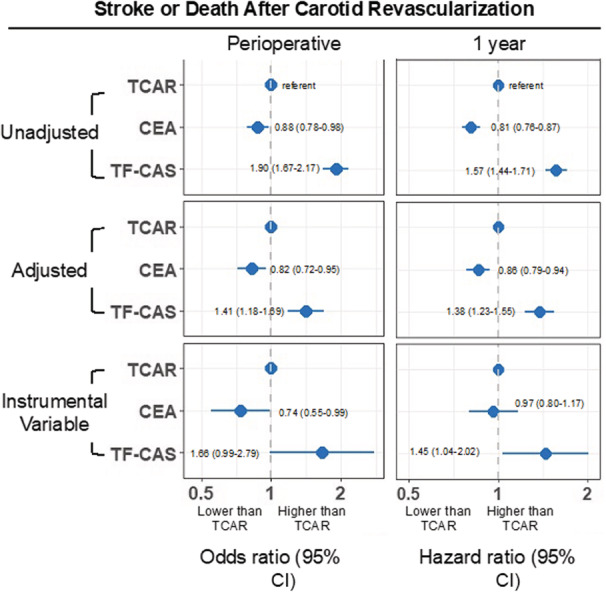
CEA indicates carotid endarterectomy; TCAR, transcarotid artery revascularization; and TF‐CAS, transfemoral carotid artery stenting.
Stroke or Death: 1‐Year
The 1‐year rate of stroke or death was 6.4% for TCAR, 5.2% for CEA, and 9.7% for TF‐CAS (log‐rank P<0.001; Figure 2). Compared with TCAR, the adjusted HR of 1‐year stroke or death was 0.86 (95% CI, 0.79–0.94) for CEA, and 1.38 (95% CI, 1.23–1.55) for TF‐CAS (Figure 1). After IV adjustment, the HR of stroke or death was 0.97 (95% CI, 0.80–1.17) for CEA, and 1.45 (95% CI, 1.04–2.02) for TF‐CAS.
Figure 2. Kaplan–Meier estimated rate of stroke or death for TCAR, CEA, and TF‐CAS.
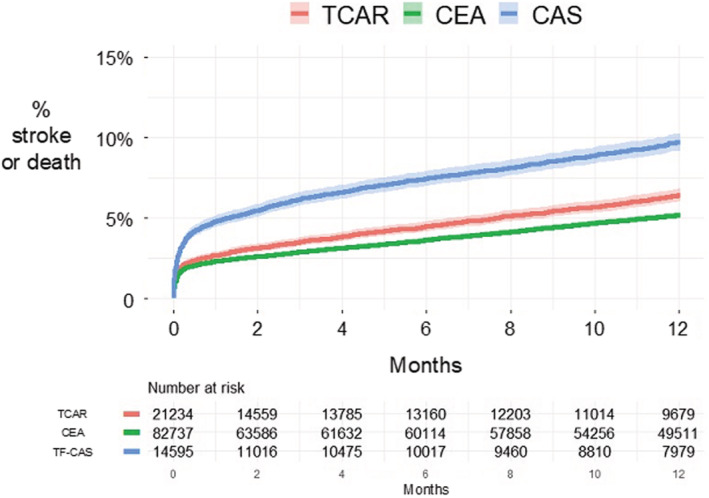
CAS indicates carotid artery stenting; CEA, carotid endarterectomy; TCAR, transcarotid artery revascularization; and TF‐CAS, transfemoral carotid artery stenting.
Stroke or Death Stratified by Presenting Symptoms: Perioperative and 1‐Year
Stroke or death was highest among patients presenting with focal neurologic symptoms. The perioperative rate of stroke or death for asymptomatic patients was 1.2% for TCAR, 1.1% for CEA, and 1.8% for TF‐CAS (P<0.001). For symptomatic patients, the perioperative rate of stroke or death was 2.7% for TCAR, 2.4% for CEA, and 4.6% for TF‐CAS (P<0.001).
Findings were similar at 1 year (Figure 3). Among asymptomatic patients, the 1‐year rate of stroke or death was 4.9% for TCAR, 3.8% for CEA, and 6.6% for TF‐CAS (log‐rank P<0.001). For symptomatic patients, the rate was 8.0% for TCAR, 6.5% for CEA, and 11.3% for TF‐CAS (log‐rank P<0.001).
Figure 3. Kaplan–Meier estimated rate of stroke after TCAR, CEA, and TF‐CAS, stratified by presenting neurologic symptom status.
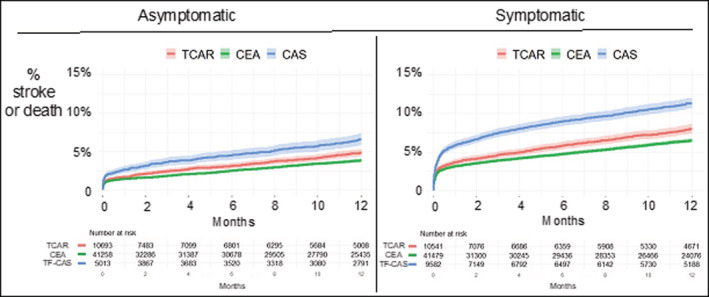
CAS indicates carotid artery stenting; CEA, carotid endarterectomy; TCAR, transcarotid artery revascularization; and TF‐CAS, transfemoral carotid artery stenting.
The adjusted and IV‐adjusted ORs and HRs of stroke or death were similar for most comparisons when stratified by presenting symptoms (Figure 4). However, in contrast to the Cox regression models, the IV‐adjusted models demonstrated that patients presenting with focal neurologic symptoms appeared to have the lowest 1‐year HR of stroke or death with TCAR. The traditional Cox regression models showed that compared with TCAR, the 1‐year HR of stroke or death among symptomatic patients who underwent CEA was 0.95 (95% CI, 0.85–1.06), and for those who underwent TF‐CAS was 1.43 (95% CI, 1.25–1.65). Conversely, when compared with TCAR, the IV‐adjusted HR among symptomatic patients who underwent CEA was 1.30, (95% CI, 1.04–1.64) and for those who underwent TF‐CAS was 1.86 (95% CI, 1.27–2.71).
Figure 4. Relative likelihood of stroke or death after TCAR, CEA, and TF‐CAS, perioperative, and at 1 year, stratified by presenting neurologic symptom status.
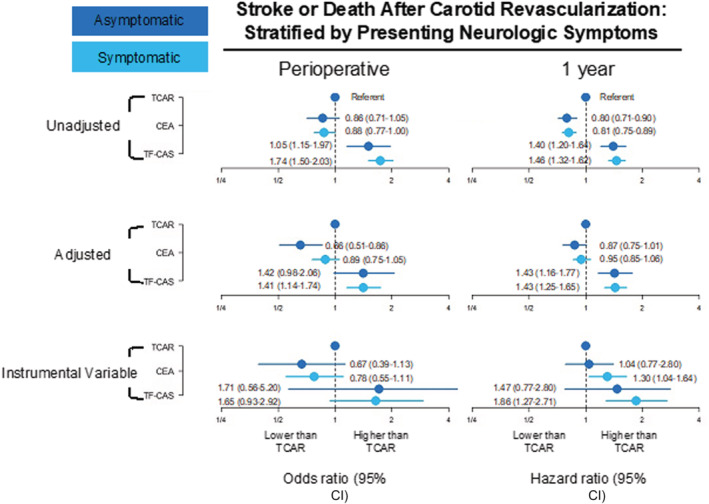
CEA indicates carotid endarterectomy; TCAR, transcarotid artery revascularization; and TF‐CAS, transfemoral carotid artery stenting.
Secondary Outcomes
The rate of perioperative myocardial infarction was low for all 3 procedures (TCAR: 0.5%, CEA: 0.7%, TF‐CAS: 0.5%, P=0.027; Table 3). Cranial nerve injury was highest for CEA (CEA: 2.5%, versus TCAR: 0.2%, TF‐CAS: 0%, P<0.001). Operative times were longest for CEA (CEA: median: 110 minutes, versus TCAR median: 66 minutes, TF‐CAS median: 61 minutes, P<0.001). The rate of technical failure was low (TCAR: 0.5%, CEA: 0%, TF‐CAS: 0.6%, <0.001).
Sensitivity Analyses
There were 3744 patients who were missing data about their percent stenosis category before surgery. Including this as a covariate in the model did not meaningfully change the point estimates of the primary analyses, with similar findings for including the proceduralist as a random effect and including both percent stenosis and the proceduralist. Results can be found in Table S1 and Table S2.
DISCUSSION
To our knowledge, this is the first large scale real‐world comparison of TCAR to CEA and TF‐CAS using IV methodology to account for selection bias and unmeasured confounding, which has been an important limitation of prior studies. We determined that the rate of perioperative stroke or death was greater following TCAR compared with CEA. However, after 1 year there was no statistically significant difference in stroke or death between the 2 procedures in the IV‐adjusted models. Moreover, TCAR and CEA demonstrated a decreased rate of stroke or death than TF‐CAS at both time points, consistent with existing evidence. 10 , 11 , 12 , 13 , 14 , 34 , 35 Approximately half of procedures were performed in symptomatic patients. Stratifying the cohort into asymptomatic and symptomatic subgroups revealed that symptomatic patients appeared to have the most favorable 1‐year result with TCAR. These findings indicate that although CEA remains the gold standard procedure for patients with carotid stenosis, TCAR appears to be a safe alternative to CEA and TF‐CAS and may be useful for symptomatic patients.
To date, there has been persistent controversy in the management of carotid artery stenosis. Historical trials of CEA versus BMT have demonstrated a stroke‐risk reduction benefit in select patient subgroups (Table 1). 5 , 6 , 7 , 8 , 9 However, advances in BMT have almost certainly led to a reduction in baseline stroke risk from carotid artery stenosis. 36 , 37 , 38 As such, which patients benefit from CEA over BMT alone remains a focus of ongoing debate. 24 , 36 , 38 , 39 , 40
Interestingly, in the backdrop of this ongoing controversy, the Food and Drug Administration approved TCAR in 2015 as an additional procedural option to treat patients with carotid artery stenosis. Somewhat surprisingly, approval was authorized without supporting evidence from a randomized clinical trial, under the stipulation that all procedures be entered into the VQI registry, which captures >95% of TCARs performed. Despite this lack of level 1 evidence, TCAR has been rapidly adopted in the United States, with ≈21 000 implants across nearly 500 centers. Therefore, it is imperative to compare the safety profile of TCAR to the more established CEA and TF‐CAS, recognizing that such comparisons provide no insights into TCARs effectiveness over BMT alone.
Prior investigators comparing TCAR to CEA and TF‐CAS have reported results, but with methodologic limitations. 19 , 20 Schermerhorn et al. compared TCAR to TF‐CAS in 3286 pairs of propensity‐matched patients, reporting a statistically significant lower risk of perioperative in‐hospital stroke or death with TCAR (reported relative risk, 0.51 [95% CI, 0.37–0.72]). 19 Malas et al. then compared TCAR to CEA in 6384 pairs of propensity‐matched patients, reporting no statistically significant difference in perioperative in‐hospital stroke or death (reported relative risk, 1.01 [95% CI, 0.77–1.33]). 20 Although they demonstrate favorable results for TCAR, these studies have important methodologic limitations. Propensity matching can be performed only on known factors contained in the data set. However, clinicians are privy to a myriad of individual patient characteristics that cannot be, or are not, accurately recorded with a registry variable. These include, for example, clinician selection effects on the intended procedure, proceduralist quality, characteristics of the center and postoperative care, varying severities of comorbidities that may wax and wane over the disease course, anatomic nuances pertinent to the revascularization procedure, and characteristics of the carotid lesion (eg, echolucency, thrombus) that may affect stroke risk. To date, these unmeasured factors have not been sufficiently accounted for in the published literature, and the grade of evidence surrounding TCAR remains low. 3 , 4
It was our objective to address the limitations of prior reports and improve our understanding of TCAR's safety profile compared with CEA and TF‐CAS. To do this we compared the 3 procedures using an IV method for risk adjustment. 41 IV techniques are the optimal way to account for unmeasured confounding when randomization is not available. 22 , 28 , 29 , 32 Using this methodology, we found that stroke or death after TCAR was higher than CEA in the perioperative period. However, after 1 year of follow‐up, there was no statistically significant difference between the 2 procedures. In addition, TCAR was superior to TF‐CAS at both time points. Interestingly, Although the unadjusted 1‐year rate of stroke or death was higher for TCAR than CEA among symptomatic patients, we found that TCAR had a lower 1‐year HR of stroke or death than CEA after IV adjustment. We believe the reason for this is that symptomatic patients may be particularly prone to confounding factors that are difficult to measure, including the presence of crescendo transient ischemic attacks, the severity and/or duration of the transient ischemic attack, and time from the neurologic event to the procedure, among a variety of others, although these findings require further validation. 30 , 42 , 43 , 44 These factors highlight the utility of using IV methods for situations such as TCAR, where a new procedure is rapidly adopted without randomized trial evidence. Not accounting for unmeasured confounding when studying TCAR may yield incorrect results. Therefore, investigators evaluating results after TCAR should consider both measured and unmeasured factors during risk adjustment.
Our findings highlight areas for future work. Although our results add to the growing body of literature defining the performance of TCAR versus CEA and TF‐CAS, there remains no comparison to BMT alone. Although we applied an IV method to adjust for unmeasured confounding and bias, there still remains no level 1 evidence to support the use of TCAR in contemporary practice. Until a randomized trial of TCAR determines its efficacy versus BMT and/or other procedures to treat carotid stenosis, clinical practice guidelines will be limited in its endorsement. 3 , 4 The fact that TCAR has risen to such rapid popularity despite a lack of level 1 evidence is a bit surprising, and factors related to this should be elucidated in future work with careful attention to patient safety. Furthermore, its rapid uptake highlights the importance of using procedural registries to monitor patient outcomes and inform clinical practice. To that end, we recommend that all patients with carotid stenosis who are treated with BMT alone, or who undergo TCAR, CEA, or TF‐CAS, be entered into a clinical registry for outcome assessment and quality assurance.
Our study has limitations. First, we are unable to comment on the details of neurologic symptoms that patients who were classified as symptomatic were experiencing, including the severity, number of events, and time frame before the procedure. To address this limitation, we incorporated an IV approach, which seeks to account for unmeasured or unmeasurable confounding, including these factors. However, this lack of detail limits the comparison of our findings to other published studies or historical randomized trials. Second, we are unable to comment on some details of the medical therapy among patients who underwent TCAR, CEA, or TF‐CAS, such as statin dose and blood pressure. However, we do know details on several important medications including antiplatelet therapy and beta blockers, among others, which we were able to characterize across the patient groups. Third, the IV analysis relies upon several assumptions, which we believe are met (Data S1). The IV analysis requires a larger sample size than traditional regression. This is the primary reason this study is being conducted now rather than early in TCARs development. Now that there are ≈20 000 patients who underwent TCAR, we believe that there is adequate power to provide meaningful information on the relative hazards of TCAR, CEA, and TF‐CAS using robust IV modeling techniques, improving upon the limitations of prior published reports using other methods. Finally, we cannot conclude that the unmeasured confounding accounted for in this analysis, which may have been related to treatment decisions, was appropriate with respect to selecting patients likely to benefit from a carotid artery procedure compared with current BMT. Reasons for treatment decisions regarding particular patients require ongoing evaluation at the point of care. Multispecialty teams, including academics, free of financial misincentives are a key requirement for optimizing clinical practice.
CONCLUSIONS
In this analysis using IV methodology, we found that TCAR had a greater rate of perioperative stroke or death than CEA. However, after 1 year there was no statistically significant difference in stroke or death between the 2 procedures. Moreover, TCAR and CEA demonstrated a lower stroke or death rate than TF‐CAS at both time points. Symptomatic patients had the most favorable 1‐year result with TCAR, which appears to provide a safe procedural alternative to CEA and TF‐CAS for this higher risk cohort. However, given the lack of randomized trial data comparing TCAR with CEA, TF‐CAS, or BMT, further work is needed to elucidate TCARs most appropriate role in the contemporary management of carotid occlusive disease.
Sources of Funding
This work was supported in part by the Hitchcock Foundation, and by the Patient‐Centered Outcomes Research Institute (ME‐1503‐28 261). All statements in this paper, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Hitchcock‐Foundation, the Patient‐Centered Outcomes Research Institute, its Board of Governors or Methodology Committee. The funders had no role in the design or execution of the study.
Disclosures
None.
Supporting information
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024964
For Sources of Funding and Disclosures, see page 11.
See Editorial by Abbott.
REFERENCES
- 1. Ahmad FB, Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325:1829–1830. doi: 10.1001/jama.2021.5469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, society of NeuroInterventional surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124:489–532. doi: 10.1161/CIR.0b013e31820d8d78 [DOI] [PubMed] [Google Scholar]
- 3. AbuRahma AF, Avgerinos ED, Chang RW, Darling RC III, Duncan AA, Forbes TL, Malas MB, Perler BA, Powell RJ, Rockman CB, et al. The Society for Vascular Surgery implementation document for management of extracranial cerebrovascular disease. J Vasc Surg. 2022;75:26S–98S. doi: 10.1016/j.jvs.2021.04.074 [DOI] [PubMed] [Google Scholar]
- 4. Bonati LH, Kakkos S, Berkefeld J, de Borst GJ, Bulbulia R, Halliday A, van Herzeele I, Koncar I, McCabe DJ, Lal A, et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur Stroke J. 2021;6:I–XLVII. doi: 10.1177/23969873211012121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study . Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 6. Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, Taylor DW, Haynes RB, Finan JW, Hachinski VC, et al. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30:1751–1758. doi: 10.1161/01.STR.30.9.1751 [DOI] [PubMed] [Google Scholar]
- 7. Halliday A, Harrison M, Hayter E, Kong X, Mansfield A, Marro J, Pan H, Peto R, Potter J, Rahimi K, et al. 10‐year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST‐1): a multicentre randomised trial. Lancet. 2010;376:1074–1084. doi: 10.1016/S0140-6736(10)61197-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hobson RW II, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, Wright CB. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328:221–227. doi: 10.1056/NEJM199301283280401 [DOI] [PubMed] [Google Scholar]
- 9. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European carotid surgery trial (ECST). Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- 10. Brott TG, Hobson RW II, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, et al. Stenting versus endarterectomy for treatment of carotid‐artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carotid Stenting Group , Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, et al. 30 day results from the SPACE trial of stent‐protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non‐inferiority trial. Lancet. 2006;368:1239–1247. [DOI] [PubMed] [Google Scholar]
- 12. International Carotid Stenting Study Investigators , Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, Lo TH, Gaines P, Dorman PJ, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (international carotid stenting study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–997. doi: 10.1016/S0140-6736(10)60239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, Larrue V, Lievre M, Leys D, Bonneville JF, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671. doi: 10.1056/NEJMoa061752 [DOI] [PubMed] [Google Scholar]
- 14. Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, et al. Protected carotid‐artery stenting versus endarterectomy in high‐risk patients. N Engl J Med. 2004;351:1493–1501. doi: 10.1056/NEJMoa040127 [DOI] [PubMed] [Google Scholar]
- 15. Kwolek CJ, Jaff MR, Leal JI, Hopkins LN, Shah RM, Hanover TM, Macdonald S, Cambria RP. Results of the ROADSTER multicenter trial of transcarotid stenting with dynamic flow reversal. J Vasc Surg. 2015;62:1227–1234. doi: 10.1016/j.jvs.2015.04.460 [DOI] [PubMed] [Google Scholar]
- 16. Malas MB, Leal J, Kashyap V, Cambria RP, Kwolek CJ, Criado E. Technical aspects of transcarotid artery revascularization using the ENROUTE transcarotid neuroprotection and stent system. J Vasc Surg. 2017;65:916–920. doi: 10.1016/j.jvs.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 17. Columbo JA, Martinez‐Camblor P, O'Malley AJ, Stone DH, Kashyap VS, Powell RJ, Schermerhorn ML, Malas M, Nolan BW, Goodney PP. Association of adoption of transcarotid artery revascularization with center‐level perioperative outcomes. JAMA Netw Open. 2021;4:e2037885. doi: 10.1001/jamanetworkopen.2020.37885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Food and Drug Administration . Premarket approval for Transcarotid Artery Revascularization (TCAR) in Standard‐Risk Patients. Accessed June 1st, 2022. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P140026S016.
- 19. Schermerhorn ML, Liang P, Eldrup‐Jorgensen J, Cronenwett JL, Nolan BW, Kashyap VS, Wang GJ, Motaganahalli RL, Malas MB. Association of transcarotid artery revascularization vs transfemoral carotid artery stenting with stroke or death among patients with carotid artery stenosis. JAMA. 2019;322:2313–2322. doi: 10.1001/jamanetworkopen.2020.37885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malas MB, Dakour‐Aridi H, Kashyap VS, Eldrup‐Jorgensen J, Wang GJ, Motaganahalli RL, Cronenwett JL, Schermerhorn ML. TransCarotid revascularization with dynamic flow reversal versus carotid endarterectomy in the vascular quality initiative surveillance project. Ann Surg. 2020;Publish Ahead of Print. doi: 10.1097/SLA.0000000000004496 [DOI] [PubMed] [Google Scholar]
- 21. Schermerhorn ML, Liang P, Dakour‐Aridi H, Kashyap VS, Wang GJ, Nolan BW, Cronenwett JL, Eldrup‐Jorgensen J, Malas MB. In‐hospital outcomes of transcarotid artery revascularization and carotid endarterectomy in the society for vascular surgery vascular quality initiative. J Vasc Surg. 2020;71:87–95. doi: 10.1016/j.jvs.2018.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rassen JA, Schneeweiss S, Glynn RJ, Mittleman MA, Brookhart MA. Instrumental variable analysis for estimation of treatment effects with dichotomous outcomes. Am J Epidemiol. 2009;169:273–284. doi: 10.1093/aje/kwn299 [DOI] [PubMed] [Google Scholar]
- 23. SilkRoad Medical . TCAR Surveillance Project . Accessed August 1st, 2021. Available at: https://silkroadmed.com/tcar‐surveillance‐project.
- 24. Mott M, Koroshetz W, Wright CB. CREST‐2: identifying the best method of stroke prevention for carotid artery stenosis: National Institute of Neurological Disorders and Stroke organizational update. Stroke. 2017;48:e130–e131. doi: 10.1161/STROKEAHA.117.016051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terza JV. Two‐stage residual inclusion estimation in health services research and health economics. Health Serv Res. 2018;53:1890–1899. doi: 10.1111/1475-6773.12714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cai B, Small DS, Have TR. Two‐stage instrumental variable methods for estimating the causal odds ratio: analysis of bias. Stat Med. 2011;30:1809–1824. doi: 10.1002/sim.4241 [DOI] [PubMed] [Google Scholar]
- 27. Terza JV, Basu A, Rathouz PJ. Two‐stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27:531–543. doi: 10.1016/j.jhealeco.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martinez‐Camblor P, Mackenzie T, Staiger DO, Goodney PP, O'Malley AJ. Adjusting for bias introduced by instrumental variable estimation in the Cox proportional hazards model. Biostatistics. 2019;20:80–96. doi: 10.1093/biostatistics/kxx062 [DOI] [PubMed] [Google Scholar]
- 29. Martínez‐Camblor P, MacKenzie TA, Staiger DO, Goodney PP, James O'MA. An instrumental variable procedure for estimating Cox models with non‐proportional hazards in the presence of unmeasured confounding. J Royal Stat Soc. 2019;68:985–1005. [Google Scholar]
- 30. Columbo JA, Martinez‐Camblor P, MacKenzie TA, Staiger DO, Kang R, Goodney PP, O'Malley AJ. Comparing long‐term mortality after carotid endarterectomy vs carotid stenting using a novel instrumental variable method for risk adjustment in observational time‐to‐event data. JAMA Netw Open. 2018;1:e181676. doi: 10.1001/jamanetworkopen.2018.1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramkumar N, Martinez‐Camblor P, Columbo JA, Osborne NH, Goodney PP, O'Malley AJ. Adverse events after atherectomy: analyzing long‐term outcomes of endovascular lower extremity revascularization techniques. J Am Heart Assoc. 2019;8:e012081. doi: 10.1161/JAHA.119.012081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brookhart MA, Schneeweiss S. Preference‐based instrumental variable methods for the estimation of treatment effects: assessing validity and interpreting results. Int J Biostat. 2007;3:Article 14. doi: 10.2202/1557-4679.1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kashyap VS, Schneider PA, Foteh M, Motaganahalli R, Shah R, Eckstein HH, Henao S, LaMuraglia G, Stoner MC, Melton J, et al. Early outcomes in the ROADSTER 2 study of transcarotid artery revascularization in patients with significant carotid artery disease. Stroke. 2020;51:2620–2629. doi: 10.1161/STROKEAHA.120.030550 [DOI] [PubMed] [Google Scholar]
- 34. Halliday A, Bulbulia R, Bonati LH, Chester J, Cradduck‐Bamford A, Peto R, Pan H, Group A‐C . Second Asymptomatic Carotid Surgery Trial (ACST‐2): a randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet. 2021;398:1065–1073. doi: 10.1016/S0140-6736(21)01910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, Wechsler L, Jaff MR, Gray W, Investigators AI. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med. 2016;374:1011–1020. doi: 10.1056/NEJMoa1515706 [DOI] [PubMed] [Google Scholar]
- 36. Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke. 2009;40:e573–e583. doi: 10.1161/STROKEAHA.109.556068 [DOI] [PubMed] [Google Scholar]
- 37. Howard DPJ, Gaziano L, Rothwell PM, Oxford VS. Risk of stroke in relation to degree of asymptomatic carotid stenosis: a population‐based cohort study, systematic review, and meta‐analysis. Lancet Neurol. 2021;20:193–202. doi: 10.1016/S1474-4422(20)30484-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang RW, Tucker LY, Rothenberg KA, Lancaster E, Faruqi RM, Kuang HC, Flint AC, Avins AL, Nguyen‐Huynh MN. Incidence of ischemic stroke in patients with asymptomatic severe carotid stenosis without surgical intervention. JAMA. 2022;327:1974–1982. doi: 10.1001/jama.2022.4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Columbo JA, Zwolak RM, Arous EJ, Goodney PP, Lilly MP, Welch HG. Variation in ultrasound diagnostic thresholds for carotid stenosis in the United States. Circulation. 2020;141:946–953. doi: 10.1161/CIRCULATIONAHA.119.043963 [DOI] [PubMed] [Google Scholar]
- 40. Columbo JA, Suckow BD, Griffin CL, Cronenwett JL, Goodney PP, Lukovits TG, Zwolak RM, Fillinger MF. Carotid endarterectomy should not be based on consensus statement duplex velocity criteria. J Vasc Surg. 2017;65:1029–1038 e1, 1029, 1038.e1. doi: 10.1016/j.jvs.2016.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Lemos JA, Nallamothu BK. The challenges of observational comparative effectiveness research. Circulation. 2020;141:237–239. doi: 10.1161/CIRCULATIONAHA.119.045178 [DOI] [PubMed] [Google Scholar]
- 42. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 43. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17:2265–2281. doi: [DOI] [PubMed] [Google Scholar]
- 44. Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297:278–285. doi: 10.1001/jama.297.3.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. King AH, Kumins NH, Foteh MI, Jim J, Apple JM, Kashyap VS. The learning curve of transcarotid artery revascularization. J Vasc Surg. 2019;70:516–521. doi: 10.1016/j.jvs.2018.10.115 [DOI] [PubMed] [Google Scholar]
- 46. Kashyap VS, King AH, Liang P, Eldrup‐Jorgensen J, Wang GJ, Malas MB, Nolan BW, Cronenwett JL, Schermerhorn ML. Learning curve for surgeons adopting Transcarotid artery revascularization based on the vascular quality initiative‐Transcarotid artery revascularization surveillance project. J Am Coll Surg. 2020;230:113–120. doi: 10.1016/j.jamcollsurg.2019.09.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


